Abstract
To evaluate disease dynamics, treatment results, and frequency of malignant transformation. Ten-year single center retrospective study. The study included 171 patients, 28–99 years old. Follow-up was 1–16 years. 49.5% exhibited changes in clinical presentation, with 19% yearly increase of probability for type shift. Index of extent (number of oral locations) showed a mean 40% decrease and 94.1% reported improvement. There were significant differences between treated and untreated patients (P = 0.012). Patients with or without systemic diseases had identical treatment requirements for oral lesions. The prevalence of SCC was 5.8%. Oral lichen planus constantly changes presentation and extent of involvement. The effect of systemic diseases was insignificant in the present study. There is a clear value for treatment to reduce the extent of lesions. The results indicate that all clinical forms of the disease need to be equally followed since the clinical presentation typically changes over time, while malignant transformation can occur in all forms.
Keywords: Oral lichen planus, Malignant transformation, Classification, Treatment
Introduction
Oral lichen planus (OLP) is a chronic inflammatory disease of uncertain etiology. It is considered a cell-mediated immunological process, probably occurring in a predisposed population [1]. However, specific antigens evoking the immune response have not yet been defined. The mean prevalence of OLP in reports from different countries is 1.27%, with a prevalence of 0.96% in men and 1.57% in women [2].
Typically, OLP occurs bilaterally and is usually symmetrical. Lesions have been historically classified into six clinical forms: reticular, plaque form, atrophic, erosive, annular, and bullous [2], although others have limited the classification to the reticular and erosive types [3]. OLP patients may have extra-oral manifestations, most frequently in the skin, nails, and genital mucosa. OLP tends to be chronic, with fluctuations in signs and symptoms over time. Complete spontaneous resolution is rare. OLP is considered potentially malignant, with the malignancy risk recently supported by the identification of chromosomal numerical aberrations in OLP [4]. OLP is considered a potentially malignant condition, with the chronic stromal inflammation considered a possible factor driving the malignant transformation [5].
The lack of understanding of the etiology is associated with generally unsatisfactory treatment modalities, which may address the symptoms with variable success, but have not been able to achieve resolution or cure. The concept of “field cancerization” has been applied to OLP patients in parallel with traditional head and neck cancers [6].
The objectives of the present investigation were to evaluate the clinicopathologic characteristics in OLP patients, the dynamics of the disease, the results of treatment, and the frequency of malignant transformation.
Materials and Methods
The present investigation was conducted as a retrospective study set in the Oral and Maxillofacial Surgery Department of a major medical center, which functions as a tertiary referral center with a large population base. At the time period included in the study, a licensed oral pathologist (IK) was responsible for both the microscopic diagnosis in all cases, as well as clinical treatment and follow-up. The records of patients with OLP diagnosed between 1996 and 2007 were retrieved. Reports signed out as “compatible with” or “consistent with” OLP were also included. The histologic slides were reviewed and compliance with the revised histologic criteria for OLP was confirmed for all included cases [7, 8].
The data retrieved from the files included demographic information, co-existing medical conditions, initial presentation, changes in the presentation in subsequent visits, areas of the mucosa affected, extra-oral manifestations, symptoms, treatment modalities, objective and subjective response to treatment, the presence of oral malignancy and the time from diagnosis of OLP to malignancy.
The details on specific medications used per individual were not included in the data collected for the study.
Patients were included in the study if they met the following inclusion criteria: oral lesions clinically consistent with the modifications for WHO 2003 criteria suggested by van der Meij and van der Waal [8] in at least one visit; biopsy confirming the diagnosis of OLP in accordance with said criteria [8]; and records of three or more follow-up visits, no less than 1 year after diagnosis.
