Abstract
Objectives
To evaluate retrospectively, the possible difference in diffusion tensor imaging (DTI) metric of fractional anisotropy (FA) between good and poor surgical outcome cochlear implantation (CI) patients using investigator-independent voxel-wise analysis.
Methods
Eighteen patients (11 males, 7 females; mean age, 5.9 years) with profound sensorineural hearing loss underwent DTI scans using a 3.0 Tesla magnetic resonance scanner. Among the 18 patients, 10 patients with categories of auditory performance (CAP) score over 6 were classified into the good outcome group and 8 patients with CAP score below 6 were classified into the poor outcome group. The diffusion tensor scalar measure was calculated from the eigenvalues of the tensor on a voxel-by-voxel basis from each subject and two-sample t-test evaluation between good and poor outcome subjects were performed for each voxel of FA values, across the entire brain, with a voxel-wise intensity threshold of P<0.0005 (uncorrected) and a contiguous cluster size of 64 voxels. Individual values of FA were measured by using the region-of-interest based analysis for correlation analysis with CAP scores, open sentence and open word scores.
Results
Two-sample t-test evaluation using SPM voxel-wise analysis found significantly higher FA values at the several brain areas including Broca's area, genu of the corpus callosum, and auditory tract in good outcome subjects compared to poor outcome subjects. Correlation analyses between FA and CAP scores, open sentence and open word scores revealed strong correlations at medial geniculate nucleus, Broca's area, genu of the corpus callosum and auditory tract.
Conclusion
Investigator-independent voxel-based analysis of DTI image demonstrated that good outcome subjects showed better neural integrity at brain areas associated with language and auditory functions, suggesting that the conservation of microstructural integrity of these brain areas is important. Preoperative functional imaging may be helpful for CI.
Keywords: Diffusion tensor imaging, Cochlear implantation, Hearing loss, Surgical outcome
INTRODUCTION
Cochlear implantation (CI) has become a routine surgical procedure worldwide for the management of severe-to-profound sensorineural hearing loss (1). At the time of the medical evaluation for CI, a complete neuro-otologic and otolaryngologic examination is performed (2). Typically, evaluations include pure-tone audiometry and auditory brainstem response (ABR) testing in the case of children. Otoacoustic emission (OAE) testing complements these studies. For imaging evaluation, computer tomography (CT) scanning is performed to evaluate the status of the cochlea and to establish the presence of a non-ossified cochlea or to identify a common cavity, Mondini dysplasia, enlarged vestibular aqueduct, or an ossified cochlea. In some cases, a magnetic resonance imaging (MRI) scan is used instead of CT when questions exist regarding the presence of the eighth nerve or severe ossification. However, few studies have examined the utility of functional imaging in assessing the possible outcome of CI.
Whereas conventional structural MRI and CT are relatively insensitive to functional microstructure of the central nerve system (CNS), diffusion tensor imaging (DTI), which measures the orientation of water molecules in vivo, yields an index of microstructural integrity through quantification of the directionality of water diffusion (3). Therefore, DTI provides a new in vivo tool, not only for the assessment of CNS structural integrity, but also for assessment of disease conditions that disturb tissue structural coherence (4, 5). A previous study demonstrated that the diffusion anisotropy measured by DTI is highly sensitive to otherwise subtle changes in the integrity of auditory pathway in patients with sensorineural hearing loss (6).
In the current technical study, we evaluated, retrospectively, the possible difference in DTI metric of fractional anisotropy (FA) between good and poor surgical outcome CI patients using investigator-independent statistical parametric mapping (SPM) voxel-wise analysis. Furthermore, we investigated the relationships between the clinical outcomes and FA values of DTI.
