Abstract
The concept of gametic isolation has its origins in the 1937 edition of T. Dobzhansky’s Genetics and the Origin of Species. Involving either positive assortative fertilization (as opposed to self-incompatibility) or negative assortative fertilization, it occurs after mating but prior to fertilization. Gametic isolation is generally subsumed under either prezygotic or postmating isolation and thus has not been the subject of extensive investigation. Examples of assortative fertilization in Drosophila are reviewed and compared with those of other organisms. Potential mechanisms leading to assortative fertilization are discussed, as are their evolutionary implications.
In the 1937 edition of Genetics and the Origin of Species, Dobzhansky (1) described four subtypes of “physiological” isolating mechanisms that exist when parental forms occur together. Three of these were given formal names: “sexual isolation,” “mechanical isolation,” and “inviability of the hybrids.” The unnamed mechanism was described as when the “spermatozoa fail to reach the eggs, or to penetrate into the eggs; in higher plants, the pollen tube growth may be arrested if foreign pollen is placed on the stigma of the flower.” These events were attributed to “chains of reaction that bring about the actual union of gametes, or fertilization proper.” Examples of this fourth mechanism provided in the 1937 edition were limited, confined to marine invertebrates and incompatibility between plant species, and were accompanied by speculations as to the viability requirements of sperm of various vertebrates.
In the 1951 edition, not only had this mechanism been given a formal name (2), “gametic or gametophytic isolation,” with the definition “spermatozoa, or pollen tubes, of one species are not attracted to the eggs or ovules, or are poorly viable in the sexual ducts of another species,” but also examples were included from several species of Drosophila in which the viability of stored sperm was notably decreased in interspecific crosses (3–5).
Despite the growing list of examples of potential gametic isolation, it remains inadequately understood. One reason for this has been the focus of interest on mechanisms that act either earlier or later, as exemplified by the two common terminologies employed in studies of reproductive isolation: premating vs. postmating and prezygotic vs. postzygotic. These are primarily concerned with sexual isolation vs. hybrid inviability or sterility:
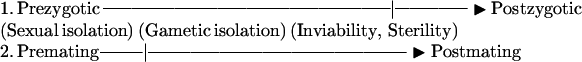 |
Gametic isolation, because it is included by default in one of these forms of isolation (6), tends not to be singled out for study. This is not surprising, given the ease of quantifying either sexual isolation or hybrid sterility as compared with tests for gametic interactions.
Gametic isolation, as a form of reproductive isolation, is equivalent to positive assortative fertilization. This, however, is only half of the story because, as with assortative mating, nonrandom union of gametes may vary between homogamy or positive assortative fertilization, and heterogamy, negative assortative fertilization, with implications for population differentiation similar to those of positive and negative assortative mating. The difference is that assortative fertilization occurs at a later place in the sequence between mating and offspring production. This presentation will concern itself with factors influencing assortative fertilization, once copulation has occurred, but prior to zygote formation, during the “chains of reactions that bring about the actual union of gametes.”
Our interest, however, is not in all barriers to fertilization, only with those that underlie assortative fertilization. Fertilization barriers may occur for a variety of reasons: sterility of the male or female, lack of oviposition sites, age of the male or female, interference from the ejaculate of another male, and genotypes of the male and female. Assortative fertilization is a subtype of fertilization barrier that depends upon characters of the female and male such that gametes from like or unlike parents have a greater or lesser than random chance of uniting. In the genus Drosophila, there is an incredible amount of variation in gametes and other internal reproductive characters with the potential to influence assortative fertilization. Evidence for assortative fertilization will be presented, including some recent observations from my own laboratory. Then, the assumptions regarding the mechanisms underlying assortative fertilization will be described. Finally, we will discuss how it may have an impact on population differentiation and speciation.
