Abstract
Purpose
Time-lapse monitoring allows for a flexible embryo evaluation and potentially provides new dynamic markers of embryo competence. Before introducing time-lapse monitoring in a clinical setting, the safety of the instrument must be properly documented. Accordingly, the aim of this study was to evaluate the safety of a commercially available time-lapse incubator.
Methods
In a two center, randomized, controlled, clinical trial 676 oocytes from 59 patients in their 2nd or third treatment cycle, age <38 years and ≥8 oocytes retrieved were cultured in the time-lapse incubator or in a conventional incubator. The primary outcome was proportion of 4-cell embryos on day 2. Secondary outcomes were proportion of 7–8 cell embryos on day 3 and proportion of blastocysts on day 5. Implantation pregnancy rates were registered based on presence of fetal heart activity visualized by ultrasound 8 weeks after embryo transfer.
Results
No significant difference was found between the time-lapse incubator (TLI) and conventional incubator (COI) in proportion of 4-cell embryos on day 2 irrespective of whether data was analyzed according to ITT (RRTLI/COI: 0.81 (0.65; 1.02)) or PP (RRTLI/COI: 0.80 (0.63; 1.01)). Nor were any significant differences detected in the secondary endpoints; i.e. proportion of 7–8-cell embryos on day three ITT (RRTLI/COI: 0.96 (0.73; 1.26)); PP (RRTLI/COI: 0.95 (0.72; 1.26)) and proportion of blastocysts on day five ITT (RRTLI/COI: 1.09 (0.84; 1.41)); PP (RRTLI/COI: 1.09 (0.83: 1.41)). We found no differences in clinical pregnancy rate or implantation rate.
Conclusion
Culture in the time-lapse incubator supports embryonic development equally to a conventional incubator.
Keywords: Time-lapse monitoring, Safety, Embryo culture, Human, ART
Introduction
Selection of the most competent embryo remains a cornerstone in improvement of the safety and efficacy in IVF and ICSI treatment. The increased demand to lower the risk of neonatal complications and maternal pregnancy-related health problems associated with multiple pregnancies is met by promoting single embryo transfer (SET). The concurrent wish for high pregnancy rates intensifies the need to introduce techniques that can improve embryo selection. Currently, assessment of embryo morphology remains the choice of method. The correlation between developmental potential and morphological parameters, such as the degree of fragmentation, presence and number of nuclei and size, number and symmetry of blastomeres per embryo at distinct inspection points is well-established [12, 31, 33, 36, 39, 43]. Present morphological evaluation, however, requires inspection outside the controlled environment of the incubator, which exposes the embryos to undesirable changes in critical parameters: temperature, humidity and pH when evaluated [42]. Using traditional incubators, inspection is therefore limited to snapshots at a few discrete points in time, reducing the amount of information that could potentially be obtained. Time-lapse monitoring overcomes this limitation without exposing the embryos to environmental changes. Moreover, several recent studies suggest that time-lapse monitoring may introduce new dynamic markers of embryonic competence ([2, 18, 23, 32, 41]).
Relevant concerns have been given to safety of time-lapse monitoring, since the recording necessitates periodical exposure to light during image acquisition. It has been shown that extensive light exposure may be detrimental to embryo development, and especially that short wavelength light exposure should be minimized [4, 27, 28, 40]. Furthermore, heat due to motion and friction of moving parts; presence of magnetic fields, sheer stress of moving culture dishes and presence of lubricants may each represent theoretical risks to embryo development. If new reproductive technologies are introduced in clinical practice without a sound evaluation of their safety and efficacy, the result can be implementation of methods that can later on prove to be inefficient or even harmful [6, 14]. Before introducing time-lapse monitoring in a clinical setting, the safety of the instrument must therefore be properly documented. Comparisons have been made between embryos, both mouse and human, cultured in conventional incubators and time-lapse instruments, without reports of any adverse effects on development or implantation rate [5, 15, 18, 24, 26, 32, 41]. However, none of the previous studies were conducted as randomized clinical trials and the included human embryos derived from oocyte donor cycles [5] may not be representative of embryos from infertile couples.
Accordingly, the aim of the present study was to evaluate the development of sibling embryos from oocytes randomized to culture either in a conventional incubator or in a time-lapse incubator.
Materials and methods
A two center randomized study was conducted at the Fertility Clinic, Aarhus University Hospital (AUH) and the Fertility Clinic, Copenhagen University Hospital Rigshospitalet, Denmark (CUH) between June 2010 and April 2011.
Written informed consent was obtained from each couple before inclusion. Patients consented to randomisation of their oocytes to time-lapse monitoring and culturing of the embryos in the time-lapse incubator (EmbryoScope™, Unisense Fertilitech, Aarhus, Denmark) or culturing in a conventional incubator (Galaxy R, RS Biotech, CM Scinetific, West Lothian, UK). The Central Denmark Region Committees on Biomedical Research Ethics and the Danish Data Protection Agency approved the study. The study was registered at the ClinicalTrial.gov, study number 25940.