MATERIALS AND METHODS
Participants
Eighteen patients (11 males, 7 females; mean age, 5.9 years) with profound sensorineural hearing loss participated in this study. All the patients were deaf prelingually and the causes of deafness were unknown. In temporal bone CT, there were no inner ear anomalies. MRI and DTI scans were performed before the surgeries. Among the 18 patients, 10 patients (mean age, 6.7±2.0 years) with categories of auditory performance (CAP) score over 6 were classified into the good outcome group and 8 patients (mean age, 5.06±0.8 years) with CAP score below 6 were classified into the poor outcome group. There was no difference in age between the groups (P>0.05). In addition to CAP, open-set sentence perception and open-set word perception test were measured, as well. In the good outcome group, the mean age at time of surgery was 40.6 months (range, 19 to 77 months). In the poor outcome group, the mean age at time of surgery was 25.1 months (range, 19 to 35 months). There was no significant difference in age at time of surgery between the groups (P>0.05). Patient demographics are summarized in Table 1. All legal guardians of participants agreed to participate in the functional MRI study and provided oral informed consent or assent where appropriate. The study protocol was approved by the local Institutional Review Board.
Table 1.
Patient demographic and clinical data
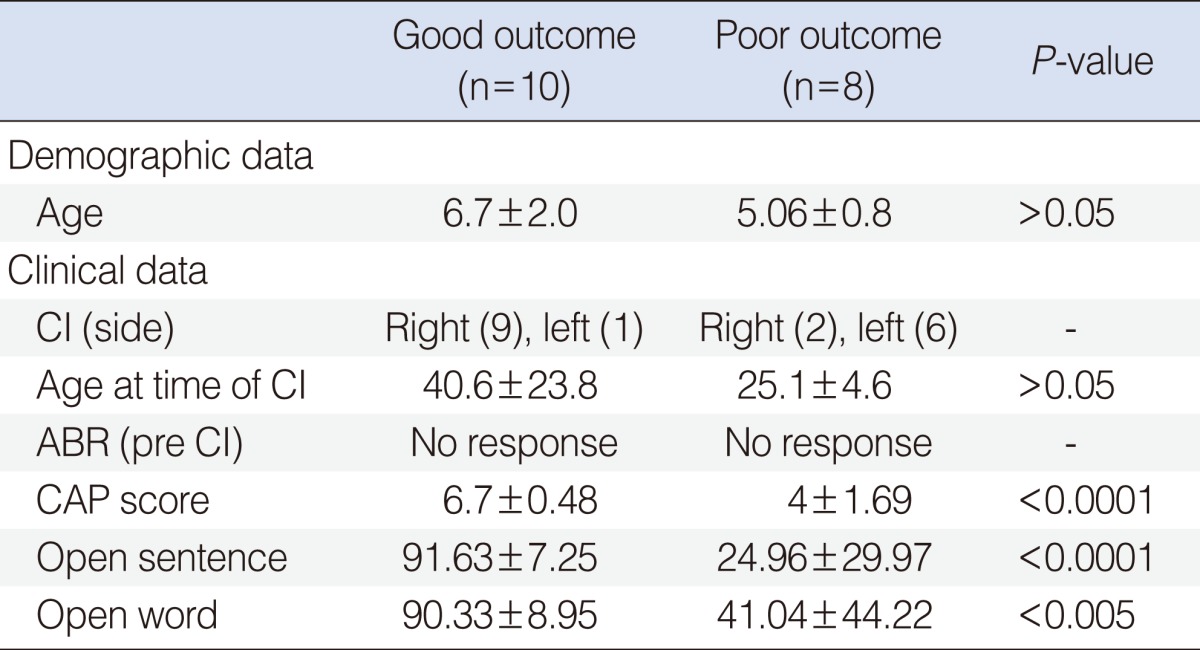
Values are presented as mean±SD.
CI, cochlear implantation; ABR, auditory brainstem response; CAP, categories of auditory performance.
