Abstract
Lingual adenocarcinomas (ADC), either primary or metastatic to the tongue are extraordinarily rare neoplasms. Primary lingual adenocarcinomas are primarily of minor salivary gland origin. Two cases of primary colonic-type adenocarcinomas of the base of the tongue were recently reported for the first time in the English literature. We present an additional case of lingual intestinal-type adenocarcinoma with mucinous features that occurred in association with cervical node metastasis and discuss the clinicopathologic features and histogenetic aspects of this rare entity.
Keywords: Adenocarcinoma, Intestinal-type, Tongue, Minor salivary glands, Histogenesis
Introduction
Lingual adenocarcinomas (ADC), either primary or metastatic to the tongue, are rare neoplasms. Primary lingual adenocarcinomas are of minor salivary gland origin [1]. Two cases of primary colonic-type adenocarcinomas of the base of the tongue were recently reported for the first time in the English literature [2]. Similarly, metastatic adenocarcinomas to the tongue are extraordinarily uncommon but may include a variety of types including those of renal, hepatic, lung, endometrial, adrenal, thyroid, bone/soft tissue, and colorectal origin. As far as we can determine, there are only 3 reported cases of metastatic colonic adenocarcinoma to the tongue [3]. We present an additional case of lingual intestinal-type adenocarcinoma with mucinous features that occurred in association with cervical node metastasis and absence of a primary neoplasm in the gastrointestinal tract and elsewhere.
Case Report
The patient is a 49-year-old male who presented with a 6 week history of irritation of the back of the tongue. Approximately 4 weeks later, the patient noted a painless right neck mass. His prior medical history was remarkable for parathyroidectomy, cholecystectomy and right shoulder surgery, all for benign lesions. The patient had no history of radiation treatment. There was no history of tobacco use or significant alcohol exposure. The patient had a long history of sawdust exposure starting 20 years ago when he worked in a lumberyard handling building materials. He also used to work on weekends with his brother who is a carpenter and even was exposed to dust as a child when on jobs with his father who was a sheet rock installer. Currently, and for the past 10 months, he has worked for a commercial hardware chain with exposure to sawdust while cutting wood.
Physical examination of the head and neck revealed a 2.5 cm lymph node at the right level 2 region of the neck and an exophytic mass at the base of the tongue that was slightly tender to palpation. Fiberoptic examination did not reveal any evidence of involvement of the epiglottis and the laryngopharynx was normal.
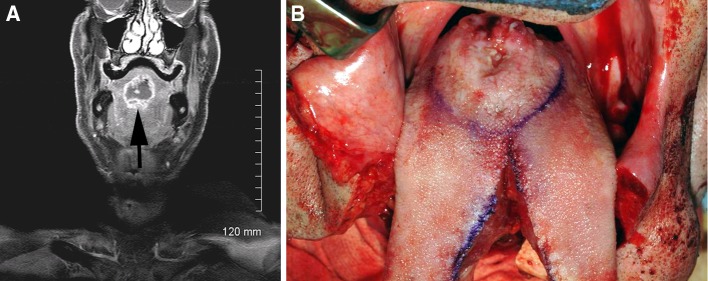
MRI Scan showed an irregular, centrally necrotic mass in the midline base of tongue, extending to the dorsal surface. The mass measured 3.6 × 2.9 × 2.5 cm radiologically (Fig. 1a). In addition, there was a prominent, centrally necrotic right level 2A lymph node. Additional enlarged right level 2 and level 3 cervical nodes were also identified. PET/CT imaging studies revealed no evidence of metastatic disease seen to chest, abdomen or pelvis. Direct laryngoscopy revealed a large, firm, exophytic midline lesion at the base of tongue (Fig. 1b). The lesion was biopsied and a diagnosis of mucinous (colloid) adenocarcinoma of minor salivary gland origin was rendered.
Fig. 1.
a Axial and coronal fat suppressed images demonstrate a multilobular midline rim enhancing and centrally necrotic oral tongue lesion (arrow). b The tumor (center) was resected through a median mandibulolabioglossotomy approach
Subsequently, the patient underwent a bilateral modified neck dissection and resection of the tongue carcinoma. The tumor was approached through a midline labiomandibulotomy approach with a complete resection of the tumor. The posterior margins did not extend to the vallecula. On completion of the ablative procedure, the right mobile tongue that had been preserved appeared to be ischemic. As a result, the right mobile tongue and the central portion of the tongue were reconstructed with a radial forearm free flap. The patient had an uneventful postoperative course with recovery of swallowing function and very favorable articulation. He is currently undergoing adjuvant radiation therapy to the oral cavity and bilateral neck.
