Abstract
Mammary analogue secretory carcinoma (MASC) was recently identified as a distinct salivary gland neoplasm, morphologically resembling intercalated duct cell predominant acinic cell carcinoma (AciCC). To determine how frequently MASC has mimicked an intercalated duct cell predominant AciCC, we reviewed AciCC diagnosed from 1956 to 1975. Nine AciCC consecutively diagnosed in that period were identified. Based on morphologic examination, one case diagnosed as AciCC in a male patient in 1960 was re-classified as MASC [confirmed by fluorescence in situ hybridization (FISH) showing ETV6 translocation]. Another case diagnosed as AciCC of the palate in a female patient in 1975 was re-classified as mucoepidermoid carcinoma (based on the lack of acinar differentiation, presence of mucous cells, and confirmed by FISH showing MAML2 translocation). In this proof-of-principle study, we show that 1 in 9 cases historically designated as AciCC represents a MASC. “Intercalated duct cell predominant AciCC”, especially among male patients, most likely represent examples of MASC. For anatomic sites outside of the parotid glands, broader differential diagnoses should be considered before accepting morphologic variants of AciCC as the final diagnosis.
Keywords: Acinic cell carcinoma, Mammary analogue secretory carcinoma, Mucoepidermoid carcinoma, ETV6, MAML2
Introduction
Acinic cell carcinoma (AciCC) was described as a distinct entity more than a century ago [1]; yet it was not recognized as a low grade adenocarcinoma until 1953 [2]. Neoplastic cells with serous acinar differentiation represent the morphologic hallmark of AciCC. In classic cases of AciCC, the tumor cells are characterized by numerous cytoplasmic basophilic zymogen granules. In addition to the prototypical acinar cells, intercalated duct type cells and cells with clear vacuolated cytoplasm were accepted as variants of AciCC. Cases with predominant intercalated duct type cells or cells with vacuolated cytoplasm are known to be diagnostically challenging [3]. For instance, the combination of cytoplasmic clearing and vacuolization with intraluminal mucin raises the diagnostic possibility of mucoepidermoid carcinoma (MEC).
Most recently, mammary analogue secretory carcinoma (MASC) was identified as a distinct salivary gland neoplasm [4]. Its microscopic features closely mimic “intercalated duct cell predominant AciCC”. A key molecular event separating MASC from AciCC is the ETV6-NTRK3 gene fusion, which generates a constitutively active tyrosine kinase EN [4]. In our own experience, 4 of 10 intercalated duct cell predominant AciCC diagnosed between 2007 and 2008 were bona fide MASC [5]. Currently, MASC appears to be a rare entity with only 21 cases reported in the literature [4–6]. One approach to estimate the incidence of MASC is to determine how frequently it has mimicked an intercalated duct cell predominant AciCC in historic cohorts.
To estimate the incidence of MASC historically labeled as AciCC, we reviewed AciCC diagnosed from 1956 to 1975. Some of the largest and most influential studies on AciCC were based on cases diagnosed during this time period [7]. Also, this time frame reflects an effort to maintain and update our historic pathology archive [8]. All changes of the original diagnoses were supported by the modern morphologic interpretation and fluorescence in situ hybridization (FISH) studies for ETV6 and MAML2 translocations.
Materials and Methods
Studied Patients
The study was approved by the University of Pittsburgh Institutional Review Board (IRB# PRO10060222). The pathology archive was searched as previously described [8] (Note: the annual case volume over the studied time period was about 6,000–10,000) [8]. Cases with available slides and/or tissue blocks were included in this study. The histological features were evaluated in accordance with the latest World Health Organization classification of head and neck tumors and the knowledge of MASC [4]. Medical records for the historic cases were not available; hence all clinical information was based on original pathology reports.
S100 immunohistochemistry was performed according to manufacturer’s recommendations (polyclonal rabbit antibody, 1:500, Dako, Carpinteria, CA, USA).
FISH for ETV6 translocation was performed with commercial break-apart ETV6 probes (Abbot Molecular, Des Plains, IL, USA) [5].
FISH forMAML2translocation was performed using commercial break-apart MAML2 probes (MEC 1 Dual Color Break-apart Probe, ZytoVision, Bremerhaven, Germany). Imaging analysis was performed with a fluorescence microscope (Nikon Eclipse E600) and Cytovysion Workstation (Applied Imaging, Santa Clara, CA, USA). Slides were counterstained with 4′-6-diamidino-2-phenylindole (DAPI) (Abbot Molecular, Des Plains, IL, USA).
