Abstract
Resting mature human B cells undergo a dynamic process of clonal expansion, followed by clonal contraction, during an in vitro response to surrogate C3d-coated antigen and innate immune system cytokines, IL-4 and BAFF. We here explore the mechanism for clonal contraction through following the time- and division-influenced expression of several pro- and anti-apoptotic proteins within CFSE-labeled cultures. Several findings, involving both human and mouse B cells, show that a mitochondria-dependent apoptotic pathway involving p53 contributes to the high AICD susceptibility of replicating blasts. Activated B cell clones exhibit elevated p53 protein and elevated mRNA/protein of pro-apoptotic molecules known to be under direct p53 transcriptional control, Bax, Bad, Puma, Bid, and pro-caspase 6, accompanied by reduced anti-apoptotic Bcl-2. Under these conditions, Bim levels were not increased. Findings that full length Bid protein significantly declines in AICD-susceptible replicating blasts, while Bid mRNA does not, suggests that Bid is actively cleaved to short-lived, pro-apoptotic tBid. AICD was diminished, albeit not eliminated, by p53 siRNA transfection, genetic deletion of p53, or Bcl-2 overexpression. DNA damage is a likely trigger for p53-dependent AICD since susceptible lymphoblasts expressed significantly elevated levels of both phospho-ATMser1980 and phospho-H2AXser139. Deficiency in activation-induced cytosine deaminase (AID) diminishes but does not ablate murine B cell AICD, indicating that AID-induced DNA damage is only in part responsible. Evidence for p53-influenced AICD during this route of TI clonal expansion raises the possibility that progeny bearing p53 mutations might undergo positive selection in peripherally inflamed tissues with elevated levels of IL-4 and BAFF.
INTRODUCTION
The mechanisms regulating the growth of antigen-stimulated B cell clones are complex and involve stimuli from surrounding cells and stroma as well as intracellular pathways for controlling cell cycle and survival. T cells are clearly important for B cell clonal expansion and memory cell development and only limited B cell memory evolves in their absence (1–4). To better understand how B cell clonal growth is regulated during T cell independent (TI)4 responses, this laboratory has probed the dynamic process of clonal expansion and ensuing clonal contraction evidenced during the in vitro response of quiescent human B cells to a set of synergistic stimuli: C3d-coated antigen (as a limiting dose of anti-IgM:anti-CD21:dextran) and the cytokines IL-4/IL-13 and BAFF (5, 6). This in vitro model may mimic the response of naïve mature human B cells as they enter inflamed tissues with C3d-coated microbes or self-antigens, e.g. IgG complexes or apoptotic cells, and IL-4 and BAFF-producing cells of the innate immune system: mast cells/basophils/eosinophils and dendritic cells/macrophages, respectively. We have reported that progeny from this response are characterized by elevated levels of CD23, CD86, CD38 and CD27 and sustained expression of CD20 (6). Interestingly, they show minimal evidence of plasmablast differentiation (6, 7) and bear some resemblance to the “marginal zone-like” cells observed within salivary glands of BAFF-overexpressing mice (8) and humans with Sjogren’s Syndrome (9). Importantly, during this TI response, dividing progeny contemporaneously upregulate activation-induced cytosine deaminase (AID) and several proteins of the cyclooxygenase 2 (COX-2) pathway (7). The latter, i.e. COX-2, downstream PGE2 synthase, mPGES-1, and the PGE2 receptor, EP2, contribute at least in part to the progressive rise in AID with each division (7). By day 5 of the response, this TI clonal expansion begins to slow and many of the progeny undergo activation-induced cell death (AICD) (5, 6).
In this study we have examined the mechanisms contributing to clonal contraction of these BCR-triggered, innate immune system-dependent clones. A motivating factor was the potential for valuable insights. Firstly, the study could help illuminate why memory cell formation to TI antigens is impaired, even when pro-survival stimuli from the innate immune system are present. Secondly, they should reveal whether AID-induced DNA damage can contribute to the clonal contraction of TI B cells clones, in a manner similar to that recently reported in responses to TD stimuli (10). Thirdly, pro-apoptotic molecules promoting clonal contraction might be targets for mutation by AID or reactive oxygen species (ROS) generated during clonal expansion. Thus, through understanding the mechanism for clonal contraction, we may be in a better position to understand the etiology of certain B cell disorders characterized by abnormal clonal growth.
Past studies from this laboratory have provided glimpses into possible mechanisms for the demise of human B cell clones during BCR-triggered, innate immune system-driven responses (5, 6). Two findings suggest that mitochondria-dependent intrinsic apoptosis is involved. Firstly, Bcl-2 levels within replicating blasts decline progressively with each division (6), in a manner reminiscent of the low levels of Bcl-2 seen in germinal centers (11, 12). The level of Bcl-2 expressed is inversely related to AICD vulnerability (6). Secondly, when BAFF, APRIL or exogenous PGE2-induced signals are available, dividing cells upregulate Mcl-1, a short-lived Bcl-2 family member, resulting in diminished AICD within replicating blasts (5, 6). Importantly, anti-apoptotic Mcl-1 binds with high affinity to several mitochondrial membrane-disrupting pro-apoptotic molecules, Bim, Puma, and truncated Bid (tBid) (13–16), suggesting that it is an important controller of mitochondria-dependent cell death.
The identity of the pro-apoptotic mediators present in the above BCR-triggered, innate immune system-driven clones remain undefined. While there has been precedent for function of a FOXO3a-driven Bim pathway in promoting B cell AICD (17–20), there are reasons to suspect that a pathway involving DNA-damage-promoted p53 contributes to AICD in certain settings. It may be important that replicating blasts in these BCR-triggered, innate immune system-driven clones display high levels of both AID and a marker of DNA damage: serine 1981-phosphorylated ataxia telangiectasia mutated (ATM) protein (7). ATM autophosphorylation at serine 1981 occurs rapidly at sites of DNA double strand breaks (21) and stabilizes p53 – a protein considered the master guardian of the genome (22). P53 can affect survival through nuclear transactivation of genes encoding death receptors, e.g. Fas and DR5 (23, 24), and genes encoding molecules that induce mitrochondria-dependent apoptosis, Bax, Puma, Bid, and Bad (13, 25–27), as well as through more direct pro-apoptotic functions in the cytoplasm (28). To clarify whether AICD in the above clones is mediated by a FOXO-driven Bim pathway involving cytokine growth factor withdrawal (29) and/or a p53 pathway, the present study began by monitoring Bim and known p53-driven pro-apoptotic molecules within primary lymphoblasts at various stages of a dynamic clonal expansion process. Prompted by our early findings, we have tested the hypothesis that a p53-driven apoptotic pathway contributes to the clonal contraction of BCR-triggered, innate immune system-driven B cell clones.
METHODS
mAb:dex conjugates
Human B cells were activated with a previously described surrogate for C3dg-coated moderately multivalent antigen: a soluble, high MW mAb:dextran conjugate, i.e. anti-human IgM:anti-human CD21:dextran (BCR:CD21-L), generated with either high affinity HB57 or intermediate affinity Mu53 anti-human IgM mAb (30). Cultures were routinely stimulated with mAb:dextran at limiting concentration of 0.01 μg mAb/ml (30, 31). Mouse B cells were activated by a distinct anti-IgM:dextran conjugate (BCR-L) constructed on a high MW (2×106 Da) scaffold of AECM(293)-dextran. For preparation of the latter, AECM-dextran, prepared as previously described (32) (and also available through Fina BioSolutions LLC, Rockville, MD) was covalently linked to streptavidin, via AECM-dextran’s amino groups, using the Lightning-Link Streptavidin Conjugation Kit (Novus Biologicals, Inc. Littleton, CO). Following conjugation, culture grade-BSA (0.2%) was added, the mixture dialyzed in PBS, sterile filtered, and stored until use. At least 30 minutes prior to use in culture, SA-AECM-dextran was pre-mixed with biotinylated rat anti-mouse IgM mAb II/41 (eBioscience catalog number 13-5790-85) yield BCR-L (each was present in culture at a final concentration of 2 μg/ml and 0.5–1 μg/ml, respectively).
Cytokines and culture reagents
The rhBAFF was kindly provided by Dr. S. Kalled (Biogen-Idec) or obtained from Alexis Biochemicals. rhBAFF and rhIL-4 (R&D Systems) were used at concentrations of 50 and 5 ng/ml, respectively. rmBAFF and rmIL-4 (R&D Systems) were used at concentrations of 50 and 10 ng/ml, respectively. PGE2 (Cayman Chemical) was stored as a 2 mM stock in ethyl alcohol at −70°C and diluted in culture medium just before use. Z-VAD-FMK (Sigma-Aldrich) stock (20 mM) was prepared in DMSO and diluted to a final concentration of 40 μM.
B cell sources, purification and culture
Human FO cells: De-identified tonsils from elective tonsillectomy were used according to institutional review board guidelines (with the cooperation of the Department of Pathology, New York Eye and Ear Infirmary, NY, NY and the Department of Pathology, North Shore University Hospital, Manhasset, NY). De-identified spleens were obtained from National Disease Research Interchange and Cooperative Human Tissue Network, processed and stored at −150°C (6). The FO subset designation is here used to represent cells of the conventional mature B cell subset, as distinguished from B1 cells and MZ cells. As we described earlier (5, 6), human FO (B2) cells were selected from human tonsil, or occasionally from normal human spleen, on the basis of their high density, and hence relatively resting state, upon Percoll density gradient centrifugation, as well as their CD27 and CD43 negativity upon depletion of CD27 and CD43 positive cells by magnetic bead separation (Miltenyi Biotech). To monitor replication, FO B cells were labeled with 1 μM CFSE and cultured in 96-well plates at a concentration of 0.5×105 to 105 cells per 200 μl, in an enriched medium (5). For lysate preparation, cells were cultured in 24 well plates at 1 to 3×106 cells/2 ml. In some experiments, purified resting B cells from the peripheral blood of one of the authors was obtained by negative depletion using a magnetic bead purification kit (Miltenyi-Biotech) Mouse B cells: Studies with mice were performed following review and in concordance with institutional animal use and care committee guidelines. Age-matched male wild-type C57BL/6nTac mice and congenic B6.129-Trp53 tmlBrd N12 mice with a homozygous p53 mutation (33) were obtained from Taconic Farms. Mice of the congenic C57BL/6 mouse strain with deletion of gene for AID (AICDA) generated in the laboratory of Dr. Tasuko Honjo (34) (also on the C57BL/6 background) were the kind gift of Dr. Matthew Scharff. Balb/c x EμBcl-2.22 mice (35) with an inserted Bcl-2 transgene (TG) were generously provided by Dr. Betty Diamond. All mouse spleen cell suspensions were subjected to density gradient centrifugation as above to obtain high density lymphocytes. B cells were subsequently purified by negative selection (Miltenyi Biotec B cell isolation kit: 130-090-862), labeled with CFSE and cultured as above.
Assessment of culture viability and replication
Methods described previously were employed (5, 6). In brief, CFSE-labeled cells were harvested into cold PBS, fixed in 1–2% formaldehyde; and analyzed by flow cytometry (FACScan or FACSCalibur with Cell Quest data analysis software or FlowJo 7.6.1 software) following exclusion of very low FSC events (debris), with an exception shown in Figure 1A. The relative yield and relative viability of cells within each division subset were calculated by gating total cells (viable and apoptotic) into various division subsets and subsequently determining % viability within each subset on the basis of FSC/SSC (3).
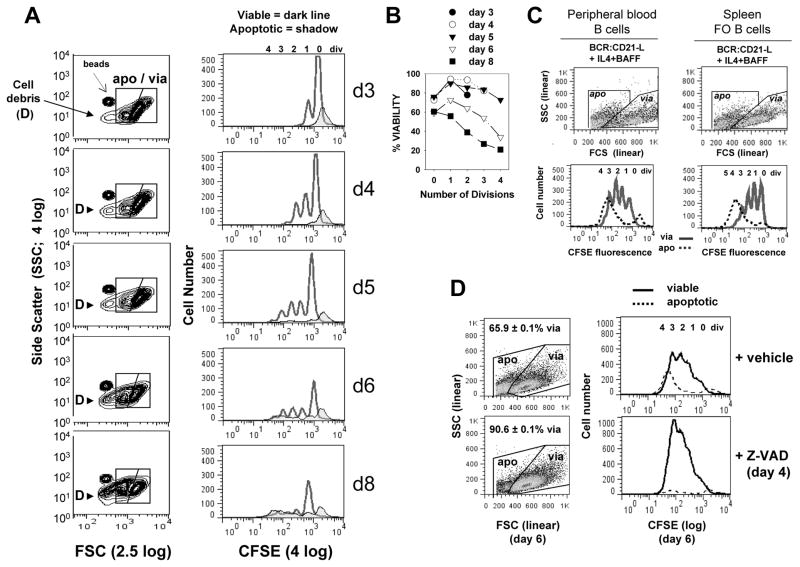
FIGURE 1. Kinetics of caspase-dependent AICD following human B cell clonal expansion in response to surrogate C3d-coated Ag (BCR:CD21-L), IL-4 and BAFF.
(A) CFSE-labeled, resting B cells were cultured with a limiting dose of BCR:CD21-L (0.01 μg/ml) + IL-4 and BAFF. Following varying culture intervals, cultures were pulsed with a known quantity of Autocomp beads, harvested, fixed with 2% formaldehyde, and analyzed by flow cytometry. 20,000 total events were acquired at each time point and FSC and SSC were evaluated on a log scale (left column, Figure 1A). This enabled detection of Autocomp beads (which have a lower FCS than the standardization beads used for assessing cell recovery in all other experiments (CountBright absolute counting beads; Invitrogen)). It also revealed that subcellular particles (debris = D) rise substantially after day 5 (6). For analysis of the divisions represented within intact viable and apoptotic cells (right column), events were gated on the basis of light scatter (as seen in left column) and analyzed for level of CFSE-fluorescence: dark lines = viable, tinted histogram = apoptotic. As described earlier (6), a fraction of undivided cells succumbs to death during the first 2 days of culture (far right apoptotic peak in CFSE histograms). These retain the high levels of CFSE present at the time of their demise and are unlikely related to activation, since they are seen at greater levels within non-stimulated cultures containing medium alone (6). (B) Calculations for % viability within each division subset of total intact cells (viable + apoptotic) were made from the cultures in Figure 1A, as described in our earlier studies (5, 6) and in the present materials and methods. It should be noted that these % viability determinations are not fully accurate because they do not account for the significant number of apoptotic cells which fragment into debris (D) and are excluded from consideration Nevertheless, the % viability calculations are valuable because they demonstrate that survival of highly divided progeny diminishes with time. (Note: % viability as determined by FSC/SSC-based gating of these cultured cells is comparable to % viability determinations on the basis of FITC-annexin V/PI staining (127) or PE-annexin/7-AAD staining (Supplementary Figure 1). (C) CFSE-labeled peripheral blood B cells and splenic FO B cells were cultured with BCR:CD21-L + IL-4 + BAFF for 5 days, gated into apoptotic and viable subsets as shown and evaluated for CFSE fluorescence. (In these and all subsequent experiments, FSC and SSC are plotted on linear scales, with the FSC threshold setting excluding most debris.) Similarly to activated tonsil FO (B2) cells, both these latter populations show evidence of significant AICD in replicating blasts (D) Cultures of activated B cells received a day 4 pulse of pan-caspase inhibitor, Z-VAD-FMK (40 μM), or DMSO vehicle control. Cultures were harvested on day 6, fixed, and analyzed by flow cytometry, Scatter plots to left show the gating of viable and apoptotic cells and the % viability values of replicate cultures within the experiment (mean ± SEM). Histograms to right represent day 6 CFSE fluorescence profiles for viable (solid) and apoptotic (dashed) cells. (Results representative of > 3 exps)
Intracellular staining of cultured CFSE-labeled B cells
Cells were fixed in EM grade formaldehyde (Polysciences Inc.) (2% in PBS, pH 7.2), washed and pre-incubated in PBS-HEPES + 30% heat-inactivated human AB serum + 0.1% saponin, as previously described (6) or exposed to Fix-Perm and Perm-Wash solutions (BD Biosciences). For detection of p53, pATM, and pH2AX cells were exposed to 90% methanol (−20°C for 2 hrs to ON) prior to washing and emersion in permeabilizing buffer (with phosphatase inhibitor in the case of pATM and pH2AX staining). Cells were stained intracellularly by exposure to specific Ab (or with control IgG) for 20–40 min, washed and if needed, exposed to secondary labeled anti-IgG for 20 to 30 min. Washed cells were re-fixed in 2% formaldehyde prior to flow cytometry. Antibodies used for the above assays included: anti-Bid (mouse mAb 7A3; Cell Signaling Technology), anti-p-H2AX (ser139/140) mouse mAb 3F2 (Thermo Scientific), followed by PE-goat F(ab′)2 anti-mouse IgG pre-absorbed against human IgG; anti-p53 [PE-anti-p53 (clone DO-7) and PE-IgG2b mAb control (clone 27–35), BD PharMingen staining kit]; PE-conjugated anti-pATM-ser1981 (mouse mAb 10H11-E12; Millipore) and anti-Mcl-1 (mouse mAb clone 22 BD Pharmingen) or MOPC-21 mouse IgG1 mAb control (directly labeled with Zenon Alexa-Fluor 647-R-PE mouse IgG1 labeling reagent).