The exclusion criteria were: indefinite diagnosis (such as lichenoid inflammation); less than 1 year of follow-up and/or less than three return visits; and medical conditions which may present with features similar to OLP (graft versus host disease, systemic, or discoid lupus erythematosus). Cases which could be clearly diagnosed clinically as drug-induced oral lichenoid lesions based on a close correlation between the onset of treatment with a particular medication and the onset of lichenoid oral lesions, or cases which microscopically presented lichenoid changes but not true OLP, were all excluded from the study group, but a methodological effort to rule out drug-related lichenoid reactions was not attempted clinically for reasons presented in the discussion section.
For a semi-quantitative analysis of the clinical severity, an index of extent (IE) was defined as the number of oral locations involved. The locations recorded were buccal, tongue, floor, or gingiva. IE values ranged between 0 and 4.
Treatment was offered only in the presence of symptoms and/or erosive lesions. Treatment modalities employed over the study period included Clobetasol 0.05%, Dexamethasone rinse (0.01–0.04%), Retinoic acid gel (0.025%), systemic Triamcinolone (4–12 mg/day), systemic Prednisone (5–20 mg/day) and Tacrolimus 1% ointment. Retinoic acid gel was usually prescribed once a day and is the only type of treatment which was used continuously, whereas all other modalities were used for periods of up to 2 weeks at a time, and repeated if lesions relapsed, but an interval of at least 2 weeks was advised, depending on the patient response and needs. Systemic corticosteroids were avoided in patients with diabetes mellitus, glaucoma and severe osteoporosis. Other than these general considerations, there was no record of the reasons for the treatment modality chosen in any particular case.
To evaluate fluctuations in disease manifestations over time, two sets of criteria were defined:
Objective Criteria: The difference in IE between initial visit and subsequent visits. Decrease of at least one area was defined as improvement, and increase of at least one area as exacerbation. In the final analysis, cases with no change or exacerbation were coded as “lack of improvement”.
Subjective Criteria: Patients’ verbal descriptions of their oral condition, as recorded in the files at each visit, were classified into four categories: (1) complete remission; (2) partial remission; (3) no change; and (4) exacerbation. For analysis, complete or partial remissions were coded as “improvement”, whereas no change or exacerbations were coded as “lack of improvement”.
Statistical Analysis
ANOVA, Chi-square, Fisher Exact Test and Multivariate Logistic Regression Model were applied respectively, using SPSS version 14.0.1.
The study was approved by the institutional IRB (Helsinki) committee.
Results
The study group included a total of 171 patients, comprised of 51 males and 120 females, with an M:F ratio of 1:2.4. There was a wide age range in the study population of 28–90 years (mean 59.1 ± 12.4). There were no significant age differences between genders.
Information on medical background was available in 114 cases. High blood pressure was recorded in 37 (32.5%) cases, hyperlipidemia in 38 (33.3%), adult type diabetes mellitus in 23 (20.2%), hypothyroidism in 17 (14.9%), osteoporosis in 17 (14.9%), non-oral malignancy in 16 (14.0%), and gastrointestinal disease in 13 (11.4%) (Note: more than one diagnosis per patient is possible).
Clinical subtypes of OLP at the initial visit were divided into three groups: (1) hyperkeratotic (reticular, plaque form, annular); (2) atrophic; and (3) erosive. Bullous OLP was not identified in any of the cases. The majority of patients (N = 108; 63.2%) had combined lesions (of more than one type) while over one-third (N = 62; 36.5%) had only one clinical type. The cases were further classified as follows: 58 (33.9%) as hyperkeratotic; 7 (4.1%) as hyperkeratotic-erosive; 29 (17%) as hyperkeratotic-erosive-atrophic; 3 (1.8%) as erosive; and 1 (0.6%) as atrophic lesions. There were no significant differences in the distribution of presentations between genders.
During follow-up, 85 (49.5%) of cases exhibited changes in the clinical presentation as compared to the initial visit. Logistic regression analysis indicated that the longer the follow-up time period the greater the likelihood of clinical change to occur from a single to a combined type lesion, reflected by an increase of 19% for each additional year (OR = 1.198, P = 0.026, 95% CI = 1.022, 1.405).
