Abstract
Interleukin 17A (IL-17) is the signature cytokine produced by TH17 cells and has been implicated in host defense against infection and the pathophysiology of autoimmunity and cardiovascular disease. Little is known, however, about the influence of IL-17 on endothelial activation and leukocyte influx to sites of inflammation. We hypothesized that IL-17 would induce a distinct pattern of endothelial activation and leukocyte recruitment when compared to the TH1 cytokine, IFNγ. We found that IL-17 alone had minimal activating effects on cultured endothelium, while the combination of TNFα and IL-17 produced a synergistic increase in the expression of both P-selectin and E-selectin. Using intravital microscopy of the mouse cremaster muscle, we found that TNFα and IL-17 also led to a synergistic increase in E-selectin dependent leukocyte rolling on microvascular endothelium in vivo. In addition, TNFα and IL-17 enhanced endothelial expression of the neutrophilic chemokines CXCL1, CXCL2, and CXCL5, and led to a functional increase in leukocyte transmigration in vivo and CXCR2-dependent neutrophil but not T-cell transmigration in a parallel-plate flow chamber system. By contrast, endothelial activation with TNFα and IFNγ preferentially induced the expression of the integrin-ligands ICAM1 and VCAM1, as well as the T-cell chemokines CXCL9, CXCL10, and CCL5. These effects were further associated with a functional increase in T-cell but not neutrophil transmigration under laminar shear flow. Overall, these data show that IL-17 and TNFα act in a synergistic manner to induce a distinct pattern of endothelial activation that sustains and enhances neutrophil influx to sites of inflammation.
Introduction
Interleukin 17A (IL-17) is the signature cytokine secreted by TH17 cells, a recently discovered subset of pro-inflammatory CD4+ T cells that were first described in 2005 (1, 2). IL-17 production is characteristic of TH17 cells in the setting of chronic inflammation, but it can also be secreted by a wide variety of cell types associated with innate immune responses, including γδ T cells, NK cells, and neutrophils (3–5). Given the ubiquitous expression of the IL-17 receptor complex, IL-17 signaling has relevance for a range of cellular targets and organ-specific immune functions (6, 7). For example, IL-17 has an important physiologic role in mucosal immunity and host defense against extracellular bacteria and fungi, as was demonstrated in studies showing increased susceptibility to staphylococcal infection and candidiasis in mice lacking IL-17 (8–10). By contrast, aberrant IL-17 production is highly associated with inflammatory pathology in the setting of autoimmune disease. Elevated IL-17 expression has been observed in inflamed tissues of patients with rheumatoid arthritis, psoriasis, and multiple sclerosis, and IL-17 deletion attenuates disease severity in mouse models of autoimmunity (11–16). Interestingly, recent studies have also proposed a pathologic role for IL-17 in atherosclerosis, plaque destabilization, and other inflammatory vascular disorders, including cardiac allograft vasculopathy and Kawasaki’s disease (17–24). Accordingly, targeted IL-17-depletion has already been adopted as a therapeutic strategy and is currently being tested in clinical trials for several human autoimmune diseases (25, 26).
Despite the heightened interest in IL-17 over recent years, relatively little is known from a mechanistic standpoint as to its unique effects in directing and sustaining leukocyte influx to sites of inflammation, particularly in relation to other T-cell associated cytokines, such as IFNγ. In addition, although there are many putative cellular targets of IL-17, there is little information on which cell types are most important in mediating its pro-inflammatory effects. As was demonstrated in early studies by Gimbrone and colleagues, activation of the vascular endothelium by pro-inflammatory cytokines, such as TNFα and IL-1β, results in the induction of adhesion molecules (e.g. E-selectin) and chemokines (e.g. CXCL8) that play a central role in the now well-known cascade of leukocyte tethering, slow rolling, firm adhesion, and transendothelial migration (27–30). Differences in the expression of these molecules by vascular endothelium in response to different cytokines can contribute to the unique temporal and spatial patterns of leukocyte subset recruitment to sites of inflammation. For example, the TH1 cytokine IFNγ has been shown to modulate endothelial activation and antigen-presenting function, and can also synergize with cytokines such as TNFα to augment endothelial expression of specific adhesion molecules and chemokines (31, 32). Recent data from our laboratory has demonstrated the inhibitory effect of TGF-β secretion by regulatory T cells on endothelial activation and leukocyte recruitment in response to activation by TNFα (33). Currently, however, it is unclear whether IL-17 stimulates endothelial activation or modulates the effects of other pro-inflammatory cytokines. One prior study has suggested that IL-17 alone is capable of potently activating human umbilical vein endothelial cells in vitro, although this finding contrasts with the experience of our own laboratory, in which IL-17 alone had little effect on the activation of this cell type (34). In addition, studies in non-endothelial cell types have suggested that the predominant effect of IL-17 activity relates to the synergistic induction of genes such as CXCL1 and IL-6 in response to co-treatment with TNFα (35, 36).
In the current study, we examined the effects of IL-17 on endothelial activation by evaluating its ability to stimulate the expression of selectins, integrin-ligands, and chemokines, and to potentiate the effects of the classical pro-inflammatory cytokine, TNFα. In addition, we have compared the impact of IL-17 on endothelial activation to that of the signature TH1 cytokine, IFNγ. Furthermore, we have tested the functional effects of endothelial activation with IL-17 by using two widely validated models of leukocyte recruitment; namely, an in vitro parallel plate flow chamber system, which models leukocyte-endothelial interactions under physiologically relevant levels of laminar shear flow, as well as intravital microscopy of leukocyte rolling on microvascular endothelium in vivo. Finally, using blocking antibodies and transgenic mice, we attempted to elucidate the specific adhesion molecules and chemokine pathways that serve as functional mediators of IL-17 pro-inflammatory activity. We report that IL-17 and TNFα promote a distinct pattern of endothelial activation that sustains and enhances neutrophil influx to sites of inflammation, which is primarily mediated through the synergistic induction of endothelial selectins and CXCR2-activating chemokines.
Materials and Methods
Mice
All mice were maintained in a pathogen-free facility at the New Research Building of Harvard Medical School in accordance with the animal research guidelines established by the Committee of Animal Research and the National Institutes of Health. C57BL/6 (wild-type [WT]) mice were purchased from Charles River Laboratories (Wilmington, MA). CXCR2 deficient mice (Cxcr2−/−) on the BALB/cJ background and age-matched controls were purchased from Jackson Laboratories (Bar Harbor, ME). All mice were used at 8–12 wk of age.
Primary isolation and culture of mouse heart endothelial cells
Mouse heart endothelial cells (MHEC) were prepared from cell suspensions of cardiac digests by a sequential positive-selection technique using immunomagnetic beads specific for PECAM-1 (CD31) and ICAM-2 (CD102), as previously described (37). Isolated cells were cultured in gelatin-coated tissue culture plates in growth medium containing 20% FBS, 100 mg/ml porcine heparin, and 100 mg/ml EC mitogen from Biomedical Technologies (Stoughton, MA). Cells were harvested at >80% confluence with trypsin-EDTA and either cultured for experimental assay or frozen in liquid nitrogen for future use.
Flow cytometry for adhesion molecule expression
Confluent MHEC were stimulated for 0.5–16h with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml). Cells were harvested with trypsin-EDTA and stained with fluorescently labeled antibodies (5 µg/ml) against endothelial adhesion molecules, including P-selectin, E-selectin, ICAM1, ICAM2, VCAM1, MAdCAM1, PECAM1, and VE-cadherin. Cells were then analyzed by flow-cytometry without prior fixation on a FACScalibur cytometer by BD Biosciences (San Jose, CA) and FlowJo 9.3.1 software by TreeStar (Ashland, OR).
Quantitative RT-PCR analysis
Confluent MHEC were activated with recombinant cytokines for 8h and RNA was extracted with an RNeasy kit from Qiagen (Valencia, CA), according to the manufacturers instructions. RNA was reverse-transcribed using the ThermoScript system by Invitrogen (Grand Island, NY) and amplified by RT-PCR with SYBR Green and a Step-One Detection System from Applied Biosystems (Carlsbad, CA). Primer sequences are available upon request.
Intravital microscopy of leukocyte recruitment during microvascular inflammation in vivo
Intravital microscopy (IVM) of leukocyte recruitment to post-capillary venules of the mouse cremaster muscle was performed at various time points following intrascrotal injection of IL-17A (1000ng/mouse) and/or TNFα (100ng/mouse). Mice were anesthetized and surgical exteriorization of the cremaster muscle was performed, as previously described (38, 39). In some studies, a cannula was also inserted into the jugular vein to allow for the injection of specific anti-E-selectin (9A9) blocking antibodies, as previously described (40). The entire cremaster preparation was typically accomplished in <15 min. Microvessel data was obtained using a specialized Olympus FV 1000 intravital microscope (Center Valley, PA) fitted with an Olympus 40x water immersion objective. Centerline RBC velocity (Vcl) in each vessel was measured in real-time with a dual photodiode velocimeter (Texas A&M, College Station, TX). Leukocyte rolling interactions were recorded from 5–8 vessels per mouse using an Olympus DP71 CCD video camera and Olympus FluoView 1000 imaging software. Systemic leukocyte counts for each mouse were determined from a 50 µl retroorbital blood sample and analyzed with a Hemavet 950FS by Drew Scientific (Waterbury, CT). Videos were analyzed off-line with the NIH software package ImageJ (Bethesda, MD). Wall shear rate (Wsr) was determined according to the equation Wsr=2.133[(8x0.625xVcl)/Dv)], where Dv is the measured vessel diameter (µm). The volumetric blood flow (Q) was calculated from the equation Q=(Vcl)(0.625)(Acs), where Acs is the cross-sectional area of the cylindrical vessel and 0.625 is an empirical correction factor. The rolling leukocyte flux was determined by counting the number of cells passing through a plane perpendicular to the vessel axis per min. The average leukocyte rolling velocity was calculated by measuring the distance traveled and elapsed time for 10 leukocytes in each vessel. The total number of rolling leukocytes per 100 µm vessel segment was determined by dividing the rolling leukocyte flux (cells/min) by the average rolling velocity (expressed as µm/min) and multiplying by 100 µm, as previously described (41). The number of transmigrated leukocytes per vessel was determined by counting the average number of perivascular cells in a 50 × 100 µm area adjacent to the vessel wall, as previously described (42). All parameters were also expressed as a ratio normalized for differences in vessel diameter, volume, and systemic leukocyte count.
