Abstract
Purpose
To assess the longevity of ovarian grafts in five cancer patients who underwent heterotopic autotransplantation of frozen-thawed ovarian tissue.
Method(s)
Five cancer survivors underwent heterotopic ovarian transplantation between 2001and 2011. Stored ovarian tissue (for 1–10 years) was rapidly thawed and transplanted into the space between the rectus sheath and the rectus muscle (8–20 cortical sections per patient). Endocrine function was assessed by monthly blood tests (FSH, LH, E2, progesterone and testosterone) and ultrasound after transplantation. The monitoring was continued until the cessation of endocrine function by consecutive blood tests (E2 < 20 pg/ml; FSH ≥ 35 IU/L).
Result(s)
Endocrine function was restored in all patients between 12–20 weeks after transplantation. Four patients required the second transplantation one to two years after the first transplantation. The duration of endocrine function after the second transplantation was much longer (9 months–84 months). The longest duration of endocrine function was seen in a woman who underwent ovarian transplantation in 2003 and 2004 after radiotherapy for cervical cancer. Even more than seven years after transplantation, endocrine function has not ceased (FSH 9.5, E2 108, on July 1, 2011). Of note, this patient underwent three IVF cycles in 2004 which resulted in four embryos.
Conclusion(s)
Long-term endocrine function lasting for seven years can be established with heterotopic transplantation of cryobanked human ovarian tissue. Re-establishment of long-term endocrine function after ovarian transplantation will benefit young cancer survivors with premature ovarian failure.
Keywords: Fertility preservation, Ovarian transplantation, Heterotopic transplantation, Endocrine function, Cancer, Cryopreservation, Ovarian tissue
Introduction
One of the promising and practical strategies for fertility preservation is cryopreservation of ovarian tissue followed by transplantation. Human ovarian transplantation is not a new technology, but rather has a long history dating back to 1895. The initial interest in human ovarian transplantation in the early 20th century waned gradually, as there were no good clinical indications for ovarian transplantation until the development of new technologies for assisted reproduction and cryopreservation. Almost 100 years after the first human ovarian transplantation by Robert Morris in New York [1], the interest in ovarian transplantation re-ignited in 1994 when restoration of fertility after grafting frozen-thawed ovarian tissue in sheep was demonstrated by Roger Gosden in Edinburgh [2].
Since then, ovarian tissue cryopreservation and transplantation has become one of the most important topics in reproductive medicine, and the feasibility of clinical applications of ovarian transplantation has been entertained (especially for fertility preservation in cancer patients). In 2004, the first live birth in a woman with Hodgkin’s lymphoma was reported after transplantation of frozen-thawed human ovarian tissue by Jacques Donnez in Belgium [3]. Although there was initial skepticism, it has been quickly replaced by enthusiasm as more reports of live births followed. To date, 18 healthy babies have been born after transplantation of frozen-thawed human ovarian tissue worldwide [4].
Even with remarkable advances in technology and rising enthusiasm for clinical applications, human ovarian transplantation is still considered as an investigational procedure, and will remain experimental until the efficacy and safety of this technology can be proven. Indeed, there are many uncertainties and unanswered questions related to human ovarian tissue transplantation. One of clinically relevant questions with this technology is “how long would the ovarian graft be expected to function?”
The longevity of ovarian grafts has always been debatable. It has been estimated that follicular loss after transplantation of frozen-thawed ovarian tissue is over 70 % [5]. Thus, in hypothesis, the duration of ovarian function after transplantation should be relatively short, but there are not sufficient clinical data to support or refute it. In the present study, we monitored endocrine function of five cancer patients after heterotopic ovarian transplantation (longitudinal follow-up from 2001 to 2011) to assess the longevity of ovarian grafts.
