Abstract
In biomedical research, one key stage of translating basic science knowledge to clinical practice is the reconciliation of phenotypes employed for laboratory animal studies with those important for the clinical condition. Alcohol dependence (AD) is a prototypic complex genetic trait. There is a long history of behavior genetic studies of AD in both human subjects and various genetic animal models. This review assesses the state of the art in our understanding of the genetic contributions to AD. In particular, it primarily focuses on the phenotypes studied in mouse genetic animal models, comparing them to the aspects of the human condition they are intended to target. It identifies several features of AD where genetic animal models have been particularly useful, and tries to identify understudied areas where there is good promise for further genetic animal model work.
Keywords: Alcoholism, genetic animal models, tolerance, withdrawal, drinking, adverse consequences
Introduction
Alcohol dependence (AD) is defined categorically in the Diagnostic and Statistical Manual of Mental Disorders, Fourth Edition (DSM-IV) and represents a complex syndrome with numerous and varied symptoms. In many respects, AD is a prototypic complex genetic trait and there is clear evidence from human genetic studies that 50–60% of risk for an AD diagnosis can be inherited (Goldman et al. 2005; Stacey et al. 2009). Genetic sources of risk ultimately must be traceable to specific genes, and there are numerous environmental factors of known importance to risk (e.g., family situation and dynamics, peer interactions, life stressors, comorbidities with other psychiatric disorders, etc.). There is also clear evidence of gene-environment interaction in risk for AD (Cloninger 1987; Kendler et al. 2003; Sher et al. 2010; van der Zwaluw et al. 2009).
In an experimental attempt to identify and understand genetic and environmental influences on a behavioral phenotype, we first need to be able to define what we mean by three things: genetic influences, the environment, and the behavioral target (the phenotype). I consider each of these in turn with respect to genetic risk for AD. To focus this enquiry, I concentrate on one key stage of translating basic science knowledge to clinical practice: namely, the reconciliation of phenotypes employed for laboratory animal studies with those important for the clinical condition. What is the appropriate level for defining the “genetic influences” on AD risk? When the Central Dogma still reigned, this was a relatively simple question. We could basically only ascertain which genes were associated with a specific trait, and perhaps with luck identify a sequence variant such as a mutation with a marked effect. Now, we must determine whether sequence variation is relevant at all, and if so, at what level (SNPs, common variants, rare variants, haplotypes?). Or, is it the transcriptome that we should focus on? Or, networks of co-expressed genes and/or their associated proteomes? There are now data linking AD risk with variation at all the above levels, and it will take some time to sort out which genetic level of analysis is most apposite.
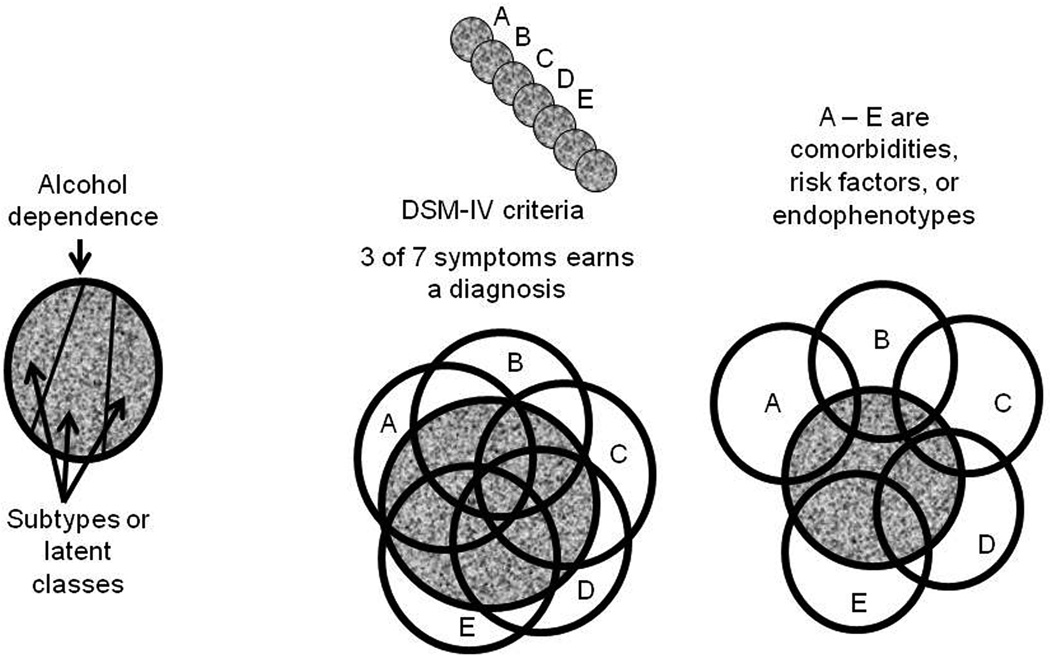
Defining the environment is equally important, and equally difficult. Good discussions of these issues can be found elsewhere (Sher et al. 2010; van der Zwaluw et al. 2009). Genes are expressed differently in different environments. For example, AD is a developmental disorder, and may reflect the influence of different sets of active genes during adolescence than during adulthood. Genes are expressed differently in different brain areas. Epigenetic regulation is important, but little studied to date. The environment for a human genetic study includes family members, peers, nutrition, and work status, just to name a few. For laboratory animals, work is generally irrelevant, and nutrition is accounted for, but the laboratory environment is extremely complex and extends clear through the details of any experimental protocols employed. Laboratory environment can have strong effects on gene-behavior relationships (Cabib et al. 2000; Chesler et al. 2002; Crabbe et al. 1999). Finally, consider the phenotype itself, the main topic of this review. As Gerald McClearn pointed out many years ago, any trait as behaviorally/biologically complex as AD cannot likely be represented adequately with a single genetic animal model. Rather, the human diagnosis, a categorical phenotype, can better be fractionated and represented by multiple animal models, each targeted to a specific constellation of important phenotypic characteristics (McClearn 1988). Figure 1 presents a few ways of thinking about the complex trait, AD. AD is defined in the DSM-IV as the occurrence of any three of the following 7 symptoms occurring during the same 12 month period: a. tolerance; b. withdrawal; c. use greater than intended; d. persistent desire/repeated attempts to quit drinking; e. devotion of much time/activity to obtaining, using, or recovering from alcohol; f. giving up or reducing important social, occupational or recreational activities; and g. continued use despite knowledge of adverse consequences (Hasin 2003). Each of these specific symptoms may be targeted separately. Practically speaking, they tend to be correlated, as depicted in the center panel of Figure 1. Of course, diagnosing AD by any group of 3 symptoms from a list of 7 inevitably means that any population of AD subjects is diagnostically, hence biologically heterogeneous. Thus, there is also a long history in alcohol research of seeking taxonomically distinct subgroups of AD individuals. The presumption is that restricting analysis of biological and genetic correlates of AD to specific subtypes may reveal a more clear path to gene-behavior relationships (Babor et al. 1986; Jellinek 1960; Vaillant 1983). Mining the generally voluminous data taken from subjects in a human genetics experiment, investigators have sought other types of latent classes of subdiagnoses as well [e.g. (Bucholz et al. 1996)]. Subtyping approaches do not reveal a structure as simple as that depicted in the left panel of Figure 1, although they can improve the strength of gene-diagnosis relationships detected.
Figure 1.
Three ways to partition the construct represented by a diagnosis of alcohol dependence (AD), represented by the gray space. The left panel represents a case where three distinct and independent subtypes might exist. These could emerge as latent classes from clusters of symptoms or traits. Such a clear result is unlikely to occur. The middle panel depicts how multiple traits (A – E) might interact with AD. The circles represent the individual variabilities associated with each trait, and their overlap with each other and with AD indicate covariance. Here, A – E might represent 5 of the 7 symptoms used by the DSM-IV to assign diagnosis. Each symptom covaries substantially with AD and tends to covary with most other symptoms. Finally, the right panel depicts a more realistic pattern of covariances for such things as comorbidities, risk markers, and endophenotypes. The traits differ in the proportion of covariance shared with AD (for C a little, for E a lot). E covaries with AD independently from A, B, and C, but shares some covariance with both D and AD. Most of the covariance between D and E is also shared with AD, while most of the covariance between B and C is independent of AD.
A related way of thinking about how to pursue the complexity of AD genetics focuses on endophenotypes or intermediate phenotypes (Gottesman et al. 2003). For example, electrophysiological signals have been associated with AD diagnosis (Edenberg et al. 2004). Various polymorphisms in candidate genes such as GABRA2, OPRM1 and the CRHR1 receptors have shown reasonably consistent associations with AD (Anton et al. 2008; Enoch 2008; Treutlein et al. 2006). The intent of such studies is not necessarily primarily to implicate the gene product of the associated candidate gene, or the brain process underlying an electrophysiological signal, but rather to provide a surrogate marker that has an inheritance pattern presumed to be more simple than AD itself. Finally, there is an extensive literature on comorbidity of AD with ADHD, major depression, anxiety disorders, and other drug dependencies, as well as with such personality characteristics as impulsivity and risk-taking. This genetic epidemiology literature shows that each such comorbidity has some individual sources of genetic influence but also some sources apparently shared with AD (Kendler et al. 2003; Sher et al. 2005; Volkow et al. 2005). How such risk factors and surrogate genetic markers might covary with AD diagnosis is depicted in the right panel of Figure 1.