Immunoblotting studies
Lysates were prepared in M-PER lysis solution (Pierce) or in RIPA lysis buffer + 1 mM EDTA + protease/phosphatase inhibitor mix (Roche). Lysates were quantified for protein, electrophoresed and blotted onto nitrocellulose or PVDF membranes as described (6). Western blotting was performed with the following: rabbit anti-Bax and rabbit anti-Bim pAbs (BD Pharmingen); rabbit anti-Bad pAb and mouse anti-Bid (mAb 7A3) (Cell Signaling Technology); anti-Puma (rabbit mAb EP512Y; Epitomics); anti-p53 (mouse mAb DO-1) (a kind gift of Dr. Roger Grand); anti-total H2AX (rabbit mAb D17A3; Cell Signaling); anti-phospho-H2AX-S139 (mouse mAb 3F2; Thermo Scientific) and loading control anti-actin mAb (Novus Biologicals) or anti-catalase pAb (6). In each experiment, blots were sequentially stripped and reanalyzed for additional pro-apoptotic molecules and loading control protein. The latter was detected with standard ECL detection reagent; other blots utilized SuperSignal Pico or Femto High Sensitivity Substrate (Pierce). In analysis of Bax, Bad, Bid, Bim and Puma, standardized values of a given protein were obtained as described (36) and then expressed as a % of the maximum level attained within that experiment.
Quantitative RT-PCR of Bim, Bid, Bax, and p53 mRNA levels
For quantitative analysis of mRNA levels within B cells, mRNA was isolated from harvested cells (≥ 106) using a Mini-prep Qiagen kit. From each mRNA, cDNA synthesis was performed using Oligo dT primers from a cDNA kit (Invitrogen). Primers and probes were designed from universal probe library (Roche). Bim: probe # 63 and primer sequence (Forward 5′ gctgtggaggctgaatcc 3′ and Reverse 5′ tcggctgcttggtaattattc 3′). Bid: probe # 44 and primer sequence (Forward 5′ tgtgaaccaggagtgagtcg 3′ and Reverse 5′ ggctggaaccgttgttga 3′). Bax: probe # 55 and primer sequence (Forward 5′ caagaccagggtggttgg 3′ and Reverse 5′ cactcccgccacaaagat 3′). p53: probe # 12 and primer sequence (Forward 5′ aggccttggaactcaaggat 3′ and Reverse 5′ ccctttttggacttcaggtg 3′). Q-RT-PCR was executed at least in triplicates using cDNA as a template with Eurogentec master mix. The conditions for qPCR were as follows: initial uracil-N-glycosylase activation at 50°C for 2 min; HotGoldStar activation (and uracil-N-glycosylase inactivation) at 95°C for 10 min; followed by 45 cycles, each at 95°C for 15 s (denaturation) and 60°C for 1 min (annealing & extension). Fold change was calculated by comparing treated versus untreated groups. Endogenous control for assay was β-actin, used as described previously (10). Data was analyzed using RQ manager version 1.2. Fold change was calculated by comparing treated versus untreated groups.
p53 siRNA treatment
Prior to siRNA transfection, B cells were pre-activated with low dose BCR:CD21-L + IL-4 (± BAFF) for 2–3 d. Recovered cells (in some cases enriched for viable cells by Ficoll-Hypaque centrifugation) were washed, and 1–5×106 cells subjected to nucleofection with 2 μg p53 siRNA (Qiagen validated siRNA Hs_TP53_9) or 2 μg All Stars control siRNA (Qiagen) (or alternatively 1.5 μg siRNA representing p53 Stealth RNAi (TP53HSS110905-7) and GC-rich Stealth Control RNAi (Invitrogen)) using Program U-15 of an Amaxa nucleofection device. Following re-equilibration (6–12h in RPMI + 10% FCS + Glutamax + PenStrep), nucleofected cells were restimulated with BCR:CD21-L + IL-4 + BAFF for an additional 3 days in medium containing Primocin (Amaxa). At that time, cell cultures were assessed by flow cytometry for viability and cell size (light scatter). Cell recovery was determined in most experiments by introducing a known number of standardizing Calibrite beads into each assay tube prior to analysis. In early optimization experiments, nucleofection of Amaxa pmaxGFP plasmid under the above conditions resulted in 40–70% GFP+ cells within the gated viable subset at 24 hrs following nucleofection.
RESULTS
Kinetics of AICD in human FO lymphocytes stimulated by surrogate C3d-coated Ag and innate immune system cytokines
The representative experiment in Figure 1A reveals the time course for the dynamic clonal expansion/contraction response of CFSE-labeled quiescent human tonsil FO (B2) cells upon culture with a limiting dose of surrogate C3d-Ag (anti-IgM:anti-CD21:dextran = BCR:CD21-L) and IL-4 + BAFF. The histograms confirm our prior findings that the greatest proportional yield of viable progeny in this TI response is seen between days 4 and 5 of the response (6). At day 5, apoptotic progeny (shrunken cells with low CFSE) are clearly evident. Following day 5 of activation, the proportion of gated intact apoptotic cells increases with time, as does the detection of fragmented cells of very low FSC (debris = D) (Figure 1A). The rise in debris indicates that % viability values, calculated on the basis of gated intact viable and apoptotic cells (Figure 1B), are undoubtedly an overestimate at late culture intervals. Nevertheless, as we have described earlier (5, 6), through evaluating % viability as a function of division it is readily apparent that AICD affects primarily cells with ≥ 2 divisions (Figure 1B). The AICD of progeny is not unique to tonsil FO cells following in vitro activation, but is also evidenced during BCR:CD21-L + IL-4 + BAFF-induced clonal expansion of purified peripheral blood B cells and isolated splenic FO B cells (Figure 1C) (6). In an earlier study we demonstrated that progeny gated as apoptotic, on the basis of FSC/SSC, express high levels of active caspase 3 (23). The importance of intracellular caspases in mediating the above AICD is presently shown by the significantly greater recovery of viable progeny in cultures pulsed on day 4 with Z-VAD, a pan-caspase inhibitor (Figure 1D).
Importantly, several possibilities for this in vitro AICD have been excluded. The AICD is not due to limiting nutrients following the burst of proliferation: cultures pulsed with CD40-L or with TLR-9-engaging CpG (ODN-2006) manifest considerably greater cycling and substantially lesser AICD through day 7 (data not shown). Furthermore, supplementing cultures with fresh medium or reducing the cell density does not affect AICD (data not shown). Additionally, membrane death receptors, Fas, TNF-R, and DR do not appear relevant: in several experiments, Fas was undetectable by immunostaining with Ab that detected Fas on CD40-L-stimulated cells (36) and AICD was unaffected by neutralizing anti-TNF-α mAb or TRAIL-Fc (data not shown). In the following experiments, we explore other explanations for the declining viability of replicating blasts in these TI clones.
Expression of several pro-apoptotic Bcl-2 family members rises during BCR-triggered, innate immune system-driven clonal expansion
To obtain greater insights into the mechanism for the above AICD, we monitored cultures prior to (day 3–4) and after (day 5–6) AICD was evident for expression of several molecules implicated in intrinsic, mitochondria-dependent apoptosis. This involved immunoblotting and/or immunostaining for FOXO-regulated Bim (37, 38) and p53-regulated Bax, Bid, Bad, Puma and Caspase 6 (13, 25–27). Expression of pro-apoptotic molecules within lysates of cells exposed to low dose BCR:CD21-L plus growth-promoting IL-4 ± BAFF was compared to that within cultures exposed to BCR:CD21-L alone (Figure 2A–D). B cells exposed solely to this low dose of BCR:CD21-L typically manifest sustained viability through day 6, but minimal growth. (6, 31). We additionally examined pro-apoptotic molecules, alongside anti-apoptotic Bcl-2 and Mcl-2, by two-color intracellular immunofluorescent staining of CFSE-labeled cultures (Figure 2E–G). In this case, we compared relative expression of apoptosis-regulating proteins within dividing, apoptosis-vulnerable blasts and non-dividing, apoptosis-resistant blasts (6).
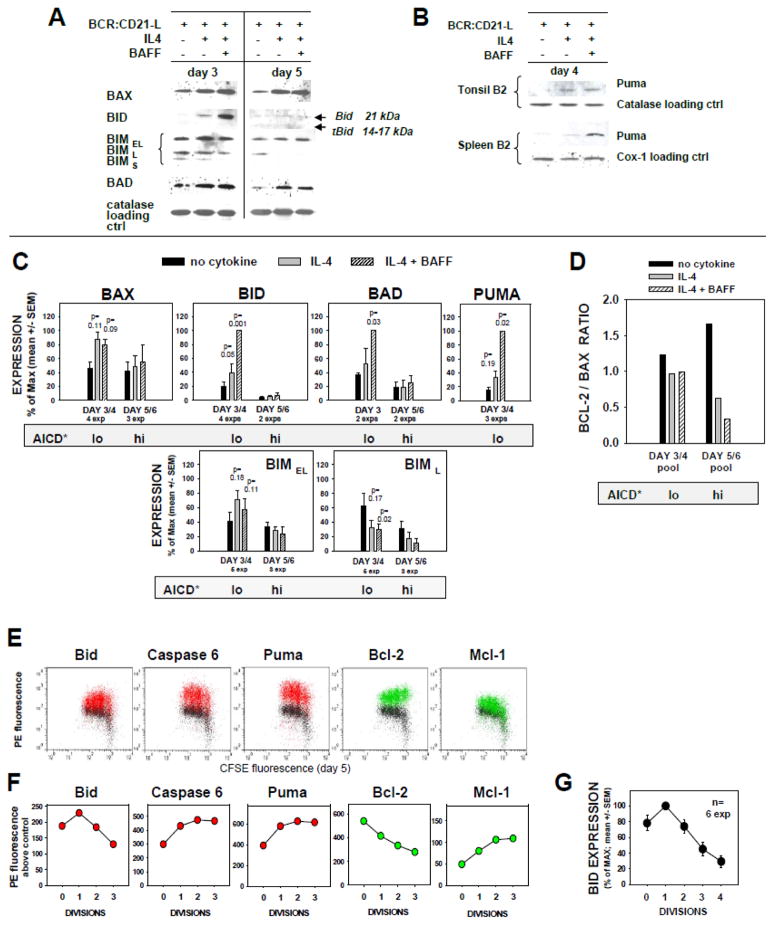
FIGURE 2. Profile of pro- and anti-apoptotic molecules displayed in BCR:CD21-L triggered B cells receiving co-stimuli from IL-4 ± BAFF.
(A–D) Immunoblotting analyses of SDS-PAGE separated proteins in lysates of human FO cells cultured for 3–4 d or 5–6 d with BCR:CD21-L and medium, IL-4, or IL-4 + BAFF. (A) Lysates from a representative single experiment were analyzed for Bax, Bid, Bim, and Bad by sequential stripping and reblotting. In this experiment, d3 and d5 lysates were contemporaneously transferred to different blots, with subsequent simultaneous analysis. (B) Immunoblots from 2 separate experiments evaluating Puma within day 4 lysates. (C) Pooled analysis of the relative expression of Bax, Bid, Bad, Puma, and BimEL and BimL isoforms, within multiple experimental lysates collected at day 3–4 (prior to AICD) and at day 5–6 (AICD evident). Within each experiment, densitometric data for expression of the test molecule and loading control protein was obtained; values for the given test molecule were standardized on the basis of loading control in each experiment and calculated as % of maximum observed; and finally, all the latter values were from multiple experiments are expressed as mean ± SEM. P values for significance from Student’s t test are shown. The shaded bar below each set of pooled data indicates the relative propensity of cultures to display AICD, as shown in Figure 1 and elsewhere (6). (D) Bcl-2/Bax expression ratios were obtained for the differing culture conditions by dividing the mean values for Bcl-2 expression (as % of max from a past analysis (6) of the same lysates here analysed for Bax) by the mean values (% of max) for Bax expression. (E–F) Immunocytofluorimetric analysis of selected pro-apoptotic and anti-apoptotic molecules within CFSE-labeled blasts in 5 day cultures activated by BCR:CD21-L + IL-4 + BAFF. (E) Following intracellular staining with mAb to intact Bid, Caspase 6, Puma (red), or Bcl-2 or Mcl-1 (green), or alternatively IgG control (black), viable-gated cells were analyzed for expression of each protein within cells of differing division status by two color flow cytometry. (F) Shown are the calculated values for PE fluorescence intensity above IgG control background, for each CFSE-determined division. (Results representative of a minimum of 3 exps). (G) Mean ± SEM of % of max values for Bid expression in diverse division subsets (n=6 experiments).
Profile prior to AICD
Despite the relatively high cell viability at day 3–4 in cultures supplemented with growth-promoting cytokines (Figure 1) (6), these cells showed a pronounced upregulation of several pro-apoptotic molecules reported to be downstream of p53, i.e. Bax, Bid, Bad, and Puma (Figure 2A–C). As discussed in an earlier report (6), it is highly likely that apoptosis is thwarted in these day 3 lymphoblasts by the concomitant high expression of several anti-apoptotic members of the Bcl-2 family: Bcl-2, Bcl-xL, and Mcl-1. The levels of FOXO-regulated Bim were not significantly increased. Indeed, the most pro-apoptotic isoforms, BimL and BimS, were less evident in stimulated cultures containing growth-promoting cytokines, than those without (Figure 2A, C bottom row).
