Abstract
Background
The long-term outcome of patients with chronic phase chronic myeloid leukemia treated with imatinib after failure of interferon alpha therapy has not been detailed.
Patients and Methods
368 patients were analyzed. Univariate and multivariate analyses for survival were conducted using standard statistical methods.
Results
Overall, 247 patients (67%) achieved complete cytogenetic response (CCyR). Of 327 patients studied, 207(63%) achieved major molecular response (MMR), and 99 (30%) had undetectable BCR-ABL levels at some time on therapy. The estimated 10-year survival rate was 68%, progression-free survival rate 67%, and event-free survival rate 51%. By multivariate analysis, age ≥ 60 years, hemoglobin < 10g/dl, marrow basophils ≥ 5%, any peripheral blasts, and clonal evolution were independent adverse factors for survival. The estimated 7-year survival by the presence of none (n=154), 1-2 (n=190), or ≥ 3 factors (n=24) were 93%, 70%, and 25% respectively (p <0.01). Achievement of MMR, CCyR, or partial cytogenetic response at 12 months were associated with significantly better 10-year survival rate by landmark analysis (10-year survival 80-90%) vs. achieving minor cytogenetic response or complete hematologic response (10-year survival 55-65%) vs. other response (10-year survival 10%). Using landmark analysis to include imatinib response at 12 months, achievement of major cytogenetic response or better (hazard ratio 0.12; p< 0.001) and complete hematologic response or minor cytogenetic response (hazard ratio 0.36; p=0.003) were significant favorable prognostic factors.
Conclusions
The estimated 10-year survival rate of 68% in patients with chronic myeloid leukemia receiving imatinib after interferon failure has improved.
INTRODUCTION
Imatinib mesylate, a Bcr-Abl selective tyrosine kinase inhibitor (TKI), has revolutionized the treatment and prognosis of patients with Philadelphia-chromosome (Ph)-positive chronic myeloid leukemia (CML). Imatinib is now frontline standard of care in CML (1-4). With imatinib therapy, a complete cytogenetic response (CCyR) is obtained in 80-85% of patients and is durable at 5 years in 67%. The estimated 8-year survival of patients is 84% - 93% depending on whether non-CML deaths are included or censored (2,4).
The incidence of CML in the United States is about 5000 new cases annually (5). With a median survival of 3-6 years prior to the widespread use of imatinib, the prevalence of CML was estimated to be about 15,000 to 30,000 cases. These patients would have been treated either with allogeneic stem cell transplant (SCT) or with interferon alpha-based therapy. Thus, it is estimated that about 15,000 – 20,000 patients in the U.S. might have been treated with imatinib after interferon. The short-term follow-ups of these patients showed favorable results (6-8), but it is important to determine their long-term prognosis to decide whether such therapy continues to be beneficial and which patients should be considered for alternative therapies including second generation TKIs and allogeneic SCT. This analysis focuses on the very long-term results of patients with chronic phase CML treated with imatinib after interferon failure at our institution. Compared with multi-institutional or pharmaceutical-sponsored studies, single institutional studies may follow up better the course of patients for events or progression after they are taken off the protocol imatinib regimen.
MATERIALS AND METHODS
Adults with Ph-positive CML in chronic phase treated at our institution with imatinib therapy following interferon failure were included in this analysis. These patients were entered on protocols available at our institution during the time period and approved by the institution review board (IRB). Informed consent was obtained according to institutional guidelines and in compliance with the declaration of Helsinki. The details of eligibility criteria, treatment plan and modifications have been previously detailed (6-8).
