Abstract
Lung infections represent a tremendous disease burden and a leading cause of acute lung injury. STAT3 signaling is essential for controlling lung injury during pneumonia. We previously identified leukemia inhibitory factor (LIF) as a prominent STAT3-activating cytokine expressed in the airspaces of pneumonic lungs, but its physiological significance in this setting has never been explored. To do so, Escherichia coli was intratracheally instilled into C57BL/6 mice in the presence of neutralizing anti-LIF IgG or control IgG. Anti-LIF completely eliminated lung LIF detection and markedly exacerbated lung injury compared to control mice as evidenced by airspace albumin content, lung liquid accumulation, and histological analysis. Although lung bacteriology was equivalent between groups, bacteremia was more prevalent with anti-LIF treatment, suggestive of compromised barrier function rather than impaired antibacterial defense as the cause of dissemination. Inflammatory cytokine expression was also exaggerated in anti-LIF-treated lungs, albeit after injury had ensued. Interestingly, alveolar neutrophil recruitment was modestly but significantly reduced compared to control mice despite elevated cytokine levels, indicating that inflammatory injury was not a consequence of excessive neutrophilic alveolitis. Lastly, the lung transcriptome was dramatically remodeled during pneumonia, but far more so following LIF neutralization, with gene changes implicating cell death and epithelial homeostasis amongst other processes relevant to tissue injury. From these findings, we conclude that endogenous LIF facilitates tissue protection during pneumonia. The LIF-STAT3 axis is here identified as a critical determinant of lung injury with clinical implications for pneumonia patients.
INTRODUCTION
Lung infections result in the greatest burden of infectious disease worldwide, regardless of socioeconomic status (1). Moreover, lung infections account for the largest number of infection-related deaths and disability-adjusted life years (1, 2), demanding further insight regarding the pathophysiology of this important disease. Pulmonary innate immunity, however, is a complex and robust response to invading pathogens that must be delicately balanced to ensure adequate host defense while minimizing tissue injury. Indeed, pneumonia is the most common cause of acute lung injury (ALI) including its most severe form, acute respiratory distress syndrome (ARDS) (3). Therefore, the inflammatory response to pneumonia is both a necessary and dangerous consequence of respiratory infection, and the mechanisms whereby this response is tilted in favor of the host remain enigmatic.
Signal transducer and activator of transcription-3 (STAT3) has emerged as a signaling hub that may be uniquely poised to both promote host defense and limit injurious inflammation in the lungs (4). This transcription factor is activated in the lungs by multiple stimuli (4), and its presence in alveolar epithelial cells is protective in several contexts of inflammatory injury (5–10). During pneumonia, we have shown that mice lacking alveolar epithelial STAT3 exhibit defects in both innate immunity and tissue protection (10), suggesting that synthesis of factors upstream of STAT3 in the lungs may be particularly influential on outcome. For instance, multiple STAT3-activating cytokines have been linked to host defense and/or lung injury, including IL-6, IL-11, IL-10, IL-22, IL-23, and G-CSF amongst others (11–15). Whether and which of these factors converge to govern the inflammatory response to pneumonia through STAT3 is unclear.
The IL-6 family of cytokines includes at least 10 biologically diverse members, all of which signal through STAT3 and share gp130 as a common receptor subunit (16). Leukemia inhibitory factor (LIF) is amongst the IL-6 family members significantly induced during pneumonia, and appears to be particularly important for epithelial STAT3 activation (10). Yet, the influence of LIF expression on STAT3-mediated tissue protection or other host responses to pneumonia is unknown. This pleiotropic cytokine is most widely recognized for its ability to preserve the totipotency of embryonic stem cells (17). Other well-appreciated functions include roles in fertility, hematopoiesis, endocrine regulation, hepatic acute phase protein expression, and development (18). LIF has also been linked to inflammation, albeit inconsistently, with evidence that it can exacerbate or diminish this process depending on the biological context (19). The administration of excess recombinant LIF by pharmacologic or genetic engineering approaches suggests a potential protective ability of this cytokine in response to intrapulmonary LPS (20) or hyperoxia (21), confirming LIF’s capacity to signal in the lung environment. Although LIF protein is detectable in pleural effusions (22) and increased in the lungs of patients with ARDS (23), its functional relevance in the setting of lung injury is entirely unknown. In the present study, we sought to determine whether and how endogenous LIF influenced acute lung injury and host defense during pneumonia.
MATERIALS AND METHODS
Mice
Experiments were performed using C57BL/6 mice at 6–12 weeks of age. All animal protocols were approved by the Institutional Animal Care and Use Committee at Boston University.
Pneumonia
Mice were anesthetized by intraperitoneal administration of a cocktail containing ketamine (50 mg/kg) and xylazine (5 mg/kg). For intratracheal (i.t.) instillations, a 24-gauge angiocatheter was placed into surgically exposed tracheas and guided into the left bronchus. A 50 µl bolus of saline containing approximately 106 CFU E. coli (serotype 06:K2:H1; ATCC #19138) or 25 ng of recombinant murine LIF (rmLIF; R&D Systems) was instilled through the angiocatheter into the left lung lobe. The bacterial inoculum was estimated by optical density and confirmed by plating serial dilutions on 5% sheep blood agar plates. Where indicated, bacteria or rmLIF was co-instilled with 10 µg of normal goat IgG (R&D Systems) or a neutralizing goat polyclonal IgG targeting murine LIF (R&D Systems). At the indicated time points, mice were euthanized by isoflurane overdose and tissues were harvested for the measurements described below.
Bronchoalveolar Lavage
Bronchoalveolar lavage fluid (BALF) was collected as previously described for the indicated outcomes (10, 24). Briefly, isolated lungs were cannulated with a 20-gauge blunted stainless steel catheter and washed 10 times with 1 ml of PBS. Supernatants from the first wash were aliquoted following centrifugation (300 × g for 5 min at 4°C) and archived at −80°C for subsequent analyses. Total cell counts were calculated using a hemacytometer. Differential counts were performed on cells stained with Diff-Quik (Dade-Behring) following cytocentrifugation. For alveolar macrophage studies, lungs of uninfected mice were lavaged 10 times with ice-cold HBSS (no Ca2+ or Mg2+) containing 1M HEPES, Pen/Strep, and 0.5M EDTA. Following centrifugation, cell pellets (>99% alveolar macrophages) were resuspended in serum-free RPMI 1640 medium and cultured as described below.
Protein Measurements
LIF, G-CSF, GM-CSF, IL-10, IL-17, IL-1β, IL-6, KC, and MIP-2 concentrations were determined using a LiquiChip 200 workstation (Qiagen) in conjunction with a Bio-Plex cytokine bead array (Bio-Rad). Mouse albumin (Bethyl), receptor for advanced glycation end products (RAGE; R&D Systems), and in some cases LIF concentrations (R&D Systems) were measured by ELISA.
Immunoblots
Snap-frozen lungs were homogenized using a Bullet Blender (Next Advance) and total protein concentrations were determined in extraction buffer as previously described (24). Immunoblots were performed for Y705-phosphorylated STAT3 (pSTAT3) and total STAT3 as described (10, 24) using antibodies from Cell Signaling Technology (catalog #’s 9131 and 9132, respectively).
Lung Histology and Immunohistochemistry
Following euthanasia, hearts were ligated to maintain pulmonary blood volume, and isolated lungs were instilled with buffered 6% gluteraldehyde or 4% paraformaldehyde solutions at 23 cm H2O pressure for fixation. Hematoxylin-eosin stained sections were prepared from gluteraldehyde-fixed lungs for a qualitative visual assessment of pulmonary inflammation. Immunohistochemistry for pSTAT3 was performed on paraformaldehyde-fixed lungs using an Intellipath FLX Autostainer and pre-formulated processing and detection reagents provided by the manufacturer (Biocare Medical). Briefly, sections were blocked with peroxidase solution following deparaffinization and washed in TBS containing 0.05% Tween-20 (which preceded every subsequent step). Slides were trypsinized for 15 minutes and blocked with Rodent Block M solution for 30 minutes to eliminate effects of endogenous IgG. After blocking, sections were exposed to rabbit monoclonal anti-pSTAT3 (diluted 1:100; Cell Signaling Technology #9145) or an equal concentration of isotype control antibody (Cell Signaling Technology #3900) for 1.5 hrs. Slides were then exposed to secondary antibody (Rabbit HRP Polymer) for 30 min and pSTAT3 immunoreactivity was visualized following DAB exposure and dehydration.
Lung Wet:Dry Weight
Wet:dry ratios were calculated from freshly isolated and desiccated lungs as described previously (10).
Bacteriology
Blood or lung homogenates were serially diluted in sterile H2O and grown overnight at 37°C on 5% sheep blood agar plates. Viable bacteria were enumerated by colony counts and expressed as total CFU per lung or per ml of blood.
Cell Culture
RAW 264.7 and MLE-12 cells were purchased from American Type Culture Collection and maintained as recommended by the provider. 2×105 (RAW 264.7) or 1×105 (MLE-12) cells were seeded in 48-well tissue culture plates. Alveolar macrophages were harvested by lavage as described above and 2×105 cells were allowed to adhere in 48-well plates for 30 minutes in serum-free RPMI 1640 medium. After the incubation, medium was replaced with RPMI 1640 containing 10% FBS and Pen/Strep. In all cases, cells were stimulated on the following day with vehicle or LPS (5 µg/ml; Sigma) for 2 hrs in the absence or presence of rmLIF (1 µg/ml), and cell lysates were collected for mRNA extraction.
qRT-PCR
Cell lysate or lung mRNA induction was quantified for IL-6, Fas, and VEGFa using quantitiative RT-PCR (qRT-PCR). For in vivo experiments, left (infected) lung lobes were homogenized using a Bullet Blender (Next Advance) and total RNA was purified using the RNeasy kit (Qiagen). For in vitro experiments, cell lysates were collected using Trizol reagent (Life Technologies) and total RNA was purified based on the manufacturer’s protocol. qRT-PCR was performed using the CFX96 Real-Time System (Bio-Rad) and the TaqMan RNA-to-CT 1-step kit (Applied Biosystems). Primer and probe sequences for IL-6 were published elsewhere (14). Other sequences were as follows: Fas = Fwd 5’-TGTCCTGCCTCTGGTGCTT-3’, Rev 5’-AATGGGCCTCCTTGATATAATCCT-3’, TaqMan probe 5’-CACGAACCCGCCTCCTCAGCTTTAAACTCT-3’; and VEGFa = Fwd 5’-CTCCCTCCCTCTACAGATCAT-3’, Rev 5’-CTGGCTTTGTTCTGTCTTTCTT-3’, Taqman probe 5’-ATGTGCTGGCTTTGGTGAGGTTTGATCCG-3’. All probes were labeled with 6-carboxyfluorescein (5’) and Iowa Black quencher (3’) and purchased from Integrated DNA Technologies. Expression values were normalized to the content of 18s rRNA (14) and expressed as fold induction.