Progress in human genetic studies of AD
Most human genetic studies of AD have targeted the diagnosis in case-control studies. That is, they have ascertained populations of individuals with the diagnosis and populations matched for various environmental sources of influence thought to be important. Thus, they have sought to limit the comparison of cases vs controls on the dependent variables of interest to genetic factors to the extent possible. The results from such candidate gene and association studies have thus far been somewhat disheartening, probably because most such studies have limited statistical power to detect associations (Foroud et al. 2010; Heath et al. 2011). The usual finding is of LOD scores of around 2 for the strongest signals detected, followed by subsequent studies that fail to find a significant signal. There are however exceptions where strong associations have been reported [e.g. (Dick et al. 2010a)]. Recently, hope has turned to genome-wide association studies (GWAS), but the first generation of GWAS studies has also failed to find genome-wide significance. Heath and colleagues attempted to approach AD and heavy consumption using multivariate methods to achieve greater power than is achievable by focusing specifically on diagnostic category or specific symptoms. They examined 12000 Australian twins and family members, 2000 of which had an AD diagnosis. 8700 individuals were genotyped for SNPs throughout the genome. Factor scores for AD and heaviness of drinking were surveyed. They found no significant genome-wide linkages. Even more sobering, the effect size for each of the modestly associated SNPs averaged about 0.25% of QTL heritability, implying that perhaps hundreds of very small effect genetic variants affected AD risk. They state that meta-analysis of multiple GWAS studies may be needed to provide sufficient statistical power to delineate specific genetic contributions (Heath et al. 2011). Indeed, a report on combined data from two earlier studies recently achieved genome-wide significance (Zuo et al. 2012). Progress with human genetic studies of AD has been reviewed elsewhere (Edenberg et al. 2006; Foroud et al. 2010; Goldman et al. 2005; Kohnke 2008; Treutlein et al. 2011).
Contributions from genetic animal models
Animal modelers have mostly employed mice or rats, and I limit my discussion henceforth mostly to mice. They typically have targeted specific DSM symptoms, or attempted to identify or develop an animal that fulfills all 7 DSM criteria. For convenience, I turn to the DSM criteria and consider each to assess progress with employing genetic animal models.
Tolerance
Tolerance is alternatively defined as a reduction in response to a repeated equivalent dose of alcohol (ethanol), or the need to raise the alcohol dose in order to maintain the same level of effect (Kalant et al. 1971). Tolerance is very easily established with rodents in the laboratory using nearly any reliably measured sign of alcohol intoxication. Most frequently, measures of impaired motor performance are employed, but tolerance to ethanol hypothermia is also seen. There are multiple types of tolerance, differentiated based on the speed with which they develop. Inbred strains of mice and rats differ markedly in their degree of tolerance of various sorts, and lines of mice have been selectively bred for two forms of tolerance, rapid tolerance (Rustay et al. 2003; Rustay et al. 2004) and acute functional tolerance (Erwin et al. 1996). Tolerance also varies across lines of rats selectively bred for alcohol preference drinking (Waller et al. 1983). The genetic studies of different forms of alcohol tolerance in rodents were recently reviewed (Crabbe et al. 2011b).
Thus, tolerance is a clear example where genetic animal models have been successfully developed and studied. Tolerance is not an especially unambiguous symptom of AD, however. In a general population sample of patients with a DSM-IV AD diagnosis, 49% displayed tolerance (Grant 2000). To earn an AD diagnosis, it is necessary to ingest a lot of alcohol on a regular basis, which explains the frequency of this symptom. But, many individuals are demonstrably tolerant to alcohol who do not show enough other symptoms to qualify as dependent - thus, the specificity of tolerance is not high. In addition, there is another sense in which tolerance has been suggested to play a role in AD. It has been reported that individuals with low level of response to an alcohol challenge are at greater risk for earning an AD diagnosis later in life (Schuckit 1985; Schuckit et al. 1996). Close examination of these data suggests that such “low level of response” individuals may actually display high levels of acute functional tolerance to alcohol (Newlin et al. 1990). Here, a propensity to develop tolerance is taken as a risk factor for chronic drinking. However, tolerance has rarely been measured in the human studies, so little is known about whether a genetic risk for enhanced tolerance development is actually the key predictor of subsequent abuse. Here is an area where rodent research could contribute more to our understanding of this AD symptom’s role. Mouse lines were selectively bred to show high (HAFT) or low (LAFT) acute functional tolerance (Erwin et al. 1996). When these animals were compared for voluntary ethanol drinking, HAFT drank more alcohol than LAFT, though both lines drank very little (Erwin et al. 2000). Similarly, lines of mice were selected for high (HRT) vs low LRT) rapid tolerance to ethanol’s intoxicating effects. Unfortunately, they were never tested for drinking before cryopreservation (Rustay et al. 2004). Although there are scattered data on tolerance in lines of rats and mice bred for high vs low preference, we could benefit from studies where an attempt is made to relate tolerance to the intoxicating effects of drinking to future risk for excessive drinking. For a discussion of the potential application of the low level of response phenotype and the role of tolerance as a predictor of AD in mice, see (Crabbe et al. 2010a).
Withdrawal
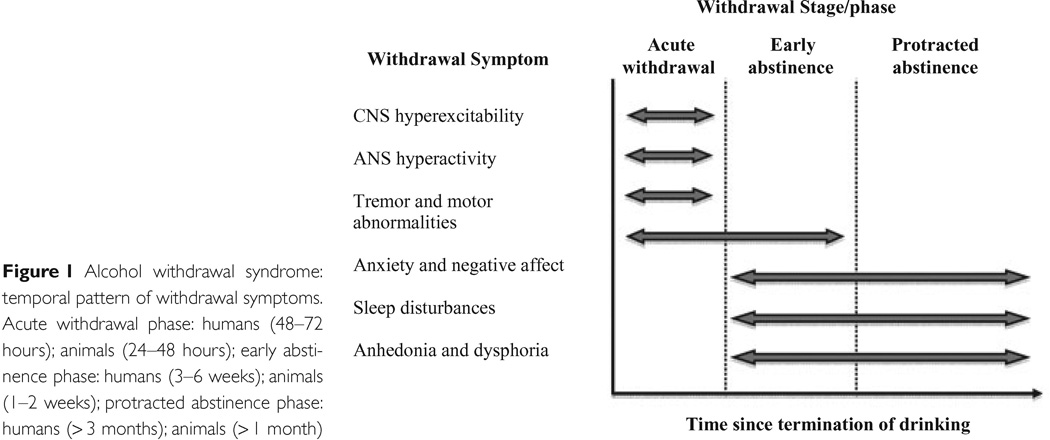
When alcohol administration is discontinued, a characteristic withdrawal reaction ensues comprising dysregulation of many central and autonomic system functions. The reactions are very similar across mammalian species, and follow similar time courses (see Figure 2). Withdrawal severity has been one of the aspects of AD most frequently modeled in rodents. Mice have shown all of the symptom groups shown in Figure 2 (Crabbe et al. 2011b), although sleep disturbances have been rarely studied (Veatch 2006). The motor disturbances used to assess alcohol tolerance can also be used to quantify withdrawal severity (Philibin et al. 2008; Philibin et al. 2011). Interestingly, the mouse genetic data show that tolerance and withdrawal seem to be largely distinct in genetic etiology [(Crabbe et al. 2011b); see next section].
Figure 2.
From Heilig et al. (2010) Addiction Biol 15:169–184.
Mouse studies of withdrawal have a long history in the alcohol research field, largely because Dora Goldstein invented a vapor inhalation method for rapidly inducing dependence in mice, as well as a simple scale for quantifying the central nervous system hyperexcitability of withdrawal using a handling-induced convulsion, or HIC (Goldstein et al. 1971). Curiously, rats do not show this sign of withdrawal. She showed that withdrawal HICs were heritable (Goldstein 1973), and we subsequently used her methods to develop lines of mice that were Withdrawal Seizure-Prone (WSP) or -Resistant (WSR) following chronic vapor inhalation (Crabbe et al. 1985). There is now a rather large literature on the genetics of alcohol withdrawal HIC, which has been reviewed elsewhere (Crabbe et al. 2011b; Finn et al. 2004; Metten et al. 1996).
The HIC is a very sensitive sign of withdrawal disturbance and can be seen to be exacerbated even a few hours following an acute intraperitoneal injection of alcohol or other depressant drugs (Metten et al. 1994). Acute alcohol and pentobarbital withdrawal HIC have been subjected to an extensive gene mapping effort by Kari Buck and her group (Buck et al. 1997). Using inbred strains, selected lines, recombinant inbred strains, congenics, and many other methods, she has successfully identified Mpdz as a quantitative trait gene (Shirley et al. 2004) and continues to pursue its function (Chen et al. 2009a; Chen et al. 2011a). Interestingly, human genetic studies have identified markers in the region of human chromosome 9 that is syntenic with the mapped region in mouse, and these studies show signs of association with alcohol-related phenotypes [for review, see (Ehlers et al. 2010)].
Thus we have a great deal of genetic data from mice addressing alcohol withdrawal severity, but it is nearly all about withdrawal HIC. I discuss later some other withdrawal characteristics of importance, and offer here one example of why more knowledge of withdrawal may be important. Several studies with different mouse genetic animal models suggest that genotypes that display high alcohol preference drinking tend to show modest withdrawal HIC after being made physically dependent. The converse is also true - low drinking is associated with high withdrawal HIC (Metten et al. 1998). These data suggest that susceptibility to severe withdrawal may be protective against AD, and some studies are beginning to explore other aspects of withdrawal that may mediate this genetic relationship.
The relationship between tolerance and withdrawal
Any animal that displays an alcohol withdrawal sign can be demonstrated to be tolerant to alcohol, if a sensitive enough assay is employed. The converse, however, is not true. As noted earlier, the notion of multiple AD typologies has a long history, and this concept implies that there may not be genetic uniformity of risk for AD. However, the design of human genetic risk studies almost always assumes that DSM-IV AD represents a single phenotype to which each of the multiple symptoms contributes (Saha et al. 2006). The genetic animal model literature, on the other hand, suggests that genetic contributions to alcohol responses differ from response to response. For example, systematic studies have shown that genotypes sensitive to one intoxicating effect of an acute ethanol injection are not necessarily sensitive to another (Crabbe et al. 2005). A recent genetic epidemiological study in twins explored whether a single or multiple genetic factors contributed to DSM-IV risk for AD by exploring the factor structure of genetic contributions to the individual diagnostic criteria using structural equation modeling. This exploration revealed that three independent genetic factors were needed to capture risk. Furthermore, the symptoms “withdrawal” and “tolerance” loaded on different factors (Kendler et al. 2011). Tolerance was genetically correlated with a screening question assessing excessive quantity and frequency of drinking, as well as the symptom of allocating much time to use-related behaviors. Withdrawal severity was genetically correlated with continued use despite complications and with reducing important life activities. A systematic review of the rat and mouse genetic animal model literature also found little evidence for genetic correlation between laboratory studies of ethanol tolerance and withdrawal. The evidence adduced mostly comprised correlations between mean phenotypic values for standard inbred strains or BXD recombinant inbreds on tolerance and withdrawal measures (Crabbe et al. 2011b). Earlier data had also found that the WSP and WSR mouse selected lines did not differ in alcohol tolerance magnitude (Crabbe et al. 1986).
