Abstract
Purpose
This study aims to determine if in-vitro maturation (IVM) of human immature oocytes should be performed before or after vitrification.
Methods
A total of 184 immature oocytes were randomly divided into two different groups: 100 were vitrified at metaphase II (MII) stage 24 h-48 h after IVM (group 1) and 84 were immediately vitrified at germinal vesicle (GV) or metaphase I (MI) stages and in vitro matured after warming (group 2).
Results
Survival rate after warming was similar in both groups (86.9% versus 84.5%). However, oocyte maturation rate per collected oocyte was significantly higher for oocytes matured before vitrification (group 1, 46%) than for oocytes vitrified before IVM (group 2, 23.8%) (p < 0.01). Consequently, the number of MII oocytes inseminated per oocyte collected was significantly higher for group 1 (40%) than for group 2 (23.8%) (p < 0.05).
Conclusion
IVM procedure is more efficient when it is performed before oocyte vitrification.
Keywords: Human oocyte vitrification, In vitro maturation rate, Survival rate, Fertilization and development rates
Introduction
The development of an effective cryopreservation program for immature oocytes may have a major impact on in vitro fertilization (IVF) clinical practice, especially to preserve the fertility of women who will be treated with cytotoxic drugs [1,2] or for women with polycystic ovarian syndrome (PCOS) at risk of ovarian hyper-stimulation syndrome (OHSS) [3–5]. Moreover, for cancer patients, transplantation of cryopreserved ovarian tissue is not always conceivable because of the risk of retransmission of the disease due to the presence of neoplasic cells in the tissue. In these cases, combining IVM and vitrifcation offers a novel approach for fertility preservation, especially when the risk and the delay due to classical gonadotrophin stimulation before collecting mature oocytes is not indicated, as in advanced breast cancer patients [6].
Healthy infants have been born following IVM [7–9]. However, improving the IVM success rate remains an important challenge to obtain better efficiency for fertility preservation. Moreover, vitrification is a widely applied and highly successful approach for cryopreservation in reproductive biology, including storage of human oocytes [10–12]. Recently, studies reported high morphological survival rates after vitrification of mature oocytes, with in vitro embryo development, implantation and pregnancy rates comparable to those achieved with fresh oocytes [13–15]. The timing to vitrify the oocytes for long-term storage may however significantly affect the efficiency of the IVM process. Importantly, the maturation stage at which oocytes are vitrified may impact the efficiency of the IVM process. We previously showed that there was no significant difference in survival rates between oocytes vitrified at GV and oocytes vitrified at MI (86.3% vs 76.5%, respectively). Oocytes vitrified at GV stage gave, however, significantly worse results of in vitro maturation (6.8%) than the oocytes vitrified at MI stage (43.2%) (p < 0.01) [16].
In the present study, we assessed the efficiency of IVM before and after vitrification of human immature oocytes.
Materials and methods
Experimental design and patients
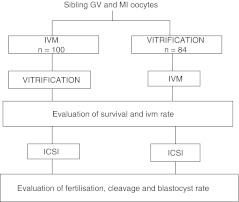
Between May 2009 and February 2010, a total of 184 immature oocytes at the GV or MI stages were obtained from 83 patients who underwent an intracytoplasmic sperm injection (ICSI) cycle. All MII oocytes retrieved were used for patients’ treatments, while immature oocytes, according to a previously prepared randomization list established on a weekly basis, were randomly allocated to one of the groups and vitrified after in-vitro maturation (group 1) or immediately (group 2) (Fig. 1).
Fig. 1.
Experimental design used to test the efficiency of vitrification before or after in vitro maturation (IVM) of oocytes collected at germinal vesicle (GV) and metaphase I (MI) stages
All patients included were thoroughly informed and signed written consent forms. The study was accepted by the ULB University Ethical Committee, by the Erasme Hospital Ethical Committee and by the Belgian Federal Research and Scientific Committee.