Microarray
Total lung RNA was isolated and purified as described above for qRT-PCR. RNA quality was validated using an Agilent bioanalyzer and microarrays were performed for each sample using Affymetrix GeneChip Mouse Gene 1.0 ST Arrays. Probes on the array were mapped to the mouse genome using a custom CDF file as described (25), which can be found at http://brainarray.mbni.med.umich.edu/Brainarray/Database/CustomCDF/genomic_curated_CDF.asp. Following data normalization, filtering, and preprocessing, fold changes were calculated between experimental groups. Clustering and heat maps were generated using the Cluster 3.0 and TreeView software (http://rana.lbl.gov/eisen/?page_id=42). Identification of functional categories was achieved using Ingenuity Pathway Analysis software (content version 11631407; Ingenuity Systems). All data have been deposited in the NCBI Gene Expression Omnibus (accession #: GSE34901; http://www.ncbi.nlm.nih.gov/geo/).
Statistics
Statistical analyses were performed using GraphPad Prism (GraphPad Software). Data are presented as means ± SEM or medians, as indicated in figure legends. Comparisons between two groups were performed using a Student’s t test or Mann-Whitney test (for bacteriology data only). Comparisons of multiple groups to a single control group were performed using a one-way analysis of variance (ANOVA) followed by a Dunnett’s post hoc test. Comparisons amongst multiple groups were performed using a 2-way ANOVA followed by Holm-Sidak test for multiple comparisons. Values were log-transformed prior to analysis if they were expressed on a log scale and/or did not pass the F test for equal variance. Comparisons were considered significant when p < 0.05. P values were adjusted to false discovery rate (FDR) for microarray studies to account for multiple comparisons.
RESULTS
LIF expression and blockade during pneumonia
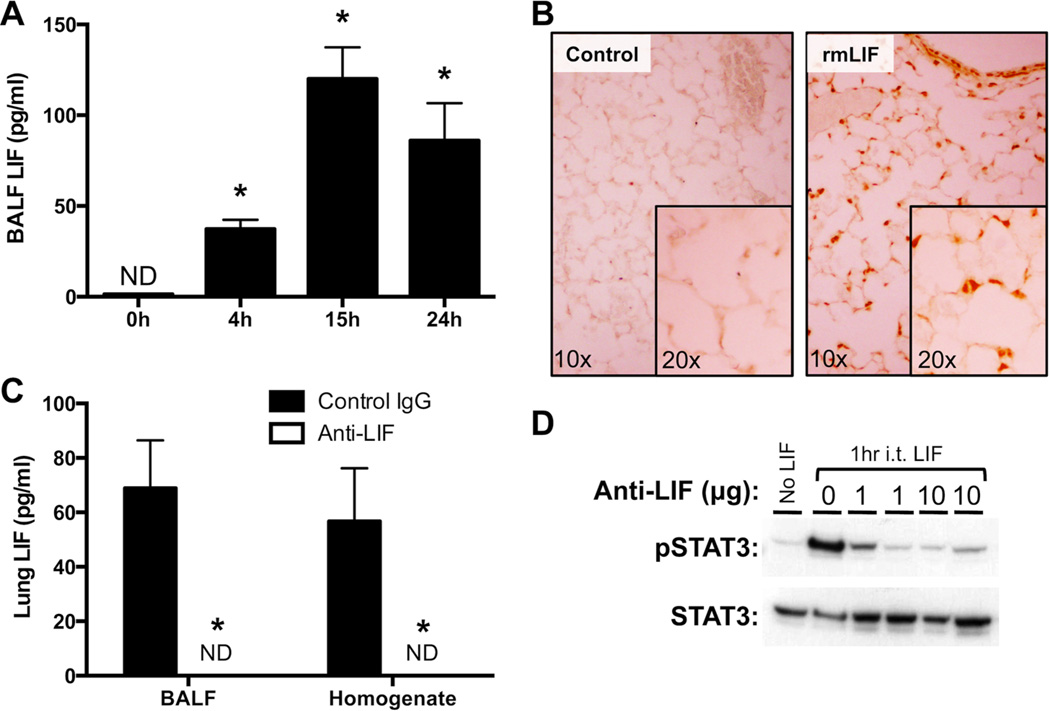
We have previously shown that LIF mRNA is amongst the IL-6 family members significantly induced in the lungs of mice during pneumonia (10). Of those cytokines, which also included IL-6, oncostatin M (OSM), and IL-11, LIF was particularly important for the epithelial STAT3 activating capacity of pneumonic alveolar lining fluid. To determine the dynamics of LIF protein expression during pneumonia, BALF LIF concentrations were measured 0–24 hrs after i.t. E. coli. While undetectable at baseline, LIF was significantly expressed by 4 hrs of infection, increased further over ensuing hours, and remained elevated through 24 hrs (Figure 1A). Although our previous ex vivo studies were designed to determine the degree to which airspace, as defined by the lavageable fraction, of LIF contributes to STAT3 activation in alveolar epithelial cells (10), it is entirely possible that LIF functions in multiple lung cell types. In order to identify lung cell populations that are directly responsive to LIF, tyrosine-phosphorylated STAT3 was visualized in lung sections using immunohistochemistry 1 hr after i.t. rmLIF. Results reveal that airway epithelial cells and type 2 alveolar epithelial cells are amongst those highly responsive to LIF stimulation (Figure 1B).
Figure 1.
LIF expression and biological activity in the lungs during pneumonia. (A) LIF protein concentrations were quantified in bronchoalveolar lavage fluid (BALF) 0–24 hrs after intratracheal (i.t.) instillation of Escherichia coli. Values are expressed as means ± SEM (n = 4–5). * p < 0.05 compared to uninfected (0h) controls. (B) Immunohistochemistry was used to visualize Y705-phosphorylated STAT3 in histological lung sections prepared from mice treated for 1h with or without i.t. recombinant murine LIF (rmLIF). Background staining was undetectable on sections from i.t. rmLIF mice exposed to an isotype control antibody (not shown). Representative images are shown at 10× and 20× magnification. (C) LIF protein concentrations were quantified in BALF 24 hrs after i.t. E. coli co-instilled with anti-LIF or control IgG. Values are expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 4–5). (D) Y705- phosphorylated STAT3 immunoreactivity was measured by immunoblot in lung homogenates collected from mice 1h after i.t. rmLIF in the presence of 0–10 µg anti-LIF.
To establish an in vivo loss-of-function model for lung LIF during pneumonia, E. coli was co-instilled with equal concentrations of neutralizing LIF antibody or control IgG. Following 24 hrs of infection, LIF protein remained completely undetectable in BALF and whole lung homogenates from anti-LIF-treated mice (Figure 1C), confirming that the antibody delivered in this fashion effectively bound all LIF protein endogenously produced. To determine blockade of LIF signaling to endogenous cells in the lungs, we tested the effect of anti-LIF on rmLIF-induced lung STAT3 activation. Confirming blocking efficacy, the phosphorylation of STAT3 in the lungs was completely neutralized by anti-LIF (Figure 1D). Overall, these results indicate that LIF is induced during pneumonia, that it activates STAT3 in type II epithelial cells, and that anti-LIF administration is an effective strategy to interrogate the roles of endogenous lung LIF during pneumonia.