Together, this suggests that laboratory animal models may be very useful for addressing the genetic complexities of AD symptom clusters (Kendler et al. 2011). If risk for different AD subtypes reflects different constellations of genes, new genetic animal models might improve our ability to identify those risk and protective genes. Animal models to date have generally targeted simple, discrete phenotypes in an attempt to increase the reliability of phenotypic assessment and to reduce the genetic complexity. Perhaps [following (Kendler et al. 2011)] we should try to identify or develop genotypes that display both severe withdrawal and persistent alcohol drinking despite negative consequences.
Use greater than intended, and persistent desire/repeated attempts to quit drinking
Despite our best efforts, we cannot fathom the intent of a mouse or rat. We can observe the strength of association between a stimulus and an animal’s response, and manipulate the strength of that association, but there is no conceivable way to model “use greater than intended” in a laboratory rodent. For the same reason, neither is it reasonable to characterize a rodent’s behavior as an “attempt to quit drinking”-- the statement implies an interoceptive state (“desire”) or intent (“attempts”) and neither is observable.
Devotion of much time/activity to obtaining, using, or recovering from alcohol; giving up or reducing important social, occupational or recreational activities
These seem like reasonable targets for genetic animal modeling. Although they are dissociable in humans and their real world contexts, they seem related when applied to laboratory settings with rodents. If we ignore “occupational” and “recreational,” replacing these descriptors with “other,” then certainly the time allocation of a rodent’s behavior can be monitored. A limited number of behaviors can be assessed in the home cage and display genetic variation both quantitatively and temporally (Goulding et al. 2008). Videotracking systems have the capacity to make very fine-grained assessments of small genetic differences in behavior (Drai et al. 2001; Fonio et al. 2006). I will return to this in the context of seeking alcohol versus other available rewards.
Continued use despite knowledge of adverse consequences
This, too, seems like a straightforward behavior of humans with AD. Can rodents be shown to engage in analogous self-destructive behavior? The subtle point here is the term “knowledge of,” which may be self-reported by humans, but is difficult to demonstrate indirectly in rodents. First, let us consider the most extreme adverse consequence of alcohol seeking - overdose and death. There are two lines of data regarding such persistence of which I am aware. In the late 1970's, Anthony Deutsch’s group showed that after passive intragastric exposure, rats would learn to drink a flavored solution in order to obtain an intragastric infusion of ethanol (Deutsch et al. 1980). These early experiments reported that it was necessary to limit access to intragastric alcohol consumption to prevent the rats from overdosing and dying. The intragastric model has recently been revived by Tara Fidler and Chris Cunningham, who have shown similar effects in mice of several genotypes (Fidler et al. 2011b; Fidler et al. 2011a). They, too have found that animals will overdose by this route of administration. While it may be argued that the animals are unaware of these adverse consequences, adverse they certainly are, and intragastric alcohol consumption persists for several days even when water is freely available. This model is being actively pursued (see below).
A second example is a line of early research with mice that explored the role of alcohol metabolism and blood alcohol levels in controlling intake. Working initially with Vincent Dole, Tom Gentry showed that C57BL/6J mice, known to be genetic alcohol preferrers, would continue to drink alcohol even when they were administered the long-acting alcohol dehydrogenase inhibitor, 4-methyl pyrazole. Half the population of mice in one study had to be rescued from overdose after a week of persistently drinking enough to reach blood alcohol levels in excess of 200 mg% (Gentry 1985). This interesting approach could also be pursued in other genotypes to explore its generality and relationship to other genetic predispositions.
There has been a good bit of recent interest in another approach to achieving adverse consequences, application of a punisher to an animal engaged in voluntary alcohol drinking. It has clearly been shown for rats self-administering cocaine intravenously that they will develop an insensitivity to coupled foot shocks that would suppress self-administration in an earlier stage of the animal’s drug history. The phenomenon has been linked to the shift from ‘goal-directed” to “habitual” responding and the neural bases are under active investigation (Vanderschuren et al. 2004). Alcohol researchers have not published papers that I am aware of showing development of insensitivity to shock, which suggests that it has not been possible thus far to see this in the laboratory. Instead, investigators have proposed that the willingness of animals to continue to ingest alcohol solutions even though they are adulterated with bitter-tasting quinine is evidence of “drinking despite knowledge of adverse consequences.” Initial use of this method was applied to rats drinking alcohol with chronic access for several months and experiencing repeated periods of forced abstinence. When alcohol access was reinstated, these animals achieved relatively high intakes. Wolffgramm and others state that these rats have transitioned to a state of “behavioral dependence” (Wolffgramm et al. 1991) or “loss of control” characteristic of “addiction” (Wolffgramm et al. 1995) (Wolffgramm et al. 2000) because when quinine was added to the ethanol solutions, animals with long prior experience with drinking ethanol continued to drink ethanol, while animals previously naive to ethanol avoided the adulterated solutions. Subsequent use of this technique has labeled the behavior “inflexible” or “indifferent” alcohol drinking and it has been reported in Sardinian Preferring rats and C57BL/6J mice (Lesscher et al. 2010; Loi et al. 2010).
These studies are not entirely convincing as currently performed. In the older rat studies, the authors interpret the continued intake of alcohol in the long-term chronic drinkers as evidence of insensitivity to quinine. However, the degree of reduction of intake in the chronically exposed groups was quite similar to the degree of attenuation of intake seen in the previously naive individuals. That is, quinine did not appear to be any more potent in chronic drinkers. In the study with C57BL/6J mice (Lesscher et al. 2010), a dose-response analysis of quinine’s attenuating effects on ethanol drinking was performed, but studies to date cannot rule out the possibility that the effect of prior experience with alcohol is simply to change the perceived taste of quinine. Quinine at low concentrations is preferred by rodents under some conditions (Hopf et al. 2010; Tordoff et al. 2008; Wayner et al. 1972). The combined flavors of ethanol and quinine mixtures have not been subjected to rigorous analysis of taste preference, and the change in drinking mixed solutions after experience with ethanol over time may represent reduced quinine avoidance and not the increased rewarding value of ethanol that is the investigators’ preferred interpretation. The exception to this may be a recent study of Wistar rats (Hopf et al. 2010). These authors used multiple doses of quinine or quinine plus alcohol offered under different conditions to show that after long experience with alcohol under intermittent access conditions, quinine did not reduce alcohol intake but was able to affect sucrose preference and water intake.
Challenges and opportunities
Thus, certain aspects of human AD have been successfully modeled in rats and mice. In the last part of this review, I turn to some areas where I believe further work could be targeted that would have a good likelihood of achieving progress. One frustrating limitation of animal models is the struggle to infer motivation from their behavior. Motivation to consume alcohol is such a central feature of AD, but, lacking self report, we still do not understand why some mice and rats ingest a great deal of alcohol while others avoid it. Are mice and rats choosing to drink alcohol simply because they are bored? We know that taste is important, but cannot figure out exactly how important it is. Below, I raise a series of questions and discuss some approaches the field is considering or that I believe we should consider.
1. How can we get mice or rats to drink enough to sustain a state of intoxication?
We don’t understand why it is so difficult to engage a rodent in self-destructive behavior regarding alcohol. A good example of this is that despite more than 50 years of research with genetically preferring rats and mice, it is rare that even high preferrers will drink enough alcohol to become intoxicated. Some of the protocols scientists have used to overcome this barrier are reviewed elsewhere [e.g., see (Rhodes et al. 2005)]. In general, they require substantial food and/or water restriction, or schedules of access or other conditions that are manipulated over many weeks or even months.
At least three recent approaches appear to show promise. In our laboratory, we have offered mice alcohol for a limited period during their circadian dark phase and found that some genotypes will ingest enough alcohol to reach intoxicating blood alcohol levels (Rhodes et al. 2005; Rhodes et al. 2007). We have successfully selectively bred two mouse lines, the High Drinking in the Dark 1 and 2 (HDID-1,-2) genotypes, that reach intoxicating blood alcohol levels (>100 mg%) during these DID sessions (Crabbe et al. 2009) (Crabbe et al. 2010b). These animals do not show particularly high ethanol preference during continuous access, suggesting that the genetic contributions to DID and preference are somewhat distinct (Crabbe et al. 2011c), and are not strikingly different from the control line in sensitivity to, tolerance to, or dependence on ethanol (Crabbe et al., 2012, in press a, b). They reach higher blood alcohol levels than control mice during DID sessions by drinking in larger bouts than controls (Barkley-Levenson et al. 2011). The DID binge-like drinking phenotype itself is proving to be a convenient method to study the neurobiology of excessive consumption [for review, see (Crabbe et al. 2011a); for a bibliography, see http://www.scripps.edu/cnad/inia/methodology.html].
A recent study has examined drinking during standard two-bottle preference tests in lines of mice selectively bred for High (HAP) or Low (LAP) Alcohol Preference using much the same protocol used for selection of multiple rat lines such as P/NP and AA/ANA. By studying overnight drinking and collecting data each 2 hr, these investigators found that HAP-1 mice, and a new line created by crossing HAP-1 and HAP-2 mice to introduce new genetic variability, both drink substantial amounts of ethanol (Matson et al. 2011). Both lines drink more than 20 g/kg ethanol/day, and sustain overnight blood alcohol levels exceeding 200 mg%, greater than the high preferring C57BL/6J inbred strain [14 g/kg/day, 60 mg%; (Matson et al. 2011)]. These animals always have water freely available, and must surely be intoxicated, although this remains to be demonstrated.
