Abstract
During their development as myelinating cells oligodendrocyte progenitors (OPC) undergo dramatic changes in the organization of their cytoskeleton. These changes involve an increase in cell branching and lamella extension, which are important for the ability of oligodendrocytes to myelinate multiple axons in the CNS. We have previously shown that the levels of the actin-associated motor protein non-muscle myosin II (NMII) decrease as oligodendrocyte differentiate and that inhibition of NMII activity increases branching and myelination, suggesting that NMII is a negative regulator of oligodendrocyte differentiation. In agreement with this interpretation, we have found that overexpression of NMII prevents oligodendrocyte branching and differentiation, and that OPC maturation is accelerated in NMII knockout mice as shown by a significant increase in the percentage of mature MBP+ cells. Although several pathways have been implicated in oligodendrocyte morphogenesis, their specific contribution to the regulation of NMII activity has not been directly examined. We tested the hypothesis that the activity of NMII in OPC is controlled by Fyn kinase via downregulation of RhoA-ROCK-NMII phosphorylation. We found that treatment with PP2 or knockdown of Fyn using siRNA, prevents the decrease in myosin phosphorylation normally observed during OPC differentiation, and that the inhibition of branching induced by overexpression of constitutively active RhoA can be reversed by treatment with Y27632 or blebbistatin. Taken together our results demonstrate that Fyn kinase downregulates NMII activity thus promoting oligodendrocyte morphological differentiation.
Keywords: Cytoskeleton, CNS, Myelination, RhoGTPases, Fyn
Introduction
The mechanism whereby oligodendrocytes extend multiple processes that wrap around central axons is still poorly defined but it involves active remodeling of their cytoskeleton. Oligodendrocytes in culture differentiate through a sequence of stages characterized by the expression of specific molecular markers and a dramatic increase in cell branching and membrane extension (Pfeiffer et al. 1993). These morphological changes have been linked to differentiation and myelin formation both in vivo and in vitro (Kachar et al. 1986; Kim et al. 2006; Sloane and Vartanian 2007).
We have previously shown that inhibition of the motor protein non-muscle myosin II (NMII), a key regulator of cytoskeleton dynamics, enhances oligodendrocyte branching, differentiation and myelin formation in culture (Wang et al. 2008). The molecular mechanism behind these effects is not known, but we hypothesize that cytoskeletal “relaxation” or downregulation of NMII-mediated cell contraction is a signal that favors oligodendrocyte branching and myelin formation. In support of this idea our group and others have reported that the expression levels and activity of NMII are downregulated as oligodendrocyte differentiate and myelinate (Cahoy et al. 2008; Dugas et al. 2007; Wang et al. 2008).
Although several pathways have been implicated in oligodendrocyte branching morphogenesis (Liang et al. 2004; Rajasekharan et al. 2009), their specific contribution to regulation of NMII activity and expression in oligodendrocytes has not been directly examined. In non-muscle cells, NMII is activated by phosphorylation of myosin light chain (MLC) (Conti and Adelstein 2008). Several kinases can phosphorylate MLC, including Rho-associated kinase (ROCK), a major downstream effector of RhoGTPase (Amano et al. 1996). We have shown that in the PNS, inhibitors of ROCK downregulate MLC phosphorylation and affect the coordinated wrapping of Schwann cells around axons and their domain organization (Melendez-Vasquez et al. 2004). In the CNS, activation of ROCK by RhoA has been implicated in myelin-mediated inhibition of axonal outgrowth and OPC differentiation following nerve injury (Baer et al. 2009; Bito et al. 2000; Niederost et al. 2002).
Activation of Fyn kinase downstream of integrin β1 is a key regulator of oligodendrocyte survival, morphological differentiation and myelination (Colognato et al. 2004; Laursen et al. 2009). Fyn kinase has also been shown to inhibit RhoA activity, thus promoting oligodendrocyte branching (Wolf et al. 2001). We have tested the hypothesis that the activity of NMII in OPC is controlled by Fyn via downregulation of RhoA-ROCK-MLC phosphorylation. We now report that inhibition or downregulation of Fyn activity prevents the decrease in phosphorylated MLC levels normally observed during OPC differentiation (Wang et al. 2008). Moreover the inhibition of OPC branching induced by over-expression of constitutively active RhoA (Liang et al. 2004), can be reversed by pharmacological inhibition of ROCK or NMII. Furthermore and in agreement with a negative role for NMII in oligodendrocyte differentiation, we have found that oligodendrocyte maturation is accelerated in NMII null mice, as shown by a significant increase in the number of MBP+ cells in cultures derived from these mice. Taken together our results confirm that downregulation of NMII promotes oligodendrocyte branching and maturation and suggest that upstream activation of Fyn kinase acts as a negative regulator of NMII activity promoting active cytoskeleton remodeling.
Materials and Methods
Animals
All rats and mice were cared and euthanized for tissue collection in accordance with the guidelines published in the NIH Guide for the Care and Use of Laboratory Animals for the humane treatment of laboratory animals (Publication No. 85-23, revised 1985).
Purified OPC cultures
A2B5+ oligodendrocyte precursors (OPC) were purified by immunopanning from mixed glial cultures of postnatal day 1 rat cerebral cortices as previously described (Wang et al. 2008). Purified OPC were seeded onto poly-lysine coated glass coverslips and maintained in either proliferation media with PDGF (10 ng/ml) and bFGF (10 ng/ml) or induced to differentiate in media containing T3 (30 ng/ml). For inhibitor studies Y27632, PP2 or blebbistatin (Calbiochem) were added to the media at the concentration specified and maintained throughout the experiment (1–3 days).
Generation of mice OPC
Neurospheres were prepared from E14.5 mouse cortices as previously described (Chen et al. 2007). Formation of OPC was induced by feeding neurospheres with B104-conditioned media for 20 days. Oligospheres were treated with trypsin and dissociated to single cell suspension. OPC (150,000 cells) were plated on coverslips coated with poly-lysine, and maintained in either proliferation media with growth factors or induced to differentiate in media containing T3. Myosin IIB knockout mice (Tullio et al. 1997)(Strain: B6;129S4-Myh10tm2Rsad/Mmnc, Stock: 016991-UNC) were obtained from Mutant Mouse Regional Resource Center (MMRRC, University of Missouri).
Transfections
For transfection of primary OPC, cells were resuspended in the Amaxa nucleofection solution (Lonza) to a final concentration of 1–5×106 cell/100µl. The cell suspension was mixed with 1–2.5 µg of DNA or siRNA duplex and transfected using the O-17 nucleofection program. Transfected OPCs were plated on poly-lysine coated glass coverslips and maintained in either proliferation or differentiation media for 1–5 days. Pools of siRNA duplex targeting rat myosin IIA (NM_013194), myosin IIB (NM_031520) and Fyn (NM_012755) were purchased from Dharmacon (siGENOME ON-TARGETplus SMARTpool duplex). A non-targeting duplex pool (ON-TARGETplus siCONTROL Non-Targeting Pool D-001810-10-05) and siGloRed Transfection Indicator (D-001630-01) were used as controls. Plasmids expressing wild type (pcDNA3-EGFP-RhoA), constitutively active (pcDNA3-EGFP-RhoA-Q63L) and dominant negative (pcDNA3-EGFP-RhoAT19N) RhoA as well as GFP-tagged NMIIB (CMV-EGFP-NMIIB) were obtained from Addgene.
Immunofluorescence
OPC cultures were fixed in 4% PFA and processed for immunocytochemistry as previously described (Wang et al. 2008). Cultures were examined by epifluorescence on a Leica DMI4000 microscope or on a Zeiss LSM 510 confocal microscope. Image analysis (see below) was performed using ImageJ 1.38v and Adobe Photoshop CS8. Adjustment of image brightness or contrast was performed in some cases but without misrepresenting data.