Effect of LIF neutralization on acute lung injury
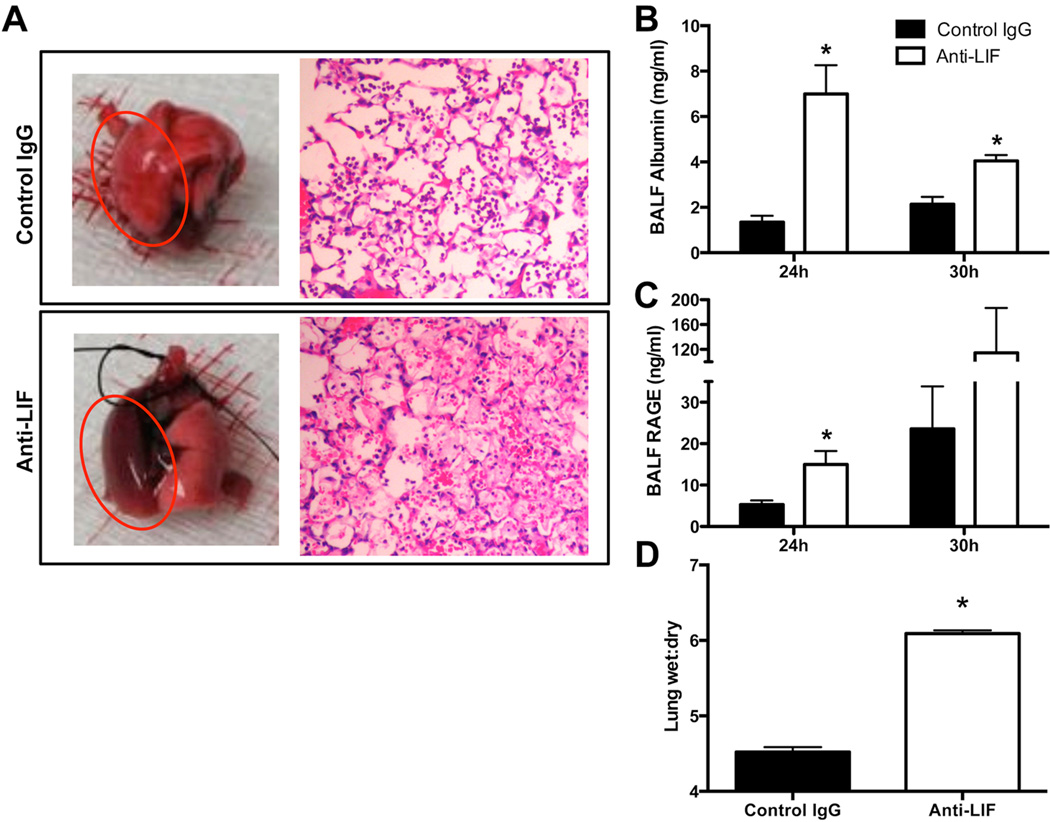
Although LIF administration (20) or overexpression (21) can limit pulmonary inflammation in response to LPS or hyperoxia, respectively, the influence of endogenous LIF on lung responses to infection or any other intra-pulmonary challenge is yet to be examined. To determine the functional significance of LIF during pneumonia, E. coli was intratracheally instilled into mice in the presence of anti-LIF or control IgG. By 24 hrs of infection, anti-LIF-treated mice had considerably more gross and histological evidence of lung injury compared to mice treated with control IgG (Figure 2A). BALF albumin, a metric of plasma protein exudate in the alveoli, was nearly 4-fold higher in the absence of LIF 24 hrs after i.t. E. coli, and remained elevated through at least 30 hrs, the latest time point analyzed (Figure 2B). BALF levels of soluble RAGE, a marker of type 1 alveolar epithelial cell death and index of alveolar-specific injury (26), were approximately 3 times higher in mice treated with anti-LIF at 24 hrs (Figure 2C). Similar results were observed at 30 hrs, although, the difference between groups did not reach statistical significance. Pulmonary edema was further confirmed by calculating lung wet:dry weight ratios, which were markedly higher in anti-LIF-treated mice (Figure 2D). No histological evidence of lung injury was present in uninfected mice treated with anti-LIF as compared to those treated with control IgG, which was expected due to the absence of detectable LIF protein under baseline conditions (Figure 1A). These data strongly suggest that LIF is a functionally relevant cytokine whose expression is causally linked to the pathophysiology of bacterial pneumonia.
Figure 2.
Effect of LIF neutralization on acute lung injury. Lungs were collected from mice 24 hrs after intratracheal inoculation of Escherichia coli co-instilled with anti-LIF or control IgG. (A) Representative images are shown for intact freshly isolated lungs and hematoxylin/eosin-stained lung sections. Red circles are used to denote infected left lung lobes. Bronchoalveolar lavage fluid concentrations of (B) mouse albumin and (C) receptor for advanced glycation end products (RAGE), as well as (D) lung wet:dry weight ratios were determined and expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 3–5).
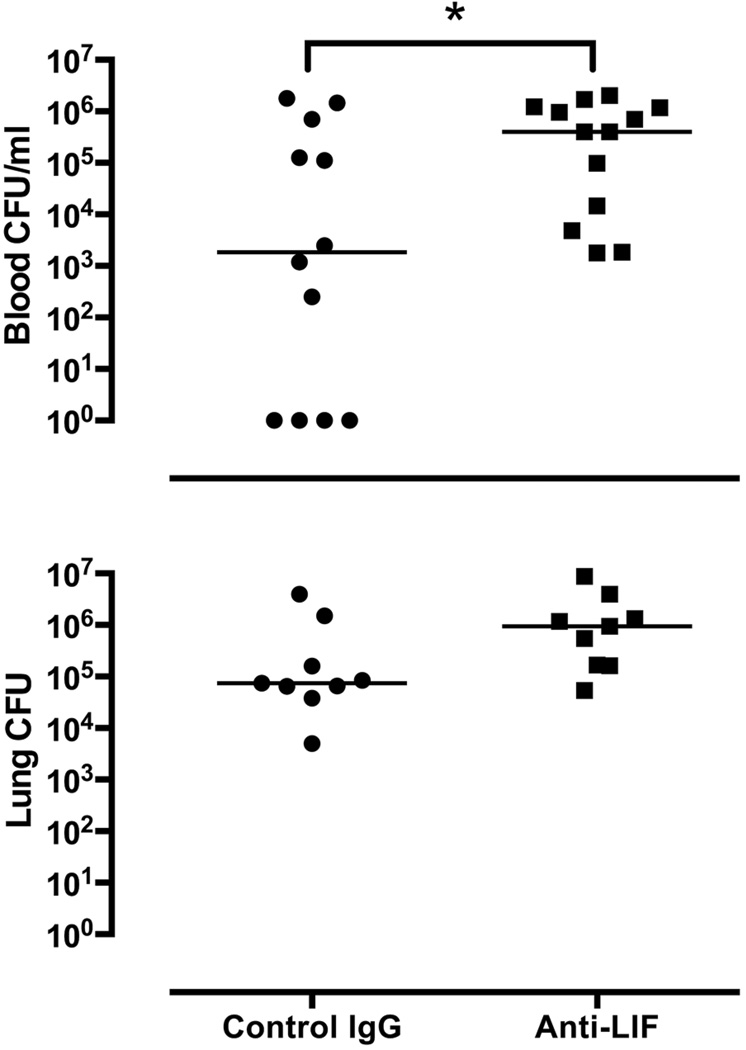
Additional experiments were performed to determine the effects of anti-LIF on pulmonary and systemic host defense. 24 hrs after i.t. E. coli, lung bacterial burdens were not significantly affected by LIF blockade (Figure 3). In contrast, bacteremia was significantly more frequent and more severe in the absence of LIF (Figure 3), suggesting defects in systemic host defense and/or increased dissemination from injured lungs.
Figure 3.
Effect of LIF neutralization on bacterial clearance. Escherichia coli colony-forming units (CFU) were enumerated in blood and lungs after 24 hrs of pneumonia in the presence of anti-LIF or control IgG. CFU/ml (blood) and total lung CFU are shown for individual mice with horizontal lines indicating the median value within each experimental group. * p < 0.05 compared to mice treated with control IgG (n = 9–13).
LIF neutralization and pulmonary innate immunity
Neutrophil recruitment and cytokine synthesis are essential mediators of pulmonary host defense (27). These robust responses to infection, however, reflect both a consequence and a cause of acute lung injury, as excessive neutrophil recruitment, cytokine expression, and injured cells (through recognition of damage-associated molecular patterns) can directly promote tissue injury (28, 29). To determine whether LIF influences innate immunity during pneumonia, we performed differential cell counts and cytokine analyses in BALF collected from pneumonic mice treated with anti-LIF or control IgG.
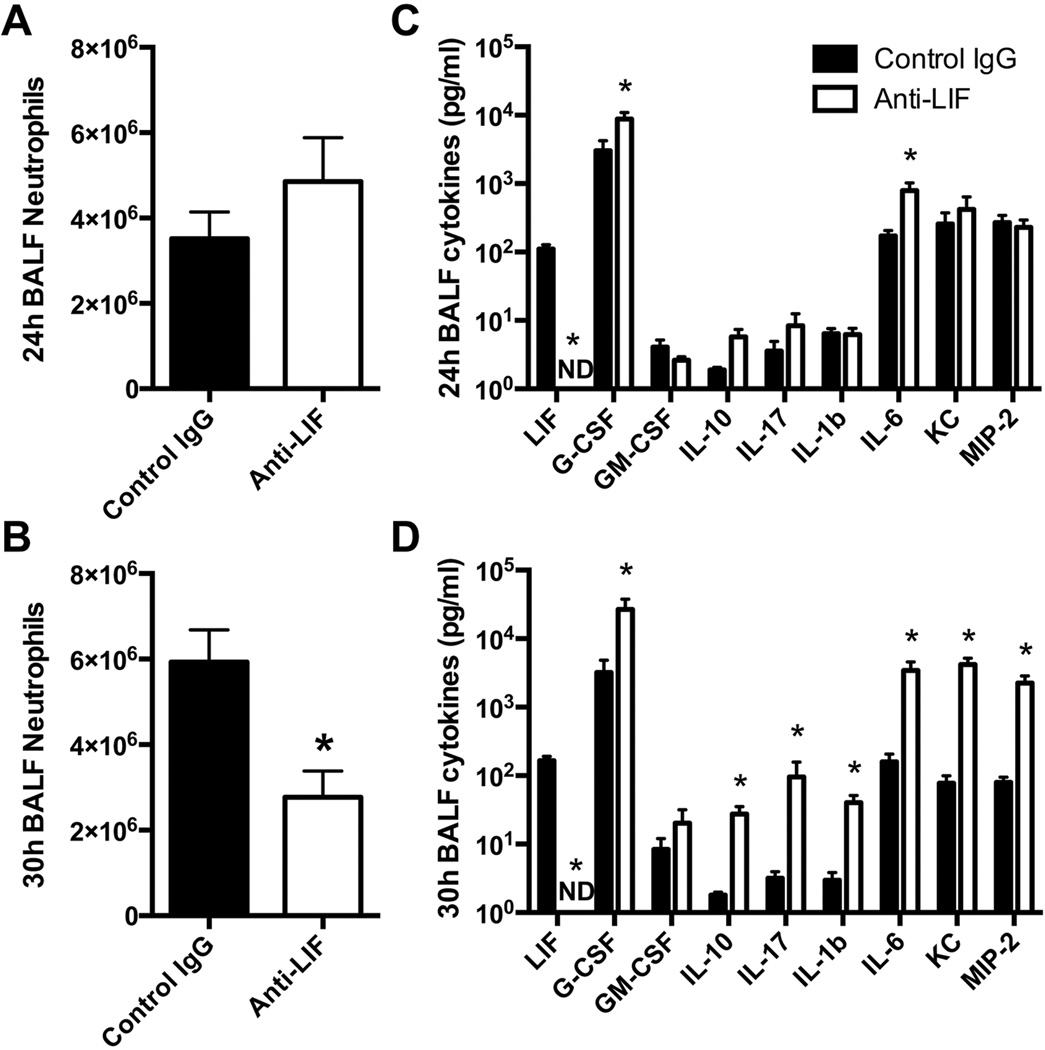
At the 24 hour time point, when severe lung injury was already observed, alveolar neutrophil counts were unaffected by LIF neutralization (Figure 4A). At later times, in the even more severely injured lungs, neutrophil counts in the BALF were significantly decreased in the anti-LIF group (Figure 4B). Thus, endogenous LIF does not limit neutrophil recruitment and the exacerbated lung injury after LIF blockade is not a result of excess neutrophils.