Results
Eleven cases of AciCC diagnosed between 1956 and 1975 were identified. Glass slides and/or tissue blocks were available for 9 cases. Seven of 9 cases were characterized by tumor cells with unequivocal abundant basophilic granular cytoplasm (Fig. 1a). One of these cases demonstrated neoplastic cells with cytoplasmic clearing in addition to numerous cells with acinar differentiation (Fig. 1b).
Fig. 1.
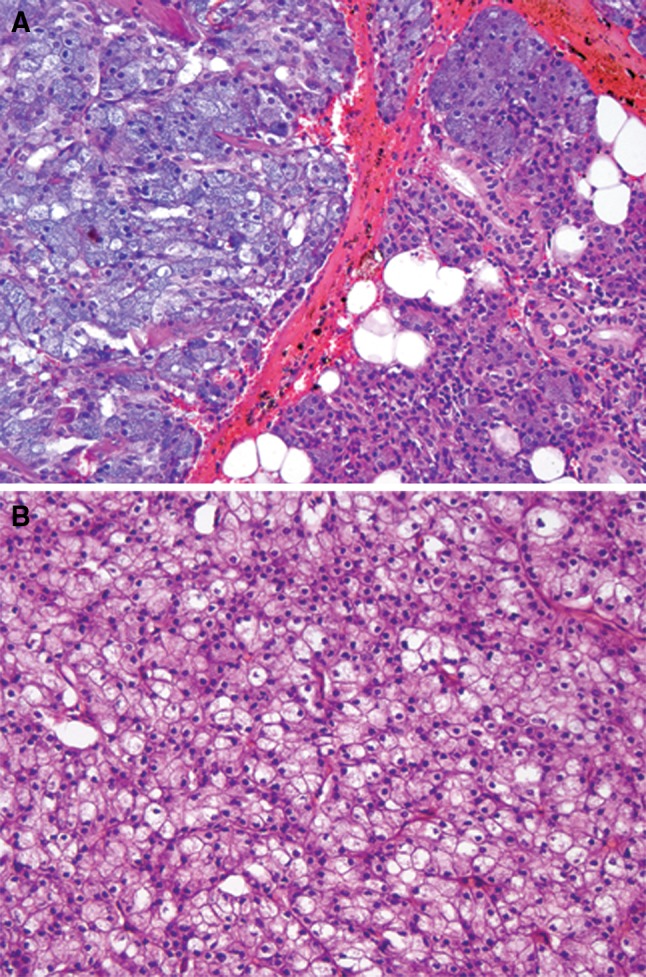
a Classic acinic cell carcinoma. Tumor cells (left half of the field) are well demarcated from normal parotid gland tissue (right half of the field) and show serous acinar cells with abundant zymogen granules, H&E, 200×. b Acinic cell carcinoma, a subset of cells demonstrate cytoplasmic clearing, H&E, 200×
Two cases did not fit the current diagnostic criteria of AciCC.
Case 1
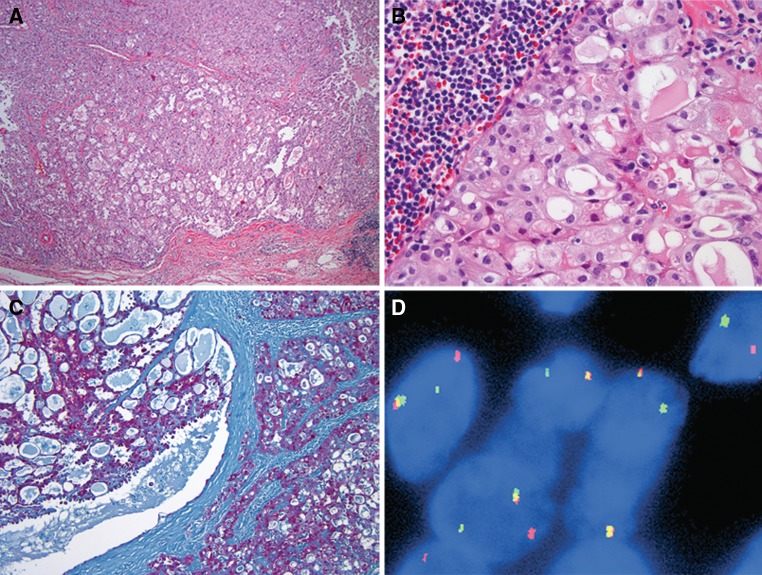
A man of unknown age presented with a parotid gland mass in March 1960. The 3.0 × 2.0 × 1.0 cm superficial parotidectomy revealed a 2.2 cm well circumscribed mass with mixed solid and microcystic growth patterns (Fig. 2a). The microcystic spaces were lined by cuboidal cells and filled with colloid-like secretions (Fig. 2b). Intracellular zymogen granules were absent. One mitotic figure in 10 high power fields was identified. Fifty years ago this tumor was designated as an “acinic cell carcinoma”; however, today, these features are more suggestive of MASC. Immunohistochemical stain for S100 was positive (Fig. 2c). This histological impression was confirmed by ETV6 FISH, which showed 63 of 66 (95.5%) analyzed cells harbor the ETV6 translocation (Fig. 2d).