Pathology
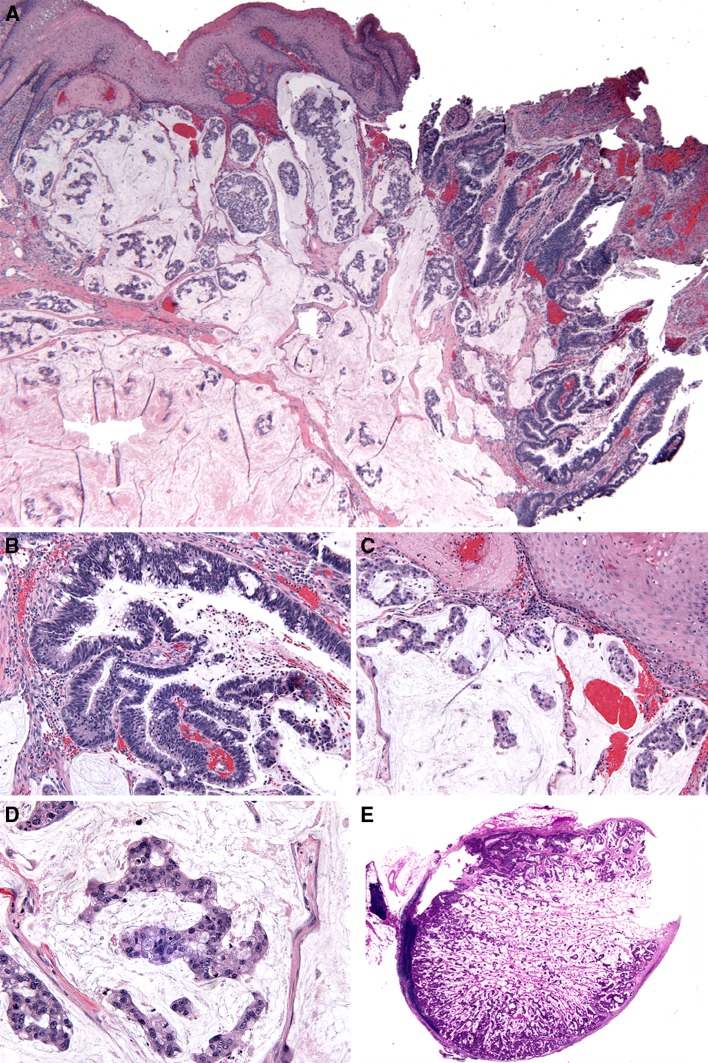
The initial biopsy revealed an infiltrative tumor composed of scattered small nests and isolated tumor cells floating in mucin pools, suggesting the diagnosis of a mucinous (colloid) adenocarcinoma. The overlying squamous mucosa showed no evidence of dysplastic changes. The surgical resection showed the presence of a submucosal invasive adenocarcinoma with tubulo-glandular growth pattern with mucinous features. Architecturally, the tubulo-glandular component showed pseudostratification of cells, minimal loss of polarity and a mild increase in mitotic figures, while cytologically it demonstrated increased nuclear to cytoplasmic ratio, elongated (cigar shaped) nuclei with a homogenous chromatin pattern and inconspicuous nucleoli. Abundant, apically located mucocytes were also observed (Fig. 2a, b). In addition, copious, variably-sized mucin lakes separated by fibrous septa were present. Within the mucin lakes, there were detached tumor cells floating individually and in clusters (Fig. 2a, c). Cytologically, these tumor cells showed increase in the nuclear-to-cytoplasmic ratio with intermediate grade nuclei that exhibited a fine chromatin pattern with central conspicuous nucleoli and eosinophilic cytoplasm (Fig. 2d). There was mild to moderate nuclear pleomorphism and identifiable mitotic figures; atypical mitoses and necrosis were not identified (Fig. 2d). Although the submucosal neoplastic proliferation focally approximated the surface epithelium, there was no evidence of direct continuity to the surface epithelium and no evidence of intraepithelial dysplasia. Focally, surface erosion/ulceration were present (Fig. 2a). Histologic examination of the lymph nodes recovered from the bilateral neck dissection revealed mucinous (colloid) metastasis in 4 of 64 lymph nodes on the right, with extracapsular extension in one of them, and no metastatic disease in the left neck (Fig. 2e).