Materials and methods
Study patients
The study was approved by the institutional review board, and informed consent was obtained from each patient. We recruited five women with cancer for this study (three with cervical cancer, one with breast cancer, and one with Hodgkin’s lymphoma). Cryopreservation of ovarian tissue was done between 1999 and 2004. All frozen ovarian tissue was stored in liquid nitrogen until use. The age of patients at the time of ovarian cryopreservation was between 29–37 years old (Table 1). Ovarian transplantation was performed in five patients between 2001–2011 (one to 10 years after ovarian cryopreservation). The first patient who underwent ovarian transplantation had a relapse of cancer and deceased soon after transplantation, therefore, she was eliminated from a long-term follow up, although the return of ovarian function was confirmed 5 months after transplantation. Of remaining four patients, three patients desired fertility restoration with ovarian transplantation, but one patient was more interested in restoration of endocrine function.
Table 1.
Summary of characteristics & outcomes of heterotopic ovarian transplantation
| Age* (year) | Para | Diagnosis | Duration of storage before transplant (year) | Number of cortical sections transplanted: 1st/ 2nd | The year of transplantation: 1st/ 2nd | Duration of endocrine function: 1st/ 2nd (month) | |
|---|---|---|---|---|---|---|---|
| A | 37 | G2P2 | cervical cancer | 2 | 20/20 | 2002/2003 | 4/14 |
| B | 28 | G0P0 | cervical cancer | 2 | 9/8 | 2003/2004 | 6/84 |
| C | 29 | G0P0 | breast cancer | 5 | 11/8 | 2005/2006 | 5/9 |
| D | 30 | G0P0 | Hodgkin’s lymphoma | 10 | 10/8 | 2010/2011 | 9/ongoing |
Age* is at the time of ovarian tissue cryopreservation
Ovarian tissue harvest and cryopreservation
Before chemotherapy and/or radiotherapy, a whole ovary was removed by laparoscopic oophorectomy in three patients and by laparotomy in two patients (during surgery for cervical cancer). An ovarian biopsy specimen was sent to pathology to screen minimal residual disease in the harvested ovary. The collected ovary was transported to the laboratory in Leibovitz L-15 medium (Sigma, St. Louise, MO) in an hour at room temperature. Preparation and cryopreservation process have been described previously [6]. Briefly, the ovary was bisected and processed into thin slices of ovarian cortex (5 mm × 5 mm × 1 mm) after removing the medulla. Slices of ovarian cortex were equilibrated in 1.5M dimethyl sulfoxide (DMSO) with 0.1 M sucrose for 30 min at 25 °C. One to two slices of ovarian cortex were loaded in 1.8 ml cryogenic vials containing cryoprotectant, cooled in a programmable freezer per our slow freezing protocol (cooled at 2 °C/min to −7 °C, seeding at −7 °C, 0.3 °C/min to −40 °C, 10 °C/min to −80 °C), and stored in liquid nitrogen.
Transplantation of thawed ovarian tissue
Ovarian tissue had been stored for 1–10 years before the patient requested ovarian transplantation (Table 1). Three women with cervical cancer underwent ovarian transplantation approximately 2 years after cancer therapy (one recurred, two free of disease). A woman with breast cancer (stage II) who was treated with surgery followed by adjuvant chemotherapy underwent ovarian tissue transplantation after the completion of 5 years of tamoxifen treatment. A woman with Hodgkin’s lymphoma waited for 10 years before making a decision for ovarian transplantation.
Cryopreserved ovarian tissue in a vial was rapidly thawed (~100 °C/min) in a warm water bath for 2–3 min, and washed in a stepwise manner (1.0 M DMSO + 0.1 M sucrose, 0.5 M DMSO + 0.1 M sucrose, and then 0.1 M sucrose). Each washing step took 3 min at room temperature (25 °C). After washing, slices of ovarian cortex were incubated in a culture medium containing antibiotics and 100 μg/ml ascorbic acid for 30 min at 37 °C. To facilitate angiogenesis after grafting, gonadotropin (300 IU daily) was administered subcutaneously two days before and three days after transplantation in all patients.
Eight to 10 slices of thin ovarian cortex were threaded onto a 3-0 Vicryl suture (Ethicon, Edinburg, UK), and transplanted into the heterotopic site (between the rectus muscle and the rectus sheath) through a small skin incision (2 cm) (Fig. 1). All procedure required only conscious sedation with local anesthesia.
