Abstract
Primary sinonasal tract mucoepidermoid carcinomas (MEC) are uncommon tumors that are frequently misclassified, resulting in inappropriate clinical management. The design of this study is retrospective. Nineteen cases of MEC included 10 females and 9 males, aged 15–75 years (mean, 52.7 years); males, on average were younger by a decade than females (47.2 vs. 57.7 years). Patients presented most frequently with a mass, obstructive symptoms, pain, and/or epistaxis present for a mean of 12.6 months. The majority of tumors involved the nasal cavity alone (n = 10), maxillary sinus alone (n = 6), or a combination of the nasal cavity and paranasal sinuses (n = 3) with a mean size of 2.4 cm. Most patients presented at a low clinical stage (n = 15, Stage I & II), with only 4 patients presenting with Stage III disease. Histologically, the tumors were often invasive (bone or perineural invasion), with invasion into minor mucoserous glands. Surface involvement was common. The neoplastic cells were composed of a combination of squamoid cells, intermediate cells, and mucocytes. Cystic spaces were occasionally large, but the majoritywere focal to small. Pleomorphism was generally low grade. Necrosis (n = 5) and atypical mitotic figures (n = 6) were seen infrequently. Over half of the tumors were classified as low grade (n = 11), with intermediate (n = 4) and high grade (n = 4) comprising the remainder. Mucicarmine was positive in all cases tested. Immunohistochemical studies showed positive reactions for keratin, CK5/6, p63, CK7, EMA, and CEA in all cases tested, while bcl-2 and CD117 were rarely positive. GFAP, MSA, TTF-1, and S100 protein were non-reactive. p53 and Ki-67 were reactive to a variable degree. MEC need to be considered in the differential diagnosis of a number of sinonasal lesions, particularly adenocarcinoma and necrotizing sialometaplasia. The patients were separated into stage I (n = 9), stage II (n = 6), and stage III (n = 4), without any patients in stage IV at presentation. Surgery occasionally accompanied by radiation therapy (n = 2) was generally employed. Six patients developed a recurrence, with 5 patients dying with disease (mean, 2.4 years), while 14 patients are either alive (n = 9) or had died (n = 5) of unrelated causes (mean, 14.6 years). MEC probably arises from the minor mucoserous glands of the upper aerodigestive tract, usually presenting in patients in middle age with a mass. Most patients present with low stage disease (stage I and II), although invasive growth is common. Recurrences develop in about a third of patients, who experience a shorter survival (mean, 6.5 years). The following parameters, when present, suggest an increased incidence of recurrence or dying with disease: size ≥4.0 cm (P = 0.034), high mitotic count (P = 0.041), atypical mitoses (P = 0.007), mixed anatomic site (P = 0.032), development of recurrence (P = 0.041), high tumor grade (P = 0.007), and higher stage disease (P = 0.027).
Keywords: Sinonasal tract; Mucoepidermoid carcinoma; Nasal cavity; Maxillary sinus, ethmoid sinus; Frontal sinus; Review; Meta-analysis; Immunohistochemistry; Prognosis; Outcome; Staging; Differential diagnosis; Carcinoma
Introduction
Carcinomas arising within the sinonasal tract (nasal cavity and paranasal sinuses) are not infrequent occurrences. Adenocarcinoma, specifically, is separated into salivary gland-type and non-salivary gland-type (Table 1) [1–4]. The latter is divided into two major categories: intestinal-type and non-intestinal type. Further, intestinal type adenocarcinoma is separated into a variety of different subtypes, the separation yielding a clinical significant difference in outcome. Adenocarcinomas of sinonasal tract can originate from the respiratory epithelium or the underlying mucoserous glands, with the majority arising from the mucoserous glands (60%) [5]. Specifically, mucoepidermoid carcinoma (MEC) is thought to arise from the minor mucoserous glands which lie within the mucosa, below the respiratory-type epithelium of the nasal cavity and paranasal sinuses. However, MEC in these anatomic sites is uncommon, resulting in potential diagnostic dilemmas because of their varied clinical and histological manifestations. MEC in this anatomic region is seldom seen by general surgical pathologists, accounting for <0.1% of primary malignant neoplasms in this anatomic region. The varied clinical behavior, treatment alternatives, and long-term patient prognosis of the different grades of MEC play a role in diagnosis and management for major salivary gland sites. While there have been a few series, the information is often included with general discussions about carcinoma of the sinonasal tract, about MEC in the head and neck [6–15], or is limited to case reports in the English literature (Table 2) [16–31]. Therefore, there is no large comprehensive evaluation of primary sinonasal tract MECs with respect to their histomorphology, immunohistochemical reactivity, clinical behavior, and treatment outcomes. We undertook this study in an attempt to identify any specific features that can be used to separate tumors within the sinonasal tract or suggest a specific clinical management or prognostic outcome.
Table 1.
| Salivary gland-type adenocarcinoma | Includes mucoepidermoid carcinoma, adenoid cystic carcinoma, acinic cell carcinoma, epithelial-myoepithelial carcinoma, clear cell carcinoma, polymorphous low-grade adenocarcinoma, among others | |
|---|---|---|
| Intestinal type adenocarcinoma | Papillary type | Papillary tubular cylinder cell type I |
| Colonic type | Papillary tubular cylinder cell type II | |
| Solid type | Papillary tubular cylinder cell type III | |
| Mucinous type | Alveolar goblet type | |
| Signet ring type | ||
| Mixed | Transitional | |
| Non-intestinal type adenocarcinoma | Low-grade | |
| High-grade | ||
Table 2.
| Characteristics | Mucoepidermoid carcinoma |
|---|---|
| Total: N = 19a | |
| Genderb | |
| Women | 7 |
| Men | 11 |
| Age (in years)b | |
| Range | 40–83 |
| Mean | 61 |
| Women (mean) | 61 |
| Men (mean) | 59 |
| Symptom duration (in months)b | |
| Range | 0.5–12 |
| Mean | 6.2 |
| Women (mean) | 7.0 |
| Men (mean) | 4.8 |
| Symptoms at presentationb,c | |
| Mass, facial swelling | 11 |
| Obstructive symptoms | 8 |
| Pain | 7 |
| Drainage, discharge, crusting, bleeding | 6 |
| Headaches | 5 |
| Nerve changes (paralysis, palsy, paresthesias, numbness, dysphagia, trismus, tingling) | 4 |
| Other (proptosis, ptosis, fistula, saddle nose, diplopia) | 5 |
| Locationb | |
| Mixed (more than one anatomic site) | 10 |
| Maxillary sinus only | 4 |
| Nasal cavity only | 3 |
| Ethmoid sinus only | 2 |
| Lateralityb | |
| Right | 8 |
| Left | 6 |
| Bilateral | 1 |
| Midline | 1 |
| Tumor grade | |
| Grade 1 (low-grade) | 5 |
| Grade 2 (intermediate-grade) | 5 |
| Grade 3 (high-grade) | 9 |
| Tumor stage | |
| Stage 1 | 4 |
| Stage 2 | 4 |
| Stage 3 | 4 |
| Stage 4 | 7 |
| All patients with follow-up (n = 15) (mean years of survival) | |
| Follow-up range | 0.5–6 |
| Alive, no evidence of disease (n = 7) | 2.7 |
| Alive, with disease (n = 4) | 2.5 |
| Dead, no evidence of disease (n = 1) | 6.0 |
| Dead, with disease (n = 3) | 2.1 |
| Alive/Dead, no evidence of disease (n = 8) | 3.1 |
| Alive/Dead, with disease (n = 7) | 2.3 |
| Patients with recurrence (n = 8) | 3.0 |
| Patients without recurrence (n = 6) | 2.8 |
aThis table does not include the series reported in this study
bParameter was not stated in all cases
cPatients may have experienced more than one symptom
Materials and Methods
The records of 26 patients with tumors diagnosed as MEC of the nasal cavity and paranasal sinuses (sphenoid, maxillary, ethmoid, and frontal sinuses) were selected. The cases were retrieved from the files of the Otorhinolaryngic-Head & Neck Tumor Registry of the Armed Forces Institute of Pathology (AFIP), Washington, DC, between 1970 and 2002 and from the authors’ consultation cases. However, 7 patients were excluded from further consideration because either: (1) paraffin blocks were unavailable for additional sections or immunophenotypic analysis; or (2) the original submitted case did not have sufficient demographic information supplied from which to obtain adequate follow-up information. The 19 patients that comprised the subject of this study were chosen from a review of 25,269 (0.075%) benign or malignant primary sinonasal tract tumors seen in consultation during this time. Needless to say, these tumors are rare. Fourteen cases were obtained from civilian sources, including university medical centers and foreign contributors, 3 cases from military hospitals, and 2 cases from Veterans Administration medical centers.
Patient files were supplemented by a review of: demographics (gender, age); symptoms, physical findings, and duration at presentation, including mass, nasal obstruction, polyps, difficulty breathing, changes in breathing, epistaxis, discharge, chronic rhinosinusitis, pain, headaches, nerve paralysis, visual changes; and past medical and surgical historyies In addition, we reviewed radiographic, surgical pathology, and operative reports and obtained follow-up information by direct written or oral communication with the referring pathologist, the patient’s physician, oncology data services and tumor registries, or the patient (or patient’s relatives). Follow-up data, available for all patients, included information regarding tumor location, presence of recurrent disease, treatment modalities used, and the patient’s current clinical status. Since most samples were submitted in a fragmented fashion, definitive margins were not assessed nor were margins identified by the surgeons. Furthermore, as many of the cases were consultations, margin status remained unreliable even if inking had been performed. Patients who were found to have a salivary gland primary (minor mucoserous glands of the palate, parotid gland, or orbit) were excluded from further consideration. No patients in this series were part of a syndrome associated kindred (no familial cancer syndrome). It is important to add that as a tertiary pathology review center, conducting a retrospective review of these patients, we did not treat the patients. This clinical investigation was conducted in accordance and compliance with all statutes, directives, and guidelines of the Code of Federal Regulations, Title 45, Part 46, and the Department of Defense Directive 3216.2 relating to human subjects in research.