Response criteria were as previously described. A cytogenetic response is categorized as: complete (CCyR) if Ph positive metaphases were reduced to 0%; partial (PCyR), if Ph-positive metaphases were reduced to 1-35%; and minor, if Ph-positive metaphases were reduced to 36-90% (6-8). A major cytogenetic response (MCyR) included CCyR and PCyR. Survival was calculated from the start of therapy until death from any cause. Time to progression to accelerated or blastic phase (progression-free survival: PFS) was calculated from the start of therapy until the development of accelerated or blastic phase or until death from any cause on or off TKI therapy. Event-free survival (EFS) was calculated from the start of therapy until the development of any event leading to the patient being off the study, including loss of MCyR, loss of hematologic response, unacceptable toxicity, death from any cause or transformation to accelerated or blastic phase. Survival, PFS and EFS curves were analyzed by the Kaplan-Meier method and compared by the log-rank (9). The cumulative incidence rates for CCyR, complete molecular response (CMR), and major molecular response (MMR) were estimated by taking into account the competing risks for events. Univariate and multivariate analyses survival utilized standard statistical methods (10).
Follow up studies included marrow studies, cytogenetics and molecular analyses were performed every 3 months in the first year, every 6 months in years 2-5, and at least there after every 6-12 months. Of note, 95 patients were not followed up regularly at our institution after deciding not to continue on the imatinib study protocol; their subsequent outcome is followed only for death as an event. Therefore, they are censored at the time of last follow-up at M.D. Anderson Cancer Center unless they die, at which time they would be updated as death event for survival, PFS, and EFS.
RESULTS
A total of 368 patients with Ph positive CML in chronic phase following interferon alpha failure treated on imatinib therapy were analyzed. Their characteristics are detailed in Table 1. Their median age was 54 years; 121 patients (33%) were 60 years or older. The median follow up of patients on study is 114 months (range 1+ to 132 + months).
Table 1.
Characteristics of the Study Group (n=368)
| Characteristic | Category | No (%); or median (range) |
|---|---|---|
| Age (years) | ≥60; median | 121(33) 54 (21-82) |
| Splenomegaly | Present | 52/366 (14) |
| Hemoglobin (g/dl) | < 12; median | 149 (41) 12.3 (7.3 – 16.9) |
| WBC (×109/1) | > 50; median | 38 (10) 9.1 (1.8 – 240.5) |
| Platelets (×109/l) | > 450; median | 72 (20) 241 (81-1116) |
| % marrow blasts | ≥ 5 | 33 (9) |
| Peripheral blasts | Present | 67 (18) |
| % marrow basophils | ≥ 5 | 44 (12) |
| % peripheral basophils | ≥ 7 | 36 (10) |
| Cytogenetic clonal evolution | Yes | 66 / 368 (18) |
| Duration of chronic phase (mos) | < 12 | 36 (10) |
| 12-35 | 163 (44) | |
| ≥ 36 | 169 (46) | |
| % Philadelphia-positive pretreatment | < 90 | 94 (26) |
| Interferon alpha failure | Hematologic | 47 (13) |
| Cytogenetic | 171 (46) | |
| Intolerance | 148 (40) |
Overall, 247 patients (67%) achieved CCyR as their best cytogenetic response. Of 326 patients who had molecular studies, 207 (63%) achieved a major molecular response (MMR; BCR – ABL ratio on International scale ≤ 0.1%), and 99 (30%) had undetectable levels at sometime on therapy. The cumulative incidence of MMR by 7 years was 55% (95% C.I. 45-64) and the cumulative incidence of undetectable BCR levels was 35% (95% C.I. 29-42). Considering the total denominator of 368 patients, by intent-to-treat analysis at 7 years, 129 patients (35%) were in CCyR, 116 (32%) were in MMR, and 40(11%) had undetectable BCR-ABL levels.
With the current follow-up, the estimated 10-year survival rate is 68 %, PFS rate 67%, and EFS rate 51 % (Figure 1).
Figure 1.