Morphological analysis
OPC were counted in micrographs from 10–12 random low-power fields/coverslip, using ImageJ 1.38v (total of 20–30 fields per condition per experiment; total of 2–3 experiments). For transfection experiments GFP+OPC were counted and classified according to their branching complexity as follows: (0): no branching; (1) low complexity: cells with at least one or two branches extending directly from the cell body; (2) medium complexity: cells with processes extending from primary branches and (3) high complexity: cells with processes extending from secondary branches. Fractal dimension and process length were calculated using ImageJ software as previously described (Wang et al. 2008). Statistical tests (t-test and ANOVA) were performed using Graph Pad Prism software.
Time-lapse microscopy
Videos were acquired with a Leica DMI4000B microscope system fitted with a stage incubator and a temperature and C02 digital controller (CTI Controller 37000 Pecon). OPC were plated on poly-lysine-coated glass-bottomed 35-mm tissue culture dishes (MatTek). Phase images were acquired using a N PLAN L 20x/0.40 objective. Cultures were maintained at 37 °C and 5% C02 throughout the observation period.
Cell extracts and Western blotting
Lysates from OPC were prepared as previously described (Wang et al. 2008) subjected to SDS-PAGE and blotted onto nitrocellulose. Appropriate regions of the blots were cut and incubated with specific antibodies and developed using chemiluminescent substrate (Pierce). For estimation of changes in protein levels, X-ray films from 2–3 independent experiments were scanned and the relative intensity of each protein band was calculated in Adobe Photoshop by dividing the absolute intensity of each protein band (the area of the band by the number of pixels contained in that area) by the absolute intensity of the corresponding actin band.
Antibodies
Antibodies used in these studies included those reactive to: myosin IIA and IIB (Covance), MLC2 and phosphorylated MLC2 (Cell Signaling Technology); phalloidin-FITC, phalloidin-coumarin, GAP43 and actin (Sigma); Olig2 and Fyn (Millipore); MBP and GAP43 (Chemicon). Secondary antibodies conjugated to rhodamine, fluorescein, coumarin, or cyanin 5 were obtained from Jackson Laboratories.
Immunoelectron microscopy
NMII was localized immunocytochemically in OPC cultures using the avidin-biotin complex (ABC) peroxidase method (Hsu et al. 1981). In brief, OPC maintained in PDGF-containing media for 6 days on poly-lysine/laminin-coated Aclar coverslips were fixed in 0.1M phosphate buffer (PB; pH 7.4) containing 2% paraformaldehye/3.75% acrolein (Polysciences) for 30 minutes at room temperature. Cultures were washed in PB and incubated in PB containing 1% sodium borohydride for 30 min. After multiple PB washes the coverslips were incubated in two changes of 0.1M Tris-saline (TS; PH 7.6) and then blocked in TS containing 0.5% BSA for 30 minutes. The cultures were then washed several times in 0.1M TS and incubated in anti-NMIIA or IIB antibody (Covance) diluted in TS containing 0.1% BSA and 0.025% Triton X-100 overnight at room temperature. Control cultures were incubated in the same solution without primary antibody. Subsequent steps involving incubation with biotin-conjugated secondary antibody and peroxidase-avidin complex and processing for electron microscopy were performed as described previously (Einheber et al. 1996). Specimens were examined on a Philips (Eindhoven, The Netherlands) CM10 electron microscope.
Results
Overexpression of NMIIB prevents OPC branching and differentiation
We have previously shown that downregulation of NMII activity results in enhanced myelination in OPC-DRG cocultures (Wang et al. 2008). In order to elucidate the NMII isoform(s) responsible for these effects we conducted siRNA experiments and found that silencing of NMII isoforms A or B reproduces the increase in branching and myelination observed after total NMII inhibition (Fig. 1A). This is in agreement with data showing that expression of NMIIA and NMIIB is downregulated during oligodendrocyte development (Wang et al. 2008), and with immunocytochemistry studies (Song et al. 2001) showing that both isoforms are expressed by oligodendrocytes, with NMIIB being predominant.
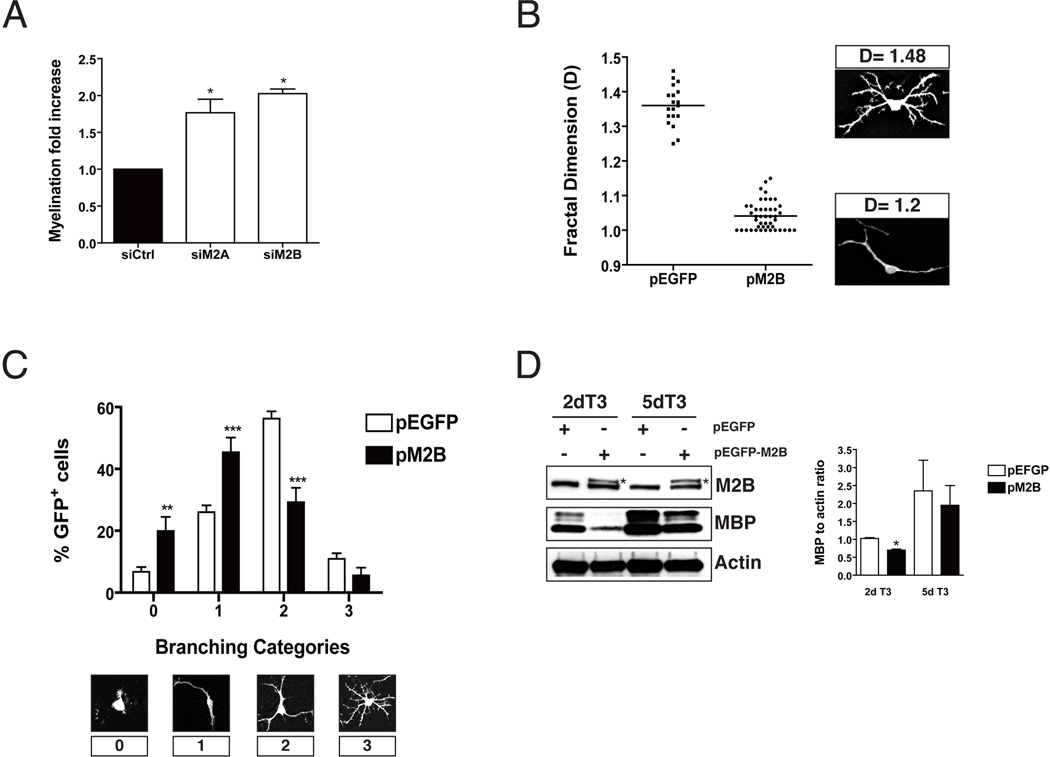
Figure 1. Overexpression of NMII impairs OPC branching and differentiation.
A. OPC transfected with siRNA targeting NMIIA (M2A) or NMIIB (M2B) isoforms, or non-targeting control (siCtrl) were placed in coculture with neurons and allowed to myelinate as previously described (Wang et al. 2008). After 14 days cocultures were stained with antibodies to MBP and the total number of MBP+ segments was quantified. Silencing of either isoform resulted in 1.8-2 fold increase in myelination (p<0.05; t- test). Data on graph represents the mean ± SD of two separate experiments (2 cultures/per condition/per experiment). B. Fractal analysis of OPC cultures transfected with pEGFP-N1 (pEGFP) or pEGFP-NMIIB (pM2B) and maintained in proliferating media (PDGF+bFGF) for 2 days. The mean fractal dimension (D) in control cultures was significantly higher than in OPC overexpressing NMIIB (pEFGP 1.36 ± 0.06 vs. pM2B 1.04 ± 0.04; mean ± SD; ***p<0.0001; t-test), indicating a less complex cytoskeletal branching. Representative examples of GFP+-OPC with low and high fractal dimension are shown. C. Semi-quantitative analysis of OPC branching complexity. GFP+-OPC were classified in four categories according to the extent of branching. Overexpression of NMIIB resulted in a reduction of the percentage of OPC exhibiting medium (2) or high complexity branching (3), while increasing the percentage of cells exhibiting low complexity (1) or no branching (0). Data represent mean +/− SEM from 2 experiments (2 cultures per condition/per experiment, **p<0.01; ***p<0.0001, t-test). Representative examples of low and high complexity GFP+- OPC are shown. D. Western blot of transfected OPC cultures kept in differentiating media (T3) for 2 and 5 days. A significant reduction (*p<0.05, t-test) in the levels of MBP expression was found in OPC overexpressing NMIIB-GFP (asterisk) compared to control cultures (pEGFP).
