Summary
Purpose
To determine the effect of seizure focus location within the left hemisphere on the expression of regional language dominance.
Methods
In this cross sectional study we investigated 90 patients (mean age 23.3±12.9 years) with left hemisphere focal epilepsy (mean age onset 11.7 ±8.3 years). 18 patients had a frontal lobe focus, 72 temporal lobe focus (43 mesial; 29 neocortical). Subjects performed an auditory word definition language paradigm using 3T BOLD EPI fMRI. Data was analyzed in SPM2. Regional laterality indices (LI) for inferior frontal gyrus (IFG), and Wernicke’s area (WA), were calculated using a bootstrap method. Categorical language dominance and mean LI were analyzed.
Key Findings
Mean WA LI was lower for subjects with a mesial temporal focus compared to a frontal focus (p=0.04). There was a greater proportion of atypical language in WA for subjects with a mesial temporal focus compared to a frontal focus (χ2=4.37, p=0.04). WA LI did not differ for subjects with a neocortical focus compared to a mesial focus or a frontal focus. Mean IFG LI and proportion of atypical language in IFG were similar across seizure focus groups. Age and age of onset were not correlated with mean laterality in WA or IFG. Epilepsy duration tended to be negatively correlated with WA LI (r=−0.18, p=0.10), but not IFG LI.
Significance
Temporal lobe foci have wide-ranging effects on the distributed language system. In contrast, the effects of a frontal lobe focus appear restricted to anterior rather than posterior language processing areas.
Keywords: Reorganization, Language dominance, Childhood epilepsy
Introduction
Atypical language dominance, common in patients with chronic focal epilepsy (Gaillard et al., 2007; Woermann et al., 2003), may exhibit several patterns with varying degrees of laterality in the frontal (Broca’s) and temporal (Wernicke’s) areas that sustain language processing (Gaillard, 2004). Age of seizure onset, age of brain insult, and underlying pathologic substrate are important factors that may affect language network representation (Gaillard et al., 2007). Patients with a left-hemisphere seizure focus have a high likelihood of atypical or reduced language dominance; however, the effect of the location of seizure focus within the left hemisphere on the regional organization of language functions is unclear. A left temporal focus may exert a remote effect on regional activation laterality in inferior and middle frontal gyrus (IFG & MFG) (Berl et al., 2005); a left mesial temporal focus, though remote from neocortical language processing cortex, is associated with altered language laterality in temporal neocortical and frontal regions (Gaillard et al. 2007; Janszky et al. 2006; Weber et al., 2006). However, there is mixed evidence for the effect of a frontal focus on regional language dominance (Weber et al. 2006; Liegeois et al., 2004; Anderson et al., 2006).
We examined the effect of seizure focus on the expression of regional language laterality by comparing patients with frontal and temporal seizure foci. We further compared the effect of neocortical versus mesial seizure focus in those patients with a temporal focus. As temporal brain regions reach structural and functional maturity before frontal areas (Holland et al., 2001; Sowell et al., 2004; Gogtay et al., 2004; Berl et al., 2010; Shaw et al., 2008; Sowell et al., 2001; Paus et al., 1999) we predicted that temporal lobe seizures would have a greater effect on hemispheric language lateralization than frontal lobe seizures. We predicted that patients with a temporal seizure focus would have a higher incidence of atypical language and reduced language lateralization in both frontal and temporal areas than patients with a frontal seizure focus. For patients with a frontal seizure focus, atypical language would occur in frontal areas but not temporal areas.
Methods
In this cross sectional study conducted at a tertiary care epilepsy referral center we investigated 90 English speaking patients with left focal epilepsy (50 males, 40 females) who were evaluated between December 2003 and December 2009. All consecutive patients with a left frontal, left temporal neocortical, or left mesial temporal focus who had successfully completed fMRI were identified for this study. Seizure focus determination was based on clinical features, neurological examination, standard EEG/ictal video-EEG, subdural recording, an epilepsy imaging protocol including high resolution structural 1.5 T MRI, and, when available, intracranial EEG and surgery. Handedness was characterized by clinical assessment and, when available, based on the Edinburgh Handedness Inventory (n=54): right handed or atypical handedness (left handed or mixed handedness). Clinical characteristics are presented in Table 1. Patients had mean age of 23.3±12.9 years (range 4.5 to 57 years), mean age of seizure onset 11.7±8.3 years (range 0.8 to 38 years), and mean duration of epilepsy 11.6±11.6 years (range 0 to 55 years). Eighteen patients had a frontal focus. Seventy-two had a temporal focus: 43 mesial and 29 neocortical. MRI was normal in 26; 22 had mesial temporal sclerosis (MTS, two of these with possible hippocampal volume loss without signal change), 29 had tumors (all developmental/low grade, except for two with glioblastoma multiforme), five focal cortical dysplasia, five vascular lesions, and three focal encephalomalacia/gliosis (Table 1). Age of pathological injury was categorized as occurring either before or after age six, (57 subjects had injury before age six). The study was approved by the institutional review board of the National Institute of Neurological Disorders and Stroke, NIH. Informed consent was obtained from adult patients/parents of pediatric patients and assent from minors.