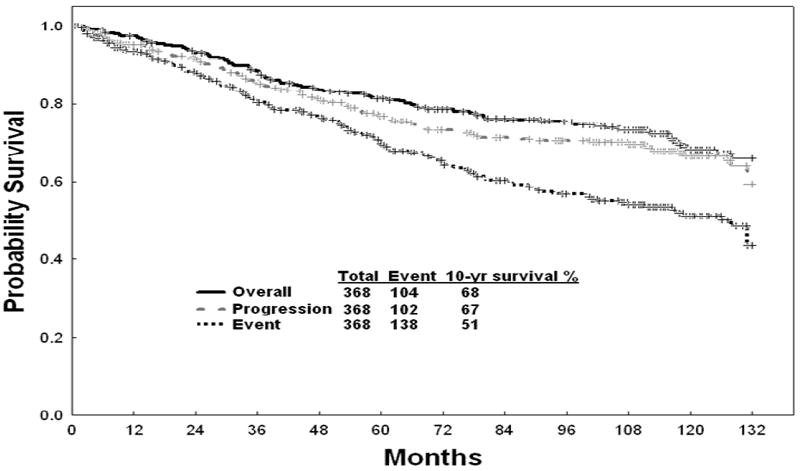
Survival, progression-free survival (PFS), and event-free survival (EFS)
At present, 104 patients (28 %) have died. The causes of death are shown in Figure 2. Death in transformation was caused by accelerated phase disease complications in 13 patients and by blastic phase disease in 39 patients. Among the latter, blastic phase was lymphoid in 9 patients, myeloid in 24 patients, and mixed lineage in 1 patient (5 patients with unknown blastic phase morphology).
Figure 2.
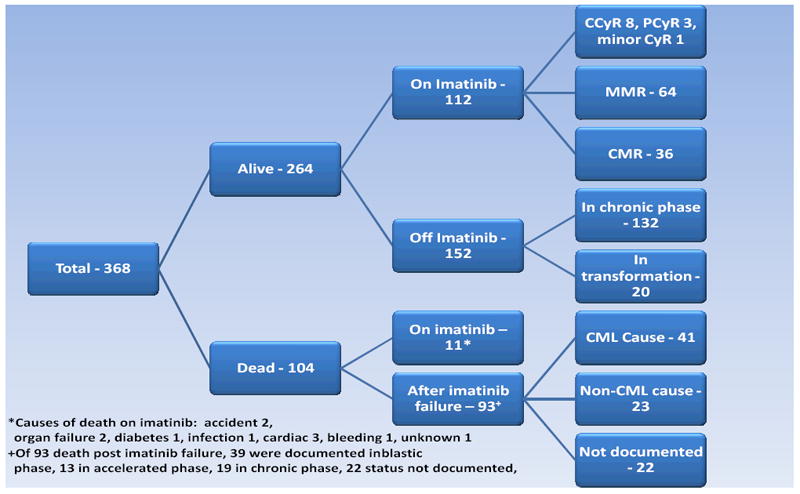
Patient Status
Among 256 patients (70%) who stopped imatinib therapy, 11 died on imatinib therapy from non-CML causes (Figure 2). One hundred eighteen patients (35%) stopped the study imatinib for reasons other than imatinib resistance: no further follow-up at our institution (n=95), patient request (n=4), physician decision (n=3), socio-economic factors (n=5), non-compliance (n=3), other malignancies or diseases (n=8). One hundred twenty seven patients failed imatinib: 86 remain in chronic phase, 41 have transformed (blastic phase, n=14; accelerated phase, n=27) (Figure 2).
Sixty-two of 127 patients (49%) who failed on imatinib therapy underwent salvage therapy with second-generation TKIs as first salvage (nilotinib 14 patients; dasatinib 34 patients; others 14 patients) and 27 patients underwent second salvage therapy with second-generation TKIs. The estimated 5-year survival of patients from the time of second generation TKIs as salvage therapy is 60%.
A total of 10 patients were referred for allogeneic SCT. Referral to transplant was after imatinib resistance in chronic phase in 4 patients and in transformation in 6 patients. At present, 3 patients (30%) are alive without evidence of disease. (1 relapsed and treated by dasatinib).
Prognostic factors associated with survival are shown in Table 2. A multivariate analysis identified older age (≥ 60 years), severe anemia (hemoglobin < 10g/dl), marrow basophils ≥ 5%, presence of any peripheral blasts, and clonal evolution at the start of imatinib therapy to be independent adverse factors. Overall survival by the number of adverse factors is shown in Figure 3 and Table 3. Three risk groups were generated to predict for long-term survival.