Prior to commencement of the randomized trial, the time-lapse incubator was tested on discarded human embryos with 1- or 3- pronuclei (PN) observed 18–20 h after insemination. All patients were eligible for this assessment. Based on the assumption that 30 % of all 1- or 3- PN embryos would develop into 4-cell embryos (based on clinical data, AUH and CUH 2008) 30 fertilized oocytes would be needed in each group to detect a 50 % decrease in 4-cell embryo proportion with 80 % power and a significance level of 0.05 (two-tailed tests). A 4-cell embryo proportion >15 % in the time-lapse incubator was a requirement for initiating the randomized study. Thirty-one (31) 1- and 3-PN embryos were eventually cultured in the time-lapse incubator. Of these, 11 (35 %) developed into 4-cell embryos on day two, allowing initiation of the randomized study.
Patients were eligible for randomization of their oocytes if they fulfilled the following criteria: (i) 2nd or third treatment cycle with a normal fertilization rate (≥ 50 %) and embryo development in the first cycle (ii), age <38 years, (iii) ≥8 oocytes retrieved. Patients with endometriosis were excluded.
Randomization
Following retrieval, oocytes were randomized to culture in a conventional incubator or in the time-lapse incubator. Block randomization was done 1:1 using random numbers from sealed envelopes. All oocytes retrieved for standard IVF were randomized immediately following insemination. In case of ICSI, only mature oocytes (MII) were randomized after the ICSI procedure. At the Fertility Clinic, CUH, all retrieved oocytes were randomized and immature oocytes (GV or MI) were later excluded from the analysis.
Ovarian stimulation and oocyte retrieval
Patients underwent ovarian stimulation in either a long down regulation protocol or a short antagonist protocol using urinary or recombinant FSH stimulation according to the guidelines of each clinic. FSH doses were adjusted individually to the patient’s ovarian response. A dose of 10.000 IU of hCG was administered when at least 3 follicles measured ≥17 mm and ultrasound guided oocyte retrieval was conducted 36 h later.
In vitro fertilization and embryo culture
Insemination was performed using standard IVF or ICSI procedures according to treatment indications. Micro inseminated oocytes were immediately after injection placed in individual wells within a special culture slide (EmbryoSlide™, Unisense Fertilitech, Aarhus, Denmark) in the time-lapse incubator or a standard culture dish (Nunc® multidish 4 wells, Roskilde, Denmark) in the conventional incubator according to randomization. IVF oocytes randomized to time-lapse incubation were cultured 20 h in the conventional incubator followed by transfer to EmbryoSlides™ for further culturing in the time-lapse incubator. All embryos were cultured in sequential culture medium (Sydney IVF Fertilization/Cleavage/Blastocyst Medium, COOK®, Sydney, Australia) under oil at 37 °C, 20 % O2, and 6 % CO2. One or two blastocysts were selected for transfer based on blastocyst score on the morning of day five.
Morphological assessment of embryos
In both groups, embryos were removed from the incubators and evaluated morphologically 44–46 h after insemination (day 2), 66–68 h after insemination (day 3) and 115–117 h after insemination (day 5). On day 2 and 3 embryos were evaluated on the basis of number and symmetry of blastomeres, degree of fragmentation and presence of multi-nucleated cells. On day 5 blastocyst assessment was based on expansion of the blastocele cavity (1–6) and number and cohesiveness of the inner cell mass and trophectoderm (A-C)[7]. The technician evaluating and selecting the embryos for transfer on day 5 was blinded to incubation method. The primary endpoint was proportion of 4-cell embryos on day 2. Secondary end-points were proportion of 7–8 cell embryos on day 3 and proportion of blastocysts (score 1–6) on day 5.
Embryo transfer
One or two embryos were transferred on day five according to embryo quality, patient characteristics and patient wishes. Clinical pregnancy rate was registered as number of ongoing pregnancies per patient, based on presence of fetal heart activity visualized by ultrasound 8 weeks after embryo transfer. Implantation rate was recorded as total number of ongoing pregnancies per total number of embryos transferred.
All data was recorded on a case report form and approved by a study monitor. Two individuals manually entered all data twice into an electronic database (EpiData; www.epidata.dk). A computer-based comparison of the two datasets was performed and errors identified were corrected according to the original data.
Sample size calculation and statistical analysis
Assuming that 50 % of all inseminated oocytes would develop into 4-cell embryos (based on clinical data, CUH and AUH 2008), 200 fertilized oocytes would be needed in each group to detect a decrease in 4-cell embryo proportion of 30 % with 80 % power and a significance level of 0.05 (two-tailed tests).
Continuous data following a normal distribution was analyzed with Students t-test. Continuous data not fulfilling the assumption of normality was analyzed with Wilcoxon rank-sum test. For categorical data Chi-squared or Fisher’s exact test was used. Two-tailed p-values < 0.05 were considered significant. All statistical analyses were performed in STATA, version 11.0 (StataCorp, USA).
