Abstract
Abnormalities in cell cycle regulation, tumor suppressor gene functions and apoptosis are frequent events in tumorigenesis. Their role in the pathogenesis and prognosis of primary mucosal melanomas (MM) of the upper aerodigestive tract remains unknown. Sixty-four patients (40 men, 24 women, median age 64 years) with MM were included in this study; 32 had tumors in the nasal/paranasal cavities, 28 in the oral cavity and 4 in the pharynx. Archival tissues from 47 initial mucosal tumors, 17 mucosal recurrences, and 13 nodal/distant metastases were subjected to immunohistochemistry using antibodies against p16, p53, and bcl-2. The results were correlated with histological features and survival data. Expressions of p16, p53, and bcl-2 proteins were seen in 25% (N = 19/76), 21% (N = 16/76), and 74% (N = 56/76) of all tumors, respectively. bcl-2 expression in the initial tumors was associated with significantly longer overall and disease specific survival (3.3 vs. 1.5 years, P ≤ 0.05). Expression of p16 was increasingly lost, from 32% in initial tumors to 12% in recurrent and 15% in metastatic tumors (P = 0.06). Tumors comprised of undifferentiated cells were significantly more p53 positive than epithelioid or spindle cells (80% vs. 33%, P = 0.02). Expression of these markers did not correlate with necrosis, or vascular and/or deep tissue invasion. Expression of bcl-2 is associated with better survival in MM. Loss of p16 was seen with tumor progression whereas aberrant p53 expression was frequent in undifferentiated tumor cells.
Keywords: Mucosal melanoma, Head and neck, p16, p53, bcl-2
Introduction
Primary mucosal melanomas (MM) of the head and neck are rare tumors comprising 0.7% of 84,836 melanomas in the National Cancer Data Base [1]. Their etiopathogenesis is poorly understood. MM is more aggressive than cutaneous melanomas. It frequently presents in advanced stage with regional and distant metastases, and it is less amenable to complete surgical resection. Well-defined histologic prognostic criteria comparable to Breslow thickness and Clark levels in cutaneous melanoma are lacking in MM. The aim of this study was to identify molecular markers for predicting prognosis, and for possible targeted therapy.
The G1/S phase regulator p16INK4a is a member of the cyclin-dependent kinase inhibitor (CDK4I, INK4a) protein family encoded by multiple tumor suppressor gene 1 (MTS1). Inactivation of p16 propels cells from G1 to S phase, and has been observed frequently in carcinomas, and reported in uveal melanomas [2, 3]. Franchi et al. [4] reported loss of p16 expression by immunohistochemistry in 73% of sinonasal melanomas and reported an association with morphology but not with prognosis. Loss of p16 expression has also been reported in a small number of oral melanomas, however, their prognostic significance is unknown [5].
In the presence of excessive DNA damage, the wild type p53 protein causes G1 arrest, up-regulates DNA repair genes, and promotes apoptosis. Genetic alterations, e.g. homozygous deletions and mutations in the p53 gene, are the most common events affecting nearly 50% of solid tumors [6–9]. The mutated p53 renders the cell resistant to chemo- and radiotherapy [10, 11]. Interestingly, cutaneous melanomas infrequently demonstrate aberrant p53 expression. Inactivation of p16INK4a and mutation in p53 tumor suppressor genes are early events in oncogenesis and appear to adversely influence prognosis in carcinomas of the breast, pancreas, colon, lung, and cutaneous melanoma [12].
The bcl-2 oncogene, located on chromosome 18q21, encodes a family of anti-apoptotic proteins that prolong cell survival. bcl-2 is over-expressed in several malignant neoplasms including cutaneous and ocular melanoma. Kostov et al. [13] investigated the expression of p16, p53, and bcl-2 in cutaneous melanomas and correlated it to histologic prognostic predictors. They reported that increasing proliferative index, Breslow thickness and Clark levels had a positive correlation with p53 expression but negative correlation with bcl-2 and/or p16. In cutaneous melanomas arising from benign nevi, expression of p53 was seen in the malignant component only, whereas expression of p16 and bcl-2 was strong in benign but weak or negative in the malignant component [14]. The prognostic significance of p53, p16, and bcl-2 proteins in a large series of well-characterized primary mucosal melanomas of the head and neck has not been investigated before.