MRI and DTI acquisition
MRI and DTI scans were performed using a Signa Exite HD 3.0 Tesla MR scanner (General Electric, Waukesha, WI, USA) with an eight-channel head coil. Three-dimensional (3D) fast spoiled gradient-echo (FSPGR) T1-weighted images were obtained with a 180° inversion recovery prepared pulse (repetition time 7.8 msec, echo time 3.0 msec, inversion time 450 msec, flip angle 20°, slice thickness 1.3 mm, partition number 120, no gap, 256×256 matrix size, number of average unity, and a 24 cm field-of-view). Total scan times were 4 minutes 42 seconds and 5 minutes 16 seconds. DTI was performed using the single-shot, spin-echo, echo-planar imaging technique employing Stejskal-Tanner diffusion-sensitizing pulses. DTI imaging parameters were 220×220 mm field-of-view, 128×128 matrix size, 12-16 axial slices, 4 mm slice thickness, repetition time of 8,000 msec, and echo time of 71 msec. Diffusion was measured in 25 non-collinear directions. For each slice and gradient direction, 2 images were acquired: one with no diffusion weighting (b, 0 second/mm2) and one with diffusion weighting (b, 1,000 seconds/mm2).
Voxel-wise analysis of DTI metrics
The diffusion tensor scalar measure (FA) was calculated from the eigenvalues of the tensor on a voxel-by-voxel basis from each subject using MedINRIA software (Asclepios Research Project, INRIA Sophia Antipolis, Cedex, France). SPM was performed using the software package SPM5 (Wellcome Department of Cognitive Neurology, London, UK) implemented in Matlab 7.01 (Mathworks, Sherborn, MA, USA). Diffusion tensor images in dicom format were converted into NIfTI format using dcm2nii, a tool included in mricron (http://www.mricro.com, mricron v7; Chris Rorden, Columbia, SC, USA). Template images of FA were created for spatial normalization. To be accurate, FA template images were resampled to an isotropic voxel size of 2×2×2 mm. SPM5 was used for subsequent bias correction, segmentation, spatial normalization, and co-registration of FA images with T1 anatomic images. A Gaussian kernel (8×8×8 mm) was convolved with the spatially normalized parametric images for smoothing, to accommodate inter-individual anatomic variability and to improve the signal-to-noise ratio prior to statistical analysis. To reduce partial volume errors by incorrect sampling, and 'bleeding' errors at the gray matter-white matter boundary, all voxels were thresholded with a voxel-wise FA value >0.25. Two-sample t-test evaluation between good and poor outcome subjects were performed for each voxel of FA values, across the entire brain, with a voxel-wise intensity threshold of P<0.0005 (uncorrected) and a contiguous cluster size of 64 voxels. The resulting statistical map was overlaid with T1 anatomic images.
Correlation and statistical analysis
Individual values of FA were measured by using the region-of-interest (ROI) based analysis for correlation analysis with CAP scores, open sentence, and open word scores. ROI was defined as a sphere shape with radius of 5 mm using the voxel of interest (VOI) function of SPM5 and applied to the medial geniculate nucleus, Broca's area, genu part of corpus callosum, and auditory tract near primary auditory cortex. We assessed the effects of FA by multiple regression analysis, using CAP scores, open sentence, and open word scores as dependent variables. We used Pearson correlation analyses to determine correlations among FAs and CAP score, open sentence, and open word scores. The correlations between CAP scores and open sentence and open word scores were also determined. SPSS ver. 14 (SPSS, Chicago, IL, USA) was used for all statistical analyses.