Next, we tested if overexpression of NMII interferes with OPC differentiation. To this end, we transfected rat OPC with constructs expressing a GFP-tagged version of NMIIA or NMIIB prior to treatment with T3 hormone. We found that overexpression of both isoforms in OPC results in decreased morphological complexity of OPC. Since both isoforms appear to be functionally equivalent in terms of their role in oligodendrocyte morphological differentiation, and due to the availability of knockout mice for the IIB isoform [(Tullio et al. 1997)(see below)] but not for IIA (Conti et al. 2004) we focused our analysis on NMIIB. First, we found that the mean fractal dimension (D) (Behar 2001) of OPC in control cultures was significantly higher than in cultures overexpressing NMIIB (pEFGP: 1.36 ± 0.06 vs. pNMIIB: 1.04 ± 0.04; mean ± SD; p<0.0001; t-test), indicating less complex cytoskeleton branching (Fig 1B). This finding was also corroborated by a semi-quantitative approach, where GFP+OPC were classified in four branching categories. Thus a significant reduction (p<0.0001) in the percentage of OPC exhibiting medium (29.2±4.6% vs. 56.3±2.3% in controls) or higher (5.6±2.5% vs. 10.9±1.8% in controls) order branching was found in cultures overexpressing NMIIB, together with a concomitant increase in the percentage of OPC that did not branch (19.9±4.6% vs. 6.7±1.5% in controls) or that only elaborated primary processes (45.4±4.7% vs. 26±2.1% in controls; (Fig. 1C). In addition, Western blotting analysis showed a significant reduction (p<0.05, t-test) in the levels of MBP in cells overexpressing NMIIB-GFP (Fig. 1D). Taken together these results indicate that NMIIB inhibits OPC branching and differentiation.
Oligodendrocyte differentiation is accelerated in NMIIB knockout mice
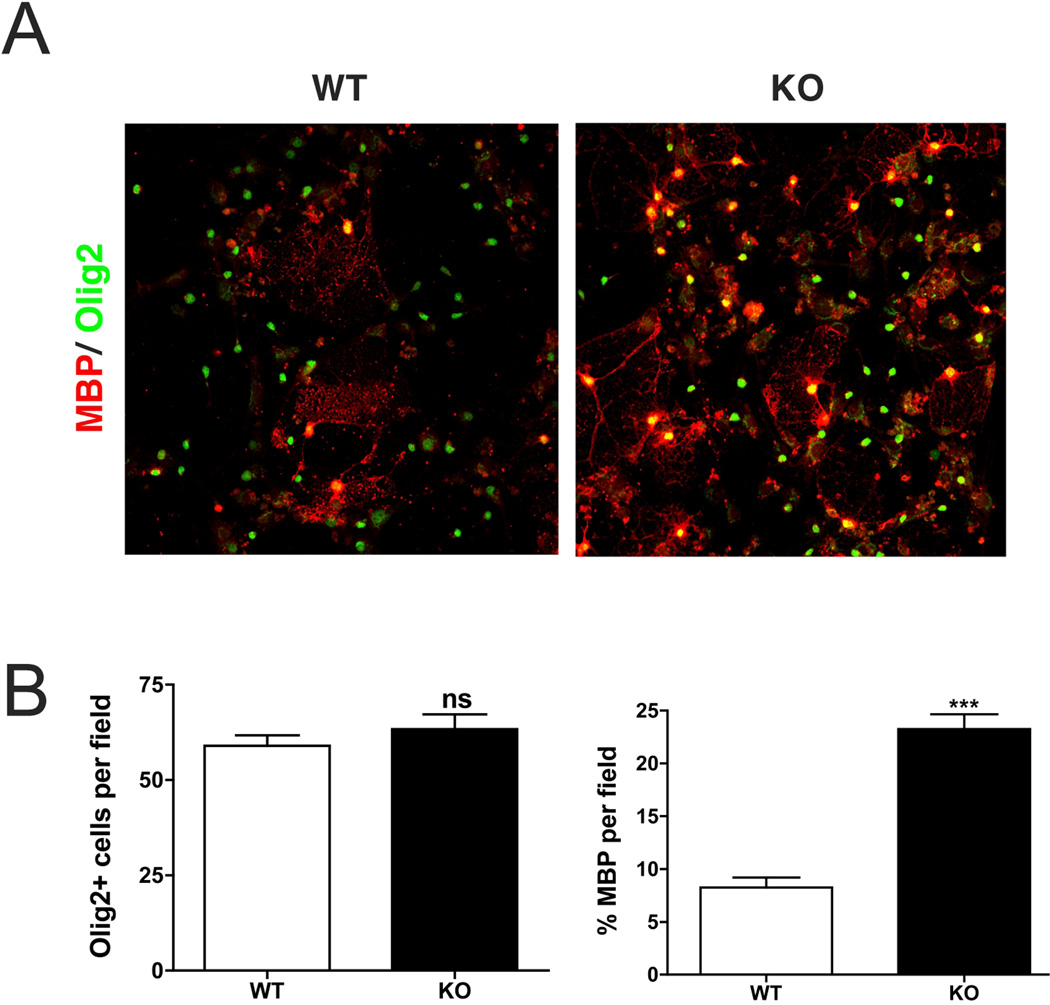
To test the relevance of our in vitro findings we analyzed the differentiation of OPC in NMIIB knockout mice. Ablation of NMIIB results in severe hydrocephalus and abnormal cardiac development (Tullio et al. 1997; Tullio et al. 2001). Approximately 65% of the knockout embryos die prior to birth, and those that are born die during the first day due to congestive heart failure. Therefore, in order to overcome the embryonic lethality of NMIIB ablation and study oligodendrocyte development, we isolated OPC from embryonic mouse brains (E13.5 to E14.5). After 3 weeks, cultures were fixed and stained for Olig2 and MBP, in order to estimate the total number of oligodendrocyte lineage cells and their extent of maturation. No differences in the number of Olig2+ cells per field were observed between wild type (63±3.9 cells) and knockout (58±2.7 cells) cultures (Fig 2B). However, we found that oligodendrocyte maturation was accelerated in NMIIB null mice compared to wild type (Fig 2A�B), as shown by a significant increase (p<0.0001, t-test) in the percentage of mature MBP+ cells in cultures derived from knockout animals (23.3±1.4% vs. 8.3±0.9% in controls). These results further confirm that NMIIB is a negative regulator of oligodendrocyte differentiation.
Figure 2. Accelerated maturation of OPC in cultures derived from NMIIB knockout mice.
A. Cortical OPC cultures derived from wild type (WT) and NMIIB knockout (KO) E14.5 embryos and kept for 2 days in differentiation promoting media (T3). Although, a similar number of oligodendrocytes (Olig2+, green) were observed in WT and KO cultures; staining for MBP (red) revealed a significant increase in the percentage of mature oligodendrocyte in NMIIKO cultures. Scale bar 50µm. B. Data in graphs represent the mean +/− SEM of 3 independent experiments (***p<0.0001, t-test).