Materials and Methods
The archives of Pathology at the Memorial Sloan Kettering Cancer Center were searched for primary mucosal melanoma (MM) arising in the head and neck mucosa from 1956 to 2000 following approval of the study by the institutional review board. A database of 99 well-characterized MMs was created by the authors. All available hematoxylin and eosin stained slides from biopsies and/or resections were reviewed by a head and neck pathologist (MLP) and a dermatopathologist (KJB). The mucosal origin of the tumors was determined from clinical information, i.e. absence of primary melanoma in any other body site or absence of disseminated melanoma, and by detecting in-situ mucosal melanoma associated with a mucosal tumor, when present. The melanocytic nature of the tumors was supported by intracytoplasmic melanin, and/or by immunohistochemistry with melanocytic markers, e.g. MART-1/Melan A (A103), glycoprotein 100 (HMB45), tyrosinase (T311), S100 protein, microphthalmia-associated transcription factor (D5) as published elsewhere [15]. This database has been used for clinical, pathologic, and protein expression profiling of oral and sinonasal melanomas in the past [16–21]. The current study includes a subset of cases where formalin-fixed paraffin-embedded (FFPE) tumor tissue was available for additional study of prognostic markers. Cases with insufficient tumor tissue were excluded. Thus, this study includes 64 cases: 32 patients with tumors arising in the sinonasal cavities, 28 in the oral cavity, and 4 in the pharynx (2 in oropharynx and 2 in nasopharynx).
Clinical Information
Clinical information was collected through chart review and is summarized in Table 1. Tumor size was known in 33 patients, including 16 oral cases (0.6 mm to 3.2 cm; median 1.2 cm) and 17 sinonasal cases (6 mm to 7 cm; median 2 cm). In 4 patients (2 oral and 2 sinonasal), the tumors were simply documented as ‘extensive’. Tumor progression, e.g. local recurrence, was noted in 21 patients, cervical lymph node involvement were noted in 9 (6 sinonasal and 3 oral MM) and distant metastases in 25 (11 sinonasal and 14 oral) patients, respectively. Follow-up was available until death in 48 patients and from 4 months to 19.5 years in the 14 remaining patients who were alive at the end of the study (median 1.6 years). Thirty eight patients died due to MM 1 month to 16.5 years after diagnosis (median survival 2.3 years). The most common cause of death was disseminated MM (N = 16).
Table 1.
Clinical characteristics of 64 patients with primary head and neck mucosal melanoma
| Age, sex, site | Patients |
|---|---|
| Age | Range 23–93 years (median 64) |
| Sex | Male 40:Female 24 (1.6:1) |
| Site | |
| Nasal/paranasal sinuses | 32 |
| Hard palate | 11 |
| Alveolus | 9 |
| Lip | 4 |
| Buccal | 2 |
| Floor of mouth | 1 |
| Tongue | 1 |
| Oropharynx/tonsil | 2 |
| Nasopharynx | 2 |
| Size (N = 33) | 0.6 mm to 7 cm (median 1.6 cm) |
| Stage (N = 54)a | |
| Stage I | 42 |
| Stage II | 9 |
| Stage III | 3 |
| Therapy (N = 61) | |
| Surgery | 57 |
| Radiotherapy | 18 (3 definitive, 15 adjuvant) |
| Chemotherapyb | 6 (1 definitive, 5 adjuvant) |
| Adjuvant immunotherapyc | 6 |
| End status (N = 62) | |
| DWD | 38 |
| DOO | 10 |
| AWD | 4 |
| AFD | 10 |
DWD died with disease, DOO died of other causes, AWD alive with disease, AFD alive free of disease
aStage I local disease, Stage II regional metastasis, Stage III distant metastasis
bVincristine, nimustine and/or dimethyltriazenoimidazole-caroxamide (DTIC)
cBacillus Calmette-Guerin or α-interferon
Review of Pathology
The tumor was amelanotic in 22 patients. In-situ MM was observed in 47 of 58 patients. Tumor thickness varied from 0.5 mm to 2 cm (median 5.5 mm) in 63 cases. Depth of invasion could be assessed in 55 cases: 2 arising in oral mucosa showed microinvasion only (defined as invasive individual or clusters of <10 atypical melanocytes near the epithelial–subepithelial junction) [19, 23] tumors invaded into the lamina propria, and 30 tumors (54.5%) invaded more deeply into muscle, bone, cartilage, orbit, and/or skin. Tumor morphology was recorded as epithelioid, spindled, or undifferentiated. The undifferentiated tumor cells were typically “small round blue cells” (lymphoma-like) or severely atypical tumor cells with bizarre multinucleated giant cells reminiscent of high-grade pleomorphic sarcoma [18]. The tumor was comprised of epithelioid and/or spindle cells in 49 (76.5%) cases, and of predominantly undifferentiated cells in 15 (23.4%) of 64 patients (Fig. 1). Tumor necrosis was present in 45 (70%), and vascular invasion was noted in 18 (28%) of 64 patients.
