Abstract
Purpose
To evaluate the family environments of children with cochlear implants and to examine relationships between family environment and post-implant language development and executive function.
Method
Forty-five families of children with cochlear implants completed a self-report family environment questionnaire (FES) and an inventory of executive function (BRIEF/BRIEF-P). Children’s receptive vocabulary (PPVT-4) and global language skills (PLS-4/CELF-4) were also evaluated.
Results
The family environments of children with cochlear implants differed from those of normal-hearing children, but not in clinically significant ways. Language development and executive function were found to be atypical, but not uncharacteristic of this clinical population. Families with higher levels of self-reported control had children with smaller vocabularies. Families reporting a higher emphasis on achievement had children with fewer executive function and working memory problems. Finally, families reporting a higher emphasis on organization had children with fewer problems related to inhibition.
Conclusions
Some of the variability in cochlear implantation outcomes that have protracted periods of development is related to family environment. Because family environment can be modified and enhanced by therapy or education, these preliminary findings hold promise for future work in helping families to create robust language-learning environments that can maximize their child’s potential with a cochlear implant.
Contribution of Family Environment to Pediatric Cochlear Implant Users’ Speech and Language Outcomes: Some Preliminary Findings
Most of the research on family factors related to children’s success after cochlear implantation has focused on the use of oral language at home, provision of support, the family’s role in therapy, family size, education, socioeconomic status (SES), and maternal influences. Little is currently known, however, about the direct or indirect impact of more structural and functional dimensions of the family environment on vocabulary, language ability, and executive function. The purpose of this investigation was three-fold: (a) to examine the social climate of the family which includes interpersonal relationships, personal growth, and family structure in a sample of families of young cochlear implant users using a psychometrically rigorous, self-report questionnaire (the Family Environment Scale [FES] – 4th Edition [Moos & Moos, 2009]); (b) to compare the sample’s self-reported family environments with those of typically developing, normal-hearing children; and (c) to examine relations between self-reported family environment and post-implant language skills and executive function.
Family Environment and Language Development
The search for factors that contribute to cochlear implant success in children has led many investigators and clinicians to examine the role of the child’s immediate family. The family is a reasonable place to look because it is generally believed to play a crucial role in many areas of child development, including cognition and social development (e.g., Belsky, 1981). The majority of research on sources of variability in pediatric cochlear implant outcomes that has focused on families have concentrated efforts in the areas of the choice and use of communication modality (e.g., Geers, Brenner, & Davidson, 2003; Geers, Strube, Tobey, Pisoni & Moog, 2011; Holt & Svirsky, 2008; Kirk et al., 2002), provision of support (e.g., Edwards, Thomas, & Rajput, 2009; Nikolopoulos, Gibbin, & Dyar, 2004), the family’s role in therapy (Bertram & Päd, 1995; Moeller, 2000), and the family’s size (Geers et al., 2003, 2011), education level (Geers et al., 2003) and SES (Geers et al., 2003, 2011; Holt & Svirsky). In addition, several maternal factors have been investigated in children with sensory aids, including maternal attachment and sensitivity (e.g., Lederberg & Mobley, 1990; Pratt, 1991; Pressman, Pipp-Siegel, Yoshinaga-Itano, & Deas, 1999), and maternal involvement and self-efficacy (DesJardin, 2005; DesJardin & Eisenberg, 2007). Finally, maternal linguistic input has been widely implicated in language and literacy development in children with cochlear implants (e.g., DesJardin, Ambrose, & Eisenberg, 2008; DesJardin & Eisenberg, 2007). Each of these areas of family environment and their contribution to pediatric cochlear implant language outcomes are described below.
Communication Modality
For cochlear implanted children, the family’s choice of communication modality typically is limited to one that emphasizes oral/aural communication (oral or auditory-verbal communication) or one that combines oral language with signing (Total communication). Although the influence of communication modality on speech and language outcomes is admittedly complicated (Kirk et al., 2002), most studies have concluded that children who are reared and educated in environments that strongly emphasize the use of oral language have better speech and language outcomes than those whose communication partners use supplementary sign cues while speaking (e.g., Geers et al., 2003; Holt & Svirsky, 2008; Kirk et al., 2002). For example, Geers et al. (2003) consistently found that the most important rehabilitative factor in having good spoken word recognition with a cochlear implant at age 8 and 9 years was the choice of communication modality: children reared and educated in oral environments had better spoken word recognition skills than children in Total communication environments, despite controlling for many contributing factors to spoken language outcomes including those related to family, education, device, the child, and therapy received. In a recent study that followed most of these same children through adolescence, Geers et al. (2011) reported that although communication mode does not directly influence speech perception and intelligibility in the teenage years, it does strongly influence verbal rehearsal speed, which influences speech outcomes in the elementary years, which in turn strongly influences outcomes during adolescence (see too Pisoni, Kronenberger, Roman, & Geers, 2011).
Family Support
Family structure and support have long been identified by clinicians and cochlear implant teams as critical components to successful cochlear implantation outcomes (Bertram & Päd, 1995), although the exact definition of support is often poorly defined or is underspecified. Family structure and support was valued enough as a contributing factor to cochlear implant outcomes that it was included in a survey tool, the children’s implant profile (ChIP; Hellman et al., 1991), that can be used to guide cochlear implant candidacy. Of the 11 factors used in the original ChIP, family structure/support accounted for a significant amount of variance in the post-implant speech perception (Nikolopoulos et al., 2004).
Using a revised and expanded version of the tool, the Great Ormond Street Hospital ChIP (GOSHChIP), Edwards et al. (2009) reported that children in families who the cochlear implant team had greater concerns about their ability to provide support and optimize the use of the cochlear implant had poorer speech perception after 1 year, but not 2 or 3 years, of device use than children whose families were rated as likely to provide support and maximize use of the device. Further, family support predicted speech intelligibility 1, 2 and 3 years post-implant. In sum, there is evidence that family support/structure, albeit underspecified, plays an important role in the spoken language development of children following cochlear implantation.
Family’s Role in Therapy
Moeller (2000) quantified family involvement, which is likely related to family structure/support, using a rating scale developed specifically for the purpose of retrospectively examining the role of family involvement in language development of 112 deaf and hard-ofhearing (over half had severe to profound losses) 5-year-olds who wore hearing aids. Early interventionists who had worked semiweekly with families for between two and four years rated families’ levels of participation in their hard-of-hearing/deaf child’s intervention. Controlling for various family and individual factors, the level of family involvement explained the most variance in children’s language skills of all of the factors studied (Moeller, 2000).
Family Size and Socioeconomic Status
In addition to the communication modality effect discussed earlier, Geers et al. (2003) also reported that when non-verbal intelligence was accounted for, the only family factor (including SES) that influenced spoken word recognition was family size – children from smaller families had better spoken word recognition. Partially supporting Geers et al.’s (2003) findings, Holt and Svirsky (2008) did not find a relationship between estimated family income (a measure of SES) and spoken word recognition of children with cochlear implants. However, they did find a relationship between estimated income and receptive and expressive language: relative to children from families with lower estimated incomes, children from families with higher estimated incomes had faster rates of receptive language development, but slower rates of expressive language development.
Maternal Influences
The influence of SES on language development in typically developing children has been of interest at least since Hart and Risley’s (1985) seminal work on the effect of early linguistic experience. However, the relationship between SES and language development is not straightforward; there are likely numerous mediating factors, such as maternal language input (Hoff, 2003). Maternal effects on children’s language development are of special interest because in normal-hearing children, the quality and quantity of communication directed at the child facilitates language acquisition (e.g., Bates, Bretherton, Beeghly-Smith, & McNew, 1982). Deaf infants born into hearing families, approximately 96% of those born deaf according to Ross and Karchmer (2004), are at risk for suboptimal language input primarily because their hearing loss limits the oral language they have access to, but also because of the hearing status mismatch and resulting communication difficulties between the mother-child dyad. Hearing mothers tend to be more rigid, negative, intrusive, and less likely to respond to their deaf and hard-of-hearing children than hearing parents of hearing children (e.g., MacTurk, Meadow-Orlans, Koester, & Spencer, 1993; Meadow-Orlans & Steinberg, 1993). They also use less complex language structures and fewer expansions with their deaf and hard-of-hearing children (Cross, Johnson- Morris, & Nienhuys, 1980; Nienhuys, Horsborough, & Cross, 1985).
Although Lederberg and Mobley (1990) did not find that hearing mothers are more rigid, negative and less likely to respond to their deaf children, they did find that the interactions were shorter, more likely to be interrupted due to break downs in communication, and the communication was likely to be controlled by the parent. Vaccari and Marschark (1997) also have reported that hearing parents are more directive and controlling in their interactions with their deaf children. When the child’s initial language level, as well as known influences on language development (e.g., communication mode), were controlled for, maternal sensitivity (the ability to read a child’s cues and respond appropriately, to resolve parent-child misunderstandings or conflict, and to tolerate various affective states of the child while keeping interactions positive) contributed positively to expressive language gains approximately 1 year after the initial assessment, but not to performance at the initial language assessment (Pressman et al., 1999).
Maternal self-efficacy in the ability to help one’s child develop language skills, as well as maternal involvement, was found to be positively related to mothers’ qualitative and quantitative linguistic input to their implanted children, specifically, facilitative language techniques, such as parallel talk and expansion (DesJardin & Eisenberg, 2007). In turn, mothers’ use of higher-level facilitative language techniques (e.g., recast and open-ended questions) was positively related to children’s spoken language skills, whereas mothers’ use of lower-level techniques (e.g., label and directive) negatively influenced children’s language abilities. Furthermore, mother’s mean length of utterance accounted for most of the variance in children’s language skills (DesJardin & Eisenberg). Mothers’ use of facilitative techniques (such as open-ended questions) even extends to literacy development in children with cochlear implants (DesJardin et al., 2008). Therefore, maternal factors play an important role in the development of speech, language and literacy in children with hearing loss who use sensory aids.
The literature reviewed thus far has focused on the impact of family factors on language outcomes in children who use sensory aids. Recently, however, there has been a new line of outcomes research in this population related to core underlying neurocognitive processes that might be affected by deafness and spoken language delay. The following section discusses the literature on the influences of family factors on executive function and their relevance to this clinical pediatric population.