Inhibition of oligodendrocyte branching by RhoA is rescued by blebbistatin treatment
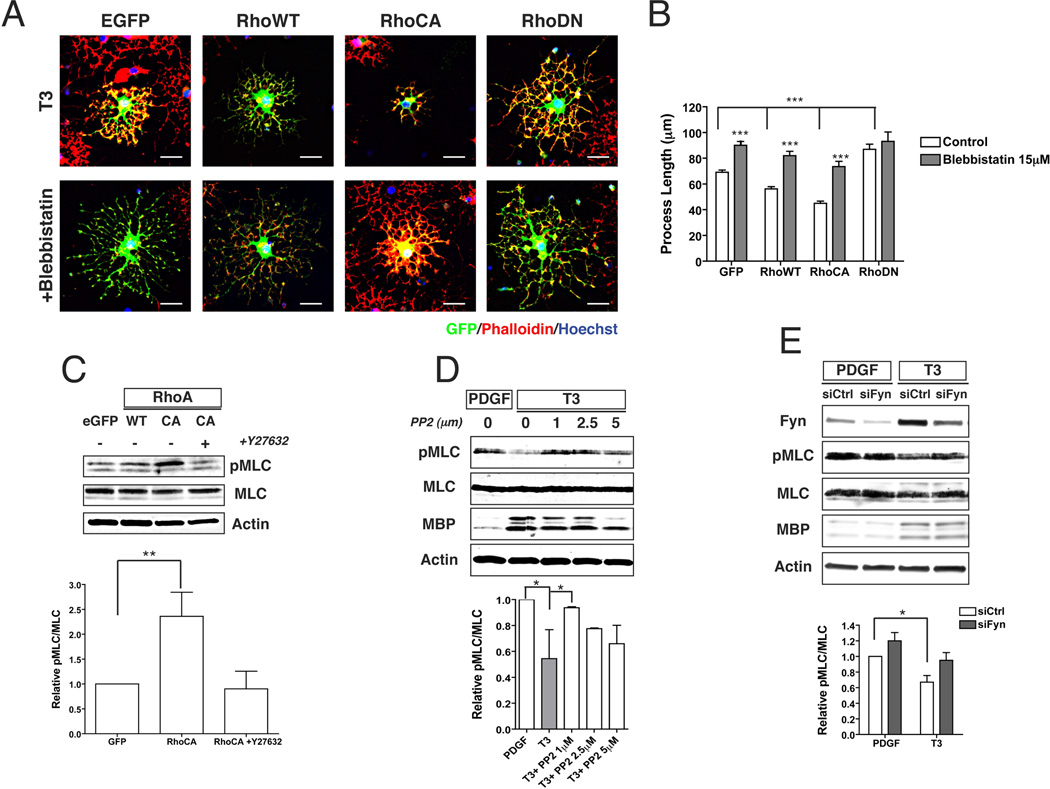
Next we investigated if there is a direct link between Rho and NMII activity in oligodendrocytes. Although it is well established that Rho activity is downregulated by Fyn during oligodendrocyte development (Liang et al. 2004), the role of NMII in the inhibition of oligodendrocyte branching induced by Rho overexpression has not been directly investigated. We found that treatment with the specific NMII inhibitor blebbistatin (Straight et al. 2003), rescues the inhibition of process extension observed in cells overexpressing wild type (WT) or constitutively active (CA) RhoA (Fig 3A). Quantitation of actin process extension showed a significant 19% reduction in process length for RhoWT (56±1.7 mm) and of 35% for RhoCA (45±1.7 mm) compared to controls (69±1.7 mm; mean ±SEM; p<0.001). By contrast, overexpression of dominant negative (DN) Rho caused an increase in process length of about 26% (87±4 mm). Treatment with blebbistatin (Fig. 3B) increased process length significantly in all conditions but the DN RhoA expressing cells (range: 74±4 to 93±7 mm), thus suggesting that inhibition of NMII activity restores process outgrowth in cells overexpressing Rho.
Figure 3. Overexpression of RhoA and/or inhibition of Fyn increase NMII phosphorylation and inhibit branching of oligodendrocytes.
A. Representative images of OPC transfected with control plasmid (EGFP) or plasmids expressing GFP-tagged wild type (WT), constitutively active (CA) or dominant negative (DN) RhoA. Overexpression of WT or CA RhoA resulted in decreased process extension (***p<0.0001, t-test). This effect can be reversed by inhibition of NMII with blebbistatin. Overexpression of DN Rho also increases oligodendrocyte branching. Scale bar 25µm. B. Quantitation of process length by transfected OPC with or without blebbistatin. Cultures were stained with phalloidin and process length of transfected GFP+ was measured using Image-J from the cell body to the end of the actin network (EGFP n=192 cells; EGFP+ Bleb n=155 cells; RhoWT n=162 cells; RhoWT+Bleb n=132 cells; RhoCA n=117 cells; RhoCA+Bleb n=102 cells; RhoDN n=50 cells; RhoDN+Bleb n=39 cells). C. Western blot of OPC cultures transfected with EGFP, RhoWT or RhoCA and kept in differentiation media (T3) for 1 day. Increased levels of phosphorylated MLC were observed in cultures overexpressing RhoA (**p<0.01, t-test). This increase was reversed by treatment with the ROCK inhibitor Y27632. D, E. Western blots of OPC cultures maintained in proliferating media (PDGF) or differentiation media (T3) for 1 day. Pharmacological inhibition of Fyn (+PP2) or silencing with siRNA (syFyn) resulted in increased levels of phosphorylated MLC (*p<0.05, t-test). Expression of MBP was not significantly affected by inhibition/silencing of Fyn.
Phosphorylation of MLC is controlled by Fyn-Rho signaling
NMII is a downstream target of RhoA through Rho-kinase (ROCK), which phosphorylates the regulatory myosin light chain (MLC) and activates NMII (Somlyo and Somlyo 2000). In agreement with this model we found that overexpression of CA RhoA in OPC significantly increases the levels of phosphorylated MLC (pMLC) compared to control cells. This increase in activity is dependent on ROCK, as treatment of OPC overexpressing CA RhoA with the specific inhibitor Y-27632 (Hirose et al. 1998), restores pMLC levels to that of controls (Fig 3C). It has also been shown that p190 RhoGAP, a GTPase activating factor, is a substrate of Fyn and that its activity increases during the differentiation of oligodendrocytes (Wolf et al. 2001). We found that inhibition of Fyn prevents MLC dephosphorylation observed during OPC development (Wang et al. 2008). Thus, OPC maintained in proliferating conditions (+PDGF) have higher levels of pMLC compared to mature oligodendrocytes (+T3) (Fig 3D). Treatment with PP2 (Osterhout et al. 1999) resulted in increased MLC phosphorylation in T3-treated cultures, to levels that were comparable to those seen in proliferating conditions (Fig. 3D). Similarly, knockdown of Fyn using siRNA also resulted in increased levels of pMLC in OPC maintained in either PDGF or T3 (Fig. 3E). Taken together these results indicate that inactivation of NMII downstream of Fyn/RhoA/ROCK signaling promotes oligodendrocyte process extension via downregulation of MLC phosphorylation. Interestingly, although high levels of pMLC resulting from Fyn inactivation or downregulation prevent process extension, the expression of differentiation markers such as Rip (data not shown) or MBP (Fig 3E and D) was no affected by these treatments. These results are in agreement with previous data (Osterhout et al. 1999), showing that oligodendrocyte morphological and molecular differentiation can be uncoupled in vitro.