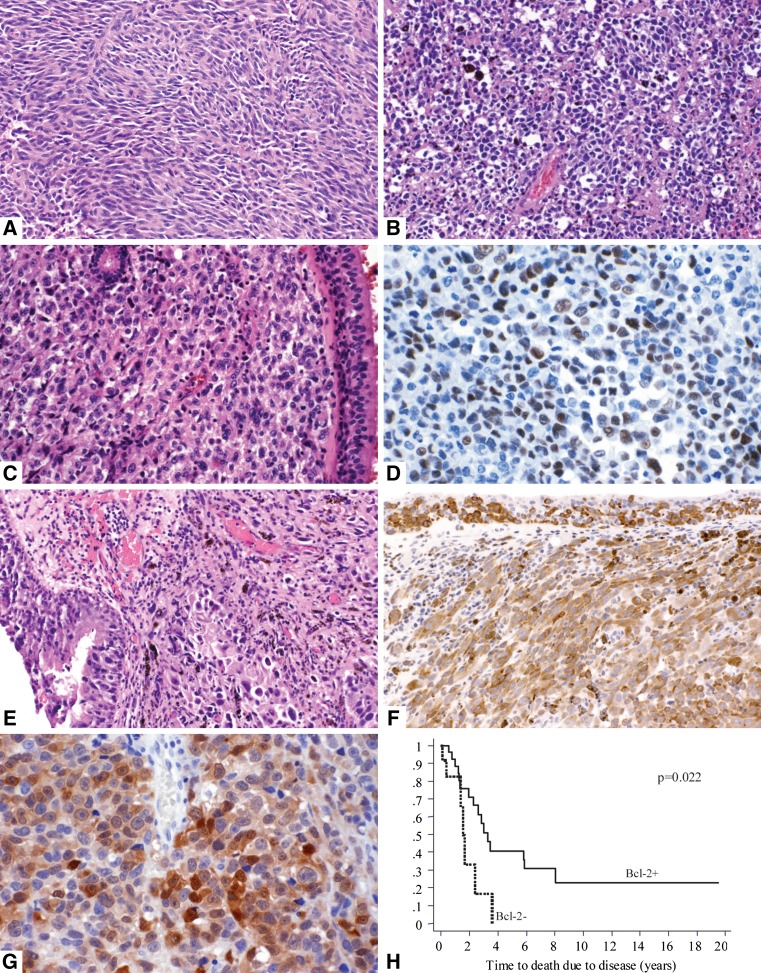
Fig. 1.
a 40-year-old woman with amelanotic spindle cell melanoma arising in the maxillary antrum. b 50-year-old woman with melanoma involving the right nasal cavity and turbinate. The tumor cells are undifferentiated “small round blue cells” and show focal melanin pigment. c–d 66-year-old man with melanoma in the right maxillary sinus comprised of amelanotic epithelioid tumor cells (c ×20) showing nuclear expression of p53 (d ×40). e–f 77-year-old woman with melanoma in the left nasal cavity. The tumor is comprised of epithelioid cells and shows in-situ tumor cells within the respiratory mucosa. Melanin pigment is focally present (e ×20). Invasive and in-situ melanoma cells are strongly and diffusely positive for bcl-2 (f ×20). g 68-year-old woman with a nodular mass in the right maxillary antrum comprised of epithelioid cells showing nuclear and cytoplasmic expression of p16 (×40). h Kaplan–Meier curve for disease-specific survival for bcl-2 expression in initial mucosal melanomas. The y-axis shows the proportion of surviving patients
Immunohistochemistry
Seventy-seven formalin-fixed paraffin-embedded tumor tissues were available from the 64 patients, and represented 47 initial, 17 recurrent, and 13 metastatic events. The metastases involved lymph nodes (N = 6), lung (N = 3), gastrointestinal tract (N = 2), liver (N = 1) and brain (N = 1). Tumor tissues from multiple separate clinical events were available in 13 patients, including initial and recurrent tumors (N = 4), initial and metastatic tumors (N = 6), and recurrent and metastatic tumors (N = 3).
Five micrometre thick full histologic sections were collected on charged slides (Superfrost, Fisher Scientific, Pittsburgh, PA, USA). Heat induced antigen retrieval was carried out by steaming the sections in citrate buffer (10 mM, pH 6) at 90°C for 30 min. Immunohistochemistry was performed manually with mouse monoclonal antibodies to bcl-2 (clone 124, 1:50, Dako, Carpinteria, CA, USA), p53 (clone DO-7, 1:500, Dako), and p16 (clone DCS-50, 1:200, Oncogene Science, MA, USA). Positive controls included a normal lymph node for bcl-2, a p53 positive colonic adenocarcinoma, and a p16 positive alveolar rhabdomyosarcoma. Negative controls without primary antibodies were used in each staining batch.
