Abstract
Background and Objectives
Left atrial (LA) fibrosis is a main determinant of LA remodeling and development of atrial fibrillation. However, non-invasive prediction of LA fibrosis is challenging. We investigated whether preoperative LA strain as measured by speckle tracking echocardiography could predict the degree of LA fibrosis and LA reverse remodeling after mitral valve (MV) surgery.
Subjects and Methods
Speckle tracking echocardiography and LA volume measurements were performed in 50 patients one day before MV surgery. LA tissues were obtained during the surgery, and the degrees of their interstitial fibroses were measured. LA volume measurements were repeated within 30 days after surgery (n=50) and 1-year later (n=39).
Results
Left atrial global strain was significantly correlated with the degree of LA fibrosis (r=-0.55, p<0.001), and its correlation was independent of age, underlying rhythm, presence of rheumatic heart disease and type of predominant MV disease (B=-1.37, 95% confidence interval -2.32 - -0.41, p=0.006). The degree of LA fibrosis was significantly correlated with early (r=-0.337, p=0.017) and 1-year (r=-0.477, p=0.002) percent LA volume reduction after MV surgery, but LA global strain was not significant.
Conclusion
Left atrial strain as measured by speckle tracking echocardiography might be helpful for predicting the degree of LA fibrosis in patients with MV disease.
Keywords: Echocardiography, Fibrosis, Left atrium
Introduction
Patients with severe mitral valve (MV) disease can develop alterations in left atrial (LA) geometry and function, a process known as LA remodeling. LA remodeling refers to a time-dependent adaptive regulation of cardiac myocytes to maintain homeostasis against external stressors such as valvular disease.1) LA remodeling is often accompanied by a change in LA function with a progressive increase in interstitial fibrosis. Increased LA size and change in LA function with fibrosis are associated with subsequent development of atrial fibrillation (AF), stroke, acute myocardial infarction, congestive heart failure, as well as cardiac and all-cause mortality.2-5) However, LA remodeling is reversible, and MV surgery can relieve LA pressure and volume overload, thereby reducing LA size and improving LA function; i.e., LA reverse remodeling.6),7) The direct impact of reversing LA remodeling on cardiovascular outcomes remains to be seen, but the evidence, at least indirectly, suggests that the risk of certain outcomes, such as AF and stroke, can be significantly reduced. If the factors associated with LA fibrosis and reverse remodeling can be determined, especially by non-invasive methods, they could be valuable for clinical evaluation, risk stratification, and earlier therapeutic intervention for patients with MV disease. This study was conducted to determine predictors for the degree of LA fibrosis as determined by histopathology and reverse remodeling after MV surgery using speckle tracking echocardiography.
Subjects and Methods
Patients and study protocol
From May 2007 to July 2008, 53 cases were enrolled. Exclusion criteria included previous MV replacement or repair, concomitant severe aortic valve disease, congenital heart disease, coronary artery disease, chronic liver disease, chronic renal disease, uncontrolled thyroid disease, poor echocardiographic window, and insufficient myocardial tissue. Among them, three cases were excluded due to poor echo image qualities (n=2) and insufficient tissue to be analyzed (n=1). Finally, 50 patients (mean age, 56±12 years) scheduled for MV surgery due to severe MV stenosis {mitral stenosis (MS), n=28} or regurgitation {mitral regurgitation (MR), n=22} with primary causes of rheumatic disease (n=32) and degenerative disease (n=18) were studied. All patients provided written informed consent, and the institutional review board at the University Health System approved this study. Atrial tissue samples were obtained from the LA free wall near the interatrial septum during MV surgery, were quickly frozen in liquid nitrogen, and were stored at -80℃ until use. Conventional transthoracic echocardiography and two-dimensional (2D) speckle tracking echocardiography were performed preoperatively on the patients. All the patients underwent repeated echocardiography including LA volume measurements within 30 days after surgery and LA volume measurements at 1.4±0.5 years after surgery were taken for 39 patients.
Conventional transthoracic echocardiography
Each patient underwent a complete standard transthoracic echocardiography study using a Vivid 7 apparatus (GE Medical Systems, Milwaukee, WI, USA) with a 2.5-MHz phased-array transducer. Left ventricular (LV) dimensions and LV ejection fraction were measured as recommended by the American Society of Echocardiography guidelines.8) According to these guidelines, LA maximal volume was measured using the prolate ellipsoidal method8),9) at the point of MV opening when the LA size was maximum and LA minimal volume was measured at the point of MV closure when LA size was minimum. LA reservoir fraction was calculated as (LA maximal volume-LA minimal volume)÷LA minimal volume.10) We estimated the degree of LA reverse remodeling as the percent change in LA maximal volume index (LAVImax) after MV surgery. In the case of LA volume increase after surgery, we used the negative value of percent LA volume reduction. All LA volume values were obtained as the mean of three consecutive beats and were adjusted for body surface area, due to the influence of body size on LA volume.
