Abstract
Bizarre parosteal osteochondromatous proliferation (BPOP) also eponymically called “Nora’s lesion”, is a rare benign reactive bone lesion first reported in 1983. BPOP occurs classically on the bones of the hands and feet and long bones. This lesion can easily be confused, both clinically and microscopically, with other benign and malignant lesions of bone, including osteochondroma, parosteal osteosarcoma, myositis ossificans and reactive periostitis. BPOP has been reported to have a high rate of recurrence. Only 3 cases of BPOP of the head and neck have been reported in the literature, of which one involved the maxilla. We present a rare case of BPOP involving the mandible in a 10 year old African American male. Microscopically, a fibro-cartilaginous cap giving rise to a proliferation of variably mineralized osteophytic finger-like projections of bone was seen. Multiple trabeculae of “blue bone” were noted as well as numerous atypical appearing chondrocytes. The lesion recurred within 4 months following the initial excision but has not recurred to date after the second local excision. To the best of our knowledge, this is the first report of BPOP arising in the mandible. In addition, we discuss the clinical and microscopic features, differential diagnosis, and prognosis of this rare entity. We present a case of BPOP of the mandible and believe this is the first report of such a case in the mandible.
Keywords: Bizarre parosteal osteochondromatous proliferation, Nora’s lesion, Mandible
Introduction
Bizarre parosteal osteochondromatous proliferation (BPOP) was first described by Nora et al. [1] in 1983 in which 35 histologically and radiographically distinctive bony lesions involving the proximal phalanges, metatarsals, or metacarpals were reported. The authors believed this lesion was a reactive process. Since then several other authors have reported this lesion on the skull and long bones (including humerus, ulna, radius, fibula, and femur) [2, 3]. These lesions arise in the periosteum and histologically resemble osteochondroma or osteosarcoma. BPOP typically presents as a broad based calcific mass attached to the underlying cortex [1–3]. The pathogenesis of BPOP is not clearly understood; however, most reports consider the lesion to be reactive in nature [4–6]. BPOP has been reported to exhibit a high recurrence rate after surgical resection [1, 3, 7, 8]. Microscopically BPOP demonstrates three distinct zones, including: (1) a hypercellular cartilage with calcification and ossification that demonstrates a characteristic basophilic staining pattern (“blue bone”); (2) central portions with variably mineralized and calcified osteophyte like bone; and (3) a spindle cell predominant stroma [2]. Some of these features may lead to a mistaken diagnosis of parosteal osteosarcoma [2, 3]. The microscopic differential diagnosis includes both benign and malignant processes including osteochondroma, parosteal osteosarcoma, myositis ossificans, and florid reactive periostitis. We document a rare case of BPOP of the mandible in a 10-year-old African American male. The lesion was conservatively excised only to recur within a month. This is the first report of BPOP of the mandible and furthermore, only three cases to date have been reported in the bones of the head and neck.