The protein expression was cytoplasmic for bcl-2, nuclear for p53, and both nuclear and cytoplasmic for p16. The intensity of staining was uniformly strong for bcl-2 and p16, irrespective of the number of positive tumor cells. The staining intensity for p53 was weak in tumors staining 25% or less tumor cell nuclei, and increased in intensity to moderate and strong with higher percentage of positive tumor cells. The expression was graded semi-quantitatively on a score of 0–5 based on the percentile of tumor cells showing immunoreactivity, as follows: negative or 0 representing 0–5% positive tumor cells, and 1+ to 4+ representing 6–25, 26–50, 51–75 and >75% of positive tumor cells respectively [13]. The scoring was done by consensus between two observers (MLP, KJB) on a double-headed microscope.
Statistical Analysis
The immunohistochemical results were converted to binary data for analysis: for p16 and bcl-2, tumors showing >5% positive tumor cells were considered positive and those with ≤5% expression were considered negative. For p53, tumors with >25% positive tumor cells were considered positive for aberrant p53 expression. These results were then correlated with pathologic features previously determined to indicate poor prognosis, e.g. undifferentiated tumor cells, vascular invasion, neural invasion and necrosis [21], disease progression, and overall and disease-specific survival. Correlation of protein expression to survival was restricted to the initial mucosal melanomas, in order to recapitulate the disease management scenario for some of the most frequent malignancies, e.g. breast carcinoma, where prognostic markers are queried at the initial diagnosis. For disease-specific survival, patients that died of other causes were censored at the time of death. Kaplan–Meier curves and Log-rank tests were calculated with α level set at P < 0.05. Simple univariate analyses were performed using Chi-square, t test and analysis of variance with the software package STATA (Version 6, Stata Corp, College Station, TX, USA).
Results
The expression of p16, p53, and bcl-2 proteins in the initial and recurrent mucosal, and metastatic tumors is shown in Table 2 (Fig. 1d, f, g). The expression of p16 was seen in 15 initial, 2 recurrent, and 2 metastatic tumors, however, only 3 tumors showed strong and diffuse expression in >75% of the tumor cells. Overall, 75% of the tumors lost p16 expression, and a trend to increasing loss from initial mucosal tumors to recurrent/metastatic tumors (P = 0.06) was noted. No significant difference in p53 and bcl-2 expression was seen in initial and advanced (recurrent/metastatic) tumors (Table 2). No p53 expression (N = 37) or weak expression in ≤25% tumor cells (N = 23) was seen in 60 of 76 tumor tissues. Expression of p53 was significantly (P = 0.002) associated with undifferentiated tumor cell morphology, resembling small round blue cell tumors (lymphoma-like) or undifferentiated high grade sarcoma (Table 3). No correlation was noted between all three markers and vascular invasion, necrosis, and deep tissue (bone, cartilage or skeletal muscle) invasion (Table 3). None of the markers were helpful in predicting concurrent or subsequent distant metastases seen in 16 patients (Table 4).
Table 2.
Protein expression in initial, recurrent, and metastatic tumors
| Protein | Initial N = 47 (%) | Recurrent N = 17 (%) | Metastatic N = 13 (%) | Total (%) N = 77 (%) |
|---|---|---|---|---|
| p16* | 15/47 (32%) | 2/16 (12.5%) | 2/13 (15%) | 19/76 (25%) |
| 1+ | 4 | 0 | 1 | 5 |
| 2+ | 3 | 2 | 0 | 5 |
| 3+ | 5 | 0 | 1 | 6 |
| 4+ | 3 | 0 | 0 | 3 |
| p53** | 10/46 (22%) | 3/17 (17.6%) | 3/13 (23%) | 16/76 (21%) |
| 1+ (considered negative) | 15 | 3 | 5 | 23 |
| 2+ | 5 | 1 | 1 | 7 |
| 3+ | 4 | 2 | 1 | 7 |
| 4+ | 1 | 0 | 1 | 2 |
| bcl-2 | 35/47 (75%) | 12/16 (75%) | 9/13 (69%) | 56/76 (74%) |
| 1+ | 2 | 0 | 2 | 4 |
| 2+ | 5 | 2 | 2 | 9 |
| 3+ | 7 | 4 | 2 | 13 |
| 4+ | 21 | 6 | 3 | 30 |
The difference in total numbers is due to insufficient tissue for immunohistochemistry in some tumors
* p16 was increasingly lost from initial to recurrent and metastatic tumors (P = 0.06)
** Only tumors that showed staining reaction in >25% of tumor cells (2+ to 4+) were considered positive for p53
Table 3.
