Abstract
Purpose
We recently described ovarian genotypes and sub-genotypes of the FMR1 gene with distinctly associated ovarian aging patterns, which in infertile women follow a typical X-linked distribution pattern. Whether normally fertile women, however, also demonstrate the same distribution, is unknown.
Methods
We, therefore, investigated ovarian FMR1 genotype and sub-genotype distribution in 182 oocyte donor candidates in comparison to 339 infertile controls. As previously reported, genotype designation was made, based on a normal range of CGG n = 26–34 (median 30), defining women as normal (norm), heterozygous (het) or homozygous (hom). Het and hom genotypes were further subdivided into sub-genotypes, based on whether abnormal alleles were above (high) or below normal range (low).
Results
Oocyte donors presented with 47.8% norm, 45.1% het and 7.1% hom genotypes, confirming a typical X-linked distribution pattern. They, however, still subtly differed from infertility patients, especially in het sub-genotypes.
Conclusion
These findings validate recently newly described ovarian genotypes and sub-genotypes, reaffirming their relevance to female fertility/infertility.
Keywords: Fragile X mental retardation 1 gene (FMR1), Ovarian reserve, Genotypes, Sub-genotypes, Oocyte donors, Infertility
Introduction
We previously reported ovarian FMR1 genotypes and sub-genotypes, which were based on statistical analyses of the gene’s CGG triple nucleotide repeats (CGG n) in exclusively infertile patients [1–3]. These newly defined genotypes and sub-genotypes have to be differentiated from traditional FMR1 genotypes, historically defining primarily neuro-psychiatric risks, including the fragile X syndrome [4].
Traditional FMR1 genotypes include common (normal) at CGG n < 44, intermediate (or gray zone) at CGG n = 45–54, premutation range at CGG n = 55–200 and full mutation (fragile X syndrome) at CGG n > 200 [4]. In contrast, recently reported ovarian genotypes of FMR1 are based on the recognition that CGG n in female populations is distributed in typical X-linked fashion, defined by a normal range of CGG n = 26–34 (median 30) [1–3]. This median has previously been reported as switching point between positive and negative message, and as point of peak translation for the gene [5].
Depending on CGG n on both X chromosomes, a woman, thus, can be normal (norm) if both alleles are in normal range, heterozygous (het) if one allele is outside normal range or homozygous (hom) if both alleles are outside range.
Het and hom genotypes can be further sub-divided into sub-genotypes, depending on whether abnormal counts are above (high) or below (low) normal range. Het can, thus, be sub-divided into het-norm/high and het-norm/low, while hom can be sub-divided into hom-high/high, hom-high/low and hom-low/low [2, 3]. Prior studies in 339 infertile patients demonstrated that approximately half demonstrated the norm genotype, around 40% the het genotype, with slightly more showing het-norm/low than het-norm/high, and remaining women demonstrating the various hom sub-genotypes [2]. Genotype and sub-genotype distribution to a degree varies between races/ethnicities, with women of African descent demonstrating disproportionally het-norm/low sub-genotypes, while Asian women demonstrate excessive het-norm/high sub-genotypes in comparison to Caucasian women [3].
CGG triple nucleotide repeats in infertile women are, however, to some degree skewed since low as well as high repeat numbers, have been associated with increased risk towards prematurely diminished ovarian reserve, also given the acronym of premature ovarian aging (POA) [6]. Since POA patients represent as significant portion of infertility patients [7], their distribution of CGG repeats may somewhat differ from the distribution in normally fertile women.
Carefully screened oocyte donors, of course, should represent normally fertile women. Validation of distributions of FMR1 genotypes and sub-genotypes in oocyte donors, therefore, would reaffirm in infertile women established criteria for definition of ovarian FMR1 genotypes and sub-genotypes and, in addition, validate these newly described ovarian FMR1 genotypes and sub-genotypes as physiologically relevant.
The purpose of this study was, therefore, a comparison of distribution patterns of FMR1 genotypes and sub-genotypes in normal oocyte donors and in, previously reported, infertile women [2, 3].
Materials and methods
This study was performed at the Center for Human Reproduction in New York City. In the U.S., the law permits anonymous oocyte donation, and oocyte donors are allowed to receive reasonable remuneration for time and efforts. Our center, therefore, maintains a large pool of prescreened oocyte donors to allow recipients choice between donors, and a quick recipient/donor matching process.
