Abstract
Inflammation increases with age and is associated with many chronic diseases that are prevalent among older adults. Persistent pathogens such as latent herpesviruses and chronic bacterial infections can act as a source of inflammation. Herpesviruses, including Epstein-Barr virus (EBV) and cytomegalovirus (CMV), establish latent infections following primary infection and reactivate when the cellular immune system is compromised. EBV and CMV replication can induce proinflammatory cytokine production and thus could influence systemic inflammation. The present study addressed relationships among EBV and CMV antibody titers, and levels of C-reactive protein (CRP) and interleukin-6 (IL-6) in a sample of 222 community dwelling older adults (meanage= 64.1 ± 14.1 years). Participants were divided into two groups based on whether they were EBV seropositive and CMV seronegative (EBV+CMV−), or EBV and CMV seropositive (EBV+CMV+). Among individuals who were EBV+CMV−, EBV antibody titers were not associated with either CRP or IL-6 levels. However, among those who were EBV+CMV+, higher EBV antibody titers were related to elevated levels of CRP and IL-6 in those individuals with higher CMV antibody titers; there was no relationship between EBV antibody titers and CRP or IL-6 levels in those participants with lower CMV antibody titers. These data suggest that the combination of latent EBV and CMV reactivation (indexed by antibody titers) may boost CRP and IL-6 production. Thus, reactivation of multiple herpesviruses may drive inflammation and could contribute to poorer health among older adults.
Keywords: cytomegalovirus (CMV), Epstein-Barr virus (EBV), C-reactive protein (CRP), interleukin-6 (IL-6), aging, latent reactivation, immunosenescence
1. Introduction
Inflammation predicts morbidity and all-cause mortality in older adults (Bruunsgaard and Pedersen, 2003; De Martinis et al., 2006). Elevated proinflammatory cytokines are associated with many age-related diseases including Type 2 diabetes, cancer, Alzheimer’s disease, and cardiovascular disease (Ershler and Keller, 2000). Although levels of C-reactive protein (CRP) and interleukin-6 (IL-6) increase with age (Ferrucci et al., 2005; Shurin et al., 2007), factors other than aging contribute to inflammation.
Persistent pathogens that are acquired across the lifespan include herpesviruses and chronic bacterial infections. Being seropositive (previously infected) with multiple persistent pathogens (i.e., pathogen burden) does not always lead to symptomatic illness; however, individuals with a greater pathogen burden can have higher CRP and IL-6 levels (Nazmi et al., 2010; Zhu et al., 2000). Greater pathogen burden with higher inflammation has been linked to an increased risk for cardiovascular disease and cardiovascular-related mortality, especially in those seropositive for CMV or EBV (Waldman et al., 2008; Zhu et al., 2000; Zhu et al., 1999).
Herpesviruses are commonplace among adults. Approximately 60% of U.S. adults have antibody to CMV by their forties (Staras et al., 2006), and about 95% of adults worldwide are EBV seropositive (WHO, 2008). Following primary infection, herpesviruses establish latent infections in various cells (Glaser and Jones, 1994). Reactivation of latent herpesviruses, as indexed by higher antibody titers, occurs when the cellular immune system is compromised (Glaser and Kiecolt-Glaser, 1994).
Diminished cellular immunity may paradoxically lead to elevated inflammation. For example, depression can weaken cellular immune function and provoke herpesvirus reactivation (Kiecolt-Glaser et al., 1991). Major depression and depressive symptoms have also been associated with higher levels of IL-1, IL-6, and CRP (Bremmer et al., 2008; Maes et al., 1995). Moreover, in coronary artery disease patients, the combination of higher CRP and the presence of three latent herpesviruses infections (i.e., CMV, EBV, and herpes simplex virus) was associated with higher depressive symptoms (Miller et al., 2005). Herpesvirus reactivation may provide one mechanism leading to higher levels of inflammation; EBV and CMV replication can induce IL-6, tumor necrosis factor-alpha (TNF-α, IL-8, and IL-1β production in cultured cells (Almeida et al., 1994; Burns et al., 1999; Glaser et al., 2006). Thus, herpesvirus reactivation and the number of latent herpesvirus infections could contribute to inflammation.
CMV seropositivity may contribute to negative health outcomes in older adults. For example, CMV seropositive older women were more likely to be frail than their CMV seronegative counterparts (Schmaltz et al., 2005; Wang et al., 2010). CMV seropositive individuals have also presented with increased CRP levels compared to CMV seronegative individuals (Simanek et al., 2011; Zhu et al., 1999). Together, these studies suggest that CMV seropositivity may increase health risks because of elevated inflammation. Indeed, among women with higher serum levels of IL-6, being CMV seropositive was associated with a 20-fold increased risk for frailty compared to those who were CMV seronegative; among women with lower levels of IL-6, CMV seropositive status was not related to frailty (Schmaltz et al., 2005).
Furthermore, CMV seropositive individuals with higher levels of IL-6 and CRP were also at greater risk for cardiovascular-related and all-cause mortality compared to those individuals with lower IL-6 and CRP levels, and this association was related to higher CMV antibody titers (Blankenberg et al., 2001; Nazmi et al., 2010; Simanek et al., 2011). Another study investigated the connection between CMV antibody titers and mortality. Compared to individuals in the lower three quartiles, those with the highest CMV antibody titers were more likely to die from cardiovascular-related disease as well as all-cause events; this relationship was partially explained by IL-6 and TNF-α levels (Roberts et al., 2010). During EBV reactivation, early viral proteins induced macrophages in contact with endothelial cells to produce proinflammatory cytokines, which upregulated expression of endothelial adhesion molecules (Waldman et al., 2008). Thus, EBV reactivation may play a role in cardiovascular disease pathogenesis. Taken together, the relationship among herpesvirus seropositivity, inflammation, and poorer health appears to exist in older populations.
The combination of CMV seropositivity and higher inflammation may result in poorer health. Although CMV seropositivity is not always associated with higher levels of inflammation, herpesvirus reactivation induces proinflammatory cytokine production in vitro. Therefore, this study assessed relationships among herpesviruses reactivation (CMV and EBV) and inflammation (CRP and IL-6) in a sample of community-dwelling older adults. Regardless of whether the participant was seropositive for only EBV or for both EBV and CMV, we expected that higher levels of latent viral reactivation would be associated with higher levels of inflammation. In participants who were both EBV and CMV seropositive, we hypothesized that concurrent reactivation (indexed by antibody titers) of both viruses would be related to increased levels of CRP and IL-6. We also expected that higher EBV antibody titers would be correlated with more depressive symptoms.
2. Methods
2.1 Participants
Participants were part of a larger project on stress and health in older adults. Study recruitment occurred via community and university newspapers, Craigslist.org, senior citizen centers, a collaborating neurologist, and the Alzheimer’s Disease Association. Individuals with immunologically-related health problems such as cancer or recent surgeries were excluded during recruitment. After obtaining informed consent, participants completed questionnaires about mood, health behaviors, and sociodemographic characteristics, and provided a blood sample.
We used all participants from the parent trial who had complete serological data (i.e., CRP and IL-6 values and CMV and EBV seropositivity and antibody titers for those who were seropositive) and were not Type 2 diabetic (N=226). Because acute infections boost inflammation, participants who were visibly ill upon arrival, running a fever at the beginning of the session, or canceled due to illness were rescheduled for an appointment at least 2 weeks later. In addition, during acute viral infections CRP values can range from 20–40 mg/L (Jaye and Waites, 1997). To be conservative, we excluded 4 participants, whose CRP values were greater than 20 mg/mL. Thus, our final sample contained 222 participants including 88 caregivers who were currently providing care at least 5 hours per week for a spouse or parent with Alzheimer’s disease or another progressive dementia, and 134 noncaregivers who were demographically indistinguishable from caregivers but had no similar responsibilities.