Profile when AICD is manifest (days 5–6)
Further insights into the mechanism for AICD came from analyzing the repertoire of expressed pro-apoptotic proteins, in the period after the initial burst of replication. Firstly, Bim levels generally declined in late stage cytokine-supplemented cultures, as compared to cultures with BCR:CD21-L alone (Figure 2A, C bottom row), further suggesting that Bim is not a primary inducer of AICD late in these cultures. This was preceded by significantly diminished Bim mRNA on day 4 (Figure 3). Secondly, both Bax and Bad appear elevated compared to levels detected in cells responding to BCR:CD21-L alone (Figures 2A). Although these differences did not reach statistical significance in the pooled experiments (Figure 2C), we attribute this to (a) the notably greater cell death and ensuing protein degradation within the cytokine-supplemented cultures at this late interval (cells with most elevated levels have succumbed to apoptosis) and (b) the fewer experimental lysates analyzed at this late interval. In support of this conclusion, Bax mRNA was significantly elevated within day 4 cultures (Figure 3), just prior to obvious AICD (Figure 1). Thirdly, pro-apoptotic Puma was not only upregulated at day 4, as assessed by immunoblotting (Figure 2B, C top row), but also strongly expressed within viable day 5 replicating lymphoblasts, as discerned by intracellular staining (Figure 2E and F). Fourthly, Caspase 6, a protease reported to lower the threshold for apoptosis, cleave nuclear lamin, and activate Caspase 8 (27, 39), was found highly expressed in day 5 replicating blasts (Figure 2E & F). Finally, tBid, a truncated highly pro-apoptotic form of Bid (13) appeared to be actively formed within the replicating clones, as detailed below.
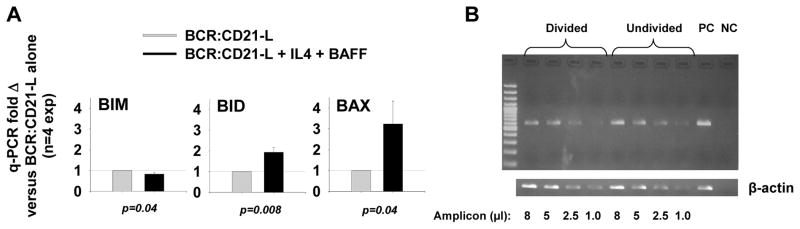
FIGURE 3. Replicating B cells stimulated with BCR:CD21-L + IL-4 + BAFF exhibit elevated mRNA levels of Bid and Bax, but repressed Bim mRNA.
(A) Cultures stimulated with BCR:CD21-L alone or with growth-promoting IL-4 and BAFF were harvested on day 4, mRNA isolated, and cDNA prepared with oligo(dT) primer. Quantitative PCR (q-PCR) was performed with primers specific for Bim, Bid, Bax, and β-actin. Δ Ct values for each of the above were obtained through comparison with β-actin. Values for fold difference (Δ) were obtained by comparing Δ Ct values for each pro-apoptotic mRNA in cytokine-supplemented cultures with the respective Δ Ct values in control cultures with BCR:CD21-L alone. The p values represent the significance of comparisons between Δ values in cytokine-supplemented cultures versus those in control cultures in a total of 4 replicate experiments, using two-tailed Students t test. (B) Bid mRNA levels in divided blasts are comparable to Bid mRNA in undivided blasts. For semi-quantitative RT-PCR of Bid, cells were stimulated for 5 days with BCR:CD21-L (0.01 μg/ml) + IL-4 + BAFF and sorted on the basis of CFSE fluorescence into two populations: undivided blasts and divided blasts (2–5 divisions). mRNA was isolated and cDNA prepared as above. PCR amplification was performed with Bid or β-actin-specific primers. Varying μl amounts of the amplicons were loaded onto 1.5% agarose gels and electrophoresed. PC=positive control and NC=negative control for PCR. (Similar results obtained from a 2nd experiment evaluating levels of Bid mRNA in undivided versus divided blasts).
Evidence for tBid generation in replicating blasts
Although indirect, there is substantial evidence suggesting that Bid is fragmented to tBid during this TI B cell clonal expansion. Firstly, prior to the proliferative burst, the non-apoptotic Bid pro-form (21 kDa) was highly expressed in cytokine-supplemented cultures (particularly those with both IL-4 and BAFF). This major 21 kDa band vanished two days later when AICD was prominent; rather, weak bands approximating tBid (14–17 kDa) were occasionally evident (Figures 2A and C). Difficulty in discerning tBid is expected due to (a) asynchrony of AICD in these cultures, (b) the short intracellular half-life of tBid (< 30 min) (40), (c) size heterogeneity in tBid fragments, due to variable protease cleavage sites (41), and (d) preferential specificity of the immunoblotting mAb for Bid over tBid. A second line of evidence supporting Bid fragmentation to tBid derives from findings that Bid mRNA levels remained high within IL-4 + BAFF-supplemented cultures on day 4, just prior to prominent AICD (Figure 3A). Furthermore, semi-quantitative RT-PCR of RNA from sorted non-divided and divided blasts on day 5 of activation showed Bid mRNA levels to be sustained during division (Figure 3B). A third line of evidence supporting the formation of tBid is that levels of intact Bid significantly decline in viable divided blasts, as compared to the undivided blasts within the same cultures (Figure 2E, F and G). Finally, there clearly exists an inverse relationship between level of intact Bid and lymphoblast susceptibility to AICD: cells with the most extensive division manifest the lowest levels of intact Bid (Figure 2G) and the greatest susceptibility to AICD (5, 6). Taken together, the above findings strongly suggest that cleavage of intact Bid into labile, but highly pro-apoptotic, tBid contributes to the high apoptosis-susceptibility of progeny within these TI human B cell clones.
Significance of diminished Bcl-2 expression for AICD
In the context of a notable upregulation of several pro-apoptotic molecules within replicating blasts, it is warranted to reconsider expression of the opposing anti-apoptotic molecules, namely Bcl-2 and Mcl-1. We have previously shown that Bcl-2 levels precipitously decline with each successive division, while Mcl-1 levels rise, in a BAFF-dependent manner (6). The opposing trends are also evident within the new experiment in Figure 2E and F. Given previous evidence that the Bcl-2 to Bax ratio is strongly linked to B cell survival (42), we here compared levels of Bcl-2 and Bax in cultures before and after AICD had commenced (day 3 versus day 5). Figure 2D shows that whereas the Bcl-2 to Bax ratio in cells exposed only to BCR:CD21-L remains high throughout 5–6 days of culture, this ratio notably drops in cytokine-supplemented cultures beginning to manifest AICD. Thus, despite the BAFF-driven elevation in Mcl-1, the decline in Bcl-2 appears to jeopardize the survival of replicating blasts under these stimulation conditions.
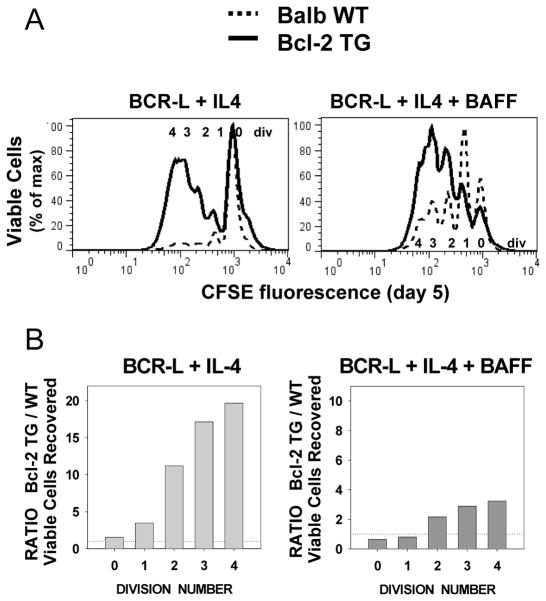
To further test this contention, we established similar culture conditions for stimulating a TI response from B cells from wild-type (WT) and Bcl-2 transgenic mice (35). In these latter experiments, cultures were stimulated with a limiting dose of BCR-L (anti-mouse IgM:dextran at 0.5 to 1 ug/ml) + IL-4 and BAFF. The data in Figure 4A,B clearly demonstrate that presence of the Bcl-2 TG augments the yield of viable day 5 progeny. The bonus effect of Bcl-2 over-expression in these mouse B cell cultures was most prominent in responses to BCR-L + IL-4 (Figure 4A and B, left panels), but nevertheless also evident in responses to BCR-L + IL-4 + BAFF (Figure 4A and B, right panels). Taken together, we conclude that a rise in pro-apoptotic molecules, combined with declining Bcl-2 expression, makes these TI clones highly susceptible to AICD.
FIGURE 4. Over-expression of Bcl-2 augments the recovery of viable lymphoblasts in mouse B cell TI clones.
CFSE-labeled splenic B cells from Balb/c wild-type mice, and Balb/c mice over-expressing Bcl-2, due to insertion of a Bcl-2 TG, were stimulated for 5 days with BCR-L + IL-4 ± BAFF. Cultures were harvested following the addition of standardization beads to each well, fixed and analyzed by flow cytometry. (A) Shown are overlays of CFSE fluorescence within the viable-gated cells recovered from each set of congenic cultures, stimulated under the indicated conditions. (Results representative of two identical experiments). (B) Bcl-2 TG/WT (wild-type) ratio for total viable cell recovery within each division subset. Total viable cell recovery was computed, with the use of Count-bright standardization beads, from each of 6 replicates. A ratio was obtained by dividing mean value for Bcl-2 TG cultures by mean value for WT cultures in a representative experiment.
p53 is upregulated during TI stimulation of human B cells
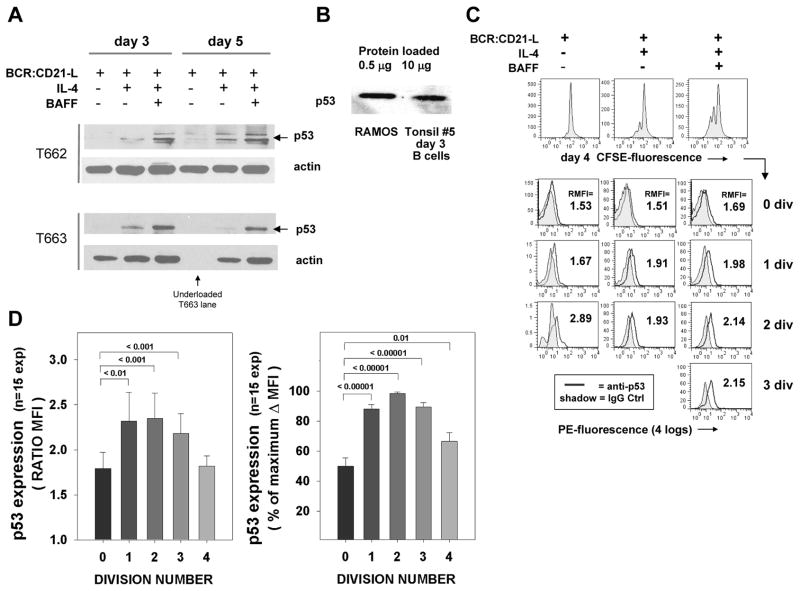
Although levels of p53 protein in normal cells are typically quite low due to ubiquitination of p53 by Mdm2 (43, 44). the evidence for heightened expression of molecules known to be direct transcriptional targets of p53 led us to monitor p53 protein expression. Elevated p53 expression was confirmed though two separate approaches. Firstly, p53 immunoblotting of day 3 and day 5 lysates showed that this transcription factor was significantly upregulated in cytokine-supplemented cultures, as compared to those exposed to BCR:CD21-L or medium alone (Figure 5A). Interestingly, when freshly isolated blots were probed with p53 and subjected to longer periods of exposure, several bands other than the p53 kDa band were evident (as seen for T662). The stack of weak bands above p53 may represent ubiquitinated forms of p53, as a pattern similar to the one we observed was attributed to multimonoubiqutinated forms of p53 in a recent study (45). The band below the major p53 band may be 46–48 kDa protein isoforms of p53 known to represent multiple splice variants, as mAb D01 used for immunoblotting can recognize some of the latter (46). These bands were less evident when the blots were reprobed with less film exposure (as seen with T663). It should be noted that the level of expressed p53 protein in activated normal human B cells was always substantially less than expressed within the Ramos human B cell line (Figure 5B), which bears a mutated, less functional p53 (47).
FIGURE 5. Levels of p53 are upregulated in human B cells triggered by BCR:CD21-L + IL-4 + BAFF.
(A) Immunoblotting evidence for p53. Top: Day 3 and 5 lysates from tonsil FO cells stimulated with BCR:CD21-L ± IL-4 ± BAFF were analyzed for p53 and for β-actin loading control (following stripping and reblotting). A fresh blot and longer film exposure was used for p53 analysis T662 lysates, while a re-stripped blot with shorter film exposure was used for p53 analysis of T663 lysates. (p53 upregulation is representative of 6 experiments). (B) Comparative p53 levels within lysates of the human Ramos B cell line (expressing mutated p53) or day 3 normal human B cell cultures stimulated with BCR:CD21-L + IL-4 + BAFF. Note that the Ramos lysate was loaded at 1/20th of the total protein as the day 3-stimulated normal B cell lysate. (C) Flow cytometric evidence for p53. Top row: histograms representing CFSE-fluorescence in cells stimulated for 4 days with BCR:CD21-L with or without IL-4 ± BAFF, prior to intracellular staining. Subsequent rows: Stimulated cells were gated on the basis of CFSE fluorescence (division status) and assessed for PE fluorescence indicative of p53 staining (line) or IgG2b control staining (tinted histogram). Shown is the ratio of mean fluorescence intensity values (RMFI) obtained when anti-p53 MFI is divided by control MFI. The proportion of viable cells following into the 0, 1, 2, and 3 division subgroups in this experiment were as follows: BCR:CD21-L only: 92, 7, 1, and 0%; BCR:CD21-L + IL-4: 72, 18, 8 and 1%; BCR:CD21-L + IL-4 + BAFF: 49, 27, 19, and 5%, respectively. (D) Pooled data from 15 experiments evaluating the relative expression of p53 within the varying division subsets. The left plot shows data expressed as RMFI; the right plot shows data expressed as % of the maximal MFI above background (Δ MFI) within each experiment. The values above the bars show the p values for level of significance between p53 expression in undivided cells and cells with the indicated division.
Heightened p53 protein in these TI cultures was further evidenced by staining CFSE-labeled cells for intracellular p53 (Figure 5C and D). Both the representative experiment shown in Figure 5C and the results from pooled experiments in Figure 5D indicate that p53 was maximally expressed in divided lymphoblasts. Cytokines clearly augmented p53 expression, but do not appear to be obligatory given that the very infrequent progeny within cultures exposed to BCR:CD21-L alone also showed elevated p53 (Figure 5C). Cells exposed solely to IL-4 did not manifest elevated p53 (data not shown). Taken together, these findings indicate that p53 protein is upregulated within low dose BCR:CD21-L-triggered B cells -- particularly those blasts undergoing IL-4 ± BAFF-driven growth.
p53 loss impairs AICD
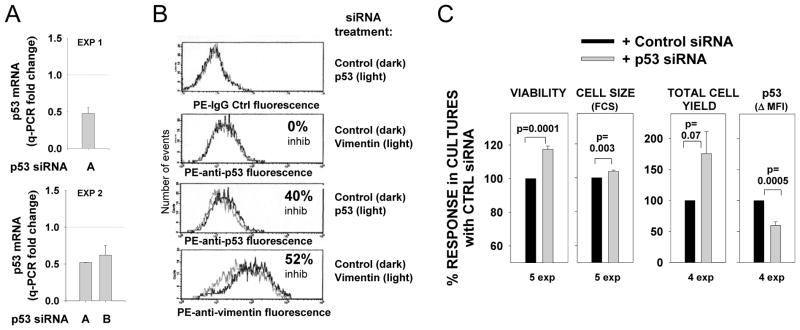
Two approaches were taken to evaluate whether p53 expression contributes to AICD observed under these TI activation conditions. As one approach, we treated activated human B cells with p53 siRNA and control siRNA. Nucleofection of day 2 pre-activated B cells with specific siRNA only partially inhibited p53 expression in re-stimulated B cells, as indicated both by diminished, but not ablated p53 mRNA expression, as assessed by quantitative PCR of cDNA (Figure 6A) and by a 40% decline in p53 protein (as reflected by MFI of PE-anti-p53 intracellular staining) (Figure 6B). Nevertheless, this partial decline in p53 expression was associated with a significant increase in culture viability, cell size, and cell yield (Figure 6B). Treatment with specific vimentin siRNA failed to affect p53 levels, while effectively downregulating vimentin (Figure 6A), and had no effects on cell viability or size, as compared to parallel cultures treated with control siRNA (data not shown). These findings are consistent with a p53 effect on B cell viability and/or division. We were unable to reliably monitor the effects of p53 siRNA transfection on CFSE-labeled cultures given that even control nucleofected cells failed to regain the strong proliferative burst seen in unmanipulated cultures.
