Abstract
Purpose
DNA damage may occur during sperm processing, thereby negatively influencing fertilizing ability of the sperm. The present study was designed to compare the effectiveness of gradient and swim-up, either alone or in combination, to eliminate sperm with DNA damage.
Methods
A total of 51 subjects visiting the University infertility clinic with normozoospermic parameters, oligozoospermia and teratozoospermia were included. Semen characteristics were analysed by standard criteria; Terminal deoxy nucelotidyl transferase mediated dUTP nick end labeling assay was employed for DNA damage assessment.
Results
The percentage of TUNEL positive sperm after sperm processing was significantly lower in normozoospermic (P < 0.05), oligozoospermic (P < 0.001) and teratozoospermic samples (P < 0.01). No difference was observed in the incidence of TUNEL positive sperm between the various techniques, suggesting that they are comparable.
Conclusions
Sperm preparation has been found to result in enrichment of sperm with intact chromatin, which is likely to improve the chances of achieving a viable pregnancy.
Keywords: Sperm DNA damage, Sperm selection, Semen preparation, Swim-up, Density gradient
Introduction
The application of assisted reproduction techniques (ART) has revolutionized the treatment of infertility. Over the years, with a shift of assisted reproduction from mere gynecological indications to more of andrological indications, there has been an surge by researchers to develop more sophisticated techniques to separate functional spermatozoa from those that are immotile, have poor morphology or are incapable of fertilizing oocytes [1]. The fact that fertilization can be achieved with very few spermatozoa by Intra Cytoplasmic Sperm Injection (ICSI) should not prevent the need for greatest care while retrieving as many normal sperm as necessary for either IUI or IVF.
An ideal sperm processing technique should be gentle and one that recovers a highly functional sperm population [1]. Serial centrifugation of the semen is known to induce sperm dysfunction mediated through production of reactive oxygen species by spermatozoa and leucocytes [2–6]. Therefore, more gentle sperm selection techniques such as double density gradient centrifugation and swim-up procedures have evolved and are widely applied in clinical practice [5, 7, 8]. The success rates associated with these procedures, however, is suboptimal perhaps because sperm selection is currently based on standard criteria such as viability, motility and morphology.
Our improved understanding of sperm physiology and emphasis on the role of integrity of the male gamete in both fertilization and embryogenesis, has led to an increased demand on sperm separation techniques. Comparative studies done earlier on sperm preparation methods have essentially investigated outcomes such as recovery rates and conventional semen parameters [9–13]. In the recent days, many studies have been conducted, attempting to identify an effective sperm preparation method that would yield the maximal number of genetically competent sperm [14–19]. The results of these studies, are however inconclusive to recommend any specific selection method [20, 21]. In view of the existing lacunae in the above mentioned area, the present study was taken up for identification of a suitable sperm wash technique to eliminate sperm carrying defective DNA.
Materials and methods
Subjects
Patients visiting the infertility clinic of Kasturba Medical College participated in the study, after provision of a written, informed consent. Semen samples were obtained from patients with normozoospermic parameters (N: 11), moderate and severe oligozoospermia (N: 20) and teratozoospermia (N: 20) to find out the effectiveness of various sperm preparation techniques. All patients were asked to provide semen samples after 3–5 days of ejaculatory abstinence. Semen specimens were produced by masturbation directly into a sterile plastic container, in a room specially provided for this purpose and located adjacent to the laboratory.
Semen analysis
After liquefaction, semen processing and analysis was performed according to the recommendations of the World Health Organization [22]. Seminal volume was determined in a graduated tube and sperm concentration was assessed by conventional method using Makler counting chamber (Sefi Medical Instruments, Israel) and expressed in millions/mL. The sperm motility was assessed in at least 100 sperm and expressed as percent of motile sperm (sum of rapid progression plus slow progression sperm). Sperm morphology was assessed by Shorr staining and sperm viability by Eosin-Nigrosin stain.
Sperm preparation techniques
The semen sample was split into three equal parts after routine examination and processed according to the three methods mentioned below:
Swim up
An aliquot of 0.5 ml of whole semen was gently mixed with 1 ml of pre-warmed sperm preparation medium, supplemented with 0.1% human serum albumin (Sigma-Aldrich, St. Louis, Catalogue # A1653) in a test tube. Centrifugation was then performed at 200 × g for 8 min. Subsequently, the supernatant was discarded and washed with pre-warmed medium at 100 × g and 45 × g for 8 min respectively. Following this, 200 μl of the sperm preparation medium was carefully added to the final pellet. The tube was inclined at an angle of 45 °C and incubated for 1 h at 37 °C in a carbon dioxide incubator. The supernatant and pellet fractions was then removed and assessed for sperm concentration, motility, viability, normal morphology and DNA integrity.