Table 2.
Prognostic Factors Associated with Survival
| Survival | |||||||
|---|---|---|---|---|---|---|---|
| Parameter | Category | n | %7-yr | %10-yr | Univariate p value | Hazard ratio | Multivariate p value |
| Age (years) | <60 | 247 | 82 | 75 | <0.001 | 2.2 | <0.001 |
| > = 60 | 121 | 66 | 55 | ||||
| CML duration (months) | <12 | 36 | 73 | 69 | 0.18 | NS | |
| 12-35 | 163 | 80 | 74 | ||||
| >=36 | 169 | 74 | 62 | ||||
| Splenomegaly | No | 314 | 80 | 73 | <0.001 | NS | |
| Yes | 52 | 50 | 34 | ||||
| Hemoglobin (g/dL) | <10 | 26 | 52 | 41 | 0.001 | 2.53 N.S. | 0.003 N.S. |
| 10-11.9 | 123 | 77 | 68 | ||||
| >=12 | 219 | 79 | 72 | ||||
| Platelet (×109/L) | <=450 | 296 | 80 | 71 | 0.001 | NS | |
| >450 | 72 | 64 | 55 | ||||
| WBC (×109/L) | <10 | 197 | 83 | 77 | <0.001 | NS | |
| 10-50 | 133 | 73 | 60 | ||||
| >50 | 38 | 53 | 49 | ||||
| %PB blasts | 0 | 301 | 82 | 74 | <0.001 | 2.45 | <0.001 |
| >=1 | 67 | 50 | 42 | ||||
| %M blasts | <5 | 335 | 78 | 69 | 0.03 | NS | |
| >=5 | 33 | 61 | 56 | ||||
| %PB baso | <7 | 332 | 78 | 69 | 0.11 | NS | |
| >=7 | 36 | 61 | 56 | ||||
| %M baso | <5 | 324 | 80 | 73 | <0.001 | 1.79 | 0.02 |
| >=5 | 44 | 48 | 33 | ||||
| CE | No | 301 | 79 | 71 | 0.006 | 1.63 | 0.04 |
| Yes | 66 | 64 | 56 | ||||
| % Ph+mets | <=90 | 111 | 89 | 79 | 0.002 | NS | |
| >90 | 256 | 71 | 64 | ||||
| IFN failure | Hematologic | 47 | 59 | 56 | <0.001 | NS | |
| Cytogenetic | 171 | 83 | 77 | ||||
| intolerance | 148 | 74 | 62 | ||||
PB =peripheral blood; M=marrow; baso = basophils; CE=clonal evolution; Ph+mets = Philadelphia + metaphases; IFN = interferon alpha;
Figure 3.
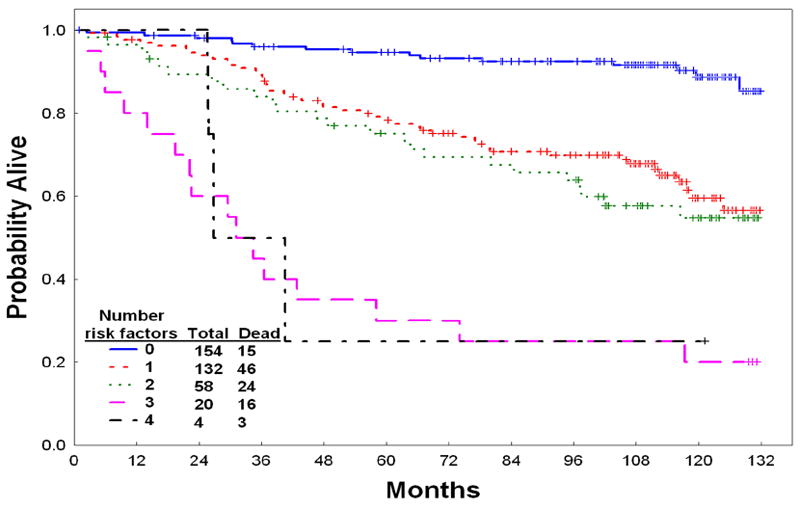
Survival by number of pretreatment adverse prognostic factors
Table 3.