FIGURE 6. p53 siRNA treatment of pre-activated human FO cells diminishes p53 protein expression but augments B cell viability, cell size and total cell yield.
Purified FO cells from tonsils or spleens were pre-activated for 2 d (with either BCR:CD21-L, BCR:CD21-L + IL-4, or BCR:CD21-L + IL-4 + BAFF) prior to nucleofection with p53 siRNA, control siRNA (or vimentin siRNA) as described in materials and methods. (A) mRNA was isolated from cells at 24–32 hours post nucleofection. cDNA was prepared and analyzed for p53 and actin levels by q-PCR with specific primers. Values for Δ Ct values were obtained as described for Figure 3A; fold-change was calculated by comparing Δ Ct values within p53 siRNA-treated cultures with respective Δ Ct values in cultures treated with control siRNA. Shown are fold-change values from transfections performed with two distinct activated B cell populations and two different sources of p53 siRNA (mean +/−SEM values from replicate qPCR assays are shown). (B) Cells were harvested at 3 days following nucleofection with the indicated siRNA and fixed, permeabilized and stained intracellularly with PE-anti-p53 antibody, PE-anti-vimentin, or PE-IgG control. Shown is PE fluorescence histograms on a 4-log scale. (C) At 3 to 4 days after nucleofection, fixed cells were analyzed for cell viability (by light scatter), cell size (by FSC of viable subset), and total cell yield (as determined with standardization beads, or in the first experiment, by comparison of “events per minute” during acquisition of cells from control and p53 siRNA-treated cultures). To standardize data from multiple experiments, values for each parameter within an experiment were expressed as a % of the value obtained with control siRNA-treated cells. Shown are the mean ± SEM values of such values. A two-tailed, non-paired Student’s t test was used to compare the responses in cultures treated with p53 siRNA versus control siRNA (p values of significance are shown).
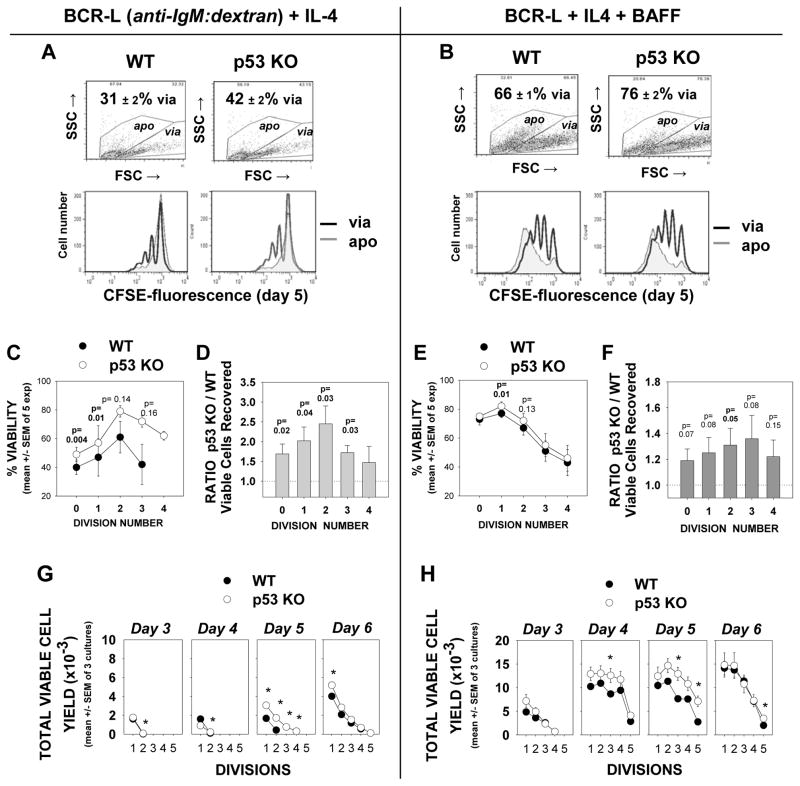
As an alternative test for assessing the functional relevance of p53 in mediating AICD we compared viability and viable cell yield within cultures of CFSE-labeled B cells from p53-deficient (p53 KO) or wild-type mice. Purified high density splenic B cells were stimulated either with mouse-specific BCR-L and IL-4 (Figures 7A,C,D, and G) or with BCR-L + IL-4 and BAFF (Figures 7B,E,F, and H). (In supplementary Figure 2, an experiment testing various BCL-L doses is shown; a dose of 0.5 or 1 ug/ml was used for all subsequent experiments.) These studies with p53 KO B cells consistently showed (5 of 5 experiments) that overall culture viability was increased when p53 was absent (Figure 7A and B). When CFSE-labeled division subsets were analyzed, heightened viability was typically seen at all divisions, but this was statistically significant only in those cells that had divided minimally (Figures 7C & E). Furthermore, the yield of viable lymphoblasts was greater within all division subsets of p53-deficient B cell cultures, as compared to wildtype B cell cultures, at levels that were either statistically significant, or of borderline significance (Figures 7D and F). Thus, overall survival is heightened if B lymphoblasts lack the p53 gene.
FIGURE 7. cultures of p53-deficient mouse B cells exhibit greater viability and greater total lymphoblast yield than similarly activated cultures of wild-type mouse B cells.
CFSE-labeled high density splenic B cells from C57BL/6 WT and p53-KO congenic mice were stimulated for 5 days with BCR-L + IL-4 ± BAFF. Cultures were harvested with standardization beads, fixed and analyzed by flow cytometry for % viability within each division subset, as well as total viable cell recovery, within each gated division subset (6). (A,B) Top dot plots: Comparisons of total culture viability in each of the treated cell groups, on the basis of FSC/SSC gating (mean ± SEM for 5 replicate cultures in an experiment is shown). Bottom histograms: Overlays of CFSE-fluorescence within gated viable (dark line) and apoptotic (grey shadow) cells from cultures of WT or p53-KO B cells in a representative experiment (of five). Cultures were seeded with equivalent numbers of cells and each plot represents a total of 40,000 events collected, using an identical scale for cell number. (C,E) % viability values for each division subset in p53 KO and WT B cell cultures were calculated for each of 5 experiments and expressed as mean ± SEM. p values were calculated with the Student’s t test. Darkened p values show the division subsets where statistical significance was reached. Lighter p values show those of borderline statistical significance. (D,F) p53 deficiency provides a bonus for viable cell yield: Total number of viable cells recovered within each division subset was determined, with the use of standardization beads. A ratio of the total cells recovered in p53 KO cultures versus the recovery in WT cultures was calculated for each experiment. Shown are the mean ± SEM of these values from 5 experiments. P values are shown for the division subsets in which the p53 KO/WT Ratio was significantly (dark) or near-significantly (light) different from the control value of “1”. (G,H) Kinetic analysis of total viable cell yield within p3 KO and WT cultures stimulated with either BCR-L + IL-4 (G) or BCR-L + IL4 and BAFF (H). Note that the greater yield of viable blasts in p53 KO cultures is most significant from days 4–5; by day 6, the number of viable highly divided blasts in the p53 KO cultures drops, possibly reflecting induction of “mitotic catastrophe” as discussed in the text.
Despite the above, it was also evident that at least half of the p53-deficient, highly divided lymphoblasts succumbed to death in cultures optimally stimulated with BCR-L + IL-4 + BAFF. This finding might suggest that p53 has only a minor pro-apoptotic function. Nevertheless, an alternative possibility is that the highly divided lymphoblasts within the p53-deficient cultures are dying by “mitotic catastrophe”. The latter is a death that occurs in cells with defective checkpoints and has been observed in replicating p53-deficient cells during mitosis, due to the lack of p53-imposed cell cycle brakes that permit repair of DNA damage (48–50).
Although not definitive, there is some support for mitotic catastrophe within p53-deficient B cell cultures activated with BCR-L + IL-4 + BAFF. Firstly, the viability bonus attributed to p53-deficiency was less apparent in day 6 cultures, as compared to day 4 and day 5 cultures (Figure 7G and 7H). Secondly, greater levels of Cyclin B1 were evident in a lysate of activated day 5 p53 KO cultures as compared to WT cultures upon immunoblotting (Supplementary Figure 3). Cyclin B1 is characteristically elevated in cells undergoing mitotic catastrophe due to loss of a cell cycle brake in G2/M (51). While, further studies are necessary before we can unequivocally conclude that p53-deficient clones die by mitotic catastrophe, this process termed the “Achilles heel” of p53-deficient cells (50) may contribute to the eventual demise of p53-deficient blasts that avoid earlier apoptosis. Taken together, our results indicate that a p53-mediated pro-apoptotic pathway is at least in part responsible for the reduced viability of lymphoblasts in wild-type cultures.
Viable replicating B lymphoblasts in TI clones express high levels of phosphorylated ATM and H2AX
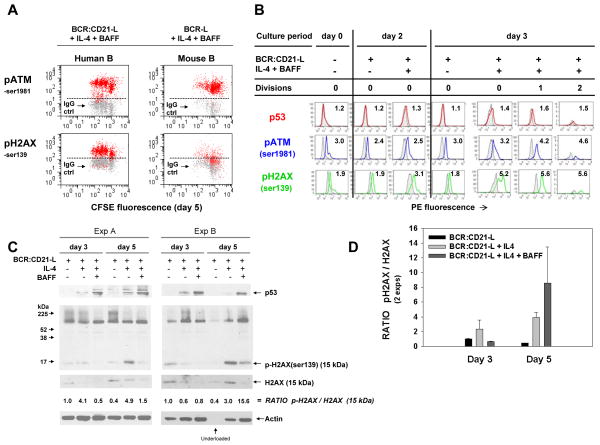
One primary route whereby p53 protein levels rise is through p53 phosphorylation (stabilization) by ATM. Once phosphorylated, p53 is resistant to ubiquitination by Mdm2(52, 53). Thus, recent findings that activated (ser-1981 phosphorylated) ATM is highly upregulated within blasts of these TI cultures (54) is consistent with an active p53 axis. Insomuch as ATM can be activated both through its detection of DNA double strand breaks and through oxidative stress (53), we have here probed TI blasts for a more definitive indicator of DNA damage: phosphorylation of the histone H2AX (55). Results from two representative experiments in which CFSE-labeled blasts in day 5 cultures were stained for pATM-ser1981 and pH2AX-ser139 are shown in Figure 8A (left panels: human B cells activated with BCR:CD21-L + IL-4 + BAFF; right panels: mouse B cells activated with BCR-L + IL-4 + BAFF). While all cells were positive for pATM and pH2AX, dividing lymphoblasts clearly exhibited the highest levels of these phospho-proteins, both in human and mouse cultures.
FIGURE 8. pATM-ser1981 and pH2AX-ser139 expression is elevated in human and mouse B lymphoblasts undergoing TI activation.
(A) Cultures of human and mouse CFSE-labeled B cells were stimulated as indicated, harvested on day 5, and stained intracellularly for molecules indicative of DNA double strand breaks (phosphorylated ATM and H2AX), in the presence of phosphatase inhibitors. The human B cell cultures were pulsed with the pan-caspase inhibitor, Z-VAD (20 μM) on day 4 to diminish apoptosis of activated B cells with DNA damage. (Z-VAD was absent in the mouse cultures.) Plots of human B cells are representative of 4 experiments; plots of mouse B cells are representative of 2 experiments. ATM is known to be autophosphorylated and activated upon binding DNA double stand breaks; activated pATM-ser1981 targets histone H2AX for phosphorylation at ser139 (55, 128). The dotted horizontal line indicate the PE intensity below which are > 95% of the cells stained with IgG control. (B) Comparison of the time course of p53, pATM-ser1981, and pH2AX-ser139 expression in CFSE-labeled cells exposed to BCR:CD21-L alone or in combination with IL-4 and BAFF. Purified unlabeled cells prior to culture, or after CFSE-labeling and 2 and 3 days of culture with the above stimuli, were frozen in an optimal DMSO:FSC-based cell freezing solution until simultaneous defrosting for analysis of p53, pATM, and pH2AX by flow cytometry. Shown are the overlaid histograms of viable-gated cells stained with the indicated specific mAb or with the respective IgG control. Values shown represent RMFI, as calculated in Figure 5. (C) Evidence for upregulation of pH2AX-ser139 by immunoblotting. Lysates from activated cultures were immunoblotted for p53, as in Figure 5A. Following stripping, they were reblotted for the ser-139 phosphorylated form of H2AX, total H2AX, and β-actin. Shown are ratiometric values for the relative degree of H2AX phosphorylation (an indicator of DNA damage) within the various cultures. Not considered in this calculation is an undefined band of high molecular weight which was quite prominent in this and a replicate experiment upon immunoblotting with the anti-pH2AX-ser139 mAb. As discussed in the text, the latter might represent a complex of p-H2AX with other protein(s) known to cluster in areas of DNA damage. It may be of relevance that both experimental sets of lysates, a low ratio in d3 cultures supplemented with both IL-4 and BAFF was accompanied by evidence of a very prominent band between the 52–225 kDa markers in the p-H2AX blots (the intensity of this upper band always exceeded that seen under other stimulation conditions, both at day 3 and day 5.) (D) Bar graph showing ratios of pH2AX densitometric intensity to total H2AX intensity (mean ± SEM of the 15 kDa band in two replicate experiments.)
To better understand the timing of DNA damage within these cultures, B cells were stained for pATM-ser1981 and pH2AX-ser139 both prior to culture and at day 2 to 3 following culture with BCR:CD21-L, with or without supplementary cytokines. The representative experiments in Figure 8B shows that while levels of pH2AX remain low in cultures exposed to BCR:CD21-L alone, expression of this indicator of DNA damage significantly rises in cultures with added IL-4 and BAFF. When a small proportion of blasts have begun to divide (day 3), a subset of undivided cells and all the divided blasts express high levels of pH2AX. Interestingly, pATM was more prominent in freshly isolated cells than pH2AX, perhaps reflecting the capacity of oxidants such as H202 to activate ATM phosphorylation (53). Levels of pATM did not further increase in cells exposed to BCR:CD21-L alone, but rose substantially in cells receiving growth-promoting stimuli from IL-4 and BAFF, particularly in dividing blasts. Taken together, the representative experiment in Figure 8B shows that B cells exhibit some ATM activation even prior to culture, but DNA damage, as shown by pH2AX, is minimal until cells begin showing signs of DNA replication/division.