The location of the lesions was also found to be related to clinical types: patients with tongue lesions were 4.5 times more likely to present combined rather than single-type lesions (OR = 1.198, P = 0.026, 95% CI = 1.022, 1.405).
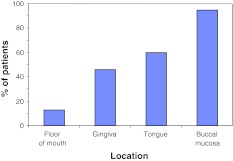
The most frequent area involved was the buccal mucosa 162 (94.7%), followed by the lingual mucosa 103 (60.5%), gingiva 78 (46.6%), and floor of mouth 22 (12.9%) (Fig. 1). There were no differences between genders, and no correlation between specific oral locations and the presence of symptoms.
Fig. 1.
Oral distribution of OLP at baseline shows the buccal mucosa and tongue to be the most frequently involved areas
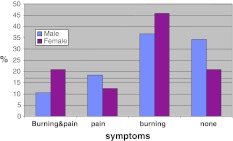
The mean IE at the initial visit prior to treatment was 2.0. Information on symptoms was available in 134 patients: 33 (24.6%) were asymptomatic, while 58 (43.3%) reported burning, 19 (14.2%) pain, and (7.9%) 24 burning and pain. There were no significant differences between genders (Fig. 2).
Fig. 2.
Comparison of symptoms shows no significant differences between genders
Extra-oral involvement included skin (N = 35; 20.5%), genitals (N = 9; 5.3%), and both skin and genitals (N = 4; 2.9%). Cutaneous involvement was 2.4 times more frequent in females (P = 0.025, RR = 2.4). Genital involvement did no show a predilection for females in the present study group.
The follow-up (FU) routine for OLP patients included return visits every 4–6 months. The FU period in the study group ranged between 1 and 16 years (mean 4.3). In 113 (66.1%) cases, the FU was longer than 3 years.
Asymptomatic patients without erosive lesions (N = 33; 19.3%) were invited for FU but no treatment was prescribed. There was a significant difference between genders in the requirement for treatment: 102 (85%) women and 36 (70.6%) men received treatment. Thus, women received treatment 1.2 times more often than men, although no differences in either the type of lesions or frequency of symptoms existed between genders (P = 0.025). Of the 57 patients with no recorded systemic diseases (which are assumed to be medication-free in the analysis), 12 (21%) did not require treatment for OLP, which is not significantly different than the treatment-needs in the remaining study population.
The treatment modalities recorded in the study group included Clobetasol 0.05% in 78 (56.7%) cases, Dexamethasone rinse (0.01–0.04%) in (43.5%) 60, Retinoic acid gel (0.025%) in 40 (28.9%), systemic Triamicinolone (4–12 mg/day) in 14 (10.1%), systemic Prednisone (5–20 mg/day) in 5 (3.6%), and Tacrolimus ointment in 18 (13.0%).
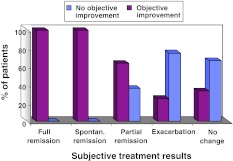
Response to treatment was evaluated by both an objective parameter, using IE, and a subjective parameter, based on the patient’s verbal report. Comparison between these parameters showed a high degree of correlation: in the group with subjective improvement, there was a high frequency of objective improvement (decrease in IE); while in the group without subjective improvement (no change or exacerbation) there was a significantly lower frequency of decrease in IE (P < 0.001) (Fig. 3). During FU, both subjective and objective parameters improved in comparison to the first visit; mean IE decreased by 0.8 areas (40% of initial mean IE); in the subjective parameters, 130 (94.2%) patients reported improvement, 5 (3.6%)spontaneous remission, 5 (3.6%) no change, and 4 (2.9%) exacerbation.
Fig. 3.
Comparison of objective and subjective indices for treatment results shows a good correlation between indices. X axis: subjective (verbal report), Y axis: objective (index of extension)
There was a significant difference in IE between patients who did or did not receive treatment (P = 0.012). A decrease of at least one area was observed in 84 (60.9%) of treated patients and only 10 (30.3%) of untreated patients. Thus, the chances of a decrease in IE were 1.9 times higher in treated patients. Differences between treated and untreated patients in the subjective report did not reach statistical significance. Comparison of treatment results between the different modalities used did not yield significant differences in the degree of IE reduction.