Table 1. Clinical Characteristics and Experimental Results Summary.
Clinical characteristics and LI data for all subjects, and divided by location of seizure focus (frontal, temporal, mesial temporal, neocortical temporal, and other/not localized temporal).
| All Subjects (n=90) Mean (SD) |
Frontal (n=18) |
Temporal (n=72) |
Mesial Temporal (n=43) |
Neocortical Temporal (n=29) |
||
|---|---|---|---|---|---|---|
| Age (years) | 23.3 (12.9) | 19.1 (12.6) | 24.3 (12.9) | 25.3 (13.3) | 23.0 (12.4) | |
|
Age of Seizure Onset (years) |
11.7 (8.3) | 9.6 (7.6) | 12.2 (8.5) | 11.0 (8.1) | 14.0 (8.7) | |
|
Epilepsy duration at study (years) |
11.6 (11.6) | 9.5 (7.5) | 12.1 (12.4) | 14.3 (12.8) | 9.0 (11.4) | |
| Age of injury | % of subjects (n) | |||||
| <6 years | 63.3% (57) | 55.6% (10) | 65.3% (47) | 76.7% (33) | 48.3% (14) | |
| ≥6 years | 26.7% (24) | 33.3% (6) | 25% (18) | 18.6% (8) | 34.5% (10) | |
| indeterminate | 10% (9) | 11.1% (2) | 9.7% (7) | 4.7% (2) | 17.2% (5) | |
| MRI | ||||||
| Normal | 28.9% (26) | 44.4% (8) | 25% (18) | 16.3% (7) | 37.9% (11) | |
| Tumor* | 32.2% (29) | 44.4% (8) | 29.2% (21) | 25.6% (11) | 34.5% (10) | |
| Focal Cortical Dysplasia |
5.6% (5) | - | 6.9% (5) | 4.7% (2) | 10.3% (3) | |
| MTS^ | 24.4% (22) | - | 30.6% (22) | 51.2% (22) | - | |
| Vascular+ | 5.6% (5) | - | 6.9% (5) | 2.3% (1) | 13.8% (4) | |
| Small focal gliosis/ encephalomalacia |
3.3% (3) | 11.1% (2) | 1.4% (1) | - | 3.4% (1) | |
| Gender | 50 M, 40 F | 12 M, 6 F | 38 M, 34 F | 20 M, 23 F | 18 M, 11 F | |
| Handedness | 80 right, 10 left/mixed |
16 right, 2 left/mixed |
64 right, 8 left/mixed |
39 right, 4 left/mixed |
25 right, 4 left/mixed |
|
|
Typical vs. Atypical Language |
68 typical, 22 atypical |
15 typical, 3 atypical |
53 typical, 19 atypical |
30 typical, 13 atypical |
23 typical, 6 atypical |
|
| IFG LI | 0.42 (0.57) | 0.54 (0.56) | 0.39 (0.57) | 0.39 (0.61) | 0.39 (0.51) | |
| WA LI | 0.52 (0.51) | 0.72 (0.29) | 0.47 (0.54) | 0.39 (0.61) | 0.57 (0.42) | |
all developmental/low-grade tumors except two glioblastoma multiforme (one mesial temporal, one neocortical temporal); and two mesial temporal lesions were unclear low-grade tumor vs. dysplasia
two of 22 had possible hippocampal volume loss without signal change; the remainder had clear MTS
all vascular findings were cavernous angiomas except one mesial temporal TBI and one neocortical temporal TBI
Blood Oxygen Level Dependent Echo Planar Imaging fMRI were acquired at 3.0 T using an auditory word definition decision task adjusted for skill level. Acquisition parameters, paradigm presentation and analysis methods have been described in detail previously (Gaillard et al., 2007). Briefly, fMRI data were acquired at 3.0 T (General Electric Medical Systems, Milwaukee, WI) using EPI blood oxygen level dependent (BOLD) techniques. Gradient echoplanar images were collected using TE 30 msec, FOV 22 by 22cm, acquisition matrix 64 by 64, and interscan interval (TR) 2,000 msec. Brain volumes consisted of 28, 4-mm-thick, axial slices. Anatomic images were collected using a three-dimensional fast SPGR sequence and brain volumes consisting of 28 axial slices, 4 mm thickness. Images were collected parallel to the anterior commissure–posterior commissure plane. We used an Auditory Description Decision Task (ADDT) adjusted for patient skill level using a block design composed of five epoch cycles; each cycle consisted of an experimental condition that alternated with a control condition (reverse speech), each hemicycle lasted 30 seconds. Total time for the paradigm was 5 minutes, during which patients were instructed to remain silent and motionless. During the experimental condition, the participant heard a sentence that described, then named, an object (“a king’s hat is a crown”; 70% were correct definitions, 30% were foils). The auditory stimuli were digitized and presented via a PC using E-Prime computer software v1.1 (Psychology Software Tools, Inc., Pittsburgh, PA) through pneumatic earphones. The fMRI tasks reliably elicits activation along the left superior temporal sulcus (Brodmann Areas 21,22,39) implicated in “receptive” speech processing (derived from comprehension of the word definition), the left inferior frontal gyrus implicated in “expressive” speech processing (BA 44,45) and semantic decision (BA 47) (derived from the task requirement for a semantic decision from matching the presented definition to the proposed answer) and left middle frontal gyrus (BA 9,46) implicated in verbal working memory (Mbwana et al., 2009; Rosenberger et al., 2009).