The immunoblotting evidence in Figures 8C and D provides further support for the conclusion that H2AX phosphorylation is most marked in BCR:CD21-L-triggered B cells exposed to growth-promoting cytokines. While the ratio of pH2AX to total H2AX was elevated in IL-4-supplemented cultures at day 3, evidence for pH2AX phosphorylation was substantially more pronounced at day 5 when many cells have divided. Interestingly, blotting with the anti-pH2AX mAb not only revealed an expected 15 kDa band for H2AX, but also a relatively intense band between the 52 and 225 kDa MW markers. In both experiments, the band was most notable within cultures exposed to IL-4 and BAFF. Although its identity remains unclear, there is a possibility that the high MW band reflects a complex of pH2AX and other protein(s) known to aggregate at sites of DNA damage (56, 57). This possibility is consistent with the decline in 15 kDa total H2AX, as well as 15 kDa pH2AX (as compared to actin loading control) within cultures supplemented with both IL-4 and BAFF (Figure 8C). While a similar high MW band was not noted with the anti-H2AX immunoblotting mAb that recognizes total H2AX (data not shown), it is possible that the epitope engaged by the latter mAb is modified or blocked in the putative complex. Taken together, while the identity of the molecule(s) in the upper MW band is unclear, both the available evidence from intracellular staining and the ratiometric analysis of monomeric (15kDa) pH2AX/H2AX levels show that B cells within cytokine-supplemented cultures exhibit greater pH2AX-ser193 phosphorylation and hence greater DNA damage. Several PI-3K-like kinases, ATM, ATR, and DNA-PK, could be involved in initiating the phosphorylation of the H2AX histone at sites of DNA damage. Evidence of the concomitant upregulation of pATM-ser1981 by both immunostaining (Figure 8A and B) (7) and immunoblotting (Supplementary Figure 4) suggests that activated ATM at a minimum is involved.
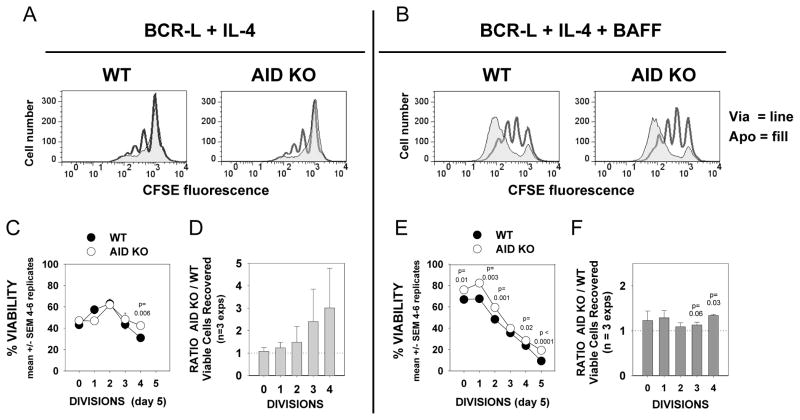
B cells genetically deficient in AID exhibit lesser, but not ablated, AICD
Although H2AX phosphorylation in part undoubtedly reflects DNA strand breaks known to occur during the process of DNA replication (58), the evidence that AID is highly expressed and active within replicating human blasts stimulated within BCR:CD21-L + IL-4 ± BAFF (7) strongly suggests that a component of the evidenced DNA damage reflects AID-induced enzymatic activity. To test the hypothesis that AID function is contributing to AICD within TI clones, we compared the in vitro responses of B cells from wild-type mice and mice with deleted Aicda (AID KO) to a similar mode of TI activation. The data in Figure 9 show that while AICD remains prominent in AID KO cultures, AID deficiency does have a minor, yet reproducible impact on the % viability of highly divided lymphoblasts and their total yield. This is weakly apparent upon the overlay of histograms from viable and apoptotic CFSE-labeled blasts (Figure 9A and B). It is further evidenced by the greater % viability (Figure 9C and E) and greater total viable cell yield (Figure 9D and F) of AID-KO cultures versus wild-type cultures. While in BCR-L + IL-4-activated cultures, the pro-survival effects of AID deficiency are consistently noted only in the most highly divided B lymphoblasts (Figure 9C,D), within cultures exposed to both IL-4 and BAFF, the pro-survival effects of AID deficiency appear to extend to both undivided and divided lymphoblasts (Figure 9E,F). This might relate to the fact that BAFF synergizes with IL-4 in promoting AID expression/activity (7, 59, 60). Taken together, these mouse B cell studies indicate that although AID function contributes to AICD within BCR-triggered, IL-4 and BAFF-driven clones, AID function is not obligatory for AICD of the replicating B lymphoblasts.
FIGURE 9. TI stimulation of AID-deficient mouse B cells yields a slight, but significant, increase in viable lymphoblast recovery as compared to wild-type mouse B cells.
CFSE-labeled high density splenic B cells from C57BL/6 WT and AID-KO congenic mice were cultured at the same density for 5 days with BCR-L + IL-4 ± BAFF. Cultures were processed as in Figure 7. (A,B) Overlays of the CFSE-fluorescence histograms from a representative experiment (of 3) with WT and AID-KO B cells, gated as viable (red) or apoptotic (black). (C and E) % viability values for cells in each gated division subset were calculated for the 4–6 replicates within one experiment. Shown is the intra-experimental mean ± SEM values for % viability in the AID-KO and WT B cell cultures. (p values show that % viability values from the two sets of cultures are significantly different.) (D and F) The total number of recovered viable cells within each division subgroup was determined for each of 3 experiments, and expressed as a ratio of the yield in AID KO versus WT cultures (as in Figure 7). Any bonus in viable cell recovery attributed to AID-deficiency is indicated by a ratio greater than 1. The p values are shown for divisions in which these inter-experimental ratios were (p=0.03), or approached (p=0.06), statistical significance, as compared to WT control values of 1. It should be noted that, although the ratios for infrequent viable cells reflecting 4 divisions was not significantly different in the cultures with BCR-L + IL-4, this reflected the wide spread in ratios between the 3 experiments (1.84, 1.28, and 6.53); in all experiments the AID KO/WT ratio was greater than the WT control value of 1. (Mean ± SEM values for the absolute number of viable cells representing 4 divisions (in 5–6 replicate cultures per each of 3 experiments) were 154 ± 69, 2205 ± 90, and 270 ± 37 (WT cultures) as compared to 284 ± 96, 2816 ± 274, and 1764 ± 222 (AID KO), respectively.
DISCUSSION
The present study provides novel evidence that p53 -- a transcription factor, coined “guardian of the genome and policeman of the oncogenes” (61, 62)–has a functional role in regulating lymphoblast survival during an in vitro TI immune response of mature B lymphocytes. Importantly, the synergistic stimuli which generate susceptible clones, antigen, C3d, IL-4 and BAFF are abundant in certain in vivo settings where B cell foci are prominent (63–65), suggesting these findings may have physiologic relevance. A functional role for p53 in promoting AICD was supported by several lines of data: (a) elevated levels of p53 protein, as well as upregulated mRNA and/or protein of several pro-apoptotic molecules known to be under the positive transcriptional control of p53: Bax, Bad, Puma, Bid, and Caspase 6 (25–27, 66); (b) augmented lymphoblast survival upon p53 siRNA treatment (human) or p53 gene deletion (mouse), and (c) correlation of p53 pathway upregulation with heightened levels of DNA damage. p53 can transactivate genes for pro-apoptotic proteins involved in mitochondria-dependent intrinsic apoptosis as well as genes for membrane death receptors, Fas and DR. Nevertheless, these studies with B lymphoblasts from TI clones showed evidence for the former but not the latter. Such findings are consistent with evidence that p53 transcriptional targeting is highly regulated (67). Importantly, in recent years it has become clear that p53 can also promote mitochondria-dependent apoptosis through its direct functions in the cytoplasm (28, 68, 69). This latter function of p53 may also influence TI clonal contraction. Future studies are needed to clarify its relative importance.
Up to now, direct evidence that p53 influences B lymphoblast survival during a normal immune response has been limited. This is perhaps surprising given the established roles of p53 in regulating cell cycle progression and survival following cell stress in many lineages (62). Certainly, p53 function is anticipated within T cell-dependent germinal centers given the high levels of AID-induced DNA damage and oxidative stress within rapidly replicating centroblasts. Quite consistent with this, a past study by Martinez-Valdez et al. found that germinal centers express elevated p53 protein as well as pro-apoptotic proteins such as Bax (12). Nevertheless, the importance of p53 in such T cell-dependent foci became obscured by other findings suggesting that p53 function within germinal centers was under strong negative control. Firstly, p53 mutations were rare in most B cell malignancies of germinal center origin compared to an average rate of ~ 50% in non-B cell malignancies (70). Secondly, the major germinal center master regulator, Bcl-6, was found to actively repress transcription of p53 mRNA (71), as well as transcription of ATR, a kinase which phosphorylates and stabilizes p53 protein following single-stranded DNA damage (72). Thirdly, there was evidence that FOXO-regulated Bim, but not p53-regulated Bid, was important in germinal center apoptosis (18). Finally, mice with genetic deficiency in p53 readily succumbed to tumors of non-B cell lineages, rather than B cell tumors (73), suggesting that p53 function in B cells is distinctly regulated. A possible reconciling explanation is that transcription from the p53 gene is significantly downregulated within germinal centers (making the p53 gene less vulnerable to mutation), but that low levels of translated p53 protein are significantly stabilized by p53-modifying kinases, ATM and DNA-PKA. The latter are not subject to Bcl-6 repression and are activated by DNA damage/cell stress (21, 74, 75).
Of relevance to TI responses and p53 activity, two earlier studies with p53-deficient murine B cells showed that p53 can slow cell cycle progression in response to LPS and IL-4 (76, 77). Because no difference was observed in the viability of cultured p53-deficient and wild-type B cells at day 3, the authors concluded that p53 did not influence mature B lymphocyte survival. Consistent with the above, we also observed no difference in viability between wild-type and p53-deficient B cells at this early culture interval. Nevertheless, significant differences emerged as clonal expansion progressed through day 5 of the response.
Thus, the present report provides what we believe to be the first direct evidence that p53 has a functional role in regulating B lymphoblast viability during clonal expansion. Findings reported by Hao et al. (78) as this manuscript was in revision are consistent with our conclusions that p53 plays a role in regulating the survival of B cells during TI B cell responses and, furthermore, suggest that this occurs in vivo. In the latter study, mice with genetic ablation of Mule (a ubiquitin ligase which targets p53, as well as other proteins such as Mcl-1 (79)) exhibited significantly diminished levels of serum IgM and IgG3 in response to the TI stimulus, TNP-Ficoll, while exhibiting relatively normal IgM and IgG responses to the TD stimulus, NP-CGG. Interestingly, cultures of mule-deficient B cells yielded lesser highly divided lymphoblasts in response to LPS + IL-4 and showed an abnormal increase in Puma mRNA as compared to WT B cells, which was not apparent in the absence of p53 (78). Taken together, the data from the present study involving both human and mouse B cells and the recent in vivo and in vitro murine studies of Hao et al, (78) strongly argue that p53 has a functional role in regulating B cell survival during a TI clonal expansion.
A predilection for TI B cell clones to succumb to p53-mediated apoptosis a result of sustained DNA damage sheds some light on several past observations. Mice deficient in p53 exhibit an expanded splenic marginal zone and increased incidence of marginal zone lymphoma (73). p53-deficient lymphoblasts switch more effectively to IgG2a than do wild-type B cells, following in vitro stimulation with LPS + cytokines or in vivo stimulation of mice with polyoma virus (80). Ig-associated gene translocations occur more frequently in LPS + IL-4-stimulated B cell cultures from p53-deficient mice than in similarly stimulated normal B cells or B cells from mice genetically deficient in both p53 and AID (81). Finally, in humans, p53 mutations are more common in malignancies that likely originate outside of germinal centers: chronic lymphocytic leukemia (B-CLL) and related pro-lymphocytic leukemia (70, 82–84), mucosa-associated lymphoid tissue (MALT) lymphoma (70, 85, 86), and MZ lymphoma (87, 88).
In the present model for TI B cell clonal expansion, it appears likely that Puma and tBid are the important triggers for the oligomerization of apoptosis-inducers, Bax or Bak, within the mitochondrial membrane and ensuing AICD (89). Although the evidence for Puma involvement is only indirect, other recent evidence shows that Puma has a critical role in determining the fate of mitogen-stimulated B cells in vitro and the development of B cell memory in vivo (16). Interestingly, while Puma is transcriptionally upregulated by p53 upon DNA damage, it can also be upregulated during cytokine growth withdrawal by FOXO3a (29). The latter pathway may be less relevant in our IL-4 and BAFF-supplemented cultures given that mRNA and protein for another FOXO3a-regulated molecule, the apoptosis-activator Bim (90), is downregulated in these cultures. Diminished Bim mRNA may represent optimal activation of the PI-3K/Akt pathway by IL-4 (91) since Akt phosphorylates FOXO3a and promotes its exclusion from the nucleus (92, 93). Consistent with the above interpretation, lysates of cultures stimulated with BCR:CD21-L + BAFF, in the absence of IL-4, expressed significantly greater levels of the various Bim isoforms (P. Mongini, unpublished observations).
The evidence for involvement of tBid in promoting AICD is also indirect, yet worth noting. Although tBid levels were difficult to reliably observe, intact Bid protein (but not mRNA) precipitously declined during successive divisions within IL-4 and BAFF-supplemented cultures. Bid cleavage to the highly pro-apoptotic, labile tBid can be achieved by number of proteases, e.g. caspases 8 and 10, calpain, granzyme B, and cathepsins (94). Importantly, all have been reported in activated B cells (95–97). Thus, although in vivo studies with Bid-deficient mice suggest that tBid has minimal to no effect on germinal center formation (18), there is a strong possibility that this molecule is relevant in precipitating the clonal contraction of TI clones.
In the context of Puma and tBid, it is undoubtedly relevant that Mcl-1 can bind both with high affinity and thereby block intrinsic apoptosis (13, 98). The significance of Mcl-1 is made more apparent by the precipitous decline of Bcl-2. While the latter anti-apoptotic protein is highly expressed in resting B cells and non-dividing activated blasts, its levels drop with each successive division (present study and (6)). This phenomenon may help explain why an in vivo-expressed Bcl-2 transgene helped prevent deletion of autoreactive mouse B cells within peripheral tissues (99, 100) and, furthermore, had a greater effect at augmenting B cell memory to TI antigens, as compared to TD antigens (101). Prior findings from this laboratory suggest that deletion of autoreactive B cells by p53-driven AICD might be averted and TI memory increased in tissues with elevated concentrations of Mcl-1-augmenting BAFF, APRIL and PGE2 (5). Such a scenario might in part explain why salivary gland-targeted Sjogren’s Syndrome is highly linked to APRIL titers (102) and why SLE autoantibody titers decline in mice treated with inhibitors of Mcl-1-upregulating COX-2 (103).
It warrants noting that p53 has been implicated in the negative regulation of Bcl-2 both through direct repressive effects at the Bcl-2 promoter (104) and through induction of Bcl-2 inhibitory micro-RNA: miR-15, miR-16, and miR-34 (105–107). Nevertheless, because Bcl-2 is subject to other means of regulation (108–110), one cannot conclude that the decline in Bcl-2 reflects an augmented p53 axis. This will require further study.
In this study, we made efforts to replicate the human TI response to low dose BCR:CD21-L + IL-4 ± BAFF with mouse cultures stimulated with higher dose BCR-L + IL-4 ± BAFF in order to more readily study the effects of AID on the process of AICD. The resulting experiments showed that AID function only slightly contributes to the clonal contraction of mouse B cell clones. Interestingly, while reduced AICD in p53 deficient cultures extended to all division subsets, the protective effect of AID deletion was noted predominantly in the most highly divided cells. This is consistent with the strong linkage between AID expression/function and cell division (7, 111).
