Abstract
Background
As advancements in cancer therapies have led to dramatic improvements in long term survival, there has been increasing interest in methods to expand fertility preservation options for cancer patients.
Methods
An experimental protocol for ovarian tissue cryopreservation was developed at the University of Pennsylvania for patients requiring gonadotoxic therapies. The protocol for adults was implemented at the Hospital of the University of Pennsylvania and for children at the Children’s Hospital of Philadelphia in collaboration with the Oncofertility Consortium and the National Physicians Cooperative (NPC).
Results
A total of twenty-one patients (age range: 8–36 years) have cryopreserved ovarian tissue as part of this study. While patients had a variety of diagnoses and treatment exposures, 10/21 (48 %) patients suffered from hematologic disorders and 43 % were anticipating stem cell transplantation. No patients have requested that the tissue be used for clinical purposes.
Conclusions
Ovarian tissue cryopreservation protocols can be implemented at pediatric and adult institutions through multi-disciplinary collaboration. While more research is needed to determine the safety and efficacy of ovarian tissue cryopreservation, this procedure provides hope for preserving the ability to have biological offspring to patients facing gonadotoxic therapies for a variety of medical conditions.
Keywords: Ovarian tissue, Cryopreservation, Fertility preservation, Cancer, Oncofertility
Introduction
Over 100,000 females less than 45 years of age are diagnosed with cancer annually. Of these, approximately 12,400 are children <20 years of age [1]. During the past 4 decades, advancements in cancer therapies, particularly chemotherapeutics, have led to dramatic improvements in survival. As a result, care has now focused on improving long term health and quality of life for survivors. One of the most important quality of life issues in reproductive age cancer survivors is the ability to have biologic children. Unfortunately, cancer therapies such as alkylating agents, local radiotherapy in proximity to the ovaries, total body irradiation and stem cell conditioning regimens increase the risk of infertility and ovarian failure. The ovary is particularly sensitive to the adverse effects of cancer treatments because of the finite number of germ cells present in the post-natal ovary [2, 3]. Ovarian failure from these agents appears to be dependent on the dose of therapy and the patient’s age at the time of treatment, with older age at exposure carrying greater risk [4–6].
There has been increasing interest in methods to expand fertility preservation options for cancer patients or others with diagnoses treated with gonadotoxic agents. While embryo cryopreservation remains the standard option for adult females with a committed sexual partner, oocyte cryopreservation and ovarian tissue cryopreservation (OTC) technologies have become clinically available experimental options for females without a partner [7]. These fertility preservation technologies have gained traction, particularly after the publication of the ASCO fertility preservation recommendations in 2006 [8]. However, currently available methods do have limitations. Embryo and oocyte cryopreservation are particularly limited by the need for ovarian stimulation and oocyte retrieval, a process which can delay cancer treatment 2–4 weeks [9]. Further, these techniques are not possible in prepubertal girls who do not have mature eggs in their ovaries. OTC eliminates the need for ovarian stimulation and does not require a sperm source. While investigational, live births have been reported following OTC and autologous transplantation in cancer patients [10]. Currently, this is the only method available for fertility preservation in prepubertal girls. In this paper, we describe our experience with OTC in children and adult females at 2 academic institutions.
Patients/methods
An experimental protocol for ovarian tissue cryopreservation was developed at the University of Pennsylvania in collaboration with the Oncofertility Consortium and the National Physicians Cooperative (NPC). Institutional Review Board (IRB) approval of Ovarian Tissue Cryopreservation study was initially obtained at Hospital of the University of Pennsylvania (HUP) in April 2007 and at the Children’s Hospital of Philadelphia (CHOP) in May 2009.
At HUP, study eligibility required that participants be generally healthy females between 18 and 42 years of age with both ovaries, and in need of imminent medical or surgical treatment likely to result in ovarian failure. Subjects were excluded from participation if they had medical problems putting them at high risk for surgical complications, serum FSH levels above 20 mlU/mL in the absence of recent administration of chemotherapy, BRCA gene mutation, suspected ovarian malignancy, and/or the presence of large ovarian masses. Eligible subjects were offered oophorectomy or ovarian biopsy. Due to concerns about the vulnerability of children in relation to the risk/benefit ratio of the proposed procedure, CHOP IRB approval was initially restricted to females at least 10 years of age but has recently been expanded to allow participation of children at least 1 year of age. At CHOP the procedure was limited to ovarian biopsy only. Eligible pediatric subjects were also limited to those at highest risk for long term ovarian dysfunction based on planned cumulative dose of alkylating agents, radiation plan or stem cell transplant conditioning. Previous therapy was not an exclusion. The ovarian surgery, cryopreservation and storage were performed at no cost to the participants. Procedure costs were subsidized by philanthropic funds.