After normalization of functional data into the MNI anatomic atlas, individual t-maps were generated with movement parameters as covariates of noninterest; individuals’ mean motion in the x, y, and z directions was less than one voxel. Using SPM2 (University College London, London UK), a group map was generated from individual activation maps using a random-effects model to obtain a whole brain activation map. Language dominance was then established by analyzing individual maps with the LI toolbox bootstrap method adapted for SPM2 (Wilke & Schmithorst, 2006). Regions of interest that comprised a distributed language network were defined using the Wake Forest PickAtlas and included: Wernicke’s area (WA) broadly defined (BA 21, 22, 39); Broca’s area (IFG, BA 44, 45, 47). For all subjects, regional laterality indices (LI) were calculated, where LI = (L−R)/(L+R). Left lateralization for each region was defined as LI≥0.20; atypical language (AL) when regional LI was right dominant (LI<-0.20), or bilateral (LI<|0.20|) (Gaillard et al., 2002).
Data were analyzed in SPSS using chi-squared analyses, independent samples t-tests, and MANOVA according to variable type (categorical vs. continuous) and number of groups.
Results
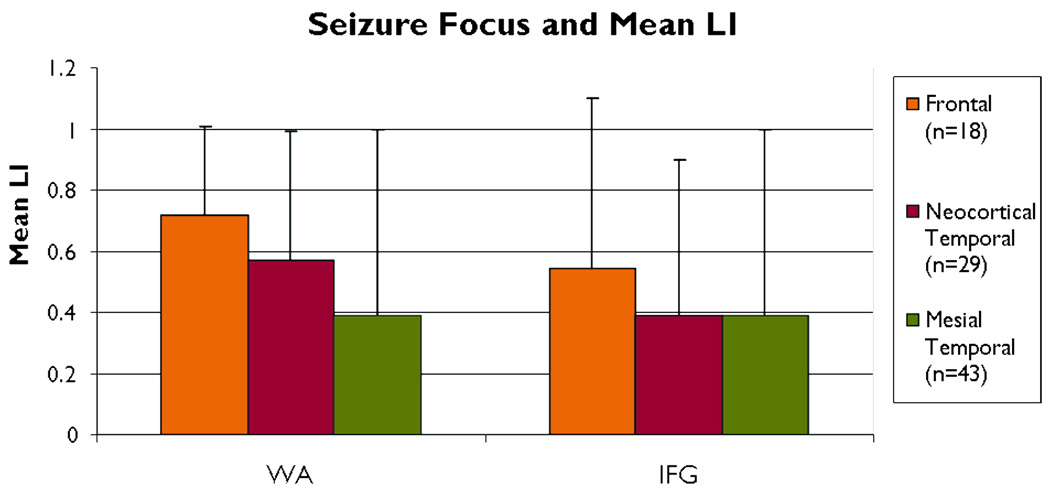
Subjects with both temporal and frontal seizure foci showed left lateralized activation in language areas (Table 1). Mean laterality in WA tended to differ by seizure focus group (F=2.68, p=0.08). However, mean WA LI was lower for subjects with a mesial temporal focus compared to a frontal focus (p=0.04) (Figure 1). Subjects with a mesial temporal focus did not differ from those with a temporal neocortical focus (p=0.12), nor did those with a frontal focus differ from those with a temporal neocortical focus (p=0.42). Mean IFG LI did not differ by seizure focus group (p>0.10).
Figure 1.
Mean regional LI by seizure focus. There are no differences in IFG. Mean LI for WA between Frontal and Mesial Temporal focus is different *p<0.04. LI =laterality index; WA=Wernicke’s Area; IFG=inferior frontal gyrus.