Isolation and purification of neutrophils from mouse bone-marrow
Neutrophils were isolated from bone marrow extracted from the femur and tibias of WT mice using an EasySep kit from Stem Cell Technologies (Vancouver, BC) according to the manufacturer’s instructions. Briefly, bones were flushed with isolation media and the resulting cell suspension was incubated with a negative-selection cocktail of biotinylated antibodies against mouse CD4, CD5, CD11c, CD45R/B220, CD49b, CD117, TER119, and F4/80, followed by sequential incubations with bispecific anti-biotin/anti-dextran antibody complexes and dextran-coated magnetic particles. Contaminating cells were then removed by magnet separation. Neutrophil purity was typically >90% as determined by flow cytometry for CD11b / Ly6G double-positive cells.
Differentiation of effector CD4+ T cells
Effector CD4+ T cells were prepared from naïve CD4+ cells isolated from the spleens of WT mice using anti-CD4 MACS beads from Miltenyi Biotec (Bergisch Gladbach, Germany). Naïve CD4+ cells were differentiated with plate-bound anti-CD3ε (5 µg/ml) as well as soluble anti-CD28 (2 µg/ml) and IL-2 (15 U/ml). On day 3, cultures were diluted 1:2 in IL-2 (15 U/ml) containing media and transferred to fresh culture plates without anti-CD3ε coating. Media containing IL-2 (15 U/ml) was supplemented again on day 5 and cells were harvested for use on day 6.
Parallel-plate flow chamber assay for leukocyte adhesion and transendothelial migration
Neutrophil and effector CD4+ T cell interactions with MHEC monolayers under laminar shear stress were measured with a parallel plate flow chamber, as previously described (37, 43). Briefly, MHEC were cultured on fibronectin-coated 25-mm glass coverslips and incubated for 48h to allow for monolayer formation. Monolayers were then stimulated for 16h with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml) before being placed in a flow chamber on the stage of a Nikon Eclipse Ti microscope (Melville, NY) controlled by MetaMorph software (Sunnyvale, CA). Neutrophils or effector CD4+ T cells were isolated as described above and suspended at 0.5–1 × 107 cells/ml in flow buffer. A bolus of 0.5–1 × 106 neutrophils or T cells was then perfused across the monolayer at 37C and allowed to bind at a shear stress of 0.2 dynes/cm2. Shear was then increased to 0.8 dynes/cm2 and subsequent interactions of perfused leukocytes with the monolayer were recorded for 10 min. In some studies, monolayers were pre-incubated for 20 min with blocking antibodies (20 µg/ml) against P-selectin (RB40.34), E-selectin (9A9), ICAM1 (YN1), and ICAM2 (3C4). Accumulated and transmigrated leukocytes were then quantified off-line using ImageJ software. Accumulated cells were defined as the average number of adherent or transmigrated cells in 5 fields after the 10 min video period. Transmigrated cells were defined as the proportion of initially adherent cells that transmigrated during the 10 min video period.
Statistical analysis
Data are expressed as the mean +/− SEM unless otherwise stated. Chi-squared and student t test were used for two-group comparisons, ANOVA with Bonferonni post-tests for multiple group comparisons, and simple linear-regression analysis to control for microvessel shear stress during the intravital microscopy studies. All statistical analyses were performed in Prism 5.0 software by GraphPad (La Jolla, CA) and considered statistically significant at p < 0.05.
Results
IL-17 and TNFα promote a synergistic increase in endothelial selectin expression
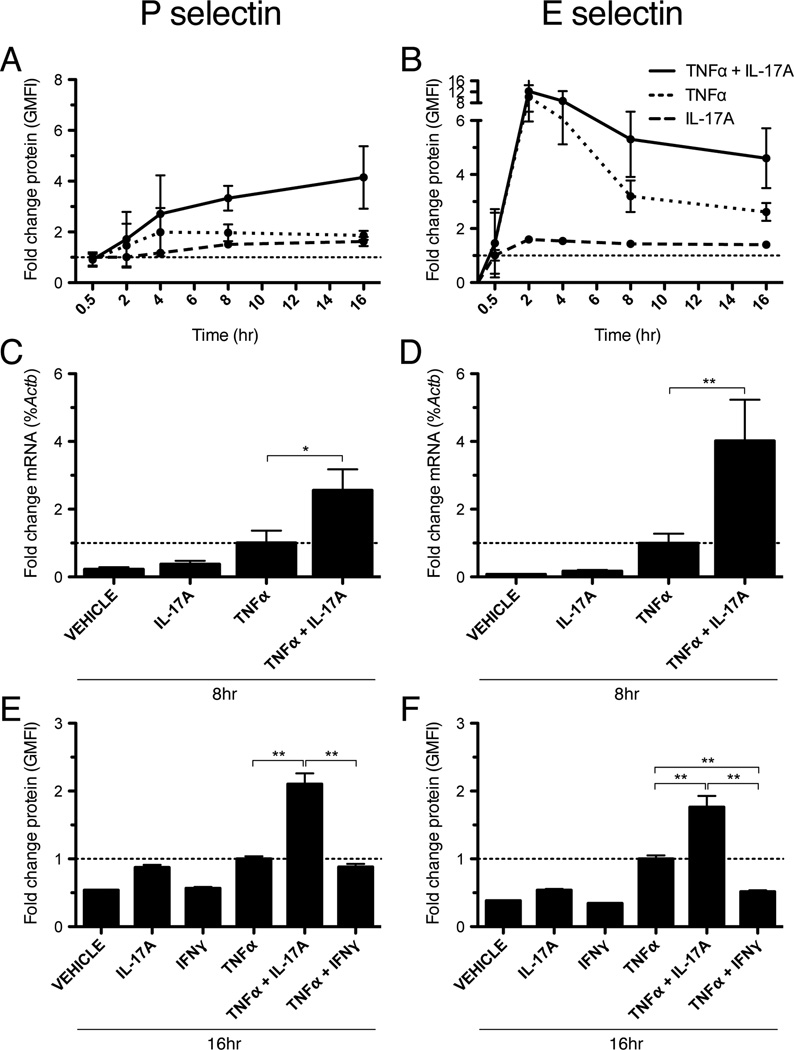
We tested the ability of IL-17 to promote endothelial selectin expression alone or in combination with TNFα. Given the distinct temporal dynamics of P-selectin and E-selectin expression during acute inflammation, we first conducted time-course experiments to evaluate the expression of these molecules following cytokine stimulation. MHECs were grown in monolayers, stimulated with recombinant IL-17 and/or TNFα, and analyzed for surface protein expression of P and E-selectin by immunofluorescence staining and flow cytometry. In these studies, we found that stimulation of MHEC with IL-17 alone had no statistically significant effect on selectin expression relative to vehicle (Fig. 1A, B). However, we found that co-treatment with IL-17 and TNFα led to a marked, synergistic increase in the surface expression of both P- and E-selectin (Fig. 1A, B). Interestingly, although there was no difference in the peak expression of E-selectin at 2h with IL-17 and TNFα versus TNFα alone, combined treatment did lead to a clear increase in the duration of E-selectin surface expression that was sustained up to 16h (Fig. 1B). Consistent with these findings, we also observed that combination IL-17 and TNFα produced a synergistic increase in Psel and Esel mRNA levels after 8h of activation, as determined by quantitative RT-PCR (Fig. 1C, D). By contrast, we found that co-treatment with TNFα and the signature TH1 cytokine IFNγ for 16h led to a reduction in E-selectin expression and had no effect on P-selectin expression relative to TNFα alone (Fig. 1E, F). Finally, we found that co-stimulation with IL-17 and IL-1β did not lead to an increase in endothelial selectin expression relative to IL-1β alone (data not shown).
Figure 1.
IL-17A and TNFα synergistically increase endothelial selectin expression in vitro. A and B, Time course (0.5 – 16h) of selectin surface protein expression by MHEC in response to IL-17A (100ng/ml) and/or TNFα (50ng/ml), as determined by immunofluorescent flow cytometry. Data are represented as the fold change in the geometric mean of the fluorescence intensity (GMFI) relative to treatment with vehicle alone. Error bars show the 95% confidence interval for each time point. C and D, qRT-PCR of selectin mRNA expression after 8h of stimulation with IL-17A (100ng/ml) and/or TNFα (50ng/ml). Data are normalized to the level of β-actin (Actb) expression and represented as the mean fold change in %Actb expression +/− SEM relative to treatment with TNFα alone. E and F, Flow cytometry of selectin expression after 16h of treatment with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml). Data are represented as the mean fold change in GMFI +/− SEM relative to treatment with TNFα alone. All data are from at least 3 independent experiments. *, p < 0.05. **, p < 0.01.