Case Report
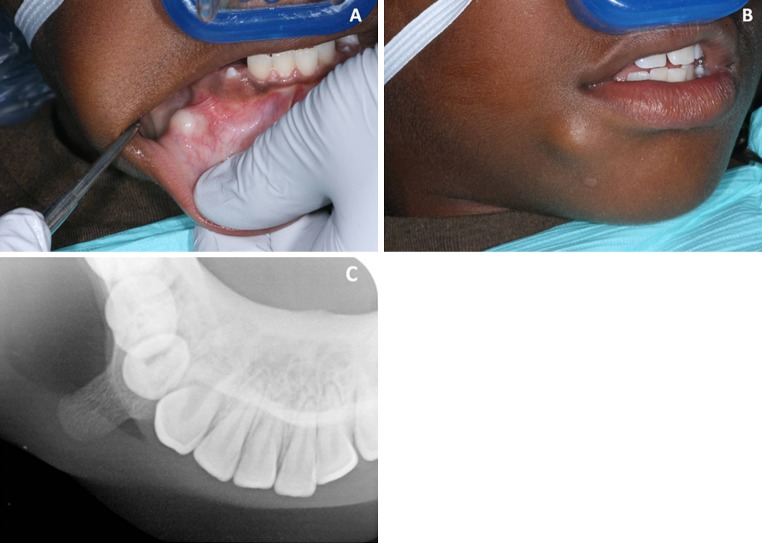
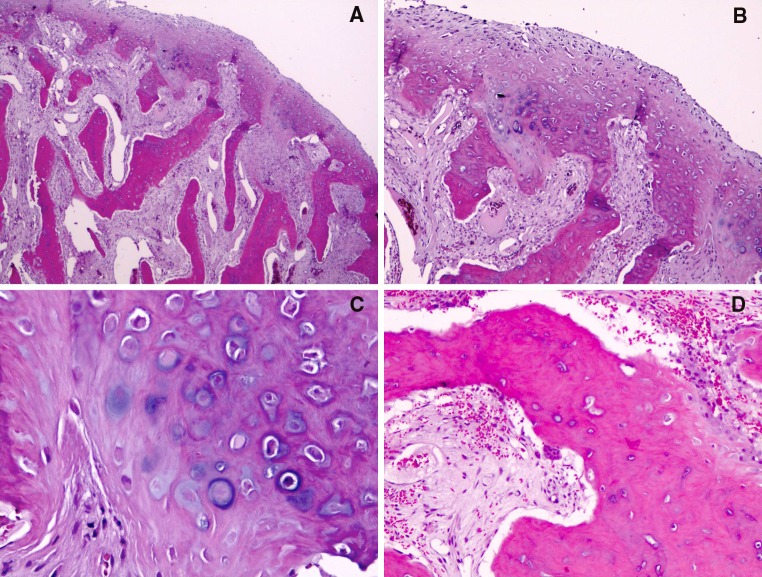
A 10-year-old African American male presented to our dental clinic for evaluation of a bony hard mass on the right lower jaw. Clinical examination revealed a bony protrusion located in the non-attached gingiva and vestibule near the mandibular right canine and first pre-molar. The lesion was asymptomatic but had been enlarging gradually to cause stretching of the overlying mucosa and was esthetically displeasing to the patient and his parents (Fig. 1a, b). The lesion was present for an unknown duration (Fig. 1c). A mandibular occlusal radiograph revealed a well-defined osseous growth with density approximating that of cancellous bone. The opacity appeared to be projecting laterally from the buccal cortical plate in the region of the right mandibular canine and first bicuspid. The base of the lesion was not clearly visible, as the partially erupted canine was superimposed upon the base (Fig. 1c). The clinical impression was bony exostosis and surgical removal was planned. A mucosal flap was laid and the lesion was completely exposed and found to be arising from the mandibular bone immediately subjacent to the right mandibular first premolar. Two partially erupted premolar teeth were seen in the vicinity. During excision it was felt that the lesion did not affect the cortical bone. The bony mass was easily removed and submitted for microscopic examination. Gross examination revealed a tan brown nodular hard tissue mass measuring 0.7 × 0.7 × 0.7 cm (Fig. 2a, b). The tissue was decalcified and microscopic examination revealed an unusual proliferation of variably mineralized and calcified bone surfaced on one end by a fibrocartilagenous rim and surrounded by vascular fibrous connective tissue. Numerous osteophytic finger-like projections of bone were noted extending downward from the cartilaginous cap. These bony trabeculae exhibited variable mineralization and osteocytes within lacunae and were rimmed by prominent plump osteoblasts. Prominent resting and reversal lines were seen throughout these bony trabeculae (Fig. 2c). Subjacent to the cartilaginous cap, multiple trabeculae of partially calcified and mineralized “blue bone” was noted. Intermixed within this cartilaginous area were numerous atypical appearing pleomorphic chondrocytes (Fig. 2d). The intervening connective tissue was highly vascular and demonstrated numerous dilated and engorged vascular channels and scattered inflammatory cells and fibroblasts. Occasional multinucleated osteoclastic giant cells were also seen within the framework. Although no osteoid formation was noted, osteochondroma and parosteal low-grade osteosarcoma were considered in the differential diagnosis. After further review of the slides, taking into consideration the clinical and radiographic presentation, a diagnosis of BPOP was rendered.