Speckle tracking echocardiography
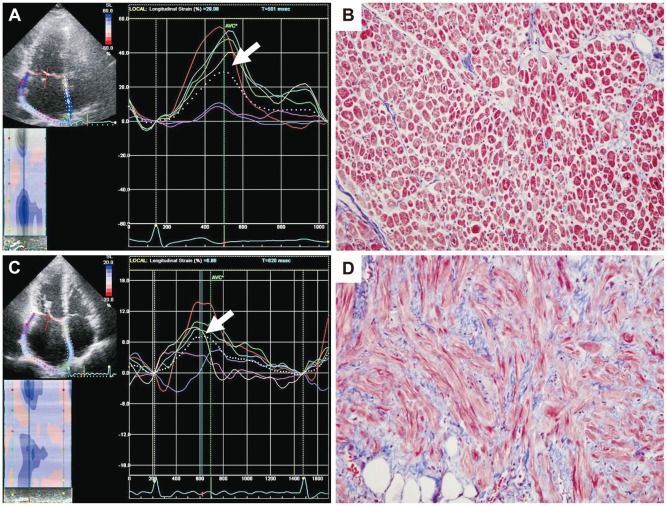
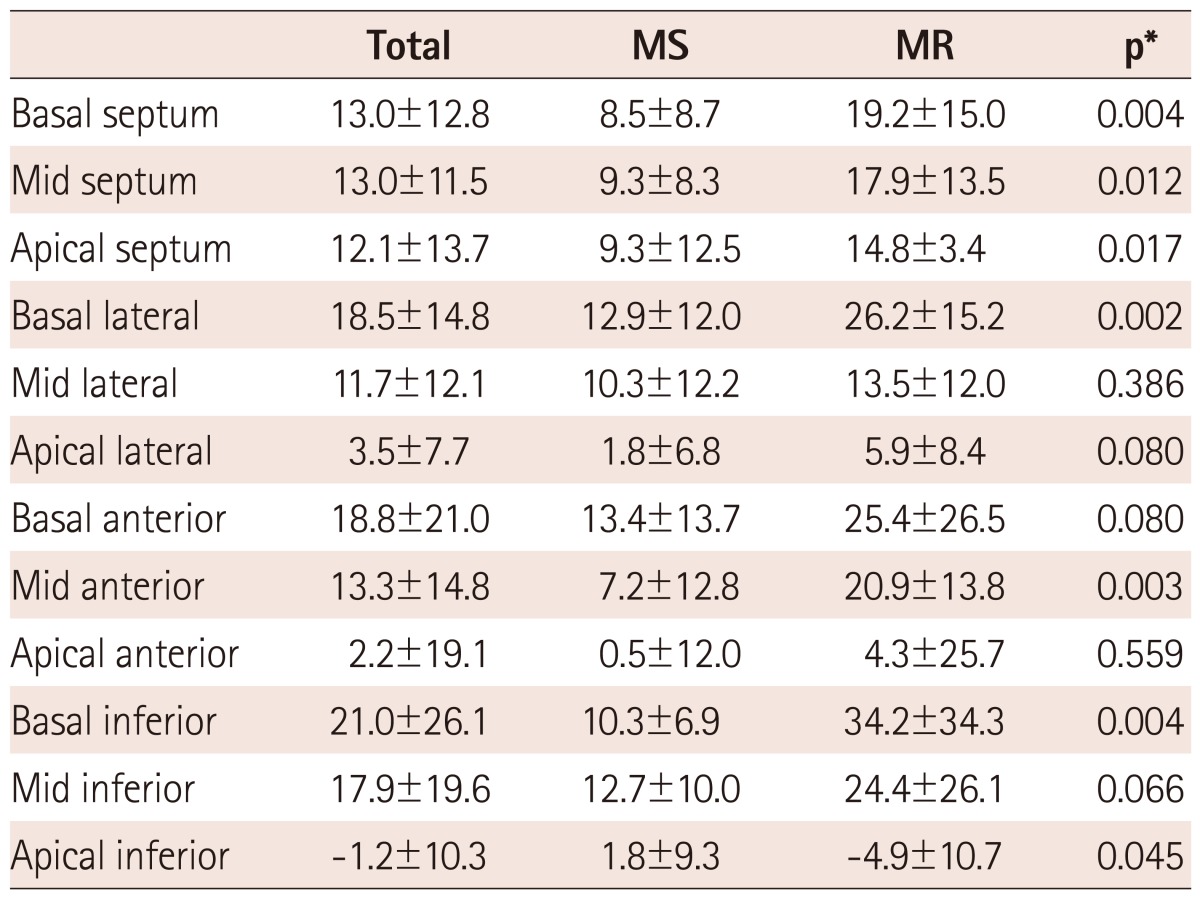
For the LV speckle tracking analysis, apical four- and two-chamber view images were obtained using conventional gray scale echocardiography (Vivid 7; GE Medical Systems). For the LA speckle tracking analysis, LA-focused images in apical four-chamber and two-chamber views were obtained. Two consecutive heart cycles were recorded and averaged, and five consecutive heart cycles were averaged for AF patients. A minimum frame rate of 40 frames per second was required for the reliable operation of this program. Recordings were processed using acoustic-tracking software (EchoPAC v8.0; GE Medical Systems), allowing offline, semi-automated analysis of speckle-based strain.11) For 2D speckle tracking strain analysis, a line was manually drawn along the LA endocardial border of the apical four- and two-chamber views after contraction, when the LA was at its minimum volume, using the point-and-click approach. The software then automatically generated additional lines near the atrial epicardium and mid-myocardial line, with the narrowest region of interest (ROI). The ROI then included the entire LA myocardial wall, and a click feature increased or decreased the widths between endocardial and epicardial line for thicker or thinner walls, respectively. The software generated strain curves for each atrial segment. A total of 12 segments were analyzed for each patient for whom the imaging results were of adequate quality. To trace the ROI in the discontinuity of the LA wall corresponding to the pulmonary veins and LA appendage, the directions of the LA endocardial and epicardial surfaces at the junction with these structures were extrapolated. The peak atrial longitudinal global strain was generated using the peaks of entire LA segments and the mean values obtained in the four- and two-chamber views (Fig. 1).