Fig. 1.
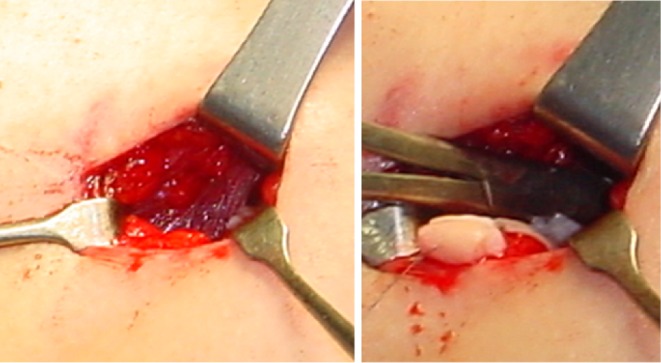
Transplantation of frozen-thawed ovarian tissue to the heterotopic site
Monitoring
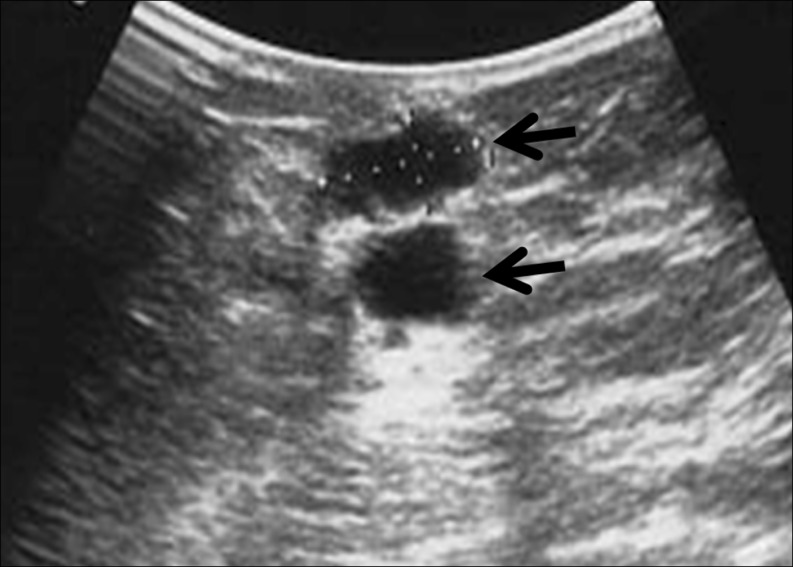
The return of endocrine function was monitored by monthly blood tests (FSH, LH, estradiol, progesterone, and testosterone) starting 4–8 weeks after transplantation. Serum hormones were measured by a solid phase chemiluminiscent assay. Once restoration of endocrine function was confirmed by hormonal profiles, the graft site was examined with ultrasound to monitor follicular development in addition to serial blood tests (Fig. 2). Many times, follicular growth was detected by palpation at the time of sonographic examination.
Fig. 2.
Ultrasound image of two growing follicles in the ovarian graft (between the skin and the rectus muscle)
Results
The serum hormone concentrations of all transplant patients were consistent with the postmenopausal level at the time of transplantation (FSH 80–110 IU/L; Estradiol <20 pg/ml). In addition, four patients complained of menopausal symptoms (especially hot flush) before transplantation which subsided with restoration of endocrine function from ovarian grafts. The age of patients at the time of ovarian tissue transplantation was between 30 and 40 (one to ten years after ovarian cryopreservation) (Table 1). Endocrine function was restored in all patients between 12–20 weeks after transplantation. We were able to conduct a long term follow-up in four patients with serial blood tests until a cessation of endocrine function (E2 < 20 pg/ml; FSH ≥ 35 IU/L). These four patients required the second transplantation one to two years after the first transplantation, because of the relatively short ovarian function (3–6 months) with the first transplantation (Table 1). It appears that restoration of endocrine function after the second transplantation was faster (only 2–4 months). Most of all, establishment of long term endocrine function was noticed after the second transplantation (lasting for 9–84 months) (Table 1). The longest duration of endocrine function was seen in a 28 year old woman who underwent ovarian transplantation in 2003 and 2004 after radiotherapy for cervical cancer. After radiation therapy, the complete cessation of menstruation with high serum FSH levels (above 90 IU/L) was noted. The FSH level at the time of second transplantation was 84.7 IU/L. Nine weeks after transplantation, the FSH level deceased to 11 IU/L and stayed below 20 IU/L. Even seven years after transplantation, endocrine function of the grafts has not ceased (FSH 9.5 IU/L, E2 108 pg/ml, AMH 0.2 ng/ml on July 1, 2011). In my knowledge, this is the longest duration of ovarian function reported in the literature after heterotopic autotransplantation of frozen-thawed human ovarian tissue. Of note, this patient also underwent three IVF cycles in 2004 which resulted in four embryos.