Family Environment and Executive Function
Executive Function in Children with Cochlear Implants
Recent research suggests that children with severe to profound hearing loss who have experienced a period of auditory deprivation and language delay may also experience delays and deficits in elementary neurocognitive processes underlying spoken language processing. In an effort to explain individual differences in speech and language outcomes after cochlear implantation, Pisoni and his colleagues have been investigating domain-general executive-organizational-integrative (EOI) processes such as executive function, cognitive control, and self-regulation in children who use cochlear implants (Pisoni, Conway, Kronenberger, Henning & Anaya, 2010). Their work suggests that some children with cochlear implants show delays in immediate memory capacity, working memory, sequence memory and learning, verbal rehearsal speed, and executive function compared to normal-hearing peers. Furthermore, these delays are associated with poorer performance on several conventional speech and language measures used to assess outcomes after cochlear implantation (Pisoni et al., 2010). Because deafness affects spoken language development, and language and neurocognitive development are interdependent, currently it is difficult to describe the specific relationship between deafness and neurocognitive development.
The auditory scaffolding hypothesis proposed recently by Conway and colleagues suggests that auditory input is necessary for the development of cognitive processes that require the encoding, learning, and manipulation of sequential information, which includes spoken language (Conway, Pisoni & Kronenberger, 2009). In fact, deaf children with cochlear implants also demonstrate atypical motor and visual sequence learning compared to age-matched normally hearing children (Conway, Pisoni, Anaya, Karpicke & Henning, 2011; Conway, Karpicke et al., 2011). Furthermore, the relationship between EOI processes and language is likely bidirectional in that the control, organizational, and mediation functions of language are necessary for the development of EOI processes, which are in turn necessary for the development of more complex language skills. Consequently, the language delay experienced by deaf children impacts higher-order cognitive functions mediated by language, including processes that require active verbal rehearsal (e.g., working memory) and verbal mediation (e.g., planning), thus placing key EOI processes at risk in this clinical population (Pisoni et al., 2010).
Executive function is an umbrella term used to describe a collection of neurocognitive processes used to guide and control thinking, behavior, and emotions. The core executive processes that can be differentiated as early as age 2 years are inhibitory control (the ability to resist a behavior or thought in favor of doing or thinking what is appropriate to the situation), working memory (the ability to hold information in mind and mentally manipulate it), and cognitive flexibility (the ability to switch attention from one focus to another quickly; Diamond, Barnett, Thomas, & Munro, 2007). Inhibitory control and working memory are associated with early math competency, literacy, and social understanding in typically developing children (Blair & Razza, 2007; Carlson, Moses & Breton 2002; Espy, 2004; Hughes & Ensor, 2007; McClelland et al., 2007). Using a parent report measure that assesses eight executive functions as they relate to day-to-day functioning, Beer, Kronenberger, and Pisoni (2011) reported that school-age children with cochlear implants had significantly more executive function difficulties related to working memory, inhibitory control, and behavior regulation than the normative sample, although the observed scores were within normal limits. In addition, children with more executive function difficulties related to working memory (e.g., having trouble remembering things and staying on task) had significantly poorer performance on tests of sentence perception in noise and complex language than children with fewer executive difficulties in this area (Beer et al., 2011). However, there were no differences between these groups on sentence perception in quiet or receptive vocabulary, suggesting that executive difficulties are more likely to impact performance on tasks with a high cognitive load, such as listening in noise.
Neurobiological Account of Executive Function
The development of executive function is typically understood with a neurobiological perspective that associates changes in executive function with neural maturation of the prefrontal system. The prefrontal system contains brain circuits associated with selective attention, emotion processing, and working memory, and is highly plastic and functionally connected to other brain regions. The prefrontal system also has a protracted post-natal course of development compared to other areas of the brain, with maturation continuing well into adolescence and early adulthood (Ciccia, Meulenbroek, & Turkstra, 2009). Although a neurobiological model of executive function development is useful for understanding the relationship between brain development and executive function, recent research with typically developing children also provides strong evidence for social contributions to the development of executive function as well, thus emphasizing experience-dependent maturation of the brain (Carlson, 2009).
Social Contributions to Executive Function: A Role for Family Environment
Bernier, Carlson & Whipple (2010) reported that the quality of mother-child interactions at 15 months of age was related to child executive function at 18 months. Specifically, mothers who were more sensitive, mind-minded (mothers’ tendency to use mental terms when talking to their child reflecting a perspective that the child is an individual with a mind rather than simply an individual with needs that must be satisfied), and more supportive of their child’s autonomous behavior had children who performed better on tasks of working memory and/or conflict executive function tasks. In addition to quality of mother-child relationship, other researchers have focused on the role of maternal verbal scaffolding on children’s language and executive function. Landry, Miller-Loncar, Smith, and Swank (2002) reported that maternal verbal scaffolding (providing conceptual links between objects, people, activities, or functions) at age 3 years directly influenced children’s early language skills at age 4 years, which in turn affected children’s executive function skills at age 6 years. In a study of the timing of elaborative and directive maternal scaffolding, Bibcok, Carpendale, and Muller (2009) reported that contingent elaborative utterances by the mother during a puzzle solving task (e.g., utterances that elaborate or evaluate the child’s present course of action) were related to executive function, but contingent directive utterances were not (e.g., those that direct the child what to do next).
Finally, Hughes and Ensor (2009) proposed a broader approach to understanding the social influences on executive function by measuring the effects of several family influences on early executive function. They found that maternal planning behavior, family chaos, and maternal scaffolding accounted for 14% of the variance in executive function at age 4 years, after controlling for executive function and verbal skills at age 2 years. In a different investigation, family chaos was found to negatively influence children’s behavior above and beyond the parenting style itself and can amplify effects of negative parenting (Coldwell, Pike, & Dunn, 2006). Kronenberger and Thompson (1990), investigating family characteristics of children with behavior problems and chronic illness using the FES (the instrument used in the current investigation), reported that children with behavior problems had families that were less supportive and had higher levels of conflict than children who did not have behavior problems.
The Need for More Research on Family Environment and Cochlear Implant Outcomes
Despite the many investigations cited above on the environmental factors that affect sensory aid (primarily cochlear implant) outcomes in children, no investigations have examined the structural and functional dimensions of family environment in detail and how they might directly or indirectly mediate language and cognitive outcomes in children with cochlear implants. This is an area in need of additional research, because family environment influences outcomes in both clinical and healthy populations. Therefore, it is reasonable to expect that these core dimensions of family environment could affect outcomes in children with cochlear implants. But what aspects of the family environment are important? And what outcome(s) might family environment moderate?
The literature reviewed above on both healthy and other clinical populations suggests that children with fewer behavior problems and better executive function tend to have families that provide support, scaffolding, consistency, and structure. These areas, as well as others, are evaluated on a widely used and psychometrically rigorous self-report questionnaire – the Family Environment Scale (FES – 4th Edition (Moos & Moos, 2009). This still leaves open the question of what outcome measures to evaluate. Noble, Norman, and Farah (2005) reported that environmental influences might be particularly strong for neurocognitive abilities with protracted post-natal development periods, such as language and executive function. Therefore, as a first attempt to examine family environment and cochlear implant outcomes, we used the FES to evaluate the possible relations between family environment of a sample of children with cochlear implants and their post-implant language development and executive function.
The purpose of this investigation was three-fold: (1) to examine the self-reported family environment of a sample of children with cochlear implants; (2) to compare the family environments of the sample with those of typically developing, normal-hearing children; and (3) to examine relations between the family environment and measures of post-implant language development and executive function. If family environment is related to cochlear implant outcomes, attention to family characteristics might be a beneficial component of early intervention and therapy for this clinical population.
Method
Participants
Parents of 45 prelingually deaf children with cochlear implants who had no additional disabilities were invited to complete the FES (described in detail below) as part of their participation in a longitudinal study investigating speech and language outcomes after cochlear implantation; two families chose not to complete the FES, stating that the questions were too personal. Children with cochlear implants in these families encompassed a wide age range (M=7.8 yrs; SD=4.3 yrs) and length of device use (M=5.5 yrs; SD=4.0 yrs); 36 used oral communication strategies; 14 were binaurally implanted; and 5 used a hearing aid on their nonimplanted ears. Table 1 displays a summary of the demographic characteristics of the children and their families. Maternal education was scored by assigning integer values (1 through 7) to levels of formal education: 1 = some high school, 2 = high school diploma, 3 = some college, 4 = Associate degree, 5 = Bachelors degree, 6 = Masters degree, 7 = Doctorate degree.
Table 1.
Characteristics of the Children and Families
| Mean | SD | Range | |
|---|---|---|---|
| Age at test (yrs) | 7.8 | 4.3 | 1.3–18.2 |
| Age at implant (yrs) | 2.4 | 1.4 | 0.7–6.8 |
| Duration of implant use (yrs) | 5.5 | 4.0 | 0.5–16.0 |
| *Unaided PTA (better ear) | 105.9 | 15.2 | 65–118.4 |
| Maternal education | 3.5 | 1.5 | 1–7 |
| Family size (members) | 3.9 | 1.2 | 2–8 |
| Percent oral communicators | 80 | — | — |
| Percent female | 42 | — | — |
| Percent married parents | 76 | — | — |
PTA = Pure tone average
re: ANSI (2004)
Materials
Family Environment Scale (FES)
The FES (Moos & Moos, 2009) is a 90-item self-report true-false questionnaire that assesses three dimensions of family environment: (a) family relationships, (b) personal growth and goals within the family, and (c) the family’s focus on system maintenance using 10 subscales. Three subscales make up the family relationship dimensions: Cohesion, Expressiveness, and Conflict. The personal growth dimensions consist of five subscales: Independence, Achievement Orientation, Intellectual-Cultural Orientation, Active-Recreational Orientation, and Moral-Religious Emphasis. Finally, the system maintenance dimensions consist of two subscales: Organization and Control. FES subscale raw scores are converted to T-scores based on a normative sample of 1432 families from all areas of the United States, representing racially diverse families, single and multi-generational families of all ages, as well as newly married families and families with children of varying ages (Moos & Moos, 2009).