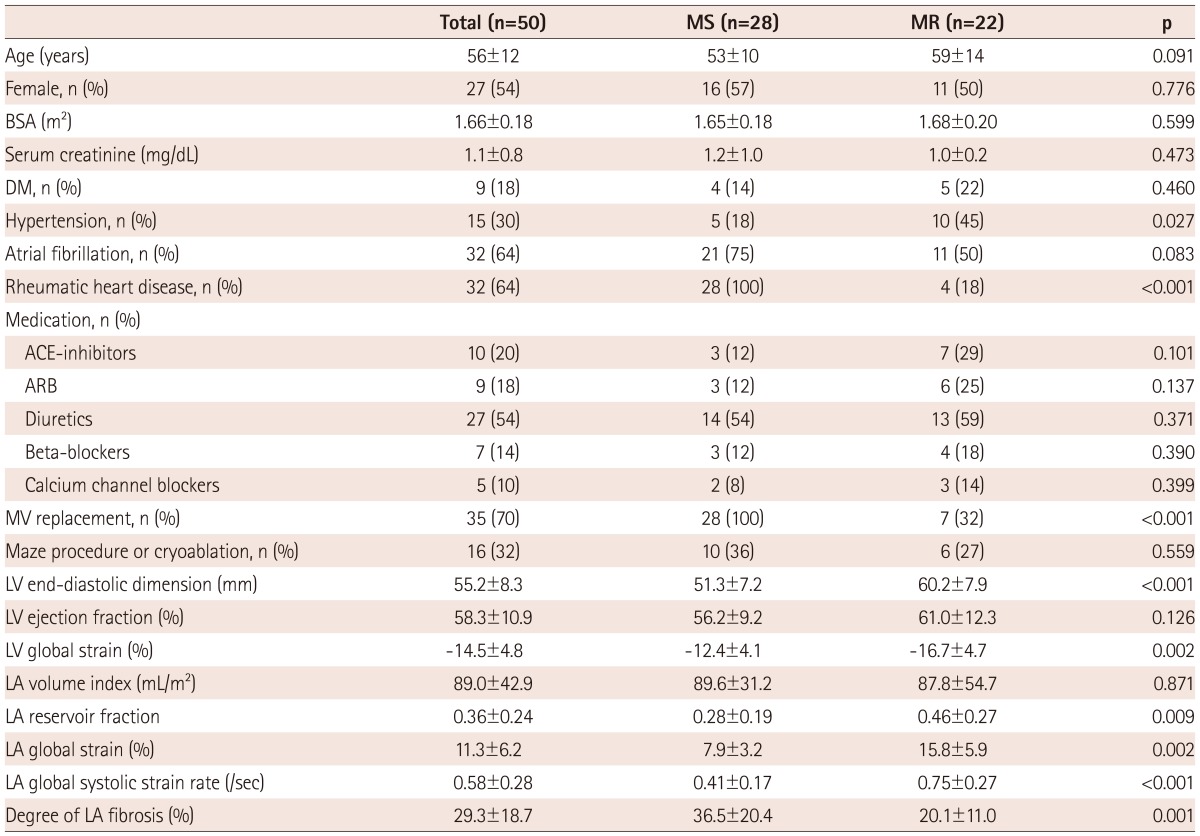
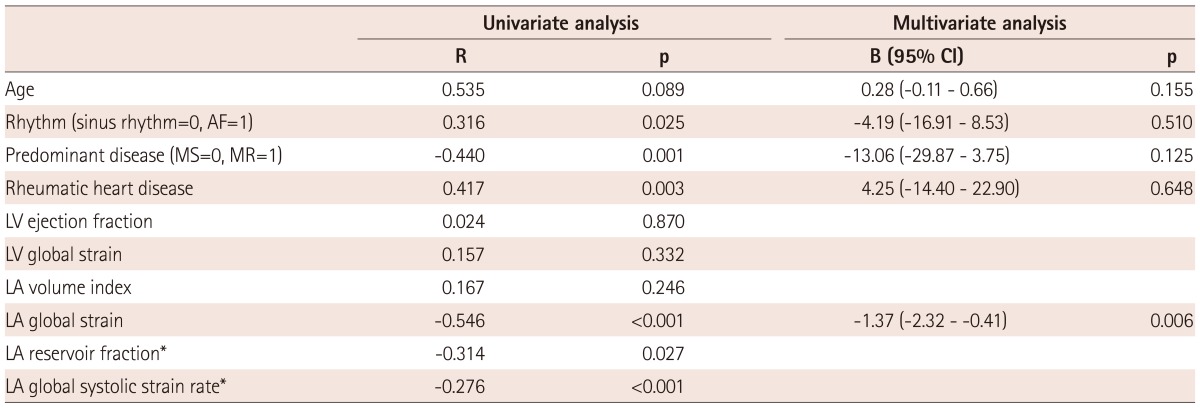
Fig. 1.