Fig. 2.
a Colonic type adenocarcinoma with mucinous differentiation, low power. Unremarkable squamous epithelial lining with focal surface ulceration, underlying tubulo-glandular and mucinous adenocarcinomatous components. b Tubulo-glandular component showing pseudostratification and loss of polarity, medium power (20×). c Variably sized mucin pools separated by fibrous septa with tumor cells floating individually and/or in clusters, medium power (20×). d The floating tumor cells show increase in the nuclear-to-cytoplasmic ratio with intermediate grade nuclei and eosinophilic cytoplasm, high power (40×). e Right neck lymph node involved by metastatic colonic type adenocarcinoma, (whole mount)
Histochemical staining showed the presence of intraluminal and intracytoplasmic mucicarmine and diastase-resistant, periodic acid Schiff positive material. The extracellular mucinous pools also were mucicarmine and diastase-resistant, periodic acid Schiff positive.
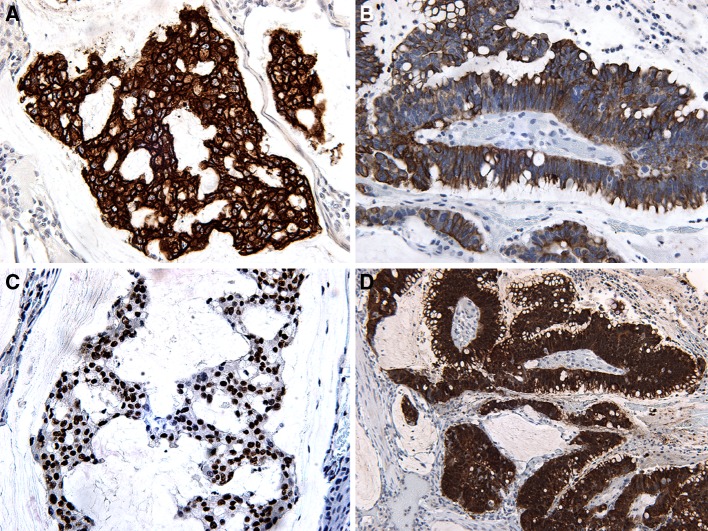
Immunohistochemical staining showed the lesional cells to be variably reactive for cytokeratins (AE1/AE3, CAM5.2, CK7, CK20), CDX-2 (nuclear staining), villin, p16, carcinoembryonic antigen (CEA), and epithelial membrane antigen (EMA), (Fig. 3a, d).
Fig. 3.
a CK7—strong membranous and cytoplasmic staining. b CK20—moderate membranous and cytoplasmic staining. c CDX-2 showing strong nuclear staining. d Villin—strong cytoplasmic staining
Discussion
Mucinous (colloid) adenocarcinoma (MAC) of salivary gland origin is very rare comprising less than 0.1% of epithelial salivary gland tumors [4]. MAC predilects to major salivary glands, specifically the submandibular gland, [4] but may also occur in minor salivary glands, particularly the palate [4]. The histologic features of MAC include the presence of malignant epithelial cells floating in pools of basophilic appearing mucinous material [4, 5]. Such were the features seen in this patient’s initial biopsy. However, following complete resection of the neoplasm it was evident that the findings were those of a colonic-type adenocarcinoma. Primary colonic-type adenocarcinomas of the head and neck are rare with the majority of such cases occurring in the sinonasal tract [6]. Intraoral primary colonic-type adenocarcinoma is an extraordinarily rare entity that, as far as we can determine, includes only two previously reported cases [2, 6]. Similar to our tumor, the cases reported by Bell et al. [2] were localized to the base of tongue with metastases to cervical lymph nodes, and occurred in the absence of a known primary colonic adenocarcinoma. Histologically, our case showed similar features to those reported by Bell et al. [2] including the presence of a submucosal-based infiltrative neoplasm comprised of an admixture of well-to moderately-differentiated colonic type adenocarcinoma characterized by tubular/glandular structures with an associated mucinous component.
Given the rarity of primary lingual colonic-type adenocarcinoma, it is imperative to exclude a metastasis to this site from a colonic primary. This imperative is further enhanced given the fact that the posterior third of the tongue, because of its rich blood supply and lymphatic drainage, is the most commonly involved site for a metastasis to localize [3, 7]. To date, there have been only 5 cases of gastrointestinal adenocarcinomas metastasizing to the tongue reported in the English literature, including 3 cases of metastatic colorectal adenocarcinoma, 1 case of gastric adenocarcinoma and 1 case of pancreatic adenocarcinoma [3]. Although there are overlapping histomorphologic and immunohistochemical features between primary colonic adenocarcinomas and primary base of tongue colonic-type adenocarcinoma, we are confident that our case represents a primary adenocarcinoma at the base of the tongue rather than a metastasis given the absence, following detailed clinical evaluation, of a primary colonic adenocarcinoma and the presence of metastatic carcinoma in a nodal distribution similar to that seen in association with base of tongue squamous cell carcinoma.