Results
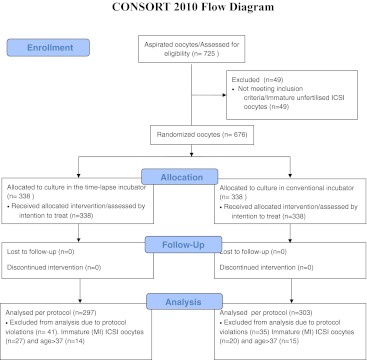
725 oocytes were retrieved from 59 patients (Fig. 1). Three hundred and forty-three (n = 343) oocytes from 32 patients were recruited from CUH, and 382 oocytes from 27 patients were recruited from AUH. Patients and cycle characteristics from the two clinics were comparable, apart from number of oocytes retrieved per patient, AUH retrieving a higher number (Table 1). According to the protocol, 49 immature (GV or MI) ICSI oocytes were excluded before randomization. The remaining 676 oocytes were randomized to the conventional incubator group or the time-lapse incubator resulting in 338 oocytes analyzed by intention-to-treat (ITT) in each group. Due to protocol violations, 76 oocytes were excluded after randomization. The excluded 76 oocytes consisted of 47 immature (GV or MI) ICSI oocytes that, according to the protocol, should have been excluded before randomization and 29 oocytes from three patients not fulfilling the inclusion criteria. No oocytes were excluded on patient request. Thus, in the time-lapse incubator group 297 oocytes were assessed per protocol (PP), while 303 oocytes were assessed PP in the standard incubator group (Fig. 1). No oocytes were lost to follow-up. Before pooling data from the two clinics, the primary and secondary endpoints were analyzed for each clinic separately. No substantial or significant differences were found, allowing data to be pooled (data not shown). No significant difference was found between the time-lapse incubator (TLI) and conventional incubator (COI) in proportion of 4-cell embryos on day 2 irrespective of whether data was analyzed according to ITT (RRTLI/COI: 0.81 (0.65; 1.02)) or PP (RRTLI/COI: 0.80 (0.63; 1.01)) (Table 2). Nor were any significant differences detected in the secondary endpoints; i.e. proportion of 7–8-cell embryos on day three and proportion of blastocysts on day five (Table 2) or in clinical pregnancy rate and implantation rate (Table 3). To ensure that the excluded immature ICSI embryos did not influence the results, a post-hoc analysis including IVF embryos only was conducted. The conclusion of no difference in embryo development between the two incubators was unchanged (Table 4).
Fig. 1.
Flow of oocytes through each stage of the trial comparing the time-lapse incubator and a conventional incubator. The Fertility Clinic, Aarhus University Hospital (AUH) and the Fertility Clinic, Copenhagen University Hospital Rigshospitalet, Denmark (CUH) between June 2010 and April 2011
Table 1.
Patient and cycle characteristics
| ITT/PP | CUH | AUH | Combined | p-value* | |
|---|---|---|---|---|---|
| No. of patients recruited | ITT | 32 | 27 | 59 | |
| PP | 29 | 27 | 56 | ||
| Patient age/years | ITT | 32.7 ± 3.3 | 31.6 ± 3.2 | 32.2 ± 3.3 | 0.20 |
| PP | 32.1 ± 2.9 | 31.6 ± 3.2 | 31.9 ± 3.0 | 0.53 | |
| No. of oocytes retrieved | ITT | n = 343 | n = 382 | n = 725 | |
| PP | n = 314 | n = 382 | n = 696 | ||
| No. of inseminated oocytes (IVF) | ITT | n = 134 | n = 126 | n = 260 | 0.74 |
| PP | n = 105 | n = 126 | n = 231 | ||
| No. of micro injected oocytes (ICSI) | ITT | n = 209 | n = 207 | n = 416 | 0.71 |
| PP | n = 162 | n = 207 | n = 369 | ||
| No. of oocytes retrieved/patient | ITT | 10.7 ± 2.5 | 14.1 ± 4.7 | 12.3 ± 4.0 | <0.001 |
| PP | 10.8 ± 2.5 | 14.1 ± 4.7 | 12.4 ± 4.0 | 0.002 | |
| Number of clinical pregnancies | ITT | 12 (37.5 %) | 8 (29.6 %) | 20/59(33.9 %) | 0.59 |
| PP | 11 (37.9 %) | 8 (29.6 %) | 19/56 (33.9 %) | 0.58 |
Continuous variables are expressed as means ± SD, differences are tested with Students t-test. Categorical variables are expressed as numbers (%), differences are tested with χ2 test
AUH Aarhus University Hospital; CUH Copenhagen University Hospital, Rigshospitalet
ITT Assessed by intention-to-treat; PP Assessed per protocol
*AUH compared with CUH
Table 2.