The multi-disciplinary investigative team was comprised of a pediatric oncologist, a reproductive endocrinologist, a pediatric surgeon, a pediatric and adult clinical research nurse and a psychosocial counselor. The pediatric oncologist was responsible for counseling potential participants and families at CHOP. The reproductive endocrinologist was responsible for counseling adult participants enrolled at HUP and for performing the surgical procedures at both hospitals. A pediatric surgeon was asked to participate in cases where small children were enrolled and when the reproductive endocrinologist was not available. Clinical nurses were responsible for coordinating care.
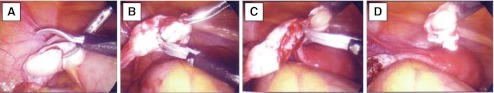
A member of the team approached eligible patients or family members and discussed the risks and potential benefits of the study. When appropriate, psychosocial counseling was provided to review informed consent with the patient and/or parents. At CHOP, a child advocate was appointed for every patient enrolled on the study. For those patients capable of assent, the advocate assures that the child’s needs for self determination are met. For cases where assent is not possible, the advocate’s role is to assess that the parent is acting in the best interest of the child, is not motivated by their own wishes and has an understanding that any future use of the cryopreserved tissue will be made by their child when they reach adulthood. Surgery was scheduled by the primary team enrolling the participant and was coordinated with the operating physician and operating room. While not required for participation, efforts were made to coordinate the procurement of ovarian tissue during another clinically required surgical procedure whenever possible. Every attempt was made to schedule the procedure in the morning so that tissue processing and cryopreservation, which can take several hours, could be completed within normal working laboratory hours. Infectious disease screening was obtained for all participants and FSH levels were determined. After obtaining informed consent and/or assent, a laparoscopy was performed under general anesthesia (unless the patient was already having an open abdominal procedure) and either an ovarian cortical biopsy or an oophorectomy was performed. For biopsy procedures, approximately one quarter to one third of the ovarian cortex of one ovary was removed using laparoscopic scissors without cautery (Fig. 1). In one case, ovarian tissue was removed at the time of a laparotomy performed for tumor resection. Safety of the procedure was assessed at a postoperative visit following surgery and by chart review.
Fig. 1.
Ovarian biopsy surgical procedure. Panels a–d depict a laparoscopic view of a surgical biopsy of ovarian cortical tissue
Tissue was transported on ice in holding media to Penn Fertility Care, which is located a few blocks from the pediatric and adult hospital. According to the Oncofertility National Physicians Cooperative (NPC) protocol which uses the Gosden method [11], ovarian cortical tissue was dissected into 5 mm × 10 mm × 1 mm thick strips and cryopreserved using 1.5 M Ethylene Glycol in MOPS-Diluent with 0.1 M Sucrose and a slow-freeze technique over 3–4 h. 80 % of the cryopreserved tissue was stored in the embryology laboratory at Penn Fertility Care for the patient’s future use. The remaining 20 % was sent to the NPC research tissue repository for studies of in vitro follicle maturation by the Oncofertility Consortium (www.oncofertility.northwestern.edu).
In one case, a small fragment of fresh cortical tissue was fixed for further histological analysis in order to determine the impact of prior chemotherapy on the ovarian follicle reserve. This was an 11 year old premenarchal Tanner Stage 1 female diagnosed at CHOP with Stage IV neuroblastoma. The patient underwent 5 cycles of chemotherapy (cyclophosphamide cumulative dose of 12.4 G/m2, topotecan, cisplatin 400 mg/m2, etoposide, doxorubicin, vincristine) and was scheduled for surgical resection of her abdominal tumor 3 weeks after her last chemotherapy treatment cycle. Her FSH level at that time was found to be remarkably high at 83.8 mlU/ml. A small portion of the ovarian cortical tissue was excised at the time of her abdominal surgery. The specimen was frozen and banked according to the methods described. Histologic sections were prepared according to established procedures, which involved fixing the tissue with neutral buffered formalin for at least 4 h, followed by washing with PBS and dehydration using graded ethanol and xylenes to then embed the tissue in paraffin. Sections of 5 μm were cut with a Microm HM355 microtome. The sections were deparaffinized and stained with Harris Hematoxylin and Eosin, and mounted with Permount. Primary and secondary follicles were counted in every fifth section in five high power fields using a Nikon TE-300 microscope.
