Abstract
Purpose
To develop an optimal method of isolation and purification of human granulosa cells from ovarian follicular fluid.
Methods
Follicular fluid was collected from patients undergoing oocyte retrieval. A series of isolation and purification techniques was performed, involving density gradient centrifugation and use of different antibody-bead complexes.
Results
The highest percent yield of live purified granulosa cells came from density gradient centrifugation using sucrose polymer followed by positive selection of granulosa cells using primary antibody to MISRII and secondary antibody coupled to iron oxide beads.
Conclusions
A novel protocol for granulosa cell purification has been developed yielding samples that are largely free of nondesirable cells. This protocol provides a purification solution, especially for patient samples that have significant RBC contamination.
Electronic supplementary material
The online version of this article (doi:10.1007/s10815-012-9739-5) contains supplementary material, which is available to authorized users.
Keywords: Human granulosa cell, Purification, Follicular fluid, Red blood cell contamination, In vitro fertilization, Immunomagnetic beads, MIS receptor
Introduction
Successful cell isolation and purification methods are essential for accurate interpretation of data resulting from experiments. One cell that is highly useful in the study of ovarian steroidogenesis and folliculogenesis is the granulosa cell, which plays a major role in production of estradiol, inhibin B, mullerian inhibiting substance, and progesterone. Although granulosa cell lines exist through which some of these processes can be studied [1], those immediately obtained from patient populations are especially valuable in translational clinical research. A common source of human granulosa cells is from follicular fluid from patients undergoing in vitro fertilization (IVF) procedures.
Several human granulosa cell isolation techniques have been described in the literature, yet many of these involve follicular fluid obtained from oophorectomized ovaries of animals [2–5] or involve collection of human granulosa cells from IVF egg retrieval specimens without visible blood contamination [6–8]. A few authors employ repeated cell washing with PBS or serum-free media and sedimentation with centrifugation to remove red blood cells (RBCs), but there is no description of how pure the cells are before their use [9, 10]. A majority of authors use density gradient separation techniques involving Percoll [11–16] or Ficoll [17–21]; however, only a few papers give the actual centrifugation speeds and times for their separation techniques. Other authors report using hemolytic buffer to purify their samples, but they do not indicate quantified granulosa cell purity [22, 23].
Some investigators proceed to plating and culturing the cells for further experiments [10, 11, 24]. Plating the cells may provide an opportunity to wash some of the nonadherent blood cells from the dishes [25], which may allow for further purification, but culture could also introduce changes in granulosa cell characteristics, as is common for many cell types [26]. Plating for purification also does not address more adherent contaminating cells, such as granulocytes, leukocytes, and epithelial cells, which are often present in follicular fluid from oocyte retrieval aspirations.
Methods to disperse granulosa cell aggregates for easier isolation have been performed by some authors who use a mechanical dispersion through a 20-gauge needle [25] or treat with enzymes, such as collagenase or hyaluraonidase [2, 18, 27]. However, this enzymatic digestion may affect the cells directly, and heavy disruption of granulosa cell-to-cell contact may cause undesirable molecular changes. Indeed, studies indicate that hyaluronic acid in the connecting extracellular matrix is important in preventing apoptosis in granulosa cells [28].
Our lab is planning future clinical studies to compare protein and mRNA levels between granulosa cell samples from IVF patients. Therefore, the goal of these experiments was to develop a protocol resulting in purified granulosa cell samples and a supplemental method to account for residual RBCs.
Materials and methods
Collection of follicular fluid
Patients undergoing egg retrievals at an IVF center in Webster, TX, were consented for collection and use of their otherwise discarded follicular fluid for research purposes. The study and consent form was approved by the IRB at the University of Texas Medical Branch (UTMB) in Galveston. Patients were treated with a variety of gonadotropin and GnRH agonist/antagonist protocols during their IVF cycles and were each given an injection of HCG approximately 36 h before egg retrieval. The follicular fluid was collected via oocyte aspiration needle into test tubes, which were then emptied into petri dishes. The embryologist isolated and removed the oocytes from the fluid and surrounding cells, the dishes were set aside. Approximately 8–10 cc follicular fluid was pipetted into 15-cc conical polypropylene tubes (BD Falcon, Becton Dickinson, Franklin Lakes, NJ) containing approximately 100 μL of heparin sodium (1,000 units/mL, APP Pharmaceuticals, Schaumburg, IL). These tubes were placed on ice and transported to the Medical Research Building at UTMB in Galveston in ~1 h for isolation and purification of granulosa cells. Data was collected for comparison of isolation methods using a total of 22 patient samples in density gradient procedures and a total of 20 patient samples for immunomagnetic bead procedures.
Initial depletion of RBCs
Percoll density gradient
For each patient, follicular fluid was pooled into a sterile container, and 50-mL conical tubes (polypropylene, Corning, Lowell, MA) were each filled with 10 mL of a 1:1 ratio mixture of phosphate buffered saline (PBS) and Percoll solution (GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom). The follicular fluid was then layered carefully with a sterile 10-mL pipette over the Percoll mixture, bringing the volume to 50 mL. The tubes were centrifuged for 10 min at 850 g at 4°C. The granulosa cells, collected at the interface of the follicular fluid and Percoll, were removed using a sterile transfer pipette. Following resuspension of these cells in DMEM/F-12 media (Gibco-BRL, Invitrogen, Carlsbad, CA) supplemented with 1x penicillin/streptomycin (Gibco-BRL, Invitrogen, Carlsbad, CA) and 5% fetal bovine serum (Es-Cult™-Tested FBS, Stem Cell Technologies, Vancouver, BC), density gradient centrifugation was repeated once.
Ficoll density gradient
Borrowing from the protocol presented by McGuckin et al. [29], which diluted cord blood before isolation of stem cells, the pooled follicular fluid volume obtained from IVF patients was measured and then diluted in ACD-A buffer [PBS with 0.6% ACD-A (Anticoagulant citrate dextrose solution A from Citra Anticoagulants, Inc., Braintree, MA) and 0.5% fraction V bovine serum albumin, equilibrated to pH 7.4 with NaOH], making a 1:3 ratio or 1:4 dilution of cells with buffer. Next, 10 mL of Ficoll-Paque Plus solution (density 1.077 g/cm3, GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) was placed into a 50-mL conical tube and carefully overlaid with 40 mL of the diluted follicular fluid. This step was repeated until the total volume of diluted follicular fluid was overlaid onto the Ficoll density gradient solution. The tubes were then centrifuged for 30 min at 1,625 g at 4°C. The interface containing the cellular layer was collected from each tube with a transfer pipette and placed into a 15-mL tube containing 3 mL of cold DMEM/F-12 media with 5% FBS and 1 × Penn/Strep. Note that the media was stored in the refrigerator and was allowed to warm to room temperature under the hood over the course of the isolation procedures. This 15-mL tube containing washed cells and media was then centrifuged for 10 min at 400 g at 10°C. The wash with DMEM/F12 media was repeated once. Before the second centrifugation, cell count and viability assessment were performed.