Endothelial activation with IL-17 and TNFα synergistically enhances leukocyte rolling in vivo
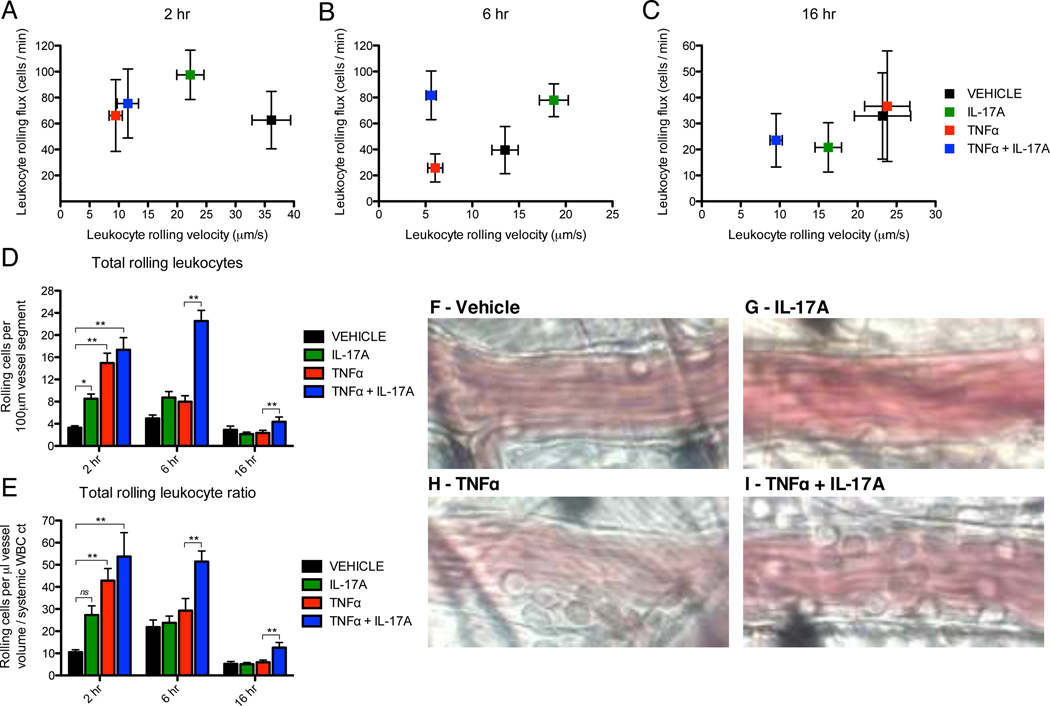
Given the marked synergistic effect on selectin expression in vitro, we further hypothesized that endothelial activation with IL-17 and TNFα would produce a functional increase in leukocyte recruitment in vivo. We chose to test this hypothesis given the established role of selectins as key mediators of leukocyte-endothelial interactions during the initial steps of the leukocyte adhesion cascade (30). Accordingly, we used intravital microscopy of the mouse cremaster muscle, which is a well-validated in vivo model of selectin-dependent leukocyte rolling on microvascular endothelium (39, 40, 44). In these studies, intrascrotal injections of IL-17 and/or TNFα were administered and then endogenous leukocyte rolling in post-capillary venules of the cremaster muscle was visualized at 2, 6, and 16h after injection. Baseline hemodynamic parameters, including systemic leukocyte count, vessel diameter (range 20–40 µm), and intravascular shear stress (range 400–1200 s−1) were similar between treatment groups (Sup Table 1). A small reduction in shear rate was seen with combination IL-17 and TNFα, which was statistically significant at early (2 and 6h) but not late (16h) time points relative to TNFα alone. Importantly, these small differences in hemodynamic status did not alter any of the central findings reported below, as linear regression analysis demonstrated that all significant treatment effects were maintained across a wide range of shear rates (Sup Fig. 1). We first analyzed the leukocyte rolling flux through each vessel (rolling cells / min) as a function of the average leukocyte rolling velocity (Fig. 2A – C). At 2h of activation, IL-17 alone produced only a marginal increase in leukocyte rolling flux relative to vehicle treatment and did not reduce average rolling velocity to levels seen with TNFα (Fig. 2A – C). In addition, there was no difference in rolling parameters at 2h when comparing TNFα alone to combination IL-17 and TNFα. After 6h of activation, however, IL-17/TNFα co-treatment produced a persistent elevation in the leukocyte rolling flux relative to TNFα alone (Fig. 2B). Importantly, this effect was not attributable to changes in average rolling velocity (Fig. 2B) or vessel shear stress (Sup Fig. 1A). Furthermore, although the leukocyte rolling flux in response to IL-17 and TNFα had decreased by 16h, the average rolling velocity remained low, suggesting that the total number of rolling leukocytes in the IL-17/TNFα treated vessels was greater than those treated with TNFα alone. This conclusion is based on the fact that more rolling leukocytes are required to maintain a given leukocyte flux as the average rolling velocity decreases. Accordingly, we quantified the total number of rolling leukocytes per 100 µm vessel segment for each treatment condition (Fig. 2D) and further expressed this number as a ratio accounting for differences in vessel diameter, volume, and the systemic leukocyte count in each mouse (Fig. 2E). Representative images of total leukocyte rolling in each vessel after 6h are also included (Fig. 2F – I). Although we did observe a trend towards a positive effect on total leukocyte rolling at 2h with IL-17 alone, it was statistically significant only in the unadjusted analysis and did not persist at 6 and 16h of activation (Fig. 2D). However, stimulation with both IL-17 and TNFα synergistically increased the total number of rolling leukocytes per vessel, which was maximal at 6h of stimulation but also remained elevated at 16h relative to TNFα alone (Fig. 2D, E). Representative images of leukocyte-endothelial rolling interactions after 6h of cytokine activation are shown in Fig. 2F–I, with maximal levels observed after stimulation with IL-17/TNFα (Fig. 2I) relative to TNFα alone (Fig. 2H), IL-17 alone (Fig. 2G), or vehicle (Fig. 2F). Importantly, these effects were not attributable to vessel hemodynamic status, as linear regression analysis showed clear differences in total leukocyte rolling with IL-17/TNFα co-treatment versus TNFα alone across a wide range of shear rates (Sup Fig. 1B).
Figure 2.
IL-17A and TNFα synergistically enhance rolling interactions of leukocytes with microvascular endothelium in vivo. Leukocyte rolling behavior in the mouse cremaster muscle was quantified by intravital microscopy at three time points (2, 6, 16h) following intrascrotal injection with IL-17A (1000ng/mouse) and/or TNFα (100ng/mouse). A – C, Leukocyte rolling flux versus average leukocyte rolling velocity for each treatment condition. Data are represented as mean +/− 95% confidence interval. D and E, Total number of rolling leukocytes per 100 µm vessel segment (D) and expressed as a ratio (E) normalized for differences in vessel diameter, volume, and systemic leukocyte count. Data are represented as mean +/− SEM. F – I, Representative still images of total rolling leukocytes for each treatment condition after 6h of stimulation. Sizes of experimental groups and hemodynamic parameters for all data are summarized in Sup Table 1. *, p < 0.05. **, p < 0.01.
Endothelial activation with IL-17 and TNFα synergistically enhances the duration of leukocyte slow-rolling in vivo
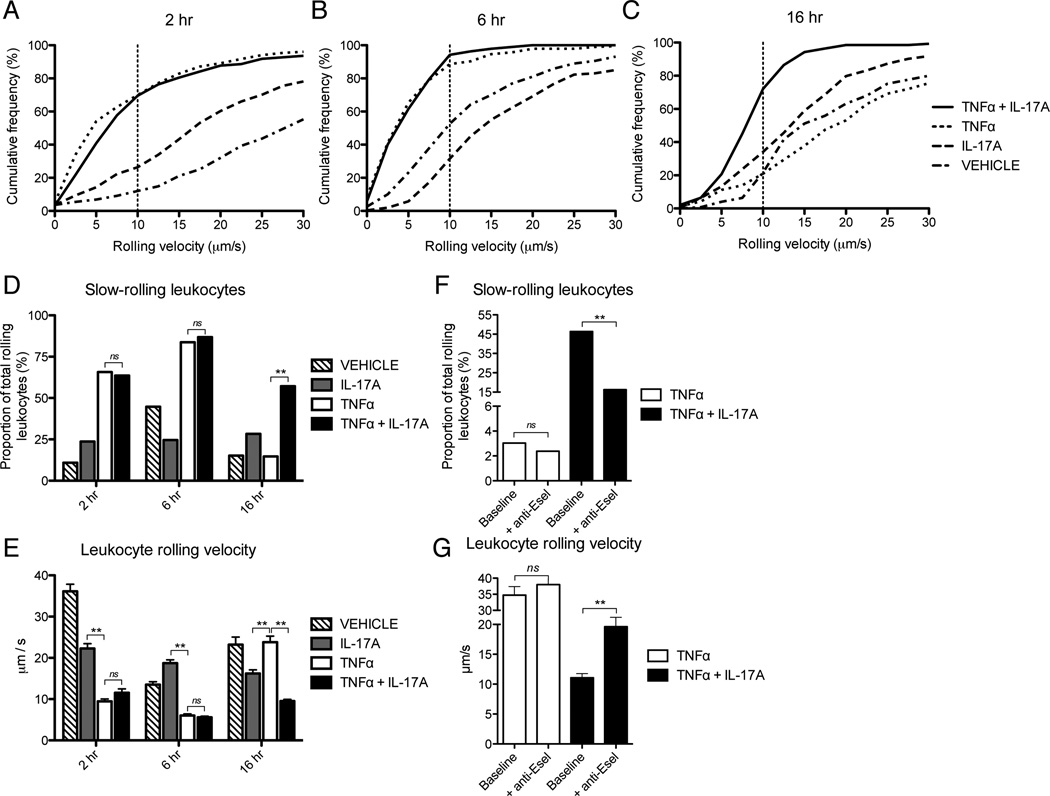
Given the synergistic effects of IL-17 and TNFα on selectin expression and leukocyte rolling demonstrated above, we anticipated that IL-17 and TNFα would also potentiate the extent and duration of leukocyte slow-rolling. The process of slow-rolling represents a key step in the leukocyte adhesion cascade, whereby reduced rolling velocity increases leukocyte exposure to chemokines presented on the apical surface of the endothelium and facilitates leukocyte integrin activation and firm adhesion (45). In these studies we analyzed the average rolling velocity of leukocytes in each vessel, as well as the proportion of slow-rolling leukocytes with velocities of less than 10 µm/sec. First, consistent with the minimal impact of IL-17 on endothelial E-selectin expression in vitro (Fig. 1), we found that treatment with IL-17 alone did not increase the proportion of slow-rolling leukocytes at any time point (Fig 3A – E). By contrast, we found that treatment with TNFα alone enhanced the cumulative frequency of slow-rolling leukocytes (Fig. 3A, B, E) and decreased the average rolling velocity at both 2 and 6h (Fig. 3D), which is consistent with published literature. At 16h of stimulation, however, the effect of TNFα on leukocyte slow-rolling had decreased back to levels seen with vehicle treatment alone (Fig. 3C – E). Interestingly, although slow-rolling and average leukocyte rolling velocity were comparable with IL-17 and TNFα treatment versus TNFα alone at both 2 and 6h, co-activation with IL-17 and TNFα significantly prolonged the duration of slow-rolling up to 16h (Fig. 3C – E). As above, this effect was not attributable to variations in hemodynamic status, as the prolongation in leukocyte slow-rolling velocity in response to IL-17 and TNFα was maintained across a wide range of shear rates (Sup Fig. 1C).