Primary and secondary endpoints
| ITT/PP | TLI | COI | RRTLI/COI | RDTLI/COI | p-value* | |
|---|---|---|---|---|---|---|
| Number of randomized oocytes | ITT | n = 338 | n = 338 | – | – | 1.0 |
| PP | n = 297 | n = 303 | – | – | 0.73 | |
| Number of four-cells day 2 | ITT | n = 92 | n = 113 | 0.81 (0.65;1.02) | −6.2 % (−13.1;0.7) | 0.08 |
| PP | n = 84 | n = 107 | 0.80 (0.63;1.01) | −7.0 % (−14.4;4.0) | 0.07 | |
| Number of 7–8 cells day 3 | ITT | n = 78 | n = 81 | 0.96 (0.73;1.26) | −0.9 % (−7.3;5.5) | 0.79 |
| PP | n = 73 | n = 78 | 0.95 (0.72;1.26) | −1.1 % (−8.1;5.8) | 0.74 | |
| Number of blastocysts day 5a | ITT | n = 88 | n = 81 | 1.09 (0.84;1.41) | 2.1 % (−4.5;8.6) | 0.53 |
| PP | n = 83 | n = 78 | 1.09 (0.83:1.41) | 2.0 % (−4.9;9.3) | 0.54 |
aBlastocyst development of embryos with delayed cleavage explained the higher number of blastocysts day 5 compared with 7–8 cell embryos day 3
RR Risk Ratio; RD Risk Difference
TLI Time-lapse incubator; COI Conventional incubator
ITT Assessed by intention-to-treat; PP Assessed per protocol
*Time-lapse incubator compared with conventional incubator
Table 3.
Clinical pregnancy and implantation rates
| TLI | COI | Mixed TLI and COI | p-value* | |
|---|---|---|---|---|
| Single embryo transfers (SET) | 17 | 13 | – | – |
| Double embryo transfers (DET) | 2 | 5 | 15 | – |
| Clinical pregnancies after SET | 5 | 3 | – | – |
| Clinical pregnancies after DET | 2 | 3 | 8 | – |
| 1 fetal heart beat | 1 | 2 | 5 | – |
| 2 fetal heart beats | 1 | 1 | 3 | – |
| Implantation rate (FHB/embryos transferred) | 38.1 % (8/21) | 30.4 % (7/23) | 36.7 % (11/30) | 0.75 |
| Clinical pregnancy rate (FHB/patient transferred) | 36.8 % (7/19) | 33.3 % (6/18) | 53.3 % (8/15) | 1 |
TLI Time-lapse incubator; COI Conventional incubator
FHB Fetal heart beat(s); *TLI compared to CO, χ2 test
Table 4.
Endpoints for IVF embryos only
| IVF only | ITT/PP | TLI | COI | RRTLI/STI | RDTLI/STI | p-value* |
|---|---|---|---|---|---|---|
| Number of randomized oocytes | ITT | n = 131 | n = 129 | – | – | – |
| PP | n = 117 | n = 114 | – | – | – | |
| Number of four-cells day 2 | ITT | n = 35 | n = 41 | 0.84 (0.57;1.23) | −5.1 % (−16.1;6.0) | 0.37 |
| PP | n = 27 | n = 35 | 0.75 (0.49;1.16) | −7.6 % (−19.0;3.8) | 0.19 | |
| Number of 7–8 cells day 3 | ITT | n = 31 | n = 29 | 1.05 (0.68;1.64) | 1.2 % (−9.1;11.4) | 0.82 |
| PP | n = 26 | n = 26 | 0.97 (0.60;1.57) | −0.6 % (−11.4;10.2) | 0.92 | |
| Number of blastocysts day 5 | ITT | n = 35 | n = 36 | 0.96 (0.64;1.42) | −1.2 % (−12.0;9.6) | 0.83 |
| PP | n = 30 | n = 33 | 0.89 (0.58;1.35) | −3.3 % (−14.8;8.2) | 0.57 |
RR Risk Ratio; RD Risk Difference
TLI Time-lapse incubator; COI Conventional incubator
ITT Assessed by intention-to-treat; PP Assessed per protocol
*Time-lapse incubator compared with conventional incubator
Discussion
The aim of this study was to evaluate the safety of a commercially available time-lapse incubator by comparing development of embryos cultured in a commercially available time-lapse incubator to embryos cultured in a conventional incubator. We found no differences in the proportion of 4-cell embryos on day two after insemination, the proportion of 7–8- cell embryos on day three or the proportion of blastocysts on day five between the two groups. Nor did we find any differences in clinical pregnancy rate or implantation rate.
Though several studies have reported similar development in time-lapse incubators compared to conventional incubators, none of these have been conducted as randomized clinical trials (RCT) [18, 24, 26, 41]. Most reports have been supplementary observations in descriptive studies investigating different aspects of development. A recent study, which compared culture in a time-lapse incubator to a conventional incubator by assessing embryo quality, blastocyst and ongoing pregnancy rates in embryos stemming from fresh donated oocytes, showed no difference between the two types of incubators [5]. However, the study was not randomized and, as pointed out by the authors, donated oocytes from fertile women, who are relatively young, may not be representative of oocytes from an infertile population. Since donated oocytes are presumably of higher quality than oocytes from a standard ART program, a better developmental potential would be expected. Our study evaluated oocytes from infertile patients, who had all demonstrated normal fertilization and development of the oocytes in one or more previous cycles. Moreover, randomization of oocytes to the two incubators was based on sample size analyses, and laboratory technicians evaluating the embryos were blinded to the type of incubator. We therefore believe the embryos in the two groups to be comparable and our results to be valid and applicable in other settings. The higher rate of blastocyst development reported in the study by Cruz et al. [5] (54.8 % in the time-lapse incubator and 50.6 % in the standard incubator) compared to our study (24.9 % in the time-lapse incubator and 23.2 % in the standard incubator) supports the assumption that the oocytes in the two studies were not of comparable quality. An additional contributing factor to the observed discrepancy in blastocyst rates was, that oocytes were allocated to the two types of incubators after confirmed fertilization in the study by Cruz et al. [5], in contrast to our study, where randomization was done before confirmed fertilization. Cruz et al. [5] consequently calculated blastocyst rates on the basis of a smaller population with higher chance of blastocyst development, which obviously results in higher blastocyst rates. We therefore consider the difference in outcome between the two trials to be explained by oocyte quality and calculation method.