From a morphological standpoint, two potential pathways could have led to malignant transformation in this case, including transformation of minor salivary duct epithelium [2, 4] or malignant transformation of a choristoma with gastrointestinal elements [8]. Supporting the likely possibility of malignant transformation of minor salivary duct epithelium is the analogous correlation seen with primary intestinal-type adenocarcinoma of the sinonasal tract, where evidence of respiratory epithelium transformation to an intestinal phenotype has been noted in adjacent nonneoplastic epithelium [2, 9–11]. Of interest is the occupational background of this patient, which suggests the possibility of this tumor being etiologically related to prolonged exposure to wood particles in a similar fashion as seen among woodworkers developing intestinal-type adenocarcinomas (ITACs) [6].
On the other hand, the possibility of this tumor deriving from heterotopic gastrointestinal tissue becomes a plausible but less likely scenario. Embryologically, the mucosa of the anterior two thirds of the tongue is of ectodermal origin, whereas the posterior one third originates from the endoderm [12]. Several proposed histogenetic routes have been hypothesized to explain the occurrence of gastrointestinal heterotopia in the tongue. One theory states that heterotopic mucosa may be derived from entrapped embryonic gastrointestinal epithelium or ectoderm from the primitive stomodeum [8]. A second theory suggests that endodermal cell rests of the stomodeum are trapped by the lateral lingual swellings and subjected to inductive influences that cause their differentiation into gastrointestinal epithelia [13, 14]. The latest and most current theory supports the idea that a disturbance in the development of the notochord and surrounding structures accounts for misplaced segments of gastrointestinal mucosa in which adherent endodermal cells are caught during the infolding of the notochordal plate [15]. Lastly, one may speculate that heterotopic gastrointestinal mucosa could result from an error of differentiation, and that pluripotent endodermal stem cells have the capability of differentiating into all types of gastrointestinal epithelium. Such an error in differentiation could potentially result in gastric mucosa being present anywhere throughout the gastrointestinal tract [16, 17]. The risk of malignant transformation of heterotopic gastric and intestinal mucosa is unknown. Although there have been case reports that specifically describe malignant transformation of heterotopic gastric mucosa of the esophagus presenting as adenocarcinoma, [18] its occurrence in other areas of the GI tract may be underestimated. Of relevance is a recent case description of an adenocarcinoma arising in a lingual foregut duplication cyst of the floor of the mouth in a 61-year-old man [19]. The findings of this case report strongly suggest that such cysts, classified as choristomas partially composed of gastrointestinal epithelia, [20] could represent the anatomic foundation for the tumor described herein, rather than a salivary gland origin. Thus, recognition of the potential malignant transformation of lingual choristomas along with a thorough clinical and immunohistochemical workup of such tumors is pivotal to resolve the differential diagnosis of challenging cases such as this one.
In conclusion, we report a rare case of colonic-type adenocarcinoma of the base of the tongue, representing only the third such case reported in the world literature. In all likelihood, this neoplasm originated from the minor salivary gland, with malignant transformation of minor salivary duct epithelium.
References
- 1.Goldblatt LI, Ellis GL. Salivary gland tumors of the tongue: analysis of 55 new cases and review of the literature. Cancer. 1987;60:74–81. doi: 10.1002/1097-0142(19870701)60:1<74::AID-CNCR2820600113>3.0.CO;2-B. [DOI] [PubMed] [Google Scholar]
- 2.Bell D, Kupferman ME, Williams MD, Rashid A, El-Naggar AK. Primary colonic-type adenocarcinoma of the base of the tongue: a previous unreported phenotype. Hum Pathol. 2009;40:1798–1802. doi: 10.1016/j.humpath.2009.01.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Hassona Y, Hughes C, Prime SS. Metastatic tumors of the tongue. Oral Oncol. 2011;47:308–311. doi: 10.1016/j.oraloncology.2011.01.010. [DOI] [PubMed] [Google Scholar]
- 4.Ellis G, Auclair P, eds. Tumors of the salivary glands. 4th ed. Washington DC: Armed Forces Institute of Pathology; 2008. p. 383–6.
- 5.Ide F, Mishima K, Tanaka A, Saito I, Kusama K. Mucinous adenocarcinoma of minor salivary glands: a high-grade malignancy prone to lymph node metastasis. Vircows Arch. 2009;454:55–60. doi: 10.1007/s00428-008-0699-1. [DOI] [PubMed] [Google Scholar]
- 6.Wenig BM ed. Atlas of head and neck pathology, 2nd ed. Philadelphia, PA: Saunders-Elsevier, 2008; 121–4.