Figure 3.
IL-17A and TNFα enhance leukocyte slow-rolling on microvascular endothelium in vivo. Leukocyte rolling velocities in the mouse cremaster muscle were quantified by intravital microscopy following intrascrotal injection with IL-17A (1000ng/mouse) and/or TNFα (100ng/mouse). A – C, Cumulative frequency distribution of leukocyte rolling velocity after 2, 6, and 16h. D and E, Proportion of slow-rolling leukocytes (< 10 µm/s) in each vessel (D) and the average leukocyte rolling velocity (E) after 2, 6, and 16h of cytokine activation. F and G, Effects of E-selectin (9A9) blocking antibody injection (50 µg/mouse) on the proportion of slow-rolling leukocytes (F) and the average leukocyte rolling velocity (G) at 16h after stimulation with TNFα alone or in combination with IL-17A. Data (D – G) are represented as mean +/− SEM. Sizes of experimental groups and hemodynamic parameters for all data are summarized in Sup Table 1. E-selectin blocking studies were performed with n = 3 mice per group. *, p < 0.05. **, p < 0.01.
Synergistic effect of IL-17 and TNFα on leukocyte slow-rolling in vivo is E-selectin dependent
We hypothesized that the potentiation of leukocyte slow-rolling in response to IL-17 and TNFα was mediated by an increase in endothelial E-selectin expression relative to treatment with TNFα alone. This hypothesis was based on the established role of endothelial E-selectin in mediating leukocyte rolling velocities less than 10 µm/sec and on our in vitro findings presented above, which demonstrated a synergistic upregulation of E-selectin in response to combined treatment with IL-17 and TNFα (40). Accordingly, we conducted intravital studies utilizing a well-validated E-selectin blocking antibody (9A9) to examine the role of E-selectin in mediating the effects of IL-17 and TNFα on leukocyte slow-rolling. In these studies, we found that injection of E-selectin blocking antibodies caused a marked reduction in IL-17 and TNFα induced leukocyte slow-rolling at 16h and led to a corresponding increase in the average leukocyte rolling velocity (Fig. 3F, G). By contrast, injection of anti-E-selectin antibodies into mice pretreated with TNFα alone for 16h had no significant effect on the proportion of slow-rolling leukocytes (Fig. 3F) or the average rolling velocity (Fig. 3G). These findings suggest that synergistic action between IL-17 and TNFα extends the duration of E-selectin dependent leukocyte rolling on microvascular endothelium in vivo.
IL-17 and TNFα do not enhance endothelial expression of integrin-ligands ICAM-1 and VCAM-1
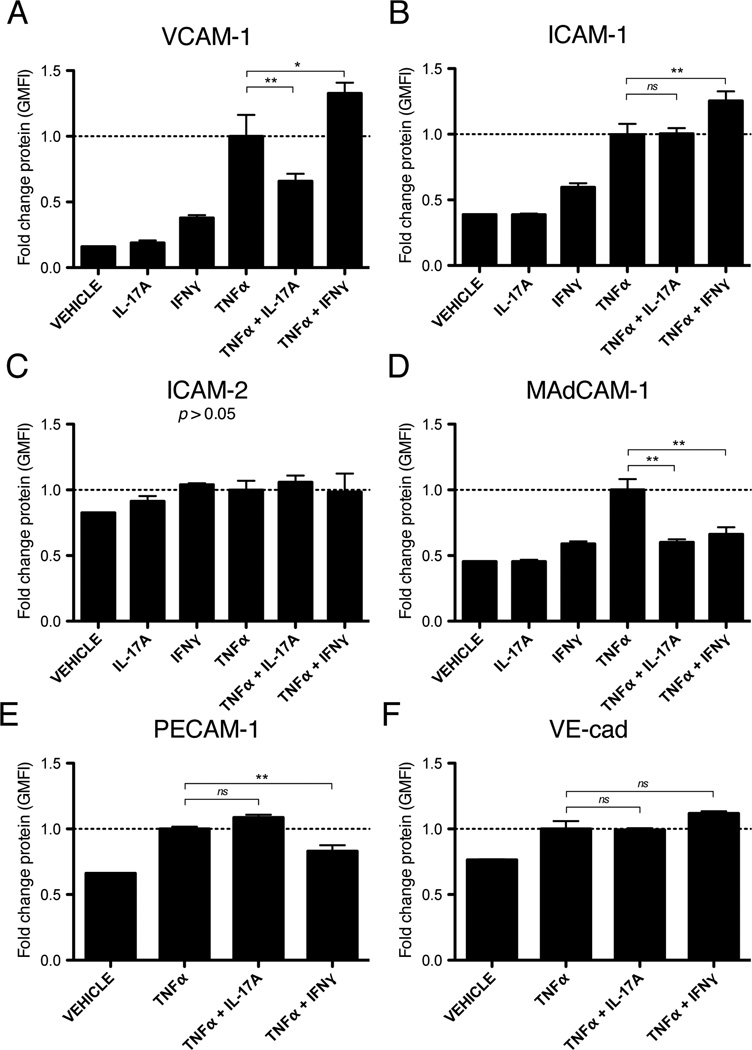
Given the synergistic effects of IL-17 and TNFα on endothelial selectin expression and selectin-dependent leukocyte rolling in vivo, we examined whether IL-17 and TNFα would also support subsequent events in the leukocyte adhesion cascade. Given the central role of endothelial integrin-ligands, such as ICAM1 and VCAM1, in leukocyte firm adhesion and transendothelial migration, we tested the ability of IL-17 and TNFα to induce the expression of these molecules in cultured endothelial cells (30). Surprisingly, we found that combined treatment with IL-17 and TNFα for 16h reduced the expression of VCAM1 and MAdCAM1 relative to TNFα alone (Fig. 4A, D), and had no enhancing effect on the expression of ICAM1, ICAM2, PECAM1, or VE-cadherin (Fig. 4B, C, E, F). Similarly, treatment with IL-17 alone had no effect on endothelial expression of any of the integrin-ligands studied (Fig. 4). By contrast, we found that co-treatment with TNFα and the TH1 cytokine IFNγ increased the expression of VCAM1 and ICAM1 relative to TNFα alone (Fig. 4A, B). Combined treatment with IFNγ and TNFα also led to a reduction in the expression of MAdCAM1 and PECAM1 (Fig. 4D, E) and had no enhancing effect on the expression of ICAM2 or VE-cadherin (Fig. 4C, F). Taken together, these data suggest that synergistic action between IL-17 and TNFα does not enhance endothelial integrin-ligand expression, which contrasts sharply to its marked effects on selectin expression (Fig 1).
Figure 4.
IL-17A does not enhance endothelial expression of integrin-ligands in vitro. A – F, Immunofluorescent flow cytometry of integrin-ligand expression after 16h of treatment with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml). Data are represented as the mean fold change in the geometric mean of the fluorescence intensity (GMFI) +/− SEM relative to treatment with TNFα alone. All data are from at least 3 independent experiments. *, p < 0.05. **, p < 0.01.