RBC lysis buffer
Subsequent to Percoll or Ficoll procedures, additional depletion of RBCs was attempted by performing an incubation with RBC lysis buffer containing ammonium chloride (Sigma-Aldrich, St. Louis, MO). The manufacturer’s instructions recommend adding 1 mL buffer to a cell pellet and mixing for 1 min. This protocol was performed with the cells on ice and then also at room temperature for up to 15 min followed by cell counting.
Gel electrophoresis and western blotting
During development of this procedure, Western blotting was used to evaluate the presence of granulosa cell markers and antibodies that might be suitable for the immunomagnetic bead positive selection of granulosa cells. Whole cell extracts were made from granulosa cells obtained using the Ficoll isolation method by using RIPA buffer [1× PBS, 1% Igepal, 0.5% sodiumdeoxycholate acid, 1% sodium dodecyl sulfate] with added protein inhibitor cocktail (Sigma-Aldrich, St. Louis, MO) plus 10 mg/mL benzamidine and phosphatase inhibitors (0.2 M sodium vanidate, 0.5 M sodium fluoride, 0.1 M sodium pyrophosphate). These extracts were then used in sample preparation, with 20 μg protein loaded in each lane, and were resolved on a 4%–12% Bis-Tris 1.5 mm gel (Invitrogen, Carlsbad, CA) with 10 wells using 1 × MOPS buffer. Gels were electroblotted onto PVDF 0.45 μm membranes (Invitrogen, Carlsbad, CA) using Tris-glycine/methanol buffer for 1.25 to 1.5 h. After treatment with antibodies, the membrane was treated with ECL (Amersham-GE Healthcare, Little Chalfont, Buckinghamshire, United Kingdom) for 1 min and developed on Kodak BioMax MR Film for 1.5 min.
Primary antibodies used for these experiments were polyclonal goat anti-human IgG FSH receptor (FSHR) (1:100 dil., Santa Cruz Biotechnology, Santa Cruz, CA) and polyclonal rabbit anti-human IgG to MISRII (1:200 dil., Santa Cruz Biotechnology, Santa Cruz, CA). Secondary antibodies used were donkey anti-goat HRP-conjugated antibody (1:5000 dil., Southern Biotech, Birmingham, AL) and goat anti-rabbit HRP-conjugated antibody (1:2000 dilution, Southern Biotech, Birmingham, AL). Rat ovary extract (Santa Cruz Biotechnology, Santa Cruz, CA) was used as a positive control for the FSH receptor at the manufacturer’s recommended 50 μg protein per lane. The KGN granulosa tumor cell line (obtained with permission from RIKEN BioResource Center cell bank in Japan) was also used in testing for the FSH receptor.
Immunocytochemistry
After using Western blotting to verify the presence of MISRII on the human granulosa cells, immunocytochemistry was used to further confirm that this receptor was indeed present on granulosa cells in isolated samples. After depleting RBCs with Percoll or Ficoll, the sample of granulosa cells was dispersed into DMEM/F12 media with 1 x penicillin/streptomycin and 10% FBS media onto sterilized coverslips lining wells of a plastic 12- well Falcon Multiwell (Becton Dickinson, Franklin Lakes, NJ) tissue culture plate. These cells were cultured for approximately 36 h. The coverslips were rinsed twice with 1 x PBS and then fixed and permeabilized with 4% paraformaldehyde and 70% EtOH. After washing with rinse buffer (0.1% Tris buffered saline with Tween (TBST)), coverslips were frozen overnight to preserve the cells for further study the following day. Coverslips were then thawed, washed with rinse buffer, and blocked with blocking buffer (5% bovine serum albumin solution in TBST). Next, the primary MISRII antibody was applied, followed by rinsing and then incubation with secondary anti-rabbit antibody tagged with florescein isothiocyanate (FITC) (Alexa Fluor 488 goat anti-rabbit IgG, Invitrogen, Carlsbad, CA). Vectashield mounting medium (Vector Laboratories, Burlingame, CA) containing 4′,6-diamidino-2-phenylindole (DAPI) was used to affix the coverslips to glass slides and stain chromatin. A fluorescent microscope (Nikon Eclipse 80i) equipped with bandpass emission filter cubes was used to obtain images.
Indirect enrichment of granulosa cells/direct depletion of RBCs
Dynabead procedure
After granulosa cell isolation with Percoll or Ficoll, the cellular pellet was resuspended in 1 mL recommended buffer (PBS with 0.1% BSA adjusted to pH 7.4 with NaOH) per 107 cells. Monoclonal mouse anti-human glycophorin A (DakoCytomation, Carpenteria, CA) was added to the cellular suspension using 0.22 μg (1.20 μL) per 106 total cells (dead and live granulosa cells + RBCs), and the mixture was incubated on ice on a rocker for 30 min. Cells were then washed with buffer (2 × 7 min) and resuspended in the original volume of buffer.
Pan mouse IgG-coated Dynabeads (4 × 108 beads/mL, Invitrogen, Carlsbad, California), in a volume of 25 μL per 107 cells, were placed into small plastic test tubes and twice rinsed to remove sodium azide preservative using PBS/BSA buffer. The EasySep magnetic separator (Stem Cell Technologies, Vancouver, BC) was used to collect the beads after washings. These beads were then added to the cellular suspension, which had been treated with primary antibody as described above. This bead and cellular solution was incubated on ice on a rocker for 30 min. The reaction vessel was then placed into the Dynal magnet (Invitrogen, Carlsbad, CA) made for a 15-mL tube. After 3–5 min, the supernatant was poured off into a new tube, and the tube containing the beads and bound cells was discarded. A cell count was performed using Trypan blue and a hemacytometer before the suspension was washed in PBS, centrifuged, and stored as a pellet in the −80°C freezer.