Figure 4.
Effect of LIF neutralization on innate immunity. (A–B) Neutrophil numbers and (C–D) cytokine protein concentrations were quantified in bronchoalveolar lavage fluid (BALF) harvested from mouse lungs 24 or 30 hrs after intratracheal Escherichia coli. Values are expressed as means ± SEM. * p < 0.05 compared to mice treated with control IgG (n = 4–5).
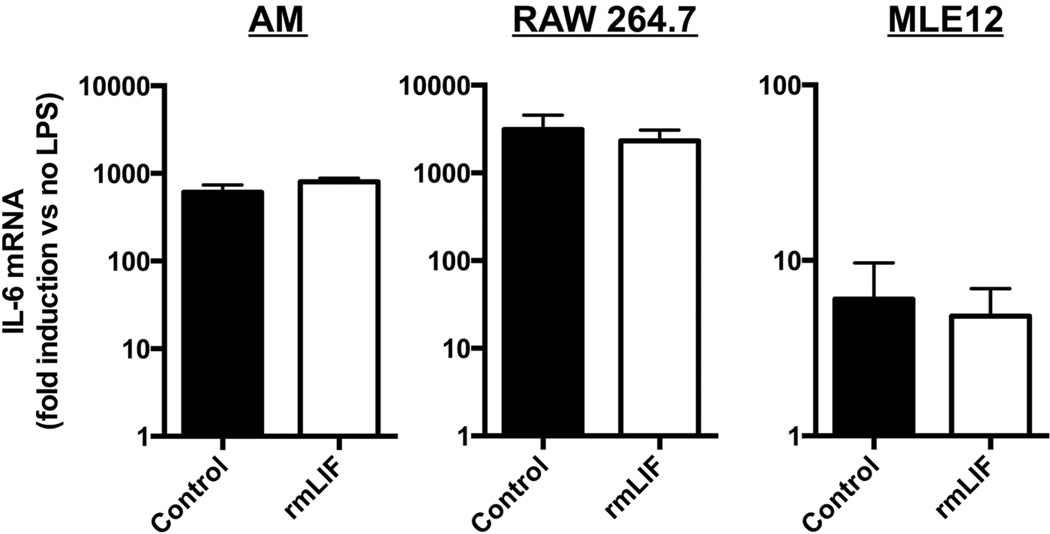
In the same mice, 9 different cytokines were measured by multiplex bead array: LIF, G-CSF, GM-CSF, IL-10, IL-17, IL-1β, IL-6, KC, MIP-2. These were chosen because each is directly relevant to acute pulmonary inflammation and injury (12, 14, 30–35). LIF remained undetectable at both time points in anti-LIF-treated mice, verifying that neutralization persisted through at least 30 hrs of infection (Figure 4C–D). The remaining cytokines were at most modestly affected by LIF blockade at 24 hrs, with significant changes observed only for G-CSF and IL-6, each of which increased by less than an order of magnitude (Figure 4C). Interestingly, logarithmic increases occurred for all cytokines measured except GM-CSF by 30 hrs (Figure 4D), such that cytokine concentrations were vastly disproportionate to neutrophil counts in the same mice. To determine whether LIF directly modulates cytokine expression, effects of rmLIF on LPS-induced IL-6 were quantified in freshly isolated alveolar macrophages along with macrophage-like (RAW 264.7) and alveolar epithelial-like (MLE-12) cell lines. IL-6 induction, which was markedly elevated by the absence of LIF in vivo, was completely unaffected by LIF supplementation in vitro (Figure 5), arguing that LIF does not directly reduce IL-6 synthesis and suggesting that amplified cytokine expression in vivo is not a primary consequence of LIF neutralization. Altogether, these results suggest exaggerated cytokine synthesis may reflect a complex immunopathology that initially results from and subsequently may contribute to the severe injury phenotype caused by LIF neutralization.
Figure 5.
Effect of LIF supplementation on IL-6 induction in vitro. IL-6 mRNA induction was determined in alveolar macrophages (AM), RAW 264.7 cells and MLE-12 cells following 2 hrs of LPS stimulation in the absence and presence of recombinant murine LIF (rmLIF). Data are expressed as fold-induction compared to cells not treated with LPS. Means ± SEM were calculated by combining data from 3 separate experiments or 3 individual mice (n = 3).
Transcriptionally profiling the effects of LIF blockade
The above results indicate that LIF neutralization exacerbates lung injury within 24 hours of lung infection, which does not appear to result from the ability of LIF to limit neutrophil recruitment or pro-inflammatory cytokine expression. To assess pathways which may be influenced by LIF during pneumonia, we used microarrays to comprehensively assess gene expression programs altered by anti-LIF treatment in infected mouse lungs. Global gene expression profiles were compared amongst three groups of mice following i.t. instillations of: 1) control IgG alone; 2) control IgG and E. coli; or 3) anti-LIF and E. coli. These groups were selected to independently interrogate the effects of pneumonia alone and pneumonia in the absence of LIF.
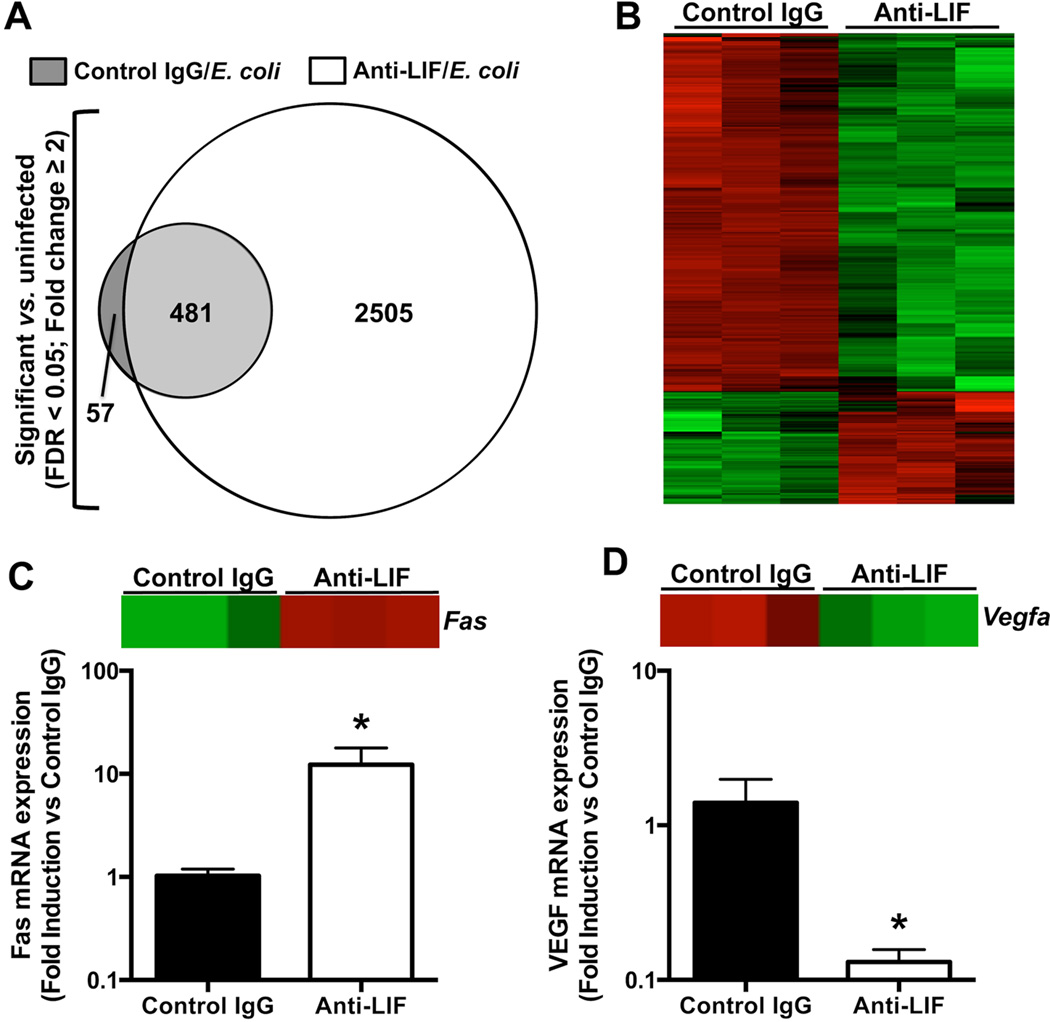
24 hrs after i.t. E.coli co-instilled with control IgG, 538 transcripts were significantly (FDR < 0.05) expressed by ≥ 2 fold compared to mice treated with control IgG alone (Figure 6A). These gene changes solely reflect those attributable to E. coli-induced lung infection, with no intervention regarding LIF signaling. 481 of these transcripts (89%) were also altered in pneumonic mice treated with anti-LIF (Figure 6A), suggesting that LIF signaling was not essential for these particular responses. Another 57 genes were altered due to pneumonia in the IgG-treated mice but not the anti-LIF mice, suggesting these as potentially LIF-dependent pneumonia-induced transcript changes. Remarkably, an additional 2505 genes were differentially expressed in infected anti-LIF treated mice compared to uninfected controls (Figure 6A). These findings support an enormous scope of biological processes downstream of LIF during pneumonia.
Figure 6.