Due to protocol violations, 76 oocytes were excluded after randomization. Of the excluded 76 oocytes, 47 were immature ICSI oocytes that, according to the protocol, should have been excluded before randomization, and 29 oocytes from three patients not fulfilling the inclusion criteria. These exclusions may potentially have disturbed the randomization, though we find this interpretation unlikely. Firstly, the exclusions were equally distributed between the two incubators, and secondly the findings were comparable both when assessment was made by intention-to-treat and per protocol. Furthermore, a post-hoc analysis excluding all ICSI embryos from the analysis did not change the results. Although the number of embryos thus analyzed was smaller, the analysis supports our interpretation that the initial inclusion of ICSI embryos did not influence the results.
In this study we evaluated the safety of the TLI by assessing embryonic development and the number of oocytes included was based on power calculations using 4-cell rate on day two as the primary endpoint. Although clinical pregnancy rate, implantation rate and live births with pediatric follow-up are the ultimate end-points of RCTs in ideal research [14], we consider the end-point used in this study to be relevant for the clinics participating in this study, since here embryo transfers are predominantly performed on day 2. To conduct a sufficiently powered study using pregnancy rate or live birth rate with pediatric follow-up would require a significantly larger number of participants. Although we recognize that such studies must be undertaken in order to continuously evaluate the safety of TLI, performing a smaller study with sufficient statistical power to detect differences for a relevant parameter, is a necessary step in hypothesis-driven basic research.
The application of elective SET in clinical practice represents the ultimate challenge to selection of the embryo with the highest chance of achieving a pregnancy. Several methods, both invasive and non-invasive, are under investigation i.e. aneuploidy screening (PGS), O2 respiration measurement, metabolic profiling and gene expression analysis [1, 3, 17, 22, 29, 35, 37]. Some of these methods, though promising, are not yet applicable in a routine clinical setting, and others, such as PGS and near infrared spectroscopy (NIR) analysis have been disappointing in their present form, when evaluated in large randomized clinical trials [10, 11, 22, 34, 38].
Time-lapse monitoring has been used for decades to study embryonic development in animals [8, 9, 19–21]. The introduction of incubators designed for clinical use enables continuous monitoring of human embryos in assisted reproduction, which allows for evaluation of all the morphological parameters already implemented in clinical practice at the corresponding points of time. However, since images are automatically recorded, the embryo assessment can be completed whenever the laboratory technician finds it convenient, allowing for a more flexible evaluation. Furthermore, recent studies [2, 18, 23, 32, 41] have addressed the potential role of time-lapse monitoring in selection of competent embryos. Apart from a more detailed and flexible embryo evaluation, these studies suggest that time-lapse monitoring may provide new dynamic markers of embryo competence. There is a well-documented correlation between embryo morphology evaluated at certain time-points and embryo competence as reviewed in a recent consensus paper by ALPHA and ESHRE [16]. However, the progressive and dynamic nature of cell cleavage and embryo development is also well known [13, 18, 30], and embryo scoring can change markedly within few hours [25]. In conventional morphological evaluation of embryo quality the number and duration of inspections outside the incubator must be restricted since changes in environment are known to induce stress [42]. Time-lapse monitoring overcomes this limitation, while providing the potential benefit of stable culture conditions during inspection. Our study evaluated the safety of a commercially available TLI by comparing embryonic development in a conventional incubator to the TLI. In order to achieve blinding of the embryologist, who assessed the embryos at the beforehand chosen time-points, embryos were removed from both incubators for evaluation. Thus, only the potential negative effects of time-lapse monitoring were evaluated, whereas embryos in the TLI were not allowed the potential positive effects of stable culture condition in the incubator during embryo assessment. We consider this design to strengthen the conclusion.
The possibility to analyze the dynamic nature of embryo development gives rise to expectations of improved embryo selection. Time-lapse monitoring enables registration of dynamic events such as the precise timing and synchrony of cell- divisions, appearance and disappearance of nuclei and pro-nuclei; events that in descriptive studies have shown correlation with developmental competence of both animal and human embryos [18, 30, 41]. In this study, we demonstrate that time-lapse monitoring can be applied safely to human embryos, thereby enabling further investigation of a promising tool for improved embryo selection.