Stimulation protocol and oocyte retrieval procedures
Pituitary down regulation was initiated with either Gonadotrophin-releasing hormone (GnRH) agonist (Buserelin acetate: Suprefact® spray; Hoechst Inc., Germany or Triptorelin: Decapeptyl®; Ipsen, France) or GnRH antagonist (Cetrorelix: Cetrotide®, Merck-Serono, Geneva, Switzerland) administration. Urinary gonadotrophins (hMG) (Menopu®r, Organon Inc., The Netherlands) or recombinant follicle-stimulating hormone (FSH) (Gonal F®, Merck-Serono, Geneva, Switzerland) were added to stimulate the ovary until at least 3 follicles reached a diameter of 17 mm. Ovulation was induced by the administration of urinary human chorionic gonadotrophin (hCG) (Pregnyl®, Organon Inc., The Netherlands) or recombinant hCG (Ovitrelle®, Merck-Serono, Geneva, Switzerland). Oocyte retrieval was performed through vaginal puncture under ultrasound guidance 36 h later. Oocyte collection and laboratory procedures are described elsewhere [17]. After the oocyte collection, the oocyte-cumulus complexes were denuded using finely drawn pipettes (170–130 μm) following 1 min of exposure to 80 IU/ml hyaluronidase solution (Sigma Aldrich SrL, UK.). The MII oocytes obtained after ovarian stimulation were used for clinical ICSI cycles, and the GV and MI stage oocytes available as discarded material for research were used for this study.
Oocyte vitrification
Oocytes were vitrified in the medium 199 (M-199) based solutions according to the manufacturer’s instructions of the vitrification Vit Kit®-Freeze (Irvine Scientific, California). First equilibration step was performed in 7.5% ethylene glycol (EG) and 7.5% dimethyl sulphoxide (DMSO) solution at room temperature for 7 min before transferring the oocyte in the vitrification solution containing 15% EG, 15% DMSO and 0.5 mol/l sucrose for 30 s. The oocytes were then loaded on in vitrification high security straws (VHS, CryoBioSystem, France) in a volume of <1 μl, and immediately submerged into liquid nitrogen.
During warming, the cryoprotectants were removed according to the manufacturer’s instructions of the devitrification kit Vit Kit®-Thaw (Irvine Scientific, California) by sequentially washing the oocytes in four different M-199 based solution containing respectively 1.0 M (mol/l) sucrose (1 min at 37°C), 0.5 mol/l sucrose (4 min at room temperature) and M-199 alone (6 min at room temperature).
After warming, all the oocytes were cultured at 37°C in a humidified atmosphere of 5%CO2-5%O2-90%N2 in the fertilization medium (Cook, Australia) and checked after 1 h for survival. Post-warming survival rate was assessed using morphological criteria, indicated by absence of overt cell degeneration, elongated shape, thick or distorted zona, expanded perivitelline space and dark pronounced cytoplasm. Subsequent oocytes competence was assessed using their ability to mature in vitro and to be fertilized.
In vitro maturation, IVF and embryo culture
Oocytes were placed in a commercial IVM medium (Sage, Copper Surgical, USA) supplemented with 0.075 IU/ml FSH and 0.075 IU/ml LH according to the manufacturer’s instructions for 24-48 h.
Matured oocytes were inseminated by ICSI using donor sperm. Oocytes were individually cultured in micro-drops of fertilization medium under paraffin mineral oil (Cook, Australia) at 37°C in 5%CO2. Fertilization was assessed 16-18 h after ICSI and success measured by the appearance of two pronuclei and two polar bodies. Embryos were cultured for 3 days in cleavage medium (Cook, Australia) and the quality was evaluated daily by scoring fragmentation, number and appearance of blastomeres. Embryos were cultured in blastocyst medium (Cook, Australia) until day 5 to evaluate their developmental stage.
Statistical analysis
Statistical analyses were performed using Chi-square or Non parametric Mann–Whitney Test as appropriate. Values p < 0.05 indicated statistical significance.
Results
100 oocytes (68 GV and 32 MI) were included in vitro matured before vitrification group (group 1), while 84 oocytes were included in the vitrified before IVM group (52 GV and 32 MI) (group 2). The proportion of GV and MI stages is similar between groups.