Fig. 1.
a Bony protrusion located in the non-attached gingiva and vestibule of the lower right mandible, note the stretching of the overlying mucosa. b An elevated bony hard mass located in the lower right side of face. c An occlusal radiograph revealed a well-defined osseous growth with density approximating that of cancellous bone. Note the partially erupted canine was superimposed upon the base of the lesion
Fig. 2.
a A fibrocartilagenous cap with an atypical proliferation of variably mineralized and calcified bony trabeculae (“blue bone”). Note the atypical interface between the cartilage cap and bony proliferation (hematoxylin-eosin, original magnification ×25). b Higher magnification of image 2A focusing on the area of interface (hematoxylin-eosin, original magnification ×100). c High magnification of cartilaginous zone with numerous atypical pleomorphic chondrocytes (hematoxylin-eosin, original magnification ×200). d Illustrates a highly vascular connective tissue, scattered inflammatory cells, fibroblasts, and occasional osteoclasts (hematoxylin-eosin, original magnification ×100)
The patient was placed under close follow up due to the known recurrence potential of these lesions. The patient reported back to the dental clinic within 5 weeks with a recurrent but smaller mass. The patient was scheduled with the oral surgery department and a cone-beam volumetric computerized tomographic scan (CT scan) was performed (Fig. 3a). The radiographic findings of the recurrent lesion were identical to the initial presentation. The base of lesion was better visible and minor disruption of the crestal third of the buccal cortical margin was observed. The lesion was excised by circumferential osteotomy under local anesthesia (Fig. 3b, c). The bone around the lesion extending to the roots of the mandibular right canine and first premolar was removed as well. The mental nerve was visualized during surgery and appeared to be intact. The recurrent lesion along with associated bone measured 1.3 × 1.0 × 0.7 cm. Microscopic examination revealed features very similar to the primary lesion except for the cartilaginous cap which was absent (most likely lost during surgery). No features of malignancy were noted. The patient was followed closely and at approximately 1 year follow-up no further recurrence was noted. The patient remains asymptomatic.
Fig. 3.
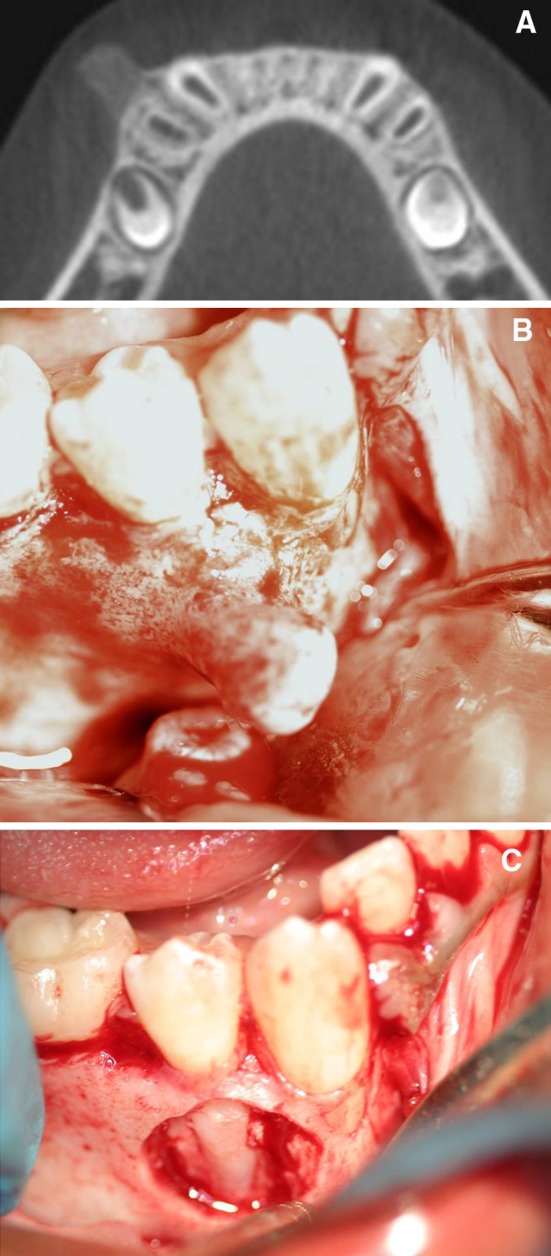
a A section from the cone-beam volumetric CT. Note the base of lesion is clearly visible. b The recurrent lesion resembles a similar bony protuberance as seen before. c The recurrent lesion was removed in addition to the associated cortical bone. Note the exposed roots of the canine and premolar
Discussion
Nora et al. in 1983 reported 35 cases, from the archives of the Mayo Clinic, of unusual peripheral skeletal osteochondromatous lesions on the bones of the hand and feet with distinctive histopathologic and radiographic features. These lesions were seen over a wide age range with no sex predilection. The patients had no history of trauma or infection. Only 5 of the 35 lesions reported were seen in the tarsal or metatarsal bones, while the other 30 were seen in the carpals and metacarpal bones of the hands [1]. The primary treatment was surgical excision and the main preoperative diagnosis was osteochondroma. Of the 35 lesions, 18 recurred and of these 8 recurred more than once. The time between first excision and recurrence ranged from 2 months to 2 years. Second recurrences were noted 3–13 years after second excision. No deaths or metastasis related to the lesions were reported [1].