Survival by Number of Adverse Factors
| Risk group | Number of adverse risk factors | Total / Dead | % 7-year Survival (95% C.I.) | % 10-year Survival (95% C.I) | Median (month) |
|---|---|---|---|---|---|
| Low | None | 154 / 15 | 93 (88-97) | 89 (83 – 95) | Not reached |
| Intermediate | 1-2 | 190 / 70 | 70 (63-77) | 58 (51-67) | Not reached |
| High | 3 or more | 24 / 19 | 25 (13-50) | 21 (10-45) | 39 (26-74) |
*Adverse factors: age ≥ 60 years, hemoglobin < 10 g / dl, marrow basophils ≥ 5%, any peripheral blasts, cytogenetic clonal evolution
The outcome of patients by their 12 months response to imatinib is shown in Figure 4. While patients achieving MMR, CCyR or PCyR by 12 months had better outcomes, still those achieving even a minor cytogenetic response or even a complete hematologic response had better outcome than non-responders.
Figure 4.
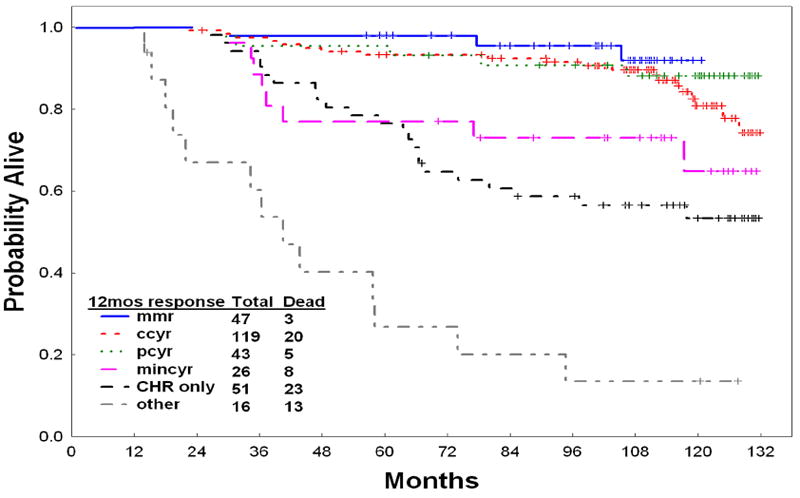
Survival by 12-month response to imatinib (landmark analysis at 12 months)
Using a landmark analysis to include imatinib response by 12 months, we identified achievement of MCyR (hazard ratio 0.12; p < 0.001), and complete hematologic response or minor cytogenetic response (hazard ratio 0.36; p = 0.003), as significant prognostic factors for long-term outcome after covariate adjusted.
DISCUSSION
This study presents the unique experience of the very long-term follow-up results from a single institution in patients treated with imatinib after interferon failure. We noted favorable results with a 10-year survival rate of 68 %, and EFS rate of 51 %.
Several recent studies have analyzed in different contexts the outcome of patients with CML on imatinib therapy, some of them including patients on imatinib post interferon failure (11, 12). In the study by Gambacorti-Passerini et al.(11), 832 patients treated on imatinib were reviewed. Among them, 478 patients received imatinib as second line therapy. The authors focus on the long-term side effects and safety profile of imatinib, emphasizing the need for multicenter independent assessment of outcomes in CML. In a population-based study of patients with CML diagnosed in Sweden from 1973 to 2008, Bjorkholm et al. demonstrate the impact of introducing imatinib therapy to a large CML population on long-term survival (12). In their analysis, the 5 year cumulative relative survival rates increased from 20-50% in the years 1973-2000 up to 80% since 2001, the year when imatinib therapy started having increased penetration into CML therapy in Sweden. The authors do not separate outcome by patients receiving imatinib as frontline versus salvage therapy, but demonstrate the overall impact of introducing imatinib therapy to the total CML population.