Survival rate after warming, IVM, fertilization rate after ICSI and developmental rate of vitrified oocytes were assessed (Table 1). Survival rate after warming was similar in both groups (86.9% versus 84.5% for groups 1 and 2, respectively) and between GV and MI stage (84.2% versus 86.6% for GV and MI, respectively). In group 1, of the 46/100 MII obtained, 26/32 were from MI and 20/68 were from GV stage; in group 2, of the 20/71 MII obtained, 20/29 were from MI and 0/42 GV stage. These results confirm our previous study [16] providing evidence that survival rate is similar between GV and MI stage and that MI stage mature better that GV stage (p < 0.001). However, oocyte maturation rate per collected oocyte was significantly higher for oocytes matured before vitrification (group 1, 46%) than for oocytes vitrified before IVM (group 2, 23.8%) (p < 0.01). Fertilization rate per injected and per included oocyte (52.5% and 21% for group 1 and 45% and 11% for the group 2, respectively) as well as cleavage rate per fertilised and per injected oocyte (52.4% and 27.5% for group 1 versus 55.5% and 25% for the group 2, respectively) were similar for both groups. Around half of the embryos reached four cells stages in both groups, but no blastocysts were obtained. In conclusion, the study shows that the IVM procedure is more efficient when it is performed before oocyte vitrification.
Table 1.
Survival and in vitro maturation (IVM) rate, fertilization and cleavage rate of vitrified oocytes after or before in vitro-maturation
| IVM before vitrification (group 1) | IVM after vitrification (group 2) | |
|---|---|---|
| Oocytes included (n) | 100 (68 GV and 32 MI) | 84 (52 GV and 32MI) |
| Oocytes vitrified (n) | 46 | 84 |
| Oocytes survived (n) | 40/46 (86.9%) | 71/84 (84.5%) |
| In vitro matured oocytes/oocytes included | 46/100 (46%)* | 20/84 (23.8%)* |
| Mature oocytes inseminated by ICSI/ oocytes included | 40/100 (40%)** | 20/84 (23.8%)** |
| Fertilization rate/oocytes injected | 21/40 (52.5%) | 9/20 (45%) |
| Fertilization rate/oocytes included | 21/100 (21%) | 9/84 (11%) |
| Cleaved embryos/oocytes fertilised | 11/21 (52.4%) | 5/9 (55.5%) |
| Cleaved embryos/oocytes injected | 11/40 (27.5%) | 5/20 (25%) |
| Developed blastocysts | 0 | 0 |
*p<0.01; **p<0.05
Discussion
Oocyte maturation is a lengthy process, during which the oocyte acquires the competence to be fertilized and to undergo embryogenesis. Significant progress has been made to improve pregnancy and implantation rates with in-vitro matured oocytes [18], but the mechanisms regulating early folliculogenesis and oocyte maturation in the human are still poorly understood [19]. Further research remains necessary to address the mechanism of oocyte maturation in order to refine culture conditions and improve the implantation rate of in vitro matured oocytes. Many factors affecting in vitro maturation rate have been reported. It has been demonstrated that priming of ovarian immature oocytes with follicle-stimulating hormone or human chorionic gonadotrophin prior to immature oocyte retrieval improves oocyte maturation rates and embryo quality as well as pregnancy rates in infertile women with polycystic ovaries or polycystic ovary syndrome [20,21]. The size of the follicles may also be important for the subsequent embryonic development, while the developmental competence of oocytes derived from the small antral follicles is not adversely affected by the presence of a dominant follicle [22]. Finally, in vitro oocyte maturation is affected by the presence of cumulus cells, the composition of IVM media and the culture conditions [23–25]. Despite the fact that implantation rate per transferred embryo using in vitro maturated oocyte doesn’t yet reach results obtained after classical IVF [26], this technique is already offered to patients at high risk of developing hyperstimulation syndrome or for fertility preservation.