In conclusion, culture in the time-lapse incubator supports embryonic development equally to a conventional incubator. Time-lapse monitoring can therefore be introduced clinically, thereby providing a tool for a potentially more refined and flexible embryo evaluation, and possibly introducing new markers of embryonic competence.
Acknowledgments
The authors wish to thank the clinical, paramedical and laboratory team of the Fertility Clinic, Aarhus University Hospital, Skejby and the Fertility Clinic, Copenhagen University Hospital Rigshospitalet. Unisense FertiliTech is thanked for providing EmbryoSlides. Inge Agerholm is thanked for scientific discussions.
Funding
Unisense FertiliTech provided EmbryoSlides.
Disclosure statement
The authors have nothing to declare
Footnotes
Capsule
Culture in a time-lapse incubator supports embryonic development equally to a conventional incubator.
Authors’ role
JI and USK designed the study. KK performed data analyses and wrote the first draft. JH and MLG were responsible for data acquisition. All authors wrote and approved the final manuscript.
References
- 1.Ahlstrøm A, Wikland M, Rogberg L, Barnett JS, Tucker M, Hardarson T. Cross-validation and predictive value of near-infrared spectroscopy algorithms for day-5 blastocyst transfer. Reprod Biomed Online. 2011;22(5):477–484. doi: 10.1016/j.rbmo.2011.01.009. [DOI] [PubMed] [Google Scholar]
- 2.Arav A, Aroyo A, Yavin S, Roth Z. Prediction of embryonic developmental competence by time-lapse observation and ‘shortest-half’ analysis. Reprod Biomed Online. 2008;17(5):669–675. doi: 10.1016/S1472-6483(10)60314-8. [DOI] [PubMed] [Google Scholar]
- 3.Assou S, Haouzi D, Vos J, Hamamah S. Human cumulus cells as biomarkers for embryo and pregnancy outcomes. Mol Hum Reprod. 2010;16(8):531–538. doi: 10.1093/molehr/gaq032. [DOI] [PubMed] [Google Scholar]
- 4.Beraldi R, Sciamanna I, Mangiacasale R, Lorenzini R, Spadafora C. Mouse early embryos obtained by natural breeding or in vitro fertilization display a differential sensitivity to extremely low-frequency electromagnetic fields. Mutat Res. 2003;538(1–2):163–170. doi: 10.1016/s1383-5718(03)00116-5. [DOI] [PubMed] [Google Scholar]
- 5.Cruz M, Gadea B, Garrido N, Pedersen KS, Martinez M, Perez-Cano I, Munoz M, Meseguer M. Embryo quality, blastocyst and ongoing pregnancy rates in oocyte donation patients whose embryos were monitored by time-lapse imaging. J Assist Reprod Genet. 2011. doi:10.1007/s10815-011-9549-1. [DOI] [PMC free article] [PubMed]
- 6.Dondorp W, Wert G. Innovative reproductive technologies: risks and responsibilities. Hum Rep. 2011;26(7):1604–1608. doi: 10.1093/humrep/der112. [DOI] [PubMed] [Google Scholar]
- 7.Gardner DK, Surrey E, Minjarez D, Leitz A, Stevens J, Schoolcraft WB. Single blastocyst transfer: a prospective randomized trial. Fertil Steril. 2004;81(3):551–555. doi: 10.1016/j.fertnstert.2003.07.023. [DOI] [PubMed] [Google Scholar]
- 8.Gonzales DS, Pinheiro JC, Bavister BD. Prediction of the developmental potential of hamster embryos in vitro by precise timing of the third cell cycle. J Reprod Fertil. 1995;105(1):1–8. doi: 10.1530/jrf.0.1050001. [DOI] [PubMed] [Google Scholar]
- 9.Grisart B, Massip A, Dessy F. Cinematographic analysis of bovine embryo development in serum-free oviduct-conditioned medium. J Reprod Fertil. 1994;101(2):257–264. doi: 10.1530/jrf.0.1010257. [DOI] [PubMed] [Google Scholar]
- 10.Hardarson T, Ahlström A, Rogberg L, Botros L, Hillensjö T, Westlander G, Sakkas D, Wikland M. Non-invasive metabolomic profiling of Day 2 and 5 embryo culture medium: a prospective randomized trial. Hum Rep. 2011. doi:10.1093/humrep/der373. [DOI] [PubMed]
- 11.Hardarson T, Hanson C, Lundin K, Hillensjo T, Nilsson L, Stevic J, Reismer E, Borg K, Wikland M, Bergh C. Preimplantation genetic screening in women of advanced maternal age caused a decrease in clinical pregnancy rate: a randomized controlled trial. Hum Rep. 2008;23(12):2806–2812. doi: 10.1093/humrep/den217. [DOI] [PubMed] [Google Scholar]