Fig. 2.
a Mammary analogue secretory carcinoma with nodular growth, well-defined border, and combination of microfollicular growth (lower half of the image) with more solid growth, H&E, 40×. b Mammary analogue secretory carcinoma, glands are filled with colloid-like secretions. The cytoplasm is vacuolated and shows no basophilic zymogen granules. The lymphoid infiltrate in the left upper corner is part of a moderate tumor-associated lymphoid response. H&E, 400×. c Neoplastic cells express S100, immunohistochemistry, 100×. dETV6 (12p13) FISH with break-apart probes. ETV6 translocation is indicated by the split of red and green signals. Chromosomes with intact ETV6 gene show a yellow signal (overlapping of green and red signals)
Case 2
A 41-year-old female presented with a right hard palate lesion in July 1975. The 0.4 × 0.4 × 0.3 cm biopsy showed a well circumscribed encapsulated cystic lesion (Fig. 3a). Mucous cells with eccentric nuclei were present (Fig. 3b). Combined with the predominance of intermediate type cells, the microscopic findings were more suggestive of MEC. Indeed, MAML2 FISH showed 34 of 39 (87.2%) cells with a classic translocation pattern (Fig. 3c).
Fig. 3.
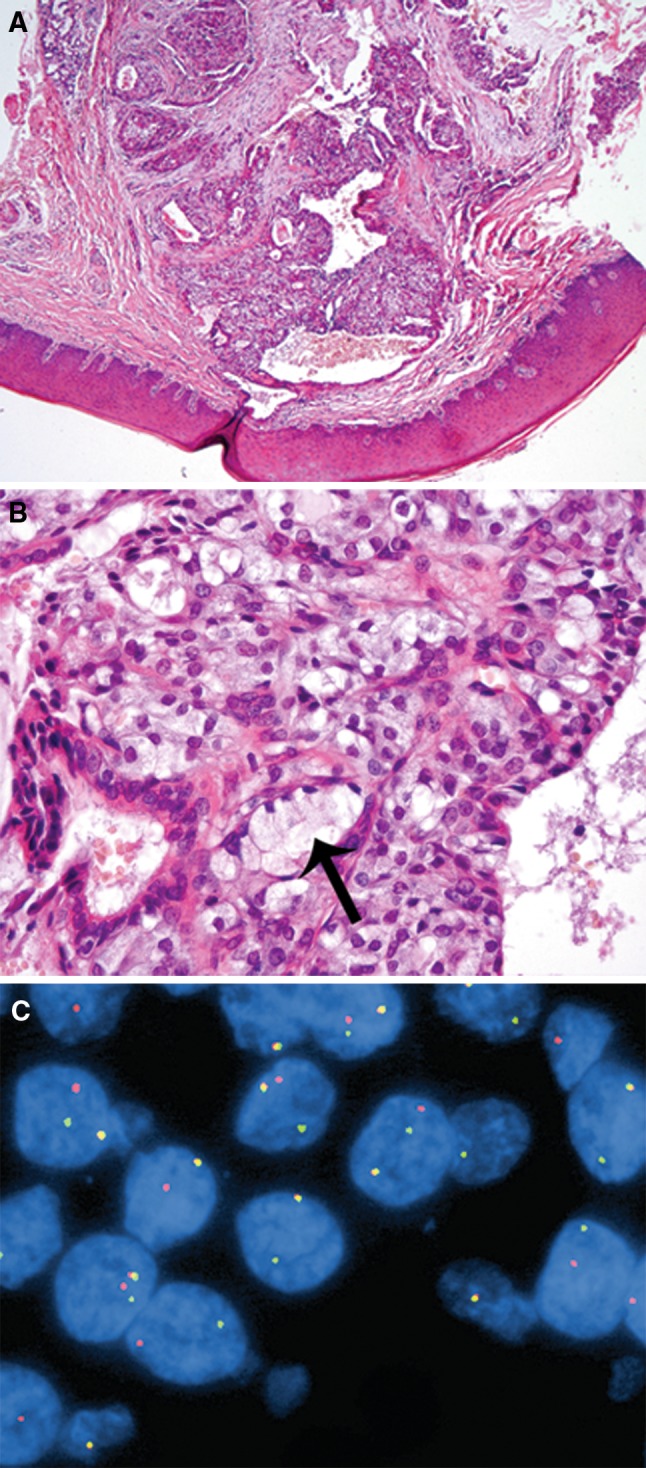
a Mucoepidermoid carcinoma. The cystic lesion involves the hard palate, original H&E prepared in 1975, 40×. b The intermediate cells show vacuolated cytoplasm with a subtle basophilic tint, mimicking zymogen granules of acinic cell carcinoma. However, rare unequivocal mucocytes can be identified (black arrow). cMAML2 FISH with break-apart probe. MAML2 translocation is indicated by the split of red and green signals. Chromosomes with intact MAML2 gene show a yellow signals (overlapping of green and red signals)
Clinicopathological Features of Patients with Confirmed Diagnosis of Acicc
In the remaining 7 cases, the original diagnosis of AciCC was confirmed. Consistent with previous reports, cases of confirmed AciCC affected more frequently female patients and most commonly involved the parotid gland [9]. Clinical follow-up was available in 3 cases. Two patients developed recurrence 9 and 49 months after resection, respectively. The third patient showed no signs of recurrence on a follow-up examination 50 months after the resection.