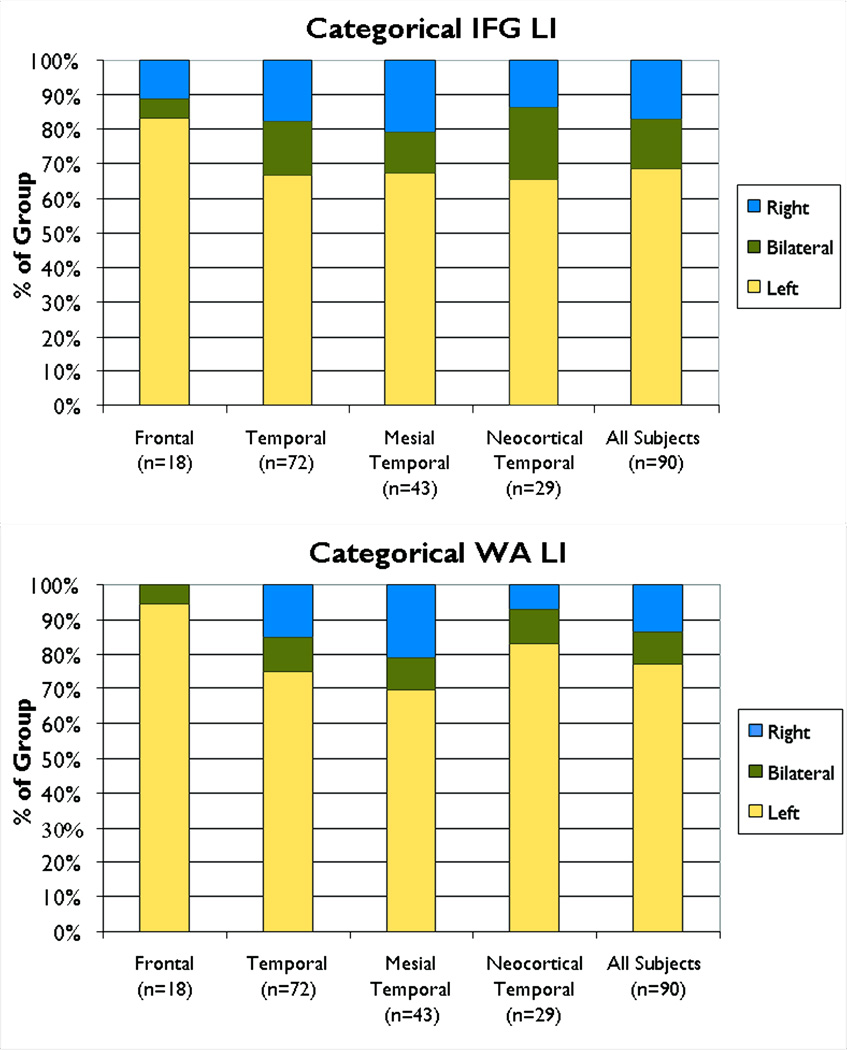
As mean LI may be influenced by the distribution of patients who reach criteria for atypical (bilateral or right) language dominance, we examined the categorical distribution of language dominance. The categorical distribution of hemispheric language dominance is shown by region in Figure 2. A larger proportion of subjects with a mesial temporal focus had atypical language in WA compared to subjects with a frontal focus (χ2=4.37, p=0.04); other group comparisons for proportion of WA atypical language were not significant (p>0.10). The proportion of atypical language in IFG did not differ by group (χ2=1.94, p>0.10).
Figure 2.
Categorical distribution of regional LI (left dominant, bilateral, or right) by seizure focus. For WA there was a greater proportion of atypical language in patients with a mesial temporal focus compared to a frontal focus *p=0.04. There were no differences in IFG. LI =laterality index; WA=Wernicke’s Area; IFG=inferior frontal gyrus.
To ensure that differences were not influenced by the patients with vascular lesions which may disrupt the BOLD response, a follow-up analysis with those five patients removed was conducted. Results were similar, but strengthened by reaching a more stringent statistical threshold. Thus, patients with a mesial temporal focus were different from patients with a neocortical temporal focus (p = .04) by having lower WA LI; the categorical distribution of atypical language was also different (p=.04) with the mesial temporal group having a greater proportion of patients with atypical language.
We conducted secondary analyses to examine the potential effects of other clinical variables first between groups and then on experimental measures. Handedness (χ2=0.35, p>0.10) and gender (χ2=2.82, p>0.10) were similar between the three groups. There were no differences in age, age of seizure onset, or length of epilepsy duration between frontal, mesial temporal, and neocortical temporal focus groups (MANOVA; p>0.10). However, pairwise comparisons revealed that frontal focus subjects tended to be younger than mesial temporal focus subjects (p=0.09) and tended to have an earlier age of onset than neocortical temporal focus subjects (p=0.08). As a consequence, subjects with a mesial temporal focus tended to have a longer epilepsy duration than those with a neocortical temporal focus (p=0.06). All other pairwise comparisons by seizure focus group for age, age of onset, and duration were not significant (p>0.10).
Age of brain insult, rather than duration, may be associated with altered representation of language. The number of subjects with a pathological injury before age six years tended to be greater in the mesial temporal seizure focus group compared to the neocortical temporal group (χ2=3.71, p=0.05); 80% of subjects with a mesial temporal focus had an age of insult before age six, compared to 58% of subjects with a neocortical temporal focus. The number of frontal focus subjects with age of insult before age six (63%) did not differ from those with a mesial (χ2=2.01, p>0.10) or neocortical focus (χ2=0.07, p>0.10).
As language laterality may be influenced by other variables independent of seizure focus, we explored the relationships between mean LI and related clinical and demographic variables. Age and age of onset were not correlated with mean laterality in WA or IFG (p>0.10). Duration tended to be negatively correlated with WA LI (r=−0.18, p=0.10), but not IFG LI. Since duration of epilepsy showed a trend with WA LI, we added duration as a covariate in the analysis of WA LI by seizure focus group. The difference in mean WA LI between the mesial temporal focus group compared to the frontal focus was p=0.05. All other pairwise comparisons for WA LI by seizure focus group remained not significant. Regional LI did not differ between subjects with an age of insult before age six compared to after age six years (WA LI: t=−1.24, p>0.10; IFG LI: t=0.39, p>0.10).