Since the first report by Nora et al. in 1983, many authors have reported this lesion in various bones throughout the skeletal system including the bones of the hands and feet, and the radius, ulna, fibula, femur, tibia, and humerus. One case each has been reported on the parietal bone of the skull, anterior maxilla, and the zygoma [2, 9, 10]. Meneses et al. [3] reviewed the largest series of BPOP from the archives of the Mayo clinic, including some originally reported by Nora et al. [1]. Of the cases in the bones of the head and neck, Shakib et al. [9] reported BPOP in a 31-year-old Afghani male where the lesion was present for 8 months before the patient sought treatment. The lesion (2 × 1.5 cm) was asymptomatic and the presenting complaint was the presence of swelling. There was no previous history of trauma or infection in the area. A CT scan on the bone demonstrated a bony protrusion with no destruction of the underlying bone and only minor surface irregularity. The microscopic examination demonstrated the features of BPOP, including dense bone with endochondral ossification, lack of bony continuity with the medullary space, and mild degree of cellular atypia. The lesion did not recur within 2 years of available follow-up information. Another case reported by Shankly et al. [10] on the maxilla is the only case to date of BPOP involving the jaw bones. This lesion occurred in a 2-year-old female and presented as an asymptomatic enlargement of the palatal gingiva lingual to the primary central incisors. The lesion had been present for 4 months and measured 4 × 5 mm. The patient presented with a diastema between the maxillary central incisors caused by the enlarging lesion but no mobility of the involved teeth was noted. Occlusal radiographs did not clearly demonstrate any involvement of the underlying bone. The radiographic density was similar to trabecular bone. A preoperative diagnosis of ossifying fibroma was considered. During surgery, the maxillary cortex was found to be extremely thin. The lesion was removed along with the two central incisors, but the base of the lesion was left in situ. Microscopic examination reported by the authors was identical to previously reported cases of BPOP. The lesion recurred after 9 months possibly due to the incomplete excision. During the second definitive surgery, the lesion appeared to be well circumscribed and separated easily from the underlying bone. The histopathologic features of the recurrent lesion were similar to the primary lesion, except for the fact that the lesion was enclosed by a fibrous capsule without attachment to the underlying cortex. Another lesion in the head and neck was reported by Meneses et al. [3] involving the parietal bone of the skull. In this large case series, Meneses et al. [3] reported that 9 of the patients reported a history of trauma to the affected area, with 3 cases also reporting a history of pain. This association with trauma was also seen in the skull case. The demographic information was not reported in this review. No case with more than one lesion has been reported to date.