Among the confounding factors, diabetes had a significant effect on treatment response: non-diabetic patients reported improvement 1.3 times more than diabetic patients (P = 0.003).
The frequency of oral SCC in OLP patients in the study group was 10 (5.8%); however, only in 6 (3.5%) of these cases did transformation take place during the FU period. The remaining 4 patients had either OLP and SCC diagnosed concomitantly, OLP diagnosed after the diagnosis of SCC (in these cases the slides were reviewed by IK), or there was no information available on when and how the initial OLP diagnosis was made (different institution). Notably, alcohol use was not recorded in any of the transforming cases. In contrast, one of the patients had a heavy smoking habit and one a past history of smoking; thus, tobacco may have been a confounding factor in only 20% of the transforming cases. Three of the transforming cases had hyperkeratotic OLP, while the remaining seven presented with either hyperkeratotic or erosive features over the follow-up period.
Of the 10 cases with SCC, the malignancy occurred in the tongue in 4 (40%) cases, the gingiva in 3 (30%), the buccal mucosa in 2 (20%), and the floor of mouth in 1(10%). In all cases, carcinoma arose in an area involved by OLP.
Due to the small number of SCC cases in the study group, correlations with other factors failed to yield significant results.
Discussion
The population included in the present study was found to be in agreement with other reports showing a predisposition for females and a mean age of 59 years [2]. There were no gender differences in any of the clinical parameters investigated, such as type of lesion, distribution in oral locations, extent of involvement, symptoms or treatment response. The only apparent difference was that female patients tended to receive treatment 1.2 times more than males, although they did not have a higher frequency of symptoms or of erosive-atrophic lesions. This is in line with reports that women generally tend to use medical services more often than men [9, 10].
The results of the present study quantitatively support the clinical impression that OLP is a dynamic disease, frequently changing in distribution, severity, and clinical type. In approximately half the cases, a change in clinical presentation over time has been recorded, with an increase of 19% per year. For many years, there was a prevalent concept that patients with atrophic-erosive-ulcerative lesions had a higher risk for transformation and needed to be monitored more closely than those with other types. The results of the present study challenge this approach, strongly suggesting that there is limited value in OLP type as a factor determining the need or frequency of FU, as there is a high probability of change over time. Similar frequency (52%) of changes has been reported by Carbone et al. [11]. In the present study, there were twice as many patients with atrophic-erosive OLP than the hyperkeratotic type, within the group that transformed to SCC, and this is in agreement with the commonly accepted view. However, the fact that a third of the patients transformed from a previous hyperkeratotic variant is a clear indication that all types of OLP are at risk for malignant transformation.
The present study has been able to quantify the dynamics of OLP, and support the need for continuous and prolonged monitoring of all patients.
Extra-oral involvement in OLP has been found in a total of 48 (23.3%) of cases, including 20.5% cutaneous, 5.3% genital, and 2.9% cutaneous and genital lesions. The frequency of cutaneous involvement is similar to that reported by Bidara et al. [12], whereas the frequency of genital lesions is lower in the present study (17.2% and 10.3% respectively) [12]). However, since this is a retrospective study, the information available was based on the patients’ own report, not on consistent examination of the genital mucosa in all patients. This was also the case in the study of Bidara et al. [12], which was based on a questionnaire survey Therefore, the true epidemiological value for the data on genital involvement in OLP is low. In comparison, a large retrospective study by Carbone et al. [12] reported a frequency of 7.8% for skin and 2.9% for genital manifestations in OLP patients—data which is more in line with the findings from the present study [11].