Protein expression in relation to poor prognostic features
| p16+ (%) | p53+ (%) | bcl-2+ (%) | |
|---|---|---|---|
| Undifferentiated tumor cells+ | 6/15 (40) | 12/15 (80)1 | 11/15 (73) |
| Undifferentiated tumor cells− | 12/49 (24) | 16/49 (33)1 | 35/48 (73) |
| Vascular invasion+ | 5/18 (28) | 6/18 (33) | 12/18 (67) |
| Vascular invasion− | 13/46 (28) | 22/46 (48) | 34/45 (76) |
| Necrosis+ | 14/45 (31) | 21/45 (47) | 34/45 (76) |
| Necrosis− | 4/18 (22) | 7/18 (39) | 12/17 (71) |
| Deep tissue invasion+a | 7/31 (23) | 14/31 (45) | 22/31 (71) |
| Deep tissue invasion− | 8/26 (31) | 11/26 (42) | 19/25 (76) |
The difference in total numbers is due to insufficient tissue for immunohistochemistry in some tumors
1P = 0.002
aLevel of invasion was evaluated in the mucosal tumors available in 57 patients only
Table 4.
Protein expression in initial mucosal melanoma and distant metastasis
| Protein expression (N = 21)a | Distant metastasis (N = 16) | Fisher’s exact P value |
|---|---|---|
| bcl-2 | ||
| Negative (5) | 5 (100%) | P = 0.278 |
| Positive (16) | 11 (69%) | |
| p53 | ||
| Negative (11) | 9 (82%) | P = 0.635 |
| Positive (10) | 7 (70%) | |
| p16 | ||
| Negative (9) | 6 (67%) | P = 0.611 |
| Positive (12) | 10 (83%) | |
aInformation regarding presence or absence of distant failure was available in 21 cases where the initial/first mucosal tumor tissues were also available
Survival Analysis
The expression of bcl-2 in initial tumors was associated with significantly improved survival (P < 0.05, Table 5, Fig. 1h) with the median overall and disease-specific survival in bcl-2 positive tumors being twice that of bcl-2 negative tumors. In general, tumors that retained p16 expression, over-expressed bcl-2 and lacked p53 (p16+/bcl2+/p53−) had a favorable overall and disease-specific survival over those that did not (p16−/bcl2−/p53+) but the difference was not significant.
Table 5.
Protein expression in initial mucosal melanoma and survival
| Protein expression (N = 45)a | Overall survival (median in years) | Disease specific survival (median in years) |
|---|---|---|
| bcl-2 | ||
| Negative (12) | 1.36 (P = 0.03) | 1.54 (P = 0.02) |
| Positive (33) | 2.6 | 3.3 |
| p53 | ||
| Negative (22) | 2.60 (P = 0.82) | 3.30 (P = 0.75) |
| Positive (23) | 1.65 | 2.29 |
| p16 | ||
| Negative (30) | 1.5 (P = 0.27) | 2.38 (P = 0.58) |
| Positive (15) | 3.01 | 3.01 |
| bcl-2+/p16+/p53− (6) | 3.30 (P = 0.17) | 3.30 (P = 0.17) |
| bcl-2−/p16−/p53+ (4) | 1.54 | 1.54 |
aSurvival information was available in 45 initial mucosal melanomas
Discussion
We have previously reported morphologic predictors of poor prognosis, e.g. undifferentiated tumor cell morphology, vascular invasion, tumor necrosis and deep tissue invasion [17, 21]. Now we extend our study to identify molecular predictors of prognosis. Molecular markers of prognosis are generally evaluated at the time of initial presentation in most solid tumors. Therefore, we studied the expression of p16, p53 and bcl-2 in 47 initial mucosal tumors to correlate it to overall and disease-specific survival. Our results show that tumors expressing bcl-2 were associated with significantly longer survival. This was unexpected. In cutaneous melanomas, Loggini et al. reported no correlation between bcl-2, and tumor thickness and level of invasion, while Kostov et al. found bcl-2 expression to be more frequent in invasive nodular melanomas [13, 22]. The association of bcl-2 expression to improved survival in the current study is similar to colorectal [23] and breast carcinoma where it correlates with better survival and favorable phenotype respectively [23, 24]. This suggests that the molecular pathogenesis of primary mucosal melanoma may be more similar to visceral malignancies, rather than its UV light-related cutaneous counterpart, and that mucosal melanomas that need to switch on the bcl-2 mediated anti-apoptotic pathway to negate programmed cell death may be less aggressive than other tumors. Simultaneous assays of apoptosis and tumor proliferation may help explain the better prognosis in bcl-2 positive tumors. Novel antisense oligodeoxynucleotide based therapy aimed at down-regulating the anti-apoptotic bcl-2 oncogene and promoting tumor cell death may be a consideration in bcl-2 positive mucosal melanomas.
Loss of p16 protein expression was observed in two-thirds of initial mucosal tumors in the current series, and increased with tumor progression. We believe that the significantly larger number of tumors in the current series, 77 tumor samples from 64 patients, provided superior statistical power to demonstrate progressive loss of p16 with tumor progression, unlike previous reports [5]. Similar to pancreatico-biliary [25, 26], hepatocellular [27], colorectal [28], and non-small cell lung carcinomas [29], we found that MM that retained p16 expression had longer overall and disease specific survival but this did not reach significance.