In order to be included into the center’s donor pool, candidates have too be under age 34 years (but usually are under age 30), and undergo a very detailed, multi-step screening process, which excludes candidates with medically relevant personal, family or genetic histories. Screening steps include an extensive written questionnaire and two face-to-face staff interviews, the latter one with a physician. Less than five percent of original applicants reach a final medical testing stage. One, therefore, cannot completely rule out the possibility that this very detailed screening process in unrecognized ways affects FMR1 genotype and sub-genotype distribution.
The candidates’ medical screening includes ovarian reserve assessments by ultrasound and measurements of anti-Müllerian hormone (AMH). Candidates with abnormally low AMH levels are usually excluded, though, in exceptional cases, may be allowed to donate with a modified ovarian stimulation protocol.
Since 2008, screening of donor candidates involves evaluation of the FMR1 gene to exclude carriers of traditional premutation range CGG triple repeats (CGG n = 55–200) and of full mutations (CGG n > 200)[4]. Donor candidates undergo FMR1 testing as part of a medical testing round, representing the last stage of screening prior to admission into the center’s oocyte donor pool.
The donors’ young age and their detailed screening virtually guarantee that none of the candidates suffers from significant ovarian function abnormalities. They, therefore, with reasonable certainty should approximate a normally fertile, young population of women.
In this study CGG n of both alleles were determined in 182 consecutive oocyte donor candidates and 339 previously reported infertility patients [2, 3]. Utilized commercial tests to assess CGG n and distribution of FMR1 genotypes and sub-genotypes in this infertile population were also reported before [2, 3]. Since oocyte donor candidates are chosen to reflect the center’s patient pool, we expected them to be similar in race/ethnicity distribution. This was confirmed (Table 1).
Table 1.
Patient characteristics of both groups
| Infertile Patients (n = 339) | Donors (n = 182) | |
|---|---|---|
| Age (years) | 37.9 ± 5.0 | 24.0 ± 3.8 * |
| BMI (kg/m²) | 24.4 ± 4.8 | 21.3 ± 2.6 * |
| AMH (ng/mL) | 1.4 ± 1.7 | 4.4 ± 2.8 * |
| FSH (mIU/mL) | 11.0 ± 6.4 | 6.5 ± 3.2 * |
| Estradiol (pg/mL) | 53.1 ± 39.9 | 54.0 ± 35.4 |
| Race (n/%) | ||
| African | 45 (13.3) | 28 (15.4) |
| Asian | 61 (18.0) | 45 (24.7) |
| Caucasian | 221 (65.2) | 98 (53.8) |
| Other | 12 (3.5) | 11 (6.0) |
Values are in means ± standard deviations unless otherwise indicated. * = P ≤ 0.001
Baseline characteristics of both groups were tabulated using means and standard deviations or percentages. Comparisons between groups were made by Chi-Square or Fisher’s Exact Test. Statistical analyses were undertaken using the Statistical Package for the Social Sciences 17.0 (SPSS, Chicago, IL, USA).
Like other patients, donors sign at time of initial consultation an informed consent that allows for use of their medical records for research purposes, as long as content of the medical record remains confidential and their identity remains protected. Both conditions were met for this study, which, therefore, qualified under the center’s Institutional Review Board (IRB) rules for expedited review. Donors, in addition, signed a specific FMR1 testing consent. Such consents are required for all genetic tests performed at the center.
Donor candidates’ ovarian FMR1 genotypes and/or sub-genotypes are currently not considered in deciding on qualification for the center’s donor pool, since FMR1 testing is performed to detect traditional premutation and full mutation range genotypes [4]. The center’s IRB, however, mandated that, based on results from prior studies [1–3, 6], donors be informed if mutations from the norm genotypes are observed.
Results
Table 1 summarizes basic patient characteristics for both study groups. As the table demonstrates, egg donors were significantly younger (P < 0.001) and had a lower BMI (P < 0.001). They also demonstrated significantly higher AMH (P < 0.001) and lower FSH values (P < 0.001).
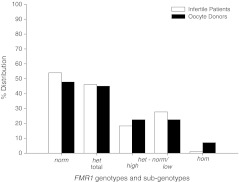
The 182 consecutive oocyte donor candidates demonstrated the following genotype and sub-genotype distribution (Fig. 1): norm 87 (47.8%); het 82 (45.1%); hom, 13 (7.1%). Het sub-genotypes were equally divided (41, 22.5%, each) between het-norm/high and het-norm/low. Hom sub-genotypes were too few in number to offer statistically valid percentages.