Interspecific Assortative Fertilization in Drosophila
There are a number of examples of postmating, prezygotic interactions between Drosophila species that result in positive assortative fertilization. In Drosophila, all published reports are of single matings by females. Examples include a failure of heterospecific sperm to enter the female storage organs, a reduction in motility of stored sperm, or both. Patterson and his associates (3) describe crosses between five different species of the virilis group in which the relative amounts and motilities of stored sperm were compared in heterospecific vs. homospecific inseminations. Motility and quantity of stored sperm were less in heterospecific crosses, suggesting an incompatibility with the female reproductive tract that caused their death. A similar observation was reported for sperm of Drosophila athabasca males in the reproductive tracts of Drosophila affinis females (4). Fuyama (7) was able to obtain a small number of inseminations of Drosophila pulchrella females by Drosophila suzukii males. While the numbers of sperm stored in the females appeared to be lower than in either conspecific cross, presumably due to the shorter duration of interspecific copulations, the viability of these sperm was elegantly demonstrated by stimulating their use with injected accessory gland products of conspecific males (7).
In many Drosophila species, primarily of the quinaria and repleta groups, homospecific mating normally is followed by the formation of a large opaque mass in the vagina, the insemination reaction, that typically lasts for 7 to 9 hr (8). Its disappearance is coincident with the onset of oviposition and subsequent remating by the female (9). In heterospecific matings this mass usually lasts considerably longer, and judging from the observations summarized by Patterson and Stone (8), and the phylogenetic relationships (10) of the species they examined, the size and duration of the mass appears to be related to the degree of divergence between the species involved. Patterson and co-workers suggested that this insemination reaction functions as an isolating mechanism, because in some crosses, they observed dead sperm within the mass and, in extreme cases, heterospecifically mated females never remated.
These examples all involve single heterospecific matings. In two other groups of insects, flour beetles (11, 12) and crickets (13–16), positive assortative fertilization was observed only under conditions of double matings, matings involving both a homo- and a heterospecific male. Heterospecific matings, both in flour beetles and in crickets, result in the production of offspring, although in all cases except crosses involving one type of Tribolium female, T. freemani, the numbers of offspring are significantly lower than in conspecific matings. When females are mated, twice, to a heterospecific and to a homospecific male, regardless of mating order, a significant majority of the offspring were sired by males of their own species. In both cases, positive assortative fertilization is the outcome of interejaculate competition dependent upon female genotype. Most of the progeny produced, regardless of mating order, were sired by the conspecific male. Because in most of these combinations, there were reduced numbers or viability of interspecific sperm after transfer, the observed effect is likely to have resulted merely from a numerical swamping out by the viable and conspecific sperm. This is not likely to be the case in T. freemani females, where sperm of both types of males appear equally viable.
Intraspecific Assortative Fertilization in Drosophila
Assortative mating, so pronounced between true species, is often examined in detail among distinct populations of the same species to identify antecedents of speciation. The same approach can be employed in treating assortative fertilization. Assortative fertilization within a species can potentially occur when a female has mated to only one male or when she has multiple mates, as seen for interspecific matings. In Drosophila, I could find no examples of positive assortative fertilization involving either single or multiple homospecific matings. This does not mean that they do not exist.
There are, however, sperm utilization patterns that clearly appear to exemplify negative assortative fertilization. Widely known in plants, the possibility of self-incompatibility in animals has received little attention. There is some evidence, such as increased recurrent spontaneous abortion among couples sharing HLA haplotypes (17), that similarities with respect to major histocompatibility complex variation may influence fertilization or implantation success, but this is poorly defined.
Investigators working with cactophilic Drosophila have long been aware that, compared with Drosophila melanogaster, it is very difficult to create isofemale lines of these species. Markow (18), in a study of inbreeding, reported the existence of a self-sterility phenomenon in Drosophila mojavensis, revealed when sib-mated lines failed to reproduce. Females mated to their brothers were full of motile sperm, and mature ovarian oocytes, but did not oviposit. The viability of these sperm was demonstrated when they were rescued from the ventral receptacles by the sperm-free ejaculates of unrelated males. The fact that these sperm could be “rescued” suggests that the responsible mechanism is a function of nonsperm ejaculate components and their interaction with females. Further analysis has been precluded by the absence of genetically marked chromosome balancers in this species.