Abnormalities in the p53 gene/protein have been extensively studied in cutaneous and uveal melanomas [30–32]. Gwosdz et al. [33] reported p53 mutations in 57–58% of MM suggesting the role of non-UV related mechanisms. We found aberrant p53 protein expression in 21% of MM, and in contrast to cutaneous melanomas where p53 over-expression increases with disease progression and metastasis [32, 34–37], we found no significant difference between initial mucosal melanomas and recurrent/metastatic tumors. Interestingly, 80% of MM comprised of undifferentiated cells were positive for p53. In a previous study, we demonstrated that undifferentiated cells were associated with an unfavorable prognosis [21]. Similarly, in uveal melanomas, abnormalities in p53 are associated with an unfavorable outcome [38]. In the current study, p53 positive tumors were associated with shorter overall and disease specific survival but it did not reach significance.
The rarity of MM restricted the current study on several fronts. The small number of cases precluded multivariate analysis and limited the power of the statistical analysis. This problem was further accentuated when the tissue samples were split into recurrent and metastatic tumors. There was significant heterogeneity among the tumors based on tumor subsite, and variable treatment protocols were received by the patients over four decades. The retrospective and single institution based data collection adds additional bias. Several of these weaknesses can only be addressed by a large scale prospective multi-institutional study. Metastasis-analysis of the SEER data will not identify biomarkers predictive of prognosis as none are routinely or consistently performed on MM.
In summary, expression of bcl-2, p53, and loss of p16 expression are frequent and early events in primary mucosal melanomas, suggesting that impairment of programmed cell death and dysregulation of the G1/S phase check-point may play a role in tumorigenesis in a subset of head and neck mucosal melanomas. Expression of bcl-2 in initial mucosal melanomas predicts better prognosis.
Acknowledgments
The authors thank Drs. James Woodruff, Victor E. Reuter and posthumously Dr. Andrew G. Huvos, Department of Pathology, Memorial Sloan Kettering Cancer Center, New York, for their help in creating this database.
Conflict of interest
The authors have no financial or commercial obligations to disclose. The study was funded with departmental funds. The materials used in this study (paraffin blocks and clinical data) were also used in several previous publications on clinicopathologic and antigenic profiling of primary mucosal melanomas as stated in the materials and methods. This work was presented as a poster at the 91st Annual Meeting of USCAP, Chicago, IL, 2002.
References
- 1.Chang AE, Karnell LH, Menck HR. The National cancer data base report on cutaneous and noncutaneous melanoma: a summary of 84, 836 cases from the past decade. The American College of Surgeons Commission on Cancer and the American Cancer Society. Cancer. 1998;83:1664–1678. doi: 10.1002/(SICI)1097-0142(19981015)83:8<1664::AID-CNCR23>3.0.CO;2-G. [DOI] [PubMed] [Google Scholar]
- 2.Clurman BE, Groudine M. The CDKN2A tumor-suppressor locus—a tale of two proteins. N Engl J Med. 1998;338:910–912. doi: 10.1056/NEJM199803263381312. [DOI] [PubMed] [Google Scholar]
- 3.Merbs SL, Sidransky D. Analysis of p16 (CDKN2/MTS-1/INK4A) alterations in primary sporadic uveal melanoma. Invest Ophthalmol Vis Sci. 1999;40:779–783. [PubMed] [Google Scholar]
- 4.Franchi A, Alos L, Gale N, et al. Expression of p16 in sinonasal malignant melanoma. Virchows Arch. 2006;449:667–672. doi: 10.1007/s00428-006-0288-0. [DOI] [PubMed] [Google Scholar]
- 5.Tanaka N, Odajima T, Mimura M, et al. Expression of Rb, pRb2/p130, p53, and p16 proteins in malignant melanoma of oral mucosa. Oral Oncol. 2001;37:308–314. doi: 10.1016/S1368-8375(00)00107-X. [DOI] [PubMed] [Google Scholar]
- 6.Brennan JA, Mao L, Hruban RH, et al. Molecular assessment of histopathological staging in squamous-cell carcinoma of the head and neck. N Engl J Med. 1995;332:429–435. doi: 10.1056/NEJM199502163320704. [DOI] [PubMed] [Google Scholar]