Results
A total of twenty-one patients participated in the ovarian tissue cryopreservation study. Characteristics of study participants are presented in Table 1. Participants ranged in age from 8 to 36 years and nine children were included. Seventeen of twenty-one (81 %) participants were Caucasian, 3/21 (14 %) were African-American, and 1/21 (5 %) was Hispanic. While diagnoses were heterogeneous, the majority of patients (10/21) suffered from hematologic disorders requiring gonadotoxic therapy. Nine (43 %) participants were exposed to chemotherapy prior to OTC and nine (43 %) were anticipating treatment with stem cell transplantation. With respect to the acquisition of ovarian tissue, three (14 %) had an entire ovary removed (all of whom were adults), while the remaining subjects had a biopsy of ovarian cortical tissue. Overall, 14/21 (67 %) had a concomitant surgical procedure, such as tumor resection or central venous access placement, at the time of OTC. Eight (38 %) attempted another method of fertility preservation either prior to or at the time of OTC. In particular, 5/21 (24 %) underwent ovarian transposition surgery or oophorpexy along with the removal of ovarian tissue. No surgical or post-operative complications have occurred. One patient (Participant #1) has died since banking due to disease relapse. No patient has requested that the tissue be used clinically.
Table 1.
Characteristics of study participants
| Age (Yrs) | Diagnosis | Gonadotoxic treatment Prior to OTC | Planned treatment | FSH mlU/mL | Specimen | Other fertility preservation options pursued | |
|---|---|---|---|---|---|---|---|
| 1 | 8 | Acute Myelogenous Leukemia | Yes | Stem Cell Transplantation | 3.8 | Biopsy | None |
| 2 | 10 | Sickle-Cell Anemia | Yes | Stem Cell Transplantation | 0.03 | Biopsy | None |
| 3 | 11 | Neuroblastoma | Yes | Stem Cell Transplantation | 83.8 | Biopsy | None |
| Abdominal Radiotherapy | |||||||
| 4 | 12 | Pineoblastoma | No | Stem Cell Transplantation | 1.3 | Biopsy | Ovarian transposition at time of ovarian biopsy |
| Craniospinal Radiotherapy | |||||||
| 5 | 14 | Acute Myelogenous Leukemia | Yes | Stem Cell Transplantation | 8.0 | Biopsy | None |
| 6 | 14 | Myelodysplastic Syndrome | No | Stem Cell Transplantation | 7.9 | Biopsy | None |
| 7 | 15 | Medulloblastoma | No | Chemotherapy (Cyclophosphamide) | 2.0 | Biopsy | Ovarian transposition at time of ovarian biopsy |
| Craniospinal Radiotherapy | |||||||
| 8 | 16 | Ewing Sarcoma | No | Chemotherapy (Ifosfamide and Cyclophosphamide) | 7.0 | Biopsy | None |
| 9 | 17 | Rhabdo-myosarcoma | Yes | Chemotherapy (Ifosfamide and Cyclophosphamide) | 6.2 | Biopsy | None |
| Radiotherapy | |||||||
| 10 | 18 | Ewing’s Sarcoma | No | Chemotherapy (Ifosfamide and Cyclophosphamide) | 0.8 | Biopsy | None |
| 11 | 18 | Central Nervous System Germinoma | No | Craniospinal Radiotherapy | 0.19 | Biopsy | Ovarian transposition at the time of ovarian biopsy |
| 12 | 20 | Hodgkin Lymphoma | Yes | Stem Cell Transplantation | 8.4 | Biopsy | Failed ovarian stimulation |
| 13 | 24 | Breast Cancer | No | Chemotherapy (ACT) | 7.3 | Biopsy | None |
| 14 | 25 | Acute lymphoblastic leukemia | Yes | Stem Cell Transplantation | 1.0 | Ovary | Failed ovarian stimulation |
| 15 | 26 | Breast Cancer | No | Chemotherapy (ACT) | 0.6 | Biopsy | None |
| 16 | 26 | Chronic Myelogenous Leukemia | Yes | Stem Cell Transplantation | 2.2 | Ovary | Ovarian stimulation with 7 oocytes banked |
| 17 | 29 | Cervical Cancer | No | Pelvic Radiotherapy and Chemotherapy (Cisplatin) | 4.7 | Biopsy | Ovarian transposition at time of ovarian biopsy |
| 18 | 32 | Hodgkin Lymphoma | Yes | Pelvic Radiotherapy | 12.1 | Biopsy | IVF with 4 embryos banked |
| Ovarian transposition at time of ovarian biopsy | |||||||
| 19 | 35 | Non-Hodgkin Lymphoma | Yes | Chemotherapy (R-CHOP) | 5.3 | Biopsy | None |
| 20 | 36 | Breast Cancer | No | Chemotherapy (ACT) | 8.8 | Ovary | None |