The results of the FES have been used as a predictor of life transitions in various clinical populations, including behavioral (e.g., ADHD), emotional (e.g., depression), and developmental disabilities, physically ill children (e.g., cancer, TBI), and congenital handicaps (e.g., cystic fibrosis, spina bifida, cerebral palsy) (Biederman et al, 1995; Loomis, Javornisky, Monahan, Burke, & Lindsay, 1997; Rice, Harold, Shelton, & Thapar, 2006; Rivara et al., 1996; Rousey, Wild, & Blacher, 2002; Varni, Katz, Colegrove, & Dolgin, 1996). Because of its widespread use, predictive validity and reliability, the FES was used in the current investigation to identify family environment variables that might explain some of the large variability in cochlear implant outcomes.
Language Measures
Peabody Picture Vocabulary Test–4 (PPVT–4) (Dunn & Dunn, 2007)
The PPVT is a norm-referenced, wide age-ranged (2.5 – 90 years), and psychometrically sound measure of receptive vocabulary routinely used with preschool and school-age children who use cochlear implants. PPVT-4 standard scores were used in this study to measure receptive vocabulary development.
Preschool Language Scales (PLS – 4) (Zimmerman, Steiner, & Pond, 2002)
The PLS provides a norm-referenced measure of receptive and expressive language. The response format of the PLS includes elicited responses, spontaneous responses, or caregiver report which allows even very young children whose spoken language and comprehension skills are just developing to receive a score. Children up to 6 years of age were tested using the PLS. In this study, PLS Auditory Comprehension (receptive language skills), Expressive Communication (expressive language skills), and Total Language (total language skills) standard scores were used to measure language performance.
Clinical Evaluation of Language Fundamentals–4 (CELF–4) (Semel, Wiig, & Secord, 2003)
The CELF is a comprehensive global measure of language ability. The Core Language Score (a standard score based on an age-based norm sample) was used to assess general language ability in children ages 5 to 18 years. Due to the overlap in the age ranges included in the PLS and the CELF, speech-language pathologists familiar with the children and experienced in testing young children with cochlear implants determined if a 5- to 6-year-old child would be best assessed with the CELF or the PLS.
Measures of Executive Function
Behavior Rating Inventory of Executive Function (BRIEF) (Gioia, Isquith, Guy & Kenworthy, 2000)
The BRIEF is an 86-item parent-report questionnaire that is used to assess everyday real-world executive function behaviors of children ages 5 to 18 years. The BRIEF measures eight core domains of executive functioning: Inhibit, Shift; Emotional Control, Initiate, Working Memory, Plan/Organize, Organization of Materials, and Monitor. These domains combine to form two indices (Behavioral Regulation and Metacognition) and one Global Executive Composite (GEC) score. Scores for the specific domains and composite indices are converted to T-scores using age- and gender-specific norms; T scores at or above 65 are considered clinically significant. The BRIEF is a psychometrically sound neurological measure of executive function with high internal consistency (α = .80 to .98), high test-retest correlations across the 8 domains over an average interval of two weeks (r = .76–.85), and evidence of convergent and divergent validity when correlated with the appropriate commonly used measures of attentional and behavioral functioning. The BRIEF has been used in several clinical populations including children with ADHD, autism spectrum disorder, TBI, and cochlear implants (Beer et al., 2011; Gilotty, Kenworthy, Sirian, Black, & Wagner, 2002; Jarratt, Riccio, & Siekierski, 2005; Mangeot, Armstrong, Colvin, Yeates, & Taylor, 2002; Pisoni et al., 2010).
The Behavior Rating Inventory of Executive Function-Preschool version (BRIEF-P) (Gioia, Espy & Isquith, 2003)
The BRIEF-P is a 63-item parent report questionnaire that is used to assess everyday executive function behaviors of children age 2;0 to 5;11 (year;month). The BRIEF-P measures a subset of the domains of executive functioning used in the BRIEF, including Inhibit, Shift, Emotional Control, Working Memory, and Plan/Organize. These domains combine to form three indices (Inhibitory Self-Control, Flexibility, and Emergent Metacognition) and a summary score, the GEC. Scores for the individual domains and composite indices are converted to T-scores using age- and gender-specific norms; T scores at or above 65 are considered clinically significant. The BRIEF-P has high internal consistency (α = .80 to .97), high test-retest reliability over an average interval of 4.5 weeks (r = .78 to .90), and the patterns of correlations between the domains and indices of the BRIEF-P and other commonly used rating scales of attention and behavior provide convergent and divergent evidence of validity.
Procedure
The test battery was administered by licensed speech-language pathologists familiar with the children and families and experienced in evaluating the development of young cochlear implant users. Caregivers were asked to complete the FES and the BRIEF or BRIEF-P, while the children were administered the PPVT and either the PLS or the CELF. Parents of children who were 5-years-old and in preschool were given the BRIEF-P, whereas parents of children who were 5-years-old and in kindergarten completed the BRIEF. Not all children received all tests due to time constraints, missed appointments, and/or child behavior and attention. Language tests were administered with auditory and visual cues for children who used oral communication; auditory, visual, and sign were used for children who used Total communication. Both spoken and signed responses were accepted for the PLS and the CELF.
Statistical Analysis
Because our sample of CI users included a wide age range, some families completed the BRIEF-P and some completed the BRIEF. In order to compare executive function across all children, only those overlapping domains of the BRIEF and BRIEF-P were used in the statistical analyses reported here (i.e., Inhibit, Shift, Emotional Control, Working Memory, Plan/Organize, and GEC).
Results
Family Environments of Children with Cochlear Implants
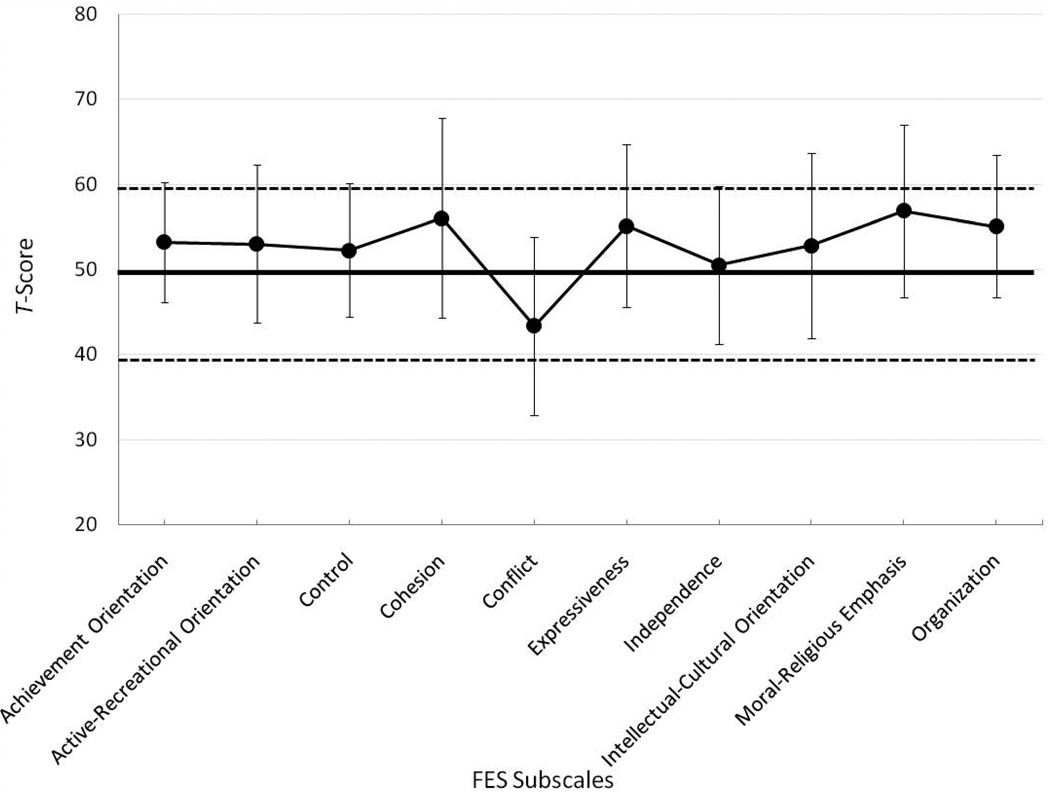
The mean T-scores (and +/− 1 SD) on each of the FES subscales are displayed in Figure 1. Parents of children in the cochlear implanted sample reported elevated scores relative to the normative sample of typically developing, normal-hearing children (mean standard score = 50) on the following subscales: Achievement Orientation (M = 53.24), t (44) = 3.096, p = .003; Active-Recreational Orientation (M = 53.02), t (44) = 2.19, p = .034; Cohesion (M = 56.044), t (44) = 3.451, p = .001; Expressiveness (M = 55.133), t (44) = 3.622, p = .001; Moral-Religious Emphasis (M = 56.911), t (44) = 4.577, p < .0001; and Organization (M = 55.067), t (44) = 4.059, p < .0001. In addition, the mean score for Conflict (43.40) was significantly lower than that for families of normal-hearing children, t (44) = −4.225, p < .0001. Although average performance was often different from typically developing, normal-hearing children, mean scores on all 10 of the subscales fell within normal limits. These results suggest that, on average, the family environments of children with CIs assessed by the FES differ from those with normalhearing children, but not in clinically significant ways.
Figure 1.
Mean T-scores (± 1 standard deviation) on each of the 10 FES subscales for the families of children with cochlear implants. The solid line indicates normative FES scores from families of typically developing children; the dashed lines indicate the boundaries of clinical significance.