A third approach is to offer ethanol in an intermittent scheduled access pattern where water and ethanol are offered only 3 days/week (or every other day). Under intermittent access, intake is increased as compared with continuous access (Wise 1973). The method is gaining in popularity [e.g., (Carnicella et al. 2009; Simms et al. 2010)]. This effect has been reported in multiple genotypes of rats. A recent paper reported high intakes in C57BL/6J mice offered intermittent access (Hwa et al. 2011). However, we have not seen such an effect in either HDID-1, HDID-2 or the HS control mice, and the degree of elevation we found in C57BL/6J mice was less than that reported by Hwa et al [Crabbe et al, submitted]. Thus, the effect of intermittency may be limited to certain genotypes. In a variant of the intermittent access protocol, water plus two different ethanol concentrations are offered for 3 X 1 hr periods during the circadian dark. This “multiple scheduled access” (MSA) procedure leads to elevated intakes in multiple genotypes of rats, and P rats reach levels exceeding 100 mg% after several weeks (Bell et al. 2009). In a recent study, both adolescent and adult P rats were found to drink intoxicating amounts of alcohol (Bell et al. 2011).
For a more extensive review of procedures used to explore excessive alcohol consumption, see (Becker 2011).
2. Will rodents drink to alleviate withdrawal?
This is an important goal, because much human relapse drinking is attributed to the negatively reinforcing effects of withdrawal self-medication. Howard Becker’s group has shown convincingly that C57BL/6J mice will increase their voluntary intake of ethanol when water is freely available in 2 hr limited access sessions during the circadian dark, offered each day during the withdrawal periods following cycles of dependence-inducing vapor inhalation exposure (Becker et al. 2004; Griffin, III et al. 2009). This paradigm has recently been tested in other genotypes, and HAP-2 male (but neither HAP-2 female nor LAP-2 male or female) mice showed escalated drinking during withdrawal (Lopez et al. 2011). The procedure is effective in rats (O'Dell et al. 2004; Rimondini et al. 2002; Roberts et al. 2000; Sommer et al. 2008). What is needed in this area is convincing evidence that the increased drinking during withdrawal is an attempt to alleviate withdrawal, although there are some indications that there is a roughly similar time course for withdrawal drinking and anxiety-like behavior (Valdez et al. 2002).
The intragastric alcohol consumption model mentioned earlier may reflect maintenance of drinking to prevent the occurrence of withdrawal. An alternative explanation for these findings is that the animals develop tolerance to post-absorptive aversive effects of alcohol. Teasing apart which phenomenon underlies the intragastric consumption presents a methodological problem somewhat similar to that faced by studies of quinine adulteration. However, the intragastric route offers the advantage of probably avoiding most of the confounding effects of taste (Cunningham et al. 2000). Two intriguing findings with this model have resulted from studies of C57BL/6J and DBA/2J mice. C57BL/6J mice (“sippers”) tend to administer their alcohol in many small bouts, while DBA/2J mice (“gulpers”) ingest alcohol in large bouts. And, during withdrawal, the normally alcohol-avoiding DBA/2J mice take in rather substantial quantities of alcohol, perhaps because the aversive taste is irrelevant (Fidler et al. 2011b).
3. Can we modify drinking environmentally?
Many aspects of the laboratory rodent’s environment do not appear to be powerful modulators of alcohol drinking. We can assume that specifics of food, bedding, light cycle, housing conditions, and many other variables are not extremely influential because inbred mouse strain surveys of ethanol preference drinking have shown remarkable similarity in strain-specific drinking across multiple laboratories and more than 50 years of data (Wahlsten et al. 2006). In a study that attempted to control every possible variable across three laboratories, multiple genotypes of mice showed remarkably similar intake patterns for ethanol drinking, even though the uncontrolled variables (including specific experimenters, tap water, air characteristics, etc) led to some substantial differences in behavior for other traits (Crabbe et al. 1999).
One aspect of the environment has been addressed because of its known importance for human AD, namely, the social environment. In a series of early studies, genetically preferring C57BL/6J mice and genetically avoiding DBA/2J mice were compared for adult ethanol preference drinking after social housing for 7 weeks starting at weaning with members of the same strain, or with companions of the opposite strain. Alcohol was available during this period. When preference was then tested in now singly housed mice, C57BL/6J mice previously housed with low-drinking DBA/2J mice drank less than animals housed with other C57BL/6Js, but the converse was not true - DBA/2J mice did not increase in the face of high drinking peers. Two other papers in this elegant series of studies explored the effect of cross fostering at weaning to high vs low drinking dams, and to the effect of intrauterine environment, achieved by ova transplantation (Randall et al. 1975a; Randall et al. 1975b; Randall et al. 1975c).
Recently, monogamous voles were studied for the possibility of exerting social modulatory effects on drinking. A number of voles were assessed for preference, and divided into high vs low drinkers. Animals were then paired socially with another in cages with a divider allowing orofacial contact but which assessed drinking separately. When housed with a cage mate who was a low drinker, high drinking voles showed less intake than those paired with another high drinker, but still higher levels than low drinkers paired with low drinkers. As in the older study with mice, the low drinking phenotype was less plastic than high drinking (Anacker et al. 2011b; Anacker et al. 2011a). An analogous, but facilitatory, influence of social interaction on nicotine self-administration has just been reported in rats, employing a novel social caging method (Chen et al. 2011b).
Genetic differences in social behaviors of many sorts have been of increasing interest in recent years (Robinson et al. 2008). Several interesting behavioral paradigms have been explored in mice (Chen et al. 2009b; Silverman et al. 2010; Yang et al. 2011). Very little work has explored social behavior as either a risk factor for, a target of alcohol, or as a potential modulator of alcohol-related behavior.
4. How is neuroadaptation to ethanol different during adolescence?
Given that adolescent onset of abusive drinking (especially if coupled with family history risk) is perhaps the worst prognostic of AD risk (Zimmermann et al. 2007), there is understandable interest in exploring alcohol-related behavior in adolescent rats and mice. Much has been learned from such studies [for reviews, see (McBride et al. 2005; Spear 2011)]. A very interesting recent study employed the multiple scheduled access protocol described above and compared drinking in adolescent vs adult female and male P rats. In adult rats of both sexes, multiple binge-like access reduced drinking as compared with continuous access. In adolescents, however, it increased intake, particularly in female rats. This suggests that in addition to being a period of heightened sensitivity to alcohol’s rewarding effects and reduced sensitivity to its aversive effects, adolescence may represent a period of more general vulnerability to a spectrum of alcohol’s effects (Bell et al. 2011). More studies are needed that target this period of brain maturation in multiple genetic animal models.
5. How does neuroadaptation to alcohol change in long term studies?
A prominent recent theoretical approach has postulated that AD represents a state of ‘allostasis,” where emotional behavior in particular is dysregulated (Koob et al. 2008). Mechanisms that normally regulate behavior homeostatically to balance between approach and use of alcohol and avoidance of overuse are driven to a new set of “basal” conditions by the chronic presence of alcohol interspersed with withdrawal states. Abstinence reveals disruptions in the limbic system, particularly those areas subserving the generalized stress response, leading to anxiety and distress which in turn drives self-medication by relapse to drinking. For AD patients, these disruptions persist for many weeks or even months into abstinence (Heilig et al. 2010).
In the animal model literature, we know a great deal about the behavioral sequelae during the initial 72 hours following cessation of chronic alcohol administration. We know a good bit less about most changes that occur during the next few days, and very little about behavioral and biological changes even 2–3 weeks after withdrawal. Even allowing for the different life spans of rodents and humans, the focus of the animal literature should be extended. For example, as Becker’s group was developing the protocols for testing increased drinking during cycles of withdrawal, they discovered that the behavioral depression that accompanies the first 2–3 days of withdrawal in mice interfered with the animals’ ingestive behavior, and work with the model progressed once they began to withhold the opportunity to return to drinking until hr 72 of withdrawal (H.C. Becker, personal communication). More work is needed to establish firmly the temporal relationships between withdrawal, anxiety-like behavior, responsiveness to stress, and drinking. Some have long advocated the need to administer alcohol to rodents for very long periods [see, e.g.,(Spanagel et al. 1999; Wolffgramm et al. 2000)]. However, most such studies have used unusual patterns of administration of the drug, and little work has been done attempting to ascertain the time course of development of behavioral changes during chronic administration and withdrawal. Given the appearance of new behavioral protocols for studying alcohol drinking, it would be productive to explore some of these newer methods in longer-term studies with the explicit goal of documenting temporal parallelisms between rodent behavior and the fairly well-understood time course of human withdrawal-related changes. Genetic animal models could be very useful for advancing such studies.
6. What are the genomic and biological contributions of behaviors other than alcohol preference drinking?
We know far more about two-bottle preference drinking than about any other alcohol-related behavior. This wealth of literature has allowed us to draw some clear biological conclusions, particularly about the sequelae of long-term moderate drinking. Long-term moderate drinking, however, is not a characteristic of AD. Many behaviors of interest have been little studied in the context of alcohol, and here are three where there are promising indications of potential genetic value.
Impulsivity
Some time ago, impulsive-like behavior in mice was suggested to be negatively genetically correlated with preference drinking across a panel of inbred mouse strains (Logue et al. 1998). Recent studies have extended the range of both genotypes and tasks and shown that there is significant genetic contribution to several types of disinhibited behavior in both rats and mice. Furthermore, disinhibition is sometimes linked with alcohol drinking or other ethanol-related behaviors, but findings thus far are not consistent (Wilhelm et al. 2007; Wilhelm et al. 2008; Wilhelm et al. 2009). The lack of consistency appears to be due in part because there are multiple forms of impulsive-like behavior which are modeled by different tasks (Dick et al. 2010b). One interesting example is the risky decision-making task, where effort to obtain food reward offers the choice between a small reward with a low probabiliy of associated shock versus a large food reward with a higher probability of shock. Although drugs of abuse including alcohol affect such risky decision making when acutely administered [(Mitchell et al. 2011; Simon et al. 2009); for review, see (Mitchell 2011)], I know of no studies where such contingencies have been offered to rodents when alcohol is the reward. It is nearly unknown whether impulsive-like behavior changes during ethanol withdrawal - if it were to be increased, it could contribute to relapse (see next section). Because virtually identical tasks can be given to humans and rodents, this general trait domain offers fertile grounds for future exploration.