| 21 | 36 | Anaplastic Large Cell Lymphoma | No | Chemotherapy (Cyclophosphamide, Adriamycin, Vincristine) | 5.4 | Biopsy | None |
ACT = Doxarubicin, Cyclophosphamide, Paclitaxel
RCHOP = Rituximab, Cyclophophamide, Doxorubicin, Vincristine
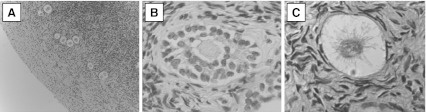
Follicle Stimulating Hormone (FSH) levels ranged from 1.0 to 83.8. The highest FSH level, 83.8 mlU/uL, was observed in an 11 year old premenarchal female with neuroblastoma who had recently been exposed to chemotherapy. Microscopic examination of fixed ovarian tissue revealed an average of 1–2 primary and 0 secondary follicles per 1.00 mm2 of tissue examined. Secondary follicles were only occasionally seen (Fig. 2).
Fig. 2.
Histologic analysis of ovarian cortical tissue. Panel a is a 10× view of remaining follicles in the patient’s ovarian cortex post cancer treatment despite her high FSH level of 83.8 mlU/ml. A single primary follicle is depicted in Panel b at 40× and a secondary follicle depicted in Panel c was visualized at 40×
Discussion
This manuscript reports our experience with OTC at the University of Pennsylvania and CHOP. To date, a total of twenty-one patients have cryopreserved ovarian tissue. Of these, 9 were less than 18 years of age. Patient characteristics, diagnoses and treatment exposures varied widely. The majority of cases were performed in patients who did not have other viable options for fertility preservation, or who did not respond to ovarian stimulation. Notably, 38 % pursued another method of fertility preservation before or at the time of OTC. To date, no transplants have been performed at our institution.
We found that ovarian tissue cryopreservation was a feasible option for children and adults facing gonadotoxic therapies. Multi-disciplinary collaboration (with a pediatric oncologist, reproductive endocrinologist, pediatric surgeon, pediatric and adult clinical/research nurse and a psychosocial counselor) was required to establish and carry out this experimental protocol at the adult and pediatric university affiliated hospitals. Not only must the investigative team identify and obtain informed consent/assent, the team must urgently coordinate preoperative testing, serologic testing for tissue storage and surgical scheduling in relation to planned cancer therapies. While scheduling surgery in the morning is ideal so that laboratory staff can be available during normal working hours to process and preserve tissue, it is often not possible due to the complex nature of OR scheduling, allocation of OR resources or unavoidable delays on the day of surgery. In our experience, tissue was received after noon in one half of the cases. This highlights the importance for surgeons and laboratory staff to be flexible. When a clinical practice makes a commitment to take care of oncofertility patients, it is important that the lab be open and available to accommodate cases outside of normal working hours.
Of particular interest is the case of an 11 year old pre-menarchal female with Stage IV neuroblastoma, status post recent chemotherapy, who elected to pursue OTC. Her remarkably high FSH level was concerning for follicular depletion; however, a complete absence of preantral follicles was not observed on histological examination of the excised ovarian tissue. This suggests that traditional measures of ovarian reserve may not accurately reflect ovarian follicle counts in pediatric patients shortly after chemotherapy. Hormones such as FSH are unreliable in prepubertal patients and those using hormonal contraception. More investigation is needed on the utility of endocrine values, particularly more novel markers such as anti-mullerian hormone levels, in the setting of pediatric cancer therapy to predict ovarian follicle reserve and ultimately determine whether OTC is of benefit to such patients. Nonetheless, at this time, OTC represents an important direction in fertility preservation for individuals who are unable to pursue other fertility preservation options such as embryo or oocyte cryopreservation.
