Abstract
Background
Contextual memory, or memory for source details, is an important aspect of episodic memory and has been implicated in alcohol-induced fragmentary blackouts (FB). Little is known, however, about how neural functioning during contextual memory processes may differ between individuals with and without a history of fragmentary blackouts. This study examined whether neural activation during a contextual memory task differed by history of fragmentary blackout and acute alcohol consumption.
Methods
Twenty-four matched individuals with (FB+; n = 12) and without (FB−; n = 12) a history of FBs were recruited from a longitudinal study of alcohol use and behavioral risks and completed a laboratory beverage challenge followed by two functional magnetic resonance imaging (fMRI) sessions under no alcohol and alcohol [breath alcohol concentration (BrAC) = 0.08%] conditions. Task performance and brain hemodynamic activity during a block design contextual memory task were examined across 48 fMRI sessions.
Results
Groups demonstrated no differences in performance on the contextual memory task, yet exhibited different brain response patterns after alcohol intoxication. A significant FB group by beverage interaction emerged in bilateral dorsolateral prefrontal cortex and posterior parietal cortex with FB− individuals showing greater BOLD response after alcohol exposure (p < .05).
Conclusions
Alcohol had differential effects on neural activity for FB+ and FB− individuals during recollection of contextual information, perhaps suggesting a neurobiological mechanism associated with alcohol-induced fragmentary blackouts.
Keywords: Alcohol, Functional MRI, Memory
Alcohol is one of the world’s most widely used drugs and has well-known deleterious effects on memory. Alcohol’s effects on memory vary in severity and range from mild deficits to alcohol-induced blackouts (for reviews see Heffernan, 2008; White, 2003). Although many studies have assessed the effects of chronic and acute alcohol consumption on memory, very little work has examined neural correlates of alcohol-induced memory impairments, particularly among individuals who have experienced alcohol-induced blackouts.
Alcohol-induced blackouts are classified as either en bloc or fragmentary (Goodwin et al., 1969a,b). En bloc blackouts involve complete memory loss for intoxicated events; whereas fragmentary blackouts (FBs) are defined as partial memory loss for intoxicated events that is resolved with provision of contextual cues. Anecdotal descriptions of FBs and recent research suggest that FBs occur more frequently than en bloc blackouts (Hartzler and Fromme, 2003a; Goodwin et al., 1969b; White et al., 2004) and likely involve alcohol-induced deficits in contextual memory (Hartzler and Fromme, 2003b; Wetherill and Fromme, 2011).
Contextual memory (often described more broadly as source memory) refers to memory for details related to a particular event (e.g., where you were and who you were with) that often facilitate recall by enabling a person to consciously re-experience a past event (Tulving, 2002). Our group previously studied the effects of alcohol consumption on contextual memory performance and found that alcohol globally impaired contextual memory and had differential effects based on history of FBs (Hartzler and Fromme, 2003b; Wetherill and Fromme, 2011). Specifically, individuals with and without a history of FBs showed no differences on memory tasks while sober; however, after alcohol consumption, individuals with a history of FBs showed poorer contextual memory than their counterparts.
Complementing the evidence described above, functional neuroimaging studies provide insight into potential neural mechanisms underlying alcohol-induced FBs. Research examining the neural correlates of contextual memory and alcohol-induced memory impairment suggests that the medial temporal lobes (MTL), prefrontal cortex (PFC), and parietal cortex are key brain regions involved in memory processes (e.g., see Dickerson and Eichenbaum, 2010; Graham et al., 2010; Mitchell and Johnson, 2009, for reviews) and are also areas affected by alcohol (Calhoun et al., 2004; Soderlund et al., 2007; Van Horn et al., 2006). For example, several studies have reported dorsolateral PFC, anterior PFC, precuneus, and cingulate activity during contextual memory tasks (Dobbins et al., 2002; Dobbins and Han, 2006; Eichenbaum et al., 2007; Uncapher et al., 2006). There is also evidence that alcohol alters activity in these brain regions (Calhoun et al., 2004; Paulus et al., 2006). As such, individuals with differential histories of FBs may show differential response to alcohol in these brain areas.
A critical unanswered question is whether contextual memory is differentially affected by acute alcohol consumption among individuals with differential FB histories. As such, we used functional magnetic resonance imaging (fMRI) to examine the effects of acute alcohol consumption on contextual memory and neural activity among individuals who have and have not previously experienced an alcohol-induced fragmentary blackout. We hypothesized that alcohol would cause changes in blood oxygenation level-dependent (BOLD) signal in PFC, MTL, and parietal brain regions associated with contextual memory (Dobbins et al., 2002; Eichenbaum et al., 2007; Mitchell and Johnson, 2009). We also predicted that those who experienced FBs would show greater alcohol-induced memory impairment and altered BOLD signal in the PFC, MTL, and parietal cortex during a contextual memory task. By identifying the acute effects of alcohol on neural correlates of contextual memory, the present study will provide insight into potential mechanisms that confer risk for alcohol-induced FBs.
Materials and Methods
Participants
Participants were students at a large public university who were part of a longitudinal study of alcohol use and behavioral risks from adolescence to adulthood (for detailed recruitment procedures, see Corbin et al., 2008; Hatzenbuehler et al., 2008, Wetherill and Fromme, 2011). Eligibility was initially determined using longitudinal data to identify individuals who were between the ages of 21 and 23 with similar drinking patterns (e.g., drinking frequency, quantity, maximum number of drinks) who had and had not experienced fragmentary blackouts. Individuals were classified as FB+ if they reported experiencing at least one alcohol-induced FB during the previous year; whereas FB− individuals denied experiencing an alcohol-induced FB during any of the semi-annual assessments over the previous four years. Subsequent telephone interviews determined final eligibility. Exclusionary criteria included history of medical or neurological disorder, psychiatric disorders, illicit substance use, being unaccustomed to alcohol consumption (less than three drinks per occasion at least twice a month), left-handedness, and contraindications to MRI or alcohol ingestion. Each FB+ participant was matched with a FB− individual on alcohol use, age, race, and gender. If deemed eligible, participants were invited to participate in two fMRI sessions (counter-balanced for beverage condition) which occurred one week apart. Participants were informed that one session would involve alcohol administration followed by fMRI scanning. The final sample included 12 FB+ and 12 FB− individuals. Groups were similar on demographics and drinking behaviors (Table 1).