The macroscopic pathology observations noted within this study were gathered from the individual gross descriptions of the neoplasms given by the contributing pathologists. Hematoxylin and eosin-stained slides from all cases were reviewed, with specific histologic features annotated as follows: exact tumor location; lateralization; tumor size (greatest dimension in centimeters); tumor encapsulation (presence or absence); tumor extension (bone or soft tissue); architectural pattern of growth (solid, papillary, cystic, infiltrating, glandular); cell population (mucocytes; epidermoid; transitional); surface origin; surface ulceration; presence or absence of necrosis; perineural invasion; lymph-vascular invasion; tumor cellularity (low, moderate, or high); cellular pleomorphism (mild, moderate, severe [anaplastic]); presence of nucleoli; mitotic figures (number of mitotic figures per 10 high-power fields [magnification at 40× with a 10× objective lens using an Olympus BX41 microscope]); atypical mitotic figures (present or absent, and defined by abnormal chromosome spread, tripolar or quadripolar forms, circular forms, or indescribably bizarre); and the presence of other microscopic pathologic findings in the remaining tissues.
Mucicarmine stain was performed using standard methodologies. Immunophenotypic analysis was performed in all cases with suitable material by a standardized Envision™ method employing 4 μm-thick, formalin-fixed, paraffin-embedded sections. Table 3 documents the pertinent, commercially available immunohistochemical antibody panel used. These antibodies were chosen specifically to characterize the epithelial components of the tumor, to test if these tumors have a similar pattern of reactivity seen in major salivary gland MECs, and to determine which antibodies may be helpful in the differential diagnosis. The analysis was performed on a single representative block for each primary tumor. Epitope retrieval was performed, as required by the manufacturer guidelines. Standard positive controls were used throughout, with serum used as the negative control. The antibody reactions were graded as absent to weak (0 to 1+), moderate (2+ to 3+) and strong (4+) staining, and the fraction of positive cells was determined by separating them into four groups: <10, 11–50, 51–90, and >90%, especially for the proliferation markers.
Table 3.
Immunohistochemical panel
| Antigen/Antibody/Clone | Type | Company | Dilution | Antigen recovery |
|---|---|---|---|---|
| Cytokeratin (AE1/AE3:M3515 and CAM5.2) | mm | Dako, Carpinteria, CA Boehringer Mannheim Biochemicals, Indianapolis, IN |
1:40 1:8 |
CC1, 30 min |
| CK5/6 (D5/16 B4) | mm | Dako | 1:25 | E2, 20 min |
| CK7 (OV-TL-12/30) | mm | Dako | 1:200 | CC1, 30 min |
| CK20 (KS20.8) | mm | Ventana Medical Systems, Tucson, AZ | Neat | CC1, 30 min |
| Epithelial membrane antigen (E29) | mm | Ventana | Neat | CC1, 30 min |
| p63 (7jul) | mm | Leica Microsystems, Buffalo Grove, IL | 1:40 | E2, 30 min |
| CEA (CLO1-cea ab-1) | mm | Lab Vision/NeoMarkers, Fremont, CA | 1:250 | CC1, 30 min |
| Vimentin (V9) | mm | Ventana Medical Systems | Neat | CC1, 30 min |
| CD117 (C-Kit) | rp | Dako | 1:400 | CC1, 30 min |
| bcl-2 (124) | mm | Dako, Carpinteria, CA | 1:40 | CC1, 30 min |
| GFAP (6F2) | mm | Dako | 1:200 | CC1, 30 min |
| Muscle specific actin (HHF35) | mm | Enzo Life Sciences, Farmingdale, NY | 1:100 | CC1, 30 min |
| TTF-1 (8G7G3/1) | mm | Ventana | Neat | CC1, 30 min |
| S100 protein | rp | Dako | 1:2000 | CC1, 30 min |
| p53 (DO-7) | mm | Dako | Neat | CC1, 30 min |
| Ki-67 (MIB-1) | mm | Dako | 1:100 | CC1, 30 min |
mm mouse monoclonal, rp rabbit polyclonal
A review of publications in English (MEDLINE, 1966–2011) was performed, with all cases reported as MEC of the sinonasal tract included in the review [16–31]. However, many cases were excluded if the lesion arose primarily in the oral cavity or parotid gland and extended into the sinonasal tract, or represented a different tumor type (hamartoma, squamous cell carcinoma, primary sinonasal tract adenocarcinoma), or if the information was too generalized and non-specific to make a meaningful interpretation of the demographics, histologic features, or patient outcome [8, 9, 11, 13, 32–41].
Statistical evaluation was performed using a standard statistics software package with categorical variables analyzed using Chi-square tests and Fisher’s Exact tests to compare observed and expected frequency distributions. Comparison of means between groups were made with independent t tests (including 1-tailed and 2-tailed tests with degrees of freedom) or one-way analysis of variance, depending on whether there were two groups or more than two groups, respectively. Confidence intervals of 95% were generated for all positive findings. The alpha level was set at P < 0.05.
Results
Clinical
The patients included 10 women and 9 men (Table 4). Their ages ranged from 15 to 75 years of age, with an overall mean age at presentation of 52.7 years (median, 58.0 years). The average age at presentation for women was older than men, at 57.7 and 47.2 years, respectively, but it was not statistically significant (P = 0.236). Patients most frequently presented with a mass within the nasal cavity/paranasal sinuses (n = 10), with the majority (n = 6) also showing obstructive symptoms, including difficulty breathing, and chronic sinusitis. Other symptoms included pain (n = 5), ophthalmologic symptoms (n = 4), and epistaxis (n = 1). Diplopia, proptosis, and visual field changes encompassed the reported ophthalmologic symptoms. Most patients presented with more than one symptom while there were no asymptomatic patients. By definition, none of the tumors were centered in the oral cavity or parotid gland. The duration of symptoms ranged from 1 to 60 months, with an average of 12.6 months. On average, women (mean, 13.5 months) experienced a slightly longer duration of symptoms than men (mean, 11.8 months), but this finding was not significant (P = 0.842). When separated by anatomic site, the mean duration of symptoms varied significantly: Nasal cavity alone: 8.2 months; maxillary sinus alone: 12.4 months; combination of nasal cavity and sinuses: 33 months. However, due to the limited number of cases in each group, there was no statistical significance (P = 0.167). One of the nasal cavity alone tumors had epistaxis, a symptom which dictates immediate clinical assessment. Mixed site tumors tended to be associated with non-specific obstructive symptoms (such as sinusitis), which progressed to a mass lesion eventually. There was no statistically significant difference in survival based on duration of symptoms (P = 0.318).
Table 4.
Clinical characteristics of our study group
| Clinical characteristics | Number |
|---|---|
| Gender | |
| Females | 10 |
| Males | 9 |
| Age (in years) (P = 0.236) | |
| Range | 15–75 |
| Mean | 52.7 |
| Women (mean) | 57.7 |
| Men (mean) | 47.2 |
| Symptomsa (P = 0.317) | |
| Duration (range, in months) | 1–60 |
| Duration (mean, in months) | 12.6 |
| Mass | 10 |
| Obstructive symptoms | 6 |
| Pain | 5 |
| Ophthalmologic symptoms | 4 |
| Epistaxis | 1 |
| Anatomic site (P = 0.089) | |
| Nasal cavity alone | 10 |
| Maxillary sinus alone | 6 |
| Combination of nasal cavity/sinuses | 3 |
| Laterality (P = 0.234) | |
| Right | 9 |
| Left | 9 |
| Bilateral | 1 |
| Size (cm) | |
| Range | 0.7–4.6 |
| Mean | 2.4 |
| Female (mean) (P = 0.478) | 2.2 |
| Male (mean) | 2.6 |
| Stage | |
| I | 9 |
| II | 6 |
| III | 4 |
aPatients may have experienced more than one symptom
Pathologic Features
Macroscopic
The tumors ranged from 0.7 cm up to 4.6 cm in greatest single linear dimension (Table 4), with an average size of 2.4 cm. Tumors were submitted in multiple fragments in most cases, precluding an evaluation of margin status. There were 9 each of right- and left-sided tumors, respectively, with a single tumor involving both sides. Left-sided tumors (mean, 2.8 cm) were larger than right-sided tumors (mean, 2.1 cm), but this was not statistically significant (P = 0.234). Similarly, male patients had larger tumors than female tumors (mean, 2.6 versus 2.2 cm), but this was also not significant (P = 0.482). The tumors were on average progressively larger as more anatomic sites were involved, a difference that approached statistical significance: nasal cavity alone: mean, 2.0 cm; maxillary sinus alone: mean, 2.4 cm; mixed: mean, 3.7 cm (P = 0.084). The majority of cases presented with tumors confined to the nasal cavity (n = 10) or maxillary sinus alone (n = 6), with the remaining three cases involving more than one area (maxillary sinus, nasal cavity, and orbit). Based on the clinical presentation, endoscopic evaluation, radiographic findings, and intraoperative observations, the cases were placed in appropriate stage, utilizing current staging criteria [42]. Nine patients had stage I disease, 6 patients stage II, and 4 patients stage III. No patients at presentation were included in stage IV. Due to the anatomic site of involvement, specific tumor macroscopic features were not well-described. The tumors were described as pale, white, yellow to reddish-tan, showing a glistening, mucinous, myxoid to honey-comb appearance. Some of the fragments were gritty, no doubt related to fragments of bony tissue normally present in turbinate tissue or samples removed via curettage of the sinuses.