2.2 Measures
Participants answered questions about their age, sex, race, highest level of education, and smoking status. Educational level was used to assess socioeconomic status because our sample included a number of older women who had not worked outside the home, as well as many retired people. Body mass index (BMI; kg/m2) was calculated from height and weight data collected during the session.
The Pittsburgh Sleep Quality Index (PSQI) assessed sleep quality and sleep disturbances (Buysse et al., 1989). The scale has good internal consistency (Cronbach α of 0.85) and high test-retest reliability (r = .86) over a 45 day period (Backhaus et al., 2002).
The Center for Epidemiological Studies-Depression Scale (CES-D) is a widely used 20-item scale assessing depressive symptomatology (Radloff, 1977). Population norms provide cutoffs for varying levels of depression, with higher scores signifying more depressive symptoms (Basco et al., 1997; Roberts and Vernon, 1983).
Physical health questions from the Older Adult Resources Survey (OARS) assessed underlying chronic medical conditions and medication use (Fillenbaum and Smyer, 1981). Several studies found excellent agreement between self-reports and hospital or physician records for the specific conditions of interest, including myocardial infarction, stroke, and diabetes (Bush et al., 1989; Kehoe et al., 1994). A dichotomous variable aggregated the use of inflammation-related medications including statins, nonsteroidal anti-inflammatory drugs, corticosteroids, estrogen medications, and antidepressants (Danese et al., 2008).
2.3 Immunological Assays
Blood samples were drawn between 8 AM and 10 AM. Serum and plasma samples were frozen and remained at −80C until assayed.
Plasma was assayed using Euroimmun EBV ELISA plates that measure EBV virus capsid antigen (VCA) antibody titers (Morris Plains, NJ). CMV IgG antibody titers were also determined using Euroimmun CMV ELISA plates (Morris Plains, NJ). CMV and EBV VCA IgG antibody titers were assessed following company instructions with some modifications. Specifically, for each ELISA plate three controls that were included in each kit (one positive sample, one negative sample, and three calibrators) were run in duplicate. Plasma samples were initially diluted 1:101 with a dilution buffer according to the recommended protocol provided by the company. Six serial two-fold dilutions of each sample were assayed. The last dilution factor with a positive IgG value determined the IgG antibody titer. Calculated viral titers for each sample were plotted and samples were rerun if the end point did not fall within the linear range (± 15%). Samples were initially screened for CMV seropositive status and only CMV seropositive samples were serially diluted to assess the CMV antibody titer. If EBV and/or CMV antibodies were present following the initial 1:101 dilution, then the sample was considered seropositive for EBV and/or CMV. If the plasma sample had no EBV and/or no CMV antibody present, then it was considered seronegative for the respective herpesvirus(es). Antibody titers were treated as continuous variables in all of our analyses based on the extant literature showing that latent virus reactivation occurs to varying degrees, and therefore should be represented as continuous (Glaser and Jones, 1994).
IL-6 levels were determined using Quantikine High Sensitivity Immunoassay kits (R&D Systems, Minneapolis, MN) according to the kit instructions. Samples were run undiluted in duplicate. The sensitivity of the kit to detect IL-6 is 0.039 pg/mL. Intra-assay coefficient of variation ranges from 6.9–7.8% and interassay coefficient variation ranges from 6.5–9.6%.
High-sensitivity C-reactive protein (hsCRP) assays were performed, using chemiluminescence methodology with Immulite 1000 (Siemens Medical Solutions, Los Angeles, California). The lowest level of detection is 0.3 mg/L. Intra-assay coefficient of variation is 5.1% and interassay coefficient variation is 7.3%.
2.4 Statistical Analyses
Subjects were categorized by their EBV and CMV seropositive status into two groups: EBV seropositive and CMV seronegative (EBV+CMV−; N=90) or EBV and CMV seropositive (EBV+CMV+; N=124). Five subjects were seronegative for EBV and CMV and three subjects were CMV seropositive, but EBV seronegative. Thus, these 8 individuals were removed from the analyses.
Due to skewed data, a log10 transformation was conducted on EBV and CMV antibody titers, as well as CRP and IL-6 levels. Zero-order correlations assessed relationships between all continuous variables. We also conducted zero-order correlations between all continuous variables within each herpesvirus seropositive group (i.e., EBV+CMV− vs. EBV+CMV+). Chi-square tests were conducted to assess EBV and CMV seropositive status differences among dichotomous variables. Unadjusted analyses of variance (ANOVAs) tested for herpesvirus seropositive group (i.e., EBV+CMV− vs. EBV+CMV+) differences on all continuous variables.
Using ordinary least squares multiple regression models, we addressed the question of whether EBV antibody titers in the EBV+CMV− group were associated with CRP and IL-6 in two separate analyses. In EBV+CMV+ participants, we investigated whether CMV antibody titers moderated the association between EBV antibody titers and CRP and IL-6 in two separate multiple regression analyses. Using similar linear regression models within each herpesvirus seropositive group, we examined whether herpesvirus antibody titers were related to depressive symptoms. All independent variables were grand mean centered. We examined residuals to confirm that they distributed normally.
Models predicting CRP and IL-6 were adjusted for age, BMI, chronic conditions, education, sleep, sex (1=male, 2=female), smoking status (1= smoker, 0=nonsmoker), race (1=Caucasian, 0=non-Caucasian), caregiver status (1= caregiver, 0=noncaregiver) and inflammation-related medications (1=user, 0=nonuser). In the models predicting depression, all covariates in the inflammation models were used with exception of sleep. Sleep was highly associated with depression (r (214) = .59, p< .05); therefore, it was not included in the models.
3. Results
3.1 Demographic, clinical, and health behaviors
As shown in Table 1, herpesvirus seropositive groups did not differ on EBV antibody titers, IL-6 levels, age, education, BMI, chronic conditions, sleep, or depressive symptoms. EBV+CMV+ individuals had higher CRP levels compared to those who were EBV+CMV−. Non-Caucasians and nonsmokers were more likely to be EBV and CMV seropositive (all χ2s> 4.48, all ps< .05). Sex, inflammation-related medication use, and caregiver status were not related to EBV and CMV seropositive status (all χ2s< .64, all ps> .05).
Table 1.