There is a strong possibility that the present findings with mouse B cells do not adequately mirror the AICD-inducing effects of functional AID within human B cell clones. Firstly, dividing mouse lymphoblasts recovered from BCR-L, IL-4 and BAFF-activated cultures manifest little, if any, intracellular staining with a anti-mouse AID mAb (clone ZA001) (H. Lee and P. Mongini, unpublished results), while replicating blasts of human B cell cultures show high AID expression, upon staining with the same mAb or an additional human AID-specific rat mAb (7). One possible explanation for these findings is that stronger BCR engagement in the mouse B cell cultures may have dampened AID transcription (112). (Mouse cultures were stimulated with 1 μg/ml of anti-IgM:dextran, while human B cell cultures were stimulated with 0.01 μg/ml of anti-IgM:anti-CD21:dextran). Secondly, in addition to diminished AID protein, AID function is less evident in the mouse cultures than human cultures: substantially fewer IgG+ switched progeny are typically recovered from the above TI-activated mouse B cell cultures (P. Mongini, unpublished results) than from activated human B cell cultures (7). Thus, taken together, it remains quite possible that AID activity has a significantly greater contribution to p53-mediated AICD within the studied TI human B cell clones than revealed in the present mouse cultures. Is it important to note that replicating mouse B cells are not resistant to AICD following AID-induced DNA damage. Zaheen et al. recently demonstrated that AID activity significantly constrains clonal size within BCR and CD40-stimulated mouse B cell cultures (10).
In conclusion, the present evidence that a p53 axis governs the survival of replicating B lymphoblasts during the above TI response may illuminate the stimulatory environment where pre-malignant or transformed B cells with altered genes of the p53 axis arise. We propose that inflamed peripheral tissues laden with C3dg-coated foreign or self antigen and IL-4(IL-13)- and BAFF-releasing cells of the innate immune system (63, 113–119) may be such a milieu. In these sites, TI B cell clonal expansion would be promoted, but there would be strong positive selection pressure for B lymphocytes to escape p53-mediated AICD. Both the high AID expression/activity elicited by these stimuli (7) as well as the oxidative stress of the proliferative burst should induce mutations. Those within p53 and other genes of the p53 axis will be selected for and may represent one step in a series toward full malignancy, or alternatively the defining event. Such a stimulatory milieu might explain why the p53 gene is deleted in ~ 30% of patients with benign CD5− monoclonal B cell lymphocytosis (120). It might also help explain why a high proportion of CD5+ B-CLL clones display alterations in genes encoding p53 pathway proteins (84, 121, 122) and/or have lost one of several p53-regulated miRNA genes that negatively control Bcl-2 (123–126).
Supplementary Material
Acknowledgments
This study is dedicated to the memory of the late Dr. Leon T. Rosenberg, who inspired both creative thought and tenacity and remained captivated by life’s complexities to his end.
We are grateful to Dr. Betty Diamond for the Bcl-2 mice and Dr. Matthew Scharff for the AID-KO mice used in the study. We additionally acknowledge the assistance of Bill Kennedy in obtaining tonsil specimens. Comments from Dr. Tatjana Stankovic regarding mitotic catastrophe and Drs. Janet Stavnezer and Betty Diamond concerning the manuscript were most useful.
Footnotes
Work was supported in part by NIH R01 AI052189, M01 General Clinical Research Center Grant (RR018535) from the National Center for Research Resources, Laboratory of Immunology, NIAID intramural program, and grants from The Karches Foundation, the Peter Jay Sharp Foundation, and the Marks Family Foundation.
Non-standard abbreviations: AICD (activation-induced cell death); AID (activation-induced cytosine deaminase); BCR:CD21-L (anti-IgM:anti-CD21:dextran conjugate); BCR-L (anti-IgM:dextran conjugate); cyclooxygenase 2 (COX-2); prostaglandin E2 (PGE2); ROS (reactive oxygen species)
References
- 1.de Vinuesa CG, Cook MC, Ball J, Drew M, Sunners Y, Cascalho M, Wabl M, Klaus GG, MacLennan IC. Germinal centers without T cells. J Exp Med. 2000;191:485–494. doi: 10.1084/jem.191.3.485. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.El Shikh ME, El Sayed RM, Szakal AK, Tew JG. T-independent antibody responses to T-dependent antigens: a novel follicular dendritic cell-dependent activity. J Immunol. 2009;182:3482–3491. doi: 10.4049/jimmunol.0802317. [DOI] [PubMed] [Google Scholar]
- 3.Baumgarth N. A two-phase model of B-cell activation. Immunol Rev. 2000;176:171–180. doi: 10.1034/j.1600-065x.2000.00606.x. [DOI] [PubMed] [Google Scholar]
- 4.Defrance T, Taillardet M, Genestier L. T cell-independent B cell memory. Curr Opin Immunol. 2011;23:330–336. doi: 10.1016/j.coi.2011.03.004. [DOI] [PubMed] [Google Scholar]
- 5.Mongini PK, Inman JK, Han H, Fattah RJ, Abramson SB, Attur M. APRIL and BAFF promote increased viability of replicating human B2 cells via mechanism involving cyclooxygenase 2. J Immunol. 2006;176:6736–6751. doi: 10.4049/jimmunol.176.11.6736. [DOI] [PubMed] [Google Scholar]
- 6.Mongini PK, Inman JK, Han H, Kalled SL, Fattah RJ, McCormick S. Innate immunity and human B cell clonal expansion: effects on the recirculating B2 subpopulation. J Immunol. 2005;175:6143–6154. doi: 10.4049/jimmunol.175.9.6143. [DOI] [PubMed] [Google Scholar]
- 7.Lee H, Trott JS, Haque S, McCormick S, Chiorazzi N, Mongini PK. A cyclooxygenase-2/prostaglandin E2 pathway augments activation-induced cytosine deaminase expression within replicating human B cells. J Immunol. 2010;185:5300–5314. doi: 10.4049/jimmunol.1000574. [DOI] [PubMed] [Google Scholar]
- 8.Groom J, Kalled SL, Cutler AH, Olson C, Woodcock SA, Schneider P, Tschopp J, Cachero TG, Batten M, Wheway J, Mauri D, Cavill D, Gordon TP, Mackay CR, Mackay F. Association of BAFF/BLyS overexpression and altered B cell differentiation with Sjogren’s syndrome. J Clin Invest. 2002;109:59–68. doi: 10.1172/JCI14121. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Daridon C, Pers JO, Devauchelle V, Martins-Carvalho C, Hutin P, Pennec YL, Saraux A, Youinou P. Identification of transitional type II B cells in the salivary glands of patients with Sjogren’s syndrome. Arthritis Rheum. 2006;54:2280–2288. doi: 10.1002/art.21936. [DOI] [PubMed] [Google Scholar]
- 10.Zaheen A, Boulianne B, Parsa JY, Ramachandran S, Gommerman JL, Martin A. AID constrains germinal center size by rendering B cells susceptible to apoptosis. Blood. 2009;114:547–554. doi: 10.1182/blood-2009-03-211763. [DOI] [PubMed] [Google Scholar]
- 11.Krajewski S, Bodrug S, Krajewska M, Shabaik A, Gascoyne R, Berean K, Reed JC. Immunohistochemical analysis of Mcl-1 protein in human tissues. Differential regulation of Mcl-1 and Bcl-2 protein production suggests a unique role for Mcl-1 in control of programmed cell death in vivo. Am J Pathol. 1995;146:1309–1319. [PMC free article] [PubMed] [Google Scholar]
- 12.Martinez-Valdez H, Guret C, de Bouteiller O, Fugier I, Banchereau J, Liu YJ. Human germinal center B cells express the apoptosis-inducing genes Fas, c-myc, P53, and Bax but not the survival gene bcl-2. J Exp Med. 1996;183:971–977. doi: 10.1084/jem.183.3.971. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Clohessy JG, Zhuang J, de Boer J, Gil-Gomez G, Brady HJ. Mcl-1 interacts with truncated Bid and inhibits its induction of cytochrome c release and its role in receptor-mediated apoptosis. J Biol Chem. 2006;281:5750–5759. doi: 10.1074/jbc.M505688200. [DOI] [PubMed] [Google Scholar]
- 14.Opferman JT, Letai A, Beard C, Sorcinelli MD, Ong CC, Korsmeyer SJ. Development and maintenance of B and T lymphocytes requires antiapoptotic MCL-1. Nature. 2003;426:671–676. doi: 10.1038/nature02067. [DOI] [PubMed] [Google Scholar]
- 15.Han J, Goldstein LA, Hou W, Gastman BR, Rabinowich H. Regulation of mitochondrial apoptotic events by p53-mediated disruption of complexes between antiapoptotic Bcl-2 members and Bim. J Biol Chem. 2010;285:22473–22483. doi: 10.1074/jbc.M109.081042. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Clybouw C, Fischer S, Auffredou MT, Hugues P, Alexia C, Bouillet P, Raphael M, Leca G, Strasser A, Tarlinton DM, Vazquez A. Regulation of memory B-cell survival by the BH3-only protein Puma. Blood. 2011 doi: 10.1182/blood-2011-04-347096. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Craxton A, Draves KE, Gruppi A, Clark EA. BAFF regulates B cell survival by downregulating the BH3-only family member Bim via the ERK pathway. J Exp Med. 2005;202:1363–1374. doi: 10.1084/jem.20051283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Fischer SF, Bouillet P, O’Donnell K, Light A, Tarlinton DM, Strasser A. Proapoptotic BH3-only protein Bim is essential for developmentally programmed death of germinal center-derived memory B cells and antibody-forming cells. Blood. 2007;110:3978–3984. doi: 10.1182/blood-2007-05-091306. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Goodyear CS, Corr M, Sugiyama F, Boyle DL, Silverman GJ. Cutting Edge: Bim is required for superantigen-mediated B cell death. J Immunol. 2007;178:2636–2640. doi: 10.4049/jimmunol.178.5.2636. [DOI] [PubMed] [Google Scholar]
- 20.Mouhamad S, Besnault L, Auffredou MT, Leprince C, Bourgeade MF, Leca G, Vazquez A. B cell receptor-mediated apoptosis of human lymphocytes is associated with a new regulatory pathway of Bim isoform expression. J Immunol. 2004;172:2084–2091. doi: 10.4049/jimmunol.172.4.2084. [DOI] [PubMed] [Google Scholar]
- 21.So S, Davis AJ, Chen DJ. Autophosphorylation at serine 1981 stabilizes ATM at DNA damage sites. J Cell Biol. 2009;187:977–990. doi: 10.1083/jcb.200906064. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Menendez D, Shatz M, Azzam K, Garantziotis S, Fessler MB, Resnick MA. The Toll-like receptor gene family is integrated into human DNA damage and p53 networks. PLoS Genet. 2011;7:e1001360. doi: 10.1371/journal.pgen.1001360. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Muller M, Wilder S, Bannasch D, Israeli D, Lehlbach K, Li-Weber M, Friedman SL, Galle PR, Stremmel W, Oren M, Krammer PH. p53 activates the CD95 (APO-1/Fas) gene in response to DNA damage by anticancer drugs. J Exp Med. 1998;188:2033–2045. doi: 10.1084/jem.188.11.2033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Takimoto R, El-Deiry WS. Wild-type p53 transactivates the KILLER/DR5 gene through an intronic sequence-specific DNA-binding site. Oncogene. 2000;19:1735–1743. doi: 10.1038/sj.onc.1203489. [DOI] [PubMed] [Google Scholar]
- 25.Strasser A. The role of BH3-only proteins in the immune system. Nat Rev Immunol. 2005;5:189–200. doi: 10.1038/nri1568. [DOI] [PubMed] [Google Scholar]
- 26.Li CQ, Robles AI, Hanigan CL, Hofseth LJ, Trudel LJ, Harris CC, Wogan GN. Apoptotic signaling pathways induced by nitric oxide in human lymphoblastoid cells expressing wild-type or mutant p53. Cancer Res. 2004;64:3022–3029. doi: 10.1158/0008-5472.can-03-1880. [DOI] [PubMed] [Google Scholar]
- 27.MacLachlan TK, El-Deiry WS. Apoptotic threshold is lowered by p53 transactivation of caspase-6. Proc Natl Acad Sci U S A. 2002;99:9492–9497. doi: 10.1073/pnas.132241599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Green DR, Kroemer G. Cytoplasmic functions of the tumour suppressor p53. Nature. 2009;458:1127–1130. doi: 10.1038/nature07986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.You H, Pellegrini M, Tsuchihara K, Yamamoto K, Hacker G, Erlacher M, Villunger A, Mak TW. FOXO3a-dependent regulation of Puma in response to cytokine/growth factor withdrawal. J Exp Med. 2006;203:1657–1663. doi: 10.1084/jem.20060353. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Mongini PK, Vilensky MA, Highet PF, Inman JK. The affinity threshold for human B cell activation via the antigen receptor complex is reduced upon co-ligation of the antigen receptor with CD21 (CR2) J Immunol. 1997;159:3782–3791. [PubMed] [Google Scholar]
- 31.Mongini PK, Tolani S, Fattah RJ, Inman JK. Antigen receptor triggered upregulation of CD86 and CD80 in human B cells: augmenting role of the CD21/CD19 co-stimulatory complex and IL-4. Cell Immunol. 2002;216:50–64. doi: 10.1016/s0008-8749(02)00512-9. [DOI] [PubMed] [Google Scholar]
- 32.Mongini PK, Blessinger CA, Highet PF, Inman JK. Membrane IgM-mediated signaling of human B cells. Effect of increased ligand binding site valency on the affinity and concentration requirements for inducing diverse stages of activation. J Immunol. 1992;148:3892–3901. [PubMed] [Google Scholar]
- 33.Donehower LA, Harvey M, Slagle BL, McArthur MJ, Montgomery CA, Jr, Butel JS, Bradley A. Mice deficient for p53 are developmentally normal but susceptible to spontaneous tumours. Nature. 1992;356:215–221. doi: 10.1038/356215a0. [DOI] [PubMed] [Google Scholar]
- 34.Muramatsu M, Kinoshita K, Fagarasan S, Yamada S, Shinkai Y, Honjo T. Class switch recombination and hypermutation require activation-induced cytidine deaminase (AID), a potential RNA editing enzyme. Cell. 2000;102:553–563. doi: 10.1016/s0092-8674(00)00078-7. [DOI] [PubMed] [Google Scholar]