Improvements in cancer treatments have increased survival rates; however, many therapies increase the risk of infertility and premature ovarian failure [5, 6, 12]. Interest in fertility preservation has increased and more efforts have been made to offer fertility preservation options to cancer patients as part of treatment. Fertility preservation strategies for females include embryo cryopreservation, oocyte cryopreservation and OTC. While embryo banking is currently considered the only standard (non-experimental) procedure, improved success with experimental techniques such as oocyte banking and ovarian tissue banking offer single females the possibility of having biological children with a future partner [10, 13, 14]. Selection of an appropriate technology depends on a variety of factors including the patient’s age and health at diagnosis, the overall gonadoxicity of the intended therapy, the urgency of treatment, cost, and the availability of a sexual partner. Many cancer patients do not have enough time to complete ovarian stimulation before starting cancer treatment, or have other concerns about this process. OTC is an attractive technique for fertility preservation because it avoids ovarian stimulation and remains the only option for pre-pubertal females. Rather than freezing individual oocytes, biopsy of the ovarian cortex theoretically represents an efficient way of preserving thousands of primordial follicles at one time. As described in this report, ovarian cortical tissue (whole ovary or biopsy) is generally obtained laparoscopically and dissected into small fragments and cryopreserved. The only method that has resulted in live human births is autotransplantation of cryopreserved tissue at the site of the native ovary that still included ovarian cortex. Sixteen human live births have been reported from previously frozen thawed tissue and are described in Table 2 [10]. Pregnancies have occurred with and without assistance after transplantation. It is important to note that all of the patients were adults at the time of tissue acquisition, most had a diagnosis of cancer, and all had remaining portions of ovarian cortex at the ovarian site at the time of transplantation of the thawed tissue.
Table 2.
Live births from orthotopic transplantation of frozen-thawed ovarian tissue
| Disease | Age at cryo (years) | Surgical method | Chemotherapy before cryopreservation | Pregnancy | Reference |
|---|---|---|---|---|---|
| Hodgkin Lymphoma | 25 | Ovarian biopsy | No | Spontaneous live birth | [24] |
| Neuro ectodermic tumor | 19 | Ovarian biopsy | No | Spontaneous live birth | [25] |
| Hodgkin Lymphoma | 20 | Ovarian biopsy | No | Spontaneous live birth | [10] |
| Non Hodgkin Lymphoma | 28 | Ovarian biopsy | Yes | IVF, live birth | [26] |
| Hodgkin Lymphoma | 24 | Unilateral oophorectomy | Yes | 2 spontaneous live births | [27] |
| Microscopic polyangitis | 27 | Unilateral oophorectomy | Yes | IVF, live birth | [10] |
| Breast Cancer | 36 | Ovarian biopsy | No | IVF, 2 live births (twins) | [28] |
| Premature Ovarian Failure | 24 | Ovarian biopsy | No | Spontaneous live birth | [17] |
| Hodgkin Lymphoma | 27 | Unilateral oophorectomy | Yes | IVF, live birth | [29] |
| Ewing sarcoma | 27 | Unilateral oophorectomy | No | IVF, 2 live births | [29] |
| Sickle Cell | 20 | Unilateral oophorectomy | No | Spontaneous, live birth | [30] |
| Hodgkin Lymphoma | 25 | Ovarian biopsy | Yes | Spontaneous, live birth | [31] |
| Thalassemia | 19 | Unilateral oophorectomy | No | IVF, live birth | [32] |
Potential limitations of OTC must be acknowledged. Most importantly, there is a significant concern regarding the potential for reseeding tumor cells following ovarian transplantation procedures in cancer survivors with tumors that might involve the ovaries. Although many types of cancer virtually never metastasize to the ovaries, leukemias are systemic in nature and therefore pose a significant risk. A recent study of 18 patients with leukemia (CML or ALL) showed that leukemic tumors occurred (4/18 cases) after thawed human ovarian cortical tissue was xenografted to mice [15]. Therefore, patients with leukemia and other tumors which involve the ovaries are counseled that cryopreserved ovarian tissue should not be used in the future for transplantation. In order to achieve pregnancy without transplantation, it would be ideal if oocytes could be matured and fertilized in vitro and embryos transferred to a woman in order for her to achieve pregnancy after cancer treatment. This technology has been successful in the mouse model and advancements have been made in the primate [16]. Ongoing studies are being conducted to move this technology forward. While auto transplantation of cryopreserved ovarian tissue also has the potential benefit of restoring temporary endocrine function to cancer survivors who develop premature ovarian failure, the duration of endocrine function is very limited [17].