Loss of cognitive flexibility
As noted earlier, a shift from goal-directed to habit-driven cognitive processing is thought to accompany the development of addictive-like behavior. A recent study suggests that during alcohol withdrawal, premature responding in the 5-choice serial reaction time task persists longer across weekly tests while it decays in control mice (Walker et al. 2011). Current data could be explained either by persistent impulsive behavior, or by a failure to adapt responding with repeated testing. Many cognitive-behavioral domains can now be approached in mice with new and sophisticated methodologies, and these methods are sensitive to genetic differences across strains [see, e.g., (Bussey et al. 2011; Lederle et al. 2011)].
Value of alcohol as a reward
Consumption of alcohol cannot directly indicate the value to the organism of alcohol as a reinforcer. Does a genotype that drinks more than another do so because the alcohol is more rewarding? Or, is the alcohol less rewarding and therefore greater intake is required to achieve the desired end point? How best to measure consumption in rodents for translational studies is discussed elsewhere (Leeman et al. 2010). How best to determine the reward value of alcohol in comparison with alternative rewards is a very difficult problem, also discussed elsewhere [(Stephens et al. 2010), and see also (Cantin et al. 2010; Lenoir et al. 2007)]. Continuing to pursue the knotty puzzles presented in interpreting data from such protocols addresses the heart of motivation for alcohol, and genetic animal models may be of value here. Particularly useful here would be studies that could convincingly demonstrate a progressive over-valuation of alcohol versus other rewards.
Conclusions
I have made no attempt to be exhaustive in this review. I have for the most part ignored comorbidities and endophenotypes, each of which domains has a large genetic animal model literature of its own (e.g., anxiety-like behaviors). Many of the topics I have touched upon escape the organizational boundaries to which I confined them for convenience. Just to give one example of this, more studies of the genetics of murine impulsive behavior (or is it loss of cognitive flexibility?) during adolescence could address its value as a genetic risk factor. Similar studies during protracted withdrawal may help us understand relapse better. Social influences during either of these developmental time periods may be important, and behavioral disinhibition as a trait may influence sensitivity to social influences. I believe an honest answer to the question posed in the title of this review is, “No.” But I also believe that we have a much better idea now than even a few years ago about where we are trying to go, and the transportation tools at our disposal continue to improve.
Acknowledgments
Dr. Crabbe’s research is supported by the NIAAA [Integrative Neuroscience Initiative on Alcoholism (INIA-West) grant AA13519 and Center grant AA10760], and the US Department of Veterans Affairs. I thank Chris Cunningham for his helpful comments on a draft of this MS.
Footnotes
This review is based on the 16th Mark Keller Honorary Lecture from the National Institute on Alcohol Abuse and Alcoholism, delivered October 25, 2011 at the National Institutes of Health.
Dr. Crabbe reports no conflicts of interest.
References
- 1.Anacker AM, Loftis JM, Kaur S, Ryabinin AE. Prairie voles as a novel model of socially facilitated excessive drinking. Addict Biol. 2011a;16:92–107. doi: 10.1111/j.1369-1600.2010.00234.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Anacker AM, Loftis JM, Ryabinin AE. Alcohol intake in prairie voles is influenced by the drinking level of a peer. Alcohol Clin Exp Res. 2011b;35:1884–1890. doi: 10.1111/j.1530-0277.2011.01533.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Anton RF, Oroszi G, O'Malley S, Couper D, Swift R, Pettinati H, Goldman D. An evaluation of mu-opioid receptor (OPRM1) as a predictor of naltrexone response in the treatment of alcohol dependence: results from the Combined Pharmacotherapies and Behavioral Interventions for Alcohol Dependence (COMBINE) study. Arch Gen Psychiatry. 2008;65:135–144. doi: 10.1001/archpsyc.65.2.135. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Babor T, Lauerman R. Classification and forms of inebriety: Historical antecedents of alcoholic typologies. In: Galanter M, editor. Recent Developments in Alcoholism. Vol. 4. New York: Plenum Press; 1986. pp. 113–144. [DOI] [PubMed] [Google Scholar]
- 5.Barkley-Levenson AM, Crabbe JC. Ethanol drinking microstructure of a High Drinking in the Dark selected mouse line. Alcohol Clin Exp Res. 2012 doi: 10.1111/j.1530-0277.2012.01749.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Becker HC. Animal models of excessive alcohol consumption. In: Spanagel R, Sommers WH, editors. Curr Topics Behav Neurosci. Berlin: Springer-Verlag; 2012. Epub ahead of print. [Google Scholar]
- 7.Becker HC, Lopez MF. Increased ethanol drinking after repeated chronic ethanol exposure and withdrawal experience in C57BL/6 mice. Alcohol Clin Exp Res. 2004;28:1829–1838. doi: 10.1097/01.alc.0000149977.95306.3a. [DOI] [PubMed] [Google Scholar]
- 8.Bell RL, Kimpel MW, McClintick JN, Strother WN, Carr LG, Liang T, Rodd ZA, Mayfield RD, Edenberg HJ, McBride WJ. Gene expression changes in the nucleus accumbens of alcohol-preferring rats following chronic ethanol consumption. Pharmacol Biochem Behav. 2009;94:131–147. doi: 10.1016/j.pbb.2009.07.019. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Bell RL, Rodd ZA, Smith RJ, Toalston JE, Franklin KM, McBride WJ. Modeling binge-like ethanol drinking by peri-adolescent and adult P rats. Pharmacol Biochem Behav. 2011;100:90–97. doi: 10.1016/j.pbb.2011.07.017. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Bucholz KK, Heath AC, Reich T, Hesselbrock VM, Kramer JR, Nurnberger JI, Jr, Shuckit MA. Can we subtype alcoholism? A latent class analysis of data from relatives of alcoholics in a multicenter family study of alcoholism. Alcohol Clin Exp Res. 1996;20:1462–1471. doi: 10.1111/j.1530-0277.1996.tb01150.x. [DOI] [PubMed] [Google Scholar]
- 11.Buck KJ, Metten P, Belknap JK, Crabbe JC. Quantitative trait loci involved in genetic predisposition to acute alcohol withdrawal in mice. J Neurosci. 1997;17:3946–3955. doi: 10.1523/JNEUROSCI.17-10-03946.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Bussey TJ, Holmes A, Lyon L, Mar AC, McAllister KA, Nithianantharajah J, Oomen CA, Saksida LM. New translational assays for preclinical modelling of cognition in schizophrenia: The touchscreen testing method for mice and rats. Neuropharmacology. 2012;62:1191–1203. doi: 10.1016/j.neuropharm.2011.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Cabib S, Orsini C, Le Moal M, Piazza PV. Abolition and reversal of strain differences in behavioral responses to drugs of abuse after a brief experience. Science. 2000;289:463–465. doi: 10.1126/science.289.5478.463. [DOI] [PubMed] [Google Scholar]
- 14.Cantin L, Lenoir M, Augier E, Vanhille N, Dubreucq S, Serre F, Vouillac C, Ahmed SH. Cocaine is low on the value ladder of rats: possible evidence for resilience to addiction. PLoS One. 2010;5:e11592. doi: 10.1371/journal.pone.0011592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Carnicella S, Amamoto R, Ron D. Excessive alcohol consumption is blocked by glial cell line-derived neurotrophic factor. Alcohol. 2009;43:35–43. doi: 10.1016/j.alcohol.2008.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Chen G, Cuzon CV, Wang J, Beck A, Heinz A, Ron D, Lovinger DM, Buck KJ. Striatal involvement in human alcoholism and alcohol consumption, and withdrawal in animal models. Alcohol Clin Exp Res. 2011a;35:1739–1748. doi: 10.1111/j.1530-0277.2011.01520.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Chen G, Reilly MT, Kozell LB, Hitzemann R, Buck KJ. Differential activation of limbic circuitry associated with chronic ethanol withdrawal in DBA/2J and C57BL/6J mice. Alcohol. 2009a;43:411–420. doi: 10.1016/j.alcohol.2009.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Chen H, Sharp BM, Matta SG, Wu Q. Social interaction promotes nicotine self-administration with olfactogustatory cues in adolescent rats. Neuropsychopharmacology. 2011b;36:2629–2638. doi: 10.1038/npp.2011.149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Chen Q, Panksepp JB, Lahvis GP. Empathy is moderated by genetic background in mice. PLoS One. 2009b;4:e4387. doi: 10.1371/journal.pone.0004387. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chesler EJ, Wilson SG, Lariviere WR, Rodriguez-Zas SL, Mogil JS. Identification and ranking of genetic and laboratory environment factors influencing a behavioral trait, thermal nociception, via computational analysis of a large data archive. Neurosci Biobehav Rev. 2002;26:907–923. doi: 10.1016/s0149-7634(02)00103-3. [DOI] [PubMed] [Google Scholar]
- 21.Cloninger CR. Neurogenetic adaptive mechanisms in alcoholism. Science. 1987;236:410–416. doi: 10.1126/science.2882604. [DOI] [PubMed] [Google Scholar]