- 35.Strasser A, Whittingham S, Vaux DL, Bath ML, Adams JM, Cory S, Harris AW. Enforced BCL2 expression in B-lymphoid cells prolongs antibody responses and elicits autoimmune disease. Proc Natl Acad Sci U S A. 1991;88:8661–8665. doi: 10.1073/pnas.88.19.8661. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Mongini PK, Jackson AE, Tolani S, Fattah RJ, Inman JK. Role of complement-binding CD21/CD19/CD81 in enhancing human B cell protection from Fas-mediated apoptosis. J Immunol. 2003;171:5244–5254. doi: 10.4049/jimmunol.171.10.5244. [DOI] [PubMed] [Google Scholar]
- 37.Huntington ND, Puthalakath H, Gunn P, Naik E, Michalak EM, Smyth MJ, Tabarias H, Degli-Esposti MA, Dewson G, Willis SN, Motoyama N, Huang DC, Nutt SL, Tarlinton DM, Strasser A. Interleukin 15-mediated survival of natural killer cells is determined by interactions among Bim, Noxa and Mcl-1. Nat Immunol. 2007;8:856–863. doi: 10.1038/ni1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Amin AR, Paul RK, Thakur VS, Agarwal ML. A novel role for p73 in the regulation of Akt-Foxo1a-Bim signaling and apoptosis induced by the plant lectin, Concanavalin A. Cancer Res. 2007;67:5617–5621. doi: 10.1158/0008-5472.CAN-07-0655. [DOI] [PubMed] [Google Scholar]
- 39.Inoue S, Browne G, Melino G, Cohen GM. Ordering of caspases in cells undergoing apoptosis by the intrinsic pathway. Cell Death Differ. 2009;16:1053–1061. doi: 10.1038/cdd.2009.29. [DOI] [PubMed] [Google Scholar]
- 40.Breitschopf K, Zeiher AM, Dimmeler S. Ubiquitin-mediated degradation of the proapoptotic active form of bid. A functional consequence on apoptosis induction. J Biol Chem. 2000;275:21648–21652. doi: 10.1074/jbc.M001083200. [DOI] [PubMed] [Google Scholar]
- 41.Billen LP, Shamas-Din A, Andrews DW. Bid: a Bax-like BH3 protein. Oncogene. 2008;27(Suppl 1):S93–104. doi: 10.1038/onc.2009.47. [DOI] [PubMed] [Google Scholar]
- 42.Pepper C, Hoy T, Bentley DP. Bcl-2/Bax ratios in chronic lymphocytic leukaemia and their correlation with in vitro apoptosis and clinical resistance. Br J Cancer. 1997;76:935–938. doi: 10.1038/bjc.1997.487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Vassilev LT, Vu BT, Graves B, Carvajal D, Podlaski F, Filipovic Z, Kong N, Kammlott U, Lukacs C, Klein C, Fotouhi N, Liu EA. In vivo activation of the p53 pathway by small-molecule antagonists of MDM2. Science. 2004;303:844–848. doi: 10.1126/science.1092472. [DOI] [PubMed] [Google Scholar]
- 44.Brown CJ, Lain S, Verma CS, Fersht AR, Lane DP. Awakening guardian angels: drugging the p53 pathway. Nat Rev Cancer. 2009;9:862–873. doi: 10.1038/nrc2763. [DOI] [PubMed] [Google Scholar]
- 45.Thalappilly S, Feng X, Pastyryeva S, Suzuki K, Muruve D, Larocque D, Richard S, Truss M, von Deimling A, Riabowol K, Tallen G. The p53 Tumor Suppressor Is Stabilized by Inhibitor of Growth 1 (ING1) by Blocking Polyubiquitination. PLoS One. 2011;6:e21065. doi: 10.1371/journal.pone.0021065. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Bourdon JC, Fernandes K, Murray-Zmijewski F, Liu G, Diot A, Xirodimas DP, Saville MK, Lane DP. p53 isoforms can regulate p53 transcriptional activity. Genes Dev. 2005;19:2122–2137. doi: 10.1101/gad.1339905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Farrell PJ, Allan GJ, Shanahan F, Vousden KH, Crook T. p53 is frequently mutated in Burkitt’s lymphoma cell lines. EMBO J. 1991;10:2879–2887. doi: 10.1002/j.1460-2075.1991.tb07837.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Fragkos M, Beard P. Mitotic catastrophe occurs in the absence of apoptosis in p53-null cells with a defective G1 checkpoint. PLoS One. 2011;6:e22946. doi: 10.1371/journal.pone.0022946. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Vakifahmetoglu H, Olsson M, Zhivotovsky B. Death through a tragedy: mitotic catastrophe. Cell Death Differ. 2008;15:1153–1162. doi: 10.1038/cdd.2008.47. [DOI] [PubMed] [Google Scholar]
- 50.Levesque AA, Eastman A. p53-based cancer therapies: Is defective p53 the Achilles heel of the tumor? Carcinogenesis. 2007;28:13–20. doi: 10.1093/carcin/bgl214. [DOI] [PubMed] [Google Scholar]
- 51.Ianzini F, Bertoldo A, Kosmacek EA, Phillips SL, Mackey MA. Lack of p53 function promotes radiation-induced mitotic catastrophe in mouse embryonic fibroblast cells. Cancer Cell Int. 2006;6:11. doi: 10.1186/1475-2867-6-11. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Harris SL, Gil G, Robins H, Hu W, Hirshfield K, Bond E, Bond G, Levine AJ. Detection of functional single-nucleotide polymorphisms that affect apoptosis. Proc Natl Acad Sci U S A. 2005;102:16297–16302. doi: 10.1073/pnas.0508390102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Guo Z, Kozlov S, Lavin MF, Person MD, Paull TT. ATM activation by oxidative stress. Science. 2010;330:517–521. doi: 10.1126/science.1192912. [DOI] [PubMed] [Google Scholar]
- 54.Mongini PKA, Chiorazzi Nicholas, Haque Shabirul, Trott Joshua. PGE2 upregulates activation-induced cytosine deaminase (AID) in human B lymphoblasts. The Journal of Immunology. 2009:183. [Google Scholar]
- 55.Tanaka T, Huang X, Halicka HD, Zhao H, Traganos F, Albino AP, Dai W, Darzynkiewicz Z. Cytometry of ATM activation and histone H2AX phosphorylation to estimate extent of DNA damage induced by exogenous agents. Cytometry A. 2007;71:648–661. doi: 10.1002/cyto.a.20426. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Chowdhury D, Keogh MC, Ishii H, Peterson CL, Buratowski S, Lieberman J. gamma-H2AX dephosphorylation by protein phosphatase 2A facilitates DNA double-strand break repair. Mol Cell. 2005;20:801–809. doi: 10.1016/j.molcel.2005.10.003. [DOI] [PubMed] [Google Scholar]
- 57.Krum SA, la Rosa Dalugdugan E, Miranda-Carboni GA, Lane TF. BRCA1 Forms a Functional Complex with gamma-H2AX as a Late Response to Genotoxic Stress. J Nucleic Acids. 2010;2010 doi: 10.4061/2010/801594. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Ward IM, Chen J. Histone H2AX is phosphorylated in an ATR-dependent manner in response to replicational stress. J Biol Chem. 2001;276:47759–47762. doi: 10.1074/jbc.C100569200. [DOI] [PubMed] [Google Scholar]
- 59.Yamada T, Zhang K, Yamada A, Zhu D, Saxon A. B lymphocyte stimulator activates p38 mitogen-activated protein kinase in human Ig class switch recombination. Am J Respir Cell Mol Biol. 2005;32:388–394. doi: 10.1165/rcmb.2004-0317OC. [DOI] [PubMed] [Google Scholar]
- 60.Frasca D, Riley RL, Blomberg BB. Aging murine B cells have decreased class switch induced by anti-CD40 or BAFF. Exp Gerontol. 2007;42:192–203. doi: 10.1016/j.exger.2006.09.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Lane DP. Cancer. p53, guardian of the genome. Nature. 1992;358:15–16. doi: 10.1038/358015a0. [DOI] [PubMed] [Google Scholar]
- 62.Efeyan A, Serrano M. p53: guardian of the genome and policeman of the oncogenes. Cell Cycle. 2007;6:1006–1010. doi: 10.4161/cc.6.9.4211. [DOI] [PubMed] [Google Scholar]
- 63.Chen K, Xu W, Wilson M, He B, Miller NW, Bengten E, Edholm ES, Santini PA, Rath P, Chiu A, Cattalini M, Litzman J, Huang BBJB, Meini A, Riesbeck K, Cunningham-Rundles C, Plebani A, Cerutti A. Immunoglobulin D enhances immune surveillance by activating antimicrobial, proinflammatory and B cell-stimulating programs in basophils. Nat Immunol. 2009;10:889–898. doi: 10.1038/ni.1748. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Puga I, Cols M, Barra CM, He B, Cassis L, Gentile M, Comerma L, Chorny A, Shan M, Xu W, Magri G, Knowles DM, Tam W, Chiu A, Bussel JB, Serrano S, Lorente JA, Bellosillo B, Lloreta J, Juanpere N, Alameda F, Baro T, de Heredia CD, Toran N, Catala A, Torrebadell M, Fortuny C, Cusi V, Carreras C, Diaz GA, Blander JM, Farber CM, Silvestri G, Cunningham-Rundles C, Calvillo M, Dufour C, Notarangelo LD, Lougaris V, Plebani A, Casanova JL, Ganal SC, Diefenbach A, Arostegui JI, Juan M, Yague J, Mahlaoui N, Donadieu J, Chen K, Cerutti A. B cell-helper neutrophils stimulate the diversification and production of immunoglobulin in the marginal zone of the spleen. Nat Immunol. 2012;13:170–180. doi: 10.1038/ni.2194. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Brandt E, Woerly G, Younes AB, Loiseau S, Capron M. IL-4 production by human polymorphonuclear neutrophils. J Leukoc Biol. 2000;68:125–130. [PubMed] [Google Scholar]
- 66.Sax JK, Fei P, Murphy ME, Bernhard E, Korsmeyer SJ, El-Deiry WS. BID regulation by p53 contributes to chemosensitivity. Nat Cell Biol. 2002;4:842–849. doi: 10.1038/ncb866. [DOI] [PubMed] [Google Scholar]
- 67.Menendez D, Inga A, Resnick MA. The expanding universe of p53 targets. Nat Rev Cancer. 2009;9:724–737. doi: 10.1038/nrc2730. [DOI] [PubMed] [Google Scholar]
- 68.Chipuk JE, Kuwana T, Bouchier-Hayes L, Droin NM, Newmeyer DD, Schuler M, Green DR. Direct activation of Bax by p53 mediates mitochondrial membrane permeabilization and apoptosis. Science. 2004;303:1010–1014. doi: 10.1126/science.1092734. [DOI] [PubMed] [Google Scholar]
- 69.Mihara M, Erster S, Zaika A, Petrenko O, Chittenden T, Pancoska P, Moll UM. p53 has a direct apoptogenic role at the mitochondria. Mol Cell. 2003;11:577–590. doi: 10.1016/s1097-2765(03)00050-9. [DOI] [PubMed] [Google Scholar]
- 70.Gaidano G, Ballerini P, Gong JZ, Inghirami G, Neri A, Newcomb EW, Magrath IT, Knowles DM, Dalla-Favera R. p53 mutations in human lymphoid malignancies: association with Burkitt lymphoma and chronic lymphocytic leukemia. Proc Natl Acad Sci U S A. 1991;88:5413–5417. doi: 10.1073/pnas.88.12.5413. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Phan RT, Dalla-Favera R. The BCL6 proto-oncogene suppresses p53 expression in germinal-centre B cells. Nature. 2004;432:635–639. doi: 10.1038/nature03147. [DOI] [PubMed] [Google Scholar]
- 72.Ranuncolo SM, Polo JM, Dierov J, Singer M, Kuo T, Greally J, Green R, Carroll M, Melnick A. Bcl-6 mediates the germinal center B cell phenotype and lymphomagenesis through transcriptional repression of the DNA-damage sensor ATR. Nat Immunol. 2007;8:705–714. doi: 10.1038/ni1478. [DOI] [PubMed] [Google Scholar]
- 73.Ward JM, Tadesse-Heath L, Perkins SN, Chattopadhyay SK, Hursting SD, Morse HC., 3rd Splenic marginal zone B-cell and thymic T-cell lymphomas in p53-deficient mice. Lab Invest. 1999;79:3–14. [PubMed] [Google Scholar]
- 74.Guo Z, Deshpande R, Paull TT. ATM activation in the presence of oxidative stress. Cell Cycle. 2010;9:4805–4811. doi: 10.4161/cc.9.24.14323. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Panta GR, Kaur S, Cavin LG, Cortes ML, Mercurio F, Lothstein L, Sweatman TW, Israel M, Arsura M. ATM and the catalytic subunit of DNA-dependent protein kinase activate NF-kappaB through a common MEK/extracellular signal-regulated kinase/p90(rsk) signaling pathway in response to distinct forms of DNA damage. Mol Cell Biol. 2004;24:1823–1835. doi: 10.1128/MCB.24.5.1823-1835.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 76.Schmidt NW, Mayo LD, Donner DB, Kaplan MH. p53 regulates Btk-dependent B cell proliferation but not differentiation. J Leukoc Biol. 2006;79:852–859. doi: 10.1189/jlb.0705402. [DOI] [PubMed] [Google Scholar]
- 77.Shick L, Carman JH, Choi JK, Somasundaram K, Burrell M, Hill DE, Zeng YX, Wang Y, Wiman KG, Salhany K, Kadesch TR, Monroe JG, Donehower LA, el-Deiry WS. Decreased immunoglobulin deposition in tumors and increased immature B cells in p53-null mice. Cell Growth Differ. 1997;8:121–131. [PubMed] [Google Scholar]
- 78.Hao Z, Duncan GS, Su YW, Li WY, Silvester J, Hong C, You H, Brenner D, Gorrini C, Haight J, Wakeham A, You-Ten A, McCracken S, Elia A, Li Q, Detmar J, Jurisicova A, Hobeika E, Reth M, Sheng Y, Lang PA, Ohashi PS, Zhong Q, Wang X, Mak TW. The E3 ubiquitin ligase Mule acts through the ATM-p53 axis to maintain B lymphocyte homeostasis. J Exp Med. 2012;209:173–186. doi: 10.1084/jem.20111363. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Chen D, Kon N, Li M, Zhang W, Qin J, Gu W. ARF-BP1/Mule is a critical mediator of the ARF tumor suppressor. Cell. 2005;121:1071–1083. doi: 10.1016/j.cell.2005.03.037. [DOI] [PubMed] [Google Scholar]
- 80.Guikema JE, Schrader CE, Brodsky MH, Linehan EK, Richards A, El Falaky N, Li DH, Sluss HK, Szomolanyi-Tsuda E, Stavnezer J. p53 represses class switch recombination to IgG2a through its antioxidant function. J Immunol. 2010;184:6177–6187. doi: 10.4049/jimmunol.0904085. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.Ramiro AR, Jankovic M, Callen E, Difilippantonio S, Chen HT, McBride KM, Eisenreich TR, Chen J, Dickins RA, Lowe SW, Nussenzweig A, Nussenzweig MC. Role of genomic instability and p53 in AID-induced c-myc-Igh translocations. Nature. 2006;440:105–109. doi: 10.1038/nature04495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 82.Lens D, De Schouwer PJ, Hamoudi RA, Abdul-Rauf M, Farahat N, Matutes E, Crook T, Dyer MJ, Catovsky D. p53 abnormalities in B-cell prolymphocytic leukemia. Blood. 1997;89:2015–2023. [PubMed] [Google Scholar]
- 83.Malcikova J, Smardova J, Pekova S, Cejkova S, Kotaskova J, Tichy B, Francova H, Doubek M, Brychtova Y, Janek D, Pospisilova S, Mayer J, Dvorakova D, Trbusek M. Identification of somatic hypermutations in the TP53 gene in B-cell chronic lymphocytic leukemia. Mol Immunol. 2008;45:1525–1529. doi: 10.1016/j.molimm.2007.08.017. [DOI] [PubMed] [Google Scholar]