Effects of pneumonia and LIF blockade on the lung transcriptome and markers of cell death. (A–B) Microarrays were performed on total lung RNA 24 hrs after intratracheal (i.t.) instillations of the following 3 combinations: 1) saline and control IgG; 2) Escherichia coli and control IgG; and 3) Escherichia coli and anti-LIF (n = 3). (A) Values indicate the number of differentially expressed genes in each group compared to uninfected mice treated with control IgG alone. (B) A heat map illustrates the expression profile of the 1313 genes significantly different between pneumonic mice treated with control IgG (left 3 columns) or anti-LIF (right 3 columns). For each row (gene), green and red annotations denote decreased or increased expression, respectively. Data in panels (A) and (B) include significantly altered transcripts (FDR < 0.05) with an expression difference of at least 2 fold. qRT-PCR was used to interrogate mRNA fold-induction (compared to control IgG-treated mice) for (C) Fas and (D) VEGF, both of which were significantly affected by anti-LIF treatment as determined by microarray. Individual heat map data are shown for both transcripts to illustrate relative expression levels determined by microarray analysis. * p < 0.05 compared to mice treated with control IgG (n = 3).
In addition to comparing gene expression profiles between infected (IgG or anti-LIF) and uninfected (IgG only) mice as described above, we also compared transcript changes between pneumonic mice of either treatment group (IgG vs anti-LIF). Filtering results to analyze differences equaling or exceeding 2 fold, 1313 transcripts were significantly (FDR < 0.05) altered (Figure 6B). Most (1000/1313) were less abundant in the lungs from the more injured anti-LIF treated group. Ingenuity Pathway Analysis was performed on all differentially expressed genes to identify significantly represented functional categories. The 10 most represented groups are listed in Table I. “Cell Death” was the category comprising the most genes (398), suggesting that increased lung injury may be attributable to damaged or lost cells required for intact barrier function. Other categories such as “Cellular Assembly and Organization,” “Molecular Transport,” and “Cell Cycle” implicate additional basic cellular processes as being downstream of LIF.
Table I.
Top functional categories represented by differentially expressed genes in lungs from anti-LIF treated mice compared to controls during pneumonia1
| Molecular and Cellular Functions | p-value | Gene # |
|---|---|---|
| Cell Death | 8.8E-06–1.69E-02 | 398 |
| Small Molecule Biochemistry | 3.84E-09-1.69E-02 | 349 |
| Post-Translational Modification | 2.82E-12-1.49E-02 | 338 |
| Cellular Assembly and Organization | 2.86E-06-1.5E-02 | 331 |
| Molecular Transport | 3.31E-05-1.49E-02 | 308 |
| Cell Cycle | 7.57E-07-1.68E-02 | 291 |
| Lipid Metabolism | 3.84E-09-1.57E-02 | 274 |
| Cellular Function and Maintenance | 2.86E-06-1.5E-02 | 245 |
| Protein Degradation | 3.64E-05-9.9E-03 | 95 |
| Energy Production | 1.07E-07-8.9E-03 | 71 |
Transcriptional profiling was performed on lung RNA collected 24 hrs after i.t. E. coli in mice co-treated with anti-LIF or control IgG (n = 3). Ingenuity Pathway Analysis was used to determine molecular and cellular functions significantly represented (p < 0.05) by all differentially expressed transcripts (FDR < 0.05; no fold cutoff). The 10 most significant categories are shown along with the number of differentially expressed genes comprising the indicated function.
The 10 most strongly induced genes and the 10 most strongly repressed due to LIF neutralization are listed in Tables II and III, respectively. These are poorly understood in the context of lung injury, but it is reasonable to infer functional significance (presented in the Discussion). In order to validate select transcripts, we performed qRT-PCR on Fas and Vegfa, both of which represent differentially expressed transcripts in the array that are recognized as important modulators of both cell death and lung injury (36, 37). Gene expression changes measured by qRT-PCR were consistent with those identified by microarray (Figure 6C–D). The combination of Ingenuity analyses and candidate mediator measurements together support a role for LIF in tissue protection, likely at the level of cytotoxicity, cell biology, and the alveolar epithelium.
Table II.
Top 10 induced genes in anti-LIF treated mice compared to controls during pneumonia1
| Symbol | Name | FDR | Fold Change |
|---|---|---|---|
| Fap | fibroblast activation protein | 0.015 | ↑7.51 |
| Mx2 | myxovirus (influenza virus) resistance 2 | 0.017 | ↑6.83 |
| Fosb | FBJ osteosarcoma oncogene B | 0.042 | ↑5.70 |
| Stfa1 | stefin A1 | 0.035 | ↑5.18 |
| Dio2 | deiodinase, iodothyronine, type II | 0.039 | ↑4.72 |
| Tas2r135 | taste receptor, type 2, member 135 | 0.030 | ↑4.67 |
| Tnfrsf11b | tumor necrosis factor receptor superfamily, member 11b (osteoprotegerin) | 0.026 | ↑4.25 |
| Sprr1a | small proline-rich protein 1A | 0.016 | ↑3.98 |
| Il24 | interleukin 24 | 0.033 | ↑3.96 |
| Tnfsf15 | tumor necrosis factor (ligand) superfamily, member 15 | 0.011 | ↑3.75 |
Transcriptional profiling was performed on lung RNA collected 24 hrs after i.t. E. coli in mice co-treated with anti-LIF or control IgG (n = 3). Fold change was ranked amongst all differentially expressed transcripts (FDR < 0.05). The 10 most up-regulated genes are shown.
Table III.
Top 10 reduced genes in anti-LIF treated mice compared to controls during pneumonia1
| Symbol | Name | FDR | Fold Change |
|---|---|---|---|
| Sntn | sentan, cilia apical structure protein | 0.015 | ↓11.77 |
| Fmo3 | flavin containing monooxygenase 3 | 0.011 | ↓9.15 |
| Cckar | cholecystokinin A receptor | 0.009 | ↓7.36 |
| Rsph4a | radial spoke head 4 homolog A (Chlamydomonas) | 0.014 | ↓6.98 |
| Hsd11b2 | hydroxysteroid 11-beta dehydrogenase 2 | 0.007 | ↓6.91 |
| Cmbl | carboxymethylenebutenolidase-like (Pseudomonas) | 0.019 | ↓6.76 |
| Atp6v0d2 | ATPase, H+ transporting, lysosomal V0 subunit D2 | 0.034 | ↓6.48 |
| l7Rn6 | lethal, Chr 7, Rinchik 6 | 0.020 | ↓6.24 |
| Mettl4 | methyltransferase like 4 | 0.024 | ↓6.06 |
| Clec1a | C-type lectin domain family 1, member a | 0.017 | ↓5.82 |
Transcriptional profiling was performed on lung RNA collected 24 hrs after i.t. E. coli in mice co-treated with anti-LIF or control IgG (n = 3). Fold change was ranked amongst all differentially expressed transcripts (FDR < 0.05). The 10 most down-regulated genes are shown.
DISCUSSION
To our knowledge, these findings represent the first evidence that LIF production is a critical determinant of tissue homeostasis in acutely inflamed lungs. During pneumonia, LIF neutralization markedly exacerbated lung injury, inflammatory cytokine expression, and bacterial dissemination. Furthermore, these changes occurred concomitantly with a vastly altered lung transcriptome in anti-LIF treated mice, supporting an expansive scope of biological processes downstream of this single cytokine. These results suggest that increased concentrations of LIF in patients with ARDS (23) serve as more than a biomarker of disease severity, but rather reflect a critical protective signaling pathway.
Previous studies have demonstrated therapeutic potential for LIF in other models of lung injury. Ulich and colleagues reported that intratracheal LPS induced lung LIF expression in rats, and that co-administration of recombinant LIF reduced cytokine expression and neutrophilic alveolitis (20). This work is consistent with our own in that it supports a protective role for LIF, but differs in several regards, such as the animal model, stimulus, and use of a pharmacologic and gain-of-function strategy. The inverse relationship between LIF and neutrophil recruitment also differs from our data, for which we found similar (24h) or even decreased (30h) neutrophil counts in the absence of LIF (Figures 4A–B). This contradiction likely stems from fundamental differences in study design, such that the biology of endogenous LIF may not be purely consistent with that suggested by gain-of-function approaches. Wang et al. reported that targeted transgene overexpression of LIF in mouse airway epithelial cells was significantly protective during hyperoxia, with improved survival and decreased pulmonary edema (21). Similar findings have been reported for IL-6 and IL-11 (38, 39), suggesting that IL-6 family cytokines may converge to elicit protective responses in acutely inflamed lungs. While all 3 aforementioned cytokines are expressed in pneumonic lungs, LIF is possibly the most bioactive in the airspaces with regards to STAT3 signaling (10), and our current results indicate that it has unique roles which are functionally indispensible.
We elected to employ LIF neutralization as a means to determine its function during pneumonia. Our results indicate that LIF detection is completely abolished through at least 30h of pneumonia in the presence of anti-LIF. Moreover, LIF-induced STAT3 is completely blocked by antibody treatment, confirming functional blockade. But the phenotype itself represents perhaps the most compelling indication of antibody efficacy. While this strategy benefits from coinciding with the spatial and temporal characteristics of our pneumonia protocol, extended time courses are far less feasible. In this case, Lif−/− mice represent a more amenable and robust approach, but are limited by infertility (in females) (40), imprecision (deletion is global and permanent), and other minor compensatory and developmental complications (18). Using this model, Weber et al. investigated the response to endotoxemia and reported increased morbidity and mortality in mice genetically devoid of LIF (41). Importantly, the authors reported increased cytokine expression and inflammatory injury, including that in the lungs, supporting our current findings in an entirely different experimental setting (41).
While our results identify the expression of LIF as a means to counter inflammatory injury during pneumonia, signals up- and downstream of this process remain unclear. Regarding the former, LIF can be induced by multiple inflammatory stimuli, including cytokines relevant to pulmonary host defense such as TNFα, IL-1β, and IL-6 (42, 43). In vitro studies have identified a variety of lung cell types capable of expressing LIF, including epithelial cells, fibroblasts, and airway smooth muscle cells (43). These findings are corroborated by positive LIF immunoreactivity in human and guinea pig airway epithelial cells (44). During pneumonia, however, pulmonary cellularity is dramatically transformed, introducing many potential sources of LIF synthesis which are yet to be determined.