Our results and those of others (8, 11, 12) are encouraging and suggest a major change in the natural course of CML after interferon failure, which is of course partly expected from the frontline results of imatinib therapy. This clarifies the favorable outcome of patients post-interferon failure, a group whose median survival was 3 years when imatinib therapy was not available (7). In the current study, the estimated 10-year survival of patients is 68% compared with 20-30% among the historical group of patients who had failure on interferon therapy and had no access to imatinib (7). This suggests that most patients can be managed safely and effectively with imatinib post-interferon failure and do not have to consider the option of allogeneic SCT as long as they are in cytogenetic response. Even if they demonstrate imatinib resistance in chronic phase, subsequent salvage therapy with second generation TKIs (second salvage in the context of this analysis; interferon therapy frontline, imatinib therapy as salvage) can be safely entertained as a durable therapeutic approach, as long as the patients are sensitive and responsive to imatinib therapy.
In multi-institutional studies, patients on TKI therapy may often not be followed adequately for events occurring after being taken off the study TKI, except for death (1, 2). This is due to limited resources as well as difficulties in following patients (for example with marrow studies or even routine blood test which are not pre-specified in the protocol requirements). Our single institutional study offers the opportunity of more precise estimations of events following failure of TKI therapy, but it is still limited by lack of detailed follow up in 95 of 368 patients (26%). The variability in the definitions of EFS and progression-free survival and its impact on perceived differences in outcome has been highlighted recently (13, 14). In our study, we used the most conservative definition of EFS where all events are counted including the event of taking a patient off TKI therapy for any reason. Therefore, a 10-year EFS rate of 51% represents a reasonably favorable outcome for this study group.
In summary, this analysis confirms the efficacy and safety of continued imatinib therapy in patients with Ph-positive CML post interferon failure, and reassures patients who are currently on such therapy in the community oncology, and their treating physicians, that continuation of this therapy is reasonable. Undertaking allogeneic SCT should be considered only in patients who develop imatinib resistance.
Footnotes
AUTHOR CONTRIBUTIONS Hagop Kantarjian and Jenny Shan designed research, performed research, contributed vital new reagents or analytical tools, analyzed data and wrote the paper. Susan O’Brien, Guillermo Garcia-Manero and Jorge Cortes designed research, performed research, analyzed data and wrote the paper. Stefan Faderl, Farhad Ravandi, and Elias Jabbour performed research, analyzed data and wrote the paper.
DISCLOSURES Hagop Kantarjian and Jorge Cortes are consultants for Novartis and have research funding from Novartis, BMS and Pfizer. Elias Jabbour is a speaker for Novartis. Susan O’Brien, Guillermo Garcia-Manero, Stefan Faderl, Farhad Ravandi and Jenny Shan have nothing to disclose.