Density gradient
The 80/40 gradients (Pureception, SAGE, USA) was prepared in a 14 ml tube, followed by layering of 0.5 ml semen and centrifugation at 200 × g for 20 min. The gradient was removed, keeping the pellet, undisturbed. The pellet was washed twice (200 × g, 5 min) in 1 ml of pre-warmed sperm preparation medium and the final pellet was overlaid with 200 μl of the sperm preparation medium, followed by incubation at 37 °C in a carbon dioxide incubator for 30 min. The supernatant and pellet fractions was then removed and assessed for sperm concentration, motility, viability, normal morphology and DNA integrity.
Density gradient and swim up
The 80/40 gradients (Pureception, SAGE, USA) was prepared in a 14 ml tube, followed by layering of 0.5 ml semen. The first centrifugation was carried out at 200 × g for 20 min. The gradient was removed, keeping the pellet, undisturbed. Three washes of the pellet were carried out with 1 ml of pre-warmed sperm preparation medium at 200 × g, 100 × g and 45 × g respectively for 8 min. The final pellet was overlaid with 200 μl of the sperm preparation medium. The tube was then inclined at an angle of 45 °C and incubated for 30 min at 37 °C in a carbon dioxide incubator. The supernatant and pellet fractions was then removed and assessed for sperm concentration, motility, viability, normal morphology and DNA integrity.
TUNEL assay
The commercial kit that uses fluorescein-dUTP to label sites of DNA fragmentation was used in this study (Apoalert DNA Fragmentation Assay Kit, Cat # 630108; Clonetech, Japan). Sperm suspension was spread on a slide and fixed in 4% paraformaldehyde and permeabilized with 0.1% Triton X-100. This was followed by TUNEL labeling at 37 °C for 1 h at dark. The negative and the positive control were also performed, respectively, by omitting the TdT enzyme following the kit instructions and by preincubating fixed and permeabilized sperm samples with DNase I (40 IU/mL) for 10 min at room temperature to produce DNA breaks. TUNEL positive cells exhibited a strong nuclear green fluorescence which was observed under fluorescence microscope (Imager-A1, Zeiss, Germany) equipped with a 490 nm excitation filter (Fig. 1). A total of 500 spermatozoa were assessed in random fields and DNA damage was expressed as percentage of TUNEL positive spermatozoa.
Fig. 1.
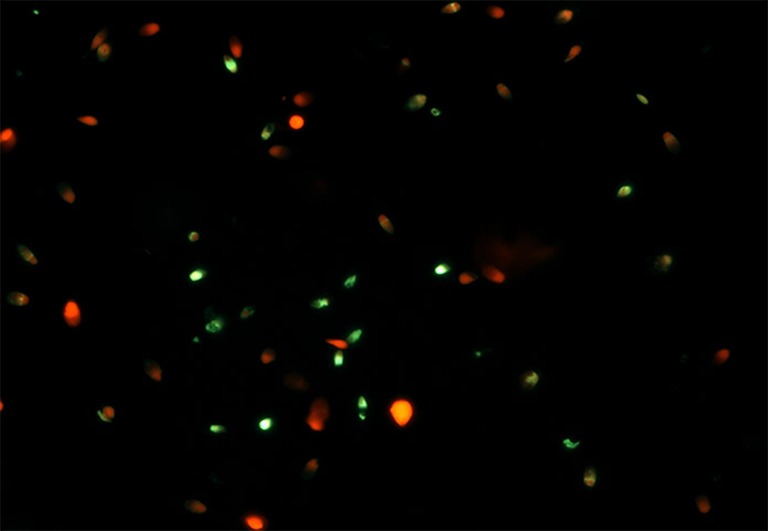
TUNEL assay in human spermatozoa, 40× magnification Red - intact sperm; Green - sperm with DNA fragmentation
Statistical analysis
Basic descriptive statistics (mean ± standard error) were calculated for different parameters such as sperm count, total motility, rapid progressive motility, normal sperm morphology and percentage of TUNEL positive sperm of the different groups using Statistical Package for Social Sciences (SPSS) and is summarized in Table 1. Statistical analysis of the means between different study groups was performed using generalized linear equations (GEE). A P-value < 0.05 was considered statistically significant.
Table 1.