Summary of Sample Characteristics
| Total Sample (N=214) |
EBV seropositive (N=90) |
EBV and CMV seropositive (N=124) |
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Variable | % | M | SD | % | M | SD | % | M | SD | F |
| †CRP (mg/L) | 3.34 | 3.73 | 2.99 | 3.71 | 3.60 | 3.74 | 4.02* | |||
| †IL-6 (pg/mL) | 1.93 | 1.50 | 2.00 | 1.72 | 1.87 | 1.32 | .018 | |||
| EBV (log10) | 3.14 | 0.43 | 3.17 | 0.37 | 3.11 | 0.47 | 1.03 | |||
| CMV (log10) | -- | -- | -- | -- | 2.96 | 0.41 | -- | |||
| Age (yrs) | 64.14 | 14.06 | 62.18 | 14.17 | 65.56 | 13.86 | 3.06 | |||
| BMI (kg/m2) | 27.86 | 5.68 | 27.45 | 4.38 | 28.15 | 6.47 | .519 | |||
| Sleep | 5.55 | 3.06 | 5.19 | 2.76 | 5.82 | 3.25 | 1.99 | |||
| Chronic conditions | 0.98 | 1.04 | 1.00 | 0.96 | 0.97 | 1.10 | .050 | |||
| Depression | 8.69 | 8.65 | 7.79 | 7.93 | 9.35 | 9.11 | 1.71 | |||
| Sex (female) | 74 | 77 | 72 | |||||||
| Race (Caucasian) | 87 | 94 | 82 | |||||||
| Caregiver (yes) | 40 | 43 | 38 | |||||||
| Smoker | 5 | 9 | 2 | |||||||
| Inflammation-related | ||||||||||
| medications | 59 | 57 | 61 | |||||||
| Education | .892 | |||||||||
| High school or less | 13 | 12 | 14 | |||||||
| Some college | 22 | 22 | 22 | |||||||
| College graduate | 38 | 35 | 40 | |||||||
| Graduate/Professional | 27 | 31 | 24 | |||||||
Raw values displayed for clarity; analyses conducted on log10 transformed data.
p < .05
Table 2 provides correlations for all continuous variables. Higher EBV antibody titers were associated with higher CMV antibody titers. Individuals with higher CRP levels also had elevated IL-6 levels, greater BMI, and poorer sleep. Higher CRP levels were also associated with less education. Higher IL-6 levels were related to aging, higher BMI, and more chronic conditions.
Table 2.
Correlations among continuous study variables
| Variable | 1 | 2a | 3 | 4 | 5 | 6 | 7 | 8 | 9 |
|---|---|---|---|---|---|---|---|---|---|
| 1. EBV (log10) | |||||||||
| 2. CMV (log10)a | .25** | ||||||||
| 3. CRP (log10) | .02 | −.01 | |||||||
| 4. IL-6 (log10) | .13 | .06 | .43*** | ||||||
| 5. Age | .04 | −.01 | −.01 | .21** | |||||
| 6. BMI | −.06 | −.07 | .41*** | .32*** | −.25*** | ||||
| 7. Chronic conditions | −.01 | .04 | .09 | .24** | .37*** | −.06 | |||
| 8. Education | −.09 | .04 | −.15* | −.13 | −.14* | −.07 | −.04 | ||
| 9. Sleep | .06 | −.07 | .15* | .12 | −.08 | .21** | .22** | −.11 | |
| 10. Depression | .16* | −.05 | .11 | .04 | −.19** | .17* | .07 | −.11 | .59*** |
CMV correlations’ sample size is 124; all other correlations’ sample sizes are 214.
p < .05
p < .01
p < .001
3.2 Associations among herpesvirus antibody titers and inflammation
Table 3 summarizes the analyses that assessed the relationship between EBV and CMV antibody titers and CRP and IL-6 levels by herpesvirus seropositivity. Although EBV antibody titers were not related to CRP or IL-6 levels in the EBV+CMV− group, the interaction between EBV and CMV antibody titers significantly predicted CRP and IL-6 levels among those EBV+CMV+. Specifically, CRP and IL-6 levels were significantly associated with EBV antibody titers when CMV antibody titers were higher (p< .05); however, this was not the case when CMV antibody titers were lower. Greater BMI was strongly associated with higher CRP and IL-6 levels; consistent with previous reports (Aronson et al., 2008; Mohamed-Ali et al., 1997; Visser et al., 1999). In the regression predicting IL-6 levels among EBV+CMV+ participants, caregiver status was a significant covariate. Higher order interactions between caregiver status and the reported associations were not significant; therefore, higher order interactions were not included in the models.
Table 3.
Summary of Multiple Linear Regressions Predicting CRP and IL-6 Using Separate Analyses by Herpesvirus Seropositive Group
| CRP | IL-6 | ||||||
|---|---|---|---|---|---|---|---|
| Model | Variable | B | SE | 95% CI | B | SE | 95% CI |
| EBV Seropositive | Age (yrs) | .001 | .004 | [−.008, .010] | .005** | .002 | [.002, .009] |
| BMI (kg/m2) | .051*** | .012 | [.027, .074] | .015** | .005 | [.006, .024] | |
| Chronic conditions | .160** | .059 | [.043, .276] | .043 | .023 | [−.003, .089] | |
| Sex | .184 | .113 | [−.040, .408] | .012 | .044 | [−.076, .100] | |
| Race | .036 | .205 | [−.372, .444] | −.099 | .080 | [−.259, .060] | |
| Smoking status | −.259 | .172 | [−.602, .083] | .110 | .067 | [−.024, .244] | |
| Education | −.027 | .050 | [−.125, .072] | −.004 | .019 | [−.043, .034] | |
| Inflammation-related medications | .053 | .098 | [−.143, .248] | −.056 | .038 | [−.132, .021] | |
| Sleep | −.010 | .019 | [−.049, .028] | .008 | .008 | [−.007, .023] | |
| Caregiver status | .016 | .099 | [−.182, .214] | −.018 | .039 | [−.095, .060] | |
| EBV (log10) | −.088 | .130 | [−.346, .170] | −.008 | .051 | [−.109, .093] | |
| R2 | .341 | .321 | |||||
| F (11,78) | 3.672*** | 3.355** | |||||
| EBV and CMV Seropositive | Age (yrs) | .003 | .003 | [−.003, .009] | .003** | .001 | [.001, .006] |
| BMI (kg/m2) | .028*** | .006 | [.015, .040] | .012*** | .002 | [.008, .016] | |
| Chronic conditions | −.005 | .040 | [−.083, .074] | .022 | .013 | [−.003, .047] | |
| Sex | .244** | .087 | [.072, .416] | .052 | .028 | [−.003, .107] | |
| Race | −.073 | .114 | [−.300, .154] | −.070 | .036 | [−.142, .002] | |
| Smoking status | .333 | .259 | [−.181, .847] | .175* | .083 | [.012, .339] | |
| Education | −.046 | .039 | [−.123, .031] | .005 | .012 | [−.020, .029] | |
| Inflammation-related medications | −.003 | .085 | [−.172, .166] | .011 | .027 | [−.043, .065] | |
| Sleep | −.001 | .013 | [−.026, .024] | −.004 | .004 | [−.012, .004] | |
| Caregiver status | .000 | .086 | [−.170, .170] | −.062* | .027 | [−.116, .007] | |
| EBV (log10) | .063 | .087 | [−.110, .235] | .070* | .028 | [.015, .125] | |
| CMV (log10) | −.031 | .098 | [−.225, .164] | −.004 | .031 | [−.066, .058] | |
| EBV (log10)×CMV (log10) | .513* | .218 | [.082, .945] | .144* | .069 | [.007, .282] | |
| R2 | .295 | .397 | |||||
| F (13,110) | 3.549*** | 5.568*** | |||||
p < .05
p < .01
p < .001
The table presents the unstandardized beta coefficient (B) and the standard error and 95% confidence interval for B.