- 22.Crabbe JC, Bell RL, Ehlers CL. Human and laboratory rodent low response to alcohol: is better consilience possible? Addict Biol. 2010a;15:125–144. doi: 10.1111/j.1369-1600.2009.00191.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Crabbe JC, Colville AM, Kruse LC, Cameron AJ, Spence SE, Schlumbohm JS, Huang LC, Metten P. Ethanol tolerance and withdrawal in High Drinking in the Dark selectively bred mice. Alcohol Clin Exp Res. 2012b doi: 10.1111/j.1530-0277.2011.01715.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Crabbe JC, Harris RA, Koob GF. Preclinical studies of alcohol binge drinking. Ann NY Acad Sci. 2011a;1216:24–40. doi: 10.1111/j.1749-6632.2010.05895.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Crabbe JC, Kendler KS, Hitzemann RJ. Modeling the diagnostic criteria for alcohol dependence with genetic animal models. In: Spanagel R, Sommer WH, editors. Curr Topics Behav Neurosci. Berlin: Springer-Verlag; 2011b. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Crabbe JC, Kosobud A. Sensitivity and tolerance to ethanol in mice bred to be genetically prone or resistant to ethanol withdrawal seizures. J Pharmacol Exp Ther. 1986;239:327–333. [PubMed] [Google Scholar]
- 27.Crabbe JC, Kosobud A, Young ER, Tam BR, McSwigan JD. Bidirectional selection for susceptibility to ethanol withdrawal seizures in Mus musculus. Behav Genet. 1985;15:521–536. doi: 10.1007/BF01065448. [DOI] [PubMed] [Google Scholar]
- 28.Crabbe JC, Kruse LC, Colville AM, Cameron AJ, Spence SE, Schlumbohm JS, Huang LC, Metten P. Ethanol sensitivity in High Drinking in the Dark selectively bred mice. Alcohol Clin Exp Res. 2012a doi: 10.1111/j.1530-0277.2012.01735.x. Epub ahead of print. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Crabbe JC, Metten P, Cameron AJ, Wahlsten D. An analysis of the genetics of alcohol intoxication in inbred mice. Neurosci Biobehav Rev. 2005;28:785–802. doi: 10.1016/j.neubiorev.2004.08.002. [DOI] [PubMed] [Google Scholar]
- 30.Crabbe JC, Metten P, Rhodes JS, Yu C-H, Brown LL, Phillips TJ, Finn DA. A line of mice selected for high blood ethanol concentrations shows drinking in the dark to intoxication. Biol Psychiat. 2009;65:662–670. doi: 10.1016/j.biopsych.2008.11.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Crabbe JC, Phillips TJ, Belknap JK. The complexity of alcohol drinking: studies in rodent genetic models. Behav Genet. 2010b;40:737–750. doi: 10.1007/s10519-010-9371-z. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Crabbe JC, Spence SE, Brown LL, Metten P. Alcohol preference drinking in a mouse line selectively bred for high drinking in the dark. Alcohol. 2011c;45:427–440. doi: 10.1016/j.alcohol.2010.12.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Crabbe JC, Wahlsten D, Dudek BC. Genetics of mouse behavior: interactions with laboratory environment. Science. 1999;284:1670–1672. doi: 10.1126/science.284.5420.1670. [DOI] [PubMed] [Google Scholar]
- 34.Cunningham CL, Fidler TL, Hill KG. Animal models of alcohol's motivational effects. Alcohol Res Health. 2000;24:85–92. [PMC free article] [PubMed] [Google Scholar]
- 35.Deutsch JA, Cannis JT. Rapid induction of voluntary alcohol choice in rats. Behav Neural Biol. 1980;30:292–298. doi: 10.1016/s0163-1047(80)91174-7. [DOI] [PubMed] [Google Scholar]
- 36.Dick DM, Meyers J, Aliev F, Nurnberger J, Jr, Kramer J, Kuperman S, Porjesz B, Tischfield J, Edenberg HJ, Foroud T, Schuckit M, Goate A, Hesselbrock V, Bierut L. Evidence for genes on chromosome 2 contributing to alcohol dependence with conduct disorder and suicide attempts. Am J Med Genet B Neuropsychiatr Genet. 2010a;153B:1179–1188. doi: 10.1002/ajmg.b.31089. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Dick DM, Smith G, Olausson P, Mitchell SH, Leeman RF, O'Malley SS, Sher K. Understanding the construct of impulsivity and its relationship to alcohol use disorders. Addict Biol. 2010b;15:217–226. doi: 10.1111/j.1369-1600.2009.00190.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Drai D, Golani I. SEE: a tool for the visualization and analysis of rodent exploratory behavior. Neurosci Biobehav Rev. 2001;25:409–426. doi: 10.1016/s0149-7634(01)00022-7. [DOI] [PubMed] [Google Scholar]
- 39.Edenberg HJ, Dick DM, Xuei X, Tian X, Almasy L, Bauer LO, Crowe RR, Goate Al, Hesselbrock V, Jones K, Kwon J, Li T-K, Nurnberger JI, Jr, O'Connor SJ, Reich T, Rice J, Schuckit MA, Porjesz B, Foroud T, Begleiter H. Variations in GABRA2, encoding the [alpha]2 subunit of the GABA[subA] receptor, are associated with alcohol dependence and with brain oscillations. Am J Hum Genet. 2004;74:705–714. doi: 10.1086/383283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Edenberg HJ, Foroud T. The genetics of alcoholism: identifying specific genes through family studies. Addict Biol. 2006;11:386–396. doi: 10.1111/j.1369-1600.2006.00035.x. [DOI] [PubMed] [Google Scholar]
- 41.Ehlers CL, Walter NAR, Dick DM, Buck KJ, Crabbe JC. A comparison of selected quantitative trait loci associated with alcohol use phenotypes in humans and mouse models. Addict Biol. 2010;15:185–199. doi: 10.1111/j.1369-1600.2009.00195.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Enoch MA. The role of GABA (A) receptors in the development of alcoholism. Pharmacol Biochem Behav. 2008;90:95–104. doi: 10.1016/j.pbb.2008.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Erwin VG, Deitrich RA. Genetic selection and characterization of mouse lines for acute functional tolerance to ethanol. J Pharmacol Exp Ther. 1996;279:1310–1317. [PubMed] [Google Scholar]
- 44.Erwin VG, Gehle VM, Deitrich RA. Selectively bred lines of mice show response and drug specificity for genetic regulation of acute functional tolerance to ethanol and pentobarbital. J Pharmacol Exp Ther. 2000;293:188–195. [PubMed] [Google Scholar]
- 45.Fidler TL, Dion AM, Powers MS, Ramirez JJ, Mulgrew JA, Smitasin PJ, Crane AT, Cunningham CL. Intragastric self-infusion of ethanol in high- and low-drinking mouse genotypes after passive ethanol exposure. Genes Brain Behav. 2011;10:264–275. doi: 10.1111/j.1601-183X.2010.00664.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Fidler TL, Powers MS, Ramirez JJ, Crane A, Mulgrew J, Smitasin P, Cunningham CL. Dependence induced increases in intragastric alcohol consumption in mice. Addict Biol. 2012;17:13–32. doi: 10.1111/j.1369-1600.2011.00363.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Finn DA, Ford MM, Wiren KM, Roselli CE, Crabbe JC. The role of pregnane neurosteroids in ethanol withdrawal: behavioral genetic approaches. Pharmacol Ther. 2004;101:91–112. doi: 10.1016/j.pharmthera.2003.10.006. [DOI] [PubMed] [Google Scholar]
- 48.Fonio E, Benjamini Y, Sakov A, Golani I. Wild mouse open field behavior is embedded within the multidimensional data space spanned by laboratory inbred strains. Genes Brain Behav. 2006;5:380–388. doi: 10.1111/j.1601-183X.2005.00170.x. [DOI] [PubMed] [Google Scholar]
- 49.Foroud T, Edenberg HJ, Crabbe J. Who is at risk for alcoholism? Alcohol Res Health. 2010;33:64–75. [PMC free article] [PubMed] [Google Scholar]
- 50.Gentry RT. An experimental model of self-intoxication in C57 mice. Alcohol. 1985;2:671–675. doi: 10.1016/0741-8329(85)90145-4. [DOI] [PubMed] [Google Scholar]
- 51.Goldman D, Oroszi G, Ducci F. The genetics of addictions: uncovering the genes. Nat Rev Genet. 2005;6:521–532. doi: 10.1038/nrg1635. [DOI] [PubMed] [Google Scholar]
- 52.Goldstein DB. Inherited differences in intensity of alcohol withdrawal reactions in mice. Nature. 1973;245:154–156. doi: 10.1038/245154a0. [DOI] [PubMed] [Google Scholar]
- 53.Goldstein DB, Pal N. Alcohol dependence produced in mice by inhalation of ethanol: grading the withdrawal reaction. Science. 1971;172:288–290. doi: 10.1126/science.172.3980.288. [DOI] [PubMed] [Google Scholar]
- 54.Gottesman II, Gould TD. The endophenotype concept in psychiatry: etymology and strategic intentions. Am J Psychiat. 2003;160:636–645. doi: 10.1176/appi.ajp.160.4.636. [DOI] [PubMed] [Google Scholar]
- 55.Goulding EH, Schenk AK, Juneja P, MacKay AW, Wade JM, Tecott LH. A robust automated system elucidates mouse home cage behavioral structure. Proc Natl Acad Sci USA. 2008;105:20575–20582. doi: 10.1073/pnas.0809053106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Grant BF. Theoretical and observed subtypes of DSM-IV alcohol abuse and dependence in a general population sample. Drug Alcohol Depend. 2000;60:287–293. doi: 10.1016/s0376-8716(00)00115-0. [DOI] [PubMed] [Google Scholar]
- 57.Griffin WC, III, Lopez MF, Becker HC. Intensity and duration of chronic ethanol exposure is critical for subsequent escalation of voluntary ethanol drinking in mice. Alcohol Clin Exp Res. 2009;33:1893–1900. doi: 10.1111/j.1530-0277.2009.01027.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Hasin D. Classification of alcohol use disorders. Alcohol Res Health. 2003;27:5–17. [PMC free article] [PubMed] [Google Scholar]