On the other hand, vitrification has strongly improved the efficiency of oocytes cryopreservation technique and the survival rate is similar whatever the oocytes maturation stage (GV or MII) [4]. However, cryopreserving immature oocytes required in vitro maturation after warming. As shown in previous studies, the cryopreservation procedures may have detrimental effects on the maturation capacity [27,28]. Developmental failure of cryopreserved oocytes is related to critical disturbances of various cell components, such as the chromosome segregation apparatus, the intracellular calcium signalling system, and the cytoskeleton [29]. Cao et al. confirmed these findings reporting that, despite the spectacular improvement of both survival rate and oocyte ultrastructure at warming after vitrification, oocyte maturation rates are significantly reduced when oocytes were vitrified at immature GV stage followed by IVM (50.8%) in comparison with the group first in vitro matured and then vitrified (70.4%) [30]. After ICSI, there was no difference in the fertilization (62.1% versus 58.8%), cleavage (69.5% versus 67.5%) and blastocyst development rates between these two groups. In this study, immature oocytes were obtained from women with PCOS who underwent IVM treatment. The patients were primed with clomiphene citrate and hMG starting on day 3 of their menstrual cycle. When the size of the leading follicles in the ovaries reached 8–10 mm in diameter as observed by ultrasound scan, hCG was given and retrieval immature oocytes was performed 36 h later. The different starting material may account for the better IVM rate obtained in this study. Our study has been performed with a less optimal material (failed-matured oocytes) and results are not comparable as those obtained in PCOS patients. Recently, Asimakopoulos et al. [31] reported the efficiency of vitrified immature human oocytes before undergoing in vitro maturation, fertilization, and embryo development. For two women (34 and 36 years old) undergoing IVF cycle, immature oocytes were vitrified. The patients were treated using a gonadotrophin agonist protocol with hMG as ovarian stimulation. Final maturation was induced with hCG. In the first case, 17 oocytes were retrieved: six at MII, six at GV stage, and five with multiple vacuoles or abnormal shape. ICSI was performed with the six MII oocytes and the GV oocytes were vitrified. One week later, the GV oocytes were successfully thawed and placed in IVM media. Four oocytes reached metaphase II after 22 h, one oocyte after 38 h, and the sixth oocyte after 48 h. Meanwhile, the woman became pregnant from the transfer of the three fresh embryos; therefore, the embryos produced by GV oocytes remain in storage. In the second case, only three oocytes were retrieved: one at MI and two at GV stage and they were vitrified. Two week later, the oocytes were thawed; two of them survived (one MI and one GV) and were placed in IVM media. After 23 h, both oocytes reached MII stage and were fertilized by ICSI. At that time, the woman was not prepared for embryo transfer and the embryos were vitrified. Two months later, both embryos were successfully thawed and transferred. There was no pregnancy. These cases demonstrate that vitrified immature oocytes can undergo post-thaw in vitro maturation and fertilization and that the produced embryos are capable to undergo vitrification and thawing. The authors however did not compare the efficiency of this strategy with the vitrification of in vitro matured oocytes. On the other hand, Zhang et al. [32] suggested that, as cryopreservation method, vitrification of immature oocytes better preserves the microtubule organization and reduces the cytoskeletal spindle damage. In another recent study, Versieren et al. [33] reported that cryopreserved GV oocytes showed decreased and delayed maturation after warming but activation potential was not affected. In this study, oocytes at different developmental stages of maturation (GV, MI, or MII) and oocytes that failed to fertilize after IVF or ICSI were cryopreserved by slow freezing. Warmed oocytes were artificially activated to verify activation potential and compared with oocytes that were not cryopreserved. They concluded that immature oocytes should be cryopreserved after in vitro maturation. Here, we confirmed that the number of matured oocytes available for IVF is higher when vitrification is performed before IVM than after IVM. However, fertilization and cleavage rates are similar whether the oocytes are vitrified before or after IVM. Furthermore, we showed that the vitrification procedure has a negative impact on oocyte maturation competence after warming. Whatever the timing, MI oocytes are more competent to reach MII stage than GV oocytes, suggesting that the first step of maturation is critical and particularly affected by vitrification. The poor developmental competence to blastocyst stage of the matured oocytes suggests that vitrification affects the oocyte at both GV and MI stage compared to MII stage when it is combined with IVM. Nevertheless, discarded oocytes, failed-matured in vivo coming from stimulated patients in ICSI cycle, were used for this study. Despite the fact that these denuded oocytes are not fully developmentally competent, they constitute a very interesting and useful source of human material, limiting the randomization of patients’ oocytes for studies. As suggested by several authors, immature oocytes from stimulated cycles can be matured in vitro creating a potential source of human oocytes for experimental research despite their inferior quality [34,35]. Additional studies are required, however, to confirm our results, to improve the oocyte developmental competence after IVM and vitrification procedures and to explore new methods in human oocyte cryopreservation techniques.