Microscopic
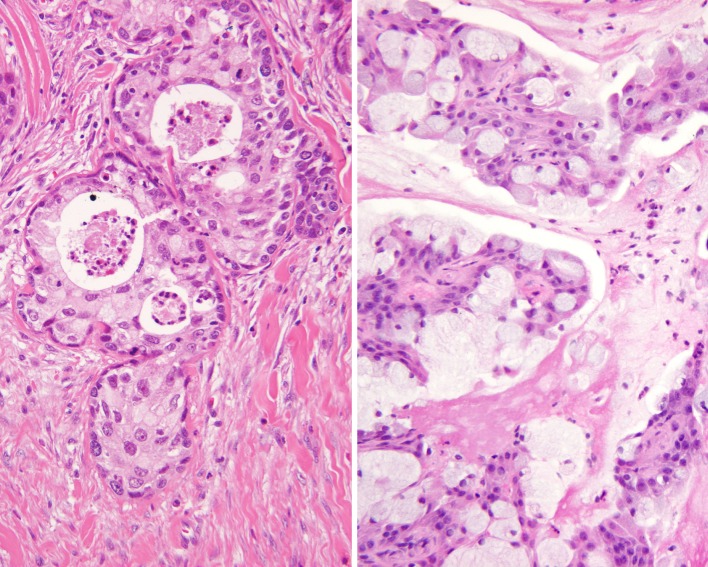
The presence of both mucocytes and an epidermoid component is requisite for the diagnosis of MEC. Further, there is an intermediate cell component that is present in these tumors, and is often the more common finding (Fig. 1). These cells are thought to be between basal cells and the epidermoid/polygonal cells. They are usually slightly larger than lymphocytes, containing scant cytoplasm, but can present as larger forms. At the outset, it is important to note that well-defined “squamous” differentiation is not a feature of MEC. Intercellular bridges, obvious keratinization, dyskeratosis, and keratin pearl formation is generally not present. Therefore, the term “epidermoid” is used because it describes the features resembling squamous epithelium, without being identical.
Fig. 1.
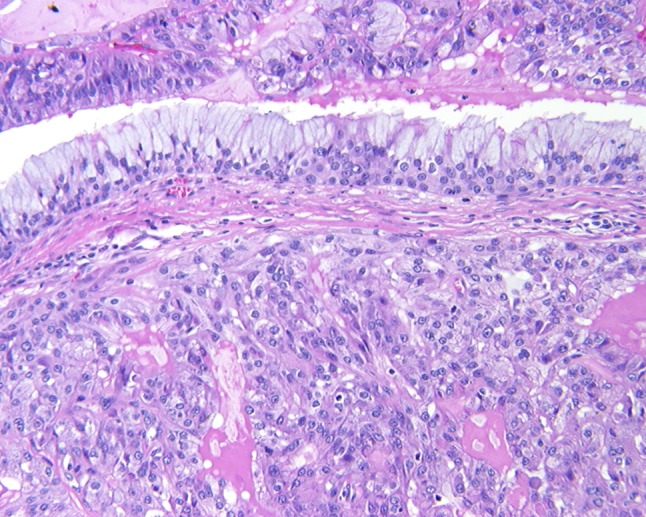
An infiltrative epidermoid neoplasm is noted beneath an intact respiratory epithelium
Only one case showed a suggestion of encapsulation; otherwise, all of the remaining cases failed to show any capsule formation (Table 5). This is an expected finding in a minor salivary gland location. Most of the tumors showed areas of invasion (n = 15), while 2 cases did not and another 2 could not be assessed for invasion due to the fragmented nature of the specimens. Perineural invasion was present in 4 cases and lymph-vascular invasion was identified in only 1 case. The overlying surface mucosa showed ulceration and erosion in a few cases, with surface involvement in 6 cases. It is difficult to determine with certainty whether the tumor was arising from the surface or invading into the epithelium. Pseudoepitheliomatous hyperplasia (PEH) was quite extensive in 3 cases as a well-defined hyperplastic mucosal squamous epithelial proliferation immediately overlying the tumor. Interestingly, the tumors invaded into and destroyed the adjacent minor mucoserous glands (n = 8), while in others it pushed or surrounded the glands without destruction (n = 6). The remaining cases did not show a specific relationship to the mucoserous glands (n = 5). Cysts were present characterized as: large spaces filled with mucinous material and lined by epithelium (n = 5); small spaces showing mucinous debris in the lumen (n = 8); or dilated duct-like spaces containing mucinous and inflammatory debris (n = 6; Fig. 2). Needless to say, a well-defined definition of what constitutes the size of a cyst is lacking in the literature. In general, if 10 or more tumor cells would fit into the space, it was interpreted to be a “cyst” (this is an arbitrary definition). Rarely, papillary or pseudopapillary structures could be seen within the cystic spaces. If the cysts were only focal, these cases were classified as being <20% cystic (as defined in the World Health Organization modified grading scheme [43], Table 6). Extravasation of the mucin into the adjacent tissue was not uncommon, focally eliciting an inflammatory and fibroblastic-type response (Fig. 3). Occasionally, pools of mucin were detected several high-power fields away from the main proliferation. A true “foreign-body” type giant cell reaction was not detected. Necrosis was present in five cases, comprised of necrotic debris, similar to comedo-type necrosis, but it was not statistically significantly correlated with patient outcome (P = 0.100). Ghost outlines of cells were not a feature seen. Fibrosis and desmoplastic stroma were present but tended not to be a dominant or significant finding (no sclerotic or hyalinized variants were identified). A prominent lymphoid infiltrate was not seen (i.e., no tumor associated lymphoid proliferation [TALP]).
Table 5.
Pathologic features
| Microscopic characteristic | Number of cases |
|---|---|
| Invasion | |
| Invasion/destruction of parenchyma | 15 |
| Perineural invasion (P = 0.765) | 4 |
| Bone invasion on histology | 2 |
| Vascular invasion (P = 0.305) | 1 |
| Surface invasion/involvement | 6 |
| Cystic structures | |
| Focal | 6 |
| Small | 8 |
| Large | 5 |
| Mucus cells present | 19 |
| Intermediate cells present | 19 |
| Squamous cells present | 19 |
| Pleomorphism | |
| Mild | 5 |
| Moderate | 9 |
| Severe | 5 |
| Necrosis present (P = 0.100) | 5 |
| Mitotic figures | |
| Present | 11 |
| Mean (per 10 HPF) | 6 |
| Range | 0–31 |
| Atypical figures (present) (P = 0.007) | 6 |
| >20/10 HPF (P = 0.041) | 4 |
| Extensive pseudoepitheliomatous hyperplasia | 3 |
| Mucoepidermoid grade (P = 0.081) | |
| Low grade | 11 |
| Intermediate grade | 4 |
| High grade | 4 |
Fig. 2.
Goblet cell formation could be seen within transitional epithelium and forming small, papillary projections
Table 6.
Grading mucoepidermoid carcinoma [43]
| Histopathologic feature | Point value |
|---|---|
| Cystic component < 20% | 2 |
| Neural invasion | 2 |
| Necrosis | 3 |
| 4 or more mitoses/10 HPFs | 3 |
| Anaplasia | 4 |
| Tumor grade | Point score |
|---|---|
| Low | 0–4 |
| Intermediate | 5–6 |
| High | 7 or more |
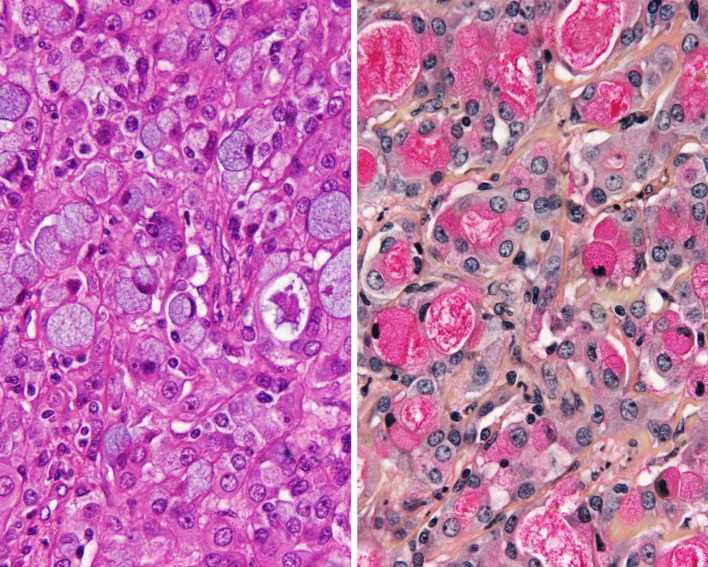
Fig. 3.
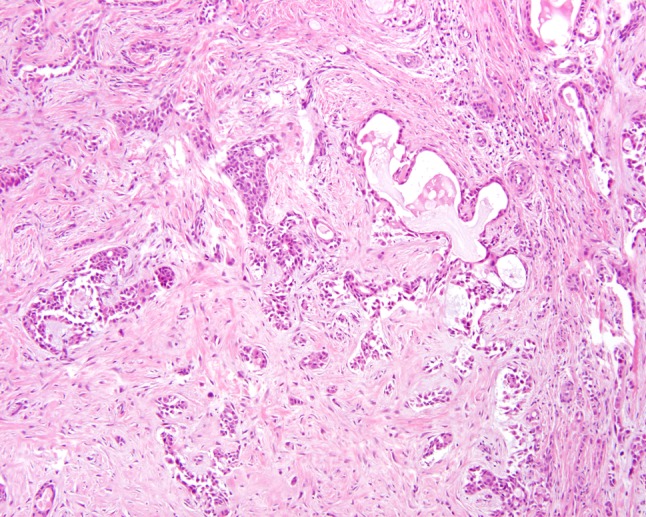
Heavy sclerosis and a desmoplastic-type stroma is noted between islands of epidermoid-intermediate epithelium and mucocytes. There is a hint of mucin extravasation
Mucocytes were present in all cases, as required for the diagnosis. These cells contain epithelial mucin within their cytoplasm (Fig. 4). The classic appearance is that of a mucocyte, showing a goblet shaped cell with a nucleus compressed to the periphery by abundant foamy to cleared cytoplasm. The cytoplasm is frequently slightly bluish, with a bubbly appearance. In a number of instances, special stains (mucicarmine [Fig. 5], Alcian blue) were required to highlight the intracytoplasmic mucin (not extracellular mucin). Mucocytes comprised <10% of the overall volume of the neoplastic cells, a significant finding if evaluating limited or incisional biopsies. The mucocytes were frequently identified lining the cystic spaces, but were also present within the duct-like structures, in isolation within the tumoral proliferation, and on the surface. Obviously, mucocytes on the surface epithelium can be identical to mucocytes within respiratory type epithelium. Needless to say, mucocytes alone on the surface should not be interpreted as part of a neoplasm without having other histologic features present.