- 84.Fabbri G, Rasi S, Rossi D, Trifonov V, Khiabanian H, Ma J, Grunn A, Fangazio M, Capello D, Monti S, Cresta S, Gargiulo E, Forconi F, Guarini A, Arcaini L, Paulli M, Laurenti L, Larocca LM, Marasca R, Gattei V, Oscier D, Bertoni F, Mullighan CG, Foa R, Pasqualucci L, Rabadan R, Dalla-Favera R, Gaidano G. Analysis of the chronic lymphocytic leukemia coding genome: role of NOTCH1 mutational activation. J Exp Med. 2011;208:1389–1401. doi: 10.1084/jem.20110921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Tapinos NI, Polihronis M, Moutsopoulos HM. Lymphoma development in Sjogren’s syndrome: novel p53 mutations. Arthritis Rheum. 1999;42:1466–1472. doi: 10.1002/1529-0131(199907)42:7<1466::AID-ANR21>3.0.CO;2-L. [DOI] [PubMed] [Google Scholar]
- 86.Du M, Peng H, Singh N, Isaacson PG, Pan L. The accumulation of p53 abnormalities is associated with progression of mucosa-associated lymphoid tissue lymphoma. Blood. 1995;86:4587–4593. [PubMed] [Google Scholar]
- 87.Baldini L, Fracchiolla NS, Cro LM, Trecca D, Romitti L, Polli E, Maiolo AT, Neri A. Frequent p53 gene involvement in splenic B-cell leukemia/lymphomas of possible marginal zone origin. Blood. 1994;84:270–278. [PubMed] [Google Scholar]
- 88.Royer B, Cazals-Hatem D, Sibilia J, Agbalika F, Cayuela JM, Soussi T, Maloisel F, Clauvel JP, Brouet JC, Mariette X. Lymphomas in patients with Sjogren’s syndrome are marginal zone B-cell neoplasms, arise in diverse extranodal and nodal sites, and are not associated with viruses. Blood. 1997;90:766–775. [PubMed] [Google Scholar]
- 89.Ren D, Tu HC, Kim H, Wang GX, Bean GR, Takeuchi O, Jeffers JR, Zambetti GP, Hsieh JJ, Cheng EH. BID, BIM, and PUMA are essential for activation of the BAX- and BAK-dependent cell death program. Science. 2010;330:1390–1393. doi: 10.1126/science.1190217. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 90.Furukawa-Hibi Y, Kobayashi Y, Chen C, Motoyama N. FOXO transcription factors in cell-cycle regulation and the response to oxidative stress. Antioxid Redox Signal. 2005;7:752–760. doi: 10.1089/ars.2005.7.752. [DOI] [PubMed] [Google Scholar]
- 91.Carey GB, Semenova E, Qi X, Keegan AD. IL-4 protects the B-cell lymphoma cell line CH31 from anti-IgM-induced growth arrest and apoptosis: contribution of the PI-3 kinase/AKT pathway. Cell Res. 2007;17:942–955. doi: 10.1038/sj.cr.2007.90. [DOI] [PubMed] [Google Scholar]
- 92.Chandramohan V, Jeay S, Pianetti S, Sonenshein GE. Reciprocal control of Forkhead box O 3a and c-Myc via the phosphatidylinositol 3-kinase pathway coordinately regulates p27Kip1 levels. J Immunol. 2004;172:5522–5527. doi: 10.4049/jimmunol.172.9.5522. [DOI] [PubMed] [Google Scholar]
- 93.Yusuf I, Zhu X, Kharas MG, Chen J, Fruman DA. Optimal B-cell proliferation requires phosphoinositide 3-kinase-dependent inactivation of FOXO transcription factors. Blood. 2004;104:784–787. doi: 10.1182/blood-2003-09-3071. [DOI] [PubMed] [Google Scholar]
- 94.Yin XM. Bid, a BH3-only multi-functional molecule, is at the cross road of life and death. Gene. 2006;369:7–19. doi: 10.1016/j.gene.2005.10.038. [DOI] [PubMed] [Google Scholar]
- 95.Matsumoto F, Saitoh S, Fukui R, Kobayashi T, Tanimura N, Konno K, Kusumoto Y, Akashi-Takamura S, Miyake K. Cathepsins are required for Toll-like receptor 9 responses. Biochem Biophys Res Commun. 2008;367:693–699. doi: 10.1016/j.bbrc.2007.12.130. [DOI] [PubMed] [Google Scholar]
- 96.Olson NE, Graves JD, Shu GL, Ryan EJ, Clark EA. Caspase activity is required for stimulated B lymphocytes to enter the cell cycle. J Immunol. 2003;170:6065–6072. doi: 10.4049/jimmunol.170.12.6065. [DOI] [PubMed] [Google Scholar]
- 97.Jahrsdorfer B, Blackwell SE, Wooldridge JE, Huang J, Andreski MW, Jacobus LS, Taylor CM, Weiner GJ. B-chronic lymphocytic leukemia cells and other B cells can produce granzyme B and gain cytotoxic potential after interleukin-21-based activation. Blood. 2006;108:2712–2719. doi: 10.1182/blood-2006-03-014001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Mei Y, Du W, Yang Y, Wu M. Puma(*)Mcl-1 interaction is not sufficient to prevent rapid degradation of Mcl-1. Oncogene. 2005;24:7224–7237. doi: 10.1038/sj.onc.1208873. [DOI] [PubMed] [Google Scholar]
- 99.Lang J, Arnold B, Hammerling G, Harris AW, Korsmeyer S, Russell D, Strasser A, Nemazee D. Enforced Bcl-2 expression inhibits antigen-mediated clonal elimination of peripheral B cells in an antigen dose-dependent manner and promotes receptor editing in autoreactive, immature B cells. J Exp Med. 1997;186:1513–1522. doi: 10.1084/jem.186.9.1513. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Kuo P, Bynoe MS, Wang C, Diamond B. Bcl-2 leads to expression of anti-DNA B cells but no nephritis: a model for a clinical subset. Eur J Immunol. 1999;29:3168–3178. doi: 10.1002/(SICI)1521-4141(199910)29:10<3168::AID-IMMU3168>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 101.Chattopadhyay G, Khan AQ, Sen G, Colino J, DuBois W, Rubtsov A, Torres RM, Potter M, Snapper CM. Transgenic expression of Bcl-xL or Bcl-2 by murine B cells enhances the in vivo antipolysaccharide, but not antiprotein, response to intact Streptococcus pneumoniae. J Immunol. 2007;179:7523–7534. doi: 10.4049/jimmunol.179.11.7523. [DOI] [PubMed] [Google Scholar]
- 102.Jonsson MV, Szodoray P, Jellestad S, Jonsson R, Skarstein K. Association between circulating levels of the novel TNF family members APRIL and BAFF and lymphoid organization in primary Sjogren’s syndrome. J Clin Immunol. 2005;25:189–201. doi: 10.1007/s10875-005-4091-5. [DOI] [PubMed] [Google Scholar]
- 103.Zhang L, Bertucci AM, Smith KA, Xu L, Datta SK. Hyperexpression of cyclooxygenase 2 in the lupus immune system and effect of cyclooxygenase 2 inhibitor diet therapy in a murine model of systemic lupus erythematosus. Arthritis Rheum. 2007;56:4132–4141. doi: 10.1002/art.23054. [DOI] [PubMed] [Google Scholar]
- 104.Wu Y, Mehew JW, Heckman CA, Arcinas M, Boxer LM. Negative regulation of bcl-2 expression by p53 in hematopoietic cells. Oncogene. 2001;20:240–251. doi: 10.1038/sj.onc.1204067. [DOI] [PubMed] [Google Scholar]
- 105.Cimmino A, Calin GA, Fabbri M, Iorio MV, Ferracin M, Shimizu M, Wojcik SE, Aqeilan RI, Zupo S, Dono M, Rassenti L, Alder H, Volinia S, Liu CG, Kipps TJ, Negrini M, Croce CM. miR-15 and miR-16 induce apoptosis by targeting BCL2. Proc Natl Acad Sci U S A. 2005;102:13944–13949. doi: 10.1073/pnas.0506654102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Boominathan L. The guardians of the genome (p53, TA-p73, and TA-p63) are regulators of tumor suppressor miRNAs network. Cancer Metastasis Rev. 2010;29:613–639. doi: 10.1007/s10555-010-9257-9. [DOI] [PubMed] [Google Scholar]
- 107.Ji Q, Hao X, Meng Y, Zhang M, Desano J, Fan D, Xu L. Restoration of tumor suppressor miR-34 inhibits human p53-mutant gastric cancer tumorspheres. BMC Cancer. 2008;8:266. doi: 10.1186/1471-2407-8-266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Saito M, Novak U, Piovan E, Basso K, Sumazin P, Schneider C, Crespo M, Shen Q, Bhagat G, Califano A, Chadburn A, Pasqualucci L, Dalla-Favera R. BCL6 suppression of BCL2 via Miz1 and its disruption in diffuse large B cell lymphoma. Proc Natl Acad Sci U S A. 2009;106:11294–11299. doi: 10.1073/pnas.0903854106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Eischen CM, Packham G, Nip J, Fee BE, Hiebert SW, Zambetti GP, Cleveland JL. Bcl-2 is an apoptotic target suppressed by both c-Myc and E2F-1. Oncogene. 2001;20:6983–6993. doi: 10.1038/sj.onc.1204892. [DOI] [PubMed] [Google Scholar]
- 110.Kim YM, Kim TH, Seol DW, Talanian RV, Billiar TR. Nitric oxide suppression of apoptosis occurs in association with an inhibition of Bcl-2 cleavage and cytochrome c release. J Biol Chem. 1998;273:31437–31441. doi: 10.1074/jbc.273.47.31437. [DOI] [PubMed] [Google Scholar]
- 111.Rush JS, Liu M, Odegard VH, Unniraman S, Schatz DG. Expression of activation-induced cytidine deaminase is regulated by cell division, providing a mechanistic basis for division-linked class switch recombination. Proc Natl Acad Sci U S A. 2005;102:13242–13247. doi: 10.1073/pnas.0502779102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.Hauser J, Sveshnikova N, Wallenius A, Baradaran S, Saarikettu J, Grundstrom T. B-cell receptor activation inhibits AID expression through calmodulin inhibition of E-proteins. Proc Natl Acad Sci U S A. 2008;105:1267–1272. doi: 10.1073/pnas.0708220105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Ekstrom Smedby K, Vajdic CM, Falster M, Engels EA, Martinez-Maza O, Turner J, Hjalgrim H, Vineis P, Seniori Costantini A, Bracci PM, Holly EA, Willett E, Spinelli JJ, La Vecchia C, Zheng T, Becker N, De Sanjose S, Chiu BC, Dal Maso L, Cocco P, Maynadie M, Foretova L, Staines A, Brennan P, Davis S, Severson R, Cerhan JR, Breen EC, Birmann B, Grulich AE, Cozen W. Autoimmune disorders and risk of non-Hodgkin lymphoma subtypes: a pooled analysis within the InterLymph Consortium. Blood. 2008;111:4029–4038. doi: 10.1182/blood-2007-10-119974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 114.Landgren O, Rapkin JS, Caporaso NE, Mellemkjaer L, Gridley G, Goldin LR, Engels EA. Respiratory tract infections and subsequent risk of chronic lymphocytic leukemia. Blood. 2007;109:2198–2201. doi: 10.1182/blood-2006-08-044008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 115.Mellemkjaer L, Pfeiffer RM, Engels EA, Gridley G, Wheeler W, Hemminki K, Olsen JH, Dreyer L, Linet MS, Goldin LR, Landgren O. Autoimmune disease in individuals and close family members and susceptibility to non-Hodgkin’s lymphoma. Arthritis Rheum. 2008;58:657–666. doi: 10.1002/art.23267. [DOI] [PubMed] [Google Scholar]
- 116.Smedby KE, Hjalgrim H, Askling J, Chang ET, Gregersen H, Porwit-MacDonald A, Sundstrom C, Akerman M, Melbye M, Glimelius B, Adami HO. Autoimmune and chronic inflammatory disorders and risk of non-Hodgkin lymphoma by subtype. J Natl Cancer Inst. 2006;98:51–60. doi: 10.1093/jnci/djj004. [DOI] [PubMed] [Google Scholar]
- 117.De Vita S, Dolcetti R, Ferraccioli G, Pivetta B, De Re V, Gloghini A, D’Agosto A, Bartoli E, Carbone A, Boiocchi M. Local cytokine expression in the progression toward B cell malignancy in Sjogren’s syndrome. J Rheumatol. 1995;22:1674–1680. [PubMed] [Google Scholar]
- 118.Quartuccio L, Fabris M, Moretti M, Barone F, Bombardieri M, Rupolo M, Lombardi S, Pitzalis C, Beltrami CA, Curcio F, De Vita S. Resistance to Rituximab Therapy and Local BAFF Overexpression in Sjogren’s Syndrome-Related Myoepithelial Sialadenitis and Low-Grade Parotid B-Cell Lymphoma. Open Rheumatol J. 2008;2:38–43. doi: 10.2174/1874312900802010038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 119.Mitsias DI, Tzioufas AG, Veiopoulou C, Zintzaras E, Tassios IK, Kogopoulou O, Moutsopoulos HM, Thyphronitis G. The Th1/Th2 cytokine balance changes with the progress of the immunopathological lesion of Sjogren’s syndrome. Clin Exp Immunol. 2002;128:562–568. doi: 10.1046/j.1365-2249.2002.01869.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 120.Amato D, Oscier DG, Davis Z, Mould S, Zheng J, Kolomietz E, Wang C. Cytogenetic aberrations and immunoglobulin VH gene mutations in clinically benign CD5-monoclonal B-cell lymphocytosis. Am J Clin Pathol. 2007;128:333–338. doi: 10.1309/WB56ET6AGM44TM5T. [DOI] [PubMed] [Google Scholar]
- 121.Fabris S, Mosca L, Todoerti K, Cutrona G, Lionetti M, Intini D, Matis S, Colombo M, Agnelli L, Gentile M, Spriano M, Callea V, Festini G, Molica S, Lambertenghi Deliliers G, Morabito F, Ferrarini M, Neri A. Molecular and transcriptional characterization of 17p loss in B-cell chronic lymphocytic leukemia. Genes Chromosomes Cancer. 2008;47:781–793. doi: 10.1002/gcc.20579. [DOI] [PubMed] [Google Scholar]
- 122.Stankovic T, Stewart GS, Fegan C, Biggs P, Last J, Byrd PJ, Keenan RD, Moss PA, Taylor AM. Ataxia telangiectasia mutated-deficient B-cell chronic lymphocytic leukemia occurs in pregerminal center cells and results in defective damage response and unrepaired chromosome damage. Blood. 2002;99:300–309. doi: 10.1182/blood.v99.1.300. [DOI] [PubMed] [Google Scholar]
- 123.Calin GA, Cimmino A, Fabbri M, Ferracin M, Wojcik SE, Shimizu M, Taccioli C, Zanesi N, Garzon R, Aqeilan RI, Alder H, Volinia S, Rassenti L, Liu X, Liu CG, Kipps TJ, Negrini M, Croce CM. MiR-15a and miR-16-1 cluster functions in human leukemia. Proc Natl Acad Sci U S A. 2008;105:5166–5171. doi: 10.1073/pnas.0800121105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Hermeking H. p53 enters the microRNA world. Cancer Cell. 2007;12:414–418. doi: 10.1016/j.ccr.2007.10.028. [DOI] [PubMed] [Google Scholar]
- 125.Dijkstra MK, van Lom K, Tielemans D, Elstrodt F, Langerak AW, van ’t Veer MB, Jongen-Lavrencic M. 17p13/TP53 deletion in B-CLL patients is associated with microRNA-34a downregulation. Leukemia. 2009;23:625–627. doi: 10.1038/leu.2008.264. [DOI] [PubMed] [Google Scholar]
- 126.Zenz T, Mohr J, Eldering E, Kater AP, Buhler A, Kienle D, Winkler D, Durig J, van Oers MH, Mertens D, Dohner H, Stilgenbauer S. miR-34a as part of the resistance network in chronic lymphocytic leukemia. Blood. 2009;113:3801–3808. doi: 10.1182/blood-2008-08-172254. [DOI] [PubMed] [Google Scholar]
- 127.Mongini PK, Vilensky MA, Highet PF, Inman JK. Membrane IgM-stimulated human B lymphocytes succumb to activation-related apoptosis at a G1-->S transition: influence of ligand affinity and valency. Cell Immunol. 1998;188:137–150. doi: 10.1006/cimm.1998.1359. [DOI] [PubMed] [Google Scholar]
- 128.Zha S, Sekiguchi J, Brush JW, Bassing CH, Alt FW. Complementary functions of ATM and H2AX in development and suppression of genomic instability. Proc Natl Acad Sci U S A. 2008;105:9302–9306. doi: 10.1073/pnas.0803520105. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.