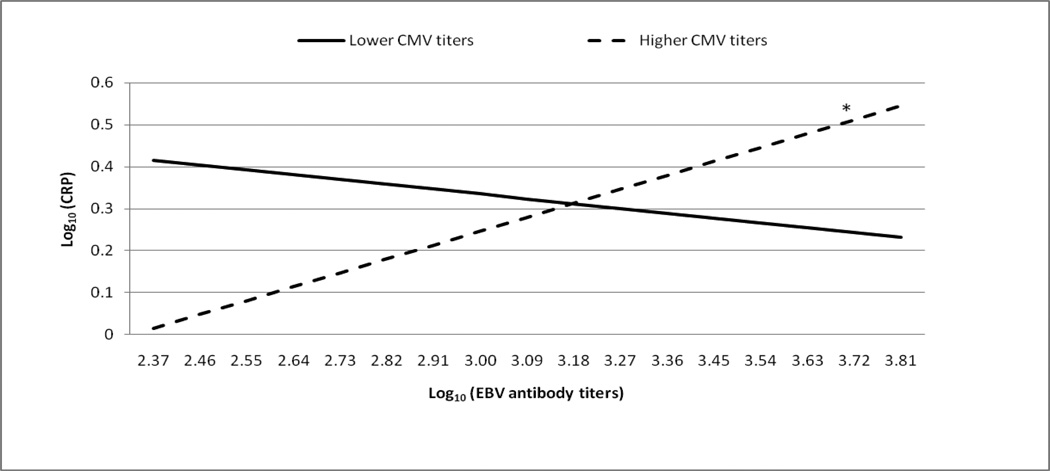
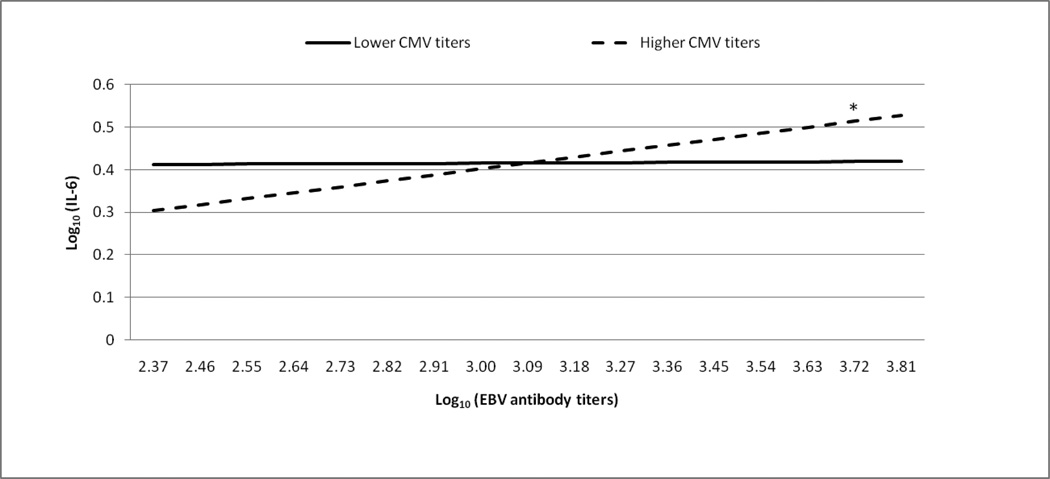
Figures 1 and 2 graphically illustrate the significant EBV antibody titer by CMV antibody titer interaction predicting CRP and IL-6 levels, respectively. As recommended by Aiken and West (1991), we provided lines for +/− 1 standard deviation from the mean to represent CMV antibody titer. We also analyzed the region of significance for the interaction. Using all possible log10 transformed CMV antibody titers (range= 2.01–3.91), we determined when the relationships between EBV antibody titers and CRP or IL-6 levels were statistically different from zero. The region of significance includes CMV antibody titers that correspond to statistically significant relationships between EBV antibody titers and CRP or IL-6 levels.
Figure 1. The moderating role of CMV antibody titers in the relationship between EBV antibody titers and CRP.
*simple slope, b=.27, t(110)=2.02, p < .05
The regression analyses used the full range of EBV and CMV antibody titers as continuous variables. CMV antibody titers were stratified for graphical purposes using +/− 1 standard deviation from the mean. The line representing those with lower CMV antibody titers does not significantly differ from zero (simple slope, b=−.15, t(110)=−1.29, p = .20).
Figure 2. The moderating role of CMV antibody titers in the relationship between EBV antibody titers and IL-6.
*simple slope, b=.13, t(110)=3.13, p < .05
The regression analyses used the full range of EBV and CMV antibody titers as continuous variables. CMV antibody titers were stratified for graphical purposes using +/− 1 standard deviation from the mean. The line representing those with lower CMV antibody titers does not significantly differ from zero (simple slope, b=.01, t(110)=.28, p = .78).
Elevated EBV antibody titers remained significantly associated with greater CRP levels when CMV antibody titers were greater than or equal to 3.51 (ps<.05). For the lowest CMV antibody titer, EBV antibody titers were negatively associated with CRP levels (p=.05). However, we were cautious to interpret the clinical significance of this negative association because only 3 of 124 participants had the lowest CMV antibody titer. The significant positive relationship between EBV antibody titers and IL-6 levels was present when CMV antibody titers were greater than or equal to 2.91 (ps<.05). For CMV antibody titers below 2.91, the relationship between EBV antibody titers and IL-6 levels were not different from zero.
3.3 Associations among herpesvirus antibody titers and depression
Caregivers were more depressed on average than controls (F(1, 210) = 18.66, p<.05); however, caregivers did not have significantly higher herpesvirus antibody titers compared to controls. Using zero-order correlations, more depressive symptoms were associated with higher EBV antibody titers across the whole sample (see Table 2). CMV antibody titers were not related to CES-D scores. When stratifying the sample by herpesvirus seropositive status, the relationship between EBV antibody titers and depression differed across subgroups. For the EBV+CMV+ group, higher EBV antibody titers were related to higher CES-D scores (r(124) = .19, p<.05), while for the EBV+CMV− group, EBV antibody titers and CES-D scores were not related (r(90) = .12, p>.05). In the EBV+CMV+ group, the interaction between EBV and CMV antibody titers was not associated with depressive symptoms.
In the fully adjusted model, the relationship between higher EBV antibody titers and higher depressive symptoms among all participants trended toward significance (b=2.24, t(204)=1.69, p = .09). In addition, within the EBV+CMV+ group, EBV antibody titers were no longer related to CES-D scores and the relationship between CMV antibody titers and CES-D scores remained nonsignificant in adjusted regression models. When caregiver status and higher order interactions were included in the regression models, the results were unchanged.
4. Discussion
Chronic inflammation can lead to poorer health, especially in older adults. Among our EBV+CMV+ participants, higher EBV antibody titers were associated with higher CRP and IL-6 levels, but only in those who had higher CMV antibody titers. EBV antibody titers were not related to CRP or IL-6 levels in the EBV+CMV− group. Consistent with previous findings (Kiecolt-Glaser et al., 1991), higher EBV antibody titers were associated with more depressive symptoms.
Greater pathogen burden is associated with elevated inflammation (Nazmi et al., 2010; Zhu et al., 2000). In our sample, those who were EBV+CMV+ had higher CRP levels than those who were EBV+CMV−. This finding supports previous data linking pathogen burden and inflammation. However, IL-6 levels did not differ between the EBV+CMV− and EBV+CMV+ groups. Several labs have shown that CMV seropositive individuals have higher CRP levels compared to those who are seronegative (Simanek et al., 2011; Zhu et al., 1999). However, the relationship between CMV seropositivity and higher IL-6 levels has not been as consistent (Blankenberg et al., 2001; Schmaltz et al., 2005).