Four cases showed a predominantly solid growth pattern, 2 cases displayed a microfollicular growth pattern and 1 case had a papillary cystic pattern. Five cases of confirmed AciCC demonstrated tumor associated lymphoid infiltrate.
Discussion
Pathology archives represent valuable resources to study the evolution of diagnostic criteria and morphology of diseases over time [10, 11]. To maximize the functionality of a pathology archive, it has to be annotated to reflect the latest diagnostic standards. Revisiting cases diagnosed half a century ago is most informative when new entities and novel molecular markers are described.
Several years ago it was suggested that the morphology of intercalated duct type cell rich AciCC closely resembles that of the secretory carcinoma of the breast [12]. The ETV6 gene translocation (t12;15) was shown to be a key genetic feature of secretory carcinoma [13], shared with congenital (infantile) fibroscarcoma [14, 15], mesoblastic nephroma [14, 16], and adult acute myeloid leukemia [17].
In a comprehensive study employing expert morphologic evaluation combined with immunohistochemical and genetic studies, Skálová et al. [4] showed that a subset of intercalated duct type cell predominant AciCC and adenocarcinomas, not otherwise specified, represent a distinct entity—mammary analogue secretory carcinoma of salivary glands.
While the number of cases reviewed here may limit our conclusions, the difficulties of studying archived material are well known and future reviews of larger AciCC series will most likely cover a more “modern” time period. Our results may serve as a historic baseline for the number of AciCC mimicking MASC and should prompt a more critical reading of the most influential AciCC series based on material diagnosed more than half a century ago [7, 9].
As expected, intercalated duct type cells were predominant in the only case re-classified from AciCC to MASC. Interestingly, in one of the largest series of AciCC, intercalated duct cells predominated in about 32% of the tumors (90/285) [7].