The most interesting aspect of BPOP are the radiographic and microscopic features which may be confused with several other osteochondromatous lesions, including both benign and malignant entities, such as osteochondroma, low grade parosteal osteosarcoma, myositis ossificans, and florid reactive periostitis. As the name implies, BPOP has an osteochondroma-like configuration. Radiographically, BPOP has a typical “stuck on” appearance, and lacks the cortical and medullary continuity seen in osteochondroma [11]. Osteochondroma, on the other hand, presents with a narrow or broad-based stalk that merges directly with the underlying cortex and medullary cavity [12]. Histopathologically, both lesions are covered peripherally by a cartilaginous cap; however, none of the atypical features, such as bizarre, binucleated, and enlarged chondrocytes, prominent proliferative activity, or irregular osteocartilagenous interface are seen in osteochondromas [12, 13]. The bony trabeculae are oriented at ninety degrees in osteochondromas [13], while BPOP demonstrates an irregular orientation of the trabecular bone, which also has a characteristic “bluish” appearance [11].
Low-grade parosteal osteosarcoma can also have features similar to BPOP both histologically and radiographically, although parosteal osteosarcomas are exceedingly rare in the small bones of the hands and feet. Parosteal osteosarcomas very commonly contain either a cartilage cap or islands of cartilage deeper within the tumor; however, this low-grade variant of osteosarcoma typically contains relatively mature bone deposited in an organized, variably cellular fibrous stroma. BPOP, on the other hand, displays the typical “blue bone”, especially at the interface with cartilage [3]. BPOP tend to exhibit a modest rate of recurrence based on previous reports in the literature. Usually recurrences manifest between 2 months and 2 years [1, 3, 8]. The presence of cartilage is not uncommon in parosteal osteosarcoma and typically is seen either superficially or deep within the lesion [12]. Radiographically, parosteal osteosarcoma arise from the outer layer of the periosteum and are often separated from the underlying cortex by a thin radiolucent cleft. Although they appear well-circumscribed, careful examination often reveals infiltration of the surrounding soft tissues. Higher stage lesions may also erode cortical bone [14] and can cause periosteal elevation [15]. Although one case of BPOP with infiltration and periosteal elevation has been reported, it is considered to be an extremely rare finding [16]. Rybak et al. in 2007 reported four cases of BPOP with radiographic evidence of medullary involvement but these cases were histologically proven to represent BPOP.
Myositis ossificans is a benign reactive lesion and may be considered in the radiographic differential diagnosis of BPOP. A periosteal reaction can often be observed radiographically in the bone subjacent to myositis ossificans [3]. Myositis ossificans is primarily a soft tissue lesion which is clinically easily distinguished from BPOP since it typically presents with pain and is precipitated by previous injury or infection [17]. Dorfman and Czerniak also propose that reactive lesions adjacent to the bone surface such as myositis ossificans share the same pathogenesis as reactive lesions of the bone surface and may develop a cortical attachment in the later stages [17].
Florid reactive periostitis, another lesion considered in the differential diagnosis of BPOP, demonstrates a periosteal reaction and juxtacortical soft tissue calcification [13] which is not seen in BPOP. Some authors have also suggested that BPOP may actually represent an intermediary stage between florid reactive periostitis and acquired osteochondroma that is either subungual or the so-called “turret” exostosis of the phalanges [17].
Periosteal osteosarcoma was not included in the differential diagnosis since radiographically it typically demonstrates soft tissue invasion with “sunburst” appearance and histologically resembles chondroblastic osteosarcoma [18].