Downstream of LIF-dependent signaling, we postulate that LIF activates STAT3 in lung epithelial cells in order to promote the expression of tissue-protective genes. Several lines of evidence implicate epithelial STAT3 as a central hub in this axis. First, i.t. LIF administration caused robust STAT3 activation in lung epithelium. Second, ex vivo epithelial STAT3 activation by pneumonic airspace constituents is largely dependent on LIF (10). Third, functional deletion of STAT3 in epithelial cells has injurious consequences during pneumonia (10) and other models of acute pulmonary inflammation (5–10). Contrary to this working model, lung pSTAT3 content was unaffected during pneumonia in the presence of anti-LIF (data not shown). Our previous approach for interrogating the influence of LIF on STAT3 activation relied on neutralizing LIF in BALF after it was harvested from normal pneumonic lungs (10). This approach did not subject BALF to the dramatic inflammatory changes imposed by LIF blockade in vivo. In the present study, however, detecting effects of LIF blockade on STAT3 activity in living lungs was likely confounded by a combination of several outcomes, including cell heterogeneity, LIF-independent STAT3 activity downstream of inflammatory injury, and perhaps most importantly, increased concentrations of alternative STAT3-activating cytokines (IL-6, IL-10 and G-CSF) in response to anti-LIF (Figures 4C–D). Yet, these STAT3-signaling cytokines fail to compensate for LIF neutralization during pneumonia, meaning that LIF fulfills non-redundant roles in this setting. We also recognize that LIF may signal through alternative transcription factors or signaling intermediates such as STAT1 or PI(3)K (16, 45), which may function along with or independent of STAT3 during pneumonia. Finally, LIF signaling may not be restricted to alveolar epithelial cells, since mice lacking STAT3 throughout these cells do not exhibit as severe a phenotype as those described here during pneumonia ((10) and unpublished observations).
Regardless of where and how LIF initiates signal transduction, our data unequivocally demonstrate its influence on lung injury. This conclusion is directly supported by changes in lung histology, lung liquid accumulation, and BALF albumin content. Lung injury was also associated with increased bacteremia, perhaps due to loss of epithelial barrier integrity, which is further evidenced by increased levels of BALF RAGE. RAGE is enriched in type I alveolar epithelial cells, and has emerged as a marker of lung injury (26). Interestingly, neutrophil recruitment was not positively associated with injury, despite pronounced increases in cytokine expression in the same mice. Based on this collection of results, we find it exceedingly unlikely that increased injury resulted from excessive inflammatory responses. In fact, large differences in cytokine expression were not detected until 30 hrs, a time by which increased lung injury was well established and exacerbated in anti-LIF-treated mice. Alternatively, we interpret these results as evidence that the function and/or survival of LIF target cells (perhaps epithelial cells) is directly compromised by LIF neutralization.
It is notable that IL-17 was amongst the cytokines enhanced in injured lungs following LIF blockade. It was recently described that LIF and IL-6 have opposing roles in balancing the development of Treg and Th17 cells, such that LIF inhibits Th17 and promotes Treg cell differentiation (46). Our finding that IL-17 expression is enhanced following LIF blockade, therefore, is consistent with the concept that LIF opposes Th17 development. Th17 cytokines such as IL-17 itself and IL-22 are critical for antibacterial host defense during pneumonia, including that against Gram-negative pathogens (11, 35, 47). However, while memory Th17 cells represent an important source of IL-17 under immunized conditions (48), the degree to which Th17 cells contribute to innate IL-17 production in the absence of a prior challenge is unclear. In fact, multiple innate sources of IL-17 have now been identified, including but not limited to γδ T cells, iNKT cells, neutrophils, and macrophages (49). Since increased injury at 24 hrs occurred prior to differences in IL-17, IL-10, and most other cytokines, we find it unlikely that the balance of Treg and Th17 cells is responsible for inflammatory injury following LIF blockade, especially since bacterial clearance was unchanged. Yet, the degree to which this axis exacerbates injury at later time points or under different experimental circumstances remains an interesting avenue of future research.
Our microarray studies revealed an astonishing number of transcript changes downstream of LIF during pneumonia. Many of these changes may be directly linked to LIF signaling. Yet, it is notable that these gene changes were recorded at a time by which anti-LIF treatment had already exacerbated injury. Therefore, it is difficult to distinguish differentially expressed transcripts that caused lung injury from those that resulted from it, both of which are likely represented. Regardless of this consideration, these data begin to reveal the vast scope of biological processes downstream of LIF during pneumonia.
While the precise mechanisms by which LIF modulates lung tissue integrity represent an exciting avenue of future investigation, the gene changes reported herein implicate cell death as a distinctly relevant possibility. For example, Fas expression, which can directly elicit epithelial apoptosis and lung injury (36), was significantly higher in the absence of LIF. Likewise, VEGF, which is both STAT3-dependent (50) and protective during lung injury (37), was decreased in the lungs following LIF neutralization. For more unbiased perspectives on LIF-dependent gene changes, we assembled lists of the 10 most up- and down-regulated transcripts in LIF-deficient injured lungs. Particularly relevant processes linked to up-regulated genes (Table II) include apoptosis (Fap, Il24, and Tnfsf15), immunology/infection (Mx2 and Sprr1a), and lung injury (Fosb, Dio2, and Tnfrsf11b) (51–59). The 10 most reduced genes (Table III) were also interesting, albeit poorly understood. For instance, inhibition of Atp6v0d2 has been shown to increase apoptosis in hepatoblastoma cells, a function possibly extending to pulmonary cells during pneumonia (60). Also, l7Rn6 deletion has been linked to respiratory distress (61). Interestingly, multiple down-regulated genes represent markers and/or structural components of epithelial cells, including Sntn, Fmo3, Rsph4a, Hsd11b2, and l7Rn6 (61–65), suggesting that epithelial cell loss may result from LIF blockade. Additional epithelial-specific markers on the array supported this trend but were not significant, such as Nkx2-1 (FDR = 0.054; 2.93 fold decrease) and Pdpn (FDR = 0.09; 1.27 fold decrease), although some epithelial-specific genes showed no such trend.
These data implicate LIF induction during pneumonia as a means to limit cell loss and/or promote cell proliferation, furthered by the findings that both “Cell Death” and Cell Cycle” were identified by Ingenuity analyses of transcript changes as amongst the top functions altered by LIF blockade. STAT3 can directly drive the expression of anti-apoptotic genes (66), including in the alveolar epithelium (67). STAT3 can also elicit gene programs driving migration and proliferation (66), both of which are driven by STAT3 in lung epithelial cells (9). Perhaps the most widely recognized function of LIF regards its ability to enhance the maintenance of stem cells (17), which may underlie an important reservoir for tissue regeneration and repair (68). Altogether, we interpret these previous findings and current results to suggest that, rather than being a determinant of inflammatory responses, the LIF-STAT3 axis is more specifically a tissue-protective pathway, preventing cell death and promoting the proliferation and differentiation programs essential to regeneration and repair in the infected lung.
To our knowledge, these findings constitute the first evidence that LIF expression is a physiologically significant, indispensable response to lung infection. The mechanisms controlling LIF expression along with those coordinating its tissue protective effects are yet to be determined. We anticipate that a more complete understanding of this signaling network may reveal novel therapeutic or prognostic targets in patients with or at risk for acute lung injury.
ACKNOWLEDGMENTS
The authors would like to thank the Boston University Microarray Resource Core, particularly Yuriy Alekseyev and Marc Lenburg, and the Immunohistochemistry Core for their expert technical assistance.
Footnotes
This work was supported by the National Institutes of Health grants R00-HL092956 (LJQ) and R01-HL079392 (JPM).