Fig. 4.
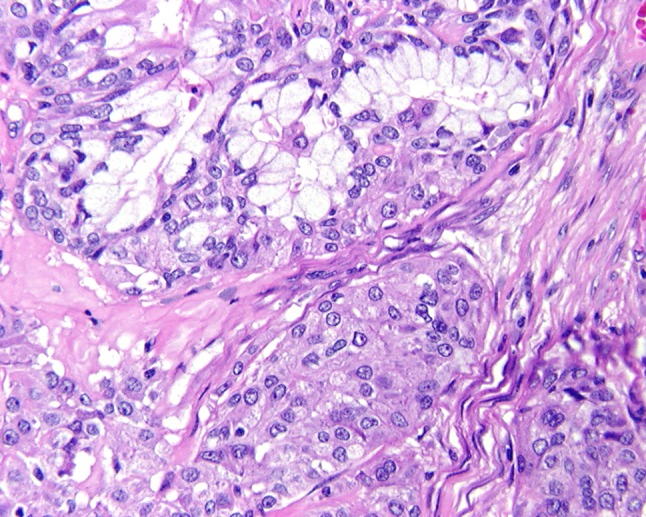
The epidermoid component has a sheet-like distribution of neoplastic cells, immediately juxtaposed with mucocytes (goblet cells)
Fig. 5.
Mucocytes contained a basophilic, granular mucus within the cytoplasm and within glandular spaces (left). Mucicarmine highlighted the mucus with a strong magenta reaction (right)
The epidermoid cells were present in a variety of patterns. Small nests, solid cords, islands and sheets were present in intimate association with the transitional cells. The cells were larger than the transitional cells, showing a polygonal appearance, along with opacified cytoplasm. Isolated and randomly scattered pleomorphic cells could be seen in the tumors. A single tumor showed areas of frank keratin pearl formation, but it was difficult to be certain if this area was a surface epithelium phenomenon blended into the tumor, or part of the tumor itself. The intermediate cells were the dominant finding, slightly smaller than the epidermoid cells. A few cells showed clear cytoplasm, which did not show mucinous material with special stains. Therefore, it may be these cleared cells contain glycogen or another substance. Cellular pleomorphism was separated into mild (n = 5), moderate (n = 9), and severe (n = 5); and only severe pleomorphism was interpreted to represent anaplasia (Fig. 6). Mitotic figures were present, ranging from 0 to 31 mitoses per 10 high-power fields (HPFs). However, no mitoses (per 10 HPFs) were seen in 8 cases and only 1 mitosis/10 HPFs was seen in 5 cases, suggesting that mitotic figures are not frequent. There were 4 cases with high mitotic counts (>20 mitoses/10 HPFs), a finding which was statistically correlated to a worse outcome (P = 0.041). Atypical mitotic figures were present in 6 cases, a finding which was also statistically significantly correlated with a worse outcome (P = 0.007). Bone invasion was detected in two cases, but this finding was not associated with a poor outcome.
Fig. 6.
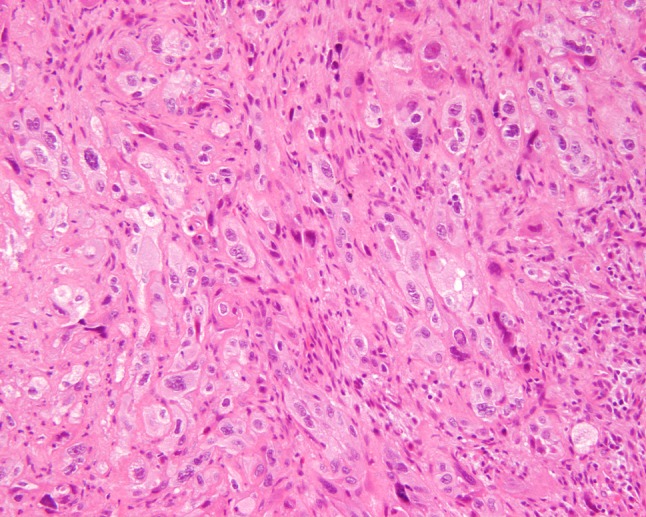
High grade MEC showing an infiltrating tumor composed by cells with profound pleomorphism. Mucocytes were rare in this neoplasm
Utilizing the accepted grading criteria (Table 6), the tumors were separated into low (n = 11), intermediate (n = 4), and high grade (n = 4). The majority of tumors were in the low grade category, and, when combining low and intermediate grade, 15 cases fell within this designation. However, when separated in this fashion, there was only a trend towards a statistically significant association with an overall worse outcome (P = 0.081). However, when the groups were stratified by Grade 1 versus Grade II and III combined, there was a statistically significant difference in outcome (P = 0.007).
Immunohistochemistry
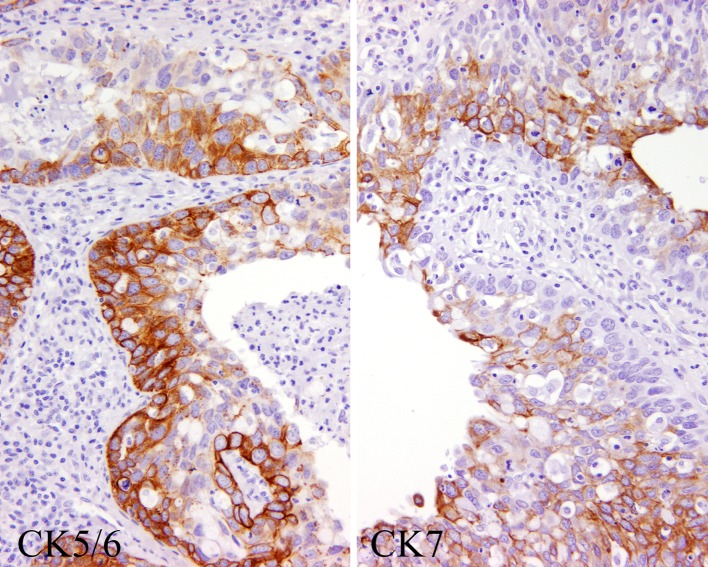
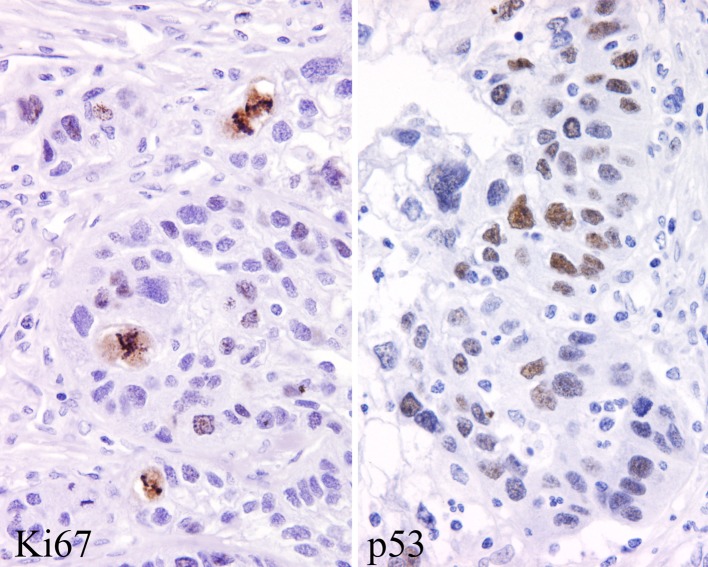
The neoplastic cells were immunoreactive with pan-keratin in all cases tested (Table 7). Further, all of the cases showed strong diffuse reactivity with CK5/6 (Fig. 7). There was a topographic distribution of the reactivity, with the more basal and parabasal zones of the tumor (basal to intermediate cell population) being preferentially highlighted. In contrast, CK7 showed a more random staining pattern (Fig. 7). Two cases showed isolated CK20 positive cells, but this was not a dominant or diffuse finding. p63 was strongly and diffusely immunoreactive in the nuclei of the epidermoid cells in 92% of the cases tested. Epithelial membrane antigen was strongly positive in most cases (93%), while the goblet-type mucinous epithelium was highlighted with CEA. One case showed isolated cells positive with GFAP. CD117 was present in a few of the intermediate cells in 5 cases, while blc-2 was positive in 3 cases. S100 protein was positive in 2 cases. Vimentin was positive in the epithelial cells (intermediate type) in 6 cases. There was a lack of staining with TTF-1 and with muscle specific actin. The nuclei of the neoplastic cells were highlighted with p53 in about 5–10% of cells with a heavy deposition in 6 cases (Fig. 8). A >5% ki-67 index was detected with a 4+ nuclear immunoreactivity in 3 cases (Fig. 8).
Table 7.