RESULTS
Voxel-wise analysis of DTI metrics
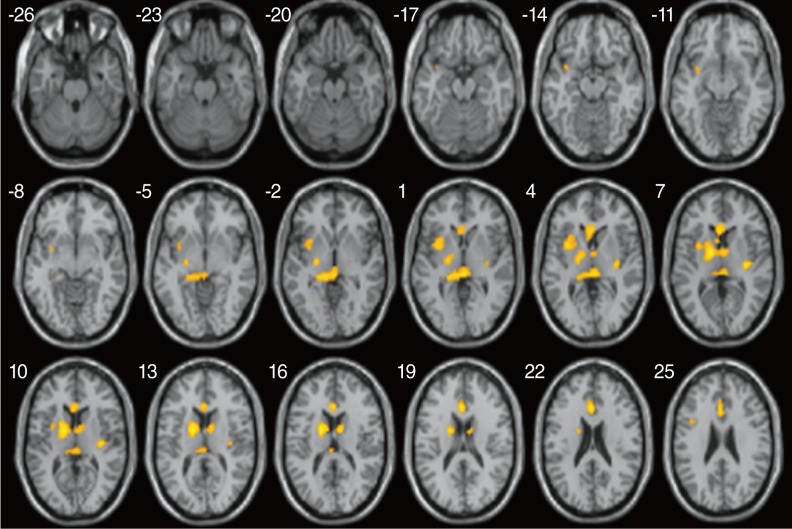
Two-sample t-test evaluation using SPM voxel-wise analysis found significantly higher FA values at the several brain areas including Broca's area, genu of the corpus callosum, and auditory tract (Fig. 1), in good outcome subjects compared to poor outcome subjects (P<0.0005). No brain areas with significantly higher FA values were found in poor outcome subjects compared with good outcome subjects.
Fig. 1.
Two sample statistical parametric mapping (SPM) axial maps rendered on to a normalized T1-weighted MR image, shows areas of significant decrease in fractional anisotropy (FA) values in poor outcome subjects compared to good outcome subjects (thresholded at uncorrected P<0.0005 and the extent of 16 clusters).
Correlation analysis
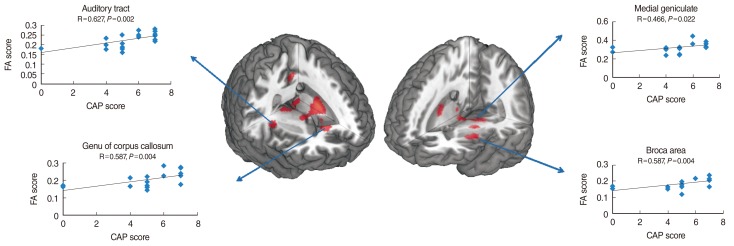
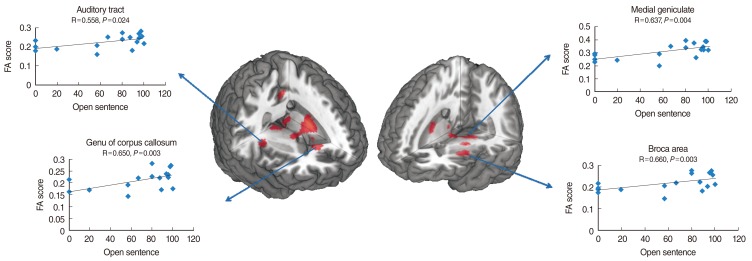
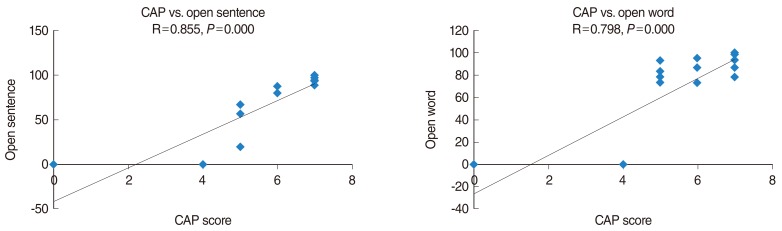
Fig. 2 depicts the correlations between FA and CAP scores at the medial geniculate nucleus, Broca's area, genu of the corpus callosum, and auditory tract. CAP scores showed statistically significant positive correlations with FA at medial geniculate nucleus (P=0.022), Broca's area (P=0.004), genu of the corpus callosum (P=0.004), and auditory tract (P=0.002). Fig. 3 displays the positive correlations between FA and open sentence scores at medial geniculate nucleus (P=0.004), Broca's area (P=0.003), genu of the corpus callosum (P=0.003), and auditory tract (P=0.024). Fig. 4 depicts the statistically significant correlations between CAP scores and open sentence and open word scores.