Language Outcomes
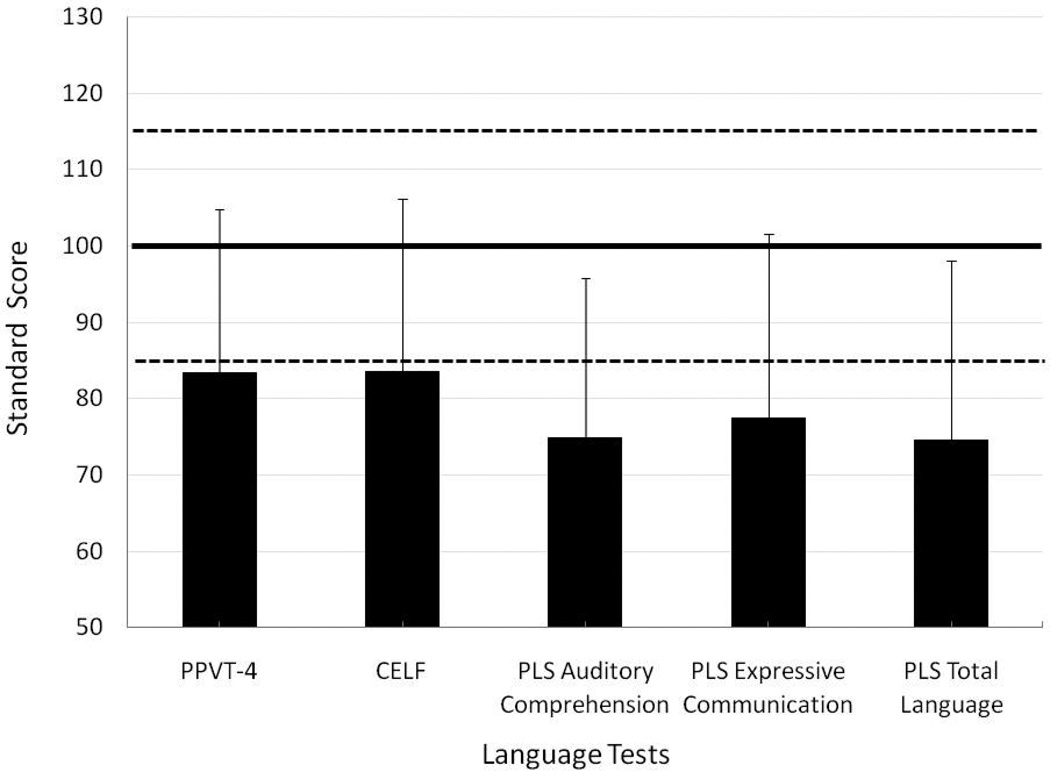
Figure 2 displays the mean standard scores (+/− 1 SD) on the PPVT-4, the CELF-4 Core Language, and the subscales of the PLS-4. As is typical in samples with cochlear implants, performance was variable across children and language abilities were both significantly lower than the normative sample mean and outside the average range: PPVT-4 (Mean = 84.43), t (37) = −4.443, p < .0001; CELF-4 Core Language (M = 83.57), t (23) = −3.484, p = .002; PLS Auditory Comprehension (M = 74.82), t (17) = −4.935, p < .0001; PLS Expressive Communication (M = 77.53), t (17) = −3.853, p = .001; and PLS Total Language (M = 74.65), t (17) = −4.444, p < .0001. In other words, as a group the children in this sample had both clinically and statistically significant delays in language development.
Figure 2.
Mean standard scores (+ 1 standard deviation) on the language measures. The solid line indicates normative language scores of typically developing children; the dashed lines indicate the boundaries of clinical significance.
Family Environment and Language Development
To determine if there were covariates that might influence the relationship between family environment, language development, and executive function, we examined correlations between FES subscale T-scores and several demographic factors including child chronological age, length of cochlear implant use, age at implantation, best pre-implant pure-tone average (at .5, 1, and 2 kHz), mothers’ education levels, and family size (number of family members living in the child’s household). The only significant correlations obtained were between age at implantation and FES Expressiveness (r = −.430, p = .003), between maternal education and FES Intellectual-Cultural Orientation (r = .295, p = .05), and between family size and FES Active- Recreational Orientation (r = .309, p = .039). Because the amount of variance accounted for is small and because controlling for these demographic factors might artificially eliminate some of the natural variability in family environment, zero-order correlations will be reported here.
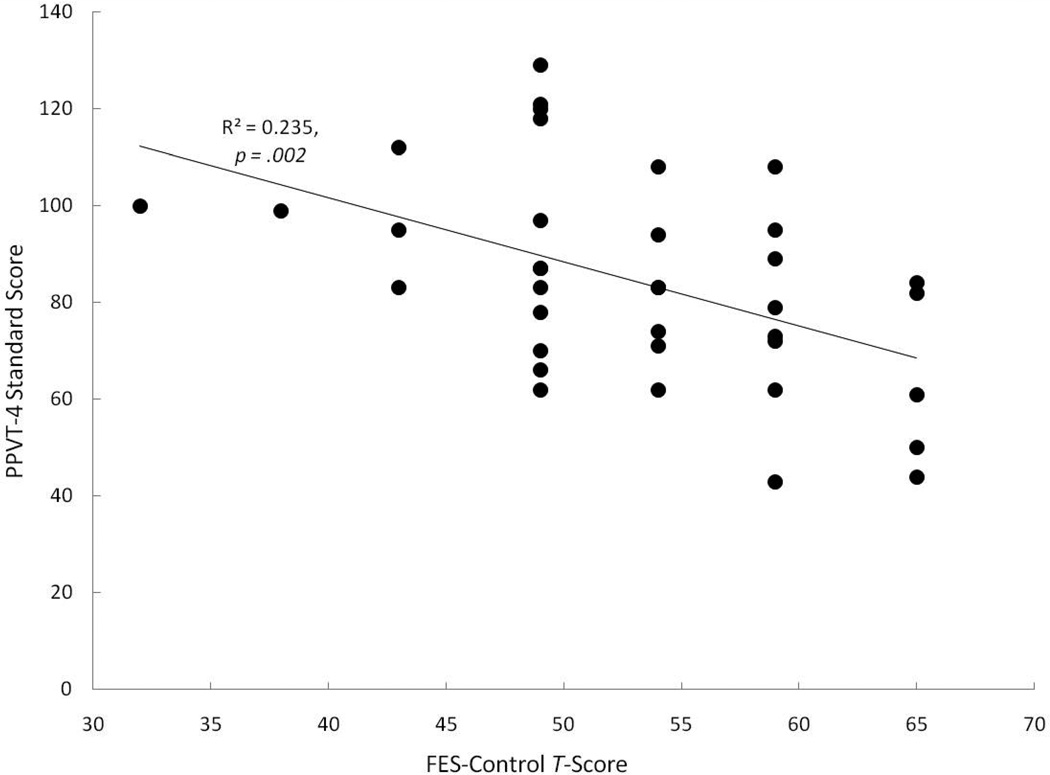
Correlations between the FES subscales and language measures indicated that families who viewed themselves as less controlling had children with larger receptive vocabularies (higher PPVT-4 scores), r = −.485, p = .002, than families who were more controlling (see Figure 3). In fact, all five children whose FES-Control scores were elevated to clinically significant levels (greater than a T-score of 60), had receptive vocabulary scores that were more than 1 standard deviation below the PPVT-4 norms. Within the normal range of variability on the FES, there were both children with near-normal receptive vocabularies and with delayed vocabularies, but there was a trend for children with lower receptive vocabulary scores to have families with higher degrees of self-rated control. None of the other FES subscales were significantly associated with receptive vocabulary. Although no other FES subscales were significantly correlated with the CELF or PLS scores, one scale approached significance with high to moderately-high r-values. Families who were less controlling tended to have children with better expressive language skills (based on the PLS Expressive Communication), r = −.451, p = .069.
Figure 3.
Correlations between FES Control Subscale T-scores and PPVT-4 measured receptive vocabulary.
Executive Function
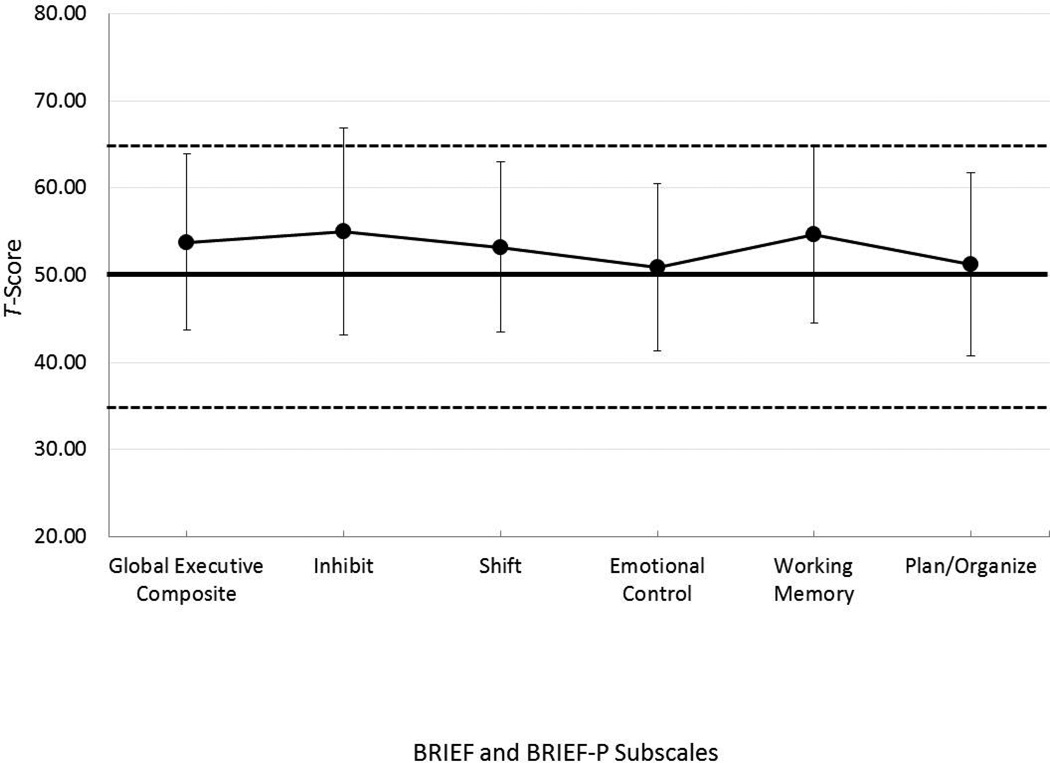
Figure 4 displays the average T-scores on domains of executive function that are common to both the BRIEF and the BRIEF-P. Parents of children in the sample reported significantly more problem behaviors than the normative sample of typically developing, normal-hearing children (mean standard score = 50) on the GEC (M = 53.81), t (42) = 2.474, p = .017, the Inhibit scale (M = 55.00), t (42) = 2.771, p = .008, and the Working Memory scale (M = 54.72), t (42) = 3.049, p = .004. However, mean scores on all of the scales fell within normal limits.