Fertility preservation technologies present a variety of ethical challenges and uncertainty, particularly in pediatric patients [18]. Issues surrounding consent are particularly important to consider, since decisions being made involve a child’s future reproductive desires [19]. Parents must consider whether to pursue fertility preservation techniques at all, and if so, also determine the disposition of the reproductive tissues in case of death. When minors are sufficiently mature to understand the risks and benefits of the procedure, they must be involved in the process of assent [20]. Moreover, because fertility preservation techniques such as OTC and oocyte cryopreservation are still considered experimental [7], enrolling minors in clinical studies presents additional challenges. Protecting vulnerable populations is paramount, and IRB’s at most institutions will only allow studies in which the expected direct benefits of the experimental treatment outweigh its risks [21]. Because of concerns regarding the protection of minors, our experimental protocol in children was limited to ovarian biopsy only (rather than oophorectomy) in patients planning to receive treatments at the highest risk for permanent gonadotoxicity. In addition, it was initially stipulated that subjects between the ages of 10 and 12 were eligible for the procedure only if they were undergoing a concomitant surgical procedure under anesthesia. Recently, after a series of reports of human births from OTC and transplantation were reviewed, the eligible age range was expanded and the procedure allowed as a stand-alone surgery in at any age (although combining with clinical care is always the preference). It is expected that the inclusion criteria and study procedures for experimental protocols in children will vary somewhat by institution depending on the human subjects concerns at the site. The role of the child advocate should be highlighted here as an effective mechanism for ensuring that children, both those who assent and those who are not capable of assent, are protected.
Cost is a major barrier to accessing many fertility preserving technologies and will likely be an impediment to widespread application of OTC. Although OTC was performed at no cost to patients at our institutions because it was being done within the context of a research protocol, charges and costs of OTC vary. Coordinating OTC at the time of other surgical procedures can not only reduce the risk of the procedure, but also decrease the overall cost in terms of charges for general supplies, OR time and anesthesia costs. Nonetheless, combined procedures are not always possible and there must be a mechanism in place to cover the costs associated with OTC. Advocates of insurance coverage for fertility preservation argue that oncofertility services are different from services for infertile couples because the subsequent infertility is directly caused by medical treatments. For example, fertility preservation services can be likened to reconstructive breast surgery, which is covered for individuals who require a mastectomy for the treatment of breast cancer but not for individuals who desire the procedure for cosmetic purposes [22]. Still, many insurance companies do not cover fertility preservation services. Research is now beginning to explore the willingness of individuals to pay for ovarian tissue cryopreservation and evidence suggests that OTC is significantly valued [23]. As evidence of the utility of the use of ovarian tissue to restore fertility continues to build in terms of the numbers of live births, insurers may begin to appreciate this technique and include it within their cadre of covered services.
There are still many unanswered questions about the safety, efficacy, and application of ovarian tissue cryopreservation and subsequent transplantation. For example, it is not yet clear whom we should target for this technology, how much cortical tissue to remove, how to best cryopreserve the tissue, whether the technology is effective in prepubertal females, and how to minimize the risk of transplanting occult malignant cells. It is also important to develop a better understanding of patient and parental beliefs about fertility at the time of diagnosis and the major decision making influences when choosing whether or not to pursue fertility preservation options like OTC. While more research is clearly needed to answer these questions, reports of success from OTC provide hope for cancer survivors without other options to have biological children after treatment. Expanding post-treatment reproductive options will no doubt improve the overall long term quality of life of cancer survivors.
Acknowledgments
Supported by grants from the National Institutes of Health KL1-CA-133839-01 (Gracia), National Institutes of Health R01HD062797-02 (Gracia), Oncofertility Consortium NIH 1 UL1 RR024926-01 NIH Roadmap Interdisciplinary Research Consortia
No company derived money was used for salaries for investigators or for talks. The first author was in charge of the data and wrote the paper.
We would like to acknowledge Gerry Knee and the andrology laboratory at Penn Fertility Care for processing and cryopreserving the ovarian tissue and Monica Mainigi, MD for preparing and examining the histologic sections of ovarian tissue.
Footnotes
Capsule A protocol for ovarian tissue cryopreservation has been successfully established at an adult and pediatric hospital through multi-disciplinary collaboration.