A similar phenomenon appears to exist in at least one other species of desert Drosophila. Drosophila nigrospiracula is a cactophilic species in which females will remate up to four times in a given morning. As with D. mojavensis, unless a female is confined with more than one of her brothers (R. L. Mangan, personal communication), attempts to inbreed this species result in most sib-mated lines failing within a few generations (T.A.M., S. Bertram, and S. Murphy, unpublished results). Dissection of adults reveals that parents are fully capable of mating and sperm transfer: in most nonreproductive pairs, females contain large numbers of motile sperm in their ventral receptacles. For some reason, however, these sperm are not being used.
Experiments were conducted to assess whether the males or females were, for some unapparent reason, sterile, and if not, whether, as with D. mojavensis, degree of relatedness acts to prevent sperm utilization. In one set of experiments, oviposition was compared between females mated once to a brother following one generation of sib mating, and females mated to a random male. There was no difference in copulation duration for females mated to sibs compared with random males. In both replications, two things are clear (Table 1). First, fewer females laid any eggs when mated to their brothers than did females mated to random males. Second, those females that did not oviposit did not fail to do so for lack of motile sperm. A high proportion of females did not utilize the sperm they carried, at least from a single mating.
Table 1.
Reproductive failure and sperm presence in inbred and random pair matings of D. nigrospiracla
| Exp. | Male | Replications | No. of females | Females not ovipositing | Females with sperm |
|---|---|---|---|---|---|
| 1×a | Brother | 1 | 45 | 34/45 (75%) | 31/34 (91%) |
| Random | 1 | 39 | 12/39 (31%) | 7/12 (58%) | |
| 1×b | Brother | 2 | 31 | 24/31 (77%) | 19/24 (79%) |
| Random | 2 | 25 | 9/25 (36%) | 5/9 (55%) | |
| 3×a | 3× | 1 | 19 | 13/19 (68%) | 11/12 (92%) |
| 2×:1× | 1 | 21 | 4/21 (19%) | 4/4 (100%) | |
| 3×b | 3× | 2 | 12 | 8/12 (67%) | 8/8 (100%) |
| 2×:1× | 2 | 17 | 2/17 (12%) | 2/2 (100%) |
Females in experiments 1×a and 1×b were mated once, to either a male chosen at random from the mass mating culture or to their brother after one generation of sib-mating. Females in experiments 3×a and 3×b were mated three times, either all three times to the same male, a brother, or twice to their brother and then to a male chosen at random from the mass culture. All matings were observed to ensure normal copulations were achieved. Mated females were separated from males and allowed to lay eggs in yeasted vials, changed daily, for 1 week. Females not ovipositing after 1 week were dissected and examined for the presence of motile sperm.
In the second experiment, females were mated three times in the same morning. Matings were either all to the same male, “random” or “sib” (a brother from a one-generation sib-mated line), or twice consecutively to a sib and then to a random male from the population (Table 1). In both replications, a mating to an unrelated male was associated with increased oviposition, suggesting that degree of relatedness is an important factor in determining whether females of this species give up their oocytes. Females mating three times produced more eggs than females mating once, but there was no difference in number of eggs between ovipositing females that mated only with related males (number of eggs per female = 96.1 ± 4.9) vs. unrelated males (number of eggs per female = 94.2 ± 7.3). About 95% of these eggs hatch, similar to what is reported in other Drosophila species.
It is clear that in both of these cactophilic species, fertilization is negatively assortative. These observations are reminiscent of self-incompatibility in plants, raising the question of whether self-incompatibility mechanisms exist in animals. They may, and simply have gone undetected. A remaining question is whether there can be positive assortative fertilization under conditions of a single intraspecific mating. If assortative fertilization within a species is contributing to the evolution of gametic isolation, we expect to also find examples in which it is positive.
In species where females remate, an interaction between overlapping ejaculates may contribute another dimension to fertilization barriers. When ejaculates of more than one male overlap, a suite of other interactions is possible. These come into play following the failure of first males to prevent remating before their sperm is used up by the female. For most Drosophila species, however, these mechanisms are not completely efficient, and in Drosophila species in which it has been measured, females typically carry sperm from more than one male (19–23).