Localization of NMII in oligodendrocytes
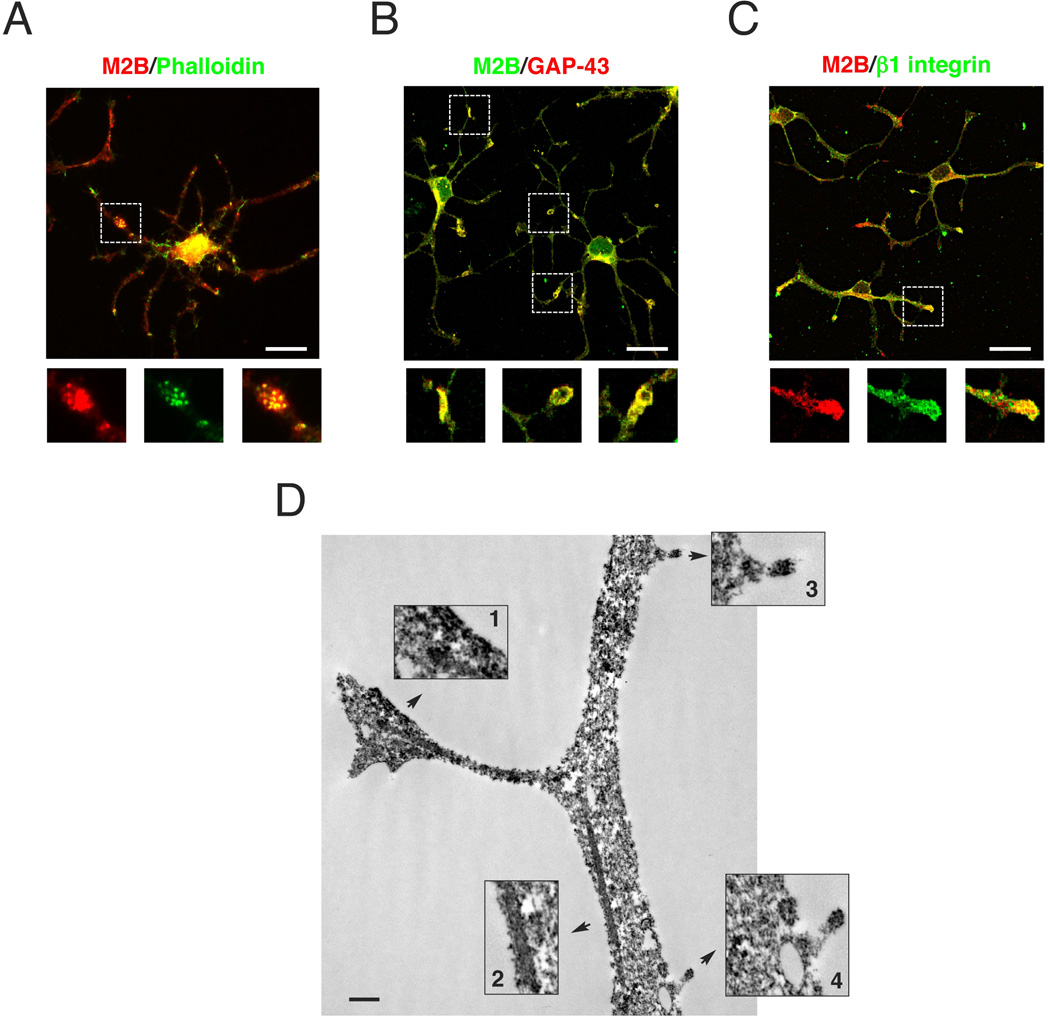
In agreement with previous reports (Song et al. 2001), we found that NMIIA and NMIIB are highly expressed in OPC, where they colocalize with F-actin (Fig. 4A) and GAP-43 (Fig. 4B) at the tip of growth cone-like (Fox et al. 2006) cell processes. Accumulation of NMIIB was also detected in thick ‘patches’ along the cell branches and at the cell cortex together with b1-integrin (Fig. 4C), suggesting a role for NMIIB in local membrane remodeling. Time-lapse video of OPC indicates that some of these patches represent nascent branching points (Supplementary Movie 1). Immunoelectron-microscopy analysis (Fig. 4D) further corroborated the enrichment of NMIIB at OPC processes in association with the cell cortex, microfilaments, at the tip of growing processes and interestingly with vesicle-like structures at the cell membrane. Collectively, these data support a role for myosin IIB in OPC branching and differentiation.
Figure 4. Localization of NMIIB in OPC.
Purified rat OPC cultures kept in proliferating media (PDGF+bFGF) for 2 days and co-stained for NMIIB and actin (A), GAP43 (B) or β1 integrin (C). NMIIB colocalize at tip of OPC processes active in cytoskeleton remodeling and in “patches” along the branches. Scale bars 20 µm. D. Electron micrograph of OPC stained with antibodies to NMIIB and processed by immunoperoxidase. Reaction product is concentrated within OPC branches and associated with the cell cortex (Box1), microfilaments (Box 2), tip of short processes (Box 3,4) and vesicle-like structures (Box 4). Scale bar 500nm.
Discussion
Studies on the role of cytoskeletal dynamics and oligodendrocyte differentiation have focused on microtubules and proteins that regulate actin polymerization (Bauer et al. 2009). However, recent studies from our laboratory and others (Kippert et al. 2009; Wang et al. 2008) have provided evidence that non-muscle myosin II (NMII), an actin-binding motor protein, also modulates key aspects of glial cell morphogenesis and myelination. In striking contrast to Schwann cell development, pharmacological inhibition of NMII or downregulation of protein levels by siRNA potentiate oligodendrocyte morphological complexity and myelin formation in vitro (Wang et al. 2008). Downregulation of NMII appears to be an obligate component of the normal differentiation program of OPC, as we and others have previously reported that there is a reverse correlation between the levels of myelin basic protein (MBP), and NMII expression and activity during oligodendrocyte development (Cahoy et al. 2008; Dugas et al. 2006; Wang et al. 2008). In the current study, we have extended these observations and provide evidence that NMII is a negative regulator of oligodendrocyte development. We have demonstrated that overexpression of NMIIB interferes with oligodendrocyte branching and differentiation and more importantly, we found that maturation of OPC is potentiated in the absence of NMII as shown by a significant increase in the number of mature, MBP+ oligodendrocytes in cultures derived from the brains of embryonic NMIIB knockout mice (Tullio et al. 1997).
The mechanisms whereby NMII mediates these effects on oligodendrocytes is not fully understood but contractile forces generated by NMII play a key role in the generation and maintenance of cell polarity, cell migration and cell adhesion (Conti and Adelstein 2008), all critical processes for oligodendrocyte maturation and myelin formation. Furthermore, in addition to its known mechanical and actin cross-linking roles, NMII might also serve as a scaffold for various signaling molecules (Conti and Adelstein 2008), including Rho GTPase guanine nucleotide exchange factors (Wu et al. 2006). Cytoskeletal remodeling by RhoGTPases plays a critical role during glial cell development (Feltri et al. 2008). Activation of Rac1 and RhoA, downstream of β1 integrin has been implicated in Schwann cell process extension, axonal segregation and myelination (Benninger et al. 2007; Nodari et al. 2007; Pereira et al. 2009). In the CNS, constitutive activation of Rho inhibits oligodendrocyte branching and maturation (Liang et al. 2004; Wolf et al. 2001), while its inactivation promotes plasma membrane condensation and differentiation (Kippert et al. 2007). The Src family tyrosine kinase Fyn has been shown to be an important regulator of oligodendrocyte development. Knockout mice are hypomyelinated (Umemori et al. 1999), and exhibit defects in the number and morphological complexity of mature oligodendrocytes (Sperber and McMorris 2001). Fyn phosphorylates p190RhoGAP (Wolf et al. 2001), a GTPase activating factor, thus reducing the levels of active GTP-bound RhoA and promoting oligodendrocyte branching (Liang et al. 2004; Rajasekharan et al. 2009). RhoA can activate several downstream targets including Rho-associated kinase (ROCK), which in turn can regulate the activity of NMII by phosphorylation of its light chain (MLC) (Kimura et al. 1996).
Despite these results pointing to an important role for Fyn-Rho signaling in the control of glial cell morphology and differentiation, the link between these processes and the regulation of NMII activity has not been addressed directly. In this study we provide evidence that downregulation of MLC phosphorylation and inhibition of NMII-contractility downstream of Fyn promotes oligodendrocyte process extension. We found that inhibition of Fyn and/or overexpression of Rho, resulted in increased levels of MLC phosphorylation and impaired oligodendrocyte branching; and that these changes can be reversed by pharmacological inhibition of ROCK or NMII. The exact mechanism(s) whereby NMII regulates oligodendrocyte branching is not known but we propose that phosphorylation of MLC promotes the assembly of contractile actomyosin fibers and restricts branching and membrane extension (Fig. 5). In support of this interpretation, it has been shown that phosphorylation of NMII by ROCK inhibits growth cone outgrowth and actin polymerization in neurons (Loudon et al. 2006; Medeiros et al. 2006) and that at high concentration NMII can act as an actin-depolymerizing agent (Haviv et al. 2008).