In conclusion, immature oocytes should be vitrified at the MII stage following IVM because oocyte maturation rates were significantly reduced when oocytes were vitrified at immature stage followed by IVM. Vitrification of in-vitro matured oocytes may be an important additional option to preserve fertility for many patients especially women who will be treated with cytotoxic drugs or women at risk of hyper-stimulation ovarian syndrome.
Acknowledgements
The study was supported by an unconditional grant from the Fonds National Recherche Scientifique (FNRS) of Belgium, and an unconditional grant from Merck Pharmaceuticals.
Footnotes
Capsule
The adequate timing for in vitro maturation was evaluated by comparing maturation and survival rates of oocyte vitrified before or after the procedure.
References
- 1.Huang JY, Chian RC, Gilbert L, Fleiszer D, Holzer H, Dermitas E, Elizur SE, Gidoni Y, Levin D, Son WY, Tan SL. Retrieval of immature oocytes from unstimulated ovaries followed by in vitro maturation and vitrification: a novel strategy of fertility preservation for breast cancer patients. Am J Surg. 2010;200:177–183. doi: 10.1016/j.amjsurg.2009.04.004. [DOI] [PubMed] [Google Scholar]
- 2.Isachenko E, Rahimi G, Isachenko V, Nawroth F. In-vitro maturation of germinal-vesicle oocytes and cryopreservation in metaphase I/II: a possible additional option to preserve fertility during ovarian tissue cryopreservation. Reprod Biomed Online. 2004;8:553–557. doi: 10.1016/S1472-6483(10)61102-9. [DOI] [PubMed] [Google Scholar]
- 3.Chian RC, Lim JH, Tan SL. State of the art in in-vitro oocyte maturation. Curr Opin Ostet Gynecol. 2004;6:211–219. doi: 10.1097/00001703-200406000-00003. [DOI] [PubMed] [Google Scholar]
- 4.Cao Y, Chian RC. Fertility preservation with immature and in vitro matured oocytes. Semin Reprod Med. 2009;27:456–464. doi: 10.1055/s-0029-1241055. [DOI] [PubMed] [Google Scholar]
- 5.Wei Z, Cao Y, Cong L, Zhou P, Zhang Z, Li J. Effect of metformin pre-treatment on pregnancy outcome of in vitro matured oocytes retrieved from women with polycystic ovary syndrome. Fertil Steril. 2008;90:1149–1154. doi: 10.1016/j.fertnstert.2007.07.1385. [DOI] [PubMed] [Google Scholar]
- 6.Demeestere I, Simon P, Emiliani S, Delbaere A, Englert Y. Orthotopic and heterotopic ovarian tissue transplantation. Human Reprod Update. 2009;15:649–665. doi: 10.1093/humupd/dmp021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Jurema MW, Nogueira D. In vitro maturation of human oocytes for assisted reproduction. Fertil Steril. 2006;86:1277–1291. doi: 10.1016/j.fertnstert.2006.02.126. [DOI] [PubMed] [Google Scholar]
- 8.Edwards RG. IVF, IVM, natural cycle IVF, minimal stimulation IVF—time for a rethink. Reprod Biomed Online. 2007;15:106–119. doi: 10.1016/S1472-6483(10)60699-2. [DOI] [PubMed] [Google Scholar]
- 9.Benkhalifa M, Demirol A, Ménézo Y, Balashova E, Abduljlil AK, Giakoumakis I, Gurgan T. Natural cycle IVF and oocyte in-vitro maturation in polycystic ovary syndrome: a collaborative prospective study. Reprod Biomed Online. 2009;18:29–36. doi: 10.1016/S1472-6483(10)60421-X. [DOI] [PubMed] [Google Scholar]
- 10.Oktay K, Cil AP, Bang H. Efficiency of oocyte cryopreservation: a meta-analysis. Fertil Steril. 2006;86:70–80. doi: 10.1016/j.fertnstert.2006.03.017. [DOI] [PubMed] [Google Scholar]