Direct enrichment of granulosa cells
BioMag bead procedure
After isolating granulosa cells from follicular fluid using Ficoll, the pelleted cells were resuspended in a microcentrifuge tube in 200 μL sterile media per 107 total cells. Rabbit mullerian inhibiting substance receptor II (MISRII) primary polyclonal antibody (200 μg in 1 mL; Santa Cruz Biotechnology, Santa Cruz, CA) was added to the cellular suspension using 6 μg (30 μL) per 107 granulosa cells. The reaction vessel was incubated at room temperature with gentle rocking for 30 min.
The volume of 500 μL anti-rabbit immunomagnetic bead solution per 107 granulosa cells (equivalent to 5 BioMag particles per target cell) was placed into a test tube. These silanized iron oxide beads, supplied as 1 × 108 beads per mg in a concentration of 1 mg/mL, were washed per manufacturer’s instructions (Quiagen BioMag, Hilden, Germany) by rinsing the beads in media and using an EasySep magnetic separator (Stem Cell Technologies, Vancouver, BC) to separate the beads from the sodium azide solution and media wash. After washing, the beads were set aside. Unbound primary antibody was removed from cells by centrifuging the cell solution at 370 g for 10 min and then washing the cells in sterile media. The pelleted cells were resuspended in 200 μL sterile media per 107 total cells.
Next, the media and anti-rabbit secondary antibody-coated beads were added to the microcentrifuge tube containing the washed cells. This solution was incubated at room temperature for 30 min. The microcentrifuge tube(s) was placed in a magnetic separator (the Invitrogen Dynal Cell Separator is one such magnet made for microcentrifuge tubes) for 7 min. After separation was complete, the supernatant was removed with careful pipetting, avoiding the beads and cells along the tube wall. The pellet(s) was resuspended in 1 mL PBS, and 10 μL of the suspension was removed for dilution and Trypan blue staining. After counting granulosa cells and assessing for viability, the suspension was aliquoted, pelleted, and stored at −80°C.
Note: A few samples were placed through the BioMag bead procedure after first subjecting them to the Dynabead procedure in an effort to achieve even better purity of granulosa cells than from the BioMag separation alone. During this experiment, the Dynabead protocol was performed, and then the pellet was resuspended in media for the subsequently performed BioMag bead procedure.
MACS microbead procedure
After isolating granulosa cells from follicular fluid with Ficoll, the pelleted cells were resuspended in 350 μL buffer (PBS with 0.5% BSA and 2 mM EDTA) per 107 cells. Polyclonal rabbit anti-human MISRII antibody (Santa Cruz Biotechnologies, Santa Cruz, CA) was added to the cellular suspension at a concentration of 5 μg (25 μL) per 107 target cells and incubated at 4°C on an end-to-end rotator for 30 min. Cells were washed of unbound antibody by adding 1–2 mL of buffer, centrifuged for 10 min at 300 g, and then washed again. The pellet was resuspended in 80 μL buffer per 107 total cells, and 20 μL MACS goat anti-rabbit IgG MicroBead solution (Miltenyi Biotech, Cambridge, MA), containing iron-dextran particles with an average diameter of 50 nm, was added per 107 total cells. Following incubation at 4°C on an end-to-end rotator for 25 min, unbound secondary antibody-bead complexes were removed with washing as performed after primary antibody incubation, and the pellet was resuspended in 500 μL per 108 cells.
Next, a sterile MACS MS column was placed into the magnetic field of a miniMACS separator and prepared with 2 mL buffer per manufacturer’s instructions. The 500 uL cellular suspension was applied to the column. The unbound cells were allowed to flow through the column and collect into microcentrifuge tubes. After rinsing with buffer to remove unbound cells, the column was removed from the magnetic field and washed with 1 mL buffer, which was pushed through the column with the provided plunger. Cells from both bound and unbound fractions were counted with hemacytometer, the solutions were centrifuged, and pellets were stored in the −80°C freezer.
Note
In an effort to improve the results from the MACS MicroBead isolations, the experiments were repeated with various, non-concurrent adjustments. The incubation temperatures were increased to room temperature, incubation times were increased, ACD-A buffer was substituted for recommended buffer, buffers were degassed per manufacturer’s instructions, and antibody concentrations were increased. In addition, the experiments were also performed with recycling of the unbound cell effluent through the column a second time.
Please see Supplement 1 for a table depicting main steps of the four bead isolation procedures.
Statistical analysis
Granulosa cell (GC) and RBC counts along with percent granulosa cell cellular purity 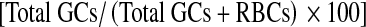 and live-to-dead GC ratio were recorded after Percoll and Ficoll isolation techniques as well as after bead purification methods. In addition, after bead procedures, the percent yield live GCs
and live-to-dead GC ratio were recorded after Percoll and Ficoll isolation techniques as well as after bead purification methods. In addition, after bead procedures, the percent yield live GCs 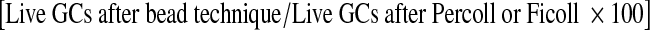 and percent reduction of RBCs
and percent reduction of RBCs 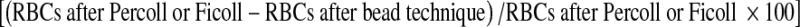 were calculated. Probability, t-test, and analysis of variance among the groups of samples to compare techniques were performed using 2004 NCSS software [30]. Statistical significance was defined as a p value of <0.05.
were calculated. Probability, t-test, and analysis of variance among the groups of samples to compare techniques were performed using 2004 NCSS software [30]. Statistical significance was defined as a p value of <0.05.
Results
Percoll and ficoll density gradients
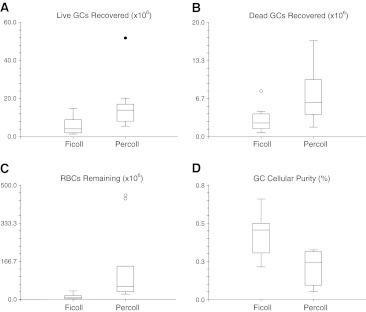
The Percoll separations (n = 11) resulted in an average of 16.05 × 106 (SE ±3.81 × 106) live granulosa cells, 7.59 × 106 (±SE 1.53 × 106) dead granulosa cells, and 132.70 × 106 (±SE 48.15 × 106) RBCs. The Ficoll separations (n = 11) resulted in an average of 5.94 × 106 (SE ±1.37 × 106) live granulosa cells, 2.95 × 106 (SE ±1.55 × 106) dead granulosa cells, and 12.11 × 106 (SE ±3.17 × 106) RBCs. Each of these values when analyzed with two-sample t-tests was statistically significant favoring Ficoll (Fig. 1a, b, c). In addition, both techniques resulted in similar live-to-dead GC ratios of approximately 2:1. However, the Ficoll method had a superior resulting cellular purity of 45.62% (SE ±4.27) compared to the Percoll method cellular purity of 22.88% (SE ±3.59, p = 0.00058; Fig. 1d).