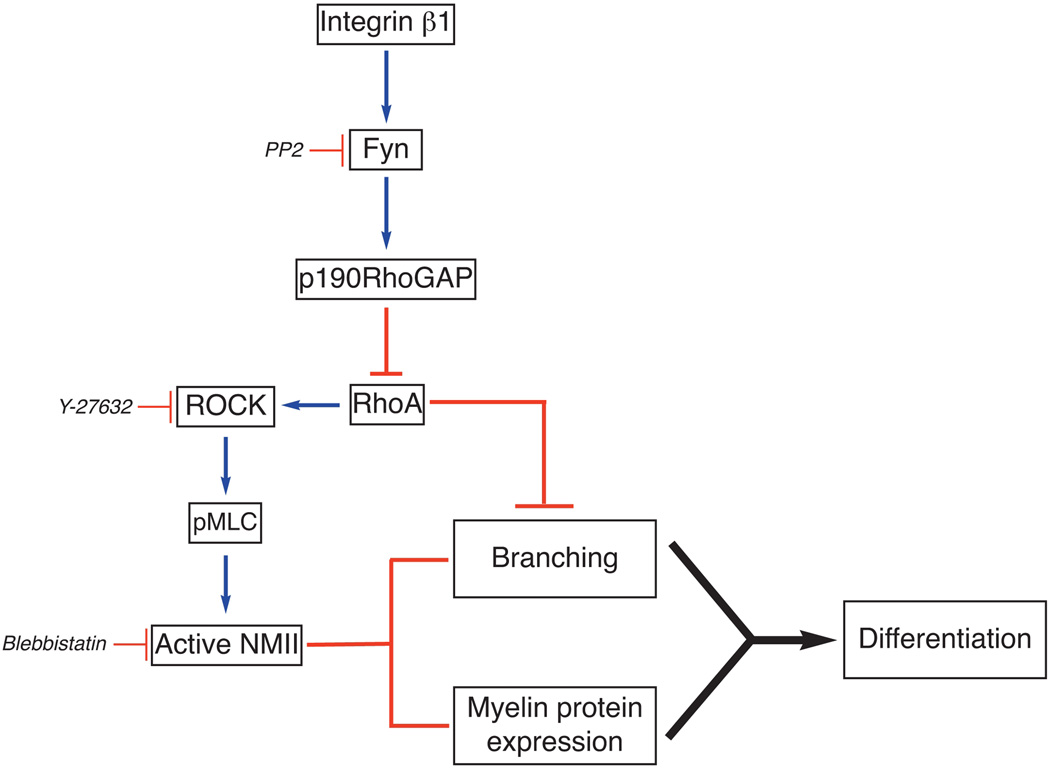
Figure 5. Model of Fyn-RhoA signaling and NMII inactivation in oligodendrocyte.
Engagement of integrins by their ECM ligands activates Fyn (Colognato et al. 2004). Fyn phosphorylates p190RhoGAP (Wolf et al. 2001), a GTPase activating factor, thus reducing the levels of active RhoA. Activation of ROCK downstream of RhoA promotes phosphorylation of MLC and activation of NMII (Somlyo and Somlyo 2000), which inhibits oligodendrocyte branching and differentiation. Inhibition of RhoA downstream of Fyn prevents NMII phosphorylation/activation by ROCK thus promoting process extension by oligodendrocytes. This model is supported by our data showing that inhibition of Fyn (PP2, siRNA) increases pMLC levels, while inhibition of ROCK (Y-27632) or NMII (blebbistatin, siRNA) reduces pMLC levels and promotes oligodendrocyte process extension. In the absence of NMII activity, expression of myelin protein and myelination are also enhanced. However, branching and myelin protein expression can be independently regulated.
Interestingly, although we have demonstrate that direct manipulation of NMII levels and/ or activity in OPC (pharmacological inhibition, protein knockdown or overexpression), affect their morphological differentiation, myelin protein expression and myelin formation [(Wang et al. 2008) and Fig 1 and 2]; we also found that inhibition of Fyn or knockdown with siRNA, while resulting in higher levels of pMLC and hence NMII activity only affects their branching but not the expression of myelin proteins (Fig. 3D and 3E). A similar result has been previously reported (Osterhout et al 1999), and indicates that in addition to Fyn other pathways are also involved in regulating myelin protein expression. Since our data indicate that NMII is a negative regulator of both oligodendrocyte branching and myelin protein expression, it will be important to establish how these processes are coordinated in vivo, and whether other pathways also regulate NMII activity. Of note, recent data from our laboratory has shown that downregulation of myosin light chain kinase (MLCK), an activator of NMII, promotes myelin protein expression in Schwann cells (Leitman et al. 2011). Whether MLCK has a similar role in OPC development is currently under study, but we hypothesize that regulation of NMII activity might be a common target for other pathways converging in oligodendrocyte cytoskeletal remodeling and differentiation (Miyamoto et al. 2007; Rajasekharan et al. 2009).
Our studies on the subcellular localization of NMII in oligodendrocytes further support a role for this molecule in active cytoskeleton remodeling. Immunocytochemistry and immunoelectromicroscopy show that NMII mostly accumulates at the cell cortex and in oligodendrocyte processes, where it colocalizes with actin and other actin-binding proteins such as GAP43 and β1 integrin. We hypothesize that following integrin engagement and Fyn activation (Fig. 5), local downregulation of NMII activity at the cell cortex releases actomyosin contraction and promotes process outgrowth and branching, changes that lead to plasma membrane extension and differentiation of oligodendrocytes. Local depletion of cortical NMII has been previously implicated in branch initiation and directional migration by endothelial cells (Fischer et al. 2009). We also observed accumulation of NMII in vesicle-like structures in association with the plasma membrane, suggesting that NMII might coordinate branching morphogenesis and local membrane remodeling by controlling the rate of membrane uptake and expansion. Data in support of this model includes the reported association between actomyosin contraction, rate of endocytosis and cell surface extension (Kippert et al. 2009), as well as the observation that downregulation of RhoA activity by neuronal signals promotes exocytosis and membrane extension by Olig-neu cells (Kippert et al. 2007). Furthermore, focal adhesion composition, assembly and maturation are also regulated by NMII-mediated contraction. A recent study by Kuo et al. (Kuo et al. 2011) showed that inhibition of NMII prevents focal adhesion maturation and promotes Rac-mediated lamellipodial extension. NMII also acts as a transducer of mechanical forces generated at focal adhesions (Clark et al. 2007). These mechanical signals are known to impact diverse cell functions, including lineage commitment and differentiation in many different cell types (Engler et al. 2006). Taken together the data strongly support a role for NMII as a central player in the coordination of integrin signaling, cytoskeleton remodeling and oligodendrocyte differentiation, and suggest that manipulation of NMII activity in oligodendrocyte progenitors might provide a novel therapeutic strategy to promote glial cell differentiation and remyelination in demyelinating conditions.
Supplementary Material
Acknowledgments
We thank Dr. Teresa Milner for her assistance with electron microscopy and Dr. Robert Adelstein for providing knockout mice tissue for our initial pilot studies. This work was supported by NIH Grant NS052259 to C. M-V; and a core facility grant (RR03037) from the NCRR. E.M. L was supported by an undergraduate fellowship from the HHMI.