Figure 4.
Mean T-scores (± 1 standard deviation) on the scales common to both the BRIEF and the BRIEF-P. The solid line indicates normative BRIEF and BRIEF-P scores from parents of typically developing children; the dashed lines indicate the boundaries of clinical significance.
Family Environment and Executive Function
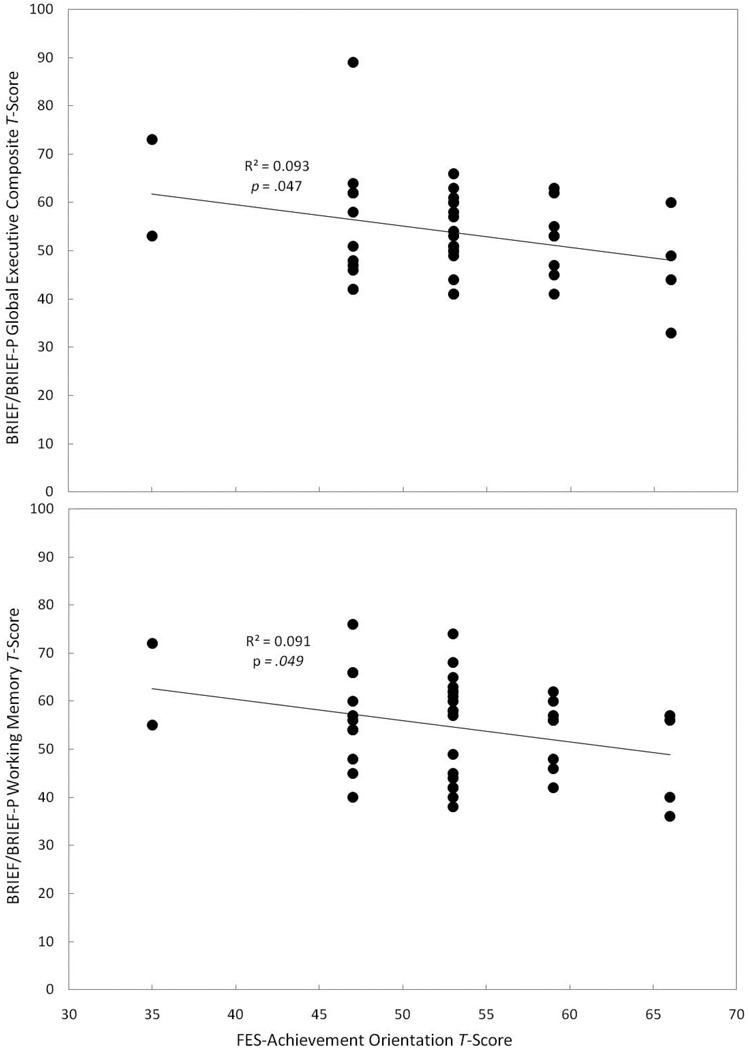
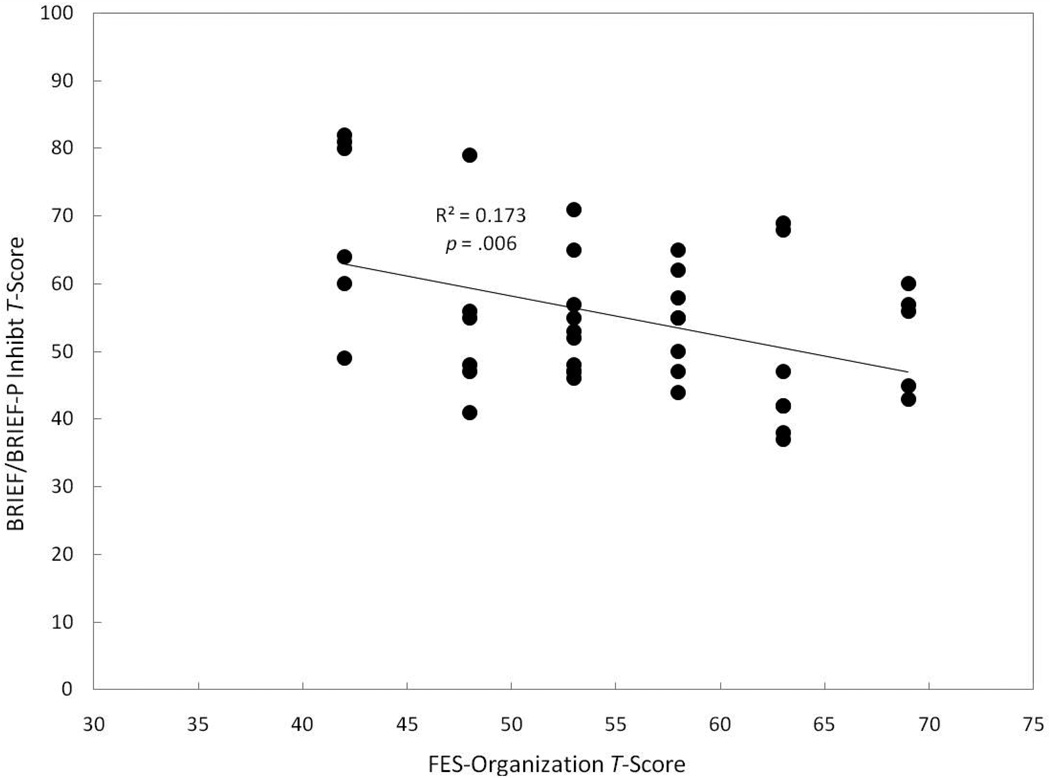
Table 2 displays both significant (p ≤ .05) and marginally significant (p < .10) correlations between the BRIEF/BRIEF-P domains and the FES Subscales. Families reporting a higher emphasis on achievement (FES Achievement Orientation Subscale) had children with fewer problems related to executive function (GEC), r = −.305, p = .047, and working memory (Working Memory Scale), r = −.302, p = .049 (see Figure 5). We also observed a trend for families who reported a higher emphasis on achievement to have children with fewer problems related to planning and organization (Plan/Organize Scale), r = −.268, p = .083. Families reporting a higher emphasis on organization (FES Organization Subscale) had children with fewer problems related to inhibition as measured by the Inhibit Scale, r = −.416, p = .006 (see Figure 6), and there was also a trend for children in these families to have fewer problems with executive function (GEC), r = −.261, p = .091 and planning and organization (Plan/Organize Scale), r = −.263, p = .088. Finally, we observed a trend for families who reported a higher emphasis on independence (FES Independence Subscale) to have children with fewer problems related to inhibition (Inhibit scale), r = −.270, p = .080.
Table 2.
Correlations between FES Subscales and BRIEF/BRIEF-P
| BRIEF/BRIEF-P Domain | ||||
|---|---|---|---|---|
| Global Executive | Inhibit | Working Memory | Planning/Organization | |
| FES -Achievement Orientation | r = −.305** | r =−.302** | r =−.268* | |
| FES -Organization | r =−.261* | r =−.416** | r =−.263* | |
| FES -Independence | r =−.270* | |||
p ≤ .05
p < .10
Figure 5.
Correlations between the degree of emphasis placed on achievement reported in the family and reported problems with children’s executive function (top panel) and working memory (bottom panel).
Figure 6.
Correlations between FES Organization subscale T-scores and BRIEF parent-reported problems with children’s inhibition.
Discussion
The results of this investigation revealed that families of children with cochlear implants differed from families of typically developing children in several different ways (higher levels of achievement orientation, active-recreational orientation, cohesion, expressiveness, moral-religious emphasis, and organization, as well as lower levels of conflict), but none of the differences were clinically significant across the sample taken as a whole. This general pattern suggests that, on average, having a child with a cochlear implant does not in and of itself create families that function in grossly different ways than those of children who are typically developing, but that a small subset of families may exist with more extreme levels of these family characteristics than was present in the norm sample. Furthermore, children in this group are delayed in other aspects language development than the normative sample, and have significantly more problem behaviors related to inhibition, working memory, and executive function than the normative sample, consistent with the body of literature on cochlear implantation in deaf children. In sum, these results suggest that there is nothing remarkable about this study sample as a whole.
Family Environment and Language Development
One of the primary goals of the present study was to extend what is currently understood about the effects of family environment (e.g., SES, maternal influences, etc.) on cochlear implant outcomes to dimensions not previously examined in this clinical population and to determine if these dimensions of family environment are related to language development and executive function. Significant relationships were revealed between family environment and both sets of outcome measures. The one area of family environment that significantly influenced language development was the degree of control in the family: families that used many set rules and procedures for running the family unit had children with smaller receptive vocabularies. In fact, all five children whose families had clinically significant elevations in the level of control in the family had receptive vocabularies that were more than one standard deviation below the test norms. Further, a trend was observed for these families to also have children with poorer expressive language skills. Although the FES-Control Subscale score is a reflection of how the family functions and is not a direct evaluation of parents’ communication style, these results are particularly interesting in light of a large body of published research on early word learning and parents’ (mothers, specifically) interaction styles (e.g., Akhtar, Dunham, & Dunham, 1991; Kaiser et al., 1996; Tomasello & Farrar, 1986).
Children’s early word-learning is enhanced more by mothers who are sensitive to what the child focuses on and who communicate with the child about that item than by mothers who redirect the child’s attention to other items in the environment (e.g., Akhtar et al., 1991; Tomasello & Farrar, 1986). Moreover, both receptive and expressive language skills develop better when parents follow the child’s lead than by redirecting the child’s selective/focused attention to a different referent and labeling it (Kaiser & Hancock, 2003; Kaiser et al., 1996; Yoder, McCathren, Warren, & Watson, 2001). And yet, mothers of children with hearing losses tend to be more controlling and directive, and dominate the interaction with their children with hearing loss than their normal-hearing children (Brinich, 1980; Henggler & Cooper, 1983; Lederberg & Mobley, 1990; Nienhuys et al., 1985; Spencer & Gutfreund, 1990; Vaccari & Marschark, 1997; Wedell-Monnig & Lumley, 1980), for example by initiating interactions and redirecting the child’s attention. Because these control strategies do not support language development in typically developing populations as effectively as strategies that capitalize on joint attention and following a child’s lead, if the same behavior pattern exists in hearing-impaired populations, it could put children with hearing loss at an even greater disadvantage for language development.