Fig. 1.
Distribution of FMR1 genotypes and sub-genotypes in oocyte donors. Here presented distribution data of 339 infertile patients have previously been reported [2, 3]. Including hom patients/donors, the distribution differed between patients and donors (Chi-Square, P < 0.0001). Individual genotype and sub-genotype distributions, however, did not differ significantly, though differences in distribution of het sub-genotypes demonstrated a trend (One tailed Fisher’s Exact Test, P = 0.08)
FMR1 genotypes and sub-genotypes for the 339 infertile women, as reported before [2, 3], were: norm in 183 (54.0%); het 156 (46.0%); and the group contained no hom patients. Het sub-genotypes were: het-norm/high 62 (18.3%); and het-norm/low 94 (27.7%).
Including hom patients, the infertile patient population differed significantly between infertility patients and donors [x2 (3,N = 521) = 27.46, P < 0.001], though individual genotypes and sub-genotypes did not differ between the two groups. The difference in distribution of het sub-genotypes demonstrated a trend (One-tailed Fisher’s Exact Test, P = 0.08).
Discussion
As this study demonstrates, ovarian FMR1 genotype and sub-genotype distribution in oocyte donor candidates reflects a typical X-linked pattern. By approximately half of all donors (47.8%) demonstrating norm genotypes, and het genotypes, equally distributed (22.5% each) between het-norm/high and het-norm/low, donors follow such an X-linked pattern even marginally closer than infertile women.
As Fig. 1 demonstrates, donor distribution of FMR1 genotypes and sub-genotypes mildly varies from that of infertile women (including hom, P < 0.0001). While individual genotype/sub-genotype distribution differences lack significance, they appear most pronounced amongst het sub-genotypes, though, even there, fail to reach statistical significance (P = 0.08).
These findings should not surprise: Small difference between supposedly normally fertile donors and an infertility population can be expected as both, abnormally low and high CGG n, has been associated with POA [6]. POA, in turn, is, of course, highly associated with infertility.
Abnormally low and high CGG n represents het and hom sub-genotypes, respectively [2, 3]. By definition, infertile women, therefore, can be expected to demonstrate a higher prevalence of het and hom genotypes.
Infertile women presented with almost identical total het genotype prevalence as donors (46% vs. 45%). Marginal differences in distribution were only seen amongst het sub-genotypes, where het-norm high represented 18.3% in patients but 22.5% in donors, and het-norm/low, with 27.7% in patients and only 22.5% in donors (P = 0.08).
The distribution of het sub-genotypes in infertile women has previously been reported [2, 3]. Hom sub-genotypes, because of their low prevalence, however, so far are not well defined in their respective distribution and functions. This study, unfortunately, also lacks adequate numbers of hom individuals, and, therefore, does not further contribute to knowledge about distribution of hom sub-genotypes of FMR1.
Almost complete absence of hom genotypes in patients (Fig. 1) may, however, offer a first hint as to the significance of the hom genotype in infertility. Its extremely low prevalence suggests two possible explanations: women with hom genotype either have normal fertility or, probably more likely, a fertility level too poor for presentation to a fertility center. They already at young ages may be so badly affected that, by more advanced age, they no longer are represented in typical infertile patient pools. Further studies of hom patients are, however, very obviously urgently needed.
Norm genotypes were marginally more frequent in patients than donors (54.0% vs. 47.8%), which has to be considered surprising. Though statistically insignificant, this finding warrants follow up to preclude unintended consequences from our current screening of donor candidates.
Considering that the het-norm/low sub-genotype is strongly associated with a reduction in IVF pregnancy chances [2, 3], the relatively high prevalence of het-norm/low among infertility patients should not surprise. An increased prevalence of het-norm/low is also observed in women of African descent, who also demonstrate diminished pregnancy chances in IVF [3].
Women with het-norm/high demonstrate better IVF pregnancy rates than those with het-norm/low FMR1 sub-genotype but lower rates than women with norm genotype [2]. Especially in older women, het-norm/high is actually associated with comparatively increased oocyte yields [8], and, therefore, likely better pregnancy chances. To find more het-norm/low and less het-norm/high in infertile women, especially older women, therefore, does not surprise.