References
- Alabed YZ, Grados-Munro E, Ferraro GB, Hsieh SH, Fournier AE. Neuronal responses to myelin are mediated by rho kinase. J Neurochem. 2006;96(6):1616–1625. doi: 10.1111/j.1471-4159.2006.03670.x. [DOI] [PubMed] [Google Scholar]
- Amano M, Ito M, Kimura K, Fukata Y, Chihara K, Nakano T, Matsuura Y, Kaibuchi K. Phosphorylation and activation of myosin by Rho-associated kinase (Rho-kinase) J Biol Chem. 1996;271(34):20246–20249. doi: 10.1074/jbc.271.34.20246. [DOI] [PubMed] [Google Scholar]
- Baer AS, Syed YA, Kang SU, Mitteregger D, Vig R, Ffrench-Constant C, Franklin RJ, Altmann F, Lubec G, Kotter MR. Myelin-mediated inhibition of oligodendrocyte precursor differentiation can be overcome by pharmacological modulation of Fyn-RhoA and protein kinase C signalling. Brain. 2009;132(Pt 2):465–481. doi: 10.1093/brain/awn334. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauer NG, Richter-Landsberg C, Ffrench-Constant C. Role of the oligodendroglial cytoskeleton in differentiation and myelination. Glia. 57(16):1691–1705. doi: 10.1002/glia.20885. [DOI] [PubMed] [Google Scholar]
- Behar TN. Analysis of fractal dimension of O2A Glial cells differentiating in vitro. Methods. 2001;24(4):331–339. doi: 10.1006/meth.2001.1203. [DOI] [PubMed] [Google Scholar]
- Benninger Y, Thurnherr T, Pereira JA, Krause S, Wu X, Chrostek-Grashoff A, Herzog D, Nave KA, Franklin RJ, Meijer D, Brakebusch C, Suter U, Relvas JB. Essential and distinct roles for cdc42 and rac1 in the regulation of Schwann cell biology during peripheral nervous system Development. J Cell Biol. 2007;177(6):1051–1061. doi: 10.1083/jcb.200610108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bito H, Furuyashiki T, Ishihara H, Shibasaki Y, Ohashi K, Mizuno K, Maekawa M, Ishizaki T, Narumiya S. A critical role for a Rho-associated kinase, p160ROCK, in determining axon outgrowth in mammalian CNS neuron. Neuron. 2000;26(2):431–441. doi: 10.1016/s0896-6273(00)81175-7. [DOI] [PubMed] [Google Scholar]
- Cahoy JD, Emery B, Kaushal A, Foo LC, Zamanian JL, Christopherson KS, Xing Y, Lubischer JL, Krieg PA, Krupenko SA, Thompson WJ, Barres BA. A transcriptome database for astrocytes, neurons, and oligodendrocytes: a new resource for understanding brain development and function. J Neurosci. 2008;28(1):264–278. doi: 10.1523/JNEUROSCI.4178-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen Y, Balasubramaniyan V, Peng J, Hurlock EC, Tallquist M, Li J, Lu QR. Isolation and culture of rat and mouse oligodendrocyte precursor cells. Nat Protoc. 2007;2(5):1044–1051. doi: 10.1038/nprot.2007.149. [DOI] [PubMed] [Google Scholar]
- Clark K, Langeslag M, Figdor CG, van Leeuwen FN. Myosin II and mechanotransduction: a balancing act. Trends Cell Biol. 2007;17(4):178–186. doi: 10.1016/j.tcb.2007.02.002. [DOI] [PubMed] [Google Scholar]
- Colognato H, Ramachandrappa S, Olsen IM, ffrench-Constant C. Integrins direct Src family kinases to regulate distinct phases of oligodendrocyte development. J Cell Biol. 2004;167(2):365–375. doi: 10.1083/jcb.200404076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Conti MA, Adelstein RS. Nonmuscle myosin II moves in new directions. J Cell Sci. 2008;121(Pt 1):11–18. doi: 10.1242/jcs.007112. [DOI] [PubMed] [Google Scholar]
- Conti MA, Even-Ram S, Liu C, Yamada KM, Adelstein RS. Defects in cell adhesion and the visceral endoderm following ablation of nonmuscle myosin heavy chain II-A in mice. J Biol Chem. 2004;279(40):41263–41266. doi: 10.1074/jbc.C400352200. [DOI] [PubMed] [Google Scholar]
- Dugas JC, Ibrahim A, Barres BA. A crucial role for p57(Kip2) in the intracellular timer that controls oligodendrocyte differentiation. J Neurosci. 2007;27(23):6185–6196. doi: 10.1523/JNEUROSCI.0628-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas JC, Tai YC, Speed TP, Ngai J, Barres BA. Functional genomic analysis of oligodendrocyte differentiation. J Neurosci. 2006;26(43):10967–10983. doi: 10.1523/JNEUROSCI.2572-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Einheber S, Schnapp LM, Salzer JL, Cappiello ZB, Milner TA. Regional and ultrastructural distribution of the alpha 8 integrin subunit in developing and adult rat brain suggests a role in synaptic function. J Comp Neurol. 1996;370(1):105–134. doi: 10.1002/(SICI)1096-9861(19960617)370:1<105::AID-CNE10>3.0.CO;2-R. [DOI] [PubMed] [Google Scholar]
- Engler AJ, Sen S, Sweeney HL, Discher DE. Matrix elasticity directs stem cell lineage specification. Cell. 2006;126(4):677–689. doi: 10.1016/j.cell.2006.06.044. [DOI] [PubMed] [Google Scholar]
- Feltri ML, Suter U, Relvas JB. The function of RhoGTPases in axon ensheathment and myelination. Glia. 2008;56(14):1508–1517. doi: 10.1002/glia.20752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischer RS, Gardel M, Ma X, Adelstein RS, Waterman CM. Local cortical tension by myosin II guides 3D endothelial cell branching. Curr Biol. 2009;19(3):260–265. doi: 10.1016/j.cub.2008.12.045. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox MA, Afshari FS, Alexander JK, Colello RJ, Fuss B. Growth conelike sensorimotor structures are characteristic features of postmigratory, premyelinating oligodendrocytes. Glia. 2006;53(5):563–566. doi: 10.1002/glia.20293. [DOI] [PubMed] [Google Scholar]
- Haviv L, Gillo D, Backouche F, Bernheim-Groswasser A. A cytoskeletal demolition worker: myosin II acts as an actin depolymerization agent. J Mol Biol. 2008;375(2):325–330. doi: 10.1016/j.jmb.2007.09.066. [DOI] [PubMed] [Google Scholar]
- Hirose M, Ishizaki T, Watanabe N, Uehata M, Kranenburg O, Moolenaar WH, Matsumura F, Maekawa M, Bito H, Narumiya S. Molecular dissection of the Rho-associated protein kinase (p160ROCK)-regulated neurite remodeling in neuroblastoma N1E-115 cells. J Cell Biol. 1998;141(7):1625–1636. doi: 10.1083/jcb.141.7.1625. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hsu SM, Raine L, Fanger H. Use of avidin-biotin-peroxidase complex (ABC) in immunoperoxidase techniques: a comparison between ABC and unlabeled antibody (PAP) procedures. J Histochem Cytochem. 1981;29(4):577–580. doi: 10.1177/29.4.6166661. [DOI] [PubMed] [Google Scholar]
- Kachar B, Behar T, Dubois-Dalcq M. Cell shape and motility of oligodendrocytes cultured without neurons. Cell Tissue Res. 1986;244(1):27–38. doi: 10.1007/BF00218378. [DOI] [PubMed] [Google Scholar]
- Kim HJ, DiBernardo AB, Sloane JA, Rasband MN, Solomon D, Kosaras B, Kwak SP, Vartanian TK. WAVE1 is required for oligodendrocyte morphogenesis and normal CNS myelination. J Neurosci. 2006;26(21):5849–5859. doi: 10.1523/JNEUROSCI.4921-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kimura K, Ito M, Amano M, Chihara K, Fukata Y, Nakafuku M, Yamamori B, Feng J, Nakano T, Okawa K, Iwamatsu A, Kaibuchi K. Regulation of myosin phosphatase by Rho and Rho-associated kinase (Rho-kinase) Science. 1996;273(5272):245–248. doi: 10.1126/science.273.5272.245. [DOI] [PubMed] [Google Scholar]