Biological differences between IL-6 and CRP may explain the discordant results of CMV seropositivity on IL-6 and CRP levels. For example, IL-6 follows a diurnal rhythm and has a relatively short half-life (Bauer et al., 1994; Moldofsky et al., 1986; Waage et al., 1989), while CRP does not vary across the day and has a half-life 4–5 times longer than IL-6 (Meier-Ewert et al., 2001; Ridker, 2003). In addition, many cells throughout the body can produce IL-6 including immune cells, adipocytes, and muscle cells (Mohamed-Ali et al., 1997; Pedersen and Febbraio, 2008). However, only the liver produces CRP (Pepys and Hirschfield, 2003).
In our older adult sample, concurrent reactivation of EBV and CMV (as indexed by antibody titers) was related to higher CRP and IL-6; these data suggest that enhanced viral reactivation of both EBV and CMV, not simply herpesvirus seropositive status or reactivation of either EBV or CMV alone, may be linked to elevated inflammation and possibly other health consequences such as Type 2 diabetes, cancer, Alzheimer’s disease, and cardiovascular disease. Our findings extend a recent report suggesting that higher antibody titers to multiple persistent pathogens were better predictors of elevated CRP than any one single pathogen alone (Nazmi et al., 2010).
EBV reactivation was associated with higher inflammation values when CMV reactivation occurred simultaneously. Data from in vitro studies show that EBV and CMV replication can induce IL-6, TNF-α, IL-8, and IL-1β production (Almeida et al., 1994; Burns et al., 1999; Glaser et al., 2006). Higher CMV antibody titers have also been associated with elevated levels of CRP, IL-6, and TNF-α in both healthy adults and cardiovascular patients (Blankenberg et al., 2001; Roberts et al., 2010; Simanek et al., 2011); however, until now no data have suggested that in vivo EBV reactivation was related to systemic inflammation in a healthy older population.
CMV seropositivity in the face of elevated inflammation may be a particularly detrimental combination during old age. For example, older women with the highest CMV antibody titers had a greater incidence of frailty 3 years later and mortality 5 years later compared to CMV seronegative women (Wang et al., 2010). In an influenza vaccination study, vaccine nonresponders (those without a 4-fold antibody response) had greater CMV reactivation and higher levels of TNF-α and IL-6 compared to vaccine responders (Trzonkowski et al., 2003). In addition, CMV seropositive individuals with higher IL-6, TNF-α, or CRP levels were also at greater risk for cardiovascular-related and all-cause mortality compared to their counterparts who had lower levels of IL-6, TNF-α, or CRP (Blankenberg et al., 2001; Roberts et al., 2010; Simanek et al., 2011). In our data, greater CMV reactivation was associated with higher CRP and IL-6 levels when EBV antibody titers were elevated; therefore, EBV reactivation might shed insight on when greater inflammation accompanies CMV seropositivity.
Consistent with previous literature (Nazmi et al., 2010; Roberts et al., 2010; Zhu et al., 2000), the presence of two herpesviruses and concurrent reactivation may be associated with a greater inflammatory effect compared to the effect of only one herpesvirus and its reactivation. In addition, this concurrent reactivation of more than one herpesvirus may be tapping into a larger biological process known as immunosenescence that reactivation of only one herpesvirus does not provide (Pawelec et al., 2009; Stowe et al., 2007). Following young adulthood, the function of the immune system normally declines as one ages. Diminished cellular immunity and increased inflammation characterize immunosenescence (Franceschi et al., 2000). Thus, our data linking greater herpesvirus reactivation to higher inflammation aligns with previous data on the aging immune system.
In vitro studies suggest that EBV and CMV reactivation may lead to greater CRP and IL-6 production (Almeida et al., 1994; Burns et al., 1999; Glaser et al., 2006). For example, our laboratory has previously shown that at least one EBV-encoded early protein, deoxyuridine triphosphate nucleotidohydrolase (dUTPase), can induce macrophages and dendritic cells to synthesize proinflammatory cytokines, including IL-6, IL-8, and TNF-α (Glaser et al., 2006). In a follow-up study, we found that EBV-encoded dUTPase activates nuclear factor-kappa B (NFκB) through the TLR2 and the MyD88-dependent signaling pathways, which subsequently induces proinflammatory cytokine production (Ariza et al., 2009).
The study design does not allow us to exclude the possibility that higher levels of inflammation may be driving EBV and CMV reactivation. Latent herpesvirus reactivation occurs when cellular immune function is compromised (Glaser and Kiecolt-Glaser, 1994). Innate immunity can regulate cellular immunity (Iwasaki and Medzhitov, 2010). Excessive production of proinflammatory cytokines such as IL-6 may disrupt normal cellular immune function. Therefore, higher inflammation could increase the likelihood of latent EBV and CMV reactivation. Future experimental studies should investigate the direction of this association.
Given the differential prevalence of EBV and CMV in older adults, we were able to investigate the relationship between EBV reactivation and inflammation in those EBV+CMV− participants and the cumulative effect of concurrent EBV and CMV reactivation in those who were EBV+CMV+. However, we could not assess the independent effect of CMV reactivation on inflammation because EBV seropositivity is highly prevalent (approximately 95%) in older adults (WHO, 2008). In our sample, only 3 participants (1%) were EBV-CMV+. Thus, the natural prevalence of EBV seropositivity limited our ability to examine the independent effect of CMV reactivation on inflammation.
We chose to remove participants with CRP values greater than 20 mg/L because CRP values ranging from 20–40 mg/L could reflect an acute viral infection (Jaye and Waites, 1997). CRP levels greater than 10 mg/L can also indicate the presence of an acute illness (Pearson et al., 2003), among a young and healthy population. However, this cutoff could be less serviceable among older adults. For example, CRP values greater than 10 mg/L during late-middle age have been related to a greater risk of cardiovascular events and all-cause mortality (Cook et al., 2006; Hamer and Chida, 2009; Hamer et al., 2010; Ridker and Cook, 2004). Thus, very high CRP values may not equate solely to an acute infection in older populations, and could indicate risk for future health events and/or psychosocial adversity. In addition, our sample’s CRP values fall within the range reported in older populations (Ballou et al., 1996; Ferrucci et al., 2005).
Throughout data collection, we rescheduled visibly ill participants to eliminate the effect of acute infections on inflammation. However, there are additional health issues in older adults that could have influenced CRP and IL-6 levels in our study. For example, individuals with periodontal disease have greater systemic inflammation than those without dental problems (Amar et al., 2003; Tonetti et al., 2007). Frail older adults are more likely to fall due to muscle wasting, have increased disease vulnerability, and present with higher inflammation compared to age-matched nonfrail adults (Cannon, 1995; Ferrucci et al., 2002; Walston et al., 2002). We did not collect data on oral health or number of illnesses or falls in the last year, therefore, we cannot rule out the presence of these health issues that can influence inflammation in our sample.
Previous data suggest that distressed caregivers may have elevated herpesvirus antibody titers compared to age-matched controls (Glaser and KiecoltGlaser, 1997; Kiecolt-Glaser et al., 1991). Although caregivers (meanCES-D= 11.54±8.63) were more depressed on average than noncaregivers (meanCES-D= 6.79±8.15), herpesvirus antibody titers were not elevated in caregivers compared to noncaregivers. The fact that we did not find this relationship may be related to characteristics of our control group. For example, a score of 16 or higher on the CES-D identifies individuals with mild depression and score of 25 or higher can indicate severe depression (Haringsma et al., 2004; Radloff, 1977). Comparing the distribution of CES-D scores between caregivers and controls, there were 19 caregivers and 13 controls with a score of 16 or higher on the CES-D, and 8 caregivers and 6 controls with a score of 25 or higher. Thus, our control group included similar extreme CES-D levels as the caregiver group, and limited our ability to detect caregiver/noncaregiver differences with regard to herpesvirus reactivation and inflammation.