Fig. 1.
a–dT-test comparison of samples purified with Ficoll and Percoll: a number of live granulosa cells (GCs) recovered, p = 0.022; b number of dead GCs recovered, p = 0.011; c number of RBCs remaining, p = 0.021; d GC purity [Total GCs/(Total GCs + RBCs)], p = 0.00058. Mild outliers are denoted with an open circle (in B and C), and a severe outlier (in A) is noted with a solid circle. Significance for differences set at p < 0.05
RBC lysis buffer
Despite following the recommended protocol and then repeating the protocol with the cellular suspension on ice and then also at room temperature for up to 15 min, there was no demonstrable decrease in RBC contamination after treatment. Cellular counts also demonstrated minor increases in granulosa cell death after treatment.
Western blotting
The antibody we selected to the N-terminus of the FSHR did not detect the FSHR protein in our patient samples nor in a granulosa tumor cell line (KGN) growing in our lab. We used a positive control of rat ovary extract as recommended by the manufacturer, and this control was validated. However, the MISRII protein was shown to be present in the IVF patient granulosa cells with Western blotting (Fig. 2) and was therefore selected for further use.
Fig. 2.
Western blot of granulosa cells from 7 IVF patients showing presence of MISRII, with the characteristic 63 Kda band
Immunocytochemistry
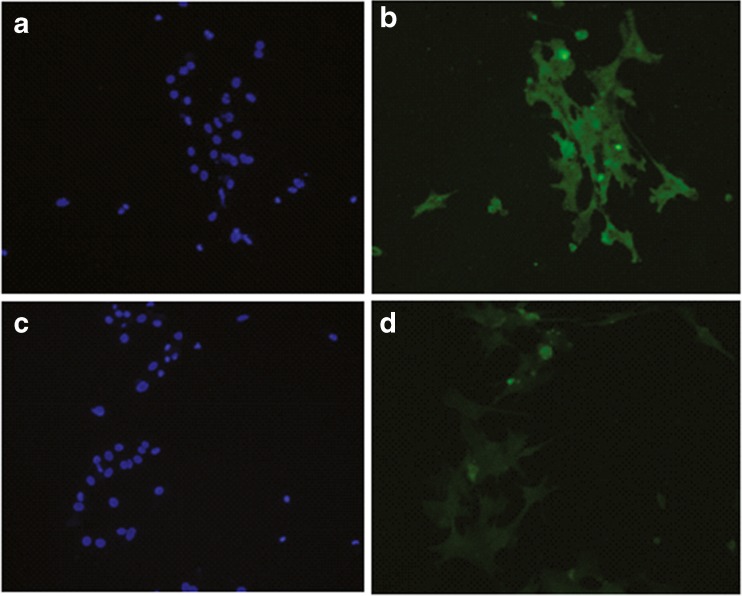
Immunocytochemistry demonstrated that most, if not all, human granulosa cells express the MIS receptor, as all of our cells plated on the cover slips showed presence of MISRII (Fig. 3). These immunocytochemistry results encouraged use of this antibody with immunomagnetic beads for separation.
Fig. 3.
a–d ICC of IVF patient granulosa cells plated for 36 h in DMEM/F12 media: a DAPI stain of MISRII probed cells; b FITC stain of MISRII probed cells; c DAPI stain of IgG probed cells; d FITC stain of IgG probed cells
Bead procedures
Dynabead procedure
The indirect enrichment of granulosa cell samples (n = 6) using secondary antibody-coated Dynabeads and glycophorin A antibody magnetically removed 91.5%(SE ±2.27%) of RBCs from the sample solution and resulted in a mean live granulosa cell count of 2.55 × 106 (SE ±0.90 × 106), a mean dead granulosa cell count of 4.96 × 106 (SE ±1.82 × 106), and mean RBC count of 4.16 × 106 (SE ±0.83 × 106). Mean granulosa cell purity from this method was 60.59% (SE ±8.31%), and the mean percent increase in purity with this method from the starting purity after Percoll/Ficoll was 34.41% (SE ±9.46). However, the live-to-dead ratio of recovered granulosa cells was very low at 0.65 (SE ±0.15). The percent yield live granulosa cells was 24.935% (SE ±7.28%).
BioMag procedure
The direct enrichment of granulosa cell samples (n = 7) using secondary antibody-coated BioMag particles and MISRII antibody to magnetically separate granulosa cells from the sample solution reduced RBC contamination by 95.40% (SE ±2.27%) and resulted in a mean live granulosa cell count of 3.37 × 106 (SE ±1.13 x 106), a mean dead granulosa cell count of 1.11 × 106 (SE ±0.28 × 106), and a mean RBC count of 1.19 × 106 (SE ±0.32 × 106). These results were achieved using 1/10th the amount of BioMag beads recommended. We found that this concentration of beads prevented the large removal of plated cells during washing that occurred at the recommended concentrations. Moreover, the lower concentration of beads allowed for better visualization of granulosa cells during counting and immunocytochemistry, ie, less obscurity from the beads (Fig. 4). Mean granulosa cell cellular purity from this method was 79.07% (SE ±3.09%), and the mean percent increase in purity with this method from the starting purity after Percoll/Ficoll was 44.05% (SE ±5.70%). More desirable, the live-to-dead ratio of recovered granulosa cells was 1.73 (SE ±0.28), and the percent yield live granulosa cells was 37.75% (SE ±10.32%).
Fig. 4.