Immunohistochemistry results
| Antibody | Number of cases with positive reactions |
|---|---|
| Cytokeratin | 13/13 (100%) |
| CK5/6 | 13/13 (100%) |
| CK7 | 10/13 (77%) |
| CK20 | 2/13 (15%) |
| Epithelial membrane antigen (EMA) | 13/14 (93%) |
| p63 | 12/13 (92%) |
| CEA | 13/14 (93%) |
| Vimentin | 6/14 (43%) |
| CD117 | 5/14 (36%) |
| bcl-2 | 3/14 (21%) |
| Glial fibrillary acidic protein (GFAP) | 1/14 (7%) |
| Muscle specific actin (myoepithelial) | 0/14 (0%) |
| TTF-1 | 0/14 (0%) |
| S100 protein (myoepithelial) | 2/14 (14%) |
| p53 (4+, ~5%) | 6/14 (43%) |
| Ki67 (4+, >5%) | 3/14 (21%) |
Fig. 7.
The neoplastic cells were highlighted in the basal zones with CK5/6 (left), while more randomly with CK7 (right)
Fig. 8.
The proliferation index was often increased, highlighted with a Ki67 (left). Many of the cell nuclei were p53 positive (right)
Treatment and Follow-up
All patients were managed with surgery as the initial management modality. The types of procedures varied based on the specific anatomic site of involvement. Excision, wide local excision, maxillectomy, and exenteration were employed to remove the primary tumor. Two cases had an initial excision followed by wide excision a few weeks later; this was still interpreted to be part of the initial management. Two patients were further managed with radiation a few weeks after surgery (600 cGy each). No patients were managed with chemotherapy. Six patients (5 males and 1 female) developed a recurrence (Table 8). The recurrences developed within 1 month to as long as 76 months later. Of these, 1 involved the nasal cavity, 2 involved the maxillary sinus, and 3 involved multiple sites. These six cases were evenly divided between grade 1 (n = 2), grade 2 (n = 2), and grade 3 (n = 2) tumors. The tumors were predominantly stage III (n = 4), with one each of stage I and stage II. Four of the patients died with disease (mean, 1.8 years), while 2 were alive without evidence of disease. Interestingly, these two patients were both young (26 and 33 years, respectively). The development of recurrence was statistically significantly associated with a worse outcome (P = 0.041).
Table 8.
Outcome for patients with recurrence (P = 0.041)
| Characteristic | 1 | 2 | 3 | 4 | 5 | 6 |
|---|---|---|---|---|---|---|
| Gender | F | M | M | M | M | M |
| Age | 75 | 26 | 33 | 52 | 67 | 74 |
| Site | X | X | N | X | Max | Max |
| Grade | 2 | 1 | 1 | 2 | 3 | 3 |
| Stage | 3 | 2 | 1 | 3 | 3 | 3 |
| Management | S, R | S | S | S | S | S |
| Time to develop recurrence (years) | 1.1 | 6.4 | 5 | 0.1 | 0.3 | 1.0 |
| Outcome | D, D | A, NED | A, NED | D, D | D, D | D, D |
| Follow-up interval (years) | 2.0 | 8.9 | 22.7 | 0.2 | 3.3 | 1.9 |
F female; M male; X mixed site; N nasal cavity alone; Max maxillary sinus alone; S surgery; S, R surgery and radiation; A,NED alive, no evidence of disease; D, D dead with disease
Overall, there was an excellent long-term survival, with overall 11.3 years of average follow-up for the 19 patients (Table 9). Five patients died with disease (local or with distant metastases), an average of 2.4 years after diagnosis. This is in sharp contrast to those who were either alive or had died but without evidence of disease at last follow-up (average, 14.6 years). There was a difference in patient outcome when stratified by age, with patients <60 having a slightly better overall survival than those who were ≥60 years of age, although this was not statistically significant (P = 0.408). There was a statistically significant difference in survival when stratified by tumor size, using 4 cm as a cutoff (P = 0.034). Patients with tumors in more than one anatomic site did have a worse overall outcome (mean survival, 3.7 years), when compared to the rest of the anatomic sites (mean survival, 12.8 years), but in our series this did not reach statistical significance (P = 0.107). There was no independent increased risk of a poor outcome when there was either perineural invasion (P = 0.765) or lymph-vascular invasion (P = 0.303). Patients with high grade tumors had a worse outcome when compared to tumors that were low or intermediate grade: Grade 1 and 2 tumors had a mean follow-up of 13.2 years; Grade 3tumors had a mean survival of 4.4 years. Moreover, since not all of the patients in the Grade 1 and 2 categories had died, this survival would be potentially longer if followed to death. Fifty percent of patients with high-grade tumors died from disease, versus 20% for low and intermediate grade tumors. Furthermore, patients with higher grade tumors were more likely to die from disease: 6.25% of patients with Grade I tumors, 50% of patients with Grade II tumors, and 33% of patients with Grade III tumors, died of disease, respectively. In other words, comparing patients with Grade I tumors to a combination of patients with Grade II and III tumors, shows that of the former group, 6.3% died with tumor (4.4 years) versus 50% of the latter group dying of tumor (mean survival, 1.84 years), respectively, a finding which reached statistical significance (P = 0.007).
Table 9.
Summary of patient outcome
| All patients | A, NED | D, NED | D, D | |
|---|---|---|---|---|
| All patients with follow-up (mean years) | 19 (11.3) | 9 (15.1) | 5 (13.6) | 5 (2.4) |
| Follow-up range (years) | 0.2–29.3 | 6.4–29.3 | 4.5–26.2 | 0.2–4.5 |
| Gender (P = 0.383) | ||||
| Females | 10 (13.1) | 5 (13.1) | 4 (15.9) | 1 (2.0) |
| Males | 9 (9.4) | 4 (17.5) | 1 (4.5) | 4 (2.5) |
| Age (P = 0.408) | ||||
| < 60 years | 10 (13.0) | 7 (14.9) | 2 (12.8) | 1 (0.2) |
| ≥60 years | 9 (9.5) | 2 (15.6) | 3 (14.2) | 4 (2.9) |
| Size (P = 0.034) | ||||
| <4.0 cm | 15 (13.5) | 9 (15.1) | 4 (15.0) | 2 (3.9) |
| ≥4.0 cm | 4 (3.1) | 0 | 1 (8.3) | 3 (1.4) |
| Anatomic site (P = 0.143) | ||||
| Nasal cavity alone | 10 (13.7) | 6 (16.4) | 3 (11.3) | 1 (4.5) |
| Maxillary sinus alone | 6 (11.3) | 2 (14.1) | 2 (17.1) | 2 (2.6) |
| Mixed (P = 0.032) | 3 (3.7) | 1 (8.9) | 0 | 2 (1.1) |
| Grade (P = 0.081) | ||||
| I | 11 (13.2) | 8 (13.3) | 2 (17.2) | 1 (4.5) |
| II | 4 (13.2) | 1 (29.3) | 1 (21.1) | 2 (1.1) |
| III | 4 (4.4) | 0 | 2 (6.3) | 2 (2.6) |
| Stage (P = 0.012) | ||||
| I | 9 (14.5) | 6 (13.1) | 3 (17.3) | 0 |
| II | 6 (13.0) | 3 (19.0) | 2 (8.2) | 1 (4.5) |
| III | 4 (1.8) | 0 | 0 | 4 (1.8) |
A, NED alive, no evidence of disease; D, NED dead, no evidence of disease; D, D dead with disease
Finally, patients with high stage tumors had a much worse outcome when compared with low-stage tumors: Mean overall survival was 1.8 years for stage III tumors compared to 13.9 years for stages I and II (P = 0.012). Furthermore, 100% of patients with Stage III tumors died of disease, versus 7% for stage I and II tumors combined.
Discussion
The sinonasal tract is infrequently affected by adenocarcinoma. When present, they are separated into salivary gland-type adenocarcinomas and non-salivary type adenocarcinomas. The salivary gland-type neoplasms of the sinonasal tract are very uncommon, but include adenoid cystic carcinoma, MEC, acinic cell carcinoma, epithelial-myoepithelial carcinoma, myoepithelial carcinoma, polymorphous low-grade adenocarcinoma, clear cell carcinoma, and carcinoma ex-pleomorphic adenoma. Benign salivary gland neoplasms are less common than malignant tumors, and include pleomorphic adenoma, myoepithelioma, and oncocytoma. Of the malignant neoplasms, MEC is the second most frequent (after adenoid cystic carcinoma). Overall, MEC accounts for <0.1% of all malignant sinonasal tract neoplasms.
Clinical
Combining the 19 cases from this clinical series and the 19 cases from a review of the pertinent literature (Table 10), there does not seem to be a gender difference. However, men seemed to be more likely than women to die from their disease: 1 of 14 females died after 2 years (7%) compared to 7 of 21 males who died (mean, 2.3 years; 33%), although this did not reach a significant probability (one tailed = 0.0691). Patients ranged in age from 15 to 83 years, with a mean age at present of 56.8 years. There was no difference in mean age at presentation between the sexes. Similarly, patients who are older than 60 years of age are more likely to die from disease than younger patients: 6 of 19 (32%) patients >60 years died of disease; 2 of 19 (11%) patients <60 years died of disease, but this did not reach significance (one tailed = 0.0948). Patients presented with basically non-specific symptoms, including obstructive symptoms, a mass lesion, ophthalmologic symptoms, and epistaxis as the most frequently noted signs and symptoms. Symptoms were usually present for about 10 months, suggesting the non-specific nature of the presenting signs and symptoms. Interestingly, when symptoms were stratified by anatomic site affected, mixed sites of presentation experienced symptoms for a longer duration (33 months) than cases involving the nasal cavity alone (8.2 months). This may be accounted for by the easier access to nasal cavity lesions than sinus lesions.