Among all participants, EBV reactivation was associated with depressive symptoms. However, when analyzing this relationship within each subgroup, it was found only among the EBV+CMV+ individuals. The lack of a relationship between EBV antibody titers and depressive symptoms in the EBV+CMV− group could result from a reduction in power due to the smaller sample. When adjusting the regression models for known confounds such as age or chronic illnesses, the relationship between EBV reactivation and depression trended towards significance among all participants. Hence, the inclusion of multiple covariates resulted in reduced power, limiting our ability to detect the relationships between EBV antibody titers and depressive symptoms.
Our data suggest that reactivation of multiple herpesviruses and elevated inflammation are linked in older adults. This association could contribute to poorer health. For example, inflammation plays a critical role in important noncommunicable diseases including diabetes, cancer, chronic respiratory disease, and cardiovascular disease (Mathur and Pedersen, 2008). In addition, chronic inflammation can lead to accelerated cellular aging, indexed by telomere length (Aviv, 2004; Kiecolt-Glaser and Glaser, 2010). Telomeres—aglet-like caps that provide stability for chromosomes—shorten during replication and once the length drops below a critical threshold, the cell reaches senescence (Blackburn, 2001). Advanced cellular aging (telomere shortening) has been linked to many age-related diseases and mortality (Epel, 2009). Inflammation increases lymphocyte proliferation; leading to shorter telomeres (Aviv, 2004). Thus, herpesvirus reactivation could possibly fuel cellular aging and development of age-related diseases through its association with increased inflammation.
Research Highlight.
Our data suggest that the combination of latent EBV and CMV reactivation may boost CRP and IL-6 production in an older population.
Acknowledgments
Work on this project was supported in part by NIH grants CA131029, DE014320, UL1RR025755, and CA016058.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of interest statement: All authors declare that there are no conflicts of interest.
References
- Aiken LS, West SG. Multiple Regression: Testing and Interpreting Interactions. Newbury Park: Sage; 1991. Interactions between continuous predictors in multiple regression; pp. 9–27. [Google Scholar]
- Almeida GD, Porada C, St Jeor S, Ascensao J. Human cytomegalovirus alters interleukin-6 production by endothelial cells. Blood. 1994;83:370–376. [PubMed] [Google Scholar]
- Amar S, Gokce N, Morgan S, Loukideli M, Van Dyke TE, Vita JA. Periodontal Disease Is Associated With Brachial Artery Endothelial Dysfunction and Systemic Inflammation. Arterioscler Thromb Vasc Biol. 2003;23:1245–1249. doi: 10.1161/01.ATV.0000078603.90302.4A. [DOI] [PubMed] [Google Scholar]
- Ariza ME, Glaser R, Kaumaya PT, Jones C, Williams MV. The EBV-encoded dUTPase activates NF-kappa B through the TLR2 and MyD88-dependent signaling pathway. J Immunol. 2009;182:851–859. doi: 10.4049/jimmunol.182.2.851. [DOI] [PubMed] [Google Scholar]
- Aronson D, Avizohar O, Levy Y, Bartha P, Jacob G, Markiewicz W. Factor analysis of risk variables associated with low-grade inflammation. Atherosclerosis. 2008;200:206–212. doi: 10.1016/j.atherosclerosis.2007.12.008. [DOI] [PubMed] [Google Scholar]
- Aviv A. Telomeres and human aging: Facts and fibs. Sci Aging Knowl Environ. 2004;51:pe43. doi: 10.1126/sageke.2004.51.pe43. [DOI] [PubMed] [Google Scholar]
- Backhaus J, Junghanns K, Broocks A, Riemann D, Hohagen F. Test-retest reliability and validity of the Pittsburgh Sleep Quality Index in primary insomnia. J Psychosom Res. 2002;53:737–740. doi: 10.1016/s0022-3999(02)00330-6. [DOI] [PubMed] [Google Scholar]
- Ballou SP, Lozanski GB, Hodder S, Rzewnicki DL, Mion LC, Sipe JD, Ford AB, Kushner I. Quantitative and Qualitative Alterations of Acute-phase Proteins in Healthy Elderly Persons. Age Ageing. 1996;25:224–230. doi: 10.1093/ageing/25.3.224. [DOI] [PubMed] [Google Scholar]
- Basco MR, Krebaum SR, Rush AJ. Outcome measures of depression. In: Strupp HH, Horowitz LM, Lambert MJ, editors. Measuring patient changes in mood, anxiety, and personality disorders. Washington D.C.: American Psychological Association; 1997. pp. 207–245. [Google Scholar]
- Bauer J, Hohagen F, Ebert T, Timmer J, Ganter U, Krieger S, Lis S, Postler E, Voderholzer U, Berger M. Interleukin-6 serum levels in healthy persons correspond to the sleep-wake cycle. Clin Investig. 1994;72:315. doi: 10.1007/BF00180048. [DOI] [PubMed] [Google Scholar]
- Blackburn EH. Switching and signaling at the telomere. Cell. 2001;106:661–673. doi: 10.1016/s0092-8674(01)00492-5. [DOI] [PubMed] [Google Scholar]
- Blankenberg S, Rupprecht HJ, Bickel C, Espinola-Klein C, Rippin G, Hafner G, Ossendorf M, Steinhagen K, Meyer J. Cytomegalovirus infection with interleukin-6 response predicts cardiac mortality in patients with coronary artery disease. Circulation. 2001;103:2915–2921. doi: 10.1161/01.cir.103.24.2915. [DOI] [PubMed] [Google Scholar]
- Bremmer MA, Beekman AT, Deeg DJ, Penninx BW, Dik MG, Hack CE, Hoogendijk WJ. Inflammatory markers in late-life depression: results from a population-based study. J Affect Disord. 2008;106:249–255. doi: 10.1016/j.jad.2007.07.002. [DOI] [PubMed] [Google Scholar]
- Bruunsgaard H, Pedersen BK. Age-related inflammatory cytokines and disease. Immunol Allergy Clin North Am. 2003;23:15–39. doi: 10.1016/s0889-8561(02)00056-5. [DOI] [PubMed] [Google Scholar]
- Burns LJ, Pooley JC, Walsh DJ, Vercellotti GM, Weber ML, Kovacs A. Intercellular adhesion molecule-1 expression in endothelial cells is activated by cytomegalovirus immediate early proteins1. Transplantation. 1999;67:137–144. doi: 10.1097/00007890-199901150-00023. [DOI] [PubMed] [Google Scholar]
- Bush TL, Miller SR, Golden AL, Halle WE. Self-reports and medical report agreement of selected medical conditions in the elderly. Am J Public Health. 1989;79:1554–1556. doi: 10.2105/ajph.79.11.1554. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Buysse DJ, Reynolds CF, Monk TH, Berman SR, Kupfer DJ. Pittsburgh Sleep Quality Index: A new instrument for psychiatric practice and research. Psychiatry Res. 1989;28:193–213. doi: 10.1016/0165-1781(89)90047-4. [DOI] [PubMed] [Google Scholar]