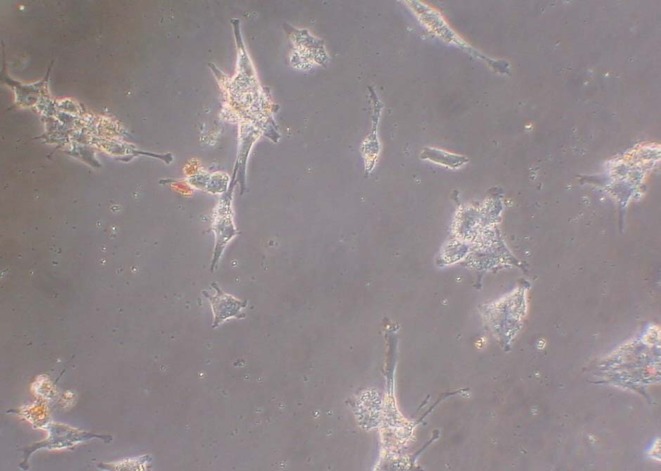
20× magnification view of GCs isolated using BioMag particles and plated on coverslips in DMEM/F12 for 24 h. These cells have been washed with PBS and are ready to stain
Double bead procedure
Utilization of the Dynal separation procedure followed by the BioMag separation procedure on the same sample (n = 2) removed 99.52% (SE ±0.37%) of RBCs and did result in higher cell purity (67.31%, SE ±0.15%) than the Dynal bead procedure alone (60.59%, SE ±8.71%), but it did not approach the purity of the BioMag procedure alone (79.07%, SE ±8.06%). Though the percent increase in cell purity beyond the Percoll/Ficoll procedures was highest of all of the methods at 58.55 (SE ±16.38%), the percent yield of live granulosa cells from the double bead method (12.00, SE ±0.14) was much less than the Dynal and BioMag procedures. Furthermore, the length of the separation procedure was increased significantly, requiring approximately 10–12 h, over the other methods, which require approximately 6 h.
MACS microbead procedure
The direct enrichment of granulosa cell samples (n = 5) using secondary antibody-coated MACS Microbead particles and MISRII antibody to magnetically separate granulosa cells from the sample solution resulted in very small numbers of cells. The yield included a mean live granulosa cell count of 0.10 × 106 (SE ±0.98 × 106), a mean dead granulosa cell count of 0.19 × 106 (SE ±1.14 × 106), and a mean RBC count of 0.17 × 106 (SE ±0.56 × 106). Mean granulosa cell purity from this method was 61.92% × 106 (SE ±9.54%), and the mean percent increase in purity with this method from the starting purity after Percoll/Ficoll was 11.76% (SE ±10.36%). The average live-to-dead ratio of recovered granulosa cells was 1.87 (SE ±43.91), and the percent yield live granulosa cells was only 2.07% (SE ±8.74%). Virtually all of the cells were collected in the unbound fraction, and recovery of bound cells was very poor, despite making changes to the protocol as described in our methods section. In addition, recycling the unbound cell effluent through the column a second time did not measurably improve bound cell counts.
Statistical comparison of bead procedures
The above three main bead procedures were compared using NCSS ANOVA software; the double bead procedure was omitted due to low numbers of samples, n = 2. There were no differences in live to dead granulosa cell ratio (p = 0.45), percent reduction in RBC (p = 0.38), or percent increase in cellular purity (p = 0.097); however, difference in mean percent yield of live granulosa cells (p = 0.041) reached statistical significance amongst the three groups (Fig. 5).
Fig. 5.
ANOVA comparison of the three bead methods regarding granulosa cell purity and yield as well as RBC reduction. Error bars depict standard error. Double bead procedure included in graphic for visualization but was not included in ANOVA due to sample size. Significance for differences between the three bead methods set at p < 0.05 and denoted by asterisks
Since the Dynal bead and the BioMag bead methods had the most favorable outcome profiles, with the highest percent yields of live granulosa cells (25% and 38% respectively), data from these two groups was further analyzed with a two-sample t-test (Table 1). T-test comparisons showed no statistically significant difference in live granulosa cell count (p = 0.59), percent reduction in RBCs (p = 0.12), percent yield live granulosa cells (p = 0.35), or increase in granulosa cell cellular purity beyond Ficoll treatment (p = 0.28), though trends favoring the BioMag procedure are evident in the data (Fig. 5). Comparisons did show a significant difference in dead granulosa cell count (Dynal>BioMag, p = 0.044), RBC count (Dynal>BioMag, p = 0.0048), resulting granulosa cell cellular purity (Biomag>Dynal, p = 0.049), and most notably live to dead granulosa cell ratio (BioMag>Dynal, p = 0.0095).
Table 1.
T-test comparison of Dynal and BioMag bead procedures
| Parameter | Bead procedure | Mean | SE | P value |
|---|---|---|---|---|
| Reduction in RBCs (%) | Dynal/GlycoA | 91.54 | ±2.46 | 0.12 |
| BioMag/MISRII | 95.79 | ±1.06 | ||
| Yield Live GCs (%) | Dynal/GlycoA | 24.93 | ±7.29 | 0.35 |
| BioMag/MISRII | 37.75 | ±10.32 | ||
| Cellular Purity (%) | Dynal/GlycoA | 60.59 | ±8.31 | 0.049 |
| BioMag/MISRII | 79.07 | ±3.10 | ||
| Increase in Cellular Purity after Ficoll/Percoll (%) | Dynal/GlycoA | 34.41 | ±6.22 | 0.28 |
| BioMag/MISRII | 44.05 | ±5.70 | ||
| Live to Dead GC Ratio | Dynal/GlycoA | 0.65 | ±0.15 | 0.0095 |
| BioMag/MISRII | 1.52 | ±0.23 |
Discussion
In order to accurately compare products of granulosa cells between patients, it is necessary to obtain purified cells reducing confounding variables from contaminating cells. Since our intent is to compare levels of granulosa cell proteins between IVF patients, a method that isolates larger numbers of cells than such techniques as fluorescence-activated cell sorting (FACS) is desired. With advancements in technology, many types of beads are commercially available for immunomagnetic sorting. Unlike FACS, which requires single cells to file through the machine for evaluation and sorting, the larger immunomagnetic beads (Dynabeads, BioMag particles) bind to granulosa cell aggregates, obviating the need for single-cell solutions, thus providing a higher yield of isolated granulosa cells and providing for future studies on granulosa cells that have maintained their intercellular connections with one another. From our experiments, we have developed a novel protocol to isolate granulosa cells from bloody follicular fluid using a MIS receptor II (MISRII) antibody and BioMag microbead particles coupled to secondary antibody and resulting in granulosa cells largely free of contaminating cells.
Our initial separations used the 50% Percoll solution described above and resulted in a large reduction in RBCs, but the process did not provide the purity we desired, nor did it address reduction in lymphocytes and epithelial cells. The Ficoll separation, which was used in later separations, resulted in better reduction of RBCs and thus improved purity, but this might be due to the protocol’s extensive dilution of the follicular fluid samples with buffer prior to overlay and centrifugation.