REFERENCES
- 1.Mizgerd JP. Lung infection--a public health priority. PLoS medicine. 2006;3:e76. doi: 10.1371/journal.pmed.0030076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Michaud CM, Murray CJ, Bloom BR. Burden of disease--implications for future research. JAMA. 2001;285:535–539. doi: 10.1001/jama.285.5.535. [DOI] [PubMed] [Google Scholar]
- 3.Matthay MA, Zemans RL. The Acute Respiratory Distress Syndrome: Pathogenesis and Treatment. Annu Rev Pathol. 2010 doi: 10.1146/annurev-pathol-011110-130158. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Quinton LJ, Mizgerd JP. NF-kappaB and STAT3 signaling hubs for lung innate immunity. Cell Tissue Res. 2010 doi: 10.1007/s00441-010-1044-y. [DOI] [PubMed] [Google Scholar]
- 5.Hokuto I, Ikegami M, Yoshida M, Takeda K, Akira S, Perl AK, Hull WM, Wert SE, Whitsett JA. Stat-3 is required for pulmonary homeostasis during hyperoxia. The Journal of clinical investigation. 2004;113:28–37. doi: 10.1172/JCI200419491. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ikegami M, Falcone A, Whitsett JA. STAT-3 regulates surfactant phospholipid homeostasis in normal lung and during endotoxin-mediated lung injury. J Appl Physiol. 2008;104:1753–1760. doi: 10.1152/japplphysiol.00875.2007. [DOI] [PubMed] [Google Scholar]
- 7.Matsuzaki Y, Xu Y, Ikegami M, Besnard V, Park KS, Hull WM, Wert SE, Whitsett JA. Stat3 is required for cytoprotection of the respiratory epithelium during adenoviral infection. J Immunol. 2006;177:527–537. doi: 10.4049/jimmunol.177.1.527. [DOI] [PubMed] [Google Scholar]
- 8.Lian X, Qin Y, Hossain SA, Yang L, White A, Xu H, Shipley JM, Li T, Senior RM, Du H, Yan C. Overexpression of Stat3C in pulmonary epithelium protects against hyperoxic lung injury. J Immunol. 2005;174:7250–7256. doi: 10.4049/jimmunol.174.11.7250. [DOI] [PubMed] [Google Scholar]
- 9.Kida H, Mucenski ML, Thitoff AR, Le Cras TD, Park KS, Ikegami M, Muller W, Whitsett JA. GP130-STAT3 regulates epithelial cell migration and is required for repair of the bronchiolar epithelium. Am J Pathol. 2008;172:1542–1554. doi: 10.2353/ajpath.2008.071052. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Quinton LJ, Jones MR, Robson BE, Simms BT, Whitsett JA, Mizgerd JP. Alveolar epithelial STAT3, IL-6 family cytokines, and host defense during Escherichia coli pneumonia. American journal of respiratory cell and molecular biology. 2008;38:699–706. doi: 10.1165/rcmb.2007-0365OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Aujla SJ, Chan YR, Zheng M, Fei M, Askew DJ, Pociask DA, Reinhart TA, McAllister F, Edeal J, Gaus K, Husain S, Kreindler JL, Dubin PJ, Pilewski JM, Myerburg MM, Mason CA, Iwakura Y, Kolls JK. IL-22 mediates mucosal host defense against Gram-negative bacterial pneumonia. Nature medicine. 2008;14:275–281. doi: 10.1038/nm1710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Gregory AD, Hogue LA, Ferkol TW, Link DC. Regulation of systemic and local neutrophil responses by G-CSF during pulmonary Pseudomonas aeruginosa infection. Blood. 2007;109:3235–3243. doi: 10.1182/blood-2005-01-015081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Happel KI, Dubin PJ, Zheng M, Ghilardi N, Lockhart C, Quinton LJ, Odden AR, Shellito JE, Bagby GJ, Nelson S, Kolls JK. Divergent roles of IL-23 and IL-12 in host defense against Klebsiella pneumoniae. The Journal of experimental medicine. 2005;202:761–769. doi: 10.1084/jem.20050193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Jones MR, Quinton LJ, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Roles of interleukin-6 in activation of STAT proteins and recruitment of neutrophils during Escherichia coli pneumonia. The Journal of infectious diseases. 2006;193:360–369. doi: 10.1086/499312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sun L, Guo RF, Newstead MW, Standiford TJ, Macariola DR, Shanley TP. Effect of IL-10 on neutrophil recruitment and survival after Pseudomonas aeruginosa challenge. American journal of respiratory cell and molecular biology. 2009;41:76–84. doi: 10.1165/rcmb.2008-0202OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Heinrich PC, Behrmann I, Haan S, Hermanns HM, Muller-Newen G, Schaper F. Principles of interleukin (IL)-6-type cytokine signalling and its regulation. The Biochemical journal. 2003;374:1–20. doi: 10.1042/BJ20030407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Williams RL, Hilton DJ, Pease S, Willson TA, Stewart CL, Gearing DP, Wagner EF, Metcalf D, Nicola NA, Gough NM. Myeloid leukaemia inhibitory factor maintains the developmental potential of embryonic stem cells. Nature. 1988;336:684–687. doi: 10.1038/336684a0. [DOI] [PubMed] [Google Scholar]
- 18.Metcalf D. The unsolved enigmas of leukemia inhibitory factor. Stem cells (Dayton, Ohio) 2003;21:5–14. doi: 10.1634/stemcells.21-1-5. [DOI] [PubMed] [Google Scholar]
- 19.Gadient RA, Patterson PH. Leukemia inhibitory factor, Interleukin 6, and other cytokines using the GP130 transducing receptor: roles in inflammation and injury. Stem cells (Dayton, Ohio) 1999;17:127–137. doi: 10.1002/stem.170127. [DOI] [PubMed] [Google Scholar]
- 20.Ulich TR, Fann MJ, Patterson PH, Williams JH, Samal B, Del Castillo J, Yin S, Guo K, Remick DG. Intratracheal injection of LPS and cytokines. V. LPS induces expression of LIF and LIF inhibits acute inflammation. The American journal of physiology. 1994;267:L442–L446. doi: 10.1152/ajplung.1994.267.4.L442. [DOI] [PubMed] [Google Scholar]
- 21.Wang J, Chen Q, Corne J, Zhu Z, Lee CG, Bhandari V, Homer RJ, Elias JA. Pulmonary expression of leukemia inhibitory factor induces B cell hyperplasia and confers protection in hyperoxia. The Journal of biological chemistry. 2003;278:31226–31232. doi: 10.1074/jbc.M301820200. [DOI] [PubMed] [Google Scholar]
- 22.Heymann D, L'Her E, JM NG, Raher S, Canfrere I, Coupey L, Fixe P, Chailleux E, De Groote D, Praloran V, Godard A. Leukaemia inhibitory factor (LIF) production in pleural effusions: comparison with production of IL-4, IL-8, IL-10 and macrophage-colony stimulating factor (M-CSF) Cytokine. 1996;8:410–416. doi: 10.1006/cyto.1996.0056. [DOI] [PubMed] [Google Scholar]
- 23.Jorens P, De Jongh R, Bossaert LL, De Backer W, Herman AG, Pollet H, Bosmans E, Taupin JL, Moreau JF. High levels of leukaemia inhibitory factor in ARDS. Cytokine. 1996;8:873–876. doi: 10.1006/cyto.1996.9999. [DOI] [PubMed] [Google Scholar]
- 24.Quinton LJ, Jones MR, Simms BT, Kogan MS, Robson BE, Skerrett SJ, Mizgerd JP. Functions and regulation of NF-kappaB RelA during pneumococcal pneumonia. J Immunol. 2007;178:1896–1903. doi: 10.4049/jimmunol.178.3.1896. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Dai M, Wang P, Boyd AD, Kostov G, Athey B, Jones EG, Bunney WE, Myers RM, Speed TP, Akil H, Watson SJ, Meng F. Evolving gene/transcript definitions significantly alter the interpretation of GeneChip data. Nucleic acids research. 2005;33:e175. doi: 10.1093/nar/gni179. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Su X, Looney MR, Gupta N, Matthay MA. Receptor for advanced glycation end-products (RAGE) is an indicator of direct lung injury in models of experimental lung injury. American journal of physiology. Lung cellular and molecular physiology. 2009;297:L1–L5. doi: 10.1152/ajplung.90546.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Mizgerd JP. Acute lower respiratory tract infection. The New England journal of medicine. 2008;358:716–727. doi: 10.1056/NEJMra074111. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Looney MR, Su X, Van Ziffle JA, Lowell CA, Matthay MA. Neutrophils and their Fc gamma receptors are essential in a mouse model of transfusion-related acute lung injury. The Journal of clinical investigation. 2006;116:1615–1623. doi: 10.1172/JCI27238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Xiang M, Fan J. Pattern recognition receptor-dependent mechanisms of acute lung injury. Mol Med. 2010;16:69–82. doi: 10.2119/molmed.2009.00097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Cai S, Batra S, Lira SA, Kolls JK, Jeyaseelan S. CXCL1 regulates pulmonary host defense to Klebsiella Infection via CXCL2, CXCL5, NF-kappaB, and MAPKs. Journal of immunology. 2010;185:6214–6225. doi: 10.4049/jimmunol.0903843. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Goodman RE, Standiford TJ. Neutralization of IL-10 increases survival in a murine model of Klebsiella pneumonia. J Immunol. 1995;155:722–729. [PubMed] [Google Scholar]
- 32.Greenberger MJ, Strieter RM, Kunkel SL, Danforth JM, Laichalk LL, McGillicuddy DC, Standiford TJ. Neutralization of macrophage inflammatory protein-2 attenuates neutrophil recruitment and bacterial clearance in murine Klebsiella pneumonia. The Journal of infectious diseases. 1996;173:159–165. doi: 10.1093/infdis/173.1.159. [DOI] [PubMed] [Google Scholar]
- 33.Jones MR, Simms BT, Lupa MM, Kogan MS, Mizgerd JP. Lung NF-kappaB activation and neutrophil recruitment require IL-1 and TNF receptor signaling during pneumococcal pneumonia. J Immunol. 2005;175:7530–7535. doi: 10.4049/jimmunol.175.11.7530. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Steinwede K, Tempelhof O, Bolte K, Maus R, Bohling J, Ueberberg B, Langer F, Christman JW, Paton JC, Ask K, Maharaj S, Kolb M, Gauldie J, Welte T, Maus UA. Local Delivery of GM-CSF Protects Mice from Lethal Pneumococcal Pneumonia. Journal of immunology. 2011;187:5346–5356. doi: 10.4049/jimmunol.1101413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Ye P, Rodriguez FH, Kanaly S, Stocking KL, Schurr J, Schwarzenberger P, Oliver P, Huang W, Zhang P, Zhang J, Shellito JE, Bagby GJ, Nelson S, Charrier K, Peschon JJ, Kolls JK. Requirement of interleukin 17 receptor signaling for lung CXC chemokine and granulocyte colony-stimulating factor expression, neutrophil recruitment, and host defense. The Journal of experimental medicine. 2001;194:519–527. doi: 10.1084/jem.194.4.519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Matute-Bello G, Liles WC, Steinberg KP, Kiener PA, Mongovin S, Chi EY, Jonas M, Martin TR. Soluble Fas ligand induces epithelial cell apoptosis in humans with acute lung injury (ARDS) J Immunol. 1999;163:2217–2225. [PubMed] [Google Scholar]