Fig. 2.
The correlations between fractional anisotropy (FA) values and categories of auditory performance (CAP) scores at the selected brain regions shown in Fig. 1. The lines are the result of a linear regression.
Fig. 3.
The correlations between fractional anisotropy (FA) values and open-set sentence perception scores at the selected brain regions shown in Fig. 1. The lines are the result of a linear regression.
Fig. 4.
The correlations between categories of auditory performance (CAP) scores and open-set sentence, open-set word perception scores. The lines are the result of a linear regression.
DISCUSSION
In this study, we undertook an investigator-independent voxel-based analysis of FA, a DTI metric, as a new in vivo imaging tool for estimating a surgical outcome of CI. To the best of our knowledge, this study is the first report to introduce voxel-based morphometric technique in a CI population using DTI. Most of the previous DTI studies explored damage to the white matter (WM) in patients with neurodegenerative or neuropsychiatric diseases, or sensorineural hearing loss using investigator-dependent ROI technique (6-8). Recently, voxel-based morphometric technique with DTI has been used to evaluate the difference in the microstructural integrity of the brain tissue (9, 10).
Voxel-based morphometry (VBM) is a new objective measure to characterize neuroanatomical differences in vivo using structural MR images (11). Using spatially normalized images, VBM compares different brains on a voxel-by-voxel basis in the entire brain, rather than a particular brain structure. Thus, the approach can examine the differences in brain gray and white matter in an unbiased and objective manner. Several recent studies have demonstrated the success of VBM in characterizing subtle changes in brain structure in a variety of diseases, including developmental disorders and in congenitally deaf adults (12, 13). In addition, VBM has also been useful in identifying gross structural abnormalities, such as those observed in Alzheimer's disease (14). In the current study, we extend the utility of VBM to the characterization of microstructural differences using FA images calculated from DTI.
The main finding of the current study was that compared to poor outcome subjects, good outcome subjects show higher FA values in areas of the brain associated with language and auditory function. Previous studies have shown that FA is a sensitive marker of the microstructural integrity of the brain (15). DTI studies found that FA was decreased in brain lesions attributable to various neuropathologic conditions, and a drop in FA may reflect edema, demyelination, or neuronal/axonal loss (16, 17). Furthermore, most previous studies used subjective ROI technique to evaluate microstructural integrity in terms of FA in brain lesions. In the current study, on the other hand, we used an investigator-independent voxel-based morphometric technique to evaluate the possible difference in microstructural integrity between poor and good outcome subjects in an unbiased and objective manner. Therefore, the relative decrease of FA in poor outcome subjects suggests that the functional integrity of brain areas associated with language and auditory functions is an important consideration factor for CI. That is, in addition to basic evaluations such as evoked response audiometry (ERA), functional imaging evaluation using DTI may play a role as a good subsidiary examination for CI. Although ERA has many advantages, it also has limitations. For example, once the structure along the auditory pathway is disrupted, more distal locations in the pathway are difficult to assess. Further, late response of ERA has a difficulty to locate the disrupted site along the pathway. The voxel-based DTI measurements, on the other hand, can assess the neuronal deficit regardless of the disrupted locations in the central auditory pathway.
Our data also shows that the conservation of language area is important. A previous study demonstrated that the formation of neural connections to the left temporal lobe speech centers is, in part, related to the language outcome of CI patients (18). These authors suggested that in a deaf patient, preoperative planning may take into consideration the possibility of some degree of altered cortical organization and, particularly in regard to language, customized preoperative functional imaging may be helpful. In line with this suggestion, the present study showed that subjects with good outcome have better neural integrity than subjects with poor outcome at the language area. Therefore, the voxel-based morphometric technique with DTI may be useful in evaluating the functional conservation of the language centers in addition to the conservation of the central auditory pathway.