OLP can not be consistently differentiated from drug-induced oral lichenoid lesions, which may overlap in both clinical and microscopic features [13]. There are multiple drug groups with occasionally reported lichenoid reactions, including the common drugs used for the diseases recorded in 114 patients in the present study. Although a methodological effort to rule out drug-related lichenoid reactions for each and every case was not attempted clinically, the fact that there were no significant differences found in the percent of patients requiring treatment for OLP between patients with or without systemic diseases (the latter group assumed to be taking medication for such conditions) serves to support the conclusion that the effect of medications as confounding factors was minor or insignificant in the present group.
The only reliable method to validate a diagnosis of drug-induced oral lichenoid lesion is to take the patient off the suspected drug, observe resolution of the signs and symptoms, then upon re-administration of the drug observe recurrence of lesions. This process is mostly impractical in a clinical setting for several reasons: patients often take multiple concomitant medications without any one particular drug that can be identified as the suspected confounding factor; in many cases both the patients and their physicians are reluctant to change medications for a condition such as hypertension or diabetes which has been stabilized under an existing drug regimen; as well as the fact that many of the alternative medications for the same condition also carry a risk for lichenoid reactions.
In addition, there is no clear protocol indicating how long one should wait for resolution to occur after cessation of treatment: are several weeks sufficient, several months, or more? There is just no reliable information available in the literature to effectively answer this question. To attempt to re-challenge with the suspected medication is even more difficult, and may not be truly justifiable outside the context of a scientific investigation. Clinically, if there is a single lichenoid lesion it may be associated with contact stomatitis due to amalgam, cinnamon hypersensitivity, or a drug-related reaction, whereas true OLP is more likely to be multifocal. However, a drug-related reaction may also present in a diffuse or multifocal pattern, and in these cases, unless there is a close correlation between the onset of treatment with a particular medication and the onset of lichenoid oral lesions, OLP and drug-induced oral lichenoid lesions are very difficult to separate.
Occasionally, these drug-induced lesions can be suspected microscopically by exhibiting a mixed inflammatory infiltrate (rather than a predominantly lymphocytic population in OLP), or an element of peri-vascular inflammation or deep inflammation in the lamina propria (rather than a band-like infiltrate), but this is not a constant finding in all drug-induced oral lichenoid lesions. Therefore, the presence of the microscopic features described can rule out OLP, but their absence does not rule-out drug-related hypersensitivity [13].
As stated in the methods section, patients with systemic diseases which can mimic OLP were excluded from the analysis. Addressing the questions on the possible effects of the systemic diseases on the response to treatment was beyond the scope of the present study, as it would require a much larger study group for statistical evaluation.
Several different scales have been used and recently validated for evaluation of signs and symptoms of OLP in research: visual analog scale (VAS), numeric rating scale (NRS), change in symptoms scale (CSS) and the modified oral mucositis index (MOMI) [14]. However, some are fairly complicated and relatively time-consuming for routine clinical use. In the present study, a retrospective study of a 10-year period, these scales have not been applied. Although less reproducible and reliable, the verbal report by the patient was used, and has proved to correlate well with the subjective criteria of IE.
The majority of OLP cases in this study did require treatment and the study results clearly support the benefit of treatment in OLP patients. Although spontaneous remissions have been recorded in a small fraction of patients, for the majority of patients improvement in the extent of the lesions was significantly more often in treated versus non-treated patients.
Conclusions
Results of this long-term study confirmed that OLP is a dynamic disease process, with constant changes in both clinical presentation and extent of lesions. Furthermore, it is suggested that there is limited value in using the classification of OLP as a factor in determining the need or frequency of follow-up, as this classification has a high probability of change over time. The effect of systemic diseases as confounding factors was minor or insignificant in the present group. In addition, results showed that there is a value for treatment of OLP in reducing the severity and extent of oral lesions.
Acknowledgments
Conflict of interest statement
None declared.