- 59.Heath AC, Whitfield JB, Martin NG, Pergadia ML, Goate AM, Lind PA, McEvoy BP, Schrage AJ, Grant JD, Chou YL, Zhu R, Henders AK, Medland SE, Gordon SD, Nelson EC, Agrawal A, Nyholt DR, Bucholz KK, Madden PA, Montgomery GW. A quantitative-trait genome-wide association study of alcoholism risk in the community: findings and implications. Biol Psychiat. 2011;70:513–518. doi: 10.1016/j.biopsych.2011.02.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Heilig M, Egli M, Crabbe JC, Becker HC. Acute withdrawal, protracted abstinence and negative affect in alcoholism: are they linked? Addict Biol. 2010;15:169–184. doi: 10.1111/j.1369-1600.2009.00194.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Hopf FW, Chang SJ, Sparta DR, Bowers MS, Bonci A. Motivation for alcohol becomes resistant to quinine adulteration after 3 to 4 months of intermittent alcohol self-administration. Alcohol Clin Exp Res. 2010;34:1565–1573. doi: 10.1111/j.1530-0277.2010.01241.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Hwa LS, Chu A, Levinson SA, Kayyali TM, DeBold JF, Miczek KA. Persistent escalation of alcohol drinking in C57BL/6J mice with intermittent access to 20% ethanol. Alcohol Clin Exp Res. 2011;35:1938–1947. doi: 10.1111/j.1530-0277.2011.01545.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jellinek EM. The Disease Concept of Alcoholism. New Haven: College and University Press; 1960. [Google Scholar]
- 64.Kalant H, LeBlanc AE, Gibbins RJ. Tolerance to dependence on some non-opiate psychotropic drugs. Pharmacol Reviews. 1971;23:135–191. [PubMed] [Google Scholar]
- 65.Kendler KS, Aggen SH, Prescott CA, Crabbe J, Neale MC. Evidence for multiple genetic factors underlying the DSM-IV criteria for alcohol dependence. Mol Psychiat. 2011 doi: 10.1038/mp.2011.153. epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Kendler KS, Prescott CA, Myers J, Neale MC. The structure of genetic and environmental risk factors for common psychiatric and substance use disorders in men and women. Arch Gen Psychiat. 2003;60:929–937. doi: 10.1001/archpsyc.60.9.929. [DOI] [PubMed] [Google Scholar]
- 67.Kohnke MD. Approach to the genetics of alcoholism: a review based on pathophysiology. Biochem Pharmacol. 2008;75:160–177. doi: 10.1016/j.bcp.2007.06.021. [DOI] [PubMed] [Google Scholar]
- 68.Koob GF, Le Moal M. Addiction and the Brain Antireward System. Annu Rev Psychol. 2008;59:29–53. doi: 10.1146/annurev.psych.59.103006.093548. [DOI] [PubMed] [Google Scholar]
- 69.Lederle L, Weber S, Wright T, Feyder M, Brigman JL, Crombag HS, Saksida LM, Bussey TJ, Holmes A. Reward-related behavioral paradigms for addiction research in the mouse: performance of common inbred strains. PLoS One. 2011;6:e15536. doi: 10.1371/journal.pone.0015536. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Leeman RF, Heilig M, Cunningham CL, Stephens DN, Duka T, O'Malley SS. Ethanol consumption: how should we measure it? Achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:109–124. doi: 10.1111/j.1369-1600.2009.00192.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Lenoir M, Serre F, Cantin L, Ahmed SH. Intense sweetness surpasses cocaine reward. PLoS One. 2007;2:e698. doi: 10.1371/journal.pone.0000698. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 72.Lesscher HM, van Kerkhof LW, Vanderschuren LJ. Inflexible and indifferent alcohol drinking in male mice. Alcohol Clin Exp Res. 2010;34:1219–1225. doi: 10.1111/j.1530-0277.2010.01199.x. [DOI] [PubMed] [Google Scholar]
- 73.Logue SF, Swartz RJ, Wehner JM. Genetic correlation between performance on an appetitive-signaled nosepoke task and voluntary ethanol consumption. Alcohol Clin Exp Res. 1998;22:1912–1920. [PubMed] [Google Scholar]
- 74.Loi B, Lobina C, Maccioni P, Fantini N, Carai MA, Gessa GL, Colombo G. Increase in alcohol intake, reduced flexibility of alcohol drinking, and evidence of signs of alcohol intoxication in Sardinian alcohol-preferring rats exposed to intermittent access to 20% alcohol. Alcohol Clin Exp Res. 2010;34:2147–2154. doi: 10.1111/j.1530-0277.2010.01311.x. [DOI] [PubMed] [Google Scholar]
- 75.Lopez MF, Grahame NJ, Becker HC. Development of ethanol withdrawal-related sensitization and relapse drinking in mice selected for high- or low-ethanol preference. Alcohol Clin Exp Res. 2011;35:953–962. doi: 10.1111/j.1530-0277.2010.01426.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Matson LM, Grahame NJ. Pharmacologically relevant intake during chronic, free-choice drinking rhythms in selectively bred high alcohol-preferring mice. Addict Biol. 2011 doi: 10.1111/j.1369-1600.2011.00412.x. epub ahead of press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 77.McBride WJ, Bell RL, Rodd ZA, Strother WN, Murphy JM. Adolescent alcohol drinking and its long-range consequences. Studies with animal models. In: Galanter M, editor. Recent Developments in Alcoholism. Vol 17. Kluwer: Plenum; 2005. pp. 123–142. [DOI] [PubMed] [Google Scholar]
- 78.McClearn GE. Animal models in alcohol research. Alcohol Clin Exp Res. 1988;12:5:573–576. doi: 10.1111/j.1530-0277.1988.tb00245.x. [DOI] [PubMed] [Google Scholar]
- 79.Metten P, Crabbe JC. Common genetic determinants of severity of acute withdrawal from ethanol, pentobarbital and diazepam in inbred mice. Behavioural Pharmacology. 1994;5:533–547. doi: 10.1097/00008877-199408000-00014. [DOI] [PubMed] [Google Scholar]
- 80.Metten P, Crabbe JC. Dependence and Withdrawal. In: Deitrich RA, Erwin VG, editors. Pharmacological Effects of Ethanol on the Nervous System. Boca Raton FL: CRC Press; 1996. pp. 269–290. [Google Scholar]
- 81.Metten P, Phillips TJ, Crabbe JC, Tarantino LM, McClearn GE, Plomin R, Erwin VG, Belknap JK. High genetic susceptibility to ethanol withdrawal predicts low ethanol consumption. Mamm Genome. 1998;9:983–990. doi: 10.1007/s003359900911. [DOI] [PubMed] [Google Scholar]
- 82.Mitchell MR, Vokes CM, Blankenship AL, Simon NW, Setlow B. Effects of acute administration of nicotine, amphetamine, diazepam, morphine, and ethanol on risky decision-making in rats. Psychopharmacology (Berl) 2011;218:703–712. doi: 10.1007/s00213-011-2363-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Mitchell SH. The genetic basis of delay discounting and its genetic relationship to alcohol dependence. Behav Proc. 2011;87:10–17. doi: 10.1016/j.beproc.2011.02.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Newlin DB, Thomson JB. Alcohol challenge with sons of alcoholics: a critical review and analysis. Psychol Bull. 1990;108:3:383–402. doi: 10.1037/0033-2909.108.3.383. [DOI] [PubMed] [Google Scholar]
- 85.O'Dell LE, Roberts AJ, Smith RT, Koob GF. Enhanced alcohol self-administration after intermittent versus continuous alcohol vapor exposure. Alcohol Clin Exp Res. 2004;28:1676–1682. doi: 10.1097/01.alc.0000145781.11923.4e. [DOI] [PubMed] [Google Scholar]
- 86.Philibin SD, Cameron AJ, Metten P, Crabbe JC. Motor impairment: a new ethanol withdrawal phenotype in mice. Behav Pharmacol. 2008;19:604–614. doi: 10.1097/FBP.0b013e32830ded27. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 87.Philibin SD, Cameron AJ, Schlumbohm JP, Metten P, Crabbe JC. Ethanol withdrawal-induced motor impairment in mice. Psychopharmacology (Berl) 2012;220:367–378. doi: 10.1007/s00213-011-2483-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 88.Randall CL, Lester D. Alcohol selection by DBA and C57BL mice arising from ova transfers. Nature. 1975a;255:147–148. doi: 10.1038/255147a0. [DOI] [PubMed] [Google Scholar]
- 89.Randall CL, Lester D. Cross-fostering of DBA and C57Bl mice. Increase in voluntary consumption of alcohol by DBA weanlings. J Stud Alcohol. 1975b;36:973–980. doi: 10.15288/jsa.1975.36.973. [DOI] [PubMed] [Google Scholar]
- 90.Randall CL, Lester D. Social modification of alcohol consumption in inbred mice. Science. 1975c;189:149–151. doi: 10.1126/science.1138373. [DOI] [PubMed] [Google Scholar]
- 91.Rhodes JS, Best K, Belknap JK, Finn DA, Crabbe JC. Evaluation of a simple model of ethanol drinking to intoxication in C57BL/6J mice. Physiol Behav. 2005;84:53–63. doi: 10.1016/j.physbeh.2004.10.007. [DOI] [PubMed] [Google Scholar]
- 92.Rhodes JS, Ford MM, Yu C-H, Brown LL, Finn DA, Garland T, Jr, Crabbe JC. Mouse inbred strain differences in ethanol drinking to intoxication. Genes Brain Behav. 2007;6:1–18. doi: 10.1111/j.1601-183X.2006.00210.x. [DOI] [PubMed] [Google Scholar]
- 93.Rimondini R, Arlinde C, Sommer W, Heilig M. Long-lasting increase in voluntary ethanol consumption and transcriptional regulation in the rat brain after intermittent exposure to alcohol. FASEB J. 2002;16:27–35. doi: 10.1096/fj.01-0593com. [DOI] [PubMed] [Google Scholar]
- 94.Roberts AJ, Heyser CJ, Cole M, Griffin P, Koob GF. Excessive ethanol drinking following a history of dependence: animal model of allostasis. Neuropsychopharmacology. 2000;22:581–594. doi: 10.1016/S0893-133X(99)00167-0. [DOI] [PubMed] [Google Scholar]
- 95.Robinson GE, Fernald RD, Clayton DF. Genes and social behavior. Science. 2008;322:896–900. doi: 10.1126/science.1159277. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 96.Rustay NR, Crabbe JC. Genetic analysis of rapid tolerance to ethanol's incoordinating effects in mice: Inbred strains and artificial selection. Behav Genet. 2004;34:441–451. doi: 10.1023/B:BEGE.0000023649.60539.dd. [DOI] [PubMed] [Google Scholar]