- 7.Davidson NGP, Wilson A. Tongue metastasis from carcinoma of the rectum. J Laryngol Otol. 1989;103:322–323. doi: 10.1017/S0022215100108825. [DOI] [PubMed] [Google Scholar]
- 8.Gorlin FJ, Jirasek JE. Oral cysts containing gastric or intestinal mucosa: unusual embryological accident of heterotopia. Arch Otolaryngol. 1970;91:594–597. doi: 10.1001/archotol.1970.00770040824018. [DOI] [PubMed] [Google Scholar]
- 9.Yom SS, Rashid A, Rosenthal DI, Elliot DD, Hanna EY, Weber RS, El-Naggar AK. Genetic analysis of sinonasal adenocarcinoma phenotypes: distinct alterations of histogenetic significance. Mod Pathol. 2005;18:315–319. doi: 10.1038/modpathol.3800315. [DOI] [PubMed] [Google Scholar]
- 10.Kennedy MT, Jordan RC, Berean KW, Perez-Odoñez B. Expression pattern of CK7, CK20, CDX-2 and villin in intestinal-type sinonasal adenocarcinoma. J Clin Pathol. 2004;57:932–937. doi: 10.1136/jcp.2004.016964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Korinth D, Pacyna-Gengelbach M, Deutschmann N, Hattenberger S, Bockmühl U, Dietel M, Schroeder HG, Donhuijsen K, Petersen I. Chromosomal imbalances in wood dust-related adenocarcinomas of the inner nose and their associations with pathological parameters. J Pathol. 2005;207:207–215. doi: 10.1002/path.1819. [DOI] [PubMed] [Google Scholar]
- 12.Lipsett J, Sparnon AL, Byard RW. Embryogenesis of enterocystomas-enteric duplication cysts of the tongue. Oral Surg Oral Med Oral Pathol. 1993;75:626–630. doi: 10.1016/0030-4220(93)90238-Y. [DOI] [PubMed] [Google Scholar]
- 13.Daley TD, Wysocki GP, Lovas GL, Smout MS. Heterotopic gastric cyst of the oral cavity. Head Neck Surg. 1984;7:168–171. doi: 10.1002/hed.2890070212. [DOI] [PubMed] [Google Scholar]
- 14.Woolgar JA, Smith AJ. Heterotopic gastrointestinal cyst of oral cavity: a developmental lesion? Oral Surg Oral Med Oral Pathol. 1988;66:223–225. doi: 10.1016/0030-4220(88)90097-7. [DOI] [PubMed] [Google Scholar]
- 15.Veeneklaas GMH. Pathogenesis of intrathoracic gastrogenic cysts. AMA Am J Dis Child. 1952;83:500–507. doi: 10.1001/archpedi.1952.02040080096010. [DOI] [PubMed] [Google Scholar]
- 16.Sauer C, Bickston SJ, Borowitz SM. Gastric heterotopia of the rectum. JPGN. 2010;50:329–333. doi: 10.1097/MPG.0b013e3181a1c476. [DOI] [PubMed] [Google Scholar]
- 17.Wolff M. Heterotopic gastric epithelium in the rectum: a report of three new gases with a review of 87 cases of gastric heterotopia in the alimentary canal. Am J Clin Pathol. 1971;55:604–616. doi: 10.1093/ajcp/55.5.604. [DOI] [PubMed] [Google Scholar]
- 18.Christensen WN, Sternberg SS. Adenocarcinoma of the upper esophagus arising in ectopic gastric mucosa. Two case reports and review of the literature. Am J Surg Pathol. 1987;11:397–402. doi: 10.1097/00000478-198705000-00009. [DOI] [PubMed] [Google Scholar]
- 19.Volchok J, Jaffer A, Cooper T, Al-Sabbagh A, Cavalli G. Adenocarcinoma arising in a lingual foregut duplication cyst. Arch Otolaryngol Head Neck Surg. 2007;133:717–719. doi: 10.1001/archotol.133.7.717. [DOI] [PubMed] [Google Scholar]
- 20.Eaton D, Billings K, Timmons C, Booth T, Biavati MJ. Congenital foregut duplication cysts of the anterior tongue. Arch Otolaryngol Head Neck Surg. 2001;127:1484–1487. doi: 10.1001/archotol.127.12.1484. [DOI] [PubMed] [Google Scholar]