Sperm use by multiply mated females, however, is rarely a random process, and other, intermale, interactions may play themselves out inside the female, serving as barriers to fertilization. In most cases, the majority of sperm recovered is from the last male to mate. Equal mixing, however, is more likely in species that transfer fewer sperm when matings are close together (24). Nonrandom recovery of sperm following multiple mating, if it depends only upon the properties of the multiple competing ejaculates, and even in some cases where it is dependent upon female genotype (25, 26), differs from assortative fertilization as defined above, although some mechanisms may be the same.
Recently, an effect similar to the negative assortative fertilization observed in D. mojavensis and in D. nigrospiracula has been inferred by Olsson et al. (27) in a sand lizard, Lacerta agilis, except that the observed assortative fertilization was detected in multiply rather singly mated females. Degree of relatedness was inferred by degree of sharing of DNA fingerprint bands. There was a reduction in the proportion of offspring, in multiply fathered clutches, sired by males that were genetically similar in DNA fingerprints to the female. This situation differs from the typically measured outcomes of sperm competition in which the genotype of the female is unimportant. In that study, the authors explain that L. agilis females “select” sperm to use and “prefer” sperm from unrelated males. It is not necessary to use the terms “select” and “prefer” here any more than it is in the case of self-incompatibility in plants. The difference here is that the mechanism is not understood at the same level as it is in plants.
In conclusion, there is evidence that assortative fertilization exists within species in Drosophila, and in a lizard, but that it is associated with heterogamy rather than homogamy. The existence of negative assortative fertilization, however, suggests the existence of the requisite mechanisms for positive assortative fertilization as well. Additional and different kinds of studies must be undertaken to define these mechanisms.
Mechanisms
In organisms such as Drosophila where fertilization is internal and females store sperm, postmating barriers to fertilization can exist at a variety of levels. These constitute the “chains of reaction that bring about the actual union of gametes … .” Sperm must successfully enter the female and be transported to the storage organs, the spermathecae or ventral receptacle. They must stay alive with adequate motility until they are utilized by the female, and later be recovered from the storage organs, activated, and enter an oocyte as it passes through the reproductive tract. Entrance of a single sperm to the egg, in the case of Drosophila, through the micropyle, must be normal and trigger the formation of a normal zygote. A failure at any of these steps prevents fertilization. To promote assortative fertilization, there must be some degree of heritable specificity in the male and female components of the above process, thus fertilization barriers that involve abnormal or subviable sperm or ejaculates are not those of interest. The relevant mechanisms are those that are associated with naturally occurring, normal variation in the population, such that the male effectively signals the female to keep his sperm alive and to utilize them in fertilization.
For the sake of simplicity, the mechanisms can be envisioned as having three levels:
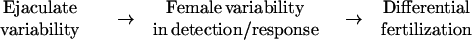 |
This general scheme will be true for cases in which females contain the ejaculate of one or of multiple males, and the potential signals and receptors may be multiple and complex.
Both the sperm and nonsperm components of the ejaculate are known to be extremely variable in Drosophila. Sperm in Drosophila species are more variable in length than in all other animal taxa combined. They range from 0.32 mm in Drosophila persimilis (28) to 58.29 mm in Drosophila bifurca (29); in the latter case sperm are about 12 times the length of the male himself. Within species, however, there is usually little variation. Pitnick and Markow (30) examined six strains of Drosophila hydei, finding a range of 23.02 mm to 25.91 mm. Snook (57) found that the long-sperm morph of North American Drosophila subobscura is 0.448 mm compared with 0.327 mm in a European strain. Species also differ with respect to how much of the sperm tail enters the egg (31); such differences could be important in postfertilization isolation.