Atypical handedness was associated with lower mean LI for IFG (t=−2.71, p=0.01), but not for WA (t=−1.48, p=0.14). When analyzed separately, subjects with a mesial or neocortical temporal focus showed the same pattern—atypical handedness had the same associations with mean LI but not as strongly; IFG LI was lower for those with atypical handedness compared to right handedness (t=−2.20, p=0.03); WA LI (t=−1.45, p>0.10) was not different.
Finally, tumors and normal MRI were different in the frontal focus group compared to the temporal lobe group (χ2 42.5, p<0.001) but not different from the temporal neocortical focus sub group.
Discussion
The proportion of patients with a left hemisphere focus who manifested atypical language dominance (25%) is similar to previous studies (Woermann et al., 2003; Lehericy et al., 2000; Adcock et al, 2003; Springer et al., 1999). We found an effect of seizure focus location within the left hemisphere on the regional expression of language. A temporal compared to a frontal focus resulted in lower laterality in Wernicke’s Area. No differences in laterality of language dominance in frontal areas were found between the different foci. We did find a modest difference in mesial temporal versus neocortical temporal seizure focus on language laterality in temporal language processing areas (Weber et al., 2006). Overall, left temporal seizure foci appear to have a more widespread effect on the distributed language processing network than do left frontal foci.
Most studies examine language dominance in temporal lobe populations. These studies find an effect on frontal and temporal regional language dominance (Berl et al., 2005; Weber et al., 2006; Briellmann et al., 2006; Thivard et al., 2005), but often do not distinguish location of focus within the temporal lobe. Studies of mesial temporal sclerosis consistently describe one quarter atypical language dominance (Gaillard et al., 2007; Woermann et al., 2003; Weber et al., 2006; Gaillard et al., 2002; Adcock et al., 2003; Thivard et al., 2005; Arora et al., 2009) even though the seizure focus may not rest in “receptive” neocortical language processing cortex. One study found a left mesial temporal, but not a left neocortical temporal, focus associated with atypical language dominance (Weber et al., 2006). We found modest differences in location and nature of seizure focus within the left temporal lobe on expression of aberrant language dominance, either in Wernicke’s or Broca’s areas. A process that disrupts local neocortical circuits might be expected to exert a direct effect on disruption of local cognitive processing circuits. A mesial temporal focus – primarily emanating from the hippocampus – may have an indirect effect on consolidation of language networks through altered verbal memory processing. These data also suggest that local/focal disruptions within a large, distributed language network may have widespread consequences (Everts et al., 2010; Binder et al., 2010). Therefore, disruption of temporal language areas where there is early (sensory/auditory) maturation may in turn influence the development of neuroanatomically distant, but functionally linked, frontal lobe areas of the language network.
In contrast to temporal lobe epilepsy, few studies examine the effect of a frontal lobe focus on the distributed language processing areas. One small series, using verbal fluency as a probe of “expressive” language, described early developmental insults to WA, but not Broca’s, had an effect on frontal language dominance (Liegeois et al., 2004). Another study, also using a semantic verbal fluency task, found local effects of frontal focus on frontal LI (Anderson et al., 2006). A third study in adults found little effect of frontal lesions on mean language laterality in temporal or frontal areas but did not report categorical results (Weber et al., 2006).
The frontal and temporal groups differed in the nature of underlying image based pathology which may have influenced results. These differences are attributable to the presence of mesial temporal sclerosis which is only found in the temporal lobe focus group and, by definition, is confined to the mesial temporal subgroup. MRI findings were comparable across the neocortical groups. A previous study did not find differences in likelihood of fMRI based atypical language dominance between MTS, focal cortical dysplasia/tumor, and MRI negative patients (Gaillard et al., 2007).
Atypical handedness is well known to be associated with increased likelihood of atypical language representation in patient populations (Gaillard et al., 2007; Rasmussen et al., 1977) and normal volunteers (Pujol et al., 1999; Szaflarski et al., 1992). However, we found no differences in handedness among our study groups that would account for the regional differences observed. We also discovered no differences in age of seizure onset or age of brain insult that might account for our findings. The mesial temporal group had longer duration epilepsy than the neocortical temporal group, which may contribute to our findings between these groups. However, previous studies find age of brain insult -- including epilepsy onset -- rather than duration of epilepsy, to be associated with atypical language dominance (Gaillard et al., 2007; Springer et al., 1999).