- 37.Mura M, Han B, Andrade CF, Seth R, Hwang D, Waddell TK, Keshavjee S, Liu M. The early responses of VEGF and its receptors during acute lung injury: implication of VEGF in alveolar epithelial cell survival. Critical care. 2006;10:R130. doi: 10.1186/cc5042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Ward NS, Waxman AB, Homer RJ, Mantell LL, Einarsson O, Du Y, Elias JA. Interleukin--induced protection in hyperoxic acute lung injury. American journal of respiratory cell and molecular biology. 2000;22:535–542. doi: 10.1165/ajrcmb.22.5.3808. [DOI] [PubMed] [Google Scholar]
- 39.Waxman AB, Einarsson O, Seres T, Knickelbein RG, Warshaw JB, Johnston R, Homer RJ, Elias JA. Targeted lung expression of interleukin-11 enhances murine tolerance of 100% oxygen and diminishes hyperoxia-induced DNA fragmentation. The Journal of clinical investigation. 1998;101:1970–1982. doi: 10.1172/JCI1337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Stewart CL, Kaspar P, Brunet LJ, Bhatt H, Gadi I, Kontgen F, Abbondanzo SJ. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79. doi: 10.1038/359076a0. [DOI] [PubMed] [Google Scholar]
- 41.Weber MA, Schnyder-Candrian S, Schnyder B, Quesniaux V, Poli V, Stewart CL, Ryffel B. Endogenous leukemia inhibitory factor attenuates endotoxin response. Laboratory investigation; a journal of technical methods and pathology. 2005;85:276–284. doi: 10.1038/labinvest.3700216. [DOI] [PubMed] [Google Scholar]
- 42.Elias JA, Zheng T, Whiting NL, Marcovici A, Trow TK. Cytokine-cytokine synergy and protein kinase C in the regulation of lung fibroblast leukemia inhibitory factor. The American journal of physiology. 1994;266:L426–L435. doi: 10.1152/ajplung.1994.266.4.L426. [DOI] [PubMed] [Google Scholar]
- 43.Knight DA, Lydell CP, Zhou D, Weir TD, Robert Schellenberg R, Bai TR. Leukemia inhibitory factor (LIF) and LIF receptor in human lung. Distribution and regulation of LIF release. American journal of respiratory cell and molecular biology. 1999;20:834–841. doi: 10.1165/ajrcmb.20.4.3429. [DOI] [PubMed] [Google Scholar]
- 44.Knight D, McKay K, Wiggs B, Schellenberg RR, Bai T. Localization of leukaemia inhibitory factor to airway epithelium and its amplification of contractile responses to tachykinins. British journal of pharmacology. 1997;120:883–891. doi: 10.1038/sj.bjp.0700965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Niwa H, Ogawa K, Shimosato D, Adachi K. A parallel circuit of LIF signalling pathways maintains pluripotency of mouse ES cells. Nature. 2009;460:118–122. doi: 10.1038/nature08113. [DOI] [PubMed] [Google Scholar]
- 46.Gao W, Thompson L, Zhou Q, Putheti P, Fahmy TM, Strom TB, Metcalfe SM. Treg versus Th17 lymphocyte lineages are cross-regulated by LIF versus IL-6. Cell Cycle. 2009;8:1444–1450. doi: 10.4161/cc.8.9.8348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Liu J, Feng Y, Yang K, Li Q, Ye L, Han L, Wan H. Early production of IL-17 protects against acute pulmonary Pseudomonas aeruginosa infection in mice. FEMS Immunol Med Microbiol. 2011;61:179–188. doi: 10.1111/j.1574-695X.2010.00764.x. [DOI] [PubMed] [Google Scholar]
- 48.Chen K, McAleer JP, Lin Y, Paterson DL, Zheng M, Alcorn JF, Weaver CT, Kolls JK. Th17 cells mediate clade-specific, serotype-independent mucosal immunity. Immunity. 2011;35:997–1009. doi: 10.1016/j.immuni.2011.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.McAleer JP, Kolls JK. Mechanisms controlling Th17 cytokine expression and host defense. Journal of leukocyte biology. 2011;90:263–270. doi: 10.1189/jlb.0211099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Xu Q, Briggs J, Park S, Niu G, Kortylewski M, Zhang S, Gritsko T, Turkson J, Kay H, Semenza GL, Cheng JQ, Jove R, Yu H. Targeting Stat3 blocks both HIF-1 and VEGF expression induced by multiple oncogenic growth signaling pathways. Oncogene. 2005;24:5552–5560. doi: 10.1038/sj.onc.1208719. [DOI] [PubMed] [Google Scholar]
- 51.Al-Lamki RS, Wang J, Tolkovsky AM, Bradley JA, Griffin JL, Thiru S, Wang EC, Bolton E, Min W, Moore P, Pober JS, Bradley JR. TL1A both promotes and protects from renal inflammation and injury. Journal of the American Society of Nephrology : JASN. 2008;19:953–960. doi: 10.1681/ASN.2007060706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Gharib SA, Liles WC, Klaff LS, Altemeier WA. Noninjurious mechanical ventilation activates a proinflammatory transcriptional program in the lung. Physiological genomics. 2009;37:239–248. doi: 10.1152/physiolgenomics.00027.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Gopalan B, Litvak A, Sharma S, Mhashilkar AM, Chada S, Ramesh R. Activation of the Fas-FasL signaling pathway by MDA-/IL-4 kills human ovarian cancer cells. Cancer research. 2005;65:3017–3024. doi: 10.1158/0008-5472.CAN-04-3758. [DOI] [PubMed] [Google Scholar]
- 54.Jin HK, Takada A, Kon Y, Haller O, Watanabe T. Identification of the murine Mx2 gene: interferon-induced expression of the Mx2 protein from the feral mouse gene confers resistance to vesicular stomatitis virus. J Virol. 1999;73:4925–4930. doi: 10.1128/jvi.73.6.4925-4930.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ma SF, Xie L, Pino-Yanes M, Sammani S, Wade MS, Letsiou E, Siegler J, Wang T, Infusino G, Kittles RA, Flores C, Zhou T, Prabhakar BS, Moreno-Vinasco L, Villar J, Jacobson JR, Dudek SM, Garcia JG. Type 2 Deiodinase and Host Responses of Sepsis and Acute Lung Injury. American journal of respiratory cell and molecular biology. 2011 doi: 10.1165/rcmb.2011-0179OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Pradervand S, Yasukawa H, Muller OG, Kjekshus H, Nakamura T, St Amand TR, Yajima T, Matsumura K, Duplain H, Iwatate M, Woodard S, Pedrazzini T, Ross J, Firsov D, Rossier BC, Hoshijima M, Chien KR. Small proline-rich protein 1A is a gp130 pathway- and stress-inducible cardioprotective protein. The EMBO journal. 2004;23:4517–4525. doi: 10.1038/sj.emboj.7600454. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Rodriguez N, Mages J, Dietrich H, Wantia N, Wagner H, Lang R, Miethke T. MyD88-dependent changes in the pulmonary transcriptome after infection with Chlamydia pneumoniae. Physiological genomics. 2007;30:134–145. doi: 10.1152/physiolgenomics.00011.2007. [DOI] [PubMed] [Google Scholar]
- 58.To M, Ito K, Ausin PM, Kharitonov SA, Barnes PJ. Osteoprotegerin in sputum is a potential biomarker in COPD. Chest. 2011;140:76–83. doi: 10.1378/chest.10-1608. [DOI] [PubMed] [Google Scholar]
- 59.Wang XM, Yu DM, McCaughan GW, Gorrell MD. Extra-enzymatic roles of DPIV and FAP in cell adhesion and migration on collagen and fibronectin. Adv Exp Med Biol. 2006;575:213–222. doi: 10.1007/0-387-32824-6_23. [DOI] [PubMed] [Google Scholar]
- 60.Morimura T, Fujita K, Akita M, Nagashima M, Satomi A. The proton pump inhibitor inhibits cell growth and induces apoptosis in human hepatoblastoma. Pediatr Surg Int. 2008;24:1087–1094. doi: 10.1007/s00383-008-2229-2. [DOI] [PubMed] [Google Scholar]
- 61.Fernandez-Valdivia R, Zhang Y, Pai S, Metzker ML, Schumacher A. l7Rn6 encodes a novel protein required for clara cell function in mouse lung development. Genetics. 2006;172:389–399. doi: 10.1534/genetics.105.048736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Castleman VH, Romio L, Chodhari R, Hirst RA, de Castro SC, Parker KA, Ybot-Gonzalez P, Emes RD, Wilson SW, Wallis C, Johnson CA, Herrera RJ, Rutman A, Dixon M, Shoemark A, Bush A, Hogg C, Gardiner RM, Reish O, Greene ND, O'Callaghan C, Purton S, Chung EM, Mitchison HM. Mutations in radial spoke head protein genes RSPH9 and RSPH4A cause primary ciliary dyskinesia with central-microtubular-pair abnormalities. American journal of human genetics. 2009;84:197–209. doi: 10.1016/j.ajhg.2009.01.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Kubo A, Yuba-Kubo A, Tsukita S, Amagai M. Sentan: a novel specific component of the apical structure of vertebrate motile cilia. Molecular biology of the cell. 2008;19:5338–5346. doi: 10.1091/mbc.E08-07-0691. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Suzuki S, Tsubochi H, Darnel A, Suzuki T, Sasano H, Krozowski ZS, Kondo T. Expression of 11 beta-hydroxysteroid dehydrogenase type 1 in alveolar epithelial cells in rats. Endocr J. 2003;50:445–451. doi: 10.1507/endocrj.50.445. [DOI] [PubMed] [Google Scholar]
- 65.Zemke AC, Snyder JC, Brockway BL, Drake JA, Reynolds SD, Kaminski N, Stripp BR. Molecular staging of epithelial maturation using secretory cell-specific genes as markers. American journal of respiratory cell and molecular biology. 2009;40:340–348. doi: 10.1165/rcmb.2007-0380OC. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Dauer DJ, Ferraro B, Song L, Yu B, Mora L, Buettner R, Enkemann S, Jove R, Haura EB. Stat3 regulates genes common to both wound healing and cancer. Oncogene. 2005;24:3397–3408. doi: 10.1038/sj.onc.1208469. [DOI] [PubMed] [Google Scholar]
- 67.Xu Y, Ikegami M, Wang Y, Matsuzaki Y, Whitsett JA. Gene expression and biological processes influenced by deletion of Stat3 in pulmonary type II epithelial cells. BMC genomics. 2007;8:455. doi: 10.1186/1471-2164-8-455. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Kotton DN, Fine A. Lung stem cells. Cell Tissue Res. 2008;331:145–156. doi: 10.1007/s00441-007-0479-2. [DOI] [PubMed] [Google Scholar]