References
- 1.Jemal A, Tiwari RC, Murray T, Ghafoor A, Samuels A, Ward E, et al. Cancer statistics, 2004. CA Cancer J Clin. 2004;54:8–29. doi: 10.3322/canjclin.54.1.8. [DOI] [PubMed] [Google Scholar]
- 2.Faddy MJ. Follicle dynamics during ovarian ageing. Mol Cell Endocrinol. 2000;163:43–48. doi: 10.1016/S0303-7207(99)00238-5. [DOI] [PubMed] [Google Scholar]
- 3.Forabosco A, Sforza C, Pol A, Vizzotto L, Marzona L, Ferrario VF. Morphometric study of the human neonatal ovary. Anat Rec. 1991;231:201–208. doi: 10.1002/ar.1092310208. [DOI] [PubMed] [Google Scholar]
- 4.Chemaitilly W, Mertens AC, Mitby P, Whitton J, Stovall M, Yasui Y, et al. Acute ovarian failure in the childhood cancer survivor study. J Clin Endocrinol Metab. 2006;91:1723–1728. doi: 10.1210/jc.2006-0020. [DOI] [PubMed] [Google Scholar]
- 5.Sklar CA, Mertens AC, Mitby P, Whitton J, Stovall M, Kasper C, et al. Premature menopause in survivors of childhood cancer: a report from the childhood cancer survivor study. J Natl Cancer Inst. 2006;98:890–896. doi: 10.1093/jnci/djj243. [DOI] [PubMed] [Google Scholar]
- 6.Green DM, Sklar CA, Boice JD, Jr, Mulvihill JJ, Whitton JA, Stovall M, et al. Ovarian failure and reproductive outcomes after childhood cancer treatment: results from the Childhood Cancer Survivor Study. J Clin Oncol. 2009;27:2374–2381. doi: 10.1200/JCO.2008.21.1839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Ovarian tissue and oocyte cryopreservation. Fertil Steril. 2008;90:S241–6. [DOI] [PubMed]
- 8.Lee SJ, Schover LR, Partridge AH, Patrizio P, Wallace WH, Hagerty K, et al. American Society of Clinical Oncology recommendations on fertility preservation in cancer patients. J Clin Oncol. 2006;24:2917–2931. doi: 10.1200/JCO.2006.06.5888. [DOI] [PubMed] [Google Scholar]
- 9.Quintero RB, Helmer A, Huang JQ, Westphal LM. Ovarian stimulation for fertility preservation in patients with cancer. Fertil Steril. 2010;93(3):865–868. doi: 10.1016/j.fertnstert.2008.10.007. [DOI] [PubMed] [Google Scholar]
- 10.Donnez J, Silber S, Andersen CY, Demeestere I, Piver P, Meirow D, et al. Children born after autotransplantation of cryopreserved ovarian tissue. A review of 13 live births. Ann Med. 2011;43:437–450. doi: 10.3109/07853890.2010.546807. [DOI] [PubMed] [Google Scholar]
- 11.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to oophorectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 12.Green DM, Kawashima T, Stovall M, Leisenring W, Sklar CA, Mertens AC, et al. Fertility of female survivors of childhood cancer: a report from the childhood cancer survivor study. J Clin Oncol. 2009;27:2677–2685. doi: 10.1200/JCO.2008.20.1541. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Noyes N, Labella PA, Grifo J, Knopman JM. Oocyte cryopreservation: a feasible fertility preservation option for reproductive age cancer survivors. J Assist Reprod Genet. 2010;27(8):495–499. doi: 10.1007/s10815-010-9434-3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Cobo A, Diaz C. Clinical application of oocyte vitrification: a systematic review and meta-analysis of randomized controlled trials. Fertil Steril. 2010;96:277–285. doi: 10.1016/j.fertnstert.2011.06.030. [DOI] [PubMed] [Google Scholar]
- 15.Dolmans MM, Marinescu C, Saussoy P, Langendonckt A, Amorim C, Donnez J. Reimplantation of cryopreserved ovarian tissue from patients with acute lymphoblastic leukemia is potentially unsafe. Blood. 2010;116(16):2908–2914. doi: 10.1182/blood-2010-01-265751. [DOI] [PubMed] [Google Scholar]
- 16.Smitz J, Dolmans MM, Donnez J, Fortune JE, Hovatta O, Jewgenow K, et al. Current achievements and future research directions in ovarian tissue culture, in vitro follicle development and transplantation: implications for fertility preservation. Hum Reprod Update. 2010;16:395–414. doi: 10.1093/humupd/dmp056. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Silber S, Kagawa N, Kuwayama M, Gosden R. Duration of fertility after fresh and frozen ovary transplantation. Fertil Steril. 2010;94(6):2191–2196. doi: 10.1016/j.fertnstert.2009.12.073. [DOI] [PubMed] [Google Scholar]
- 18.Robertson JA. Cancer and fertility: ethical and legal challenges. J Natl Cancer Inst Monogr. 2005;104–6. [DOI] [PubMed]
- 19.Nisker J, Baylis F, McLeod C. Choice in fertility preservation in girls and adolescent women with cancer. Cancer. 2006;107:1686–1689. doi: 10.1002/cncr.22106. [DOI] [PubMed] [Google Scholar]
- 20.Frosch DL, Kaplan RM. Shared decision making in clinical medicine: past research and future directions. Am J Prev Med. 1999;17:285–294. doi: 10.1016/S0749-3797(99)00097-5. [DOI] [PubMed] [Google Scholar]
- 21.Protection of human subjects. In: Code of federal regulations. vol. 45; 2004. p. 401–8.