References
- 1.Carrozzo M, Thorpe R. Oral lichen planus: a review. Minerva Stomatol. 2009;58(10):519–537. [PubMed] [Google Scholar]
- 2.McCartan BE, Healy CM. The reported prevalence of oral lichen planus: a review and critique. J Oral Pathol Med. 2008;37(8):447–453. doi: 10.1111/j.1600-0714.2008.00662.x. [DOI] [PubMed] [Google Scholar]
- 3.Neville BW, Damm DD, Allen CM, Bouquot JE. Oral and maxillofacial pathology. 3. St. Louis: Saunders; 2009. pp. 783–784. [Google Scholar]
- 4.Yarom N, Shani T, Amariglio N, Taicher S, Kaplan I, Vered M, Rechavi G, Trakhtenbrot L, Hirshberg A. Chromosomal numerical aberrations in oral lichen planus. J Dent Res. 2009;88(5):427–432. doi: 10.1177/0022034509337089. [DOI] [PubMed] [Google Scholar]
- 5.Mignogna MD, Fedele S, Lo Russo L, Lo Muzio L, Bucci E. Immune activation and chronic inflammation as the cause of malignancy in oral lichen planus: is there any evidence? Oral Oncol. 2004;40(2):120–130. doi: 10.1016/j.oraloncology.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 6.Mignogna MD, Fedele S, Lo Russo L, Mignogna C, Rosa G, Porter SR. Field cancerization in oral lichen planus. Eur J Surg Oncol. 2007;33(3):383–389. doi: 10.1016/j.ejso.2006.09.028. [DOI] [PubMed] [Google Scholar]
- 7.Kramer IR, Lucas RB, Pindborg JJ, Sobin LH. Definition of leukoplakia and related lesions: an aid to studies on oral precancer. Oral Surg Oral Med Oral Pathol. 1978;46:518–539. doi: 10.1016/0030-4220(78)90382-1. [DOI] [PubMed] [Google Scholar]
- 8.Meij EH, Waal I. Lack of clinicopathologic correlation in the diagnosis of oral lichen planus based on the presently available diagnostic criteria and suggestions for modifications. J Oral Pathol Med. 2003;32:507–512. doi: 10.1034/j.1600-0714.2003.00125.x. [DOI] [PubMed] [Google Scholar]
- 9.Bertakis KD, Azari R, Helms J, Callahan EJ, Robbins JA. Gender differences in the utilization of health care services. J Fam Pract. 2000;49:147–152. [PubMed] [Google Scholar]
- 10.Shalev V, Chodick G, Heymann AD, Kokia E. Gender differences in healthcare utilization and medical indicators among patients with diabetes. Pub Health. 2005;119:45–49. doi: 10.1016/j.puhe.2004.03.004. [DOI] [PubMed] [Google Scholar]
- 11.Carbone M, Arduino PG, Carrozzo M, Gandolfo S, Argiolas MR, Bertolusso G, Conrotto D, Pentenero M, Broccoletti R. Course of oral lichen planus: a retrospective study of 808 northern Italian patients. Oral Dis. 2009;5(3):235–243. doi: 10.1111/j.1601-0825.2009.01516.x. [DOI] [PubMed] [Google Scholar]
- 12.Bidara M, Buchanan J, Scully C, Moles DR, Porter SR. Oral lichen planus: a condition with more persistence and extra-oral involvement than suspected? J Oral Pathol Med. 2008;37:582–586. doi: 10.1111/j.1600-0714.2008.00703.x. [DOI] [PubMed] [Google Scholar]
- 13.Ismail Sb, Kumar SKS, Zain RB. Oral lichen planus and lichenoid reactions: etiopathogenesis, diagnosis, management and malignant transformation. J Oral Science. 2007;49(2):89–106. doi: 10.2334/josnusd.49.89. [DOI] [PubMed] [Google Scholar]
- 14.Chainani-Wu N, Silverman S, Reingold A, Bostrom A, Lozada-Nur F, Weintraub J. Validation of instruments to measure the symptoms and signs of oral lichen planus. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 2008;105:51–58. doi: 10.1016/j.tripleo.2007.06.022. [DOI] [PubMed] [Google Scholar]