Table 1.
Participant demographic and substance use characteristics
| FB+ (n = 12) M (SD) or % |
FB− (n = 12) M (SD) or % |
|
|---|---|---|
| Age (range 21–23) | 21.3 (0.4) | 21.4 (0.3) |
| % Female | 50.0 | 50.0 |
| % Caucasian | 66.7 | 66.7 |
| % Family history negativea | 91.7 | 91.7 |
| Current college grade point average | 3.3 (0.4) | 3.1 (0.6) |
| Rutgers Alcohol Problem Index scoreb | 3.1 (3.2) | 1.8 (1.6) |
| Drinking days per week, past 3 months | 2.8 (0.7) | 2.7 (0.8) |
| Drinks per occasion, past 3 months | 4.8 (0.7) | 4.9 (0.7) |
| Maximum number of drinks, past 3 months | 10.7 (4.3) | 10.6 (4.0) |
| Blackout episodes, past month** | 1.9 (0.6) | 0.0 |
p < .001.
No first-degree biological relative with alcohol abuse or dependence.
RAPI score without item assessing whether participant had experienced a time that they could not remember things said or done.
Measures
Alcohol consumption was measured with the Daily Drinking Questionnaire (DDQ; Collins et al., 1985). The DDQ yields estimates of the average frequency (i.e., number of drinking episodes) and quantity (i.e., number of standard drinks per drinking episode) of alcohol consumption for a typical week during the previous three month period. Alcohol-related negative consequences were assessed using the Rutgers Alcohol Problem Index (RAPI; White and Labouvie, 1989), which measures physical, psychological, and social consequences of drinking. RAPI responses served as an initial screen for blackouts, and subsequent telephone interviews further classified alcohol-induced memory loss as fragmentary or en bloc (e.g., “After drinking heavily, have you ever experienced a period of time that you could not remember things you said or did?”; “When you experienced difficulty remembering things you said or did while drinking, did you later remember when given cues or reminded later?”). Individuals who responded affirmatively to both questions were enrolled as FB+, whereas those who responded negatively to the first question were enrolled as FB−. This blackout assessment has been used in several published studies (Hartzler and Fromme, 2003a, b; Wetherill and Fromme, 2009; 2011), is similar to those used in other surveys (Nelson et al., 2004; Perry et al., 2006; White et al., 2002), and is reliably related to laboratory measures of memory (Wetherill & Fromme, 2011). Participants also completed one 30-day Timeline Followback (TLFB; Sobell and Sobell, 1992) at the time of fMRI scanning. The TLFB ascertained recent substance use as well as alcohol-induced memory loss that may have occurred between initial screen and laboratory session (Hartzler and Fromme, 2003b).
fMRI Procedures
Data were collected during two sessions on separate days. After abstaining from food for at least four hours and alcohol and drugs for 24 hours, participants arrived to the Imaging Research Center and provided informed consent, showed ID, were weighed, and completed a breath alcohol test to ensure zero breath alcohol concentration (BrAC). Women gave a urine pregnancy test that produced negative results. During each session, participants received a customized dose of beverage (alcohol or juice) based on weight and sex to reach a breath alcohol concentration (BrAC) for no alcohol (0.0% BrAC) and alcohol (0.08% BrAC). Beverages were distributed in three drinks (one every 10 minutes). Alcohol beverages contained vodka mixed with either cranberry or orange juice (combined at a 3:1 ratio of juice to vodka) and no alcohol beverages contained only cranberry or orange juice. BrAC’s were determined immediately before and after MR scanning using hand-held breathalyzers (Intoximeters, Inc., St Louis, MO).
On two separate days, one week apart, each participant completed fMRI scans under sober and intoxicated conditions. Imaging data were acquired on a 3-Tesla General Electric scanner with an 8-channel phased array head coil. Scanning included a T1-weighted spoiled gradient recalled acquisition in the steady state (SPGR; 1.2 mm slice mm thickness covering the whole brain with 1 mm2 in-plane resolution) collected in the sagittal plane that was empirically optimized for high contrast between gray matter and white matter and gray matter and cerebrospinal fluid. Functional data was collected in the axial plane using echo planar imaging (30 ms echo time, 3.125×3.125×3 mm voxels with a 0.3 mm interslice gap, 35 slices oriented for best whole-head coverage, 2,000 ms repetition time, 248 repetitions). Head position was stabilized using padding.