Table 10.
| Characteristics | Mucoepidermoid carcinoma |
|---|---|
| Total: N = 38a | |
| Genderb | |
| Women | 17 |
| Men | 20 |
| Age (in years) | |
| Range | 15–83 |
| Mean | 56.8 |
| Women (mean) | 59.2 |
| Men (mean) | 53.7 |
| Symptom duration (in months)b | |
| Range | 0.5–60 |
| Mean | 10.2 |
| Women (mean) | 11.7 |
| Men (mean) | 9.0 |
| Symptoms at presentationb,c | |
| Mass, facial swelling | 21 |
| Obstructive symptoms | 14 |
| Pain | 9 |
| Drainage, discharge, crusting, bleeding | 9 |
| Headaches | 5 |
| Nerve changes (paralysis, palsy, paresthesias, numbness, dysphagia, trismus, tingling) | 5 |
| Other (proptosis, ptosis, fistula, saddle nose, diplopia, weakness) | 5 |
| Location | |
| Nasal cavity only | 13 |
| Mixed (more than one anatomic site) | 13 |
| Maxillary sinus only | 10 |
| Ethmoid sinus only | 2 |
| Lateralityb | |
| Right | 17 |
| Left | 15 |
| Bilateral | 2 |
| Midline | 1 |
| Tumor grade | |
| Grade 1 (low grade) | 16 |
| Grade 2 (intermediate grade) | 9 |
| Grade 3 (high grade) | 13 |
| Tumor stage | |
| Stage 1 | 13 |
| Stage 2 | 10 |
| Stage 3 | 8 |
| Stage 4 | 7 |
| All patients with follow-up (n = 34) (mean years of survival) | 34 (7.6) |
| Follow-up range | 0.2–29.3 |
| Alive, no evidence of disease (n = 16) | 9.6 |
| Alive, with disease (n = 4) | 2.5 |
| Dead, no evidence of disease (n = 6) | 12.4 |
| Dead, with disease (n = 8) | 2.3 |
| Alive/Dead, no evidence of disease (n = 22) | 10.4 |
| Alive/Dead, with disease (n = 12) | 2.4 |
| Patients with recurrence (n = 14) | 4.5 |
| Patients without recurrence (n = 19) | 10.2 |
aThis table includes the current reported cases in combination with the literature
bParameter was not stated in all cases
cPatients may have experienced more than one symptom
Tumors involved a single site (nasal cavity, maxillary sinus, or ethmoid sinus) more often than multiple sites. In general, tumors were on average 2.4 cm in greatest dimension, without a side predilection. As would be expected, nasal cavity tumors were smaller (mean, 2.0 cm) than mixed site tumors (mean, 3.7 cm). Not surprisingly, patients with other than nasal cavity alone tumors (whether maxillary sinus alone or mixed locations) tended to have a worse outcome than patients with nasal cavity only tumors (P = 0.032). Likewise, patients who had a tumor ≥4 cm were more likely to die of their disease than patients with tumors <4 cm (P = 0.034). In general, most patients presented with low-grade tumors: 16 patients with Grade I tumors; 9 patients with Grade II tumors; 13 patients with Grade III tumors (combined with cases from the literature). Patients with higher grade tumors were more likely to die from disease: 6.25% of patients with Grade I tumors, 50% of a patients with Grade II tumors, and 33% of patients with Grade III tumors, died of disease, respectively. When combined, Grade I tumors compared to a combination of Grade II and III tumors, then 6.3% died with tumor (4.4 years) versus 50% dying of tumor (mean survival, 1.84 years), a finding which reached statistical significance (P = 0.007).
Most patients presented with low stage disease. The higher the stage of disease, the more likely patients were to die from disease, a finding with statistical significance (P = 0.027): No patients with Stage I tumors died of disease compared to 20% of patients with Stage II tumors, 57% of patients with Stage III tumors, and 50% of patients with Stage IV tumors, respectively. When combined, 8.7% of low stage (Stage I and II) patients died of disease, while 54.5% of high stage (Stage III and IV) patients died of disease (combined with literature cases).
If a patient developed recurrence, they were also more likely to die from disease, a finding which reached statistical significance (P = 0.041). Fourteen patients developed local recurrence, while 19 patients did not. Recurrences were identified anywhere from 1 to 5 years after original presentation. Of the patients with recurrence, 50% died of disease (mean, 2 years). In contrast, only 5.3% of patients died of disease (mean, 4.5 years) in patients who did not develop a local recurrence. In our study group of patients, of the 6 patients with recurrence, 67% died from disease at a mean of 1.8 years after diagnosis (Table 8). Patients with mixed tumor sites were more likely to develop a recurrence, while 83% of patients with a recurrence were men. There were too few patients in this subset to perform a meaningful statistical evaluation.
Overall, when combined with the literature, 64.7% of patients were either alive or had died without evidence of disease, with a mean follow-up of 10.4 years (Table 11). This is in contrast to the 35.3% of our patients who were either alive or had died with disease, with a mean follow-up of 2.4 years. Overall, the disease-free survival rate at 5 years is 41.2%; however, 64.7% were alive or had died without evidence of disease at last follow-up (mean, 10.4 years). Of the 12 patients who had disease at last follow-up, there was an average of 2.4 years of follow-up. Therefore, in general, if recurrence was to develop, it would be most likely to occur within the first 2 years after intervention, and the patients who died with disease, died an average of 2.3 years after diagnosis. There is an overall raw 5-year survival rate of 44.1% and a raw 10-year survival rate of 20.6%.
Table 11.
Combined patient outcome
| All patients | A, NED | A, D | D, NED | D, D | |
|---|---|---|---|---|---|
| All patients with follow-up (mean years) | 34 (7.6) | 16 (9.6) | 4 (2.5) | 6 (12.4) | 8 (2.3) |
| Follow-up range (years) | 0.2–29.3 | 0.5–29.3 | 0.5–5 | 4.5–26.2 | 0.2–4.5 |
| Gender (P = 0.162) | |||||
| Females | 14 (9.9) | 8 (8.9) | 1 (1.6) | 4 (15.9) | 1 (2.0) |
| Males | 19 (5.9) | 8 (10.4) | 3 (2.8) | 1 (4.5) | 7 (2.3) |
| Age (P = 0.450) | |||||
| <60 years | 17 (8.6) | 11 (10.5) | 2 (1.8) | 2 (12.8) | 2 (0.5) |
| ≥60 years | 18 (6.5) | 5 (7.8) | 2 (3.3) | 4 (12.1) | 6 (2.9) |
| Size (P = 0.034) | |||||
| <4.0 cm | 19 (11.1) | 11 (12.8) | 2 (1.1) | 4 (15.0) | 2 (3.9) |
| ≥4.0 cm | 4 (3.1) | 0 | 0 | 1 (8.3) | 3 (1.4) |
| Anatomic site (P = 0.032) | |||||
| Nasal cavity alone | 13 (11.3) | 7 (14.5) | 1 (0.5) | 4 (10.0) | 1 (4.5) |
| Maxillary sinus alone | 10 (7.9) | 4 (8.8) | 1 (3.0) | 2 (17.1) | 3 (2.2) |
| Mixed | 10 (2.6) | 5 (3.5) | 1 (1.6) | 0 | 4 (1.8) |
| Ethmoid sinus alone | 1 (5.0) | 0 | 1 (5.0) | 0 | 0 |
| Grade (P = 0.084) | |||||
| 1 (L) | 16 (9.9) | 12 (9.9) | 1 (0.5) | 2 (17.2) | 1 (4.5) |
| 2 (I)a | 6 (9.3) | 1 (29.3) | 1 (1.6) | 1 (21.1) | 3 (1.2) |
| 3 (H)a | 12 (3.5) | 3 (1.9) | 2 (4.0) | 3 (6.2) | 4 (2.5) |
| Stage (P = 0.027) | |||||
| I | 13 (11.0) | 8 (10.5) | 2 (4.0) | 3 (17.3) | 0 |
| II | 10 (9.3) | 5 (13.1) | 0 | 3 (7.5) | 2 (2.9) |
| III | 7 (1.8) | 2 (1.7) | 1 (1.6) | 0 | 4 (1.8) |
| IV | 4 (1.9) | 1 (2.0) | 1 (0.5) | 0 | 2 (2.5) |
| Patients with recurrence (P = 0.041) | 14 (4.5) | 4 (9.9) | 3 (3.2) | 0 | 7 (2.0) |
| Patients without recurrence | 19 (10.2) | 12 (9.5) | 0 | 6 (12.4) | 1 (4.5) |
Although difficult to achieve given the anatomic confines of the region, wide excision is the treatment of choice. Clear margins are very difficult to assess in many cases, but are considered to be a reliable indicator of surgical extirpation. In this clinical series, no reliable information could be obtained on margin status, and so this postulation cannot be further evaluated. Radiation therapy was only employed in a very limited number of patients, with mixed results: One patient with radiation therapy died of disease in 2.0 years, while the other patient managed with radiation died without evidence of disease 21.1 years later. Therefore, a definitive statement about the effectiveness of radiation therapy in sinonasal tract disease is not reliable.
Pathology Features
MEC probably arises from the minor mucoserous glands of the upper aerodigestive tract, although there was surface epithelial involvement in six patients in this series. It is always difficult to determine with certainty whether the tumor was invading into or arising from the surface epithelium. There was also significant PEH in 3 tumors. Therefore, it would seem that the surface epithelium may be involved as a reaction to the neoplasm rather as than the source of the neoplasm.