- Kippert A, Fitzner D, Helenius J, Simons M. Actomyosin contractility controls cell surface area of oligodendrocytes. BMC Cell Biol. 2009;10(1):71. doi: 10.1186/1471-2121-10-71. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kippert A, Trajkovic K, Rajendran L, Ries J, Simons M. Rho regulates membrane transport in the endocytic pathway to control plasma membrane specialization in oligodendroglial cells. J Neurosci. 2007;27(13):3560–3570. doi: 10.1523/JNEUROSCI.4926-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kuo JC, Han X, Hsiao CT, Yates JR, 3rd, Waterman CM. Analysis of the myosin-II-responsive focal adhesion proteome reveals a role for beta-Pix in negative regulation of focal adhesion maturation. Nat Cell Biol. 2011;13(4):383–393. doi: 10.1038/ncb2216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laursen LS, Chan CW, ffrench-Constant C. An integrin-contactin complex regulates CNS myelination by differential Fyn phosphorylation. J Neurosci. 2009;29(29):9174–9185. doi: 10.1523/JNEUROSCI.5942-08.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leitman EM, Tewari A, Horn M, Urbanski M, Damanakis E, Einheber S, Salzer JL, de Lanerolle P, Melendez-Vasquez CV. MLCK regulates Schwann cell cytoskeletal organization, differentiation and myelination. J Cell Sci. 2011;124(Pt 22):3784–3796. doi: 10.1242/jcs.080200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liang X, Draghi NA, Resh MD. Signaling from integrins to Fyn to Rho family GTPases regulates morphologic differentiation of oligodendrocytes. J Neurosci. 2004;24(32):7140–7149. doi: 10.1523/JNEUROSCI.5319-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Loudon RP, Silver LD, Yee HF, Jr, Gallo G. RhoA-kinase and myosin II are required for the maintenance of growth cone polarity and guidance by nerve growth factor. J Neurobiol. 2006;66(8):847–867. doi: 10.1002/neu.20258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Medeiros NA, Burnette DT, Forscher P. Myosin II functions in actin-bundle turnover in neuronal growth cones. Nat Cell Biol. 2006;8(3):215–226. doi: 10.1038/ncb1367. [DOI] [PubMed] [Google Scholar]
- Melendez-Vasquez CV, Einheber S, Salzer JL. Rho kinase regulates schwann cell myelination and formation of associated axonal domains. J Neurosci. 2004;24(16):3953–3963. doi: 10.1523/JNEUROSCI.4920-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miyamoto Y, Yamauchi J, Chan JR, Okada A, Tomooka Y, Hisanaga S, Tanoue A. Cdk5 regulates differentiation of oligodendrocyte precursor cells through the direct phosphorylation of paxillin. J Cell Sci. 2007;120(Pt 24):4355–4366. doi: 10.1242/jcs.018218. [DOI] [PubMed] [Google Scholar]
- Niederost B, Oertle T, Fritsche J, McKinney RA, Bandtlow CE. Nogo-A and myelin-associated glycoprotein mediate neurite growth inhibition by antagonistic regulation of RhoA and Rac1. J Neurosci. 2002;22(23):10368–10376. doi: 10.1523/JNEUROSCI.22-23-10368.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nodari A, Zambroni D, Quattrini A, Court FA, D'Urso A, Recchia A, Tybulewicz VL, Wrabetz L, Feltri ML. Beta1 integrin activates Rac1 in Schwann cells to generate radial lamellae during axonal sorting and myelination. J Cell Biol. 2007;177(6):1063–1075. doi: 10.1083/jcb.200610014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Osterhout DJ, Wolven A, Wolf RM, Resh MD, Chao MV. Morphological differentiation of oligodendrocytes requires activation of Fyn tyrosine kinase. J Cell Biol. 1999;145(6):1209–1218. doi: 10.1083/jcb.145.6.1209. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pereira JA, Benninger Y, Baumann R, Goncalves AF, Ozcelik M, Thurnherr T, Tricaud N, Meijer D, Fassler R, Suter U, Relvas JB. Integrin-linked kinase is required for radial sorting of axons and Schwann cell remyelination in the peripheral nervous system. J Cell Biol. 2009;185(1):147–161. doi: 10.1083/jcb.200809008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pfeiffer SE, Warrington AE, Bansal R. The oligodendrocyte and its many cellular processes. Trends Cell Biol. 1993;3(6):191–197. doi: 10.1016/0962-8924(93)90213-k. [DOI] [PubMed] [Google Scholar]
- Rajasekharan S, Baker KA, Horn KE, Jarjour AA, Antel JP, Kennedy TE. Netrin 1 and Dcc regulate oligodendrocyte process branching and membrane extension via Fyn and RhoA. Development. 2009;136(3):415–426. doi: 10.1242/dev.018234. [DOI] [PubMed] [Google Scholar]
- Sloane JA, Vartanian TK. Myosin Va controls oligodendrocyte morphogenesis and myelination. J Neurosci. 2007;27(42):11366–11375. doi: 10.1523/JNEUROSCI.2326-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somlyo AP, Somlyo AV. Signal transduction by G-proteins, rho-kinase and protein phosphatase to smooth muscle and non-muscle myosin II. J Physiol. 2000;522(Pt 2):177–185. doi: 10.1111/j.1469-7793.2000.t01-2-00177.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Song J, Goetz BD, Baas PW, Duncan ID. Cytoskeletal reorganization during the formation of oligodendrocyte processes and branches. Mol Cell Neurosci. 2001;17(4):624–636. doi: 10.1006/mcne.2001.0974. [DOI] [PubMed] [Google Scholar]
- Sperber BR, McMorris FA. Fyn tyrosine kinase regulates oligodendro cell development but is not required for morphological differentiation of oligodendrocytes. Development. 2001;63(4):303–312. doi: 10.1002/1097-4547(20010215)63:4<303::AID-JNR1024>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Straight AF, Cheung A, Limouze J, Chen I, Westwood NJ, Sellers JR, Mitchison TJ. Dissecting temporal and spatial control of cytokinesis with a myosin II Inhibitor. Science. 2003;299(5613):1743–1747. doi: 10.1126/science.1081412. [DOI] [PubMed] [Google Scholar]
- Tullio AN, Accili D, Ferrans VJ, Yu ZX, Takeda K, Grinberg A, Westphal H, Preston YA, Adelstein RS. Nonmuscle myosin II-B is required for normal development of the mouse heart. Proc Natl Acad Sci U S A. 1997;94(23):12407–12412. doi: 10.1073/pnas.94.23.12407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tullio AN, Bridgman PC, Tresser NJ, Chan CC, Conti MA, Adelstein RS, Hara Y. Structural abnormalities develop in the brain after ablation of the gene encoding nonmuscle myosin II-B heavy chain. J Comp Neurol. 2001;433(1):62–74. doi: 10.1002/cne.1125. [DOI] [PubMed] [Google Scholar]
- Umemori H, Kadowaki Y, Hirosawa K, Yoshida Y, Hironaka K, Okano H, Yamamoto T. Stimulation of myelin basic protein gene transcription by Fyn tyrosine kinase for myelination. J Neurosci. 1999;19(4):1393–1397. doi: 10.1523/JNEUROSCI.19-04-01393.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang H, Tewari A, Einheber S, Salzer JL, Melendez-Vasquez CV. Myosin II has distinct functions in PNS and CNS myelin sheath formation. J Cell Biol. 2008;182(6):1171–1184. doi: 10.1083/jcb.200802091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf RM, Wilkes JJ, Chao MV, Resh MD. Tyrosine phosphorylation of p190 RhoGAP by Fyn regulates oligodendrocyte differentiation. J Neurobiol. 2001;49(1):62–78. doi: 10.1002/neu.1066. [DOI] [PubMed] [Google Scholar]
- Wu D, Asiedu M, Adelstein RS, Wei Q. A novel guanine nucleotide exchange factor MyoGEF is required for cytokinesis. Cell Cycle. 2006;5(11):1234–1239. doi: 10.4161/cc.5.11.2815. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.