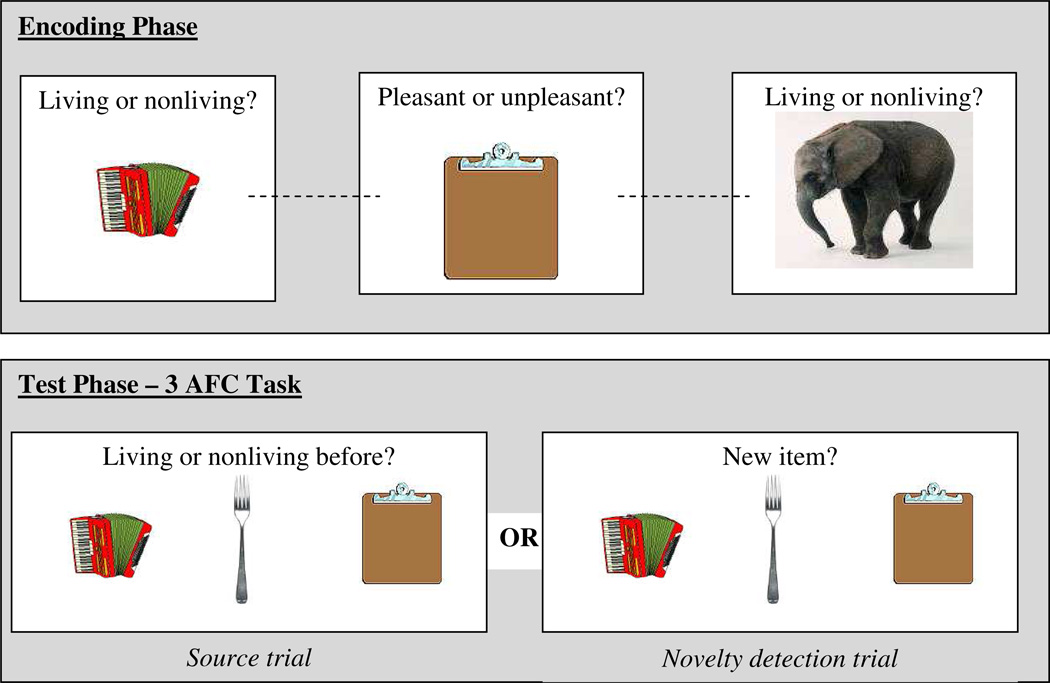
A block-design contextual memory task (modified from Dobbins et al., 2002; 2004) was presented during fMRI acquisition (Figure 1). Task stimuli consisted of 384 color pictures used in previous studies (Dobbins et al., 2004; Simons et al., 2003). Pictures portrayed single man-made (e.g. guitar) or living (e.g. fish) objects. Twelve stimuli sets were created for use in two separate study/retrieval cycles (three sets/96 images per cycle). During each 64-item study phase, participants viewed two of the three image sets while the third set of 32 novel images were presented during the subsequent three-alternative forced choice (3AFC) test. Image sets were counterbalanced across subjects and scans.
Figure 1.
Experimental design. Encoding judgments (pleasant/unpleasant or living/nonliving) were performed followed by a 3AFC test. At test, three objects (two studied and one new) were presented and either one of the two source judgments or novelty detection was required
During the study phase, participants were scanned while alternating between blocks of two different orienting tasks (i.e., “pleasant/unpleasant?” or “living/nonliving?”). Each block consisted of eight individual images that were shown for 2.5 s followed by a blank screen presented for 500 ms (3 s per image). Blocks were interspersed with fixation blocks lasting 8 s. The 3AFC test phase immediately followed each study phase and occurred within the same scan. Each test phase consisted of 32 3AFC triplets (i.e., one new image, one image from pleasant/unpleasant study block, and one image from living/nonliving study block). Retrieval cue (“pleasant-task”, “living-task”, “new”) varied across test blocks of 4 trials. During “new” blocks, participants were asked to indicate which of the images was new; during “pleasant” and “living” blocks, participants indicated which image had been encoded during “pleasant’ or “living” tasks. Each test trial lasted 5.5 s with cue and three images presented for 5 s and a blank screen presented for 500 ms between each trial. Participants’ responses and reaction times were collected for behavioral performance analyses.
Data Analyses
Behavioral analyses
Behavioral data were analyzed with a repeated measures (0.00 and 0.08 BrAC) analysis of variance with a between-subjects factor (FB+ and FB−). Two separate analyses were conducted using: (i) accuracy (percent correct during test phases) and (ii) mean reaction time as independent variables.
fMRI data processing
Imaging data were processed and analyzed using FEAT (FMRI Expert Analysis Tool) version 5.63, part of FSL (www.fmrib.ox.ac.uk/fsl) software. Head motion was corrected using FMRIB’s Linear Imaging Registration Tool (MCFLIRT; Jenkinson et al., 2002). All runs met the suggested cutoff of < 1 voxel length movement. There was no significant difference in average movement between sober and intoxicated scans [mean (SD): sober 0.153 mm (0.099), intoxicated 0.163 mm (0.093), F1,23 = 0.305, p = 0.586. Subsequent preprocessing steps included non-brain tissue removal using the Brain Extraction Tool (BET; Smith, 2002), high-pass temporal filtering with a 100 s cutoff, and spatial smoothing with a Gaussian kernel of 6 mm full width at half-maximum (FWHM).
fMRI data analyses
Statistical analyses were performed by modeling periods of context and fixation (stimulus timing files convolved with the hemodynamic response function) as explanatory variables in FEAT. Multiple linear regression analyses were performed to estimate the hemodynamic parameters for the study and test explanatory variables which subsequently were used to construct the context > fixation contrast and a corresponding t-statistic indicating significance of stimulus activation. Statistical maps were then registered to the MNI template using FLIRT (FMRIB’s Linear Image Registration Tool: Jenkinson et al., 2002; Jenkinson and Smith, 2001). Using a fixed effects model, a second-level analysis was conducted on each subject in order to combine data from their 2 runs into a single set of contrast images. Each subjects’ second level contrast was then entered into a random effects group-level analysis utilizing the FLAME (FMRIB's Local Analysis of Mixed Effects) technique implemented through FSL and threshold at Z> 3.09 cluster-corrected. In order to identify common activations across subjects, follow-up conjunction analyses were conducted in FSL (easythresh_conj) by creating an intersection of statistical maps and applying a threshold of Z > 3.09, p < 0.05.
Results
Physiological Measures of Intoxication
BrAC values pre- and post-fMRI scan during the alcohol session are reported in Table 2. There was no significant difference in BrAC values pre- or post-fMRI scan between FB+ and FB− participants.
Table 2.
Physiological measures of intoxication throughout the alcohol session
| FB+ M (SD) |
FB− M (SD) |
|
|---|---|---|
| BrAC before scan | 0.07 (0.01) | 0.07 (0.02) |
| BrAC after scan | 0.08 (0.03) | 0.08 (0.01) |
BrAC = breath alcohol concentration; FB = Fragmentary blackout
Behavioral Performance
Reaction times differed across alcohol and no alcohol test phases [F1,23 = 15.96, p < .001], with intoxicated recollection (2609 ms) taking longer than sober recollection (2494 ms). Differences in reaction time between FB+ and FB− groups while sober and intoxicated were not significant. Accuracy also differed across the alcohol and no alcohol retrieval tasks [F1,23 = 5.25, p = .03], being superior during sober conditions (73%) relative to intoxicated conditions (64%). We predicted that FB+ individuals would perform worse during intoxicated retrieval compared to FB− individuals. Analyses revealed that FB groups did not differ while sober [F1,23 = 0.09, p = .76] or after alcohol exposure [F1,23 = 1.31, p = 0.13] (Table 3).