References
- 1.Druker B, Guilhot F, O’Brien S, Gathman I, Kantarjian H, et al. Five-year follow-up of patients receiving imatinib for chronic myeloid leukemia. The New England Journal of Medicine. 2006;355:2408–17. doi: 10.1056/NEJMoa062867. [DOI] [PubMed] [Google Scholar]
- 2.Deininger M, O’Brien SG, Guilhot F, et al. International randomized study of interferon vs STI571 (IRIS) 8-year follow up: sustained survival and low risk for progression or events in patients with newly diagnosed chronic myeloid leukemia in chronic phase treated with imatinib. Blood. 2009;114(22; Suppl):462. abstract 1126. [Google Scholar]
- 3.De Lavallade H, Apperley J, Khorashad J, et al. Imatinib for newly dignosed patients with chronic myeloid leukemia: incidence of sustained responses in an intention-to-treat analysis. JCO. 2008;26(20):3358–3363. doi: 10.1200/JCO.2007.15.8154. [DOI] [PubMed] [Google Scholar]
- 4.Kantarjian H, Talpaz M, O’Brien S, Jones D, Giles F, Garcia-Manero G, Faderl S, Ravandi F, Rios MB, Shan J, Cortes J. Survival benefit with imatinib mesylate versus interferon -α-based regimens in newly diagnosed chronic-phase chronic myelogenous leukemia. Blood. 2006;108:1835–1840. doi: 10.1182/blood-2006-02-004325. [DOI] [PubMed] [Google Scholar]
- 5.Cortes J, Silver R, Kantarjian H. Chronic Myeloid Leukemia. In: Hong W, Bast R, Hait W, Kufe D, Pollack R, Weichselbaum R, Holland J, Frei E, editors. Cancer Medicine. 8. People’s Medical Publishing House-USA; Shelton, Connecticut: 2010. pp. 1582–1590. [Google Scholar]
- 6.Kantarjian HM, Cortes JE, O’Brien S, et al. Long-term survival benefit and improved complete cytogenetic and molecular response rates with imatinib mesylate in Philadelphia chromosome-positive chronic-phase chronic myeloid leukemia after failure of interferon-alpha. Blood. 2004;104:1979–1988. doi: 10.1182/blood-2004-02-0711. [DOI] [PubMed] [Google Scholar]
- 7.Kantarjian H, O’Brien S, Cortes J, Giles F, Shan J, Rios MB, Faderl S, Verstovsek S, Garcia-Manero G, Wierda W, Kornblau S, Ferrajoli A, Keating M, Talpas M. Survival advantage with imatinib mesylate therapy in chronic-phase chronic myelogenous leukemia (CML-CP) after IFN-α failure and in late CML-CP, comparison with historical controls. Clinical Cancer Research. 2004;10:68–75. doi: 10.1158/1078-0432.ccr-1035-3. [DOI] [PubMed] [Google Scholar]
- 8.Hochhaus A, Druker B, Sawyers C, Guilhot F, Schiffer C, Cortes J, et al. Favorable long-term follow up results over 6 years for response, survival, and safety with imatinib mesylate therapy in chronic-phase chronic myeloid leukemia after failure of interferon-α treatment. Blood. 2008;111:1039–1043. doi: 10.1182/blood-2007-07-103523. [DOI] [PubMed] [Google Scholar]
- 9.Kaplan EL, Maier P. Non-parametric estimation from incomplete observations. J Am Stat Assoc. 1965;53:457–81. [Google Scholar]
- 10.Cox DR. Regression models and life tables. J R Stat Soc B. 1972;34:187–220. [Google Scholar]
- 11.Gambacorti-Passerini C, Anolini L, Francois-Xavier M, et al. Multicenter independent assessment of outcomes in chronic myeloid leukemia patients treated with imatinib. JNCI. 2011;13:553–561. doi: 10.1093/jnci/djr060. [DOI] [PubMed] [Google Scholar]
- 12.Bjorkholm M, Ohm L, Eloranta S, et al. Success story of targeted therapy in chronic myeloid leukemia: a population-based study of patients diagnosed in Sweden from 1973 to 2008. JCO. 2011;29(18):2514–2520. doi: 10.1200/JCO.2011.34.7146. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Kantarjian H, Cortes J. Considerations in the management of patients with Philadelphia chromosome-positive chronic myeloid leukemia receiving tyrosine kinase inhibitor therapy. JCO. 2011;29(12):1512–1516. doi: 10.1200/JCO.2010.33.9176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Kantarjian H, O’Brien S, Jabbour E, Shan J, Ravandi F, Kadia T, Faderl S, Garcia-Manero G, Borthakur G, Cortes J. The impact of treatment endpoint definitions on perceived differences in long-term outcome with tyrosine kinase inhibitor therapy in chronic myeloid leukemia. JCO. doi: 10.1200/JCO.2010.33.4169. e-pub July 11, 2010.33.4169. [DOI] [PMC free article] [PubMed] [Google Scholar]