This study has some limitations. First and most importantly, the DTI scans were performed after surgery. Since FA scores on the patients before surgery were not available, there was some possibility that neural integrity was recovered better in the good outcome group after surgery due to unknown confounding factors. That is, having only FA scores after surgery, it is not possible to exclude the unknown confounding factors. Second, the patient population was relatively small. Due to this limitation, this study is best-considered as a preliminary study. Further studies with larger numbers of subjects are necessary. Third, as a technical point, voxel-wise analysis was performed based on the template images provided by SPM5. However, the template images of SPM5 were obtained to fit to adult brain and there is the possibility for mismatch between adult and young brains, although both good outcome and poor outcome subjects were projected to the same SPM5 templates (19). Finally, although regression analysis showed a close correlation between FA values and clinical outcomes (CAP, open sentence, open word, and closed set) from the auditory and language brain areas, these outcomes are somewhat subjective measures. Therefore, correlation analysis with more objective measures may be necessary to confirm the current findings.
In conclusion, an investigator-independent voxel-based analysis of DTI imaging is proposed as a functional imaging tool for estimating a surgical outcome of CI. Compared to poor outcome subjects, good outcome subjects showed better neural integrity at brain areas associated with language and auditory functions, suggesting that the conservation of microstructural integrity of these brain areas is important. Preoperative functional imaging may be helpful for CI.
ACKNOWLEDGMENTS
This work was supported by the Ministry of Health & Welfare, Korea (A092106).
Footnotes
No potential conflict of interest relevant to this article was reported.
References
- 1.Moore DR, Shannon RV. Beyond cochlear implants: awakening the deafened brain. Nat Neurosci. 2009 Jun;12(6):686–691. doi: 10.1038/nn.2326. [DOI] [PubMed] [Google Scholar]
- 2.Svirsky MA, Teoh SW, Neuburger H. Development of language and speech perception in congenitally, profoundly deaf children as a function of age at cochlear implantation. Audiol Neurootol. 2004 Jul-Aug;9(4):224–233. doi: 10.1159/000078392. [DOI] [PubMed] [Google Scholar]
- 3.Basser PJ, Pierpaoli C. A simplified method to measure the diffusion tensor from seven MR images. Magn Reson Med. 1998 Jun;39(6):928–934. doi: 10.1002/mrm.1910390610. [DOI] [PubMed] [Google Scholar]
- 4.Beaulieu C. The basis of anisotropic water diffusion in the nervous system: a technical review. NMR Biomed. 2002 Nov-Dec;15(7-8):435–455. doi: 10.1002/nbm.782. [DOI] [PubMed] [Google Scholar]
- 5.Hasan KM, Gupta RK, Santos RM, Wolinsky JS, Narayana PA. Diffusion tensor fractional anisotropy of the normal-appearing seven segments of the corpus callosum in healthy adults and relapsing-remitting multiple sclerosis patients. J Magn Reson Imaging. 2005 Jun;21(6):735–743. doi: 10.1002/jmri.20296. [DOI] [PubMed] [Google Scholar]
- 6.Chang Y, Lee SH, Lee YJ, Hwang MJ, Bae SJ, Kim MN, et al. Auditory neural pathway evaluation on sensorineural hearing loss using diffusion tensor imaging. Neuroreport. 2004 Aug 06;15(11):1699–1703. doi: 10.1097/01.wnr.0000134584.10207.1a. [DOI] [PubMed] [Google Scholar]