MEC in this anatomic site is invasive by definition, even though a well-demarcated periphery may be seen. Tumors infiltrate into the adjacent parenchyma, soft tissues, bone, and mucoserous glands, while infrequently displaying perineural or lymph-vascular invasion. As perineural invasion is the only one of these two criteria (lymph-vascular and/or perineural invasion) used in the grading system, this may partially explain the greater number of tumors in the low-grade category. We arbitrarily used more than 10 tumor cells’ size as a criteria for the definition of a ‘cystic space.’ If this criteria were met, we defined the tumor as having cysts. If it was not, then the tumor was considered ‘non-cystic.’ Most of the neoplasms had enlarged lumen of glands, but only 5 tumors showed large cystic spaces. Although there is no well accepted definition of cysts, we believe this more stringent criteria allows the grading of the tumors to be more meaningful.
Necrosis was identified infrequently (n = 5). However, 66% of patients developed a recurrence when necrosis was present, and 60% of patients had died with disease. Even though this did not reach statistical significance in this study, it must be viewed as a significant finding, since it is also used in the overall grading system. In general, mitoses were infrequently identified. However, when increased (by definition, > 4/10 HPFs), this finding was independently correlated to a worse outcome (P = 0.041), even though it is also used as part of the overall grading system. Similarly, atypical mitoses, when present, were correlated to a much worse clinical outcome (P = 0.007). In tumors with atypical mitoses present, 50% of patients developed recurrence; and 66.7% died from disease (mean, 2.4 years) versus those who had died without evidence of disease (mean, 6.3 years).
p53 is well known to be increased in malignant or pre-malignant neoplasms, showing a gradient of increased nuclear staining as the tumor becomes less well-differentiated. However, in this series, while six patients showed 4+ strong and diffuse reaction in the tumor nuclei, this finding did not correlate to patient outcome. However, the presence of p53 expression may help with separating other lesions in the differential diagnosis from MEC, especially on small biopsies.
The immunohistochemistry results are similar to those of major salivary gland studies, which document pan-keratin, CK5/6, and p63 reactivity, while EMA shows a more limited expression. CK7 was only focally expressed, a finding helpful in separating high-grade tumors from other lesions in the differential diagnosis. CEA and CK20 were only noted within mucocytes. The remaining antibodies were appropriately positive or negative, respectively, as would be expected for MEC in other sites and with other tumors included in the differential diagnosis.
Differential Diagnosis
Given the anatomic site, MECs must be distinguished from the more aggressive variants of squamous cell carcinoma, especially adenosquamous carcinoma, and from adenocarcinoma and necrotizing sialometaplasia (NS). Distinction can usually be made based on the pattern of growth, presence of an intermediate cell population, proclivity for small, focal cyst formation, and the presence of a blended population rather than two distinct populations. NS will usually have a lobular growth without an invasive pattern. Overlying pseudoepitheliomatous hyperplasia may blend with the areas of necrotizing sialometaplasia to yield a “pseudo-invasive” appearance. In general, NS and PEH do not have increased expression of p53, a finding which may help with separation between these lesions and MEC. This interpretation problem is usually only seen in superficial or shave-type biopsies. If a mass lesion is described clinically, it is better to be cautious and state “atypical squamoproliferative lesion” and suggest that a deeper or larger biopsy be performed before embarking on definitive therapy. Sinonasal tract adenocarcinomas, especially the intestinal type adenocarcinomas, do not have squamoid features or an intermediate cell population. Many of the intestinal type adenocarcinomas will have CK7, CDX-2 and/or CK20 immunoreactivity, while they are not usually immunoreactive with CK5/6. MEC infrequently show CK20 immunoreactivity, but are not known to express CDX-2 [44]. In addition, the two patterns of growth between these tumors are not usually a problem. Adenocarcinoma (non-intestinal type) does not have epidermoid or intermediate cells and so can usually be diagnosed without difficulty.
Adenosquamous carcinoma (ASC) is a high grade variant of squamous cell carcinoma composed of an admixture of squamous cell carcinoma and adenocarcinoma. There are some who propose that ASC is a high grade MEC [45]. By definition the tumor demonstrates biphasic components of adenocarcinoma and squamous cell carcinoma, with an undifferentiated cellular component in several tumors [46]. However, the squamous differentiation is usually confirmed by pavemented growth with intercellular bridges, keratin pearl formation, dyskeratosis or individual cell keratinization, features usually absent in MEC. The two carcinomas in ASC may be separate or intermixed, with areas of commingling and/or transition of the squamous cell carcinoma to adenocarcinoma. The “undifferentiated” areas between the two distinct carcinomas are often composed of clear cells. There is no true adenocarcinoma and distinctly separate squamous cell carcinoma in a mucoepidermoid carcinoma [47].
Finally, pleomorphic adenoma (PA) may occasionally show a mixture of squamous areas and mucocytes, although the background myxochondroid matrix material should help with the separation. It is important to remember that MEC may have S100 protein and GFAP immunoreactivity in a few cases. Therefore, when interpreting immunohistochemistry results, especially if obtained on a small biopsy, it is important to consider MEC in the differential with PA in the sinonasal tract region. However, in general, both diagnoses are usually made without the application of immunohistochemistry studies.
Summary
MECs probably arise from the minor mucoserous glands of the upper aerodigestive tract. Patients are usually in middle age, without a gender predilection, and present with a mass lesion accompanied by non-specific symptoms for usually less than 1 year. Most patients present with low stage disease (stage I and II), although invasive growth is common. Recurrences develop in about one-third of patients, usually within 2 years and these patients experience a shorter survival (mean, 6.5 years). Surgery is the treatment of choice with clear margins if possible. The differential diagnosis includes NS, adenocarcinoma, and ASC in the SNT region. The following parameters, when present, suggest an increased incidence of recurrence or dying with disease: size ≥4.0 cm (P = 0.034), high mitotic count (P = 0.041), atypical mitoses (P = 0.007), mixed anatomic site (P = 0.032), development of recurrence (P = 0.041), high tumor grade (Grade II or III; P = 0.007), and higher stage disease (P = 0.027).
Acknowledgments
The opinions or assertions contained herein are the private views of the authors and are not to be construed as official or as reflecting the views of Southern California Permanente Medical Group nor of the Department of Navy.
References
- 1.Eveson JW. Salivary gland-type carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon, France: IARC Press; 2005. P. 24–5.
- 2.Franchi A, Santucci M, Wenig BM. Adenocarcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics head and neck tumours. Lyon, France: IARC Press; 2005. P. 20–3.
- 3.Kleinsasser O, Schroeder HG. Adenocarcinomas of the inner nose after exposure to wood dust. Morphological findings and relationships between histopathology and clinical behavior in 79 cases. Arch Otorhinolaryngol. 1988;245:1–15. doi: 10.1007/BF00463541. [DOI] [PubMed] [Google Scholar]
- 4.Barnes L. Intestinal-type adenocarcinoma of the nasal cavity and paranasal sinuses. Am J Surg Pathol. 1986;10:192–202. doi: 10.1097/00000478-198603000-00006. [DOI] [PubMed] [Google Scholar]
- 5.Gnepp DR, Heffner DK. Mucosal origin of sinonasal tract adenomatous neoplasms. Mod Pathol. 1989;2:365–371. [PubMed] [Google Scholar]
- 6.Bhattacharyya N. Survival and staging characteristics for non-squamous cell malignancies of the maxillary sinus. Arch Otolaryngol Head Neck Surg. 2003;129:334–337. doi: 10.1001/archotol.129.10.1101. [DOI] [PubMed] [Google Scholar]
- 7.da Cruz Perez DE, Pires FR, Lopes MA, Almeida OP, Kowalski LP. Adenoid cystic carcinoma and mucoepidermoid carcinoma of the maxillary sinus: report of a 44-year experience of 25 cases from a single institution. J Oral Maxillofac Surg. 2006;64:1592–1597. doi: 10.1016/j.joms.2005.11.088. [DOI] [PubMed] [Google Scholar]