Female sperm storage organs show striking interspecific variability as well (32); species also differ in which of those two organs, the spermathecae or the ventral receptacle, sperm are stored. This enormous variability raises the question as to whether assortative fertilization may be mediated, in part, by a mismatch between sperm morphology and that of female storage organs. For example, in the four species of the nannoptera group, sperm differ considerably in length, as do sites of storage in the females (33). Females of Drosophila pachea and Drosophila wassermani store sperm only in the spermathecae, while Drosophila nannoptera females use only the ventral receptacle. Crosses were made among these species and the fate of sperm was examined (34). In crosses between D. pachea females and D. wassermani males, dead sperm were found in the spermathecae of 14/23 females. In D. pachea females crossed to D. nannoptera males, sperm, dead, were found in the spermathecae in 3/15 females. In this cross sperm storage location was controlled by the female, rather than the male. The lumen of the D. pachea ventral receptacle is half the diameter of the D. nannoptera receptacle (35), and while such a morphological difference may dictate storage site, sperm viability or its reduction may still result from biochemical interactions between the ejaculate and the female reproductive tract.
Several approaches might be useful in assessing the role of sperm length differences in assortative fertilization. One would be to transplant testes between species (36), to create the necessary combinations of sperm and female reproductive tracts. Another is to utilize natural differences in sperm length, in those cases in which they exist, between closely related species or divergent populations of the same species.
The existence of qualitative differences in sperm themselves has not been directly shown. Several lines of evidence, however, strongly suggest expressed genetic differences between sperm, at least at the interspecific levels. For example, it is difficult to imagine, in the case of T. freemani, where homospecific sperm are favored regardless of mating order, how positive assortative fertilization could occur without heritable, qualitative differences on the sperm themselves. Furthermore, Thomas and Singh (37) have shown significant differences in testes proteins within and between related Drosophila species, and while these were not shown to be a property of the sperm themselves, they are certainly consistent with the prediction of qualitative differences. The existence of qualitative differences on the surface of the sperm that would interact differently with the female reproductive tract or with the ejaculates of other males seems to be a prerequisite in certain cases of assortative fertilization.
On the other hand, extensive variation has been documented in the chemistry and function of the nonsperm component of the ejaculate, specifically the accessory gland proteins or Acps (38). There are approximately 80 of these proteins (M. Wolfner, personal communication), all transferred to females at the time of mating. Only 9 have been characterized in detail with respect to chemistry and function, but it is clear from studies to date that this group of substances serves to (i) alter female behavior, stimulating oviposition and delaying female remating, and (ii) facilitate the storage of sperm in the female.
It is clear that within species there is considerable sequence polymorphism at the loci encoding these proteins (39) and that the proteins show considerable sequence divergence between species (40) as well as species specificity (7, 41) of their functions. In D. melanogaster, variation in four accessory gland proteins examined appears to be associated with displacement abilities in ejaculate competition experiments, although these experiments were not designed to detect any interaction with female genotype (42).
Once the sperm is inside the female, there are a variety of reactions that must occur for successful fertilization. Some of these reactions occur within the female reproductive tract itself, but others involve the action of male-derived substances in other sites in the female.
Evidence of the reactions inside the female reproductive tract is both direct and indirect. For example, Acp36DE has been shown to localize at the entrance to the sperm storage organs, in effect corralling the sperm into storage (43). How this protein specifically identifies the appropriate site in the female tract is unknown, but it must rely on biochemical properties expressed highly locally in the female tract. If this identification is species specific, it could easily explain why in some interspecific crosses, few sperm are seen in storage (3–5).
Excellent examples of reactions that occur outside the female tract come from studies of three proteins, the sex peptide (44), esterase 6 (45), and Acp26Aa (38). These small proteins are transferred rapidly to the female hemolymph, even before copulation has terminated, where they produce, in the female, the same response: increased oviposition and decreased receptivity to remating. Their modes of transport from the reproductive tract are unknown. In the case of the sex peptide, both effects on female behavior stem from the same unknown molecular target (46). The increase in oogenesis is mediated by the resultant increase in juvenile hormone synthesis in response to the sex peptide. The exact target of Acp26Aa also is unknown, but it exerts its effect by means of the thoracic ganglion (38). Acp26Aa is a polymorphic protein, raising the question of the efficiency of different morphs in altering female behavior. An important direction for future research is the identification of the female targets of these proteins and the detection of variation in the targets that could provide mechanisms for differential fertilization.