Recent studies investigating the cytogenetics of BPOP have reported a t(1;17) (q32;q31) mutation [4], which indicates that BPOP may be a neoplastic process, while Teoh et al. reported a pericentric inversion of chromosome 6 between bands 6p25 and 6q15 and a paracentric inversion of the long arm of chromosome 7 between bands 7q22.1 and 7q31.3 [5]. This indicates that BPOP may arise due to more than one mutation or chromosomal disturbance. Zambrano et al. also compared 3 cases of subungual exostoses to 2 cases of BPOP histologically and cytogenetically and indicated that both lesions represent neoplastic entities and are not reactive proliferations [6].
The treatment of choice in BPOP is surgical excision with close follow up [19]. It is also recommended that any abnormal adjacent tissues should also be removed [19]. Wide resection is not recommended as it might impair function, especially in the hand and feet. BPOP demonstrates a fairly high rate of recurrence, ranging from 29 to 55% within a 2 year interval [1, 3, 8]. In Nora et al’s. [1] initial report, 18 of 35 cases (51%) recurred while Meneses et al. [3] and Dhont et al. [8] reported recurrence rates of 55 and 29%, respectively. To date only one case of malignant transformation in BPOP has been reported by Choi et al. [20], although it is not clear from their report whether BPOP underwent transformation to fibrosarcoma or there were two distinct entities arising in the same location. No cases of metastasis of BPOP have been reported. Michelsen et al. indicated that BPOP should be considered a neoplastic rather than a reactive process based on the atypical cellular features often found in the biopsy samples [11]. On the other hand, Horiguchi et al. reported a fibroblastic growth factor expressed by all chondrocytes in BPOP and a vascular endothelial growth factor secreted only by the large chondrocytes at the interface between the cartilage cap and bone, which suggests a reparative process since it mimics the process of endochondral ossification [21].
References
- 1.Nora FE, Dahlin DC, Beabout JW. Bizarre parosteal osteochondromatous proliferations of the hands and feet. Am J Surg Pathol. 1983;7(3):245–250. doi: 10.1097/00000478-198304000-00003. [DOI] [PubMed] [Google Scholar]
- 2.Abramovici L, Steiner GC. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion): a retrospective study of 12 cases, 2 arising in long bones. Hum Pathol. 2002;33(12):1205–1210. doi: 10.1053/hupa.2002.130103. [DOI] [PubMed] [Google Scholar]
- 3.Meneses MF, Unni KK, Swee RG. Bizarre parosteal osteochondromatous proliferation of bone (Nora’s lesion) Am J Surg Pathol. 1993;17(7):691–697. doi: 10.1097/00000478-199307000-00006. [DOI] [PubMed] [Google Scholar]
- 4.Nilsson M, Domanski HA, Mertens F, Mandahl N. Molecular cytogenetic characterization of recurrent translocation breakpoints in bizarre parosteal osteochondromatous proliferation (Nora’s lesion) Hum Pathol. 2004;35(9):1063–1069. doi: 10.1016/j.humpath.2004.02.008. [DOI] [PubMed] [Google Scholar]
- 5.Teoh KH, Shortt N, Wilkinson G, Salter DM, Robb JE, Porter DE. Bizarre parosteal osteochondromatous proliferation of the metatarsal: a pediatric case report and archival review. J Foot Ankle Surg. 2009;48(6):690 e7–90 e11. [DOI] [PubMed]