- 11.Kuwayama M, Vajta G, Kato O, Leibo SP. Highly efficient vitrification method for cryopreservation of human oocytes. Reprod Biomed Online. 2005;11:300–308. doi: 10.1016/S1472-6483(10)60837-1. [DOI] [PubMed] [Google Scholar]
- 12.Chian RC, Huang JY, Tan SL, Lucena E, Saa A, Rojas A, Ruvalcaba Castellón LA, García Amador MI, Montoya Sarmiento JE. Obstetric and perinatal outcome in 200 infants conceived from vitrified oocytes. Reprod Biomed Online. 2008;16:608–610. doi: 10.1016/S1472-6483(10)60471-3. [DOI] [PubMed] [Google Scholar]
- 13.Cobo A, Vajta G, Remohí J. Vitrification of human mature oocytes in clinical practice. Reprod Biomed Online. 2009;19(Suppl 4):4385. [PubMed] [Google Scholar]
- 14.Nagy ZP, Chang CC, Shapiro DB, Bernal DP, Kort HI, Vajta G. The efficacy and safety of human oocyte vitrification. Semin Reprod Med. 2009;27:450–455. doi: 10.1055/s-0029-1241054. [DOI] [PubMed] [Google Scholar]
- 15.Tao T, Zhang W, Valle A. Human oocyte cryopreservation. Curr Opin Obstet Gynecol. 2009;21:247–252. doi: 10.1097/GCO.0b013e328329c2d2. [DOI] [PubMed] [Google Scholar]
- 16.Fasano G, Vannin AS, Biramane J, Delbaere A, Englert Y. Cryopreservation of human failed maturation oocytes shows that vitrification gives superior outcomes to slow cooling. Cryobiology. 2010;61:243–247. doi: 10.1016/j.cryobiol.2010.08.002. [DOI] [PubMed] [Google Scholar]
- 17.Devreker F, Pogonici E, Maertelaer V, Revelard P, Bergh M, Englert Y. Selection of good embryos for transfer depends on embryo cohort size: implications for the ‘mild ovarian stimulation’ debate. Human Reprod. 1999;14:3002–3008. doi: 10.1093/humrep/14.12.3002. [DOI] [PubMed] [Google Scholar]
- 18.Chian RC, Gilbert L, Huang JY, Demirtas E, Holzer H, Benjamin A, Buckett WM, Tulandi T, Tan SL. Live birth after vitrification of in vitro matured human oocytes. Fertil Steril. 2009;91:372–376. doi: 10.1016/j.fertnstert.2007.11.088. [DOI] [PubMed] [Google Scholar]
- 19.Gougeon A. Human ovarian follicular development: from activation of resting follicles to preovulatory maturation. Ann Endocrinol. 2010;71:132–143. doi: 10.1016/j.ando.2010.02.021. [DOI] [PubMed] [Google Scholar]
- 20.Son W, Chung JT, Chian RC, Herrero B, Demirtas E, Elizur S, Gidoni Y, Sylvestre C, Dean N, Tan SL. A 38 h interval between hCG priming and oocyte retrieval increases in vivo and in vitro oocyte maturation rate in programmed IVM cycles. Human Reprod. 2008;23:2010–2016. doi: 10.1093/humrep/den210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Son WY, Tan SL. Laboratory and embryological aspects of hCG-primed in vitro maturation cycles for patients with polycystic ovaries. Human Reprod Update. 2010;16:675–689. doi: 10.1093/humupd/dmq014. [DOI] [PubMed] [Google Scholar]
- 22.Chian RC. In-vitro maturation of immature oocytes for infertile women with PCOS. Reprod Biomed Online. 2004;8:547–552. doi: 10.1016/S1472-6483(10)61101-7. [DOI] [PubMed] [Google Scholar]
- 23.Papanikolaou EG, Platteau P, Albano C, Nogueira D, Cortvrindt R, Devroey P, Smitz J. Immature oocyte in-vitro maturation: clinical aspects. Reprod Biomed Online. 2005;10:587–592. doi: 10.1016/S1472-6483(10)61665-3. [DOI] [PubMed] [Google Scholar]