Species-specific morphological and biochemical features of the female tract are easily inferred from the larger size and longer duration of the insemination reaction mass, as well as the presence of dead sperm, in certain interspecific crosses. In house flies, female accessory gland secretions have been demonstrated to activate the sperm acrosome, enabling it to penetrate the micropyle (47, 48). The actual substance involved has not been identified, nor is there any evidence as to species specificity in the activation process.
Origins and Implications for Population Differentiation and Reproductive Isolation
What are the origins of the preceding examples of assortative fertilization? The examples described above are very different and thus are likely to result from different mechanisms. All depend, however, on the specificity or its breakdown of the chain of events leading to fertilization.
One possibility is the accumulation of different mutations in geographically isolated populations, similar to the model proposed by Orr (49) to explain the asymmetries in postzygotic isolation. Most of the examples of postmating but prezygotic isolation are asymmetrical. On the other hand, interactions between the sexes taking place within the female’s reproductive tract have important fitness consequences for females, and also for males if their sperm is not used immediately. Thus males are expected to evolve seminal fluid components that are more effective in inducing oviposition and postponing female remating, while females are expected to simultaneously adapt to negate toxic effects of ejaculates (50) and to retain control over their oviposition (51, 52). These conflicting pressures are proposed as the driving force in the rapid coevolution of the signaling that occurs within the reproductive tract, potentially acting as an “engine” of speciation (53). Because this coevolution is expected to evolve differently in different populations, there is a potential for a major mismatch between opposite sexes of separate populations that can manifest itself as assortative fertilization (54).
Another potential selective force is pathogen resistance. The insemination reaction has been likened to an immune response in which females react to foreign material they receive at mating. Self-incompatibility in some plants has been shown to be a function of pistil S-proteins, RNases, and their evolutionary origin has been suggested to be the recruitment of RNases originally involved in protecting the pistil from infection (55). The incidence of sexually transmitted extracellular pathogens in Drosophila is unexplored, but the conditions inside the mated female reproductive tract that are conducive to the maintenance of sperm viability, namely the nutrient-rich environment, should also provide a suitable habitat for pathogen growth. Thus it would not be surprising if female reproductive tracts were immunoreactive.
If self-incompatibility, or negative assortative fertilization, exists within Drosophila species, its origin could also be associated with inbreeding avoidance. The two species, D. mojavensis and D. nigrospiracula, in which it appears to exist are species in which resource availability and environmental extremes conspire to create situations in which sib mating could be a common occurrence (18, 56). In both of these species, males deliver comparatively few sperm on a given mating and females remate very frequently, additional mating system features that would serve to minimize inbreeding.
The evolutionary implications of assortative fertilization resemble those for assortative mating. Gametic isolation, the extreme form of positive assortative fertilization, should prevent gene flow between populations. Whether gametic isolation can be the primary isolating mechanism, especially if other factors promote crossing, or whether it typically appears in some specific order relative to other isolating mechanisms (i.e., premating or postzygotic) has not been addressed. Negative assortative fertilization should promote outcrossing. Whether or not true self-incompatibility exists in Drosophila or other animals remains to be established, as does the nature of the interplay between negative assortative fertilization and other isolating mechanisms that act before or after fertilization.
Acknowledgments
Discussions with M. Wolfner and R. Richmond and with my former students S. Pitnick and R. Snook have been especially stimulating during the preparation of this manuscript. Bruce Wallace provided valuable editorial comments on an earlier draft. I also acknowledge the assistance of M. St. Louis, S. Murphy, S. Cleland, and S. Bertram and the support of National Science Foundation Grants INT 94-02161 and DEB 95-10645.