- 22.Campo-Engelstein L. For the sake of consistency and fairness: why insurance companies should cover fertility preservation treatment for iatrogenic infertility. Cancer Treat Res. 2010;156:381–388. doi: 10.1007/978-1-4419-6518-9_29. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Gardino SL, Sfekas A, Dranove D. Anticipating ovarian tissue cryopreservation in the health-care marketplace: a willingness to pay assessment. Cancer Treat Res. 2010;156:363–370. doi: 10.1007/978-1-4419-6518-9_27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 25.Donnez J, Squifflet J, Jadoul P, Demylle D, Cheron AC, Langendonckt A, et al. Pregnancy and live birth after autotransplantation of frozen-thawed ovarian tissue in a patient with metastatic disease undergoing chemotherapy and hematopoietic stem cell transplantation. Fertil Steril. 2011;95:1787 e1–4. doi: 10.1016/j.fertnstert.2010.11.041. [DOI] [PubMed] [Google Scholar]
- 26.Meirow D, Levron J, Eldar-Geva T, Hardan I, Fridman E, Zalel Y, et al. Pregnancy after transplantation of cryopreserved ovarian tissue in a patient with ovarian failure after chemotherapy. N Engl J Med. 2005;353:318–321. doi: 10.1056/NEJMc055237. [DOI] [PubMed] [Google Scholar]
- 27.Ernst E, Bergholdt S, Jorgensen JS, Andersen CY. The first woman to give birth to two children following transplantation of frozen/thawed ovarian tissue. Hum Reprod. 2010;25:1280–1281. doi: 10.1093/humrep/deq033. [DOI] [PubMed] [Google Scholar]
- 28.Sanchez-Serrano M, Crespo J, Mirabet V, Cobo AC, Escriba MJ, Simon C, et al. Twins born after transplantation of ovarian cortical tissue and oocyte vitrification. Fertil Steril. 2010;93:268 e11–3. doi: 10.1016/j.fertnstert.2009.09.046. [DOI] [PubMed] [Google Scholar]
- 29.Andersen CY, Rosendahl M, Byskov AG, Loft A, Ottosen C, Dueholm M, et al. Two successful pregnancies following autotransplantation of frozen/thawed ovarian tissue. Hum Reprod. 2008;23:2266–2272. doi: 10.1093/humrep/den244. [DOI] [PubMed] [Google Scholar]
- 30.Roux C, Amiot C, Agnani G, Aubard Y, Rohrlich PS, Piver P. Live birth after ovarian tissue autograft in a patient with sickle cell disease treated by allogeneic bone marrow transplantation. Fertil Steril. 2010;93:2413 e15-9. doi: 10.1016/j.fertnstert.2009.12.022. [DOI] [PubMed] [Google Scholar]
- 31.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012 epub ahead of print. [DOI] [PubMed]
- 32.Revel A, Laufer N, Ben Meir A, Lebovich M, Mitrani E. Micro-organ ovarian transplantation enables pregnancy: a case report. Hum Reprod. 2011;26:1097–1103. doi: 10.1093/humrep/der063. [DOI] [PubMed] [Google Scholar]