Semen characteristics and DNA damage by TUNEL assay in various samples
| Semen Characteristic | N | Fraction | Sperm count (Millions/ml) | Total motility (%) | Rapid Progressive (G3) motility (%) | Normal morphology (%) | TUNEL positive sperm (%) |
|---|---|---|---|---|---|---|---|
| Normozoospermia | 11 | Unprocessed | 72.55 ± 11 | 61.09 ± 4.84 | 9.27 ± 3.24 | 39.91 ± 1.10 | 7.51 ± 2.29 |
| Swim Up | 9.6 ± 1.46# | 78.91 ± 3.95‡ | 32.45 ± 3.62† | 33.73 ± 3.05 | 3.68 ± 0.94a | ||
| Density Gradient | 5.58 ± 0.89 | 79.09 ± 3.46‡ | 28.09 ± 3.55† | 30.82 ± 0.77 | 3.08 ± 0.61a | ||
| Swim up and Density Gradient | 4.78 ± 0.74 | 73.09 ± 4.76‡‼ | 30.82 ± 3.44† | 38.64 ± 1.36$ | 5.06 ± 1.61a | ||
| Oligozoospermia | 20 | Unprocessed | 13.60 ± 0.84 | 39.5 ± 4.57 | 1.15 ± 0.55 | 16.55 ± 1.51 | 9.16 ± 2.03 |
| Swim Up | 2.74 ± 0.23# | 58.35 ± 3.36‡¥ | 16.20 ± 2.38†* | 10.55 ± 1.47€ | 4.58 ± 1.43c | ||
| Density Gradient | 0.53 ± 0.17 | 49.35 ± 3.31‡ | 10.65 ± 2.32† | 17.30 ± 0.86 | 3.73 ± 0.97c | ||
| Swim up and Density Gradient | 0.51 ± 0.18 | 54.15 ± 3.94‡ | 11.70 ± 2.08† | 17.35 ± 1.16 | 5.61 ± 1.80 c | ||
| Teratozoospermia | 20 | Unprocessed | 58.55 ± 6.16 | 45.70 ± 3.33 | 1.70 ± 0.85 | 17.10 ± 1.20 | 8.01 ± 1.39 |
| Swim Up | 11.56 ± 2.25# | 63.35 ± 5.28‡¥ | 19.85 ± 3.14†* | 17.4 ± 1.79€ | 2.84 ± 0.86b | ||
| Density Gradient | 4.90 ± 0.83 | 54.80 ± 4.24‡ | 12.95 ± 3.34† | 20.95 ± 1.75 | 3.64 ± 0.89c | ||
| Swim up and Density Gradient | 5.62 ± 1.03 | 54.70 ± 4.58‡ | 16.20 ± 3.33† | 23.10 ± 1.83 | 3.61 ± 0.97 c |
#P < 0.001 compared to Density Gradient, Swim-up and Density Gradient; ‡P < 0.05 compared to unprocessed; ‼P < 0.05 compared to Swim-up and Density Gradient; ¥P < 0.05 compared to Density Gradient, Swim-up and Density Gradient; †P < 0.001 compared to unprocessed; *P < 0.05 compared to Density Gradient, Swim-up and Density Gradient; $P < 0.01 compared to Density Gradient; Swim-up
€P < 0.001 compared to Density Gradient; Swim-up and Density Gradient; aP < 0.05 compared to unprocessed; bP < 0.01 compared to unprocessed; cP < 0.001 compared to unprocessed
Results
Sperm count
Oligozoospermic samples had a significantly (P < 0.001) lower sperm count in the unprocessed fraction compared to normozoospermic and teratozoospermic samples (Table 1). With respect to sperm yield, in normozoospermic, oligozoospermic and teratozoospermic samples, swim up yielded significantly (P < 0.001) higher number of sperm compared to both density gradient and combination of density gradient and swim-up (Table 1).
Total and rapid progressive motility
In the unprocessed fraction, oligozoospermic and teratozoospermic samples had significantly lower proportion of total motile and rapid progressive sperm compared to normozoospermic samples (P < 0.001). Following sperm preparation, all the three procedures yielded a significantly higher proportion of sperm with total and rapid progressive motility compared to the unprocessed fraction (P < 0.05).
In normozoospermic samples, although all the three techniques yielded increased proportion of total motile sperm compared to the unprocessed fraction, the combination method had significantly lower proportion of total motile sperm (P < 0.05) compared to the other two methods. In oligozoospermic and teratozoospermic samples, while comparing the three techniques, swim up method had the highest percentage (P < 0.05) of total motile sperm in comparison with both density gradient and combination technique, suggesting that swim up is effective in recovering maximal number of motile sperm (Table 1).