Because RBCs are the most prevalent contaminant, we began purification subsequent to Percoll or Ficoll by performing an incubation with RBC lysis buffer containing ammonium chloride; however, this treatment did not lead to notable RBC lysis in our samples. Noteably, this buffer was officially made to remove RBCs in mice, but its composition is quite similar to that for humans. Based on our results, we abandoned the RBC lysis buffer steps for future isolations. We next performed direct, negative selection of RBCs with Dynal Dynabeads coupled to secondary antibodies that would bind glycophorin A antibodies adherent to RBCs. Glycophorin A was chosen as the antibody to directly reduce RBCs because it is a known surface molecule on the RBC [31] and had been used by this lab before in other separation procedures. This procedure did further reduce RBC contamination, but the average live-to-dead ratio of granulosa cells was undesirable at 0.65.
As positive selection of granulosa cells is the most direct and specific way to isolate them from the surrounding heterogenous follicular fluid contents [32], the next part of the experiment focused on finding a primary antibody to a surface marker expressed exclusively on granulosa cells. The FSH receptor is one such marker, but it was absent on Western blotting of our patients’ cell extracts, which were run alongside a positive control of rat ovary extract. This result was thought to be related to downregulation of the FSH receptors during gonadotropin stimulation of the patients. In an attempt to clarify, samples prepared from cultures of KGN granulosa cell tumor cell line were also run along with the postive control; however, they were also negative for this receptor. This is especially peculiar considering that the KGN cell line has been characterized as maintaining the FSH receptor [33] and has been used in many experiments in our lab where its FSH receptor has been shown to be active.
The mullerian inhibiting substance type II receptor (MISRII) was next chosen as an antibody for granulosa cell isolation, since genes for the MISRII had been shown to be present in granulosa cell samples from IVF patients in the past [34]. After we confirmed the presence this receptor in our isolated granulosa cells, first with Western blotting and then with immunocytochemistry, we decided to use our selected antibody to MISRII with either BioMag beads or MACS MicroBeads beads for purification of our cells.
The concept of the MACS MicroBeads was especially attractive because the beads have small particle diameter (0.5 nm), and their dextran composition offers fairly rapid biodegradability, which is ideal for obtaining clean monolayers in cell culture. However, in our experiments, the MACS MicroBeads did not bind to our MISRII antibody-labeled granulosa cells, despite the use of a variety of different temperatures and buffers as described. Other authors, such as Clarke [35], have also had difficulty with some types of beads, and as they note, the type of bead chosen for certain cellular isolations may be a critical factor in a protocol’s success. In their case, the Dynal Dynabeads did not bind to their antibody-coated cells despite extensive troubleshooting, whereas the MACS microbeads performed very well. In our experiments, it is unclear why there was poor binding of the MACS microbeads to the granulosa cells, when the MISRII antibody was the same one used with our effective experiment using the BioMag beads and when the secondary antibody attached to the microbeads was indeed appropriately matched to the primary antibody. It is still possible that a different MISRII antibody would have been more effective in our experiment, but since the protocol also resulted in a large proportion of dead granulosa cells in even the unbound elution which flowed through the MACS separation column, we returned to improving the BioMag bead procedure for isolation of these cells from future patients.
In contrast to the MACS microbeads, the BioMag particles were bulkier. They did successfully isolate the granulosa cells using the MISRII antibody (Fig. 4), but they washed off along with nearly all cells when plated coverslips or tissue culture dishes were washed with PBS. Therefore, a great reduction in the number of beads recommended by the manufacturer (10–50 particles per cell of the total cell population) was made (down to 5 particles per target cell) until the beads were successful in isolating sufficient numbers of cells while leaving the majority of cells adherent to the coverslips and dishes when used in tissue culture. The reduction in the concentration of the BioMag beads used also greatly improved visibility of these cells during immunocytochemistry. The average percent yield of live granulosa cells was highest with the BioMag beads compared to the other bead techniques, though this trend did not reach statistical significance compared with the Dynal bead procedure. The BioMag beads also resulted in slightly higher RBC reduction than the Dynal beads, while also maintaining a significantly higher live-to-dead ratio of granulosa cells.
For a few samples (2 for which we have data), we performed a double bead isolation. It did result in slightly higher RBC reduction (99%) compared to the Dynal bead procedure alone (92%) and the BioMag bead procedure alone (98%), and thus it did result in the highest increase in cell purity beyond Percoll and Ficoll separations. However, this procedure was quite lengthy compared to the other bead procedures, and this time factor may be the cause of its poor percent yeild live granulosa cells (12%) compared to the Dynal bead procedure (25%) and the BioMag bead procedure (38%) .
Thus, because of its supriority as described above, we have chosen the BioMag bead isolation procedure with the novel use of the MISRII antibody to effectively separate large numbers of granulosa cells, including aggregates, from the surrounding contaminants in the follicular fluid. We have also developed and are using a residual RBC quantification protocol (see Supplement 2) to assess sample purity for subsequent standardization of samples for our Western blotting comparisons. The amount of protein obtained from our isolations has been sufficient for many Western blots per patient. Furthermore, this isolation technique provides patient cells that could also be prepared for other types of analysis such as measurement of nucleic acids by PCR or be grown on tissue culture dishes and manipulated in a myriad of experiements. However, to use such granulosa cells isolated with the BioMag beads in immunocytochemistry, one has to grow the cells until the beads largely fall off during 24–48 h of culture or are washed off with gentle media changes.
There are some limitations to our study as well as areas for improvement with later studies. Our sample size for comparison of bead techniques was limited, since early in the development of our protocol many of the cell samples were simply purified with Percoll or Ficoll and since we readily switched from one bead technique to another as the protocols were improved. Trends are apparent, but additional samples would have strengthened our statistical comparisons by reducing variance amongst observations. Furthermore, though we have used the isolated granulosa cells in our own subsequent experiments, future studies to compare cultured granulosa cells isolated by this method with cultured non-purified granulosa cells would be helpful to quantify the level of functionality of these luteinized cells.
We believe this MISRII antibody with BioMag bead isolation proceedure will help researchers rapidly achieve a purified, workable cell population, even when there is significant blood contamination in the follicular fluid specimens. It is notable that the effectiveness of this isolation technique may later be subjected to further improvement as new MISRII antibodies, such as that developed by Salhi et al. [36], and possibly other immunomagnetic beads that work even better with the MISRII antibody are engineered and then made commercially available.