Discussion
Currently, more than 11 million individuals in the United States are living with cancer, and approximately 450,000 cancer survivors are of reproductive age. Furthermore, 4 % to 5 % of newly diagnosed cancer patients are younger than 35 years [7]. These epidemiologic data reflect the importance of fertility preservation, as most women of reproductive age wish to preserve fertility. Although there are several strategies to preserve fertility including embryo, oocyte and ovarian tissue cryopreservation, most of them are still considered experimental except embryo cryopreservation [8]. However, cryopreservation of ovarian tissue has unique advantages over other strategies: 1) it does not delay cancer treatment as it can be scheduled immediately, 2) it is safer for hormone dependent malignancy as hundreds of immature oocytes can be cryopreserved without the necessity for ovarian stimulation, 3) it can be done independent of menstrual cycles, 4) it is the only option for pre-pubertal girls, 5) it can restore not only fertility but endocrine function of the ovary after transplantation.
Ovarian tissue can be transplanted either orthotopically or heterotopically. To date, 18 babies have been born after orthotopic autotransplantation of frozen-thawed human ovarian tissue [4], but none with heterotopic autotransplantation. Since there is no pregnancy with heterotopic ovarian transplantation, clinical value of this technology has been questioned. It is, however, too early to discredit the value of heterotopic ovarian transplantation. The advantages of transplanting ovarian tissue to the heterotopic site include: 1) it can avoid invasive procedures, 2) it can make the recovery of oocytes easy, 3) it can be a cost effective technology when repeated transplantation is required, 4) it can be done even with severe pelvic adhesions that preclude orthotopic transplantation [9].
Transplantation of ovarian tissue has been tried to various heterotopic sites including subcutaneous tissue of the abdomen [10], forearm [11], breast tissue [12], rectus muscle, [6, 12], and subperitoneal tissue [13, 14]. Heterotopic sites may not provide an optimal environment for follicular development possibly due to differences with temperature, pressure, paracrine factors, and blood supplies. Nevertheless, restoration of endocrine function has been demonstrated consistently after heterotopic ovarian transplantation [6, 11–15]. In the present study, all five patients restored endocrine function 3 to 5 months after transplanting frozen-thawed ovarian tissue to the space between the rectus muscle and the rectus sheath. In my view, this particular site is vascular enough to facilitate angiogenesis and to minimize ischemic damage. Indeed, effective and timely angiogenesis after transplantation is critical for the survival of ovarian grafts. In addition, heterotopic transplantation to the space between the rectus muscle and the rectus sheath is more cost effective and less invasive, as it can be done under conscious sedation and/or local anesthesia without entering the peritoneal cavity, which will benefit the patients who may require multiple repeated transplantations to maintain long term endocrine function to restore fertility.
Our present study showed that long term endocrine function lasting for more than 7 years can be established with heterotopic transplantation of frozen-thawed human ovarian tissue in cancer patients. The duration of ovarian function after transplantation will be determined by the total store of follicles in grafted ovarian tissue. The majority of follicles stored in ovarian cortex are primordial follicles that are more resistant to cryoinjury. It is not uncommon to see that more than 70 % of primordial follicles survive after freezing and thawing of human ovarian tissue. However, a significant follicular loss has been observed in grafted ovarian tissue, mainly caused by tissue ischemia after transplantation while waiting for angiogenesis. It is, thus, very important to prevent ischemic tissue damage after transplantation to minimize the follicular loss and to extend the duration of ovarian function. Although there is no robust method to prevent ischemic tissue damage after transplantation, exogenous gonadotropin has been used to stimulate angiogensis [16, 17] and pre-treatment of ovarian tissue with antioxidant (ascorbic acid) before transplantation could be considered to minimize apoptosis based on our previous study [18].