- Cannon J. Cytokines in aging and muscle homeostasis. J Gerontol Ser A. 1995;50:120–123. doi: 10.1093/gerona/50a.special_issue.120. [DOI] [PubMed] [Google Scholar]
- Cook NR, Buring JE, Ridker PM. The effect of including C-reactive protein in cardiovascular risk prediction models for women. Ann Intern Med. 2006;145:21–29. doi: 10.7326/0003-4819-145-1-200607040-00128. [DOI] [PubMed] [Google Scholar]
- Danese A, Moffitt TE, Pariante CM, Ambler A, Poulton R, Caspi A. Elevated inflammation levels in depressed adults with a history of childhood maltreatment. Arch Gen Psychiatry. 2008;65:409–415. doi: 10.1001/archpsyc.65.4.409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Martinis M, Franceschi C, Monti D, Ginaldi L. Inflammation markers predicting frailty and mortality in the elderly. Exp Mol Pathol. 2006;80:219–227. doi: 10.1016/j.yexmp.2005.11.004. [DOI] [PubMed] [Google Scholar]
- Epel ES. Telomeres in a life-span perspective: a new "psychobiomarker"? Curr Dir Psychol Sci. 2009;18:6–10. [Google Scholar]
- Ershler W, Keller E. Age-associated increased interleukin-6 gene expression, late-life diseases, and frailty. Annu Rev Med. 2000;51:245–270. doi: 10.1146/annurev.med.51.1.245. [DOI] [PubMed] [Google Scholar]
- Ferrucci L, Cavazzini C, Corsi A, Bartali B, Russo CR, Lauretani F, Corsi AM, Bandinelli S, Guralnik JM. Biomarkers of frailty in older persons. J Endocrinol Invest. 2002;25:10–15. [PubMed] [Google Scholar]
- Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillenbaum GG, Smyer MA. The development, validity, and reliability of the OARS multidimensional functional assessment questionnaire. J Gerontol. 1981;36:428–434. doi: 10.1093/geronj/36.4.428. [DOI] [PubMed] [Google Scholar]
- Franceschi C, Bonafe M, Valensin S. Human immunosenescence: the prevailing of innate immunity, the failing of clonotypic immunity, and the filling of immunological space. Vaccine. 2000;18:1717–1720. doi: 10.1016/s0264-410x(99)00513-7. [DOI] [PubMed] [Google Scholar]
- Glaser R, Jones JF. Herpesvirus infections. New York: M Dekker; 1994. [Google Scholar]
- Glaser R, Kiecolt-Glaser JK. Stress-associated immune modulation and its implications for reactivation of latent herpesviruses. In: Glaser R, Jones J, editors. Herpesvirus infections. New York: M Dekker; 1994. pp. 245–270. [Google Scholar]
- Glaser R, KiecoltGlaser JK. Chronic stress modulates the virus-specific immune response to latent herpes simplex virus type 1. Ann Behav Med. 1997;19:78–82. doi: 10.1007/BF02883323. [DOI] [PubMed] [Google Scholar]
- Glaser R, Litsky ML, Padgett DA, Baiocchi RA, Yang EV, Chen M, Yeh PE, Green-Church KB, Caligiuri MA, Williams MV. EBV-encoded dUTPase induces immune dysregulation: Implications for the pathophysiology of EBV-associated disease. Virology. 2006;346:205–218. doi: 10.1016/j.virol.2005.10.034. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y. Associations of very high C-reactive protein concentration with psychosocial and cardiovascular risk factors in an ageing population. Atherosclerosis. 2009;206:599–603. doi: 10.1016/j.atherosclerosis.2009.02.032. [DOI] [PubMed] [Google Scholar]
- Hamer M, Chida Y, Stamatakis E. Association of Very Highly Elevated C-Reactive Protein Concentration with Cardiovascular Events and All-Cause Mortality. Clin Chem. 2010;56:132–135. doi: 10.1373/clinchem.2009.130740. [DOI] [PubMed] [Google Scholar]
- Haringsma R, Engels GI, Beekman ATF, Spinhoven P. The criterion validity of the Center for Epidemiological Studies Depression Scale (CES-D) in a sample of self-referred elders with depressive symptomatology. Int J Geriatr Psychiatry. 2004;19:558–563. doi: 10.1002/gps.1130. [DOI] [PubMed] [Google Scholar]
- Iwasaki A, Medzhitov R. Regulation of Adaptive Immunity by the Innate Immune System. Science. 2010;327:291–295. doi: 10.1126/science.1183021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jaye DL, Waites KB. Clinical applications of C-reactive protein in pediatrics. Pediatr Infect Dis J. 1997;16:735–747. doi: 10.1097/00006454-199708000-00003. [DOI] [PubMed] [Google Scholar]
- Kehoe R, Wu SY, Leske MC, Chylack LT. Comparing self-reported and physician reported medical history. Am J Epidemiol. 1994;139:813–818. doi: 10.1093/oxfordjournals.aje.a117078. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser J, Dura J, Speicher C, Trask O, Glaser R. Spousal caregivers of dementia victims: Longitudinal changes in immunity and health. Psychosom Med. 1991;53:345–362. doi: 10.1097/00006842-199107000-00001. [DOI] [PubMed] [Google Scholar]
- Kiecolt-Glaser JK, Glaser R. Psychological stress, telomeres, and telomerase. Brain Behav Immun. 2010;24:529–530. doi: 10.1016/j.bbi.2010.02.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maes M, Meltzer HY, Bosmans E, Bergmans R, Vandoolaeghe E, Ranjan R, Desnyder R. Increased plasma-concentrations of interleukin-6, soluble interleukin-6, soluble interleukin-2 and transferrin receptor in major depression. J Affect Disorders. 1995;34:301–309. doi: 10.1016/0165-0327(95)00028-l. [DOI] [PubMed] [Google Scholar]
- Mathur N, Pedersen BK. Exercise as a mean to control low-grade systemic inflammation. Mediators Inflamm. 2008 doi: 10.1155/2008/109502. 2008, Article ID 109502. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meier-Ewert HK, Ridker PM, Rifai N, Price N, Dinges DF, Mullington JM. Absence of Diurnal Variation of C-Reactive Protein Concentrations in Healthy Human Subjects. Clin Chem. 2001;47:426–430. [PubMed] [Google Scholar]
- Miller GE, Freedland KE, Duntley S, Carney RM. Relation of depressive symptoms to C-reactive protein and pathogen burden (cytomegalovirus, herpes simplex virus, Epstein-Barr virus) in patients with earlier acute coronary syndromes. Am J Cardiol. 2005;95:317–321. doi: 10.1016/j.amjcard.2004.09.026. [DOI] [PubMed] [Google Scholar]
- Mohamed-Ali V, Goodrick S, Rawesh A, Katz DR, Miles JM, Yudkin JS, Klein S, Coppack SW. Subcutaneous adipose tissue releases interleukin-6, but not tumor necrosis factor-alpha, in vivo. J Clin Endocrin Metab. 1997;82:4196–4200. doi: 10.1210/jcem.82.12.4450. [DOI] [PubMed] [Google Scholar]
- Moldofsky H, Lue FA, Eisen J, Keystone E, Gorczynski RM. The relationship of interleukin-1 and immune functions to sleep in humans. Psychosom Med. 1986;48:309–318. doi: 10.1097/00006842-198605000-00001. [DOI] [PubMed] [Google Scholar]