- 8.Donald PJ, Boggan JE. Sphenoid sinus malignancies. J Craniofac Surg. 1995;6:15–23. doi: 10.1097/00001665-199501000-00007. [DOI] [PubMed] [Google Scholar]
- 9.Haraguchi H, Ebihara S, Saikawa M, Mashima K, Haneda T, Hirano K. Malignant tumors of the nasal cavity: review of a 60-case series. Jpn J Clin Oncol. 1995;25:188–194. [PubMed] [Google Scholar]
- 10.Heffner DK, Hyams VJ, Hauck KW, Lingeman C. Low-grade adenocarcinoma of the nasal cavity and paranasal sinuses. Cancer. 1982;50:312–322. doi: 10.1002/1097-0142(19820715)50:2<312::AID-CNCR2820500225>3.0.CO;2-Z. [DOI] [PubMed] [Google Scholar]
- 11.Katz TS, Mendenhall WM, Morris CG, Amdur RJ, Hinerman RW, Villaret DB. Malignant tumors of the nasal cavity and paranasal sinuses. Head Neck. 2002;24:821–829. doi: 10.1002/hed.10143. [DOI] [PubMed] [Google Scholar]
- 12.Kokemueller H, Brueggemann N, Swennen G, Eckardt A. Mucoepidermoid carcinoma of the salivary glands–clinical review of 42 cases. Oral Oncol. 2005;41:3–10. doi: 10.1016/j.oraloncology.2004.01.017. [DOI] [PubMed] [Google Scholar]
- 13.Parsons JT, Mendenhall WM, Mancuso AA, Cassisi NJ, Million RR. Malignant tumors of the nasal cavity and ethmoid and sphenoid sinuses. Int J Radiat Oncol Biol Phys. 1988;14:11–22. doi: 10.1016/0360-3016(88)90044-2. [DOI] [PubMed] [Google Scholar]
- 14.Qureshi SS, Chaukar DA, Talole SD, Dcruz AK. Clinical characteristics and outcome of non-squamous cell malignancies of the maxillary sinus. J Surg Oncol. 2006;93:362–367. doi: 10.1002/jso.20500. [DOI] [PubMed] [Google Scholar]
- 15.Triantafillidou K, Dimitrakopoulos J, Iordanidis F, Koufogiannis D. Mucoepidermoid carcinoma of minor salivary glands: a clinical study of 16 cases and review of the literature. Oral Dis. 2006;12:364–370. doi: 10.1111/j.1601-0825.2005.01166.x. [DOI] [PubMed] [Google Scholar]
- 16.Davis JP, Maclennan KA, Schofield JB, Watkinson JC, Gluckman P. Synchronous primary mucosal melanoma and mucoepidermoid carcinoma of the maxillary antrum. J Laryngol Otol. 1991;105:370–372. doi: 10.1017/S0022215100116032. [DOI] [PubMed] [Google Scholar]
- 17.Schaeffer BT, Som PM, Sacher M, Lanzieri CF, Solodnik P, Lawson W, et al. Coexistence of a nasal mucoepidermoid carcinoma and sphenoid mucoceles: CT diagnosis and treatment implications. J Comput Assist Tomogr. 1985;9:803–805. doi: 10.1097/00004728-198507010-00028. [DOI] [PubMed] [Google Scholar]
- 18.Kapadia SB, Barnes L, Pelzman K, Mirani N, Heffner DK, Bedetti C. Carcinoma ex oncocytic Schneiderian (cylindrical cell) papilloma. Am J Otolaryngol. 1993;14:332–338. doi: 10.1016/0196-0709(93)90091-K. [DOI] [PubMed] [Google Scholar]
- 19.Rosdeutscher JD, Burnette R. Nasal mucoepidermoid carcinoma. Otolaryngol Head Neck Surg. 2003;129:291–292. doi: 10.1016/S0194-5998(03)00518-7. [DOI] [PubMed] [Google Scholar]
- 20.Esposito F, Kelly DF, Vinters HV, DeSalles AA, Sercarz J, Gorgulhos AA. Primary sphenoid sinus neoplasms: a report of four cases with common clinical presentation treated with transsphenoidal surgery and adjuvant therapies. J Neurooncol. 2006;76:299–306. doi: 10.1007/s11060-005-7285-z. [DOI] [PubMed] [Google Scholar]
- 21.Weinstein IR, Nagai I, Yamanaka H. Mucoepidermoid tumor of the maxilla. Report of a case. Oral Surg Oral Med Oral Pathol. 1967;23:1–11. doi: 10.1016/0030-4220(67)90476-8. [DOI] [PubMed] [Google Scholar]
- 22.Thomas GR, Regalado JJ, McClinton M. A rare case of mucoepidermoid carcinoma of the nasal cavity. Ear Nose Throat J. 2002;81:519–522. [PubMed] [Google Scholar]
- 23.Lee K, Suei Y, Yamada T, Masuda S, Ogawa I, Tanimoto K. Bone formation in a carcinoma of the maxillary antrum. Dentomaxillofac Radiol. 1999;28:375–377. doi: 10.1038/sj.dmfr.4600474. [DOI] [PubMed] [Google Scholar]
- 24.Kaznelson DJ, Schindel J. Mucoepidermoid carcinoma of the air passages: report of three cases. Laryngoscope. 1979;89:115–121. doi: 10.1288/00005537-197901000-00012. [DOI] [PubMed] [Google Scholar]
- 25.Ichimura K, Nozue M, Hoshino T, Yano J. Bilateral primary malignant neoplasms of the maxillary sinus: report of a case and statistical analysis of the reports in Japan. Laryngoscope. 1981;91:804–810. doi: 10.1288/00005537-198105000-00014. [DOI] [PubMed] [Google Scholar]
- 26.Simpson RJ, Hoang KG, Hyams VJ, Jarchow RC. Mucoepidermoid carcinoma of the maxillary sinus. Otolaryngol Head Neck Surg. 1988;99:419–423. doi: 10.1177/019459988809900412. [DOI] [PubMed] [Google Scholar]
- 27.McKee DF, Rao RN, Elliott DC, Harmon JD, Porubsky ES. Simultaneous mucoepidermoid carcinoma and Paget’s disease of the maxillary sinus. Otolaryngol Head Neck Surg. 1987;97:339–340. doi: 10.1177/019459988709700317. [DOI] [PubMed] [Google Scholar]
- 28.Peison B, Benisch B, Schwartz IS, Gordon RE. Clear-cell mucoepidermoid carcinoma arising in the nasal cavity: case report with ultrastructural observations. Mt Sinai J Med. 1988;55:417–420. [PubMed] [Google Scholar]
- 29.Bergman F. Tumors of the minor salivary glands. A report of 46 cases. Cancer. 1969;23:538–543. doi: 10.1002/1097-0142(196903)23:3<538::AID-CNCR2820230304>3.0.CO;2-7. [DOI] [PubMed] [Google Scholar]
- 30.Healey WV, Perzin KH, Smith L. Mucoepidermoid carcinoma of salivary gland origin. Classification, clinical-pathologic correlation, and results of treatment. Cancer. 1970;26:368–388. doi: 10.1002/1097-0142(197008)26:2<368::AID-CNCR2820260219>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 31.Hayashi Y, Matsuyama Z, Murai M, Shimokawa K, Amano Y, Hashizume T, et al. A case of meningeal carcinomatosis due to the ethmoid sinus mucoepidermoid carcinoma. Rinsho Shinkeigaku. 2005;45:422–425. [PubMed] [Google Scholar]
- 32.Kraus DH, Sterman BM, Levine HL, Wood BG, Tucker HM, Lavertu P. Factors influencing survival in ethmoid sinus cancer. Arch Otolaryngol Head Neck Surg. 1992;118:367–372. doi: 10.1001/archotol.1992.01880040025005. [DOI] [PubMed] [Google Scholar]
- 33.Littman MS, Kirsh IE, Keane AT. Radium-induced malignant tumors of the mastoid and paranasal sinuses. AJR Am J Roentgenol. 1978;131:773–785. doi: 10.2214/ajr.131.5.773. [DOI] [PubMed] [Google Scholar]
- 34.Carinci F, Curioni C, Padula E, Calearo C. Cancer of the nasal cavity and paranasal sinuses: a new staging system. Int J Oral Maxillofac Surg. 1996;25:34–39. doi: 10.1016/S0901-5027(96)80009-9. [DOI] [PubMed] [Google Scholar]
- 35.Suzuki S, Hanata K, Nanjo H, Ishikawa K. Adenosquamous carcinoma of maxillary sinus: case showing complete response to S-1. J Laryngol Otol 2009; 1–5. [DOI] [PubMed]
- 36.Bridger MW, Beale FA, Bryce DP. Carcinom of the paranasal sinuses—a review of 158 cases. J Otolaryngol. 1978;7:379–388. [PubMed] [Google Scholar]
- 37.Ogawa T. A clinico-pathological study of adenocarcinomas of the nasal cavity and paranasal sinuses. Nippon Jibiinkoka Gakkai Kaiho. 1989;92:317–333. doi: 10.3950/jibiinkoka.92.317. [DOI] [PubMed] [Google Scholar]
- 38.Wyllie JW, III, Kern EB, Djalilian M. Isolated sphenoid sinus lesions. Laryngoscope. 1973;83:1252–1265. doi: 10.1288/00005537-197308000-00010. [DOI] [PubMed] [Google Scholar]
- 39.Subramaniam V, Kumar P, Thahir M. Mucoepidermoid carcinoma of a nasal cavity—a rare tumour. Klin Onkol. 2010;23:354–357. [PubMed] [Google Scholar]
- 40.Thorup C, Sebbesen L, Dano H, Leetmaa M, Andersen M, Buchwald C, et al. Carcinoma of the nasal cavity and paranasal sinuses in Denmark 1995–2004. Acta Oncol. 2010;49:389–394. doi: 10.3109/02841860903428176. [DOI] [PubMed] [Google Scholar]
- 41.Loh KS, Barker E, Bruch G, O’Sullivan B, Brown DH, Goldstein DP, et al. Prognostic factors in malignancy of the minor salivary glands. Head Neck. 2009;31:58–63. doi: 10.1002/hed.20924. [DOI] [PubMed] [Google Scholar]
- 42.AJCC Cancer Staging Manual, 7th ed. New York: Springer, 2009.
- 43.Goode R, El-Naggar AK. Mucoepidermoid carcinoma. In: Barnes EL, Eveson JW, Reichart P, Sidransky D, editors. Pathology and genetics of head and neck tumours. Lyon, France: IARC Press; 2005. P. 219–20.
- 44.Barbareschi M, Murer B, Colby TV, Chilosi M, Macri E, Loda M, et al. CDX-2 homeobox gene expression is a reliable marker of colorectal adenocarcinoma metastases to the lungs. Am J Surg Pathol. 2003;27:141–149. doi: 10.1097/00000478-200302000-00001. [DOI] [PubMed] [Google Scholar]
- 45.Damiani JM, Damiani KK, Hauck K, Hyams VJ. Mucoepidermoid-adenosquamous carcinoma of the larynx and hypopharynx: a report of 21 cases and a review of the literature. Otolaryngol Head Neck Surg. 1981;89:235–243. doi: 10.1177/019459988108900218. [DOI] [PubMed] [Google Scholar]
- 46.Keelawat S, Liu CZ, Roehm PC, Barnes L. Adenosquamous carcinoma of the upper aerodigestive tract: a clinicopathologic study of 12 cases and review of the literature. Am J Otolaryngol. 2002;23:160–168. doi: 10.1053/ajot.2002.123462. [DOI] [PubMed] [Google Scholar]
- 47.Thompson LDR. Squamous cell carcinoma variants of the head & neck. Curr Diag Pathol. 2003;9:384–396. doi: 10.1016/S0968-6053(03)00069-3. [DOI] [Google Scholar]