We found that patients with a temporal focus who exhibited atypical language reorganization, assessed as both LI and categorical measures also exhibited atypical language organization in frontal regions. In contrast, patients with a frontal focus had language dominance abnormalities primarily confined to the frontal language regions with preservation of typical left language dominance in temporal regions. Categorical definitions are important for clinical assessment of language dominance as interpretation of mean LI may be influenced by skewed distribution of atypical language. Our data suggest that the effects of a frontal lobe focus, or underlying pathology, may be confined to frontal areas and have less wide ranging consequences on the distributed language processing areas than do temporal lobe foci. This observation is supported by an adult study that found extent of focal perinatal left frontal periventricular injury directly correlated with presence and strength of right frontal language homologue activation and atypical dominance (measured as a lower IFG LI) assessed by a verbal fluency task. In contrast, temporal language remained left dominant as assessed by a listening comprehension task, (Staudt et al., 2002). In our study population, the findings of “plasticity” restricted to the frontal language processing may be explained by the differential trajectory of brain maturation that may constrain the latitude for re-organization and compensation involving distinct brain regions and the portions of distributed networks they contain. The frontal lobe association areas, compared to temporal regions, are known to have a longer structural and functional developmental trajectory identified by: 1) timing and distribution of cortical thinning (Sowell et al., 2004; Gogtay et al., 2004; Shaw et al., 2008; Sowell et al., 2001; Lu et al., 2007), 2) myelination (Paus et al., 1999), 3) cerebral metabolism (Chugani et al., 1987), and 4) strength of regional asymmetry indices for language tasks (Berl et al., 2010; Holland et al., 2001; Gaillard et al., 2003).
The longer developmental maturation trajectory of frontal systems may allow greater opportunity and longer time frame during childhood development for compensation and reorganization in frontal compared to temporal regions. Regions with earlier consolidation and maturation trajectories, such as temporal receptive cortex, may also have a profound and far reaching effect on distant connected regions within a distributed network. The distant regions may then be more likely to follow the “reorganization” of the temporal areas when injured or afflicted by disease. Future investigations with larger populations may be able to stratify findings based on ages of insult/seizure onset to frontal regions during the age after which temporal regions seem to be established. Our data hint that there may be a different regional effect on the expression of language dominance that may reflect regional maturation in temporal and frontal cortex in the left hemispheric regions that mediate language processing.
Acknowledgements
Supported by the Society for Pediatric Research Summer Research Fellowship with NIH grant HD007446 (MT), and the Clinical Epilepsy Section, NINDS, NIH.
He is supported by federal funding from the NIH [NINDS 1R01NS44280-01 (PI) and NICHD 1P30HD40677-01 (IDDRC, core director), NCRR 1K12RR17613-01 (mentor), NIMH 1 R01 MH065395-01A2 (Co-I), and CDC-APTR R-03 (Paid consultant)].
Footnotes
Disclosure of Conflicts of Interest
Miss Duke reports no disclosures.
Miss Tesfaye reports no disclosures
Dr. Berl receives research support from the NIH [PCRS Scholar Award and NCRR 5K12RR017613-05 (PI)].
Miss Walker reports no disclosures.
Dr. Ritzl reports no disclosures.
Dr. Fasano reports no disclosures.
Dr. Conry reports no disclosures
Dr. Sato receives research support and salary from NINDS DIR.
Dr. Pearl reports no disclosures.
Dr. Theodore received honoraria from serving as an editor of Epilepsy Research, receives research support and salary from NINDS DIR, and holds stock options in GE.
Dr. Gaillard has served on an educational course at Boston University that was ultimately funded by Questcor Inc and he has served as a one time medical consultant for King Pharmaceuticals, neither relevant to the topic of this manuscript. His department derives clinical income from the evaluation and management of children with epilepsy, and his department also receives research support from Lundbeck Inc., King Pharmaceuticals, PRA International, Eisai Inc., and Marinus Pharmaceuticals, Inc.
We confirm that we have read the Journal’s position on issues involved in ethical publication and affirm that this report is consistent with those guidelines.
References