- 12.Hardarson T, Hanson C, Sjogren A, Lundin K. Human embryos with unevenly sized blastomeres have lower pregnancy and implantation rates: indications for aneuploidy and multinucleation. Hum Rep. 2001;16(2):313–318. doi: 10.1093/humrep/16.2.313. [DOI] [PubMed] [Google Scholar]
- 13.Hardarson T, Lofman C, Coull G, Sjogren A, Hamberger L, Edwards RG. Internalization of cellular fragments in a human embryo: time-lapse recordings. Reprod Biomed Online. 2002;5(1):36–38. doi: 10.1016/S1472-6483(10)61594-5. [DOI] [PubMed] [Google Scholar]
- 14.Harper J, Cristina Magli M, Lundin K, Barratt CLR, Brison D. When and how should new technology be introduced into the IVF laboratory? Hum Reprod. 2011;27(2):303–313. doi: 10.1093/humrep/der414. [DOI] [PubMed] [Google Scholar]
- 15.Holm P, Shukri NN, Vajta G, Booth P, Bendixen C, Callesen H. Developmental kinetics of the first cell cycles of bovine in vitro produced embryos in relation to their in vitro viability and sex. Theriogenology. 1998;50(8):1285–1299. doi: 10.1016/S0093-691X(98)00227-1. [DOI] [PubMed] [Google Scholar]
- 16.The Istanbul consensus workshop on embryo assessment: proceedings of an expert meeting. Hum Rep. 2011;doi:10.1093/humrep/der037 [DOI] [PubMed]
- 17.Jones GM, Cram DS, Song B, Kokkali G, Pantos K, Trounson AO. Novel strategy with potential to identify developmentally competent IVF blastocysts. Hum Rep. 2008;23(8):1748–1759. doi: 10.1093/humrep/den123. [DOI] [PubMed] [Google Scholar]
- 18.Lemmen JG, Agerholm I, Ziebe S. Kinetic markers of human embryo quality using time-lapse recordings of IVF/ICSI-fertilized oocytes. Reprod Biomed Online. 2008;17(3):385–391. doi: 10.1016/S1472-6483(10)60222-2. [DOI] [PubMed] [Google Scholar]
- 19.Lewis WH, Gregory PW. Cinematographs of living developing rabbit-eggs. Science. 1929;69(1782):226–229. doi: 10.1126/science.69.1782.226-a. [DOI] [PubMed] [Google Scholar]
- 20.Massip A, Mulnard J. Time-lapse cinematographic analysis of hatching of normal and frozen-thawed cow blastocysts. J Reprod Fertil. 1980;58(2):475–478. doi: 10.1530/jrf.0.0580475. [DOI] [PubMed] [Google Scholar]
- 21.Massip A, Mulnard J, Vanderzwalmen P, Hanzen C, Ectors F. The behaviour of cow blastocyst in vitro: cinematographic and morphometric analysis. J Anat. 1982;134(Pt 2):399–405. [PMC free article] [PubMed] [Google Scholar]
- 22.Mastenbroek S, Twisk M, Echten-Arends J, Sikkema-Raddatz B, Korevaar JC, Verhoeve HR, Vogel NE, Arts EG, Vries JW, Bossuyt PM, Buys CH, Heineman MJ, Repping S, Veen F. In vitro fertilization with preimplantation genetic screening. N Engl J Med. 2007;357(1):9–17. doi: 10.1056/NEJMoa067744. [DOI] [PubMed] [Google Scholar]
- 23.Meseguer M, Herrero J, Tejera A, Hilligsøe KM, Ramsing NB, Remohi J. The use of morphokinetics as a predictor of embryo implantation. Hum Rep. 2011. doi:10.1093/humrep/der256. [DOI] [PubMed]
- 24.Mio Y, Maeda K. Time-lapse cinematography of dynamic changes occurring during in vitro development of human embryos. Am J Obstet Gynecol. 2008;199(6):660 e661–665 e661. doi: 10.1016/j.ajog.2008.07.023. [DOI] [PubMed] [Google Scholar]
- 25.Montag M, Liebenthron J, Koster M. Which morphological scoring system is relevant in human embryo development? Placenta. 2011. doi:S1383-03),/j.placenta.2011.07.009. [DOI] [PubMed]
- 26.Nakahara T, Iwase A, Goto M, Harata T, Suzuki M, Ienaga M, Kobayashi H, Takikawa S, Manabe S, Kikkawa F, Ando H. Evaluation of the safety of time-lapse observations for human embryos. J Assist Reprod Genet. 2010;27(2–3):93–96. doi: 10.1007/s10815-010-9385-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Oh SJ, Gong SP, Lee ST, Lee EJ, Lim JM. Light intensity and wavelength during embryo manipulation are important factors for maintaining viability of preimplantation embryos in vitro. Fertil Steril. 2007;88(4 Suppl):1150–1157. doi: 10.1016/j.fertnstert.2007.01.036. [DOI] [PubMed] [Google Scholar]
- 28.Ottosen LD, Hindkjaer J, Ingerslev J. Light exposure of the ovum and preimplantation embryo during ART procedures. J Assist Reprod Genet. 2007;24(2–3):99–103. doi: 10.1007/s10815-006-9081-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ottosen LD, Hindkjaer J, Lindenberg S, Ingerslev HJ. Murine pre-embryo oxygen consumption and developmental competence. J Assist Reprod Genet. 2007;24(8):359–365. doi: 10.1007/s10815-007-9138-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Payne D, Flaherty SP, Barry MF, Matthews CD. Preliminary observations on polar body extrusion and pronuclear formation in human oocytes using time-lapse video cinematography. Hum Rep. 1997;12(3):532–541. doi: 10.1093/humrep/12.3.532. [DOI] [PubMed] [Google Scholar]