- 7.Kishimoto Y, Murakami Y, Shiraishi M, Hayashi K, Sekiya T. Aberrations of the p53 tumor suppressor gene in human non-small cell carcinomas of the lung. Cancer Res. 1992;52:4799–4804. [PubMed] [Google Scholar]
- 8.Kovach JS, Hartmann A, Blaszyk H, Cunningham J, Schaid D, Sommer SS. Mutation detection by highly sensitive methods indicates that p53 gene mutations in breast cancer can have important prognostic value. Proc Natl Acad Sci. 1996;93:1093–1096. doi: 10.1073/pnas.93.3.1093. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sun XF, Carstensen JM, Zhang H, et al. Prognostic significance of cytoplasmic p53 oncoprotein in colorectal adenocarcinoma. Lancet. 1992;340:1369–1373. doi: 10.1016/0140-6736(92)92558-W. [DOI] [PubMed] [Google Scholar]
- 10.Jansson T, Inganas M, Sjogren S, et al. p53 Status predicts survival in breast cancer patients treated with or without postoperative radiotherapy: a novel hypothesis based on clinical findings. J Clin Oncol. 1995;13:2745–2751. doi: 10.1200/JCO.1995.13.11.2745. [DOI] [PubMed] [Google Scholar]
- 11.Nabeya Y, Loganzo F, Jr, Maslak P, et al. The mutational status of p53 protein in gastric and esophageal adenocarcinoma cell lines predicts sensitivity to chemotherapeutic agents. Int J Cancer. 1995;64:37–46. doi: 10.1002/ijc.2910640109. [DOI] [PubMed] [Google Scholar]
- 12.Straume O, Akslen LA. Alterations and prognostic significance of p16 and p53 protein expression in subgroups of cutaneous melanoma. Int J Cancer. 1997;74:535–539. doi: 10.1002/(SICI)1097-0215(19971021)74:5<535::AID-IJC10>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 13.Kostov M, Mijovic Z, Mihailovic D, Cerovic S, Stojanovic M, Jelic M. Correlation of cell cycle regulatory proteins (p53 and p16(ink)(a)) and bcl-2 oncoprotein with mitotic index and thickness of primary cutaneous malignant melanoma. Bosn J Basic Med Sci. 2010;10:276–281. doi: 10.17305/bjbms.2010.2660. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Radhi JM. Malignant melanoma arising from nevi, p53, p16, and Bcl-2: expression in benign versus malignant components. J Cutan Med Surg. 1999;3:293–297. doi: 10.1177/120347549900300603. [DOI] [PubMed] [Google Scholar]
- 15.Prasad ML, Jungbluth AA, Iversen K, Huvos AG, Busam KJ. Expression of melanocytic differentiation markers in malignant melanomas of the oral and sinonasal mucosa. Am J Surg Pathol. 2001;25:782–787. doi: 10.1097/00000478-200106000-00010. [DOI] [PubMed] [Google Scholar]
- 16.Patel SG, Prasad ML, Escrig M, et al. Primary mucosal malignant melanoma of the head and neck. Head Neck. 2002;24:247–257. doi: 10.1002/hed.10019. [DOI] [PubMed] [Google Scholar]
- 17.Prasad ML, Patel S, Hoshaw-Woodard S, et al. Prognostic factors for malignant melanoma of the squamous mucosa of the head and neck. Am J Surg Pathol. 2002;26:883–892. doi: 10.1097/00000478-200207000-00006. [DOI] [PubMed] [Google Scholar]
- 18.Prasad ML, Busam KJ, Patel SG, Hoshaw-Woodard S, Shah JP, Huvos AG. Clinicopathologic differences in malignant melanoma arising in oral squamous and sinonasal respiratory mucosa of the upper aerodigestive tract. Arch Pathol Lab Med. 2003;127:997–1002. doi: 10.5858/2003-127-997-CDIMMA. [DOI] [PubMed] [Google Scholar]
- 19.Prasad ML, Jungbluth AA, Patel SG, Iversen K, Hoshaw-Woodard S, Busam KJ. Expression and significance of cancer testis antigens in primary mucosal melanoma of the head and neck. Head Neck. 2004;26:1053–1057. doi: 10.1002/hed.20112. [DOI] [PubMed] [Google Scholar]
- 20.Prasad ML, Patel SG, Busam KJ. Primary mucosal desmoplastic melanoma of the head and neck. Head Neck. 2004;26:373–377. doi: 10.1002/hed.10384. [DOI] [PubMed] [Google Scholar]
- 21.Prasad ML, Patel SG, Huvos AG, Shah JP, Busam KJ. Primary mucosal melanoma of the head and neck: a proposal for microstaging localized, Stage I (lymph node-negative) tumors. Cancer. 2004;100:1657–1664. doi: 10.1002/cncr.20201. [DOI] [PubMed] [Google Scholar]
- 22.Loggini B, Rinaldi I, Pingitore R, Cristofani R, Castagna M, Barachini P. Immunohistochemical study of 49 cutaneous melanomas: p53, PCNA, Bcl-2 expression and multidrug resistance. Tumori. 2001;87:179–186. doi: 10.1177/030089160108700313. [DOI] [PubMed] [Google Scholar]