The preliminary data from the current investigation did not directly evaluate mother-child linguistic dyads, however, our results do reveal that families with higher levels of self-reported control by use of many set rules and procedures have children with cochlear implants that display more impoverished language skills. We are unable to conclude from this investigation whether conversational style and family interaction style are related or are in fact different entities, but the fact that these families do not, on average, have higher levels of self-reported control than those of typically developing children suggests that the level of control in the family unit might in fact be a separate underlying causal factor from having a controlling interaction style during play. If they were intimately related, we would expect elevated control subscale scores on the FES, reflecting the widespread finding that mothers typically use a controlling style of interaction with their children who have hearing loss. In any case, the results suggest that the more control asserted in a family, the more delayed the child’s language development is likely to be. It is possible, however, that the direction of causality could be in the other direction, as well – that children who do not communicate well tend to need more oversight and control from the family.
One promising outcome of this line of work is that family environment can be modified selectively by intervention. Following family-oriented communication and education programs, increases in support and organization, reductions in conflict and control, and better family functioning have been documented (Bruce & Emshoff, 1992; Hill & Balk, 1987; Mills & Hansen, 1991). Therefore, a family’s current state of functioning need not be permanent; it can be enhanced or altered with appropriate intervention. Family-centered interventions that empower parents by emphasizing their strengths and collaborating with them to facilitate better interactions with their children have been shown to promote children’s language and cognitive development (Bailey et al., 1998; McWilliam & Scott, 2001). However, mothers’ self-efficacy in helping facilitate their children’s language development is not necessarily related to specific interaction styles that promote language development (DesJardin & Eisenberg, 2007). Therefore, a three-pronged approach that addresses parental empowerment, provision of knowledge and tools about specific strategies to promote language development, as well as addressing strategies for enhancing family function is critical for promoting robust language development in children with cochlear implants.
Family Environment and Executive Function
Children with cochlear implants had significantly more problem behaviors related to inhibition and working memory, in addition to significantly higher scores on the global measure of executive function (indicating more problems with executive function) than the normative sample. In fact, 20% of the children in our sample had T-scores in the clinically elevated range on the Inhibit scale, suggesting that inhibitory control is a high risk behavioral regulation function for some children with cochlear implants. Moreover, because inhibition allows for selective, focused, and sustained attention, it is critical for successful perception and understanding of speech in noisy and challenging contexts, which are typical and difficult environments for deaf children. Working memory also is a core foundation of executive function involving the manipulation and control of information that is the focus of immediate attention. Working memory is critical for all information processing operations, including speech and spoken language processing. Using backward digit span, a process measure of verbal working memory, Pisoni, Kronenberger, Roman and Geers (2011) reported that working memory at ages 8 and 9 years is strongly predictive of higher order language abilities such as spoken language comprehension and reading abilities eight years later in children who use cochlear implants. Using a behavior rating scale, the findings of the present study provide additional converging evidence of working memory deficits in children with cochlear implants as evidenced by their behavior in everyday real-world activities.
We found a significant relationship between two dimensions of the family environment and executive function in children with cochlear implants. First, families who describe themselves as achievement-oriented reported that their children had greater overall all executive control and fewer problems related to working memory. Families who value achievement emphasize the importance of success, competition, and being the best in school and work activities; these beliefs drive personal growth within the family. Although the precise underlying neurocognitive mechanisms of action are unknown, the finding that an emphasis on achievement in the family is associated with positive behavioral outcomes in executive function, and in particular, working memory in a clinical population of children at risk for executive difficulties, is encouraging not only for short-term habilitation for those clinicians working with families after cochlear implantation, but for long-term outcomes, including academic success. There is growing evidence that individual differences in executive skills such as working memory, attention-shifting, and inhibitory control are strongly related to achievement in math and literacy (Bull, Espy & Wiebe, 2008; Clark, Pritchard, & Woodward 2010; Hughes & Ensor, in press), academic readiness (Welsh, Nix, Blair, Bierman & Nelson, 2010), and emerging literacy (Blair & Razza, 2007; McClelland et al, 2007). It is possible that an emphasis on achievement among family members may provide affordances for the development of executive and behavioral control associated with academic achievement.
Our second significant finding between the family environment and executive function is that families who perceive themselves as organized reported that their children experienced fewer problems related to inhibitory control. Families with high levels of organization place importance on structure and planning in family activities and individual responsibilities within the home. These beliefs provide a mechanism for maintenance of the coherence of the family system. The finding that family organization is related to better inhibitory control extends what has been already reported in typically developing children (Coldwell, Pike & Dunn, 2006; Hughes & Ensor, 2009) to children with cochlear implants. In fact, using the same measures of family environment (FES) and executive function (BRIEF) as the present study, Schroeder & Kelley (2010) found that families with high levels of organization reported having children with better behavioral regulation. Similarly, other researchers have reported that family chaos is related to parent report of child problem behaviors (Coldwell et al., 2006) and executive dysfunction (Hughes & Ensor, 2009).
Recently, research on the development of executive function has evolved from a narrowly-focused neurobiological explanation to one that emphasizes the broader context within which executive function develops, namely, family interactions (Carlson, 2009). Recent studies have examined both proximal (specific types of maternal utterances and maternal scaffolding) and distal family factors (parenting and family chaos) and found that both influence performance on a wide variety of executive function tasks (Hughes & Ensor, 2009; NICHD ECCRN, 2005). Children who experienced higher quality family environments as measured by maternal sensitivity, maternal cognitive stimulation, and resources in the home that provide stimulation and support, scored significantly better on measures of sustained attention, impulsivity, and short- and long-term memory, even after controlling for family income, number of hours in childcare, and maternal vocabulary (NICHD ECCRN, 2005). The findings of the present study extend research on the social origins of executive function by providing evidence of a close link between family environment and executive function in children who are deaf and use cochlear implants—a clinical population with increased family stress due to a severe sensory impairment (Burger et al., 2005; Hintermair, 2006), and a population that has been shown to be at-risk for executive difficulties (Beer et al, 2011; Figueras, Edwards & Langdon, 2008; Pisoni et al., 2010).
Although, there is evidence of executive dysfunction in children who use cochlear implants using performance measures of executive function, the present study relied solely on parent reports of executive function and family environment. The next step in this line of research would be a multi-method, multi-trait longitudinal research design using parent reports combined with performance measures of executive function, as well as parent report and observation of both proximal and distal measures of family environment.
Summary and Conclusions
In summary, the present results suggest that the family environment influences cochlear implant outcomes in both language development and executive function – two domains with protracted post-natal developmental periods. Specifically, our findings extend those of previous work on several aspects of family environment (e.g., family support, SES, maternal influences, etc.) to previously unstudied dimensions related to the family’s social climate such as interpersonal relationships, personal growth, and family structure. The exciting promise of this work lies in its potential application to intervention. Because family dynamics are fluid and can be changed with explicit communication education and therapy, there is a real possibility that families that function in ways that do not maximize the likelihood of success with a cochlear implant could learn to function in ways more conducive to a child’s likely success. With more research in this area, these results have at least two applications in the future. First, if a child is being evaluated for a cochlear implant, assessment of family environment (e.g., using the FES) should be an important component of the overall evaluation process. If a family reports elevated or depressed scores on scales known to be related to language and executive function outcomes, pre-implant counseling to either raise family awareness or to begin addressing these atypical styles of functioning might be warranted to maximize success with the cochlear implant at an early point in development. Second, if a child has already been implanted and is struggling with her or his device, including a family component in the evaluation might be useful in identifying attributes of the family dynamics that could be strengthened or enhanced. Admittedly, benefit from a cochlear implant is a complicated and multi-faceted problem and many of the causes of success or failure with the device cannot be changed through behavioral intervention (e.g., device factors such as the number of active electrodes, etc.). However, family environment is one area that can be modified in substantial ways if the family becomes aware of these problems and is interested in addressing them. These preliminary results on the role of the family environment on cochlear implantation speech and language outcomes suggest that families and family dynamics play a critical role in the benefit obtained from a cochlear implant, a neglected domain of study that deserves further attention.
Acknowledgments
We are grateful to Shirley Henning and Bethany Colson for their contribution to data collection. This work was supported by the NIH-NIDCD T32 DC00011 and DC009581.
Footnotes
Portions of this work were presented at the 13th Symposium of Cochlear Implants in Children, Chicago, IL (July, 2011).