In contrast to AciCC, early indications suggest that MASC appears to be more frequent in males. In the largest series to date, 9 of 16 patients with MASC were male [4], and in another report 3 of 4 patients were male [5]. A recent case report of MASC in the submandibular gland affected a male patient [6]. Including the case presented here, 14 of 22 MASC cases reported to date have been in male patients. Although in most published series AciCC predominantly affects women, in the study by Ellis et al., the number of male patients was rather significant. Since “approximately one half of the cases in this series originated from civilian sources”, the number of male patients can only partially be explained by the fact that Armed Forces Institute of Pathology (AFIP) served primarily a military population [7]. It is possible that a proportion of AciCC in male patients with intercalated duct cell predominance may represent MASC. However, due to the current state of AFIP [18], it would be difficult to test this hypothesis.
Previous studies of AciCC have also documented some variations in age, local recurrence, distant metastasis and mortality rates [7, 9, 19, 20]. These differences have to be re-examined as our understanding of MASC and AciCC improves.
Another factor that may complicate the diagnosis of AciCC is intracytoplasmic and intraluminal mucicarmine positivity. Today, these findings should raise the possibility of MASC or MEC. The MAML2 translocation in MEC has been unequivocally documented in the literatures over the past two decades [21, 22]. We used MAML2 FISH in this study to substantiate our reclassification of AciCC as MEC. This case was challenging due to the predominance of intermediate type cells and may reflect the expectation for a more prominent epidermoid component in MEC, a diagnostic belief that prevailed in 1960s and 1970s [10].
In summary, this proof-of-principle study showed that 1 in 9 cases of AciCC represents a MASC. More specifically, cases historically designated as “intercalated duct cell predominant AciCC”, especially those in male patients, most likely represent examples of MASC.
Acknowledgments
We are grateful for excellent support by the Developmental Laboratory, Department of Pathology, University of Pittsburgh Medical Center.
Conflict of interest
The authors declare that there is no conflict of interests in this study.
References
- 1.Nasse D. Die Geschw¸lste der Speicheldr¸sen und verwandte Tumoren des Kopfes. Arch klin chir. 1892;44:233–302. [Google Scholar]
- 2.Buxton RW, Maxwell JH, French AJ. Surgical treatment of epithelial tumors of the parotid gland. Surg Gynecol Obst. 1953;97(4):401–416. [PubMed] [Google Scholar]
- 3.Chiosea SI, Peel R, Barnes EL, Seethala RR. Salivary type tumors seen in consultation. Virchows Archiv Int J Pathol. 2009;454(4):457–466. doi: 10.1007/s00428-009-0742-x. [DOI] [PubMed] [Google Scholar]
- 4.Skálová A, Vanecek T, Sima R, Laco J, Weinreb I, Perez-Ordonez B, et al. Mammary analogue secretory carcinoma of salivary glands, containing the ETV6-NTRK3 fusion gene: a hitherto undescribed salivary gland tumor entity. Am J Surg Pathol. 2010;34(5):599–608. doi: 10.1097/PAS.0b013e3181d9efcc. [DOI] [PubMed] [Google Scholar]
- 5.Griffith C, Seethala R, Chiosea SI. Mammary analogue secretory carcinoma: a new twist to the diagnostic dilemma of zymogen granule poor acinic cell carcinoma. Virchows Archiv Int J Pathol. 2011;459(1):117–118. doi: 10.1007/s00428-011-1098-6. [DOI] [PubMed] [Google Scholar]