- 24.Du A, Kadoch IJ, Bourcigaux N, Doumerc S, Bourrier MC, Chevalier N, Fanchin R, Chian RC, Tachdjian G, Frydman R, Frydman N. In vitro oocyte maturation for the treatment of infertility associated with polycystic ovarian syndrome: the French experience. Human Reprod. 2005;20:420–424. doi: 10.1093/humrep/deh603. [DOI] [PubMed] [Google Scholar]
- 25.Lornage J. In vitro maturation: the French experience. J Gynecol Obstet Biol Reprod. 2006;35(Suppl 2):3–7. [PubMed] [Google Scholar]
- 26.Albuz FK, Sasseville M, Lane M, Armstrong DT, Thompson JG, Gilchrist RB. Simulated physiological oocyte maturation (SPOM): a novel in vitro maturation system that substantially improves embryo yield and pregnancy outcomes. Human Reproduction. 2010;25:2999–3011. doi: 10.1093/humrep/deq246. [DOI] [PubMed] [Google Scholar]
- 27.Son WY, Park SE, Lee KA, Lee WS, Ko JJ, Yoon TK, Cha KY. Effects of 1,2-propanediol and freezing-thawing on the in vitro developmental capacity of human immature oocytes. Fertil Steril. 1996;66:995–999. doi: 10.1016/s0015-0282(16)58696-8. [DOI] [PubMed] [Google Scholar]
- 28.Wininger JD, Kort HI. Cryopreservation of immature and mature human oocytes. Semin Reprod Med. 2002;20:45–49. doi: 10.1055/s-2002-23519. [DOI] [PubMed] [Google Scholar]
- 29.Coticchio G, Bonu MA, Borini A, Flamigni C. Oocyte cryopreservation: a biological perspective. Eur J Obstet Gynecol Reprod Biol. 2004;115(Suppl 1):2–7. doi: 10.1016/j.ejogrb.2004.01.006. [DOI] [PubMed] [Google Scholar]
- 30.Cao Y, Xing Q, Zhang ZG, Wei ZL, Zhou P, Cong L. Cryopreservation of immature and in-vitro matured human oocytes by vitrification. Reprod Biomed Online. 2009;19:369–373. doi: 10.1016/S1472-6483(10)60170-8. [DOI] [PubMed] [Google Scholar]
- 31.Asimakopoulos B, Kotanidis L, Nikolettos N. In vitro maturation and fertilization of vitrified immature human oocytes, subsequent vitrification of produced embryos, and embryo transfer after thawing. Fertil Steril. 2011;95:2123. doi: 10.1016/j.fertnstert.2010.12.053. [DOI] [PubMed] [Google Scholar]
- 32.Z. Zhang, Y. Liu, Q. Xing, P. Zhou, Y. Cao, Cryopreservation of human failed-matured oocytes followed by in vitro maturation: vitrification is superior to the slow freezing method. Reproductive Biology and Endocrinology (2011) in press. [DOI] [PMC free article] [PubMed]
- 33.Versieren K, Heindryckx B, O'Leary T, Croo I, Abbeel E, Gerris J, Sutter P. Slow controlled-rate freezing of human in vitro matured oocytes: effects on maturation rate and kinetics and parthenogenetic activation. Fertil Steril. 2011;96:624–628. doi: 10.1016/j.fertnstert.2011.06.060. [DOI] [PubMed] [Google Scholar]
- 34.S.L. McElroy, J.A. Byrne, S.L. Chavez, B. Behr, A.J. Hsueh, L.M. Westphal, R.A. Pera,. Parthenogenetic blastocysts derived from cumulus-free in vitro matured human oocytes. PLoS One (5)2010 e10979. [DOI] [PMC free article] [PubMed]
- 35.Reichman DE, Politch J, Ginsburg ES, Racowsky C. Extended in vitro maturation of immature oocytes from stimulated cycles: an analysis of fertilization potential, embryo development, and reproductive outcomes. J Assist Reprod Genet. 2010;27:347–356. doi: 10.1007/s10815-010-9416-5. [DOI] [PMC free article] [PubMed] [Google Scholar]