While all the three procedures had a significantly higher percentage of rapid progressive sperm (G3) compared to the unprocessed fraction (P < 0.001), there was no difference between these techniques in normal samples. In oligozoospermic and teratozoospermic samples, swim up had higher rapid progressive sperm compared to density gradient and combination of density gradient and swim-up (P < 0.05).
Normal sperm morphology
Teratozoospermic and oligozoospermic samples had significantly (P < 0.001) lower percentage of morphologically normal sperm in the unprocessed faction compared to normozoospermic samples. After sperm processing, in normozoospermic samples, the combination technique had higher percentage of morphologically normal sperm (P < 0.01). In oligozoospermic and teratozoospermic samples, while there was no difference between density gradient and combination techniques, swim-up had significantly lower percentage of morphologically normal sperm compared to either (P < 0.001), suggesting ineffectiveness of the same in recovering morphologically normal sperm(Table 1).
TUNEL positive sperm
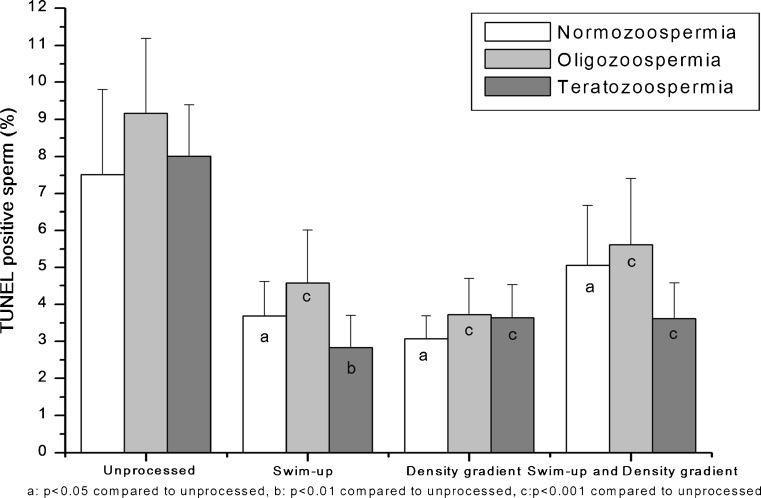
Oligozoospermic and teratozoospermic samples had a slightly increased percentage of TUNEL positive sperm in the unprocessed fraction compared normozoospermics. This difference was however statistically insignificant. The percentage of TUNEL positive sperm after sperm processing was significantly lower in normozoospermic (P < 0.05), oligozoospermic (P < 0.001) and teratozoospermic samples (P < 0.01) (Table 1, Fig. 2). Furthermore, there was no difference in the incidence of TUNEL positive sperm between the various techniques suggesting that all the three are comparable in yielding a population of sperm with low DNA damage.
Fig. 2.
Ability of various sperm wash techniques to eliminate sperms with DNA damage
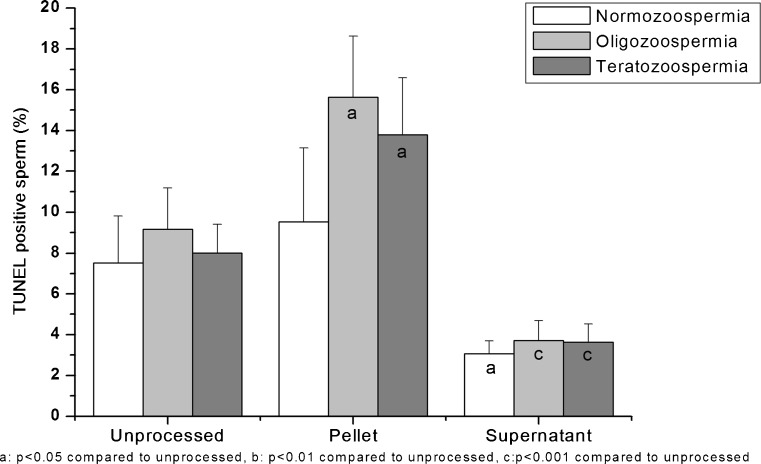
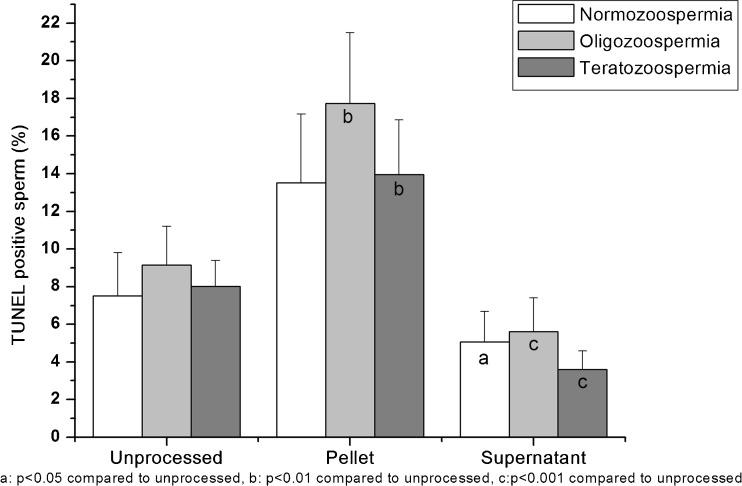
Interestingly, in oligozoospermic and teratozoospermic samples, there was a statistically significant increase in the percentage of TUNEL positive sperm in the pellet fractions after preparation by density gradient (P < 0.05) and combination technique (P < 0.01) in comparison to the unprocessed fraction (Figs. 3 and 4). The same effect was also observed in normozoospermic samples. The difference was however, statistically insignificant (Data not shown).