Left atrial (LA) strain as measured by speckle tracking echocardiography and its corresponding histopathology. A case with higher LA global strain (29%) (A) and a lower degree of LA fibrosis (9%) (B). A case with lower LA global strain (6.9%) (C) and a correspondingly high degree of LA fibrosis (40%) (D). The dotted line represents the LA global strain, and the arrow indicates the peak longitudinal strain value. On these histology slides, the purple color represents fibrosis after Masson Trichrome staining (×20).
Assessments of reproducibility
The reproducibility of 2D speckle tracking strain measurements were assessed using the data of 10 randomly selected patients. Two independent investigators analyzed patient data to obtain interobserver variability using Bland-Altman analysis and 95% limits of the equal-tailed Jeffrey's prior interval.12) The same investigators analyzed the same patient data to assess intra-observer variability.
Histology
To measure the fibrosis area, tissue was cleaned with phosphate buffered saline (Gibco BRL, Carlsbad, CA, USA) to remove the blood. Samples were fixed in 10% formalin solution (Sigma-Aldrich, St. Louis, MO, USA) for 24 hours at 4℃. Then, a paraffin block was made, and 5 µm-thick sections were prepared for Masson's trichrome staining. Slides were deparaffinized and hydrated with distilled water. Hydrated samples were mordant in Bouin's solution preheated to 58℃ for 15 minutes. After cooling at room temperature, the sections were washed in running water until the yellow color disappeared and then rinsed in two changes of distilled water. Samples were then stained with modified Weigert's iron hematoxylin for 5 minutes, washed briefly in running water, and rinsed in two changes of distilled water. Slides were placed in 0.5% hydrochloric acid in 70% alcohol for 5 seconds, washed in running tap water for 30 seconds, and rinsed in two changes of distilled water. Biebrich scarlet-acid fuchsin solution was used to treat the samples for 5 minutes. Slides were rinsed in three changes of distilled water and treated with phosphomolybdic-phosphotungstic acid solution for 5 minutes and aniline blue solution for 20 minutes. Finally, the samples were treated with acetic acid solution for 10 seconds, rinsed, dehydrated in alcohol, cleared in graded alcohols, and mounted with synthetic resin. The ratio of the purple fibrosis area to the total surface area of each section was used to assess the degree of LA fibrosis (%) as (fibrosis are-total area)×100.
Statistical analyses
Data were summarized as the mean±standard deviation for continuous variables and as frequency (percentage) for categorical variables. We compared demographic variables and medically related variables between the MS and MR groups using Student's t-test for continuous variables and Fisher's exact test for categorical variables. Simple linear regression analyses and correlation analyses were performed to examine the relationships between each predictor and LA reverse remodeling or LA fibrosis. Furthermore, a multiple linear regression was performed to determine important predictors of LA fibrosis and LA reverse remodeling. Clinical data variables such as age, rhythm, predominant disease, and operation method were included in the analysis. These variables were selected according to their clinical relevance and potential impact on LA function, as shown in earlier studies.2) The inclusion p was <0.05, and the exclusion p was >0.10. For estimating inter-observer variability and intra-observer variability, Pearson's correlation was calculated and Bland-Altman analysis was performed. All analyses were performed using Statistical Package for the Social Sciences (SPSS) ver. 13.0 (SPSS Inc., Chicago, IL, USA).
Results
Baseline characteristics
Baseline characteristics of the patients are summarized in Table 1. Of the 50 patients, 28 and 22 underwent MV surgery due to predominant MS and predominant MR, respectively. Thirty-two patients (64%) had AF, with a higher prevalence found in the MS group. Thirty-five patients (70%), mostly in the MS group, underwent MV replacement surgery and 15 patients underwent MV repair. Sixteen patients (32%) underwent the Maze procedure or cryoablation for AF during surgery.
Table 1.
Baseline clinical characteristics and echo-Doppler indices
Data are summarized as means±SD for continuous variables and as frequency (%) for categorical variables. MS: mitral stenosis, MR: mitral regurgitation, BSA: body surface area, DM: diabetes mellitus, ACE: angiotensin converting enzyme, ARB: angiotensin receptor blocker, MV: mitral valve, LV: left ventricular, LA: left atrial
Preoperative echocardiography and speckle tracking imaging
Data on conventional echo-Doppler and 2D strain measurements before MV surgery are presented in Table 1. The mean LV ejection fraction was 58.3±10.9% and the global longitudinal LV strain of MS patients was significantly lower than that of MR patients, despite similar LV ejection fractions. The mean LAVImax was 89.0±42.9 mL/m2, with no significant difference between the two groups. The mean LA global strain was 11.3±6.2%, with a higher value in MR patients than in MS patients (15.8±5.9% vs. 7.9±3.2%, p=0.002). The mean LA global systolic strain rate was 0.58±0.28 1/s. LA global strain was significantly correlated with LAVImax (r=-0.403, p=0.005), LV global strain (r=-0.681, p<0.001), LA reservoir fraction (r=0.422, p=0.003) and LA global systolic strain rate (r=0.690, p=0.001). The regional strain value was higher in the basal and mid segments than the apical segements. Lateral, anterior, and inferior segment strain values were higher than septal strain values. Like global strain values, regional strain values were higher in the MR group compared to the MS group (Table 2).
Table 2.