The longevity of grafted ovarian tissue has been debated for many years, and it is still uncertain how long endocrine function of frozen-thawed ovarian tissue can be maintained. Several factors can affect the longevity of ovarian grafts which include 1) the age of the patient at the time of cryopreservation, 2) the baseline ovarian reserve, 3) the history of cancer treatment, 4) the method of ovarian tissue preparation, 5) freezing-thawing techniques, 6) the number of cortical sections grafted, 7) transplantation techniques and graft sites, 8) the degree of ischemia after transplantation, 9) the number of follicles survived in ovarian grafts. The longevity of ovarian grafts is expected to be relatively short because of a significant follicular loss with cryopreservation and transplantation. Our results showed that there are individual variations in the duration of endocrine function after transplantation. Nevertheless, it appears that the life span of ovarian grafts is longer than expected. It has been reported that ovarian function can last for 7 years after transplanting fresh ovarian tissue and for 5 years after transplanting frozen-thawed ovarian tissue in humans [19]. The present study demonstrated that endocrine function can last more than 7 years after transplanting frozen-thawed ovarian tissue to the heterotopic site. In my knowledge, this is the longest duration of ovarian function reported after autotransplantation of frozen-thawed ovarian tissue in humans. This information is valuable for future clinical application of ovarian transplantation, especially when counseling and making a treatment plan for cancer survivors with premature ovarian failure.
Footnotes
Supported by Serono IMG grant
Capsule
Heterotopic transplantation of cryobanked human ovarian tissue can restore long-term endocrine function (up to 7 years), which will benefit young cancer survivors with premature ovarian failure.
References
- 1.Morris RT. The Ovarian Graft. New York Medical Journal 1895;62.
- 2.Gosden RG, Baird DT, Wade JC, Webb R. Restoration of fertility to ooporectomized sheep by ovarian autografts stored at −196 degrees C. Hum Reprod. 1994;9:597–603. doi: 10.1093/oxfordjournals.humrep.a138556. [DOI] [PubMed] [Google Scholar]
- 3.Donnez J, Dolmans MM, Demylle D, Jadoul P, Pirard C, Squifflet J, et al. Livebirth after orthotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;364:1405–1410. doi: 10.1016/S0140-6736(04)17222-X. [DOI] [PubMed] [Google Scholar]
- 4.Dittrich R, Lotz L, Keck G, Hoffmann I, Mueller A, Beckmann MW, et al. Live birth after ovarian tissue autotransplantation following overnight transportation before cryopreservation. Fertil Steril. 2012;97:387–390. doi: 10.1016/j.fertnstert.2011.11.047. [DOI] [PubMed] [Google Scholar]
- 5.Baird DT, Webb R, Campbell BK, Harkness LM, Gosden RG. Longterm ovarian function in sheep after ovariectomy and transplantation of autografts stored at −196 C. Endocrinology. 1999;140:462–471. doi: 10.1210/en.140.1.462. [DOI] [PubMed] [Google Scholar]
- 6.Kim SS, Lee WS, Chung MK, Lee HC, Lee HH, Hill D. Long-term ovarian function and fertility after heterotopic autotransplantation of cryobanked human ovarian tissue: 8-year experience in cancer patients. Fertil Steril. 2009;91:2349–2354. doi: 10.1016/j.fertnstert.2008.04.019. [DOI] [PubMed] [Google Scholar]