Endothelial activation with IL-17 and TNFα synergistically enhances the expression of neutrophil but not T-cell associated chemokines
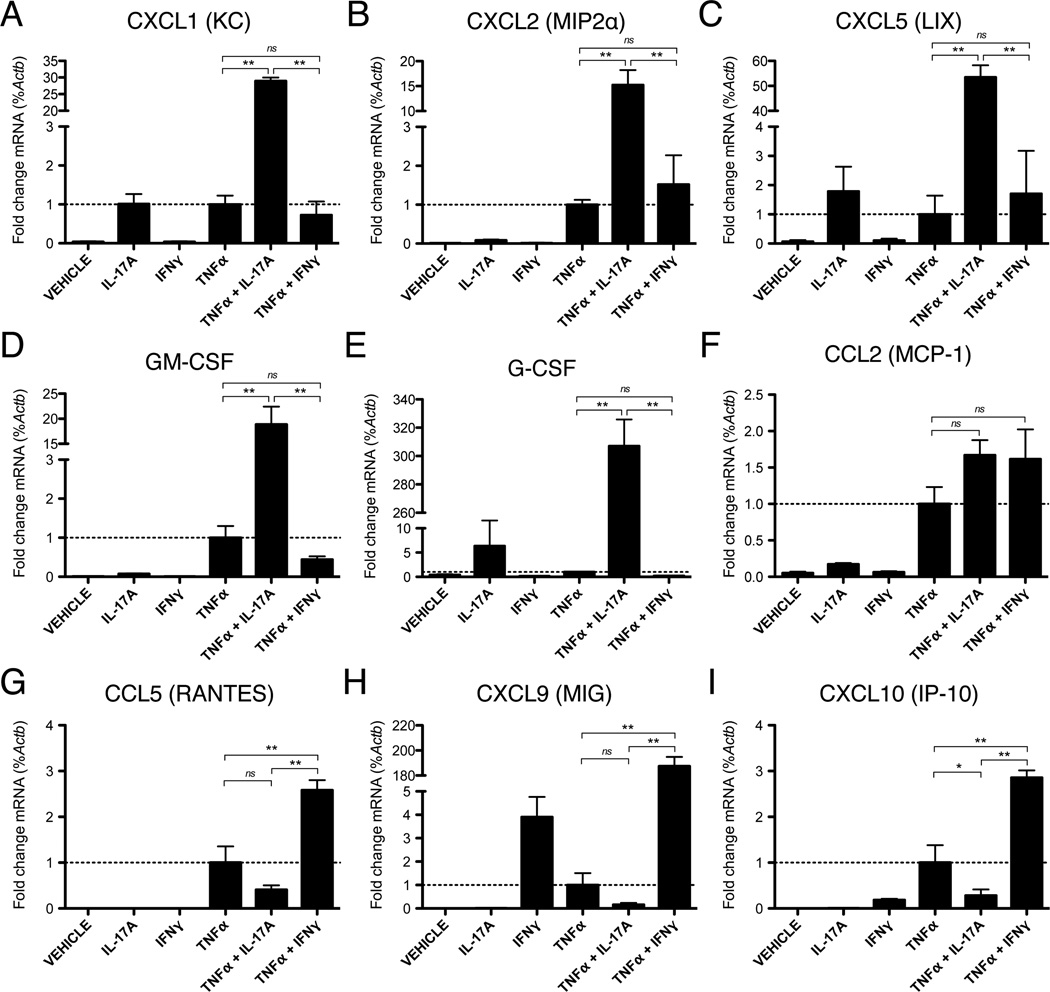
Endothelial derived chemokines are critical regulators of leukocyte integrin activation and firm adhesion, which guide the selective recruitment and chemotaxis of particular leukocyte subsets during inflammation (46). Therefore, we investigated the combined effect of IL-17 and TNFα on endothelial chemokine expression in vitro. We found that co-activation with IL-17 and TNFα synergistically increased expression of neutrophilic chemokines agonistic for the CXCR2 receptor, namely CXCL1 (KC), CXCL2 (MIP2α), and CXCL5 (LIX), which are members of the ELR+ family of chemokines (Fig. 5A – C). In addition, we found that IL-17 and TNFα synergistically induced endothelial expression of GM-CSF (Fig. 5D) and G-CSF (Fig. 5E), which have pleiotropic enhancing effects on granulopoeisis, neutrophil survival, and phagocytic activity (47–49). In contrast to its effects of neutrophilic chemokines, co-treatment with IL-17 and TNFα had no influence on the expression of the T-cell activating chemokines, CCL5 (RANTES), CXCL9 (MIG), and CXCL10 (IP-10) relative to TNFα alone (Fig. 5G – I). In order to compare potential differences in TH1 versus TH17-driven inflammation, we also tested the effects of the signature TH1 cytokine IFNγ on endothelial chemokine expression. Interestingly, we found that IFNγ alone or in combination with TNFα had no enhancing effect on the expression of neutrophilic chemokines, or levels of GM-CSF and G-CSF (Fig. 5A – E), but did strongly induce the expression of the aforementioned T-cell activating chemokines (Fig. 5G – I). Endothelial expression of the monocyte activating chemokine, MCP-1, was not significantly enhanced with either IL-17/TNFα or IFNγ/TNFα relative to TNFα alone (Fig. 5F). These data indicate that the signature cytokines produced by TH17 and TH1 cells have distinct synergistic activity with TNFα in promoting the selective expression of chemokines by endothelium that are supportive of neutrophil versus T lymphocyte migration, respectively.
Figure 5.
IL-17A and TNFα synergistically increase endothelial chemokine expression in vitro. A – I, qRT-PCR of chemokine mRNA expression after 8h of stimulation with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml). Data are normalized to the level of β-actin (Actb) gene expression and represented as the mean fold change in %Actb expression +/− SEM relative to treatment with TNFα alone. All data are from at least 3 independent experiments. *, p < 0.05. **, p < 0.01.
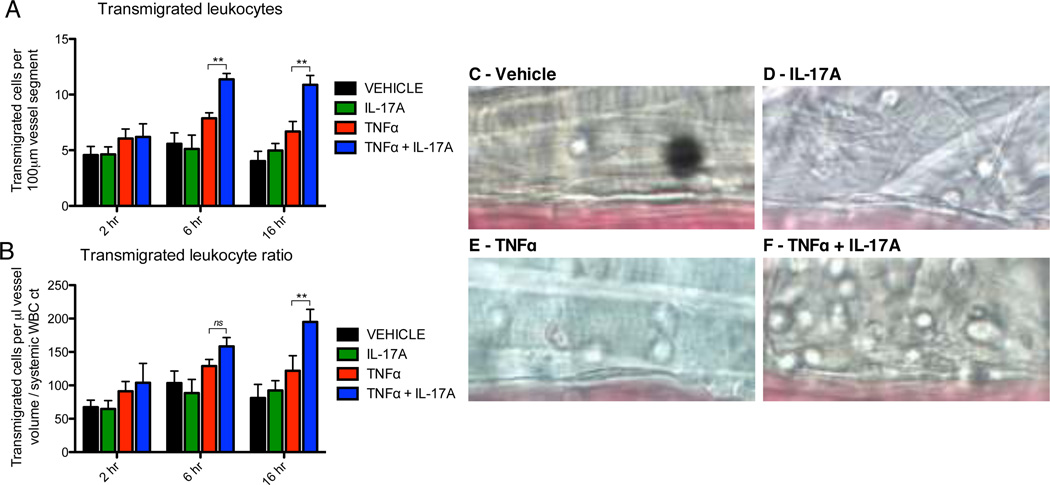
IL-17 and TNFα synergistically enhance leukocyte transendothelial migration in vivo
Having established that IL-17 and TNFα potentiate endothelial expression of neutrophilic chemokines in vitro, we hypothesized that synergistic action between IL-17 and TNFα would also have a functional impact in vivo on leukocyte transendothelial migration, which represents the physiologically critical endpoint of leukocyte recruitment to sites of infection or tissue damage. Using intravital microscopy, we quantified the number of transmigrated leukocytes in the perivascular space at 2, 6, and 16h after cytokine activation, as previously described (42). Transmigration data are presented as the absolute number of perivascular cells per 100um vessel segment (Fig. 6A) and as a ratio of absolute cells normalized for differences in vessel diameter, volume, and the systemic leukocyte count in each mouse (Fig. 6B). Representative images showing transmigrated cells in the perivascular space at 16h for each treatment condition are also shown (Fig. 6C–F). First, treatment with IL-17 alone did not enhance the number of transmigrated cells above levels observed with vehicle treatment at any of the time points studied (Fig. 6A, B). In addition, using a sub-maximal dose of TNFα (100ng/mouse) we observed a trend towards an increase in leukocyte transmigration at all time points, although this effect did not achieve statistical significance (Fig. 6A, B). Interestingly, however, co-administration of IL-17 and TNFα produced a clear, synergistic induction in the number of transmigrated perivascular leukocytes at both the 6 and 16h time-points (Fig. 6A, B). These data demonstrate that IL-17 and TNFα are capable of synergistically enhancing and sustaining leukocyte transmigration in vivo.
Figure 6.
IL-17A and TNFα synergistically increase leukocyte transendothelial migration in vivo. A – F, Transmigrated leukocytes in the perivascular space (50 × 100 µm area) were quantified at three time points (2, 6, 16h) following intrascrotal injection with IL-17A (1000ng/mouse) and/or TNFα (100ng/mouse). Data are represented as the mean +/− SEM of the total number of transmigrated cells per field of view (A) and as a ratio normalized for differences in microvessel volume and systemic leukocyte count (B). C – F, Representative still images of transmigrated leukocytes for each treatment condition after 16h of stimulation. Sizes of experimental groups and hemodynamic parameters for all data are summarized in Sup Table 1. *, p < 0.05. **, p < 0.01.
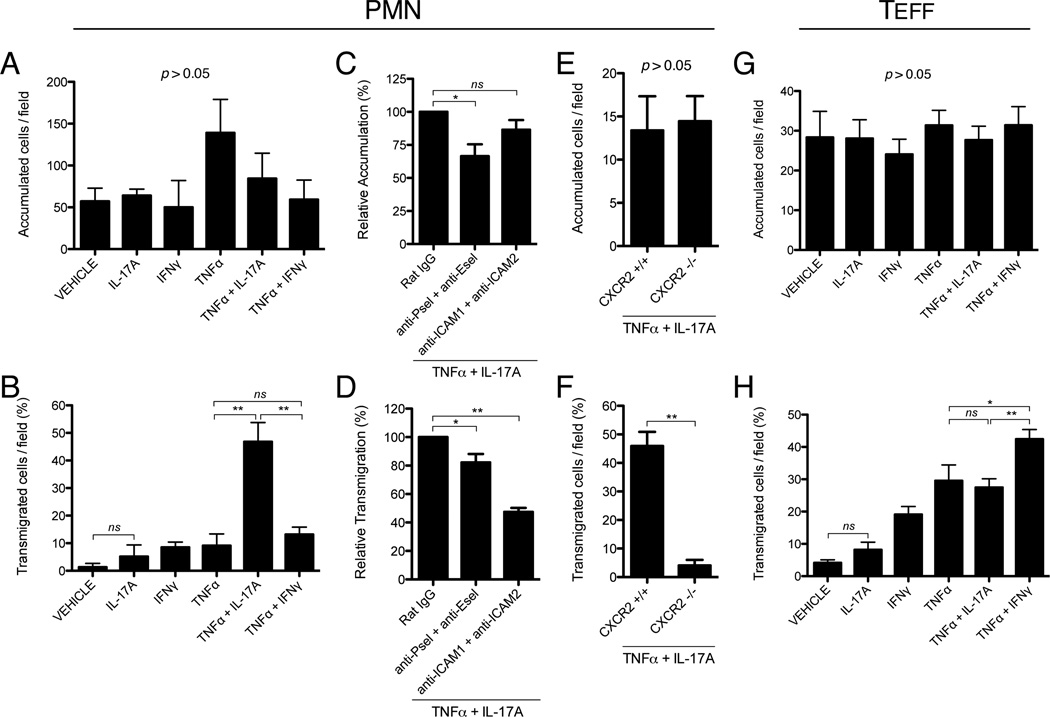
Endothelial activation with IL-17 and TNFα promotes a synergistic increase in neutrophil but not effector CD4+ T cell transmigration under laminar shear flow in vitro
Given the enhancing effect of IL-17 and TNFα on leukocyte transmigration in vivo, as well as the differential activity of IL-17 and IFNγ on endothelial expression of neutrophil and T-cell activating chemokines, respectively, we hypothesized that endothelial activation with IL-17 and TNFα would also lead to a selective increase in neutrophil but not T-cell transmigration. In order to circumvent the limitation of intravital microscopy in accurately differentiating endogenous leukocyte subsets, we chose to test this hypothesis using an in vitro parallel-plate flow chamber system, which allows for the controlled comparison of leukocyte subset recruitment under physiologically relevant levels of laminar shear flow (37, 43, 50). In these studies, neutrophils or effector CD4+ T cells were perfused across MHEC monolayers that had been pre-activated with cytokines for 16h, with the specific goal of determining if endothelial activation with IL-17 and TNFα would selectively enhance the transmigration of either subset relative to treatment with IFNγ and TNFα. The number of accumulated and transmigrated leukocytes was then recorded over a 10 min period by video microscopy. In this model, where perfused leukocytes are initially allowed to adhere at sub-physiologic levels of shear stress (<0.2 dynes/cm2) before increasing flow to experimental levels (0.8 dynes/cm2), there was no statistically significant effect on neutrophil or T-cell accumulation observed across treatment groups after 16h of activation (Fig. 7A, G). Furthermore, we found that IL-17 alone did not significantly increase the transmigration of neutrophils or effector CD4+ T cells above levels observed with vehicle treatment alone (Fig. 7B, H). However, consistent with the chemokine expression data presented above (Fig. 5), we found that endothelial activation with IL-17 and TNFα for 16h led to a marked, synergistic increase in the level of neutrophil but not CD4+ T-cell transmigration (Fig. 7B, H). By contrast, endothelial activation with IFNγ and TNFα selectively increased the transmigration of effector CD4+ T cells (Fig. 7H) but did not significantly alter neutrophil transmigration (Fig. 7B).