Segmental left atrial strain values
All the values are presented as %. *Comparison between MS and MR. MS: mitral stenosis, MR: mitral regurgitation
Degree of left atrial fibrosis and its correlates
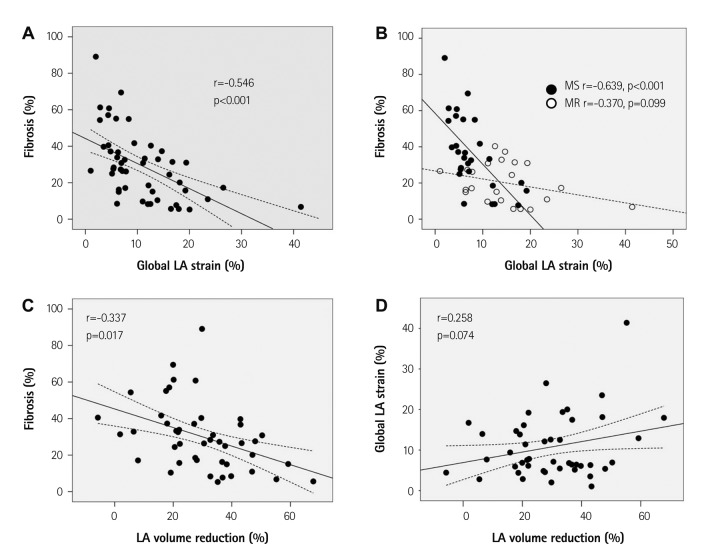
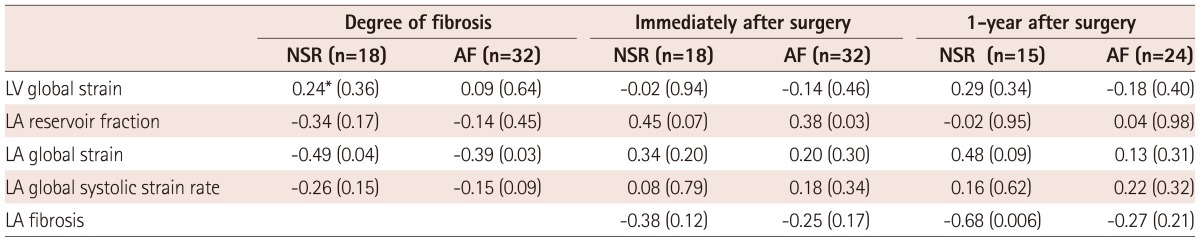
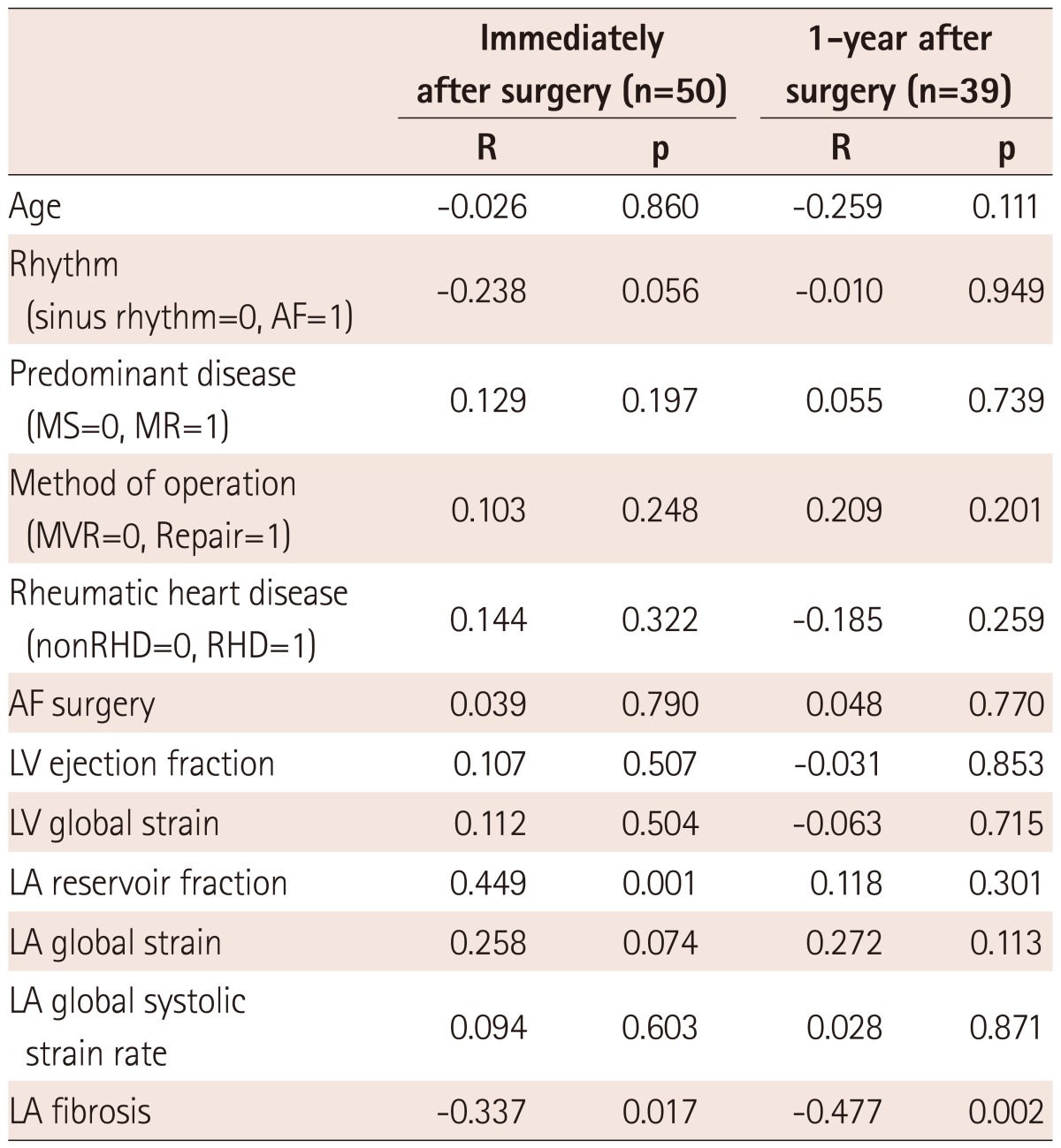
The mean degree of LA fibrosis was 29.3±18.7%, with higher degrees in the MS group than in the MR group (36.5±20.4% vs. 20.1±11.0%, p=0.001). Table 3 shows correlates of LA fibrosis. AF, MS rather than MR, rheumatic heart disease, LA reservoir fraction (r=-0.314, p=0.027), LA global strain (r=-0.546, p<0.001), and LA systolic global strain rate (r=-0.276, p<0.001) were significantly correlated with degree of LA fibrosis. But, LAVImax did not correlate with degree of fibrosis. Among 12 LA segments, mid-septal strain correlated best with the degree of fibrosis (r=-0.510, p<0.001). However, preoperative LAVImax and the LV global strain did not correlate with the degree of LA fibrosis. In multivariate analysis, the degree of LA fibrosis was significantly correlated with the LA global strain even after adjustment for age, underlying rhythm, rheumatic heart disease, and predominant disease. In subgroup analysis according to predominant disease, LA global strain was better correlated with degree of LA fibrosis in patients with predominant MS than in subjects as a whole (r=-0.639, p<0.001) (Fig. 2). However, LAVImax did not correlate with the degree of fibrosis. In MR-predominant patients, LA global strain was not significantly correlated with degree of fibrosis (r=-0.370, p=0.099). In subgroup AF and sinus rhythm subgroups, LA global strain correlated with degree of fibrosis but degree of correlation was not significantly different due to small sample size (Table 4).
Table 3.
Correlates for degree of LA fibrosis
*LA global systolic strain rate and LA reservoir fraction were not included in multivariable analysis due to significant colinearity with LA global strain. CI: confidence interval, AF: atrial fibrillation, MS: mitral stenosis, MR: mitral regurgitation, LV: left ventricular, LA: left atrial
Fig. 2.
Correlation between LA global strain and degree of LA fibrosis (A) and its subgroup analysis according to predominant mitral valve disease (B). Correlation of percent LA volume reduction after surgery with degree of LA fibrosis (C) and LA global strain (D). LA: left atrial, MS: mitral stenosis, MR: mitral regurgitation.
Table 4.
Determinants of LA fibrosis and postoperative LA volume reduction according to baseline rhythm
*Correlation coefficient (p). NSR: normal sinus rhythm, AF: atrial fibrillation, LV: left ventricular, LA: left atrial
Determinants of left atrial reverse remodeling after mitral valve surgery
Mean LAVImax decreased by 61.7±31.7 mL/m2 (immediate after surgery) and 50.7±20.7 mL/m2 (1-year follow-up; 55±12 years, 21 MS patients). The mean percent change in LAVImax was 29.4±14.8% and 36.6±16.6%, respectively. The degree of LA fibrosis was significantly correlated with percent LA volume reduction after MV surgery (r=-0.337, p=0.017 immediately after surgery; r=-0.477, p=0.002 1-year later). Table 4 and 5 shows the correlations between preoperative echocardiographic measurements and degree of LA volume reduction after MV surgery. LA reservoir fraction was related to with percent LA volume reduction immediately after MV surgery (r=0.449, p=0.001) but LA global strain failed to reach significance (r=0.258, p=0.074).