Electronic supplementary material
Isolation Protocol Summary Table (DOC 41 kb)
Western Blotting Residual RBC Contamination Quantification Protocol (DOC 33 kb)
(JPEG 1910 kb)
(DOC 111 kb)
Acknowledgment
This research was supported by NIH RO1 HD044566-01A2. Authors would like to thank Dr. Vicki Schnell (Dickinson, TX) for providing IVF patients for enrollment in this study and to thank the embryologists of her lab for facilitating collection of follicular fluid from retrievals.
Footnotes
Capsule
A protocol for isolating and purifying human granulosa cells obtained from follicular fluid is developed and discussed.
References
- 1.Havelock JC, Rainey WE, Carr BR. Ovarian granulosa cell lines. Mol Cell Endocrinol. 2004;228:67–78. doi: 10.1016/j.mce.2004.04.018. [DOI] [PubMed] [Google Scholar]
- 2.Soboloff J, Sasaki H, Tsang BK. Follicular stage-dependent tumor necrosis factor α-induced hen granulosa cell integrin production and survival in the presence of transforming growth factor α in vitro. Biol Reprod. 2001;65:477–487. doi: 10.1095/biolreprod65.2.477. [DOI] [PubMed] [Google Scholar]
- 3.Choi JY, Jo MW, Lee EY, Yoon BK, Choi DS. The role of autophagy in follicular development and atresia in rat granulosa cells. Fert Stert. 2010;93:2532–2537. doi: 10.1016/j.fertnstert.2009.11.021. [DOI] [PubMed] [Google Scholar]
- 4.Pregel P, Bolla E, Cannizzo FT, Rampazzo A, Appino S, Biolatti B. Effect of anabolics on bovice granulosa-luteal cell primary cultures. Folia Histochemica et Cytobiologica. 2007;45:265–271. [PubMed] [Google Scholar]
- 5.Murphy BD, Dobias M. Homologous and heterologous ligands downregulate follicle-stimulating hormone receptor mRNA in porcine granulosa cells. Mol Reprod Dev. 1999;53:198–207. doi: 10.1002/(SICI)1098-2795(199906)53:2<198::AID-MRD9>3.0.CO;2-K. [DOI] [PubMed] [Google Scholar]
- 6.Garrido N, Albert C, Krussel JS, O’Connor JE, Remohi J, Simon C, et al. Expression, production, and secretion of vanscular endothelial growth factor and interleukin-6 by granulosa cells is comparable in women with and without endometriosis. Fert Stert. 2001;76:568–574. doi: 10.1016/S0015-0282(01)01961-6. [DOI] [PubMed] [Google Scholar]
- 7.Qu F, Wang FF, Lu XE, Dong MY, Sheng JZ, Lv PP, et al. Altered aquaporin expression in women with polycystic ovary syndrome: hyperandrogenism in follicular fluid inhibits aquaporin-9 in granulosa cells through the phosphatidylinositol 3-kinase pathway. Hum Reprod. 2010;00:1–10. doi: 10.1093/humrep/deq078. [DOI] [PubMed] [Google Scholar]
- 8.Enien WM, Chantler E, Seif MW, Elstein M. Human ovarian granulosa cells and follicular fluid indices: the relationship to oocyte maturity and fertilization in vitro. Hum Reprod. 1998;13:1303–1306. doi: 10.1093/humrep/13.5.1303. [DOI] [PubMed] [Google Scholar]
- 9.Karamouti M, Kollia P, Kallitsaris A, Vamvakopoulos N, Kollios G, Messinis IE. Growth hormone, insulin-like growth factor 1, and leptin interaction in human cultured lutein granulosa cells steroidogenesis. Fert Stert. 2008;90:1444–1450. doi: 10.1016/j.fertnstert.2007.08.076. [DOI] [PubMed] [Google Scholar]
- 10.Rey-Ares V, Lazarov N, Berg D, Berg U, Kunz L, Mayerhofer A. Dopamine receptor repertoire of human granulosa cells. Reprod Biol Endocrinol. 2007;5:40. doi: 10.1186/1477-7827-5-40. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Quinn MCJ, McGregor SB, Stanton JL, Hessian PA, Gillett WR, Green DPL. Purification of granulosa cells from human ovarian follicular fluid using granulosa cell aggregates. Reprod Fert Dev. 2006;18:501–508. doi: 10.1071/RD05051. [DOI] [PubMed] [Google Scholar]
- 12.Liu X, Qin D, Cui Y, Chen L, Li H, Chen Z, et al. The effect of calcium phosphate nanoparticles on hormone production and apoptosis in human granulosa cells. Reprod Bio Endocrinol. 2010;8:32. doi: 10.1186/1477-7827-8-32. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Machelon V, Nome F, Durand-Gasselin I, Emilie D. Tumor necrosis factor-α induces interleukin-6 mRNA and protein in human granulosa luteinizing cells via protein tyrosine kinase without involving ceramide. Mol Cell Endocrinol. 1997;126:173–184. doi: 10.1016/S0303-7207(96)03985-8. [DOI] [PubMed] [Google Scholar]
- 14.Mayerhoffer A, Hemmings HC, Snyder GL, Greengard P, Boddien S, Berg U, et al. Functional Dopamine-1 Receptors and DARPP-32 are expressed in human ovary and granulosa luteal cells in vitro. J Clin Endocrinol Met. 1999;84:257–264. doi: 10.1210/jc.84.1.257. [DOI] [PubMed] [Google Scholar]
- 15.Brannian J, Eyster K, Mueller BA, Gietz MG, Hansen K. Differential gene expression in human granulosa cells from recombinant FSH versus human menopausal gonadotropin ovarian stimulation protocols. Reprod Bio Endocrinol. 2010;8:25–30. doi: 10.1186/1477-7827-8-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Nottola SA, Heyn RM, Camboni A, Correr S, Macchiarelli G. Ultrastructural characteristics of human granulosa cells in a coculture system for in vitro fertilization. Micr Res Tech. 2006;69:508–516. doi: 10.1002/jemt.20309. [DOI] [PubMed] [Google Scholar]
- 17.Giampietro F, Sancilio S, Tiboni GM, Rana RA, Pietro RD. Levels of apoptosis in human granulosa cells seem to be comparable after thereapy with a gonadotropin-releasing hormone agonist or antagonist. Fert Stert. 2006;85:412–418. doi: 10.1016/j.fertnstert.2005.08.020. [DOI] [PubMed] [Google Scholar]