References
- 1.Dobzhansky T. Genetics and the Origin of Species. New York: Columbia Univ. Press; 1937. [Google Scholar]
- 2.Dobzhansky T. Genetics and the Origin of Species. 2nd Ed. New York: Columbia Univ. Press; 1951. [Google Scholar]
- 3.Patterson J T. Univ Texas Publ. 1947;4720:41–77. [Google Scholar]
- 4.Miller D D. Am Nat. 1950;84:81–93. [Google Scholar]
- 5.Dobzhansky T. Am Nat. 1947;81:66–71. doi: 10.1086/281502. [DOI] [PubMed] [Google Scholar]
- 6.Coyne J A, Orr H A. Evolution (Lawrence, Kans) 1989;43:362–381. doi: 10.1111/j.1558-5646.1989.tb04233.x. [DOI] [PubMed] [Google Scholar]
- 7.Fuyama Y. Experientia. 1983;39:190–192. [Google Scholar]
- 8.Patterson J T, Stone W S. Evolution in the Genus Drosophila. New York: Macmillan; 1952. [Google Scholar]
- 9.Markow T A. Evol Biol. 1996;29:73–106. [Google Scholar]
- 10.Pitnick S, Markow T A, Spicer G. Proc Natl Acad Sci USA. 1995;92:10614–10618. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Robinson T, Johnson N, Wade M. Heredity. 1994;73:155–159. doi: 10.1038/hdy.1994.114. [DOI] [PubMed] [Google Scholar]
- 12.Wade M J, Patterson H, Chang N W, Johnson N A. Heredity. 1994;72:163–167. doi: 10.1038/hdy.1994.23. [DOI] [PubMed] [Google Scholar]
- 13.Hewitt G M, Mason P, Nichols R A. Heredity. 1989;62:343–353. [Google Scholar]
- 14.Bella J L, Bultin R K, Ferris C, Hewitt G M. Heredity. 1992;68:345–352. [Google Scholar]
- 15.Howard D, Gregory P. Phil Trans R Soc (London) 1993;340:231–236. [Google Scholar]
- 16.Gregory P G, Howard D J. Evolution (Lawrence, Kans) 1994;48:705–710. doi: 10.1111/j.1558-5646.1994.tb01355.x. [DOI] [PubMed] [Google Scholar]
- 17.Ober C, Elias S, Kostyu D, Hauck W. Am J Hum Genet. 1992;50:6–14. [PMC free article] [PubMed] [Google Scholar]
- 18.Markow T A. In: Ecological Genetics and Evolution: The Cactus–Yeast–Drosophila Model System. Barker J S F, Starmer W T, editors. New York: Academic; 1982. pp. 273–287. [Google Scholar]
- 19.Milkman R, Zeitler R R. Genetics. 1974;78:1191–1193. doi: 10.1093/genetics/78.4.1191. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Cobbs G. Am Nat. 1977;111:641–656. [Google Scholar]
- 21.Richmond R C. Am Nat. 1976;110:485–486. [Google Scholar]
- 22.Griffiths R C, McKechnie S W, McKenzie J A. Theor Appl Genet. 1982;62:89–96. doi: 10.1007/BF00276292. [DOI] [PubMed] [Google Scholar]
- 23.Marks R W, Seager R D, Barr L G. Am Nat. 1988;131:918–923. [Google Scholar]
- 24.Markow T A. Anim Behav. 1985;33:775–781. [Google Scholar]
- 25.Childress D, Hartl D L. Genetics. 1972;71:417–427. doi: 10.1093/genetics/71.3.417. [DOI] [PubMed] [Google Scholar]
- 26.Zimmering S, Fowler G L. Genet Res. 1968;12:359–363. doi: 10.1017/s0016672300011940. [DOI] [PubMed] [Google Scholar]
- 27.Olsson M, Shine R, Madsen T, Gullberg A, Tegelstrom H. Nature (London) 1996;383:585. doi: 10.1111/j.1558-5646.1996.tb02372.x. [DOI] [PubMed] [Google Scholar]
- 28.Snook R R, Markow T A, Karr T L. Proc Natl Acad Sci USA. 1994;91:1–5. doi: 10.1073/pnas.91.23.11222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Pitnick S, Spicer G, Markow T A. Nature (London) 1995;375:109. doi: 10.1038/375109a0. [DOI] [PubMed] [Google Scholar]