Contributor Information
Rachael Frush Holt, Department of Speech and Hearing Sciences, Indiana University
Jessica Beer, DeVault Otologic Research Laboratory, Department of Otolaryngology-Head and Neck Surgery, Indiana University School of Medicine
William G. Kronenberger, Department of Psychiatry, Indiana University School of Medicine
David B. Pisoni, DeVault Otologic Research Laboratory, Department of Otolaryngology- Head and Neck Surgery, Indiana University School of Medicine
Kaylah Lalonde, Department of Speech and Hearing Sciences, Indiana University
References
- Akhtar N, Dunham F, Dunham P. Directive interactions and early vocabulary development: The role of joint attentional focus. Journal of Child Language. 1991;18:41–49. doi: 10.1017/s0305000900013283. [DOI] [PubMed] [Google Scholar]
- American National Standards Institute. Specification for audiometers (ANSI S3.6-2004) New York: Acoustical Society of America; 2004. [Google Scholar]
- Bailey D, Jr, Mc William R, Darkes L, Hebbeler K, Simeonsson R, Spiker D, Wagner M. Family outcomes in early intervention: A framework for program evaluation and efficacy research. Exceptional Children. 1988;64:313–328. [Google Scholar]
- Bates E, Bretherton I, Beeghly-Smith M, McNew S. Social bases of language development: A reassessment. In: Reuse HW, Lipsitt LP, editors. Advances in Child Development and Behavior. Vol. 16. NewYork: Academic Press; 1982. pp. 8–75. [DOI] [PubMed] [Google Scholar]
- Beer J, Kronenberger WG, Pisoni DB. Executive function in everyday life: Implications for young cochlear implant users. Cochlear Implants International. 12(Suppl.):S89–S91. doi: 10.1179/146701011X13001035752570. (in press). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Belsky J. Early human experience: A family perspective. Developmental Psychology. 1981;17:3–23. [Google Scholar]
- Bernier A, Carlson SM, Whipple N. From external regulation to self-regulation: Early parenting precursors of young children's executive functioning. Child Development. 2010;81:326–339. doi: 10.1111/j.1467-8624.2009.01397.x. [DOI] [PubMed] [Google Scholar]
- Bertram B, Päd D. Importance of auditory-verbal education and parents' participation after cochlear implantation of very young children. Annals of Otology, Rhinology and Laryngology. 1995;166(Suppl.):97–100. [PubMed] [Google Scholar]
- Bibcok MB, Carpendale JM, Muller U. Parental scaffolding and the development of executive function. In: Lewis C, Carpendale JM, editors. Social Interaction and the Development of Executive Function. New Directions in Child and Adolescent Development. Vol. 123. 2009. pp. 17–34. [DOI] [PubMed] [Google Scholar]
- Biederman J, Milberger S, Faraone SV, Kiely K, Guite J, Mick E, et al. Family environment risk factors for attention-deficit hyperactivity disorder: A test of Rutter’s indicators of adversity. Archives of General Psychiatry. 1995;52:464–470. doi: 10.1001/archpsyc.1995.03950180050007. [DOI] [PubMed] [Google Scholar]
- Blair C, Razza RP. Relating effortful control, executive function, and false belief understanding to emerging math and literacy ability in kindergarten. Child Development. 2007;78:647–663. doi: 10.1111/j.1467-8624.2007.01019.x. [DOI] [PubMed] [Google Scholar]
- Brinich PM. Childhood deafness and maternal control. Journal of Communication Disorders. 1980;13:75–81. doi: 10.1016/0021-9924(80)90024-6. [DOI] [PubMed] [Google Scholar]
- Bruce C, Emshoff J. The SUPER II Program: An early intervention program. Journal of Community Psychology, OSAP Special Issue. 1992:10–21. [Google Scholar]
- Bull R, Espy KA, Wiebe S. Short-Term Memory, Working Memory, and Executive Functioning in Preschoolers: Longitudinal Predictors of Mathematical Achievement at Age 7 Years. Developmental Neuropsychology. 2008;33:205–228. doi: 10.1080/87565640801982312. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burger T, Spahn C, Richter B, Eissele S, Lohle E, Bengel J. Parental distress: The initial phase of hearing aid and cochlear implant fitting. American Annals of the Deaf. 2005;150:5–10. doi: 10.1353/aad.2005.0017. [DOI] [PubMed] [Google Scholar]
- Carlson SM. Social origins of executive function development. In: Lewis, Carpendale JIM, editors. New Directions for Child and Adolescent Development. Vol. 2009. 2009. pp. 87–98. [DOI] [PubMed] [Google Scholar]
- Carlson SM, Moses LJ, Breton C. How specific is the relation between executive function and theory of mind? Contributions of inhibitory control and working memory. Infant and Child Development. 2002;11:73–92. [Google Scholar]
- Ciccia AH, Meulenbroek P, Turkstra LS. Adolescent brain and cognitive developments. Implications for clinical assessment in traumatic brain injury. Topics in Language Disorders. 2009;29:249–265. doi: 10.1097/TLD.0b013e3181b53211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clark CAC, Pritchard VE, Woodward LJ. Preschool executive functioning abilities predict early mathematics achievement. Developmental Psychology. 2010;46(5):1176–1191. doi: 10.1037/a0019672. [DOI] [PubMed] [Google Scholar]
- Coldwell J, Pike A, Dunn J. Household chaos – links with parenting and child behavior. Journal of Child Psychology and Psychiatry. 2006;47:1116–1122. doi: 10.1111/j.1469-7610.2006.01655.x. [DOI] [PubMed] [Google Scholar]
- Conway CM, Karpicke J, Anaya EM, Henning SC, Kronenberger WG, Pisoni DB. Nonverbal cognition in deaf children following cochlear implantation: Motor sequencing disturbances mediate language delays. Developmental Neuropsychology. 2011;36:237–254. doi: 10.1080/87565641.2010.549869. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Anaya EM, Karpicke J, Henning SC. Implicit sequence learning in deaf children with cochlear implants. Developmental Science. 2011;14:69–82. doi: 10.1111/j.1467-7687.2010.00960.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conway CM, Pisoni DB, Kronenberger W. The importance of sound for cognitive sequencing abilities. Current Directions in Psychological Science. 2009;18:275–279. doi: 10.1111/j.1467-8721.2009.01651.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cross TG, Johnson-Morris JE, Nienhuys TG. Linguistic feedback and maternal speech: Comparisons of mothers addressing hearing and hearing-impaired children. First Language. 1980;1:163–189. [Google Scholar]
- Desjardin JL. Maternal Perceptions of Self-Efficacy and Involvement in the Auditory Development of Young Children with Prelingual Deafness. Journal of Early Intervention. 2005;27:193–209. [Google Scholar]
- DesJardin JL, Ambrose SE, Eisenberg LS. Literacy skills in children with cochlear implants: The importance of early oral language and joint storybook reading. Journal of Deaf Studies and Deaf Education. 2008;14:22–43. doi: 10.1093/deafed/enn011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DesJardin JL, Eisenberg LS. Maternal contributions: Supporting language development in young children with cochlear implants. Ear & Hearing. 2007;28:456–469. doi: 10.1097/AUD.0b013e31806dc1ab. [DOI] [PubMed] [Google Scholar]
- Diamond A, Barnett W, Thomas J, Munro S. Preschool program improves cognitive control. Science. 2007;318:1387–1388. doi: 10.1126/science.1151148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dunn LM, Dunn DM. Peabody Picture Vocabulary Test. Fourth Edition. Minneapolis, MN: Pearson; 2007. [Google Scholar]
- Edwards L, Thomas F, Rajput K. Use of revised children’s implant profile (GPSHChIP) in candidacy for paediatric cochlear implantation and in predicting outcome. International Journal of Audiology. 2009;48:554–560. doi: 10.1080/14992020902894533. [DOI] [PubMed] [Google Scholar]
- Espy KA. Using developmental, cognitive, and neuroscience approaches to understanding executive control in young children. Developmental Neuropsychology. 2004;26:379–384. doi: 10.1207/s15326942dn2601_1. [DOI] [PubMed] [Google Scholar]
- Figueras B, Edwards L, Langdon D. Executive function and language in deaf children. Journal of Deaf Studies and Deaf Education. 2008;13:362–377. doi: 10.1093/deafed/enm067. [DOI] [PubMed] [Google Scholar]
- Geers A, Brenner C, Davidson L. Factors associated with development of speech perception skills in children implanted by age five. Ear and Hearing. 2003;24(Suppl.):24S–35S. doi: 10.1097/01.AUD.0000051687.99218.0F. [DOI] [PubMed] [Google Scholar]
- Geers AE, Strube MJ, Tobey EA, Pisoni DB, Moog JS. Epilogue: Factors Contributing to Long-Term Outcomes of Cochlear Implantation in Early Childhood. Ear and Hearing. 2011;32(Suppl.):84S–92S. doi: 10.1097/AUD.0b013e3181ffd5b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gilotty L, Kenworthy L, Sirian L, Black DO, Wagner AE. Adaptive skills and executive function in autism spectrum disorders. Child Neuropsychology. 2002;8:241–248. doi: 10.1076/chin.8.4.241.13504. [DOI] [PubMed] [Google Scholar]
- Gioia GA, Espy KA, Isquith PK. Behavior Rating Inventory of Executive Function - Preschool Version (BRIEF-P) Psychological Assessment Resources, Inc.; 2003. [Google Scholar]
- Gioia GA, Isquith PK, Guy SC, Kenworthy L. Behavior Rating Inventory of Executive Function (BRIEF) Psychological Assessment Resources, Inc.; 2000. [Google Scholar]
- Hart B, Risley TR. In: Meaningful differences in the everyday experience of young American children. John MS, Brown DK, editors. Baltimore, MD: Brookes; 1995. [Google Scholar]
- Hellman SA, Chute PM, Kretschmer RE, Nevins ME, Parisier SC, Thurston LC. The development of a children’s implant profile. American Annals of the Deaf. 1991;136:77–81. doi: 10.1353/aad.2012.1077. [DOI] [PubMed] [Google Scholar]
- Henggeler SW, Cooper PF. Deaf child-hearing mother interaction: Extensiveness and reciprocity. Journal of Pediatric Psychology. 1983;8:83–95. doi: 10.1093/jpepsy/8.1.83. [DOI] [PubMed] [Google Scholar]
- Hill D, Balk D. The effect of an education program for families of the chronically mentally ill on stress and anxiety. Psychosocial Rehabilitation Journal. 1987;10:25–40. [Google Scholar]
- Hintermair M. Parental resources, parental stress, and socioemotional development of deaf and hard of hearing children. Journal of Deaf Studies and Deaf Education. 2006;11:493–513. doi: 10.1093/deafed/enl005. [DOI] [PubMed] [Google Scholar]