Figure 7.
IL-17A and TNFα synergistically increase neutrophil but not effector CD4+ T cell transmigration through endothelium under laminar shear flow. A and B, Bone-marrow derived neutrophils (PMN) were perfused at a defined shear stress (0.8 dynes/cm2) across MHEC monolayers that had been pre-activated for 16h with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml). Accumulated (A) and transmigrated (B) cells were then quantified by video microscopy. C and D, MHEC were pre-activated for 16h with both IL-17A (100ng/ml) and TNFα (50ng/ml) and incubated with blocking antibodies (20 µg/ml) against E-selectin (9A9), P-selectin (RB40.34), ICAM1 (YN1), and ICAM2 (3C4). Neutrophils were then perfused and accumulated (C) and transmigrated (D) cells were quantified. E and F, Neutrophils from Cxcr2−/− and Cxcr2+/+ mice were perfused over MHEC that had been activated for 16h with both IL-17A (100ng/ml) and TNFα (50ng/ml), and accumulated (E) and transmigrated (F) cells were quantified. G and H, Effector CD4+ T cells (TEFF) were differentiated in vitro from naive CD4+ cells isolated from the spleens of WT mice. Cells were then perfused over MHEC that had been activated for 16h with IL-17A (100ng/ml), IFNγ (100U/ml), and/or TNFα (50ng/ml), and accumulated (G) and transmigrated (H) cells were quantified. All data are expressed as mean +/− SEM. All data are from at least 3 independent experiments, except for E and F, which were from 2 independent experiments. *, p < 0.05. **, p < 0.01.
Synergistic effect of IL-17 and TNFα on leukocyte transendothelial migration is dependent on neutrophil CXCR2 as well as endothelial selectins and ICAMs
In order to further examine the role of specific leukocyte-endothelial interactions in mediating the synergistic effects of IL-17 and TNFα on neutrophil transmigration, we utilized blocking antibodies against endothelial P-selectin, E-selectin, ICAM-1, and ICAM-2. We chose these targets in light of their respective roles in promoting neutrophil integrin activation, firm adhesion, and transmigration (30). In these studies, MHEC monolayers that had been pre-activated with IL-17 and TNFα for 16h were incubated with blocking antibodies just prior to neutrophil perfusion through the flow-chamber. The total number of accumulated and transmigrated cells was then determined as described above. We found that pre-treatment with blocking antibodies against P- and E-selectin led to a reduction in neutrophil accumulation, as well as a small but statistically significant reduction in the rate of neutrophil transmigration (Fig. 7C, D). Importantly, although treatment with blocking antibodies against endothelial ICAM-1 and ICAM-2 did not affect neutrophil accumulation, it did lead to a significant reduction in neutrophil transmigration (Fig. 7C, D). These findings suggest that while endothelial selectins may contribute to neutrophil transmigration in response to IL-17 and TNFα, there is perhaps a larger role for selectin-independent activation of neutrophil ICAM-ligands, such as LFA-1 and Mac-1. In light of our findings that IL-17 and TNFα synergistically increase endothelial expression of the CXCR2-activating chemokines CXCL1, CXCL2, and CXCL5 (Fig. 5A – C), we further hypothesized that activation of neutrophil CXCR2 by these molecules represented the central upstream event leading to the increase in ICAM-dependent neutrophil transmigration. Accordingly, using neutrophils derived from Cxcr2−/− mice, we demonstrated that CXCR2-deficiency had no effect on neutrophil accumulation (Fig. 7E) but completely abrogated the enhancing effect of IL-17/TNFα on neutrophil transmigration (Fig. 7F). This finding indicates that the synergistic increase in neutrophil transmigration in response to IL-17 and TNFα primarily reflects enhanced endothelial expression of CXCR2 ligands.
Discussion
Recent interest in the function of IL-17 stems from its defining role in mediating TH17 effector functions and a growing awareness of its importance for innate immunity. IL-17 has established roles in host defense against infection and the pathophysiology of autoimmune disease, and may also contribute to atherosclerosis, plaque instability, and other forms of vascular inflammation. Given the beneficial effects of IL-17 gene deletion or antibody neutralization in several experimental models of inflammation, clinical trials have begun to test the therapeutic impact of IL-17 blockade in human autoimmune disease. Relatively little is known from a mechanistic perspective, however, regarding the functional importance of specific cell types or inflammatory molecules in mediating IL-17 activity. This has been particularly true for research into the role of IL-17 in endothelial activation, which plays a central part in regulating leukocyte influx to sites of inflammation. In particular, it is unclear if IL-17 is capable of directly inducing endothelial activation and leukocyte adhesion during inflammation, or if its primary function is to modulate the effects of other pro-inflammatory cytokines. Although one prior study has suggested a potent role for IL-17 alone in endothelial activation, this finding contrasts with the experience of our own laboratory and highlights the conflicting nature of published literature on the question (34). For example, several studies in non-endothelial cell types have suggested that the predominant effect of IL-17 activity during inflammation derives from its ability to synergistically upregulate pro-inflammatory gene expression when combined with TNFα or other innate cytokines such as IL-1β (35, 36). Furthermore, although TH1 and TH2-driven adaptive immune responses are typically neutrophil-poor, observational data has noted that TH17 cells and neutrophils are often associated at sites of chronic inflammation. In this regard, there has been limited investigation into how the effects of IL-17 on endothelial activation might differ from other signature T-cell cytokines, such as IFNγ, and thus contribute to functional differences in leukocyte subset recruitment during TH17 versus TH1 dominant inflammation.
We sought to better define how IL-17 contributes to inflammation by examining its role in modulating endothelial activation and leukocyte subset recruitment in vitro and in vivo. We also explored the differential roles of IL-17 and IFNγ in these processes in order to compare the potential influence of TH17 versus TH1 dominant immune responses. Our data demonstrate that IL-17 treatment alone, even at high concentrations, was less potent than TNFα alone in promoting endothelial activation and leukocyte recruitment in vivo. Furthermore, we show that combined treatment with IL-17 and TNFα promotes the synergistic activation of endothelial cells to express adhesion molecules and chemokines that specifically enhance and sustain neutrophil recruitment during inflammation. Importantly, we also show that the effects of synergistic action between TNFα and IL-17 versus TNFα and IFNγ are clearly distinct and associated with unique patterns of adhesion molecule and chemokine expression, as well as functional differences in the recruitment of neutrophils versus T lymphocytes, respectively. Our specific results clearly demonstrate that IL-17 and TNFα synergistically enhance endothelial expression of P- and E-selectin in vitro, and promote a synergistic increase in E-selectin-dependent leukocyte rolling in vivo. Furthermore, we show that endothelial activation with IL-17 and TNFα synergistically enhance leukocyte transmigration in vivo, which was associated with an increase in the expression of neutrophil but not T-cell associated chemokines in vitro and a functional increase in neutrophil but not effector CD4+ T-cell transmigration under laminar shear flow. Finally, we show that the enhancement of neutrophil transmigration in response to endothelial activation with IL-17 and TNFα is chiefly mediated by neutrophil CXCR2 and endothelial ICAM expression, suggesting an important role for the CXCR2 ligands, CXCL1, CXCL2, and CXCL5.