Table 3.
Behavioral performance
| No Alcohol | Alcohol | |||
|---|---|---|---|---|
| FB+ | FB− | FB+ | FB− | |
| Contextual recollection* | 0.73 (.08) | 0.74 (.08) | 0.61 (0.13) | 0.66 (0.09) |
| Mean reaction time (msec) ** | 2509 (124) | 2478 (165) | 2602 (92) | 2615 (225) |
FB = Fragmentary blackout,
p < 0.05,
p < 0.01.
Neural Correlates of Contextual Encoding
BOLD response during contextual encoding
As shown in Table 4, contextual encoding had a significant effect during both alcohol and no alcohol sessions in bilateral occipital cortex, left dorsolateral PFC, and left parietal cortex. Within-subject comparisons of no alcohol and alcohol conditions revealed no significant differences in BOLD response during contextual encoding.
Table 4.
Regions demonstrating activation during contextual encoding
| Region | ~BA | x | y | z | Max Z |
|---|---|---|---|---|---|
| No Alcohol | |||||
| R/L occipital cortex | 18 | 18 | −100 | 12 | 5.85 |
| L dorsolateral PFC | 46 | −44 | 26 | −22 | 5.35 |
| L parietal cortex | 7 | −30 | −58 | 50 | 3.26 |
| Alcohol | |||||
| R/L occipital cortex | 18 | −28 | −88 | −12 | 6.3 |
| L dorsolateral PFC | 6 | −4 | 6 | 50 | 5.58 |
| L parietal cortex | 7 | −28 | −58 | 50 | 4.27 |
| Within-person comparisons | |||||
| Alcohol Minus No Alcohol | No statistically significant findings | ||||
| No Alcohol Minus Alcohol | No statistically significant findings | ||||
| Between-group comparisons | |||||
| No Alcohol FB− Minus FB+ FB+ Minus FB− |
No statistically significant findings No statistically significant findings |
||||
| Alcohol FB− Minus FB+ L frontopolar PFC |
10 |
−22 |
44 |
26 |
3.86 |
| FB+ Minus FB− | No statistically significant findings | ||||
Each cluster is listed with corresponding peak voxels of activation; FB = Fragmentary blackout; BA = Brodmann’s area; L = Left; PFC = Prefrontal cortex.
Main Effects of Fragmentary Blackout History on BOLD Responses during Encoding
For the no alcohol condition, FB+ and FB− individuals showed similar patterns of BOLD response during contextual encoding with greater activity in the bilateral cingulate gyrus, left dorsolateral PFC, and left parietal cortex. Between-subject comparisons of no alcohol sessions between FB groups revealed no significant activation differences. To identify commonly active regions between FB groups, conjunction analyses were performed in FSL and revealed similar activation patterns in the cingulate gyrus, left dorsolateral PFC, and left parietal cortex (Table 5).
Table 5.
Conjunction analyses: Regions showing similar activation between FB+ and FB− groups
| Region | ~BA | x | y | z |
|---|---|---|---|---|
| Encoding | ||||
| R/L cingulate | 31 | 0 | −66 | 16 |
| L dorsolateral PFC | 6 | −28 | 18 | 46 |
| L parietal cortex | 50 | 26 | 46 | |
| Recollection | ||||
| R/L occipital cortex | 17/18 | −6 | −98 | 8 |
| R/L medial temporal lobe | 28 | −24 | −26 | −10 |
| R/L dorsolateral PFC | 46 | −52 | 28 | 20 |
| R/L ventrolateral PFC | 45 | −56 | 28 | 4 |
| R/L medial frontal cortex | 8 | −0 | 38 | 36 |
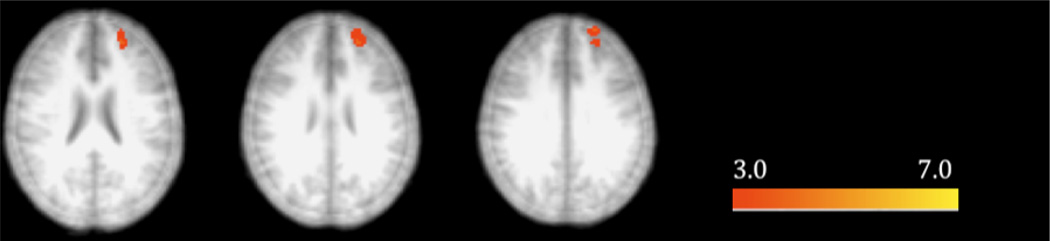
For the alcohol condition, FB+ and FB− individuals showed significant BOLD activation during contextual encoding in a large cluster spanning the occipital cortex, as well as regions in the left dorsolateral PFC and right parahippocampus. Greater activity was observed among FB− individuals relative to FB+ individuals in left frontopolar PFC (Figure 2). There were no areas of greater activation in the FB+ > FB− contrast.
Figure 2.
Significant differences in contextual encoding related BOLD response between FB+ and FB− individuals after alcohol exposure (cluster-corrected, z = 3.09). Radiological orientation with right side representing left hemisphere and left side representing right hemisphere.
Neural Correlates of Contextual Recollection
BOLD response during contextual recollection
BOLD response to contextual recollection while sober was observed in several regions, including the bilateral occipital cortex, right dorsolateral PFC, right ventrolateral PFC, right frontopolar PFC, right caudate, and MTL. BOLD response to contextual recollection after alcohol consumption was observed in bilateral occipital regions, bilateral ventrolateral PFC, right dorsolateral PFC, left frontopolar PFC, and left caudate. Within-subject comparisons of no alcohol and alcohol conditions revealed greater BOLD response during contextual recollection while sober relative to after alcohol consumption in the right frontopolar PFC. There were no areas of greater activation in the alcohol > no alcohol contrast (Table 6).