In multivariate analysis, after adjusting for underlying rhythm, the degree of LA fibrosis was significantly related to percent LA volume reduction after MV surgery (regression coefficient, B=-0.002, 95% CI -0.004-0.000, p=0.027). However, LA global strain (r=0.272, p=0.112) and reservoir fraction (r=0.118, p=0.301) did not significantly correlate with percent LA volume reduction at the 1-year follow-up.
Reproducibility of two-dimensional speckle tracking imaging
Interobserver variability coefficients of LA global strain and global strain rate were 95% CI±1.8; percent error 5.3% and 95% CI±2.4; percent error 8.2%, respectively using Bland-Altman analysis. In intraobserver variability assessment, coefficients of LA global strain and global strain rate were 95% CI±1.7; percent error 3.2% and 95% CI±1.6; percent error 6.8%, respectively. Bland-Altman analysis showed no evidence of any systematic difference regarding interobserver and intraobserver variability.
Discussion
The major finding of this study is that LA global strain measured by speckle tracking imaging was reproducible and was significantly correlated with the degree of LA fibrosis in patients with MV disease. The strain curve evaluated by speckle tracking imaging closely follows LA physiology. During the period of the LA reservoir, the LA strain increases, reaching its peak at the end of LA filling from the pulmonary venous inflow, just before the MV opening.13),14) During the conduit phase, the LA strain decreases, showing a plateau during diastasis, and achieves a negative peak at the end of LA contraction. LA global strain is a positive strain during ventricular systole, but not during atrial systole, so a positive global LA strain represents LA stretching during ventricular systole. In this study, we focused on peak positive global LA strain and systolic strain rate as an index of LA reservoir function because it is an important determinant of LA stiffness, preload of LA, and pulmonary venous pressure in MV disease.15-17) Even in patients with AF, which is frequently encountered in MV disease, these reservoir functional indices could be reliably measured. This finding suggests that LA global strain can be used as an index of LA compliance or reservoir function. We also confirmed the significant correlation of the LA global strain value with LA reservoir fraction derived from LA volume change.18) Considering the limitations of other methods for assessment of LA function,19) assessments of the LA strain and strain rate by speckle tracking imaging may represent a relatively rapid and easy-to-perform technique to explore LA function.20) This approach may be of potential clinical importance in a number of pathophysiologic conditions typically associated with abnormal LA function, such as MS or MR and cardiomyopathies.