- Nazmi A, Diez-Roux AV, Jenny NS, Tsai MY, Szklo M, Aiello AE. The influence of persistent pathogens on circulating levels of inflammatory markers: a cross-sectional analysis from the Multi-Ethnic Study of Atherosclerosis. BMC Public Health. 2010;10:706. doi: 10.1186/1471-2458-10-706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pawelec G, Derhovanessian E, Larbi A, Strindhall J, Wikby A. Cytomegalovirus and human immunosenescence. Rev Med Virol. 2009;19:47–56. doi: 10.1002/rmv.598. [DOI] [PubMed] [Google Scholar]
- Pearson TA, Mensah GA, Alexander RW, Anderson JL, Cannon RO, III, Criqui M, Fadl YY, Fortmann SP, Hong Y, Myers GL, Rifai N, Smith SC, Jr, Taubert K, Tracy RP, Vinicor F. Markers of inflammation and cardiovascular disease: Application to clinical and public health practice: A statement for healthcare professionals from the Centers for Disease Control and Prevention and the American Heart Association. Circulation. 2003;107:499–511. doi: 10.1161/01.cir.0000052939.59093.45. [DOI] [PubMed] [Google Scholar]
- Pedersen BK, Febbraio MA. Muscle as an endocrine organ: Focus on muscle-derived interleukin-6. Physiol Rev. 2008;88:1379–1406. doi: 10.1152/physrev.90100.2007. [DOI] [PubMed] [Google Scholar]
- Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radloff LS. The CES-D scale: A self-report depression scale for research in the general population. Appl Psych Meas. 1977;1:385–401. [Google Scholar]
- Ridker PM. Clinical Application of C-Reactive Protein for Cardiovascular Disease Detection and Prevention. Circulation. 2003;107:363–369. doi: 10.1161/01.cir.0000053730.47739.3c. [DOI] [PubMed] [Google Scholar]
- Ridker PM, Cook N. Clinical Usefulness of Very High and Very Low Levels of C-Reactive Protein Across the Full Range of Framingham Risk Scores. Circulation. 2004;109:1955–1959. doi: 10.1161/01.CIR.0000125690.80303.A8. [DOI] [PubMed] [Google Scholar]
- Roberts ET, Haan MN, Dowd JB, Aiello AE. Cytomegalovirus antibody levels, inflammation, and mortality among elderly Latinos over 9 years of follow-up. Am J Epidemiol. 2010;172:363–371. doi: 10.1093/aje/kwq177. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roberts R, Vernon S. The Center for Epidemiologic Studies Depression Scale: its use in a community sample. Am J Psychiatry. 1983;140:41–46. doi: 10.1176/ajp.140.1.41. [DOI] [PubMed] [Google Scholar]
- Schmaltz HN, Fried LP, Xue QL, Walston J, Leng SX, Semba RD. Chronic cytomegalovirus infection and inflammation are associated with prevalent frailty in community-dwelling older women. J Am Geriatr Soc. 2005;53:747–754. doi: 10.1111/j.1532-5415.2005.53250.x. [DOI] [PubMed] [Google Scholar]
- Shurin GV, Yurkovetsky ZR, Chatta GS, Tourkova IL, Shurin MR, Lokshin AE. Dynamic alteration of soluble serum biomarkers in healthy aging. Cytokine. 2007;39:123–129. doi: 10.1016/j.cyto.2007.06.006. [DOI] [PubMed] [Google Scholar]
- Simanek AM, Dowd JB, Pawelec G, Melzer D, Dutta A, Aiello AE. Seropositivity to cytomegalovirus, inflammation, all-cause and cardiovascular disease-related mortality in the United States. PLoS ONE. 2011:6. doi: 10.1371/journal.pone.0016103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Staras SA, Dollard SC, Radford KW, Flanders WD, Pass RF, Cannon MJ. Seroprevalence of cytomegalovirus infection in the United States, 1988–1994. Clin Infect Dis. 2006;43:1143–1151. doi: 10.1086/508173. [DOI] [PubMed] [Google Scholar]
- Stowe RP, Kozlova EV, Yetman DL, Walling DM, Goodwin JS, Glaser R. Chronic herpesvirus reactivation occurs in aging. Exp Gerontol. 2007;42:563–570. doi: 10.1016/j.exger.2007.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tonetti MS, D'Aiuto F, Nibali L, Donald A, Storry C, Parkar M, Suvan J, Hingorani AD, Vallance P, Deanfield J. Treatment of periodontitis and endothelial function. N Engl J Med. 2007;356:911–920. doi: 10.1056/NEJMoa063186. [DOI] [PubMed] [Google Scholar]
- Trzonkowski P, Mysliwska J, Szmit E, Wieckiewicz J, Lukaszuk K, Brydak LB, Machala M, Mysliwski A. Association between cytomegalovirus infection, enhanced proinflammatory response and low level of anti-hemagglutinins during the anti-influenza vaccination--an impact of immunosenescence. Vaccine. 2003;21:3826–3836. doi: 10.1016/s0264-410x(03)00309-8. [DOI] [PubMed] [Google Scholar]
- Visser M, Bouter LM, McQuillan GM, Wener MH, Harris TB. Elevated C-reactive protein levels in overweight and obese adults. JAMA. 1999;282:2131–2135. doi: 10.1001/jama.282.22.2131. [DOI] [PubMed] [Google Scholar]
- Waage A, Brandtzaeg P, Halstensen A, Kierulf P, Espevik T. The complex pattern of cytokines in serum from patients with meningococcal septic shock. Association between interleukin 6, interleukin 1, and fatal outcome. J Exp Med. 1989;169:333–338. doi: 10.1084/jem.169.1.333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Waldman WJ, Williams MV, Jr, Lemeshow S, Binkley P, Guttridge D, Kiecolt-Glaser JK, Knight DA, Ladner KJ, Glaser R. Epstein-Barr virus-encoded dUTPase enhances proinflammatory cytokine production by macrophages in contact with endothelial cells: evidence for depression-induced atherosclerotic risk. Brain Behav Immun. 2008;22:215–223. doi: 10.1016/j.bbi.2007.07.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walston J, McBurnie MA, Newman A, Tracy RP, Kop WJ, Hirsch CH, Gottdiener J, Fried LP. Frailty and activation of the inflammation and coagulation systems with and without clinical comorbidities: results from the Cardiovascular Health Study. Arch Intern Med. 2002;162:2333–2341. doi: 10.1001/archinte.162.20.2333. [DOI] [PubMed] [Google Scholar]
- Wang GC, Kao WH, Murakami P, Xue QL, Chiou RB, Detrick B, McDyer JF, Semba RD, Casolaro V, Walston JD, Fried LP. Cytomegalovirus infection and the risk of mortality and frailty in older women: a prospective observational cohort study. Amer J Epidemiol. 2010;171:1144–1152. doi: 10.1093/aje/kwq062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WHO. World Health Organization: Immunization vaccines and biologicals - Epstein-Barr virus. 2008
- Zhu J, Quyyumi AA, Norman JE, Csako G, Waclawiw MA, Shearer GM, Epstein SE. Effects of total pathogen burden on coronary artery disease risk and C-reactive protein levels. Amer J Cardiol. 2000;85:140–146. doi: 10.1016/s0002-9149(99)00653-0. [DOI] [PubMed] [Google Scholar]
- Zhu JH, Quyyumi AA, Norman JE, Csako G, Epstein SE. Cytomegalovirus in the pathogenesis of atherosclerosis - The role of inflammation as reflected by elevated C-reactive protein levels. J Am Coll Cardiol. 1999;34:1738–1743. doi: 10.1016/s0735-1097(99)00410-6. [DOI] [PubMed] [Google Scholar]