- Adcock JE, Wise RG, Oxbury JM, Oxbury SM, Matthews PM. Quantitative fMRI assessment of the differences in lateralization of language-related brain activation in patients with temporal lobe epilepsy. Neuroimage. 2003;18:423–438. doi: 10.1016/s1053-8119(02)00013-7. [DOI] [PubMed] [Google Scholar]
- Anderson DP, Harvey AS, Saling MM, Anderson V, Kean M, Abbott DF, Wellard RM, Jackson GD. fMRI Lateralization of Expressive Language in Children with Cerebral Lesions. Epilepsia. 2006;47:998–1008. doi: 10.1111/j.1528-1167.2006.00572.x. [DOI] [PubMed] [Google Scholar]
- Arora J, Pugh K, Westerveld M, Spencer S, Spencer DD, Todd Constable R. Language lateralization in epilepsy patients: fMRI validated with the Wada procedure. Epilepsia. 2009;50:2225–2241. doi: 10.1111/j.1528-1167.2009.02136.x. [DOI] [PubMed] [Google Scholar]
- Berl MM, Balsamo LM, Xu B, Moore EN, Weinstein SL, Conry JA, Pearl PL, Sachs BC, Grandin CB, Frattali C, Ritter FJ, Sato S, Theodore WH, Gaillard WD. Seizure focus affects regional language networks assessed by fMRI. Neurology. 2005;65:1604–1611. doi: 10.1212/01.wnl.0000184502.06647.28. [DOI] [PubMed] [Google Scholar]
- Berl MM, Duke ES, Mayo J, Rosenberger LR, Moore EN, VanMeter J, Ratner NB, Vaidya CJ, Gaillard WD. Functional anatomy of listening and reading comprehension during development. Brain Lang. 2010;114:115–125. doi: 10.1016/j.bandl.2010.06.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Binder JR, Swanson SJ, Sabsevitz DS, Hammeke TA, Raghavan M, Mueller WM. A comparison of two fMRI methods for predicting verbal memory decline after left temporal lobectomy: language lateralization versus hippocampal activation asymmetry. Epilepsia. 2010;51:618–626. doi: 10.1111/j.1528-1167.2009.02340.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Briellmann RS, Labate A, Harvey AS, Saling MM, Sveller C, Lillywhite L, Abbott DF, Jackson GD. Is language lateralization in temporal lobe epilepsy patients related to the nature of the epileptogenic lesion? Epilepsia. 2006;47:916–920. doi: 10.1111/j.1528-1167.2006.00513.x. [DOI] [PubMed] [Google Scholar]
- Chugani HT, Phelps ME, Mazziotta JC. Positron emission tomography study of human brain fuctional development. Ann Neurol. 1987;22:487–497. doi: 10.1002/ana.410220408. [DOI] [PubMed] [Google Scholar]
- Everts R, Harvey AS, Lillywhite L, Wrennall J, Abbott DF, Gonzalez L, Kean M, Jackson GD, Anderson V. Language lateralization correlates with verbal memory performance in children with focal epilepsy. Epilepsia. 2010;51:627–638. doi: 10.1111/j.1528-1167.2009.02406.x. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Balsamo L, Xu B, Grandin CB, Braniecki SH, Papero PH, Weinstein S, Conry J, Pearl PL, Sachs B, Sato S, Jabbari B, Vezina LG, Frattali C, Theodore WH. Language dominance in partial epilepsy patients identified with an fMRI reading task. Neurology. 2002;59:256–265. doi: 10.1212/wnl.59.2.256. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Sachs BC, Whitnah JR, Ahmad Z, Balsamo LM, Petrella JR, Braniecki SH, McKinney CM, Hunter K, Xu B, Grandin CB. Developmental aspects of language processing: fMRI of verbal fluency in children and adults. Hum Brain Mapp. 2003;18:176–185. doi: 10.1002/hbm.10091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gaillard WD. Functional MR imaging of language, memory, and sensorimotor cortex. Neuroimaging Clin N Am. 2004;14:471–485. doi: 10.1016/j.nic.2004.04.005. [DOI] [PubMed] [Google Scholar]
- Gaillard WD, Berl MM, Moore EN, Ritzl EK, Rosenberger LR, Weinstein SL, Conry JA, Pearl PL, Ritter FF, Sato S, Vezina LG, Vaidya CJ, Wiggs E, Fratalli C, Risse G, Ratner NB, Gioia G, Theodore WH. Atypical language in lesional and nonlesional complex partial epilepsy. Neurology. 2007;69:1761–1771. doi: 10.1212/01.wnl.0000289650.48830.1a. [DOI] [PubMed] [Google Scholar]
- Gogtay N, Giedd JN, Lusk L, Hayashi KM, Greenstein D, Vaituzis AC, Nugent TF, 3rd, Herman DH, Clasen Ls, Toga AW, Rapoport JL, Thompson PM. Dynamic mapping of human cortical development during childhood through early adulthood. Proc Natl Acad Sci U S A. 2004;101:8174–8179. doi: 10.1073/pnas.0402680101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holland SK, Plante E, Byars A, Strawsburg RH, Schmithorst VJ, Ball WS. Normal fMRI brain activation patterns in children performing a verb generation task. Neuroimage. 2001;14:837–843. doi: 10.1006/nimg.2001.0875. [DOI] [PubMed] [Google Scholar]
- Janszky J, Mertens M, Janszky I, Ebner A, Woermann FG. Left-sided Interictal Epileptic Activity Induces Shift of Language Lateralization in Temporal Lobe Epilepsy: An fMRI Study. Epilepsia. 2006;47:921–927. doi: 10.1111/j.1528-1167.2006.00514.x. [DOI] [PubMed] [Google Scholar]
- Lehéricy S, Cohen L, Bazin B, Samson S, Giacomini E, rougetet R, Hertz-Pannier L, Le Bihan D, Marsault C, Baulac M. Functional MR evaluation of temporal and frontal language dominance compared with the Wada test. Neurology. 2000;54:1625–1633. doi: 10.1212/wnl.54.8.1625. [DOI] [PubMed] [Google Scholar]