Table 6.
Regions demonstrating activation during contextual recollection
| Region | ~BA | x | y | z | Max Z |
|---|---|---|---|---|---|
| No Alcohol | |||||
| R/L occipital cortex | 18/19 | −34 | −86 | 18 | 7.1 |
| R dorsolateral PFC | 6 | 34 | −4 | 46 | 6.61 |
| R ventrolateral PFC | 47 | 34 | 24 | 0 | 5.46 |
| R frontopolar PFC | 10 | 32 | 64 | 4 | 4.31 |
| R caudate | 12 | 14 | 8 | 4.14 | |
| R/L MTL | 35 | 20 | −30 | −10 | 3.8 |
| Alcohol | |||||
| R/L occipital cortex | 18 | −28 | −90 | 14 | 7.05 |
| R/L ventrolateral PFC | 47 | 32 | 26 | 10 | 5.49 |
| R dorsolateral PFC | 46 | 48 | 30 | 20 | 4.96 |
| L frontopolar PFC | 10 | −28 | 68 | −10 | 4.85 |
| L caudate | −12 | −14 | 26 | 3.83 | |
| Within-person comparisons | |||||
| Alcohol Minus No Alcohol | No statistically significant findings | ||||
| No Alcohol Minus Alcohol Right frontopolar PFC |
10 |
30 |
52 |
12 |
3.64 |
| Between-group comparisons | |||||
| No Alcohol FB− Minus FB+ FB+ Minus FB− |
No statistically significant findings No statistically significant findings |
||||
| Alcohol FB− Minus FB+ R posterior parietal cortex L dorsolateral PFC R dorsolateral PFC |
7 6 6 |
12 −30 34 |
−62 −2 6 |
64 48 52 |
5.09 4.94 4.68 |
| FB+ Minus FB− | No statistically significant findings | ||||
Each cluster is listed with corresponding peak voxels of activation; FB = Fragmentary blackout; BA = Brodmann’s area; R = Right; L = Left; PFC = Prefrontal cortex.
Main Effects of Fragmentary Blackout History
In the no alcohol condition, FB+ and FB− individuals showed similar patterns of BOLD response during contextual recollection. Specifically, both groups showed greater activity during contextual recollection in several regions bilaterally, including frontopolar and dorsolateral PFC, MTL, medial frontal cortex, and occipital cortex. Between-subject comparisons (FB− versus FB+) revealed no significant activation differences in the no alcohol condition and suggest that FB+ and FB− individuals showed similar activation patterns during contextual recollection. Conjunction analyses confirmed these results and indicated similar patterns of activity in FB+ and FB− individuals in several regions including, the occipital cortex, bilateral MTL, bilateral dorsolateral and ventrolateral PFC, and medial frontal cortex (Table 5).
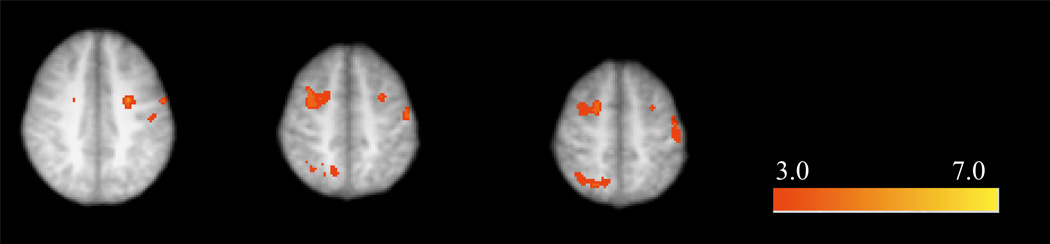
In the alcohol condition, FB− individuals showed significant BOLD activation in a large cluster spanning the occipital/temporal cortex, bilateral MTL, bilateral frontopolar PFC, and cingulate. FB+ individuals showed significant activation during contextual recollection in regions including bilateral occipital/temporal cortex, bilateral dorsolateral PFC, right hippocampus, left frontopolar PFC, and cingulate. Between-subject comparisons (FB− versus FB+) in the alcohol condition revealed greater activity during contextual recollection among FB− individuals relative to FB+ individuals in the right posterior parietal cortex and bilateral dorsolateral PFC. There were no areas of greater activation in the FB+ > FB− contrast (Figure 3).
Figure 3.
Significant differences in contextual recollection-related BOLD response between FB+ and FB− individuals after alcohol exposure (cluster-corrected; z = 3.09). FB− individuals showed greater BOLD response in bilateral dorsolateral prefrontal cortex and right posterior parietal cortex. Radiological orientation with right side representing left hemisphere and left side representing right hemisphere.
Discussion
This study examined the acute effects of alcohol on neural correlates of contextual encoding and recollection among individuals with and without a history of fragmentary blackouts. The results indicated a main effect of alcohol on overall behavioral performance as measured by contextual recollection accuracy and latency during a contextual recollection task. There were no overall FB history-related differences on task performance; however, after alcohol consumption, FB+ individuals performed marginally worse on the contextual memory task. We also found that FB groups differed on contextual memory-related brain activation during the alcohol session, but not the sober session. Following alcohol administration, individuals with a history of fragmentary blackouts showed less brain activation during contextual encoding and recollection in the PFC and posterior parietal cortex.
Alcohol Effects on Neural Activation
Consistent with previous research, alcohol intoxication (0.08 % BrAC) altered brain activity in the prefrontal cortex (Anderson et al., 2011; Gundersen et al., 2008; Soderlund et al., 2007). Specifically, we found that acute alcohol exposure attenuated contextual recollection-related brain activation in the right frontopolar PFC. The right frontopolar PFC is a key region involved in maintaining overall cognitive set while monitoring and completing another task (Boorman et al., 2009), such as memory (Christoff and Gabrielli, 2000; Sakai and Passingham, 2006) and relational reasoning tasks (Bunge et al., 2005; Wendelken et al., 2008). Thus, our findings provide additional evidence for the notion that alcohol affects neural activity in regions associated with executive cognitive functioning and higher-order processes (Van Horn et al., 2006). Furthermore, altered right frontopolar PFC activation has been observed among substance-dependent individuals (Paulus et al., 2002; 2003; Tanabe et al., 2007), perhaps suggesting that the frontopolar cortex may be particularly sensitive to the effects of alcohol and other illicit substances.