In the present study, we identify a novel role for IL-17 in potentiating classical TNFα-mediated inflammatory responses in endothelial cells. Although synergistic activity between IL-17 and TNFα has been previously reported in other cell types with regard to chemokine expression (35, 36), the present study represents the first demonstration of the functional relevancy of this mechanism for endothelial activation and leukocyte recruitment during inflammation in vivo. In particular, synergistic and protracted up-regulation of P- and E-selectin expression in response to IL-17 and TNFα has not been previously reported, and this finding represents a clear alteration in the well-characterized temporal dynamics of E-selectin expression in response to TNFα, which classically involves an early peak after 2–4h of activation followed by a gradual regression towards baseline levels by 16–24h (28, 51). As was previously demonstrated in several in vivo models, the robust induction of E-selectin at early time points during acute inflammation has a central role in driving peak levels of neutrophil influx, likely by promoting neutrophil slow-rolling and integrin-activation (40, 52–54). Interestingly, E-selectin has also been shown to enhance neutrophil integrin activation and recruitment in vivo in a cooperative manner with CXCR2-activation by chemokines such as CXCL1 (55), which has particular relevance to the present study in light of our data demonstrating a synergistic increase in endothelial expression of CXCL1, CXCL2, and CXCL5 in response to IL-17 and TNFα. Synergistic regulation of neutrophil recruitment through enhanced E-selectin-dependent rolling and increased production of CXCR2-activating chemokines may, therefore, represent critical features of IL-17 and TNFα pro-inflammatory activity on endothelium. Although beyond the scope of the present study, elucidation of the signaling pathways that drive the synergistic induction of E-selectin and other genes in response to IL-17 and TNFα represents an important avenue of future investigation. Interestingly, we have found that endothelial activation with IL-17 and TNFα does not appear to enhance nuclear translocation of the pro-inflammatory transcription factor NF-κB relative to TNFα alone (data not shown), which is consistent with prior data in other cell types and suggests that synergy between IL-17 and TNFα at the molecular level may be driven by a non-canonical mechanism of action (35, 36). Several prior studies in non-endothelial cell types have instead proposed a role for MAPK-dependent effects on the stability of specific mRNA transcripts (e.g. IL-8), although this has not yet been validated in endothelial cells and may not be relevant to all genes that are synergistically regulated by IL-17 and TNFα (35, 36, 56–59).
We believe these observations contribute to our broader understanding of IL-17 activity during both innate and adaptive immunity. For example, in the setting of acute inflammation recent studies have demonstrated that γδ T cells are capable of secreting high levels of IL-17 in response to pathogen-associated activation of TLR1, TLR2, and Dectin 1 (60, 61). Importantly, this activation occurs well prior to the development of TH17-driven adaptive immunity and takes place independently of antigen-specific TCR engagement. Neutrophils, likewise, have also been proposed as an important innate source of IL-17, as suggested by a recent study of ischemia-reperfusion injury in the kidney (4). That IL-17 is produced at high levels by innate immune cells in response to pathogens and ischemia suggests that synergistic action between IL-17 and TNFα could represent a highly conserved mechanism of host defense against infection and tissue injury. Similarly, in the setting of adaptive immunity where TH17 cells likely represent a predominant source of IL-17, synergistic action between IL-17 and TNFα could sustain neutrophilic influx to sites of chronic inflammation. In this regard, it is interesting to consider the possibility of a positive feedback loop related to TH17 and IL-17 activity, which could contribute to the indolent course of many forms of autoimmunity or the pathophysiology of acute exacerbations. For example, a recent study from our laboratory has demonstrated that TH17 cells have an enhanced ability to bind E-selectin relative to TH1 cells, which could preferentially enhance their recruitment to sites of inflammation following IL-17/TNFα mediated up-regulation of E-selectin (62). Newly recruited TH17 cells could then further amplify local production of innate cytokines once present at the site of inflammation. For example, we have observed that TH17 cells are capable of directly producing high-levels of TNFα (unpublished data) in conjunction with IL-17, which in turn could stimulate further production of TNFα and IL-1β by macrophages (63). Although the activating effects of these cytokines would undoubtedly reinforce local chemokine expression and leukocyte influx, one prior study has made the important observation that TH17 cells and neutrophils are capable of direct chemotactic crosstalk involving the secretion of the neutrophil chemokine CXCL8 and the TH17 chemokine CCL20, respectively, which could further support their mutual recruitment to sites of inflammation (64).
Finally, the present study has clear implications for the therapeutic targeting of autoimmunity and other IL-17 associated diseases. The human relevance of our in vitro and in vivo findings using the murine system is supported by the fact that we have also found synergistic regulation of E-selectin expression in human saphenous vein endothelial cells (data not shown), and by a recent report published during the preparation of this manuscript showing that TNFα and IL-17 synergistically enhance the pro-thrombotic phenotype of human umbilical vein endothelial cells (65). Although clinical trials are currently ongoing testing the efficacy of anti-IL-17 therapy in human autoimmune disease, in light of the present findings it is interesting to consider the potential impact of dual anti-IL-17 and anti-TNFα therapy, either in patients not responding to TNFα blockade alone or as an adjunct during episodes of acute exacerbation. Likewise, as more is learned about the signaling pathways relevant to IL-17 and TNFα pro-inflammatory activity, targeted approaches may become possible whereby synergistic action is selectively inhibited without interfering in the unique functions of these cytokines during host defense. Furthermore, although studies in animal models testing the role of IL-17 in atherogenesis have shown variable effects on total plaque burden, IL-17 may still have the potential to enhance atherothrombotic risk by stimulating neutrophil recruitment and inflammation within established plaques (17–20, 24, 66). This notion may be particularly relevant to patients with rheumatoid arthritis or to recipients of cardiac allografts, in whom IL-17-associated inflammatory disease is superimposed on an elevated baseline cardiovascular risk (67–70). Although clinical trials are ongoing to test the efficacy of anti-IL-17 therapy for human autoimmune disease, our data support the promise of these approaches for a variety of IL-17-associated vascular inflammatory disorders where endothelial inflammation is critically involved in the initiation and progression of disease.
Supplementary Material
Acknowledgements
We would like to thank the Dana-Farber/Harvard Cancer Center for use of the Specialized Histopathology Core, which provided complete blood count (CBC) services in conjunction with the intravital microscopy experiments. At the Brigham and Women’s Hospital, we would like to thank all members of the Vascular Research Division and the Tissue Culture Core Facility in the Department of Pathology, as well as the Intravital Microscopy Core Facility in the Cardiovascular Division of the Department of Medicine for use of the intravital microscope.
Footnotes
This work was supported by NIH grant P50HL56985 (AHL, FWL), 1K08HL086672 (KJC), K99-HL097406 (PA), and a fellowship from the Sarnoff Cardiovascular Research Foundation (GKG)
Disclosures
The authors have no financial conflicts of interest.
References
- 1.Harrington LE, Hatton RD, Mangan PR, Turner H, Murphy TL, Murphy KM, Weaver CT. Interleukin 17-producing CD4+ effector T cells develop via a lineage distinct from the T helper type 1 and 2 lineages. Nat. Immunol. 2005;6:1123–1132. doi: 10.1038/ni1254. [DOI] [PubMed] [Google Scholar]
- 2.Park H, Li Z, Yang XO, Chang SH, Nurieva R, Wang YH, Wang Y, Hood L, Zhu Z, Tian Q, Dong C. A distinct lineage of CD4 T cells regulates tissue inflammation by producing interleukin 17. Nat. Immunol. 2005;6:1133–1141. doi: 10.1038/ni1261. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Cua JD, Tato CM. Innate IL-17-producing cells: the sentinels of the immune system. Nat. Rev. Immunol. 2010;10:479–489. doi: 10.1038/nri2800. [DOI] [PubMed] [Google Scholar]
- 4.Li L, Huang L, Vergis AL, Ye H, Bajwa A, Narayan V, Strieter RM, Rosin DL, Okusa MD. IL-17 produced by neutrophils regulates IFN-gamma-mediated neutrophil migration in mouse kidney ischemia-reperfusion injury. J. Clin. Invest. 2010;120:331–342. doi: 10.1172/JCI38702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Lockhart E, Green AM, Flynn JL. IL-17 production is dominated by gammadelta T cells rather than CD4 T cells during Mycobacterium tuberculosis infection. J. Immunol. 2006;177:4662–4669. doi: 10.4049/jimmunol.177.7.4662. [DOI] [PubMed] [Google Scholar]