- 97.Rustay NR, Wahlsten D, Crabbe JC. Assessment of genetic susceptibility to ethanol intoxication in mice. Proc Natl Acad Sci USA. 2003;100:2917–2922. doi: 10.1073/pnas.0437273100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Saha TD, Chou SP, Grant BF. Toward an alcohol use disorder continuum using item response theory: results from the National Epidemiologic Survey on Alcohol and Related Conditions. Psychol Med. 2006;36:931–941. doi: 10.1017/S003329170600746X. [DOI] [PubMed] [Google Scholar]
- 99.Schuckit MA. Ethanol-induced changes in body sway in men at high alcoholism risk. Arch Gen Psychiat. 1985;42:375–379. doi: 10.1001/archpsyc.1985.01790270065007. [DOI] [PubMed] [Google Scholar]
- 100.Schuckit MA, Smith TL. An 8-year follow-up of 450 sons of alcoholic and control subjects. Arch Gen Psychiat. 1996;53:202–210. doi: 10.1001/archpsyc.1996.01830030020005. [DOI] [PubMed] [Google Scholar]
- 101.Sher KJ, Dick DM, Crabbe JC, Hutchison KE, O'Malley S, Heath AC. Consilient research approaches in studying gene × environment interactions in alcohol research. Addict Biol. 2010;15:200–216. doi: 10.1111/j.1369-1600.2009.00189.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Sher KJ, Grekin ER, Williams NA. The development of alcohol use disorders. Annu Rev Clin Psychol. 2005;1:493–523. doi: 10.1146/annurev.clinpsy.1.102803.144107. [DOI] [PubMed] [Google Scholar]
- 103.Shirley RL, Walter NA, Reilly MT, Fehr C, Buck KJ. Mpdz is a quantitative trait gene for drug withdrawal seizures. Nat Neurosci. 2004;7:699–700. doi: 10.1038/nn1271. [DOI] [PubMed] [Google Scholar]
- 104.Silverman JL, Yang M, Lord C, Crawley JN. Behavioural phenotyping assays for mouse models of autism. Nat Rev Neurosci. 2010;11:490–502. doi: 10.1038/nrn2851. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Simms JA, Bito-Onon JJ, Chatterjee S, Bartlett SE. Long-Evans rats acquire operant self-administration of 20% ethanol without sucrose fading. Neuropsychopharmacology. 2010;35:1453–1463. doi: 10.1038/npp.2010.15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Simon NW, Gilbert RJ, Mayse JD, Bizon JL, Setlow B. Balancing risk and reward: a rat model of risky decision making. Neuropsychopharmacology. 2009;34:2208–2217. doi: 10.1038/npp.2009.48. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 107.Sommer WH, Rimondini R, Hansson AC, Hipskind PA, Gehlert DR, Barr CS, Heilig MA. Upregulation of voluntary alcohol intake, behavioral sensitivity to stress, and amygdala crhr1 expression following a history of dependence. Biol Psychiat. 2008;63:139–145. doi: 10.1016/j.biopsych.2007.01.010. [DOI] [PubMed] [Google Scholar]
- 108.Spanagel R, Holter SM. Long-term alcohol self-administration with repeated alcohol deprivation phases: an animal model of alcoholism? Alcohol Alcohol. 1999;34:231–243. doi: 10.1093/alcalc/34.2.231. [DOI] [PubMed] [Google Scholar]
- 109.Spear LP. Rewards, aversions and affect in adolescence: Emerging convergences across laboratory animal and human data. Dev Cogn Neurosci. 2011;1:390–403. doi: 10.1016/j.dcn.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Stacey D, Clarke TK, Schumann G. The genetics of alcoholism. Curr Psychiat Rep. 2009;11:364–369. doi: 10.1007/s11920-009-0055-4. [DOI] [PubMed] [Google Scholar]
- 111.Stephens DN, Duka T, Crombag HS, Cunningham CL, Heilig M, Crabbe JC. Reward sensitivity: Issues of measurement, and achieving consilience between human and animal phenotypes. Addict Biol. 2010;15:145–168. doi: 10.1111/j.1369-1600.2009.00193.x. [DOI] [PubMed] [Google Scholar]
- 112.Tordoff MG, Alarcon LK, Lawler MP. Preferences of 14 rat strains for 17 taste compounds. Physiol Behav. 2008;95:308–332. doi: 10.1016/j.physbeh.2008.06.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Treutlein J, Kissling C, Frank J, Wiemann S, Dong L, Depner M, Saam C, Lascorz J, Soyka M, Preuss UW, Rujescu D, Skowronek MH, Rietschel M, Spanagel R, Heinz A, Laucht M, Mann K, Schumann G. Genetic association of the human corticotropin releasing hormone receptor 1 (CRHR1) with binge drinking and alcohol intake patterns in two independent samples. Mol Psychiat. 2006;11:594–602. doi: 10.1038/sj.mp.4001813. [DOI] [PubMed] [Google Scholar]
- 114.Treutlein J, Rietschel M. Genome-wide association studies of alcohol depndence and aubstance use disorders. Curr Psychiat Rep. 2011;13:147–155. doi: 10.1007/s11920-011-0176-4. [DOI] [PubMed] [Google Scholar]
- 115.Vaillant GE. The Natural History of Alcoholism. Cambridge, MA: Harvard University Press; 1983. [Google Scholar]
- 116.Valdez GR, Roberts AJ, Chan K, Davis H, Brennan M, Zorrilla EP, Koob GF. Increased ethanol self-administration and anxiety-like behavior during acute ethanol withdrawal and protracted abstinence: regulation by corticotropin-releasing factor. Alcohol Clin Exp Res. 2002;26:1494–1501. doi: 10.1097/01.ALC.0000033120.51856.F0. [DOI] [PubMed] [Google Scholar]
- 117.van der Zwaluw CS, Engels RC. Gene-environment interactions and alcohol use and dependence: current status and future challenges. Addiction. 2009;104:907–914. doi: 10.1111/j.1360-0443.2009.02563.x. [DOI] [PubMed] [Google Scholar]
- 118.Vanderschuren LJ, Everitt BJ. Drug seeking becomes compulsive after prolonged cocaine self-administration. Science. 2004;305:1017–1019. doi: 10.1126/science.1098975. [DOI] [PubMed] [Google Scholar]
- 119.Veatch LM. Disruptions in sleep time and sleep architecture in a mouse model of repeated ethanol withdrawal. Alcohol Clin Exp Res. 2006;30:1214–1222. doi: 10.1111/j.1530-0277.2006.00134.x. [DOI] [PubMed] [Google Scholar]
- 120.Volkow ND, Li TK. Drugs and alcohol: treating and preventing abuse, addiction and their medical consequences. Pharmacol Ther. 2005;108:3–17. doi: 10.1016/j.pharmthera.2005.06.021. [DOI] [PubMed] [Google Scholar]
- 121.Wahlsten D, Bachmanov A, Finn DA, Crabbe JC. Stability of inbred mouse strain differences in behavior and brain size between laboratories and across decades. Proc Natl Acad Sci USA. 2006;103:16364–16369. doi: 10.1073/pnas.0605342103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Walker SE, Pena-Oliver Y, Stephens DN. Learning not to be impulsive: disruption by experience of alcohol withdrawal. Psychopharmacology (Berl) 2011;217:433–442. doi: 10.1007/s00213-011-2298-0. [DOI] [PubMed] [Google Scholar]
- 123.Waller MB, McBride WJ, Lumeng L, Li TK. Initial sensitivity and acute tolerance to ethanol in the P and NP lines of rats. Pharmacol Biochem Behav. 1983;19:683–686. doi: 10.1016/0091-3057(83)90345-3. [DOI] [PubMed] [Google Scholar]
- 124.Wayner MJ, Greenberg I, Tartaglione R, Nolley D, Fraley S, Cott A. A new factor affecting the consumption of ethyl alcohol and other sapid fluids. Physiol Behav. 1972;8:345–362. doi: 10.1016/0031-9384(72)90383-6. [DOI] [PubMed] [Google Scholar]
- 125.Wilhelm CJ, Mitchell SH. Rats bred for high alcohol drinking are more sensitive to delayed and probabilistic outcomes. Genes Brain Behav. 2008;7:705–713. doi: 10.1111/j.1601-183X.2008.00406.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 126.Wilhelm CJ, Mitchell SH. Strain differences in delay discounting using inbred rats. Genes Brain Behav. 2009;8:426–434. doi: 10.1111/j.1601-183X.2009.00484.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 127.Wilhelm CJ, Reeves JM, Phillips TJ, Mitchell SH. Mouse lines selected for alcohol consumption differ on certain measures of impulsivity. Alcohol Clin Exp Res. 2007;31:1839–1845. doi: 10.1111/j.1530-0277.2007.00508.x. [DOI] [PubMed] [Google Scholar]
- 128.Wise RA. Voluntary ethanol intake in rats following exposure to ethanol on various schedules. Psychopharmacologia. 1973;29:203–210. doi: 10.1007/BF00414034. [DOI] [PubMed] [Google Scholar]
- 129.Wolffgramm J, Galli G, Thimm F, Heyne A. Animal models of addiction: models for therapeutic strategies? J Neural Transm. 2000;107:649–668. doi: 10.1007/s007020070067. [DOI] [PubMed] [Google Scholar]
- 130.Wolffgramm J, Heyne A. Social behavior, dominance, and social deprivation of rats determine drug choice. Pharmacol Biochem Behav. 1991;38:389–399. doi: 10.1016/0091-3057(91)90297-f. [DOI] [PubMed] [Google Scholar]
- 131.Wolffgramm J, Heyne A. From controlled drug intake to loss of control: the irreversible development of drug addiction in the rat. Behav Brain Res. 1995;70:77–94. doi: 10.1016/0166-4328(95)00131-c. [DOI] [PubMed] [Google Scholar]
- 132.Yang M, Silverman JL, Crawley JN. Automated three-chambered social approach task for mice. Curr Protoc Neurosci. 2011;Chapter 8(Unit 8.26) doi: 10.1002/0471142301.ns0826s56. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Zimmermann US, Blomeyer D, Laucht M, Mann KF. How gene-stress-behavior interactions can promote adolescent alcohol use: The roles of predrinking allostatic load and childhood behavior disorders. Pharmacol Biochem Behav. 2007;86:246–262. doi: 10.1016/j.pbb.2006.09.024. [DOI] [PubMed] [Google Scholar]
- 134.Zuo L, Gelernter J, Zhang CK, Zhao H, Lu L, Kranzler HR, Malison RT, Li CS, Wang F, Zhang XY, Deng HW, Krystal JH, Zhang F, Luo X. Genome-Wide Association Study of Alcohol Dependence Implicates KIAA0040 on Chromosome 1q. Neuropsychopharmacology. 2012;37:581–582. doi: 10.1038/npp.2011.229. [DOI] [PMC free article] [PubMed] [Google Scholar]