Differences between FB+ and FB− Individuals
Consistent with our prior laboratory studies (Hartzler and Fromme, 2003b; Wetherill and Fromme, 2011), FB+ and FB− individuals did not show significant contextual memory-related brain activity differences when sober; however, after alcohol administration, FB+ individuals exhibited less BOLD response during contextual encoding and recollection in the prefrontal and posterior parietal cortex, specifically the precuneus. During encoding, FB+ individuals showed less brain activation compared to FB− individuals in the left frontopolar PFC, a region involved in evaluation of self-generated information and working memory [Nyberg et al., 2003]. Similarly, FB+ individuals exhibited attenuated brain activation during recollection in the dorsolateral PFC and posterior parietal cortex. The dorsolateral PFC and posterior parietal cortex are key regions of the dorsal attention network (Corbetta and Shulman, 2002) often involved in inhibitory processing, executive control, decision-making, and working memory (Lundqvist, 2010; Squeglia et al., 2009; Tapert et al., 2004). As such, our findings suggest that FB+ individuals showed contextual-memory related BOLD response abnormalities after alcohol consumption compared to FB− individuals. Frontoparietal abnormalities have been observed in youth at risk for alcohol use disorders (Hada et al., 2001; Rangaswamy et al., 2004; Spadoni et al., 2008), and it has been suggested that alterations in frontoparietal activity may be a neurobiological marker of vulnerability (Porjesz and Rangaswamy, 2007). Although analyzing frontoparietal functional connectivity among FB+ and FB− individuals is beyond the scope of the current study, we speculate that our imaging findings might represent alcohol-induced frontoparietal functional connectivity differences between FB+ and FB− individuals.
Limitations
The current results should be considered in light of possible limitations. First, the current sample size of twelve per group limits power to detect differences in behavioral and neural response; thus, these results need replication in a larger sample. Second, we did not include a placebo condition in our alcohol administration, and therefore, were unable to examine expectancy effects on brain activity and potential differences between FB+ and FB− individuals. We plan to do this in future research. Third, it is possible that the vasoactive effects of alcohol might alter BOLD fMRI signal. Thus, it is possible that the alcohol findings reflect alcohol’s effects on blood flow rather than brain activity itself. Future research examining alcohol’s effects on brain activity will need to utilize arterial spin labeling to measure and account for blood flow.
Conclusions
In summary, the current data shows that alcohol intoxication impaired contextual memory performance and altered contextual memory-related brain activity. In addition, activation patterns of FB+ and FB− individuals differed after alcohol consumption, but not while sober. These findings indicate that acute alcohol consumption affects dorsolateral prefrontal cortex and posterior parietal cortex neural activation and suggest that frontoparietal abnormalities are a potential biomarker for alcohol-induced memory impairments.
Acknowledgments
We thank Drs. Sandra A. Brown, Susan F. Tapert, and Marsha Bates, who provided valuable advice and consultation throughout data collection and analysis. We also thank the University of Texas at Austin Imaging Research Center staff, who assisted in data collection. Funding for this study was provided by grants from the National Institute on Alcohol Abuse and Alcoholism (RO1 AA013967, F31 AA017022) and the Waggoner Center for Alcohol and Addiction Research.
References
- Anderson BM, Stevens MC, Meda SA, Jordan K, Calhoun VD, Pearlson GD. Functional imaging of cognitive control during acute alcohol intoxication. Alcohol Clin Exp Res. 2011;35:156–165. doi: 10.1111/j.1530-0277.2010.01332.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman ED, Behrens TE, Woolrich MW, Rushworth MF. How green is the grass on the other side? Frontopolar cortex and evidence in favor of alternative courses of action. Neuron. 2009;62:733–743. doi: 10.1016/j.neuron.2009.05.014. [DOI] [PubMed] [Google Scholar]
- Bunge SA, Wendelken C, Badre D, Wagner AD. Analogical reasoning and prefrontal cortex: evidence for separable retrieval and integration mechanisms. Cereb Cortx. 2005;15:239–249. doi: 10.1093/cercor/bhh126. [DOI] [PubMed] [Google Scholar]
- Calhoun VD, Altschul D, McGinty V, Shih R, Scott D, Sears E, et al. Alcohol intoxication effects on visual perception: an fMRI study. Hum Brain Mapp. 2004;21:15–26. doi: 10.1002/hbm.10145. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Christoff K, Gabrielli JDE. The frontopolar cortex and human cognition: Evidence for a rostrocaudal hierarchical organization within the human prefrontal cortex. Psychobiology. 2000;28:168–186. [Google Scholar]
- Collins RL, Parks GA, Marlatt GA. Social determinants of alcohol consumption: the effects of social interaction and model status on the self-administration of alcohol. J Consult Clin Psychol. 1985;53:189–200. doi: 10.1037//0022-006x.53.2.189. [DOI] [PubMed] [Google Scholar]
- Corbetta M, Shulman GL. Control of goal-directed and stimulus-driven attention in the brain. Nat Rev Neurosci. 2002;3:201–215. doi: 10.1038/nrn755. [DOI] [PubMed] [Google Scholar]