LA compliance, like diastolic stiffness of LV, might be related to the degree of interstitial fibrosis, although the evident to date has been scant. Presently, LA expansion or peak lengthening strain during ventricular systole, represented by positive strain, was significantly correlated with the degree of LA interstitial fibrosis. The strength of this study is that we could confirm that the degree of LA fibrosis was significantly correlated with the preoperative LA global strain and early strain rate as representatives of reservoir function. The reservoir fraction from LA volume change was also correlated with degree of fibrosis, but was not superior to the global LA strain and took more time to measure. This finding is consistent with previous findings that LA strain rather, than reservoir fraction, better predicts AF recurrence after cardioversion.15) Unlike the LA strain, preoperative LAVImax did not correlate with LA fibrosis in our study, suggesting that, although LA volume has been used as a reliable index for prognostication as a representative of long-term LV diastolic function, it is insufficient to provide mechanistic information about the left atrium itself. The applicability of LA volume in various disease entities still has some limitations. Therefore, combining LA volume with function by strain might provide better information in terms of atrial-ventricular coupling. It is also interesting that, although LA strain is significantly correlated with degree of LA fibrosis independent of predominant disease, the degree of correlation was far better in the MS group.
Although we could confirm that degree of LA fibrosis is related to degree of LA volume reduction after MV surgery, we could not find significant correlation between LA strain and LA volume reduction in spite of positive tendency. Therefore factors other than LA fibrosis, such as LV diastolic function, LV remodeling, operation method or concomitant AF surgery, might also significantly affect reverse remodeling of LA.
Study limitations
In our study, some limitations existed. First, since this study enrolled a relatively small number of patients with heterogeneous characteristics, additional recruitment of pure MR patients would be helpful to confirm the relationship within pure MR group. Our results still cannot be generalized to other disease entities other than MS. The observation that fibrosis was significantly higher in the MS group, rather than the MR group, might reflect a ehigher rheumatic involvement in the MS group and higher angiotension converting enzyme inhibitor or angiotensin receptor blocker use in MR patients. Likewise, as we had limited long-term follow-up data, larger scaled long-term follow-up studies would be helpful to achieve the statistical power necessary to ascertain the predictive value of LA strain for LA reverse remodeling. Second, consideration should be given to the difficulty in obtaining an accurate ROI close enough to the effective shape of the left atrium and to the risk of contamination by signal components arising from structures surrounding the left atrium. Higher frame rate using zoom mode might have provided a better quality of speckle tracking. Also, because there is no software specifically developed for LA strain analysis, we used software for LV analysis to assess the LA strain and strain rate. Third, only longitudinal function variables were evaluated in this study. The LA radial strain could not be calculated from apical four- and two-chamber views because the atrial wall was too thin to track the acoustic kernels associated with radial movement of the thin LA wall. Fourth, the strain rate value was not included in the segmental analysis as regional strain rate curve was too noisy to analyze properly.
In conclusion, 2D speckle tracking strain echocardiography represents a promising and reproducible non-invasive technique for assessing LA reservoir function in patients with MV disease. Analyses of strain and strain rate of the left atrium might provide information regarding degree of LA fibrosis in patients with severe MV disease, especially in MS patients.
Table 5.
Determinants of postoperative percent LA volume reduction
AF: atrial fibrillation, MS: mitral stenosis, MR: mitral regurgitation, MVR: mitral valve replacement, RHD: rheumatic heart disease, LV: left ventricular, LA: left atrial
Acknowledgments
This work was supported by the Korean Society of Cardiology Research Fund of 2007-13.
Footnotes
The authors have no financial conflicts of interest.