- 23.Grizzle WE, Manne U, Weiss HL, Jhala N, Talley L. Molecular staging of colorectal cancer in African-American and Caucasian patients using phenotypic expression of p53, Bcl-2, MUC-1 AND p27(kip-1) Int J Cancer. 2002;97:403–409. doi: 10.1002/ijc.1617. [DOI] [PubMed] [Google Scholar]
- 24.Nakopoulou L, Michalopoulou A, Giannopoulou I, et al. bcl-2 protein expression is associated with a prognostically favourable phenotype in breast cancer irrespective of p53 immunostaining. Histopathology. 1999;34:310–319. doi: 10.1046/j.1365-2559.1999.00627.x. [DOI] [PubMed] [Google Scholar]
- 25.Ichikawa K, Imura J, Kawamata H, Takeda J, Fujimori T. Down-regulated p16 expression predicts poor prognosis in patients with extrahepatic biliary tract carcinomas. Int J Oncol. 2002;20:453–461. doi: 10.3892/ijo.20.3.453. [DOI] [PubMed] [Google Scholar]
- 26.Gerdes B, Ramaswamy A, Ziegler A, et al. p16INK4a is a prognostic marker in resected ductal pancreatic cancer: an analysis of p16INK4a, p53, MDM2, an Rb. Ann Surg. 2002;235:51–59. doi: 10.1097/00000658-200201000-00007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Tannapfel A, Busse C, Weinans L, et al. INK4a-ARF alterations and p53 mutations in hepatocellular carcinomas. Oncogene. 2001;20:7104–7109. doi: 10.1038/sj.onc.1204902. [DOI] [PubMed] [Google Scholar]
- 28.Yi J, Wang ZW, Cang H, et al. p16 gene methylation in colorectal cancers associated with Duke’s staging. World J Gastroenterol. 2001;7:722–725. doi: 10.3748/wjg.v7.i5.722. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Gonzalez-Quevedo R, Iniesta P, Moran A, et al. Cooperative role of telomerase activity and p16 expression in the prognosis of non-small-cell lung cancer. J Clin Oncol. 2002;20:254–262. doi: 10.1200/JCO.20.1.254. [DOI] [PubMed] [Google Scholar]
- 30.Coupland SE, Anastassiou G, Stang A, et al. The prognostic value of cyclin D1, p53, and MDM2 protein expression in uveal melanoma. J Pathol. 2000;191:120–126. doi: 10.1002/(SICI)1096-9896(200006)191:2<120::AID-PATH591>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]
- 31.Essner R, Kuo CT, Wang H, et al. Prognostic implications of p53 overexpression in cutaneous melanoma from sun-exposed and nonexposed sites. Cancer. 1998;82:309–316. doi: 10.1002/(SICI)1097-0142(19980115)82:2<317::AID-CNCR10>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- 32.Gelsleichter L, Gown AM, Zarbo RJ, Wang E, Coltrera MD. p53 and mdm-2 expression in malignant melanoma: an immunocytochemical study of expression of p53, mdm-2, and markers of cell proliferation in primary versus metastatic tumors. Mod Pathol. 1995;8:530–535. [PubMed] [Google Scholar]
- 33.Gwosdz C, Scheckenbach K, Lieven O, et al. Comprehensive analysis of the p53 status in mucosal and cutaneous melanomas. Int J Cancer. 2006;118:577–582. doi: 10.1002/ijc.21366. [DOI] [PubMed] [Google Scholar]
- 34.Akslen LA, Morkve O. Expression of p53 protein in cutaneous melanoma. Int J Cancer. 1992;52:13–16. doi: 10.1002/ijc.2910520104. [DOI] [PubMed] [Google Scholar]
- 35.Barnhill RL, Castresana JS, Rubio MP, et al. p53 expression in cutaneous malignant melanoma: an immunohistochemical study of 87 cases of primary, recurrent, and metastatic melanoma. Mod Pathol. 1994;7:533–535. [PubMed] [Google Scholar]
- 36.Stretch JR, Gatter KC, Ralfkiaer E, Lane DP, Harris AL. Expression of mutant p53 in melanoma. Cancer Res. 1991;51:5976–5979. [PubMed] [Google Scholar]
- 37.Vogt T, Zipperer KH, Vogt A, Holzel D, Landthaler M, Stolz W. p53-protein and Ki-67-antigen expression are both reliable biomarkers of prognosis in thick stage I nodular melanomas of the skin. Histopathology. 1997;30:57–63. doi: 10.1046/j.1365-2559.1996.d01-558.x. [DOI] [PubMed] [Google Scholar]
- 38.Ragnarsson-Olding B, Platz A, Olding L, Ringborg U. p53 protein expression and TP53 mutations in malignant melanomas of sun-sheltered mucosal membranes versus chronically sun-exposed skin. Melanoma Res. 2004;14:395–401. doi: 10.1097/00008390-200410000-00010. [DOI] [PubMed] [Google Scholar]