Fig. 3.
Percentage of TUNEL positive sperms in the unprocessed, pellet and supernatant fractions after density gradient
Fig. 4.
Percentage of TUNEL positive sperms in the unprocessed, pellet and supernatant fractions after combination of density gradient - swim up
Discussion
The comparison of different semen preparation techniques in relation to sperm DNA damage has been the focus of a substantial amount of research. However, there is no consensus in the literature on this topic [20, 21]. The present study has therefore been designed to understand the extent of DNA damage in an ejaculate and how different sperm processing techniques can remove DNA-damaged sperm. As most samples encountered in infertility clinics are suboptimal in quality, in addition to normozoospermic samples, moderate oligozoospermic and teratozoospermic samples have been included.
In current clinical practice, the results of most studies on fertility and sperm DNA damage point to a greater utility of sperm DNA tests in relation to natural conception and intrauterine insemination (IUI) rather than ART treatments [23]. This could be because in techniques, such as IVF or ICSI, although a high percentage of sperm in a sample may have damaged DNA, as a consequence of sperm processing, sperm with minimal amount of DNA damage may still be selected [24]. In support of the above argument, it has recently been illustrated that a high DNA fragmentation index (DFI %) among spermatozoa in raw semen was related to low success after intrauterine insemination (IUI) [25]. A subsequent follow-up study conducted later identified that the observed effect was because the prepared sperm populations that were actually used for the insemination all had low (4–6%) and normal DFI% [26]. Thus the ‘negative impact’ of sperm DNA damage originating from an ejaculate with 30% DFI is associated with the selected sperm population but hidden to the investigator as a ‘falsely’ normal value for DFI.
On the contrary, DNA damage has also been reported to arise during sperm processing. Previous studies have shown that incubation of semen at room temperature [27] or at 37 °C [28] after their isolation by density gradient centrifugation may lead to an increase in the levels of sperm DNA fragmentation. An important relationship between sperm processing and DNA fragmentation is that, should a critical number of the sperm in the semen sample have intact chromatin, sperm processing may actually result in the enrichment of these spermatozoa, thereby increasing the chances of achieving a viable pregnancy. Hence, to improve the diagnostic capability of sperm DNA damage, further methodological work is recommended to distinguish whether elevated levels of DNA observed in an ejaculate can be truly transmitted to future generations [24].
Most studies on sperm preparation methods have explored outcomes such as recovery rates, percentage of motile sperm and other conventional semen parameters. The results of the relatively fewer number of studies that investigated the effect of sperm processing and DNA integrity are inconclusive, with insufficient evidence to recommend any specific technique. Although both swim-up and density gradient techniques have been found to be effective in obtaining a sperm population with low percentage of apoptotic sperm [19], a combination of density gradient and swim-up has been reported to be favorable for IVF as sperm prepared by this method were found to have higher rates of motility and reduced DNA fragmentation compared to other methods [29]. Percoll density gradient centrifugation for teratozoospermic samples and those with idiopathic infertility has also been recommended [17].