- Hoff E. The specificity of environmental influence: Socioeconomic status affects early vocabulary development via maternal speech. Child Development. 2003;74:1368–1378. doi: 10.1111/1467-8624.00612. [DOI] [PubMed] [Google Scholar]
- Holt RF, Svirsky MA. An exploratory look at pediatric cochlear implantation: Is earliest always best? Ear and Hearing. 2008;29:492–511. doi: 10.1097/AUD.0b013e31816c409f. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hughes C, Ensor R. Individual differences in growth in executive function across the transition to school predict externalizing and internalizing behaviors and self perceived academic success at 6 years of age. Journal of Experimental Child Psychology. 2010 doi: 10.1016/j.jecp.2010.06.005. (in press). [DOI] [PubMed] [Google Scholar]
- Hughes C, Ensor R. Executive function and theory of mind: Predictive relations from ages 2 to 4. Developmental Psychology. 2007;43:1447–1459. doi: 10.1037/0012-1649.43.6.1447. [DOI] [PubMed] [Google Scholar]
- Hughes CH, Ensor RA. How do families help or hinder the emergence of early executive function? Social interaction and the development of executive function. In: Lewis C, Carpendale JIM, editors. New Directions in Child and Adolescent Development. Vol. 123. 2009. pp. 35–50. [DOI] [PubMed] [Google Scholar]
- Jarratt KP, Riccio CA, Siekierski B. Assessment of Attention Deficit Hyperactivity Disorder (ADHD) using the BASC and BRIEF. Applied Neuropsychology. 2005;12:83–93. doi: 10.1207/s15324826an1202_4. [DOI] [PubMed] [Google Scholar]
- Kaiser A, Hancock T. Teaching parents new skills to support their young children’s development. Infants and Young Children. 2003;16:9–21. [Google Scholar]
- Kaiser A, Hemmeter M, Ostrosky M, Fischer R, Yoder P, Keeper M. The effects of teaching parents to use responsive interaction strategies. Topics in Early Childhood Special Education. 1996;16:375–406. [Google Scholar]
- Kirk KI, Miyamoto RT, Lento CL, Ying E, O’Neill T, Fears B. Effects of age at implantation in young children. Annals of Otology Rhinology and Laryngology. 2002;189(Suppl.):69–73. doi: 10.1177/00034894021110s515. [DOI] [PubMed] [Google Scholar]
- Kronenberger WG, Thompson RJ., Jr Dimensions of family functioning with chronically ill children: A higher order factor analysis of the Family Environment Scale. Journal of Clinical Child Psychology. 1990;19:380–388. [Google Scholar]
- Landry SH, Miller-Loncar CL, Smith KE, Swank PR. The Role of Early Parenting in Children's Development of Executive Processes. Developmental Neuropsychology. 2002;21:15–41. doi: 10.1207/S15326942DN2101_2. [DOI] [PubMed] [Google Scholar]
- Lederberg AR, Mobley CE. The effect of hearing impairment on the quality of attachment and mother-toddler interaction. Child Development. 1990;61:1596–1604. doi: 10.1111/j.1467-8624.1990.tb02886.x. [DOI] [PubMed] [Google Scholar]
- Loomis JW, Javornisky JG, Monahan JJ, Burke G, Lindsay A. Relations between family environment and adjustment outcomes in young adults with spina bifida. Developmental Medicine and Child Neurology. 1997;39:620–627. doi: 10.1111/j.1469-8749.1997.tb07498.x. [DOI] [PubMed] [Google Scholar]
- MacTurk RH, Meadow-Orlans KP, Koester LS, Spencer PW. Social support, motivation, language and interaction: A longitudinal study of mothers and their deaf infants. American Annals of the Deaf. 1993;139:19–25. doi: 10.1353/aad.2012.0575. [DOI] [PubMed] [Google Scholar]
- Mangeot S, Armstrong K, Colvin AN, Yeates KW, Taylor HG. Long-term executive function deficits in children with traumatic brain injuries: Assessment using the Behavior Rating Inventory of Executive Function (BRIEF) Child Neuropsychology. 2002;8:271–284. doi: 10.1076/chin.8.4.271.13503. [DOI] [PubMed] [Google Scholar]
- McClelland MM, Cameron CE, Connor CM, Farris CL, Jewkes AM, Morrison FJ. Links between behavioral regulation and preschoolers' literacy, vocabulary, and math skills. Developmental Psychology. 2007;43:947–959. doi: 10.1037/0012-1649.43.4.947. [DOI] [PubMed] [Google Scholar]
- McWilliam R, Scott S. A support approach to early intervention: A three-part framework. Infants and Young Children. 2001;13:55–66. [Google Scholar]
- Meadow-Orlans KP, Steinberg A. Effects of infant hearing loss and maternal support on mother-infant interaction at 18 months. Journal of Applied Developmental Psychology. 1993;14:407–426. [Google Scholar]
- Mills PD, Hansen JC. Short-term group interventions for mentally ill young adults living in a community residence and their families. Hospital and Community Psychiatry. 1991;42:1144–1150. doi: 10.1176/ps.42.11.1144. [DOI] [PubMed] [Google Scholar]
- Mitchell RM, Karchmer MA. Chasing the mythical ten percent: Parental hearing status of deaf and hard of hearing students in the United States. Sign Language Studies. 2004;4:138–163. [Google Scholar]
- Moeller MP. Early intervention and language development in children who are deaf and hard of hearing. Pediatrics. 2000;106:1–9. doi: 10.1542/peds.106.3.e43. [DOI] [PubMed] [Google Scholar]
- Moos RH, Moos BS. Family Environment Scale manual. 4th Ed. Menlo Park: CA: Mind Garden, Inc.; 2009. [Google Scholar]
- NICHD Early Child Care Research Network. Predicting individual differences in attention, memory, and planning in first graders from experiences at home, child care, and school. Developmental Psychology. 2005;41:99–114. doi: 10.1037/0012-1649.41.1.99. [DOI] [PubMed] [Google Scholar]
- Nienhuys TG, Horsborough KM, Cross TG. A dialogic analysis of interaction between mothers and their deaf or hearing preschoolers. Applied Psycholinguistics. 1985;6:121–140. [Google Scholar]
- Nikolopoulos TP, Gibbin KP, Dyar D. Predicting speech perception outcomes following cochlear implantation using Nottingham Children’s Implant Profile (NChIP) International Journal of Pediatric Otorhinolaryngology. 2004;68:137–141. doi: 10.1016/j.ijporl.2003.09.020. [DOI] [PubMed] [Google Scholar]
- Noble KG, Norman MF, Farah MJ. Neurocognitive correlates of socioeconomic status in kindergarten children. Developmental Science. 2005;8:74–87. doi: 10.1111/j.1467-7687.2005.00394.x. [DOI] [PubMed] [Google Scholar]
- Pisoni DB, Conway CM, Kronenberger W, Henning S, Anaya E. Executive function and cognitive control in deaf children with cochlear implants. In: Marschark, P.E. MS, editor. Oxford Handbook of Deaf Studies, Language, and Education. second edition ed. Vol. 1. New York: Oxford University Press; 2010. [Google Scholar]
- Pisoni DB, Kronenberger WG, Roman AS, Geers AE. Measures of Digit Span and Verbal Rehearsal Speed in Deaf Children After More Than 10 Years of Cochlear Implantation. Ear and Hearing. 2011;32(Suppl.):60S–74S. doi: 10.1097/AUD.0b013e3181ffd58e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pratt S. Nonverbal play interaction between hearing mothers and young deaf children. Ear & Hearing. 1991;12:328–336. doi: 10.1097/00003446-199110000-00005. [DOI] [PubMed] [Google Scholar]
- Pressman L, Pipp-Siegel S, Yoshinaga-Itano C, Deas A. Maternal sensitivity predicts language gain in preschool children who are deaf and hard of hearing. Journal of Deaf Studies. 1999;2:27–36. doi: 10.1093/deafed/4.4.294. [DOI] [PubMed] [Google Scholar]
- Rice F, Harold GT, Shelton KH, Thapar A. Family conflict interacts with genetic liability in predicting childhood and adolescent depression. Journal of the American Academy of Child and Adolescent Psychiatry. 2006;45:841–848. doi: 10.1097/01.chi.0000219834.08602.44. [DOI] [PubMed] [Google Scholar]
- Rivara JB, Jaffe KM, Polissar NL, Fay GC, Liao S, Martin KM. Predictors of family functioning and change 3 years after traumatic brain injury in children. Archives of Physical Medicine and Rehabilitation. 1996;77:754–764. doi: 10.1016/s0003-9993(96)90253-1. [DOI] [PubMed] [Google Scholar]
- Rousey AM, Wild M, Blacher J. Stability of measures of the home environment for families of children with severe disabilities. Research in Developmental Disabilities. 2002;23:17–35. doi: 10.1016/s0891-4222(01)00089-0. [DOI] [PubMed] [Google Scholar]
- Schroeder VM, Kelley ML. Family environment and parent-child relationships as related to executive functioning in children. Early Child Development & Care. 2010;180:1285–1298. [Google Scholar]
- Semel E, Wiig EH, Secord W. Clinical Evaluation of Language Fundamentals. Forth Edition. San Antonio: TX: Psychological Corporation; 2003. [Google Scholar]
- Spencer P, Gutfreund M. Directiveness in mother-infant interactions. In: Moores DF, Meadow-Orlans KP, editors. Educational and Developmental Aspects of Deafness. Washington, D.C.: Gallaudet University Press; 1990. pp. 350–365. [Google Scholar]
- Tomasello M, Farrar M. Joint attention and early language. Child Development. 1986;57:1454–1463. [PubMed] [Google Scholar]
- Vaccari C, Marschark M. Communication between parents and deaf children: Implications for social-emotional development. Journal of Child Psychology and Psychiatry. 1997;38:793–801. doi: 10.1111/j.1469-7610.1997.tb01597.x. [DOI] [PubMed] [Google Scholar]
- Varni JW, Katz ER, Colegrove R, Dolgin M. Family functioning predictors of adjustment in children with newly diagnosed cancer: A prospective analysis. Journal of Child Psychology and Psychiatry. 1996;37:321–328. doi: 10.1111/j.1469-7610.1996.tb01409.x. [DOI] [PubMed] [Google Scholar]
- Wedell-Monnig J, Lumley J. Child deafness and mother-child interaction. Child Development. 1980;51:766–774. [PubMed] [Google Scholar]
- Welsh JA, Nix RL, Blair C, Bierman KL, Nelson K. The development of cognitive skills and gains in academic school readiness for children from low-income families. Journal of Educational Psychology. 2010;102:43–53. doi: 10.1037/a0016738. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoder P, McCathren R, Warren S, Watson A. Important distinctions in measuring maternal response to communication in prelinguistic children with disabilities. Communication Disorders Quarterly. 2001;22:135–147. [Google Scholar]
- Zimmerman IL, Steiner BS, Pond R. PsychCorp.; 2002. Preschool Language Scale Fourth Edition (PLS-4) [Google Scholar]