- Corbin WR, Vaughan EL, Fromme K. Ethnic differences and the closing of the sex gap in alcohol use among college-bound students. Psychol Addict Behav. 2008;22:240–248. doi: 10.1037/0893-164X.22.2.240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dickerson BC, Eichenbaum H. The episodic memory system: neurocircuitry and disorders. Neuropsychopharmacology. 2010;35:86–104. doi: 10.1038/npp.2009.126. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dobbins IG, Foley H, Schacter DL, Wagner AD. Executive control during episodic retrieval: multiple prefrontal processes subserve source memory. Neuron. 2002;35:989–996. doi: 10.1016/s0896-6273(02)00858-9. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Han S. Cue- versus probe-dependent prefrontal cortex activity during contextual remembering. J Cogn Neurosci. 2006;18:1439–1452. doi: 10.1162/jocn.2006.18.9.1439. [DOI] [PubMed] [Google Scholar]
- Dobbins IG, Simons JS, Schacter DL. fMRI evidence for separable and lateralized prefrontal memory monitoring processes. J Cogn Neurosci. 2004;16:908–920. doi: 10.1162/0898929041502751. [DOI] [PubMed] [Google Scholar]
- Eichenbaum H, Yonelinas AP, Ranganath C. The medial temporal lobe and recognition memory. Annu Rev Neurosci. 2007;30:123–152. doi: 10.1146/annurev.neuro.30.051606.094328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goodwin DW, Crane JB, Guze SB. Alcoholic ‘blackouts’: A review and clinical study of 100 alcoholics. Am J Psychiatry. 1969a;126:191–198. doi: 10.1176/ajp.126.2.191. [DOI] [PubMed] [Google Scholar]
- Goodwin DW, Crane JB, Guze SB. Phenomenological aspects of the alcoholic ‘blackout.’. Br J Psychiatry. 1969b;115:1033–1038. doi: 10.1192/bjp.115.526.1033. [DOI] [PubMed] [Google Scholar]
- Graham KS, Barense MD, Lee AC. Going beyond LTM in the MTL: a synthesis of neuropsychological and neuroimaging findings on the role of the medial temporal lobe in memory and perception. Neuropsychologia. 2010;48:831–853. doi: 10.1016/j.neuropsychologia.2010.01.001. [DOI] [PubMed] [Google Scholar]
- Gundersen H, Specht K, Gruner R, Ersland l, Hugdahl K. Separating the effects of alchol and expectancy on brain activation: an fMRI working memory study. Neuroimage. 2008;42:1587–1596. doi: 10.1016/j.neuroimage.2008.05.037. [DOI] [PubMed] [Google Scholar]
- Hada M, Porjesz B, Chorlian DB, Begleiter H, Polich J. Auditory P300 deficits in male subjects at high risk for alcoholism. Biol Psychiatry. 2001;49:726–738. doi: 10.1016/s0006-3223(00)01049-0. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Fromme K. Fragmentary and en bloc blackouts: similarity and distinction among episodes of alcohol-induced memory loss. J Stud Alcohol. 2003a;64:547–550. doi: 10.15288/jsa.2003.64.547. [DOI] [PubMed] [Google Scholar]
- Hartzler B, Fromme K. Fragmentary blackouts: their etiology and effect on alcohol expectancies. Alcohol Clin Exp Res. 2003b;27:628–637. doi: 10.1097/01.ALC.0000062743.37558.C8. [DOI] [PubMed] [Google Scholar]
- Hatzenbuehler ML, Corbin WR, Fromme K. Trajectories and determinants of alcohol use among LGB young adults and their heterosexual peers: results from a prospective study. Dev Psychol. 2008;44:81–90. doi: 10.1037/0012-1649.44.1.81. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heffernan TM. The impact of excessive alcohol use on prospective memory: a brief review. Curr Drug Abuse Rev. 2008;1:36–41. doi: 10.2174/1874473710801010036. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Bannister P, Brady M, Smith S. Improved optimization for the robust and accurate linear registration and motion correction of brain images. Neuroimage. 2002;17:825–841. doi: 10.1016/s1053-8119(02)91132-8. [DOI] [PubMed] [Google Scholar]
- Jenkinson M, Smith S. A global optimisation method for robust affine registration of brain images. Med Image Anal. 2001;5:143–156. doi: 10.1016/s1361-8415(01)00036-6. [DOI] [PubMed] [Google Scholar]
- Lundqvist T. Imaging cognitive deficits in drug abuse. Curr Top Behav Neurosci. 2010;3:247–275. doi: 10.1007/7854_2009_26. [DOI] [PubMed] [Google Scholar]
- Mitchell KJ, Johnson MK. Source monitoring 15 years later: what have we learned from fMRI about the neural mechanisms of source memory? Psychol Bull. 2009;135:638–677. doi: 10.1037/a0015849. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EC, Heath AC, Bucholz KK, Madden PA, Fu Q, Knopik V, et al. Genetic epidemiology of alcohol-induced blackouts. Arch Gen Psychiatry. 2004;61:257–263. doi: 10.1001/archpsyc.61.3.257. [DOI] [PubMed] [Google Scholar]
- Nyberg L, Marklund P, Persson J, Cabeza R, Forkstam C, Petersson KM, Ingvar M. Common prefrontal activations during working memory, episodic memory, and semantic memory. Neuropschologia. 2003;41:371–377. doi: 10.1016/s0028-3932(02)00168-9. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Frank L, Brown GG, Schuckit MA. Decision making by methamphetamine-dependent subjects is associated with error-rate independent decrease in prefrontal and parietal activation. Biol Psychiatry. 2003;53:65–74. doi: 10.1016/s0006-3223(02)01442-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Hozack NE, Zauscher BE, Frank L, Brown GG, Braff DL, Schuckit MA. Behavioral and functional neuroimaging evidence for prefrontal dysfunction in methamphetamine-dependent subjects. Neuropsychopharmacology. 2002;26:53–63. doi: 10.1016/S0893-133X(01)00334-7. [DOI] [PubMed] [Google Scholar]
- Paulus MP, Tapert SF, Pulido C, Schuckit MA. Alcohol attenuates load-related activation during a working memory task: relation to level of response to alcohol. Alcohol Clin Exp Res. 2006;30:1363–1371. doi: 10.1111/j.1530-0277.2006.00164.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perry PJ, Argo TR, Barnett MJ, Liesveld JL, Liskow B, Hernan JM, et al. The association of alcohol-induced blackouts and grayouts to blood alcohol concentrations. J Forensic Sci. 2006;51:896–889. doi: 10.1111/j.1556-4029.2006.00161.x. [DOI] [PubMed] [Google Scholar]