- 7.Knapp CA, Quinn GP. Healthcare provider perspectives on fertility preservation for cancer patients. Cancer Treat Res. 2010;156:391–401. doi: 10.1007/978-1-4419-6518-9_30. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Kim SS. Fertility preservation in female cancer patients: current developments and future directions. Fertil Steril. 2006;85:1–11. doi: 10.1016/j.fertnstert.2005.04.071. [DOI] [PubMed] [Google Scholar]
- 9.Kim SS. General overview of ovarian cryobanking. In: Donnez J, Kim SS, editors. Principles and practice of fertility preservation. Cambridge, UK; New York: Cambridge University Press; 2011. pp. 328–341. [Google Scholar]
- 10.Callejo J, Salvador C, Miralles A, Vilaseca S, Lailla JM, Balasch J. Long-term ovarian function evaluation after autografting by implantation with fresh and frozen-thawed human ovarian tissue. J Clin Endocrinol Metab. 2001;86:4489–4494. doi: 10.1210/jc.86.9.4489. [DOI] [PubMed] [Google Scholar]
- 11.Oktay K, Economos K, Kan M, Rucinski J, Veeck L, Rosenwaks Z. Endocrine function and oocyte retrieval after autologous transplantation of ovarian cortical strips to the forearm. Jama. 2001;286:1490–1493. doi: 10.1001/jama.286.12.1490. [DOI] [PubMed] [Google Scholar]
- 12.Kim SS, Hwang IT, Lee HC. Heterotopic autotransplantation of cryobanked human ovarian tissue as a strategy to restore ovarian function. Fertil Steril. 2004;82:930–932. doi: 10.1016/j.fertnstert.2004.02.137. [DOI] [PubMed] [Google Scholar]
- 13.Rosendahl M, Loft A, Byskov AG, Ziebe S, Schmidt KT, Andersen AN, et al. Biochemical pregnancy after fertilization of an oocyte aspirated from a heterotopic autotransplant of cryopreserved ovarian tissue: case report. Hum Reprod. 2006;21:2006–2009. doi: 10.1093/humrep/del140. [DOI] [PubMed] [Google Scholar]
- 14.Stern CJ, Toledo MG, Hale LG, Gook DA, Edgar DH. The first Australian experience of heterotopic grafting of cryopreserved ovarian tissue: evidence of establishment of normal ovarian function. Aust N Z J Obstet Gynaecol. 2011;51:268–275. doi: 10.1111/j.1479-828X.2011.01289.x. [DOI] [PubMed] [Google Scholar]
- 15.Oktay K, Buyuk E, Veeck L, Zaninovic N, Xu K, Takeuchi T, et al. Embryo development after heterotopic transplantation of cryopreserved ovarian tissue. Lancet. 2004;363:837–840. doi: 10.1016/S0140-6736(04)15728-0. [DOI] [PubMed] [Google Scholar]
- 16.Imthurn B, Cox SL, Jenkin G, Trounson AO, Shaw JM. Gonadotrophin administration can benefit ovarian tissue grafted to the body wall: implications for human ovarian grafting. Mol Cell Endocrinol. 2000;163:141–146. doi: 10.1016/S0303-7207(00)00218-5. [DOI] [PubMed] [Google Scholar]
- 17.Yang H, Lee HH, Lee HC, Ko DS, Kim SS. Assessment of vascular endothelial growth factor expression and apoptosis in the ovarian graft: can exogenous gonadotropin promote angiogenesis after ovarian transplantation? Fertil Steril. 2008;90:1550–1558. doi: 10.1016/j.fertnstert.2007.08.086. [DOI] [PubMed] [Google Scholar]
- 18.Kim SS, Yang HW, Kang HG, Lee HH, Lee HC, Ko DS, et al. Quantitative assessment of ischemic tissue damage in ovarian cortical tissue with or without antioxidant (ascorbic acid) treatment. Fertil Steril. 2004;82:679–685. doi: 10.1016/j.fertnstert.2004.05.022. [DOI] [PubMed] [Google Scholar]
- 19.Silber SJ. Ovary cryopreservation and transplantation for fertility preservation. Mol Hum Reprod. 2012;18:59–67. doi: 10.1093/molehr/gar082. [DOI] [PubMed] [Google Scholar]