References
- 1.Nattel S. Electrophysiologic remodeling: are ion channels static players or dynamic movers? J Cardiovasc Electrophysiol. 1999;10:1553–1556. doi: 10.1111/j.1540-8167.1999.tb00214.x. [DOI] [PubMed] [Google Scholar]
- 2.Abhayaratna WP, Seward JB, Appleton CP, et al. Left atrial size: physiologic determinants and clinical applications. J Am Coll Cardiol. 2006;47:2357–2363. doi: 10.1016/j.jacc.2006.02.048. [DOI] [PubMed] [Google Scholar]
- 3.Cho DK, Ha JW, Chang BC, et al. Factors determining early left atrial reverse remodeling after mitral valve surgery. Am J Cardiol. 2008;101:374–377. doi: 10.1016/j.amjcard.2007.09.076. [DOI] [PubMed] [Google Scholar]
- 4.Casaclang-Verzosa G, Gersh BJ, Tsang TS. Structural and functional remodeling of the left atrium: clinical and therapeutic implications for atrial fibrillation. J Am Coll Cardiol. 2008;51:1–11. doi: 10.1016/j.jacc.2007.09.026. [DOI] [PubMed] [Google Scholar]
- 5.Barnes ME, Miyasaka Y, Seward JB, et al. Left atrial volume in the prediction of first ischemic stroke in an elderly cohort without atrial fibrillation. Mayo Clin Proc. 2004;79:1008–1014. doi: 10.4065/79.8.1008. [DOI] [PubMed] [Google Scholar]
- 6.Sanders P, Morton JB, Kistler PM, Vohra JK, Kalman JM, Sparks PB. Reversal of atrial mechanical dysfunction after cardioversion of atrial fibrillation: implications for the mechanisms of tachycardia-mediated atrial cardiomyopathy. Circulation. 2003;108:1976–1984. doi: 10.1161/01.CIR.0000091408.45747.04. [DOI] [PubMed] [Google Scholar]
- 7.Trikas A, Papathanasiou S, Tousoulis D, et al. Left atrial function, cytokines and soluble apoptotic markers in mitral stenosis: effects of valvular replacement. Int J Cardiol. 2005;99:111–115. doi: 10.1016/j.ijcard.2004.01.018. [DOI] [PubMed] [Google Scholar]
- 8.Lang RM, Bierig M, Devereux RB, et al. Recommendations for chamber quantification: a report from the American Society of Echocardiography's Guidelines and Standards Committee and the Chamber Quantification Writing Group, developed in conjunction with the European Association of Echocardiography, a branch of the European Society of Cardiology. J Am Soc Echocardiogr. 2005;18:1440–1463. doi: 10.1016/j.echo.2005.10.005. [DOI] [PubMed] [Google Scholar]
- 9.Sanfilippo AJ, Abascal VM, Sheehan M, et al. Atrial enlargement as a consequence of atrial fibrillation: a prospective echocardiographic study. Circulation. 1990;82:792–797. doi: 10.1161/01.cir.82.3.792. [DOI] [PubMed] [Google Scholar]
- 10.Spencer KT, Mor-Avi V, Gorcsan J, 3rd, et al. Effects of aging on left atrial reservoir, conduit, and booster pump function: a multi-institution acoustic quantification study. Heart. 2001;85:272–277. doi: 10.1136/heart.85.3.272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Langeland S, D'hooge J, Wouters PF, et al. Experimental validation of a new ultrasound method for the simultaneous assessment of radial and longitudinal myocardial deformation independent of insonation angle. Circulation. 2005;112:2157–2162. doi: 10.1161/CIRCULATIONAHA.105.554006. [DOI] [PubMed] [Google Scholar]
- 12.Bland JM, Altman DG. Statistical methods for assessing agreement between two methods of clinical measurement. Lancet. 1986;1:307–310. [PubMed] [Google Scholar]
- 13.Cameli M, Caputo M, Mondillo S, et al. Feasibility and reference values of left atrial longitudinal strain imaging by two-dimensional speckle tracking. Cardiovasc Ultrasound. 2009;7:6. doi: 10.1186/1476-7120-7-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Donal E, Ollivier R, Veillard D, et al. Left atrial function assessed by trans-thoracic echocardiography in patients treated by ablation for a lone paroxysmal atrial fibrillation. Eur J Echocardiogr. 2010;11:845–852. doi: 10.1093/ejechocard/jeq074. [DOI] [PubMed] [Google Scholar]
- 15.Di Salvo G, Caso P, Lo Piccolo R, et al. Atrial myocardial deformation properties predict maintenance of sinus rhythm after external cardioversion of recent-onset lone atrial fibrillation: a color Doppler myocardial imaging and transthoracic and transesophageal echocardiographic study. Circulation. 2005;112:387–395. doi: 10.1161/CIRCULATIONAHA.104.463125. [DOI] [PubMed] [Google Scholar]
- 16.Eshoo S, Boyd AC, Ross DL, Marwick TH, Thomas L. Strain rate evaluation of phasic atrial function in hypertension. Heart. 2009;95:1184–1191. doi: 10.1136/hrt.2008.156208. [DOI] [PubMed] [Google Scholar]
- 17.Shin MS, Kim BR, Oh KJ, et al. Echocardiographic assessments of left atrial strain and volume in healthy patients and patients with mitral valvular heart disease by tissue Doppler imaging and 3-dimensional echocardiography. Korean Circ J. 2009;39:280–287. doi: 10.4070/kcj.2009.39.7.280. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Donal E, Raud-Raynier P, De Place C, et al. Resting echocardiographic assessments of left atrial function and filling pressure interest in the understanding of exercise capacity in patients with chronic congestive heart failure. J Am Soc Echocardiogr. 2008;21:703–710. doi: 10.1016/j.echo.2007.10.002. [DOI] [PubMed] [Google Scholar]
- 19.Leung DY, Boyd A, Ng AA, Chi C, Thomas L. Echocardiographic evaluation of left atrial size and function: current understanding, pathophysiologic correlates, and prognostic implications. Am Heart J. 2008;156:1056–1064. doi: 10.1016/j.ahj.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 20.Vianna-Pinton R, Moreno CA, Baxter CM, Lee KS, Tsang TS, Appleton CP. Two-dimensional speckle-tracking echocardiography of the left atrium: feasibility and regional contraction and relaxation differences in normal subjects. J Am Soc Echocardiogr. 2009;22:299–305. doi: 10.1016/j.echo.2008.12.017. [DOI] [PubMed] [Google Scholar]