- 18.Jalkenen J, Ritvos O, Huhtaniemi I, Stenman UH, Laatikainen T, Ranta T. Phorbol ester stimulates human granulosa-luteal cell cyclic adenosine 3′,5′-monophosphate and progesterone production. Molec Cell Endocrinol. 1987;51:273–276. doi: 10.1016/0303-7207(87)90038-4. [DOI] [PubMed] [Google Scholar]
- 19.Fatum M, Natalie YO, David S, Joseph O, Simon A, Laufer N. Levels of steroidogenic acute regulatory protein and mitochondrial membrane potential in granulosa cells of older poor-responder women. Fert Stert. 2009;91:220–225. doi: 10.1016/j.fertnstert.2007.10.027. [DOI] [PubMed] [Google Scholar]
- 20.Kwintkiewicz J, Nishi Y, Yanase T, Giudice LC. Peroxisome proliferator-activated receptor-γ mediates bisphenol A inhibition of FSH-stimulated IGF-1, aromatase, and estradiol in human granulosa cells. Environ Health Perspect. 2010;118:400–406. doi: 10.1289/ehp.0901161. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Beckmann MW, Polacek D, Seung L, Schreiber JR. Human ovarian granulosa cell culture: determination of blood cell contamination and evaluation of possible culture purification steps. Fert Stert. 1991;56:881–887. doi: 10.1016/s0015-0282(16)54659-7. [DOI] [PubMed] [Google Scholar]
- 22.Guet P, Royere D, Paris A, Lansac J, Driancourt MA. Aromatase activity of human granulosa cells in vitro: effects of gonadotrophins and follicular fluid. Hum Reprod. 1999;14:1182–1189. doi: 10.1093/humrep/14.5.1182. [DOI] [PubMed] [Google Scholar]
- 23.Sasson R, Rimon E, Dantes A, Cohen T, Shinder V, Land-Bracha A, et al. Gonadotrophin-induced gene regulation in human granulosa cells obtained from IVF patients. Modulation of steroidogenic genes, cytoskeletal genes and genes coding for apoptotic signalling and protein kinases. Mol Hum Reprod. 2004;10:299–311. doi: 10.1093/molehr/gah041. [DOI] [PubMed] [Google Scholar]
- 24.Furger C, Pouchelet M, Zorn JR, Ferre F. Cell shape change reveals the cyclic AMP-mediated action of follicle stimulating hormone, human chorionic gonadotropin and vasoactive intestinal peptide in primary cultured human granulosa-lutein cells. Mol Hum Reprod. 1996;2:251–257. doi: 10.1093/molehr/2.4.251. [DOI] [PubMed] [Google Scholar]
- 25.Mayerhoffer A, Fohr KJ, Sterzik K, Gratzl M. Carbachol increases intracellular free calcium concentrations in human granulosa-lutein cells. J Endocrinol. 1992;135:153–159. doi: 10.1677/joe.0.1350153. [DOI] [PubMed] [Google Scholar]
- 26.Wolffe AP, Tata JR. Primary culture, cellular stress and differentiated function. Fed Euro Biochem Soc (FEBS) 1984;176:8–15. doi: 10.1016/0014-5793(84)80902-3. [DOI] [PubMed] [Google Scholar]
- 27.Richardson MC, Cameron IT, Simonis CD, Das MC, Hodge TE, Zhang J, et al. Insulin and human chorionic gonadotropin cause a shift in the balance of sterol regulatory element-binding protein (SREBP) isoforms toward the SREBP-1c isoform in cultures of human granulosa cells. J Clin Endocrinol Metab. 2005;90:3738–3746. doi: 10.1210/jc.2004-2057. [DOI] [PubMed] [Google Scholar]
- 28.Kaneko T, Saito H, Toya M, Satio T, Nakahara K, Hiroi M. Hyaluronic acid inhibits apoptosis in granulosa cells via CD44. J Assist Reprod Gen. 2000;17:162–167. doi: 10.1023/A:1009470206468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.McGuckin CP, Forraz N, Baradez MO, Navran S, Zhao J, Urban R, et al. Production of stem cedlls with embryonic characteristics from human umbilical cord blood. Cell Prolif. 2005;38:245–255. doi: 10.1111/j.1365-2184.2005.00346.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Hintze J. NCSS and PASS Number Cruncher Statistical Systems. Kaysville, Utah. www.NCSS.com 2001
- 31.Lambrecht B, Spengler HP, Nauwelaers F, Bauerfeind U, Mohr H, Müller TH. Flow cytometric assay for the simultaneous determination of residual white blood cells, red blood cells, and platelets in fresh-frozen plasma: validation and two years’ experience. Transfusion. 2009;49:1195–1204. doi: 10.1111/j.1537-2995.2008.02079.x. [DOI] [PubMed] [Google Scholar]
- 32.Daniak MB, Kuman A, Galaev IY, Mattiasson B. Methods in cell separations. Adv Biochem Engin/Biotechnol. 2007;106:1–18. doi: 10.1007/10_2007_069. [DOI] [PubMed] [Google Scholar]
- 33.Nishi Y, Yanase T, Mu Y, Oba K, Ichino I, Saito M, et al. Establishment and characterization of a steroidogenic human granulosa-like tumor cell line, KGN, that expresses functional follicle-stimulating hormone receptor. Endocrinology. 2001;142:437–445. doi: 10.1210/en.142.1.437. [DOI] [PubMed] [Google Scholar]
- 34.Chateau-Jonard S, Jamin SP, Leclerc A, Conzales J, Dewailly D, Clemente N. Anti-mullerian hormone, its receptor, fsh receptor, and androgen receptor genes are overexpressed by granulosa cells from stimulated follicles in women with polycystic ovary syndrome. J Clin Endocrinol Metab. 2008;93(11):4456–4461. doi: 10.1210/jc.2008-1231. [DOI] [PubMed] [Google Scholar]
- 35.Clarke C, Tiltley J, Davies, O’Hare MJ. An immunomagnetic separation method using superparamagnetic (MACS) beads for large-scale purification of human mammary luminal and myoepithelial cells. Epith Cell Biol. 1994;3:38–46. [PubMed] [Google Scholar]
- 36.Salhi I, Cambon-Roques S, LaMarre I, Laune D, Molina F, Pugniere M, et al. The anti-mullerian hormone type II receptor: insights into the binding domains recognized by a monoclonal antibody and the natural ligand. Biochem J. 2004;379:785–793. doi: 10.1042/BJ20031961. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Isolation Protocol Summary Table (DOC 41 kb)
Western Blotting Residual RBC Contamination Quantification Protocol (DOC 33 kb)
(JPEG 1910 kb)
(DOC 111 kb)