- 6.Petersson F, Lian D, Chau YP, Yan B. Mammary analogue secretory carcinoma: the first submandibular case reported including findings on fine needle aspiration cytology. Head Neck Pathol. 2011 doi: 10.1007/s12105-011-0283-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ellis GL, Corio RL. Acinic cell adenocarcinoma. A clinicopathologic analysis of 294 cases. Cancer. 1983;52(3):542–549. doi: 10.1002/1097-0142(19830801)52:3<542::AID-CNCR2820520326>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- 8.Smith MA, Barnes EL, Chiosea SI. Pathology archive: evaluation of integrity, regulatory compliance, and construction of searchable database from print reports. Am J Clin Pathol. 2011;135(5):753–759. doi: 10.1309/AJCP3CVA2NAVUUVU. [DOI] [PubMed] [Google Scholar]
- 9.Lewis JE, Olsen KD, Weiland LH. Acinic cell carcinoma. Clinicopathologic review. Cancer. 1991;67(1):172–179. doi: 10.1002/1097-0142(19910101)67:1<172::AID-CNCR2820670129>3.0.CO;2-X. [DOI] [PubMed] [Google Scholar]
- 10.Chenevert J, Barnes LE, Chiosea SI. Mucoepidermoid carcinoma: a five-decade journey. Virchows Archiv Int J Pathol. 2011;458(2):133–140. doi: 10.1007/s00428-011-1040-y. [DOI] [PubMed] [Google Scholar]
- 11.Chenevert J, Chiosea S. Incidence of human papillomavirus in oropharyngeal squamous cell carcinomas: now and 50 years ago. Hum Pathol. 2011 doi: 10.1016/j.humpath.2011.03.009. [DOI] [PubMed] [Google Scholar]
- 12.Hirokawa M, Sugihara K, Sai T, Monobe Y, Kudo H, Sano N, et al. Secretory carcinoma of the breast: a tumour analogous to salivary gland acinic cell carcinoma? Histopathology. 2002;40(3):223–229. doi: 10.1046/j.1365-2559.2002.01346.x. [DOI] [PubMed] [Google Scholar]
- 13.Tognon C, Knezevich SR, Huntsman D, Roskelley CD, Melnyk N, Mathers JA, et al. Expression of the ETV6-NTRK3 gene fusion as a primary event in human secretory breast carcinoma. Cancer Cell. 2002;2(5):367–376. doi: 10.1016/S1535-6108(02)00180-0. [DOI] [PubMed] [Google Scholar]
- 14.Knezevich SR, Garnett MJ, Pysher TJ, Beckwith JB, Grundy PE, Sorensen PH. ETV6-NTRK3 gene fusions and trisomy 11 establish a histogenetic link between mesoblastic nephroma and congenital fibrosarcoma. Cancer Res. 1998;58(22):5046–5048. [PubMed] [Google Scholar]
- 15.Knezevich SR, McFadden DE, Tao W, Lim JF, Sorensen PH. A novel ETV6-NTRK3 gene fusion in congenital fibrosarcoma. Nat Genet. 1998;18(2):184–187. doi: 10.1038/ng0298-184. [DOI] [PubMed] [Google Scholar]
- 16.Argani P, Fritsch M, Kadkol SS, Schuster A, Beckwith JB, Perlman EJ. Detection of the ETV6-NTRK3 chimeric RNA of infantile fibrosarcoma/cellular congenital mesoblastic nephroma in paraffin-embedded tissue: application to challenging pediatric renal stromal tumors. Mod Pathol. 2000;13(1):29–36. doi: 10.1038/modpathol.3880006. [DOI] [PubMed] [Google Scholar]
- 17.Eguchi M, Eguchi-Ishimae M, Tojo A, Morishita K, Suzuki K, Sato Y, et al. Fusion of ETV6 to neurotrophin-3 receptor TRKC in acute myeloid leukemia with t(12;15)(p13;q25) Blood. 1999;93(4):1355–1363. [PubMed] [Google Scholar]
- 18.McCook A. Death of a pathology centre: shelved. Nature. 2011;476(7360):270–272. doi: 10.1038/476270a. [DOI] [PubMed] [Google Scholar]
- 19.Bhaskar SN. Acinic-Cell Carcinoma of Salivary Glands. Report of Twenty-One Cases. Oral Surg Oral Med Oral Pathol. 1964;17:62–74. doi: 10.1016/0030-4220(64)90316-0. [DOI] [PubMed] [Google Scholar]
- 20.Abrams AM, Cornyn J, Scofield HH, Hansen LS. Acinic cell adenocarcinoma of the major salivary glands. A clinicopathologic study of 77 cases. Cancer. 1965;18:1145–1162. doi: 10.1002/1097-0142(196509)18:9<1145::AID-CNCR2820180915>3.0.CO;2-#. [DOI] [PubMed] [Google Scholar]
- 21.Horsman DE, Berean K, Durham JS. Translocation (11;19)(q21;p13.1) in mucoepidermoid carcinoma of salivary gland. Cancer Genet Cytogenet. 1995;80(2):165–166. doi: 10.1016/0165-4608(94)00187-G. [DOI] [PubMed] [Google Scholar]
- 22.Seethala RR, Dacic S, Cieply K, Kelly LM, Nikiforova MN. A reappraisal of the MECT1/MAML2 translocation in salivary mucoepidermoid carcinomas. Am J Surg Pathol. 2010;34(8):1106–1121. doi: 10.1097/PAS.0b013e3181de3021. [DOI] [PubMed] [Google Scholar]