- 30.Pitnick S, Markow T A, Spicer G. Proc Natl Acad Sci USA. 1995;92:10614–10618. doi: 10.1073/pnas.92.23.10614. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Karr T L, Pitnick S. Nature (London) 1996;379:405–406. doi: 10.1038/379405a0. [DOI] [PubMed] [Google Scholar]
- 32.Throckmorton L. Univ Texas Publ. 1962;6205:207–343. [Google Scholar]
- 33.Pitnick S, Markow T A. Am Nat. 1994;143:785–819. [Google Scholar]
- 34.Russell J S, Ward B L, Heed W B. Drosophila Inf Serv. 1977;52:70. [Google Scholar]
- 35.Ward B L, Heed W B. J Hered. 1970;61:248–258. doi: 10.1093/oxfordjournals.jhered.a108095. [DOI] [PubMed] [Google Scholar]
- 36.Kambysellis M. Univ Texas Publ. 1968;6818:71–92. [Google Scholar]
- 37.Thomas S, Singh R S. Mol Biol Evol. 1992;9:507–525. doi: 10.1093/oxfordjournals.molbev.a040738. [DOI] [PubMed] [Google Scholar]
- 38.Wolfner, M. (1997) Insect Biochem. Mol. Biol., in press. [DOI] [PubMed]
- 39.Aguadé M, Miyashita N, Langley C. Genetics. 1992;132:755–770. doi: 10.1093/genetics/132.3.755. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Coulthart M B, Singh R S. Biochem Genet. 1988;26:153–164. doi: 10.1007/BF00555496. [DOI] [PubMed] [Google Scholar]
- 41.Chen P S, Stumm-Zollinger R, Claderlari M. Insect Biochem. 1985;15:385–390. [Google Scholar]
- 42.Clark A G, Aguadé M, Prout T, Harshman L G, Langley C H. Genetics. 1995;139:189–201. doi: 10.1093/genetics/139.1.189. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Bertram, M. J., Neubaum, D. M. & Wolfner, M. F. (1997) Insect Biochem. Mol. Biol., in press. [DOI] [PubMed]
- 44.Kubli E. Adv Devel Biochem. 1996;4:99–128. [Google Scholar]
- 45.Richmond R C, Nielsen K M, Brady J P, Snella E M. In: Ecological and Evolutionary Genetics of Drosophila. Barker J S F, Starmer W T, McIntyre R J, editors. New York: Plenum; 1990. pp. 273–289. [Google Scholar]
- 46.Schmidt T, Choffat Y, Klauser S, Kubli E. J Insect Physiol. 1993;39:361–368. [Google Scholar]
- 47.Leopold R A, Degrugillier M E. Science. 1973;181:555. doi: 10.1126/science.181.4099.555. [DOI] [PubMed] [Google Scholar]
- 48.Degrugillier M E, Leopold R A. J Ultrastruct Res. 1976;56:312–325. doi: 10.1016/s0022-5320(76)90006-x. [DOI] [PubMed] [Google Scholar]
- 49.Orr H A. Genetics. 1995;139:1805–1813. doi: 10.1093/genetics/139.4.1805. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Chapman T, Liddle L F, Kalb J M, Wolfner M F, Partridge L. Nature (London) 1995;373:241–244. doi: 10.1038/373241a0. [DOI] [PubMed] [Google Scholar]
- 51.Bouletreau-Merle J, Terrier O, Fouillet P. Heredity. 1989;62:145–151. doi: 10.1038/hdy.1989.22. [DOI] [PubMed] [Google Scholar]
- 52.Bouletreau-Merle J. J Insect Physiol. 1990;36:119–124. [Google Scholar]
- 53.Rice W R. Nature (London) 1996;381:232–234. doi: 10.1038/381232a0. [DOI] [PubMed] [Google Scholar]
- 54.Markow T A, Hocutt G D. In: Endless Forms: Species and Speciation. Howard D, Berlocher S, editors. Oxford: Oxford Univ. Press; 1996. , in press. [Google Scholar]
- 55.Kao T-h, McCubbin A G. Proc Natl Acad Sci USA. 1996;93:12059–12065. doi: 10.1073/pnas.93.22.12059. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Breitmeyer, C. & Markow, T. A. (1997) Funct. Ecol., in press.
- 57.Snook, R. R., (1995) Ph.D. dissertation (Arizona State Univ., Tempe).