- 6.Zambrano E, Nose V, Perez-Atayde AR, Gebhardt M, Hresko MT, Kleinman P, et al. Distinct chromosomal rearrangements in subungual (Dupuytren) exostosis and bizarre parosteal osteochondromatous proliferation (Nora lesion) Am J Surg Pathol. 2004;28(8):1033–1039. doi: 10.1097/01.pas.0000126642.61690.d6. [DOI] [PubMed] [Google Scholar]
- 7.Bush JB, Reith JD, Meyer MS. Bizarre parosteal osteochondromatous proliferation of the proximal humerus: case report. Skeletal Radiol. 2007;36(6):535–540. doi: 10.1007/s00256-006-0236-8. [DOI] [PubMed] [Google Scholar]
- 8.Dhondt E, Oudenhoven L, Khan S, Kroon HM, Hogendoorn PC, Nieborg A, et al. Nora’s lesion, a distinct radiological entity? Skeletal Radiol. 2006;35(7):497–502. doi: 10.1007/s00256-005-0041-9. [DOI] [PubMed] [Google Scholar]
- 9.Shakib K, Kalsi H, Tsiridis E, Kumar M. Rare case of bizarre parosteal osteochondromatous proliferation presenting in the zygoma. Br J Oral Maxillofac Surg. 2010. [DOI] [PubMed]
- 10.Shankly PE, Hill FJ, Sloan P, Thakker NS. Bizarre parosteal osteochondromatous proliferation in the anterior maxilla: report of a case. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1999;87(3):351–356. doi: 10.1016/S1079-2104(99)70222-2. [DOI] [PubMed] [Google Scholar]
- 11.Michelsen H, Abramovici L, Steiner G, Posner MA. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) in the hand. J Hand Surg Am. 2004;29(3):520–525. doi: 10.1016/j.jhsa.2004.02.002. [DOI] [PubMed] [Google Scholar]
- 12.Teoh HH, Bethwaite PB, Thurston AJ. Bizarre parosteal osteochondromatous proliferation of the hand in a young man. Pathology. 1992;24(3):211–213. doi: 10.3109/00313029209063176. [DOI] [PubMed] [Google Scholar]
- 13.deLange EE, Pope TL, Jr, Fechner RE, Keats TE. Bizarre parosteal osteochondromatous proliferation vs. benign florid reactive periostitis. AJR Am J Roentgenol. 1987;148(3):650. doi: 10.2214/ajr.148.3.650-a. [DOI] [PubMed] [Google Scholar]
- 14.Tannenbaum DA, Biermann JS. Bizarre parosteal osteochondromatous proliferation of bone. Orthopedics. 1997;20(12):1186–1188. doi: 10.3928/0147-7447-19971201-16. [DOI] [PubMed] [Google Scholar]
- 15.Soon JL, Chang HC, Sim CS, Teoh LC, Low CO. A case of bizarre parosteal osteochondromatous proliferation of the hand. Singapore Med J. 2003;44(1):27–30. [PubMed] [Google Scholar]
- 16.Helliwell TR, O’Connor MA, Ritchie DA, Feldberg L, Stilwell JH, Jane MJ. Bizarre parosteal osteochondromatous proliferation with cortical invasion. Skeletal Radiol. 2001;30(5):282–285. doi: 10.1007/s002560100347. [DOI] [PubMed] [Google Scholar]
- 17.Dorfman HD, Czerniak B. Bone tumors. St. Louis: Mosby; 1998. [Google Scholar]
- 18.Unni KK, Inwards CY. Tumors of the osteoarticular system. In: Fletcher CDM, editor. Diagnostic histopathology of tumors. Philadelphia: Churchill Livingstone; 2007. pp. 1622–1623. [Google Scholar]
- 19.Gruber G, Giessauf C, Leithner A, Zacherl M, Clar H, Bodo K, et al. Bizarre parosteal osteochondromatous proliferation (Nora lesion): a report of 3 cases and a review of the literature. Can J Surg. 2008;51(6):486–489. [PMC free article] [PubMed] [Google Scholar]
- 20.Choi JH, Gu MJ, Kim MJ, Choi WH, Shin DS, Cho KH. Fibrosarcoma in bizarre parosteal osteochondromatous proliferation. Skeletal Radiol. 2001;30(1):44–47. doi: 10.1007/s002560000265. [DOI] [PubMed] [Google Scholar]
- 21.Horiguchi H, Sakane M, Matsui M, Wadano Y. Bizarre parosteal osteochondromatous proliferation (Nora’s lesion) of the foot. Pathol Int. 2001;51(10):816–823. doi: 10.1046/j.1440-1827.2001.01271.x. [DOI] [PubMed] [Google Scholar]