- Porjesz B, Rangaswamy M. Neurophysiological endophenotypes, CNS disinhibition, and risk for alcohol dependence and related disorders. Scientific World Journal. 2007;7:131–141. doi: 10.1100/tsw.2007.203. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajah MN, Languay R, Valiquette L. Age-related changes in prefrontal cortex activity are associated with behavioural deficits in both temporal and spatial context memory retrieval in older adults. Cortex. 2010;46:535–549. doi: 10.1016/j.cortex.2009.07.006. [DOI] [PubMed] [Google Scholar]
- Rangaswamy M, Porjesz B, Ardekani BA, Choi SJ, Tanabe JL, Lim KO, Begleiter H. A functional MRI study of visual oddball: evidence for frontoparietal dysfunction in subjects at risk for alcoholism. Neuroimage. 2004;21:329–339. doi: 10.1016/j.neuroimage.2003.09.018. [DOI] [PubMed] [Google Scholar]
- Sakai K, Passingham RE. Prefrontal set activity predicts rule-specific neural processing during subsequent cognitive performance. J Neurosci. 2006;26:1211–1218. doi: 10.1523/JNEUROSCI.3887-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Simons JS, Koutstall W, Prince S, Wagner A, Schacter D. Neural mechanisms of visual object priming: Evidence for perceptual and semantic distinctions in fusiform cortex. Neuroimage. 2003;19:613–623. doi: 10.1016/s1053-8119(03)00096-x. [DOI] [PubMed] [Google Scholar]
- Smith SM. Fast robust automated brain extraction. Hum Brain Mapp. 2002;17:143–155. doi: 10.1002/hbm.10062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sobell LC, Sobell MB. In: Timeline follow-back: a technique for assessing self-reported alcohol consumption, in Measuring Alcohol Consumption: Psychosocial and Biochemical Methods. Litten RZ, Allen JP, editors. Totowa, NJ: Humana Press, Inc.; 1992. pp. 41–72. [Google Scholar]
- Soderlund H, Grady CL, Easdon C, Tulving E. Acute effects of alcohol on neural correlates of episodic memory encoding. Neuroimage. 2007;35:928–939. doi: 10.1016/j.neuroimage.2006.12.024. [DOI] [PubMed] [Google Scholar]
- Spadoni AD, Norman AL, Schweinsburg AD, Tapert SF. Effects of family history of alcohol use disorders on spatial working memory BOLD response in adolescents. Alcohol Clin Exp Res. 2008;32:1135–1145. doi: 10.1111/j.1530-0277.2008.00694.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Squeglia LM, Spadoni AD, Infante MA, Myers MG, Tapert SF. Initiating moderate to heavy alcohol use predicts changes in neuropsychological functioning for adolescent girls and boys. Psychol Addict Behav. 2009;23:715–722. doi: 10.1037/a0016516. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tanabe J, Thompson L, Claus E, Dalwani M, Hutchison K, Banich MT. Prefrontal cortex activity is reduced in gambling and non-gambling substance users during decision-making. Hum Brain Mapp. 2007;28:1276–1286. doi: 10.1002/hbm.20344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tapert SF, Schweinsburg AD, Barlett VC, Brown SA, Frank LR, Brown GG, Meloy MJ. Blood oxygen level dependent response and spatial working memory in adolescents with alcohol use disorders. Alcohol Clin Exp Res. 2004;28:1577–1586. doi: 10.1097/01.alc.0000141812.81234.a6. [DOI] [PubMed] [Google Scholar]
- Tulving E. Episodic memory: from mind to brain. Annu Rev Psychol. 2002;53:1–25. doi: 10.1146/annurev.psych.53.100901.135114. [DOI] [PubMed] [Google Scholar]
- Uncapher MR, Otten LJ, Rugg MD. Episodic encoding is more than the sum of its parts: an fMRI investigation of multifeatural contextual encoding. Neuron. 2006;52:547–556. doi: 10.1016/j.neuron.2006.08.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Horn JD, Yanos M, Schmitt PJ, Grafton ST. Alcohol-induced suppression of BOLD activity during goal-directed visuomotor performance. Neuroimage. 2006;31:1209–1221. doi: 10.1016/j.neuroimage.2006.01.020. [DOI] [PubMed] [Google Scholar]
- Wendelken C, Nakhabenko D, Donohue SE, Carter CS, Bunge SA. "Brain is to thought as stomach is to??": investigating the role of rostrolateral prefrontal cortex in relational reasoning. J Cogn Neurosci. 2008;20:682–693. doi: 10.1162/jocn.2008.20055. [DOI] [PubMed] [Google Scholar]
- Wetherill RR, Fromme K. Subjective responses to alcohol prime event-specific alcohol consumption and predict blackouts and hangover. J Stud Alcohol Drugs. 2009;70:593–600. doi: 10.15288/jsad.2009.70.593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wetherill RR, Fromme K. Acute alcohol effects on narrative recall and contextual memory: An examination of fragmentary blackouts. Addict Behav. 2011;36:886–889. doi: 10.1016/j.addbeh.2011.03.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- White AM. What happened? Alcohol, memory blackouts, and the brain. Alcohol Res Health. 2003;27:186–196. [PMC free article] [PubMed] [Google Scholar]
- White AM, Jamieson-Drake DW, Swartzwelder HS. Prevalence and correlates of alcohol-induced blackouts among college students: results of an e-mail survey. J Am Coll Health. 2002;51:122–131. doi: 10.1080/07448480209596339. [DOI] [PubMed] [Google Scholar]
- White AM, Signer ML, Kraus CL, Swartzwelder HS. Experiential aspects of alcohol-induced blackouts among college students. Am J Drug Alcohol Abuse. 2004;30:205–224. doi: 10.1081/ada-120029874. [DOI] [PubMed] [Google Scholar]
- White HR, Labouvie EW. Towards the assessment of adolescent problem drinking. J Stud Alcohol. 1989;50:30–37. doi: 10.15288/jsa.1989.50.30. [DOI] [PubMed] [Google Scholar]