- 7.Bhagat YA, Beaulieu C. Diffusion anisotropy in subcortical white matter and cortical gray matter: changes with aging and the role of CSF-suppression. J Magn Reson Imaging. 2004 Aug;20(2):216–227. doi: 10.1002/jmri.20102. [DOI] [PubMed] [Google Scholar]
- 8.Silk TJ, Vance A, Rinehart N, Bradshaw JL, Cunnington R. White-matter abnormalities in attention deficit hyperactivity disorder: a diffusion tensor imaging study. Hum Brain Mapp. 2009 Sep;30(9):2757–2765. doi: 10.1002/hbm.20703. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Scherfler C, Schocke MF, Seppi K, Esterhammer R, Brenneis C, Jaschke W, et al. Voxel-wise analysis of diffusion weighted imaging reveals disruption of the olfactory tract in Parkinson's disease. Brain. 2006 Feb;129(Pt 2):538–542. doi: 10.1093/brain/awh674. [DOI] [PubMed] [Google Scholar]
- 10.Kim Y, Jeong KS, Song HJ, Lee JJ, Seo JH, Kim GC, et al. Altered white matter microstructural integrity revealed by voxel-wise analysis of diffusion tensor imaging in welders with manganese exposure. Neurotoxicology. 2011 Jan;32(1):100–109. doi: 10.1016/j.neuro.2010.11.004. [DOI] [PubMed] [Google Scholar]
- 11.Watkins KE, Paus T, Lerch JP, Zijdenbos A, Collins DL, Neelin P, et al. Structural asymmetries in the human brain: a voxel-based statistical analysis of 142 MRI scans. Cereb Cortex. 2001 Sep;11(9):868–877. doi: 10.1093/cercor/11.9.868. [DOI] [PubMed] [Google Scholar]
- 12.Eckert MA, Leonard CM, Wilke M, Eckert M, Richards T, Richards A, et al. Anatomical signatures of dyslexia in children: unique information from manual and voxel based morphometry brain measures. Cortex. 2005 Jun;41(3):304–315. doi: 10.1016/s0010-9452(08)70268-5. [DOI] [PubMed] [Google Scholar]
- 13.Emmorey K, Allen JS, Bruss J, Schenker N, Damasio H. A morphometric analysis of auditory brain regions in congenitally deaf adults. Proc Natl Acad Sci U S A. 2003 Aug;100(17):10049–10054. doi: 10.1073/pnas.1730169100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Karas GB, Burton EJ, Rombouts SA, van Schijndel RA, O'Brien JT, Scheltens P, et al. A comprehensive study of gray matter loss in patients with Alzheimer's disease using optimized voxel-based morphometry. Neuroimage. 2003 Apr;18(4):895–907. doi: 10.1016/s1053-8119(03)00041-7. [DOI] [PubMed] [Google Scholar]
- 15.Le Bihan D, Mangin JF, Poupon C, Clark CA, Pappata S, Molko N, et al. Diffusion tensor imaging: concepts and applications. J Magn Reson Imaging. 2001 Apr;13(4):534–546. doi: 10.1002/jmri.1076. [DOI] [PubMed] [Google Scholar]
- 16.Ono J, Harada K, Takahashi M, Maeda M, Ikenaka K, Sakurai K, et al. Differentiation between dysmyelination and demyelination using magnetic resonance diffusional anisotropy. Brain Res. 1995 Feb;671(1):141–148. doi: 10.1016/0006-8993(94)01335-f. [DOI] [PubMed] [Google Scholar]
- 17.Rai V, Nath K, Saraswat VA, Purwar A, Rathore RK, Gupta RK. Measurement of cytotoxic and interstitial components of cerebral edema in acute hepatic failure by diffusion tensor imaging. J Magn Reson Imaging. 2008 Aug;28(2):334–341. doi: 10.1002/jmri.21438. [DOI] [PubMed] [Google Scholar]
- 18.Shibata DK. Differences in brain structure in deaf persons on MR imaging studied with voxel-based morphometry. AJNR Am J Neuroradiol. 2007 Feb;28(2):243–249. [PMC free article] [PubMed] [Google Scholar]
- 19.Bookstein FL. "Voxel-based morphometry" should not be used with imperfectly registered images. Neuroimage. 2001 Dec;14(6):1454–1462. doi: 10.1006/nimg.2001.0770. [DOI] [PubMed] [Google Scholar]