On the other hand, although density-gradient centrifugation was comparable to swim-up technique in recovering spermatozoa with enhanced motility, spermatozoa recovered after swim-up were found to possess higher DNA integrity [15, 30]. A subsequent study which analyzed the influence of initial semen quality on sperm DNA integrity following semen processing also supported this contention, concluding that sperm processed by swim-up had superior DNA integrity [16]. Semen processing by density gradient centrifugation was not found to improve sperm apoptotic deoxyribonucleic acid fragmentation rates, leading the authors to suggest the use of other semen processing techniques in patients with underlying DNA fragmentation [31].
In our study, we found no difference in the incidence of TUNEL positive sperm in the supernatant fractions of the various techniques, in all the three type of samples, suggesting that all the three are comparable in yielding a population of sperm with low DNA damage. It has been reported that although discontinuous gradient eliminates morphologically abnormal sperm and swim-up treatment decreases DFI and HDS of spermatozoa, both methods are effective for embryo development [18]. The first study to compare the effects of gradient-density centrifugation and swim-up techniques on sperm apoptosis using flow cytometry also suggested that both the sperm preparation methods allow obtaining a sperm population with low percentage of apoptotic sperm [19]. The results of our study support the above findings.
Interestingly, in oligozoospermic and teratozoospermic samples, we found a significantly higher incidence of sperm with DNA damage in the pellet fractions of the density gradient and combination of density gradient and swim-up technique compared to the unprocessed semen. Although the same effect was observed in normozoospermic samples, it was statistically insignificant. Genetic damage in sperm may originate from a combination of both intrinsic and extrinsic factors. An excess amount of ROS produced by seminal leucocytes or as a consequence of centrifugal pelleting of unselected sperm/ density gradient centrifugation has been known to impair DNA integrity. Based on the present observation, we hypothesize that the steps of both methods, either incubation or centrifugation induces apoptosis through a ROS dependent mechanism.
Repeated centrifugation protocols used in sperm preparation are known to introduce iatrogenic DNA damage and therefore strategies for minimizing collateral DNA damage have been suggested [32]. Today, we have advanced protocols that allow sperm selection based on their ultra structural morphology or surface charges by electrophoresis and new insights into the molecular biology of spermatozoa have even prompted the development of molecular selection strategies such as hyaluronic acid mediated sperm selection, the annexin V magnetic activated cell separation (MACS), and annexin V molecular glass wool filtration. Selection of sperm based on combined density gradient and Zeta method have been suggested to improve the outcome of ICSI [33]. However, the compromised DNA integrity observed in the pellet fractions of the density gradient and the combination technique in the present study insists us to reexamine the existing sperm processing techniques and recommend the validation of similar results with studies of a large sample size, in the years to come.
As all the three methods employed in the present study yielded a population of sperm with lower DNA damage compared to the unprocessed fraction, we suggest that these methods are comparable. In the content of ART, following sperm processing, the risk of using a genetically incompetent sperm seems to be low; the choice of an ideal method would be governed by the purpose for which it is indicated. To conclude, sperm preparation has been found to result in enrichment of sperm with intact chromatin, which is likely to improve the chances of achieving a viable pregnancy.
Acknowledgment
Financial assistance provided by Kasturba Medical College, Manipal University to Ms.Varshini J (No. Accts/2008-09) is gratefully acknowledged.
Footnotes
Capsule
The crucial importance of sperm DNA integrity in fertilization/embryo development demands understanding of the extent to which sperm preparation can eliminate DNA damaged sperm. Swim-up and density gradient, both alone and in combination have been found to be effective in this regard.