- Liegeois F, Connelly A, Cross JH, Boyd SG, Gadian DG, Vargha-Khadem F, Baldeweg T. Language reorganization in children with early-onset lesions of the left hemisphere: an fMRI study. Brain. 2004;127:1229–1236. doi: 10.1093/brain/awh159. [DOI] [PubMed] [Google Scholar]
- Lu L, Leonard C, Thompson P, Kan E, Jolley J, Welcome S, Toga A, Sowell E. Normal developmental changes in inferior frontal gray matter are associated with improvement in phonological processing: a longitudinal MRI analysis. Cereb Cortex. 2007;17:1092–1099. doi: 10.1093/cercor/bhl019. [DOI] [PubMed] [Google Scholar]
- Mbwana J, Berl MM, Ritzl EK, Rosenberger L, Mayo J, Weinstein S, Conry JA, Pearl PL, Shamim S, Moore EN, Sato S, Vezina LG, Theodore WH, Gaillard WD. Limitations to Plasticity of Language Network Reorganization in Localization Related Epilepsy. Brain. 2009;132:347–356. doi: 10.1093/brain/awn329. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paus T, Zijdenbos A, Worsley K, Collins DL, Blumenthal J, Giedd JN, Rapoport JL, Evans AC. Structural maturation of neural pathways in children and adolescents: in vivo study. Science. 1999;283:1908–1911. doi: 10.1126/science.283.5409.1908. [DOI] [PubMed] [Google Scholar]
- Pujol J, Deus J, Losilla JM, Capdevila A. Cerebral lateralization of language in normal left-handed people studied by functional MRI. Neurology. 1999;52:1038–1043. doi: 10.1212/wnl.52.5.1038. [DOI] [PubMed] [Google Scholar]
- Rasmussen T, Milner B. The role of early left-brain injury in determining lateralization of cerebral speech functions. Ann N Y Acad Sci. 1977;299:355–369. doi: 10.1111/j.1749-6632.1977.tb41921.x. [DOI] [PubMed] [Google Scholar]
- Rosenberger LR, Zeck J, Berl MM, Moore EN, Ritzl EK, Shamim S, Weinstein SL, Conry JA, Pearl PL, Sato S, Vezina LG, Theodore WH, Gaillard WD. Interhemispheric and intrahemispheric language reorganization in complex partial epilepsy. Neurology. 2009;72:1830–1836. doi: 10.1212/WNL.0b013e3181a7114b. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shaw P, Kabani NJ, Lerch JP, Eckstrand K, Lenroot R, Gogtay N, Greenstein D, Clasen L, Eveans A, Rapoport JL, Giedd JN, Wise SP. Neurodevelopmental trajectories of the human cerebral cortex. J Neurosci. 2008;28:3586–3594. doi: 10.1523/JNEUROSCI.5309-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Tessner KD, Toga AW. Mapping continued brain growth and gray matter density reduction in dorsal frontal cortex: Inverse relationships during postadolescent brain maturation. J Neurosci. 2001;21:8819–8829. doi: 10.1523/JNEUROSCI.21-22-08819.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Leonard CM, Welcome SE, Kan E, Toga AW., Jr Longitudinal mapping of cortical thickness and brain growth in normal children. J Neurosci. 2004;24:8223–8231. doi: 10.1523/JNEUROSCI.1798-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Springer JA, Binder JR, Hammeke TA, Swanson SJ, Frost JA, Bellgowan Ps, Brewer CC, Perry HN, Morris GL, Mueller WM. Language dominance in neurologically normal and epilepsy subjects: a functional MRI study. Brain. 1999;122:2033–2046. doi: 10.1093/brain/122.11.2033. [DOI] [PubMed] [Google Scholar]
- Staudt M, Lidzba K, Grodd W, Wildgruber D, Erb M, Krageloh-Mann I. Right-hemispheric organization of language following early left-sided brain lesions: functional MRI topography. Neuroimage. 2002;16:954–967. doi: 10.1006/nimg.2002.1108. [DOI] [PubMed] [Google Scholar]
- Szaflarski JP, Binder JR, Possing ET, McKiernan KA, Ward BD, Hammeke TA. Language lateralization in left-handed and ambidextrous people: fMRI data. Neurology. 1992;59:238–244. doi: 10.1212/wnl.59.2.238. [DOI] [PubMed] [Google Scholar]
- Thivard L, Hombrouck J, du Montcel ST, Delmaire C, Cohen L, Samson S, Dupont S, Chiras J, Baulac M, Lehéricy S. Productive and perceptive language reorganization in temporal lobe epilepsy. Neuroimage. 2005;24:841–851. doi: 10.1016/j.neuroimage.2004.10.001. [DOI] [PubMed] [Google Scholar]
- Weber B, Wellmer J, Reuber M, Mormann F, Weis S, Urbach H, Ruhlmann J, Elger CE, Fernández G. Left hippocampal pathology is associated with atypical language lateralization in patients with focal epilepsy. Brain. 2006;129:346–351. doi: 10.1093/brain/awh694. [DOI] [PubMed] [Google Scholar]
- Wilke M, Schmithorst VJ. A combined bootstrap/histogram analysis approach for computing a lateralization index from neuroimaging data. Neuroimage. 2006;33:522–530. doi: 10.1016/j.neuroimage.2006.07.010. [DOI] [PubMed] [Google Scholar]
- Woermann FG, Jokeit H, Luerding R, Freitag H, Schulz R, Guertler S, Okujava M, Wolf P, Tuxhorn I, Ebner A. Language lateralization by Wada test and fMRI in 100 patients with epilepsy. Neurology. 2003;61:699–701. doi: 10.1212/01.wnl.0000078815.03224.57. [DOI] [PubMed] [Google Scholar]