Differences in genotypes and sub-genotypes distribution of the FMR1 gene may also be racial/ethnic: While Caucasian, African and Asian women, all, demonstrate approximately equal norm distributions in the low 50% range, they vary considerably in het sub-genotype distributions: Asian women, for example, demonstrate more het-norm/high (29.2%) than Caucasian (16.8%) and African patients (15.3%). In contrast, African women demonstrate the highest prevalence of het-norm/low (32.2%) and Asian the lowest (18.8%), with Caucasians occupying the middle ground (28.4%) [3].
Here presented data in oocyte donor candidates, thus, validate previously reported distributions in infertile women [2, 3]. Observed subtle differences between these two distinct patient populations are actually supportive of proposed physiological functions of ovarian FMR1 genotypes and sub-genotypes. Because associated with specific ovarian aging patterns [1, 2], varying in distribution amongst races/ethnicities [3], and predictive of autoimmune risk [2], they, apparently, guide important physiologic functions. Further exploration of these new ovarian genotypes of FMR1, therefore, appears indicated.
Acknowledgments
Funding
This work was supported by the Foundation for Reproductive Medicine and intramural grants from the Center for Human Reproduction.
Footnotes
Capsule
Egg donor candidates were found to have a similar, but not identical, distribution of ovarian FMR1 genotypes and sub-genotypes, compatible with previously reported functions.
N.G. and D.H.B. are listed as co-inventors on a pending U.S. patent application, which claims diagnostic benefits in infertility from assessing FMR1 genotypes and sub-genotypes.
Here presented material was in part presented in abstract form at the 67th Annual Meeting of the American Society for Reproductive Medicine in Orlando, Florida, October 15–19, 2011.
Authors’ roles
N.G. and D.H.B. contributed equally to study design, study conduct and preparation of the manuscript, with N.G, being primarily responsible for writing the manuscript and D.H.B. being primarily responsible for data analysis. A.W. contributed to study design and A.K. contributed to data analysis. All authors reviewed and approved the final manuscript.
References
- 1.Gleicher N, Weghofer A, Barad DH. Ovarian reserve determinations suggest new function of FMR1 (fragile X gene) in regulating ovarian aeging. Reprod Biomed Online. 2010;20:768–775. doi: 10.1016/j.rbmo.2010.02.020. [DOI] [PubMed] [Google Scholar]
- 2.Gleicher N, Weghofer A, Lee IH, Barad DH. FMR1 genotype with autoimmunity-associated polycystic ovary-like phenotype and decreased pregnancy chance. PLoS ONE. 2010;5:e15303. doi: 10.1371/journal.pone.0015303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gleicher N, Weghofer A, Lee IH, Barad DH. Association of FMR1 genotypes with in vitro fertilization (IVF) outcomes based on ethnicity/race. PLoS ONE. 2011;6:e18781. doi: 10.1371/journal.pone.0018781. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Willemsen R, Levenga J, Oostra B. CGG repeat in the FMR1 gene: size matters. Clin Genet. 2011;80:214–225. doi: 10.1111/j.1399-0004.2011.01723.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chen LS, Tassone F, Sahota P, Hagerman PJ. The (CGG)n repeat element within the 5’untranslated region of the FMR1 message provides both positive and negative cis effects on in-vivo translation of a downstream reporter. Hum Molec Genetics. 2003;12:307–3074. doi: 10.1093/hmg/ddg027. [DOI] [PubMed] [Google Scholar]
- 6.Gleicher N, Weghofer A, Oktay K, Barad D. Relevance of triple CGG repeats in the FMR1 gene to ovarian reserve. Reprod Biomed Online. 2009;19:385–390. doi: 10.1016/S1472-6483(10)60173-3. [DOI] [PubMed] [Google Scholar]
- 7.Barad DH, Weghofer A, Gleicher N. Age-specific levels of basal follicle-stimulating hormone assessment of ovarian function. Obstet Gynecol. 2007;109:1404–1410. doi: 10.1097/01.AOG.0000264065.37661.a0. [DOI] [PubMed] [Google Scholar]
- 8.Barad DH, Weghofer A, Kim A. The het-norm/high FMR1 sub-genotype increases oocyte yields at advanced female age. Fertil Steril 2011;Suppl:S119