References
- 1.Henkel RR, Schill WB. Sperm preparation for ART. Reprod Biol Endocrinol. 2003;1:108. doi: 10.1186/1477-7827-1-108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Aitken RJ, Clarkson JS. Significance of reactive oxygen species and antioxidants in defining the efficacy of sperm preparation techniques. J Androl. 1988;9:367–376. doi: 10.1002/j.1939-4640.1988.tb01067.x. [DOI] [PubMed] [Google Scholar]
- 3.Iwasaki A, Gagnon C. Formation of reactive oxygen species in spermatozoa of infertile patients. Fertil Steril. 1992;57:409–416. doi: 10.1016/s0015-0282(16)54855-9. [DOI] [PubMed] [Google Scholar]
- 4.Griveau JF, Lannou D. Effects of antioxidants on human sperm preparation techniques. Int J Androl. 1994;17:225–231. doi: 10.1111/j.1365-2605.1994.tb01247.x. [DOI] [PubMed] [Google Scholar]
- 5.Aitken RJ, Buckingham DW, Brindle J, Gomez E, Baker HW, Irvine DS. Analysis of sperm movement in relation to the oxidative stress created by leukocytes in washed sperm preparations and seminal plasma. Hum Reprod. 1995;10:2061–2071. doi: 10.1093/oxfordjournals.humrep.a136237. [DOI] [PubMed] [Google Scholar]
- 6.Zalata A, Hafez T, Comhaire F. Evaluation of the role of reactive oxygen species in male infertility. Hum Reprod. 1995;10:1444–1451. doi: 10.1093/humrep/10.6.1444. [DOI] [PubMed] [Google Scholar]
- 7.Mortimer D. Sperm preparation techniques and iatrogenic failures of in vitro fertilization. Hum Reprod. 1991;6:173–176. doi: 10.1093/oxfordjournals.humrep.a137300. [DOI] [PubMed] [Google Scholar]
- 8.Oehninger S, Blackmore P, Mahony M, Hodgen G. Effects of hydrogen peroxide on human spermatozoa. J Assist Reprod Genet. 1995;12:41–47. doi: 10.1007/BF02214128. [DOI] [PubMed] [Google Scholar]
- 9.Lannou D, Blanchard Y. Nuclear maturity and morphology of human spermatozoa selected by Percoll density gradient centrifugation or swim-up procedure. J Reprod Fertil. 1988;84:551–556. doi: 10.1530/jrf.0.0840551. [DOI] [PubMed] [Google Scholar]
- 10.Ng FL, Liu DY, Baker HW. Comparison of Percoll, mini-Percoll and swim-up methods for sperm preparation from abnormal semen samples. Hum Reprod. 1992;7:261–266. doi: 10.1093/oxfordjournals.humrep.a137628. [DOI] [PubMed] [Google Scholar]
- 11.Chen SU, Ho HN, Chen HF, Chao KH, Lin HR, Huang SC, et al. Comparison between a two-layer discontinuous Percoll gradient and swim-up for sperm preparation on normal and abnormal semen samples. J Assist Reprod Genet. 1995;12:698–703. doi: 10.1007/BF02212896. [DOI] [PubMed] [Google Scholar]
- 12.Chen SU, Ho HN, Chen HF, Chao KH, Wu MY, Chen CD, et al. Combination of direct swim-up technique and discontinuous Percoll gradient centrifugation for sperm preparation of oligoasthenozoospermic samples. Arch Androl. 1996;37:103–109. doi: 10.3109/01485019608988510. [DOI] [PubMed] [Google Scholar]
- 13.Prakash P, Leykin L, Chen Z, Toth T, Sayegh R, Schiff I, et al. Preparation by differential gradient centrifugation is better than swim-up in selecting sperm with normal morphology (strict criteria) Fertil Steril. 1998;69:722–726. doi: 10.1016/S0015-0282(98)00002-8. [DOI] [PubMed] [Google Scholar]
- 14.Sakkas D, Manicardi GC, Tomlinson M, Mandrioli M, Bizzaro D, Bianchi PG, et al. The use of two density gradient centrifugation techniques and the swim-up method to separate spermatozoa with chromatin and nuclear DNA anomalies. Hum Reprod. 2000;15:1112–1116. doi: 10.1093/humrep/15.5.1112. [DOI] [PubMed] [Google Scholar]
- 15.Zini A, Finelli A, Phang D, Jarvi K. Influence of semen processing technique on human sperm DNA integrity. Urology. 2000;56:1081–1084. doi: 10.1016/S0090-4295(00)00770-6. [DOI] [PubMed] [Google Scholar]
- 16.Zini A, Nam RK, Mak V, Phang D, Jarvi K. Influence of initial semen quality on the integrity of human sperm DNA following semen processing. Fertil Steril. 2000;74:824–827. doi: 10.1016/S0015-0282(00)01495-3. [DOI] [PubMed] [Google Scholar]