- 31.Pickering SJ, Taylor A, Johnson MH, Braude PR. An analysis of multinucleated blastomere formation in human embryos. Hum Rep. 1995;10(7):1912–1922. doi: 10.1093/oxfordjournals.humrep.a136206. [DOI] [PubMed] [Google Scholar]
- 32.Pribenszky C, Losonczi E, Molnar M, Lang Z, Matyas S, Rajczy K, Molnar K, Kovacs P, Nagy P, Conceicao J, Vajta G. Prediction of in-vitro developmental competence of early cleavage-stage mouse embryos with compact time-lapse equipment. Reprod Biomed Online. 2010;20(3):371–379. doi: 10.1016/j.rbmo.2009.12.007. [DOI] [PubMed] [Google Scholar]
- 33.Racowsky C, Vernon M, Mayer J, Ball G, Behr B, Pomeroy K, Wininger D, Gibbons W, Conaghan J, Stern J. Standardization of grading embryo morphology. J Assist Reprod Genet. 2010;8:437–439. doi: 10.1007/s10815-010-9443-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Schoolcraft WB, Katz-Jaffe MG, Stevens J, Rawlins M, Munne S. Preimplantation aneuploidy testing for infertile patients of advanced maternal age: a randomized prospective trial. Fertil Steril. 2009;92(1):157–162. doi: 10.1016/j.fertnstert.2008.05.029. [DOI] [PubMed] [Google Scholar]
- 35.Scott L, Berntsen J, Davies D, Gundersen J, Hill J, Ramsing N. Symposium: innovative techniques in human embryo viability assessment. Human oocyte respiration-rate measurement–potential to improve oocyte and embryo selection? Reprod Biomed Online. 2008;17(4):461–469. doi: 10.1016/S1472-6483(10)60232-5. [DOI] [PubMed] [Google Scholar]
- 36.Scott L, Finn A, O'Leary T, McLellan S, Hill J. Morphologic parameters of early cleavage-stage embryos that correlate with fetal development and delivery: prospective and applied data for increased pregnancy rates. Hum Rep. 2007;22(1):230–240. doi: 10.1093/humrep/del358. [DOI] [PubMed] [Google Scholar]
- 37.Seli E, Robert C, Sirard MA. OMICS in assisted reproduction: possibilities and pitfalls. Mol Hum Reprod. 2010;16(8):513–530. doi: 10.1093/molehr/gaq041. [DOI] [PubMed] [Google Scholar]
- 38.Staessen C, Platteau P, Assche E, Michiels A, Tournaye H, Camus M, Devroey P, Liebaers I, Steirteghem A. Comparison of blastocyst transfer with or without preimplantation genetic diagnosis for aneuploidy screening in couples with advanced maternal age: a prospective randomized controlled trial. Hum Rep. 2004;19(12):2849–2858. doi: 10.1093/humrep/deh536. [DOI] [PubMed] [Google Scholar]
- 39.Steer CV, Mills CL, Tan SL, Campbell S, Edwards RG. The cumulative embryo score: a predictive embryo scoring technique to select the optimal number of embryos to transfer in an in-vitro fertilization and embryo transfer programme. Hum Rep. 1992;7(1):117–119. doi: 10.1093/oxfordjournals.humrep.a137542. [DOI] [PubMed] [Google Scholar]
- 40.Takenaka M, Horiuchi T, Yanagimachi R. Effects of light on development of mammalian zygotes. Proc Natl Acad Sci U S A. 2007;104(36):14289–14293. doi: 10.1073/pnas.0706687104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Wong CC, Loewke KE, Bossert NL, Behr B, Jonge CJ, Baer TM, Reijo Pera RA. Non-invasive imaging of human embryos before embryonic genome activation predicts development to the blastocyst stage. Nat Biotechnol. 2010;28(10):1115–1121. doi: 10.1038/nbt.1686. [DOI] [PubMed] [Google Scholar]
- 42.Zhang JQ, Li XL, Peng Y, Guo X, Heng BC, Tong GQ. Reduction in exposure of human embryos outside the incubator enhances embryo quality and blastulation rate. Reprod Biomed Online. 2010;20(4):510–515. doi: 10.1016/j.rbmo.2009.12.027. [DOI] [PubMed] [Google Scholar]
- 43.Ziebe S, Petersen K, Lindenberg S, Andersen AG, Gabrielsen A, Andersen AN. Embryo morphology or cleavage stage: how to select the best embryos for transfer after in-vitro fertilization. Hum Rep. 1997;12(7):1545–1549. doi: 10.1093/humrep/12.7.1545. [DOI] [PubMed] [Google Scholar]