- 17.Ahmad L, Jalali S, Shami SA, Akram Z. Sperm preparation: DNA damage by comet assay in normo- and teratozoospermics. Arch Androl. 2007;53:325–338. doi: 10.1080/01485010701730963. [DOI] [PubMed] [Google Scholar]
- 18.Hashimoto S, Goda S, Akamatsu Y, Yamanaka M, Morimoto Y. Effects of sperm preparation on sperm DNA fragmentation and morphology. RBM Online. 2008;16:S28. [Google Scholar]
- 19.Ricci G, Perticarari S, Boscolo R, Montico M, Guaschino S, Presani G. Semen preparation methods and sperm apoptosis: swim-up versus gradient-density centrifugation technique. Fertil Steril. 2009;91:632–638. doi: 10.1016/j.fertnstert.2007.11.068. [DOI] [PubMed] [Google Scholar]
- 20.Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2004;3:CD004507. [DOI] [PubMed]
- 21.Boomsma CM, Heineman MJ, Cohlen BJ, Farquhar C. Semen preparation techniques for intrauterine insemination. Cochrane Database Syst Rev. 2007;4:CD004507. [DOI] [PubMed]
- 22.WHO laboratory manual for the examination of human semen and sperm-cervical mucus interaction. Cambridge: Cambridge University Press; 1999. [Google Scholar]
- 23.Spanò M, Toft G, Hagmar L, Eleuteri P, Rescia M, Rignell-Hydbom A, et al. Exposure to PCB and p, p’-DDE in European and Inuit populations: impact on human sperm chromatin Integrity. Hum Reprod. 2005;20:3488–3499. doi: 10.1093/humrep/dei297. [DOI] [PubMed] [Google Scholar]
- 24.Sakkas D, Alvarez JG. Sperm DNA fragmentation: mechanisms of origin, impact on reproductive outcome, and analysis. Fertil Steril. 2010;93:1027–1036. doi: 10.1016/j.fertnstert.2009.10.046. [DOI] [PubMed] [Google Scholar]
- 25.Bungum M, Humaidan P, Axmon A, Spano M, Bungum L, Erempreiss J, et al. Sperm DNA integrity assessment in prediction of assisted reproduction technology outcome. Hum Reprod. 2007;22:174–179. doi: 10.1093/humrep/del326. [DOI] [PubMed] [Google Scholar]
- 26.Bungum M, Spanò M, Humaidan P, Eleuteri P, Rescia M, Giwercman A. Sperm chromatin structure assay parameters measured after density gradient centrifugation are not predictive for the outcome of ART. Hum Reprod. 2008;23:4–10. doi: 10.1093/humrep/dem353. [DOI] [PubMed] [Google Scholar]
- 27.Gosálvez J, Cortés-Gutiérrez EI, Nuñez R, Fernández JL, Caballero P, López-Fernández C, et al. A dynamic assessment of sperm DNA fragmentation versus sperm viability in proven fertile human donors. Fertil Steril. 2009;92:1915–1919. doi: 10.1016/j.fertnstert.2008.08.136. [DOI] [PubMed] [Google Scholar]
- 28.Dalzell LH, McVicar CM, McClure N, Lutton D, Lewis SE. Effects of short and long incubations on DNA fragmentation of testicular sperm. Fertil Steril. 2004;82:1443–1445. doi: 10.1016/j.fertnstert.2004.04.053. [DOI] [PubMed] [Google Scholar]
- 29.Bormann C, Rocha A, Hassun P, Motta E, Serafini P, Smith G. The effect of sperm separation on sperm chromatin decondensation and motility at 0 and 24 hours of culture. Fertil Steril. 2008;90:S452–S453. doi: 10.1016/j.fertnstert.2008.07.820. [DOI] [Google Scholar]
- 30.Zini A, Mak V, Phang D, Jarvi K. Potential adverse effect of semen processing on human sperm deoxyribonucleic acid integrity. Fertil Steril. 1999;72:496–499. doi: 10.1016/S0015-0282(99)00295-2. [DOI] [PubMed] [Google Scholar]
- 31.Stevanato J, Bertolla RP, Barradas V, Spaine DM, Cedenho AP, Ortiz V. Semen processing by density gradient centrifugation does not improve sperm apoptotic deoxyribonucleic acid fragmentation rates. Fertil Steril. 2008;90:889–890. doi: 10.1016/j.fertnstert.2007.01.059. [DOI] [PubMed] [Google Scholar]
- 32.Twigg J, Irvine DS, Houston P, Fulton N, Michael L, Aitken RJ. Iatrogenic DNA damage induced in human spermatozoa during sperm preparation: protective significance of seminal plasma. Mol Hum Reprod. 1998;4:439–445. doi: 10.1093/molehr/4.5.439. [DOI] [PubMed] [Google Scholar]
- 33.Kheirollahi-Kouhestani M, Razavi S, Tavalaee M, Deemeh MR, Mardani M, Moshtaghian J, et al. Selection of sperm based on combined density gradient and Zeta method may improve ICSI outcome. Hum Reprod. 2009;24:2409–2416. doi: 10.1093/humrep/dep088. [DOI] [PubMed] [Google Scholar]