- 6.Gaffen SL. Structure and signalling in the IL-17 receptor family. Nat. Rev. Immunol. 2009;9:556–567. doi: 10.1038/nri2586. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Iwakura Y, Ishigame H, Saijo S, Nakae S. Functional specialization of interleukin-17 family members. Immunity. 2011;34:149–162. doi: 10.1016/j.immuni.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 8.Huang W, Na L, Fidel PL, Schwarzenberger P. Requirement of interleukin-17A for systemic anti-Candida albicans host defense in mice. J. Infect. Dis. 2004;190:624–631. doi: 10.1086/422329. [DOI] [PubMed] [Google Scholar]
- 9.Ishigame H, Kakuta S, Nagai T, Kadoki M, Nambu A, Komiyama Y, Fujikado N, Tanahashi Y, Akitsu A, Kotaki H, Sudo K, Nakae S, Sasakawa C, Iwakura Y. Differential roles of interleukin-17A and -17F in host defense against mucoepithelial bacterial infection and allergic responses. Immunity. 2009;30:108–119. doi: 10.1016/j.immuni.2008.11.009. [DOI] [PubMed] [Google Scholar]
- 10.Khader SA, Bell GK, Pearl JE, Fountain JJ, Rangel-Moreno J, Cilley GE, Shen F, Eaton SM, Gaffen SL, Swain SL, Locksley RM, Haynes L, Randall TD, Cooper AM. IL-23 and IL-17 in the establishment of protective pulmonary CD4+ T cell responses after vaccination and during Mycobacterium tuberculosis challenge. Nat. Immunol. 2007;8:369–377. doi: 10.1038/ni1449. [DOI] [PubMed] [Google Scholar]
- 11.Lock C, Hermans G, Pedotti R, Brendolan A, Schadt E, Garren H, Langer-Gould A, Strober S, Cannella B, Allard J, Klonowski P, Austin A, Lad N, Kaminski N, Galli SJ, Oksenberg JR, Raine CS, Heller R, Steinman L. Gene-microarray analysis of multiple sclerosis lesions yields new targets validated in autoimmune encephalomyelitis. Nat. Med. 2002;8:500–508. doi: 10.1038/nm0502-500. [DOI] [PubMed] [Google Scholar]
- 12.Ziolkowska M, Koc A, Luszczykiewicz G, Ksiezopolska-Pietrzak K, Klimczak E, Chwalinska-Sadowska H, Maslinski W. High levels of IL-17 in rheumatoid arthritis patients: IL-15 triggers in vitro IL-17 production via cyclosporin A-sensitive mechanism. J. Immunol. 2000;164:2832–2838. doi: 10.4049/jimmunol.164.5.2832. [DOI] [PubMed] [Google Scholar]
- 13.Cai Y, Shen X, Ding C, Qi C, Li K, Li X, Jala VR, Zhang HG, Wang T, Zheng J, Yan J. Pivotal role of dermal IL-17-producing gammadelta T cells in skin inflammation. Immunity. 2011;35:596–610. doi: 10.1016/j.immuni.2011.08.001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Komiyama Y, Nakae S, Matsuki T, Nambu A, Ishigame H, Kakuta S, Sudo K, Iwakura Y. IL-17 plays an important role in the development of experimental autoimmune encephalomyelitis. J. Immunol. 2006;177:566–573. doi: 10.4049/jimmunol.177.1.566. [DOI] [PubMed] [Google Scholar]
- 15.Nakae S, Nambu A, Sudo K, Iwakura Y. Suppression of immune induction of collagen-induced arthritis in IL-17-deficient mice. J. Immunol. 2003;171:6173–6177. doi: 10.4049/jimmunol.171.11.6173. [DOI] [PubMed] [Google Scholar]
- 16.Baldeviano GC, Barin JG, Talor MV, Srinivasan S, Bedja D, Zheng D, Gabrielson K, Iwakura Y, Rose NR, Cihakova D. Interleukin-17A is dispensable for myocarditis but essential for the progression to dilated cardiomyopathy. Circ. Res. 2010;106:1646–1655. doi: 10.1161/CIRCRESAHA.109.213157. [DOI] [PubMed] [Google Scholar]
- 17.Erbel C, Chen L, Bea F, Wangler S, Celik S, Lasitschka F, Wang Y, Bockler D, Katus HA, Dengler TJ. Inhibition of IL-17A attenuates atherosclerotic lesion development in apoE-deficient mice. J. Immunol. 2009;183:8167–8175. doi: 10.4049/jimmunol.0901126. [DOI] [PubMed] [Google Scholar]
- 18.Smith E, Prasad KM, Butcher M, Dobrian A, Kolls JK, Ley K, Galkina E. Blockade of interleukin-17A results in reduced atherosclerosis in apolipoprotein E-deficient mice. Circulation. 2010;121:1746–1755. doi: 10.1161/CIRCULATIONAHA.109.924886. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Erbel C, Dengler TJ, Wangler S, Lasitschka F, Bea F, Wambsganss N, Hakimi M, Bockler D, Katus HA, Gleissner CA. Expression of IL-17A in human atherosclerotic lesions is associated with increased inflammation and plaque vulnerability. Basic Res. Cardiol. 2011;106:125–134. doi: 10.1007/s00395-010-0135-y. [DOI] [PubMed] [Google Scholar]
- 20.Liang J, Zheng Z, Wang M, Han L, Peng J, Liu Z, Wei Y. Myeloperoxidase (MPO) and interleukin-17 (IL-17) plasma levels are increased in patients with acute coronary syndromes. J. Int. Med. Res. 2009;37:862–866. doi: 10.1177/147323000903700331. [DOI] [PubMed] [Google Scholar]
- 21.Yuan X, Paez-Cortez J, Schmitt-Knosalla I, D'Addio F, Mfarrej B, Donnarumma M, Habicht A, Clarkson MR, Iacomini J, Glimcher LH, Sayegh MH, Ansari MJ. A novel role of CD4 Th17 cells in mediating cardiac allograft rejection and vasculopathy. J. Exp. Med. 2008;205:3133–3144. doi: 10.1084/jem.20081937. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Itoh S, Kimura N, Axtell RC, Velotta JB, Gong Y, Wang X, Kajiwara N, Nambu A, Shimura E, Adachi H, Iwakura Y, Saito H, Okumura K, Sudo K, Steinman L, Robbins RC, Nakae S, Fischbein MP. Interleukin-17 accelerates allograft rejection by suppressing regulatory T cell expansion. Circulation. 2011;124:S187–S196. doi: 10.1161/CIRCULATIONAHA.110.014852. [DOI] [PubMed] [Google Scholar]
- 23.Sohn MH, Noh SY, Chang W, Shin KM, Kim DS. Circulating interleukin 17 is increased in the acute stage of Kawasaki disease. Scand. J. Rheumatol. 2003;32:364–366. doi: 10.1080/03009740410005034. [DOI] [PubMed] [Google Scholar]
- 24.Madhur MS, Funt SA, Li L, Vinh A, Chen W, Lob HE, Iwakura Y, Blinder Y, Rahman A, Quyyumi AA, Harrison DG. Role of interleukin 17 in inflammation, atherosclerosis, and vascular function in apolipoprotein e-deficient mice. Arterioscler. Thromb. Vasc. Biol. 2011;31:1565–1572. doi: 10.1161/ATVBAHA.111.227629. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hueber W, Patel DD, Dryja T, Wright AM, Koroleva I, Bruin G, Antoni C, Draelos Z, Gold MH, Durez P, Tak PP, Gomez-Reino JJ, Foster CS, Kim RY, Samson CM, Falk NS, Chu DS, Callanan D, Nguyen QD, Rose K, Haider A, Di Padova F. Effects of AIN457, a fully human antibody to interleukin-17A, on psoriasis, rheumatoid arthritis, and uveitis. Sci. Transl. Med. 2010;2 doi: 10.1126/scitranslmed.3001107. 52ra72. [DOI] [PubMed] [Google Scholar]
- 26.Genovese MC, Van den Bosch F, Roberson SA, Bojin S, Biagini IM, Ryan P, Sloan-Lancaster J. LY2439821, a humanized anti-interleukin-17 monoclonal antibody, in the treatment of patients with rheumatoid arthritis: A phase I randomized, double-blind, placebo-controlled, proof-of-concept study. Arthritis Rheum. 2010;62:929–939. doi: 10.1002/art.27334. [DOI] [PubMed] [Google Scholar]
- 27.Bevilacqua MP, Pober JS, Wheeler ME, Cotran RS, Gimbrone MA., Jr Interleukin-1 activation of vascular endothelium. Effects on procoagulant activity and leukocyte adhesion. Am. J. Pathol. 1985;121:394–403. [PMC free article] [PubMed] [Google Scholar]
- 28.Pober JS, Bevilacqua MP, Mendrick DL, Lapierre LA, Fiers W, Gimbrone MA., Jr Two distinct monokines, interleukin 1 and tumor necrosis factor, each independently induce biosynthesis and transient expression of the same antigen on the surface of cultured human vascular endothelial cells. J. Immunol. 1986;136:1680–1687. [PubMed] [Google Scholar]
- 29.Milstone DS, Fukumura D, Padgett RC, O'Donnell PE, Davis VM, Benavidez OJ, Monsky WL, Melder RJ, Jain RK, Gimbrone MA., Jr Mice lacking E-selectin show normal numbers of rolling leukocytes but reduced leukocyte stable arrest on cytokine-activated microvascular endothelium. Microcirculation. 1998;5:153–171. [PubMed] [Google Scholar]
- 30.Ley K, Laudanna C, Cybulsky MI, Nourshargh S. Getting to the site of inflammation: the leukocyte adhesion cascade updated. Nat. Rev. Immunol. 2007;7:678–689. doi: 10.1038/nri2156. [DOI] [PubMed] [Google Scholar]
- 31.Doukas J, Pober JS. IFN-gamma enhances endothelial activation induced by tumor necrosis factor but not IL-1. J. Immunol. 1990;145:1727–1733. [PubMed] [Google Scholar]
- 32.Marfaing-Koka A, Devergne O, Gorgone G, Portier A, Schall TJ, Galanaud P, Emilie D. Regulation of the production of the RANTES chemokine by endothelial cells. Synergistic induction by IFN-gamma plus TNF-alpha and inhibition by IL-4 and IL-13. J. Immunol. 1995;154:1870–1878. [PubMed] [Google Scholar]
- 33.Maganto-Garcia E, Bu DX, Tarrio ML, Alcaide P, Newton G, Griffin GK, Croce KJ, Luscinskas FW, Lichtman AH, Grabie N. Foxp3+-inducible regulatory T cells suppress endothelial activation and leukocyte recruitment. J. Immunol. 2011;187:3521–3529. doi: 10.4049/jimmunol.1003947. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Roussel L, Houle F, Chan C, Yao Y, Berube J, Olivenstein R, Martin JG, Huot J, Hamid Q, Ferri L, Rousseau S. IL-17 promotes p38 MAPK-dependent endothelial activation enhancing neutrophil recruitment to sites of inflammation. J. Immunol. 2010;184:4531–4537. doi: 10.4049/jimmunol.0903162. [DOI] [PubMed] [Google Scholar]
- 35.Hartupee J, Liu C, Novotny M, Li X, Hamilton T. IL-17 enhances chemokine gene expression through mRNA stabilization. J. Immunol. 2007;179:4135–4141. doi: 10.4049/jimmunol.179.6.4135. [DOI] [PubMed] [Google Scholar]
- 36.Shen F, Ruddy MJ, Plamondon P, Gaffen SL. Cytokines link osteoblasts and inflammation: microarray analysis of interleukin-17-and TNF-alpha-induced genes in bone cells. J. Leukoc. Biol. 2005;77:388–399. doi: 10.1189/jlb.0904490. [DOI] [PubMed] [Google Scholar]
- 37.Lim YC, Garcia-Cardena G, Allport JR, Zervoglos M, Connolly AJ, Gimbrone MA, Jr, Luscinskas FW. Heterogeneity of endothelial cells from different organ sites in T-cell subset recruitment. Am. J. Pathol. 2003;162:1591–1601. doi: 10.1016/S0002-9440(10)64293-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Baez S. An open cremaster muscle preparation for the study of blood vessels by in vivo microscopy. Microvasc. Res. 1973;5:384–394. doi: 10.1016/0026-2862(73)90054-x. [DOI] [PubMed] [Google Scholar]
- 39.Ley K, Bullard DC, Arbones ML, Bosse R, Vestweber D, Tedder TF, Beaudet AL. Sequential contribution of L- and P-selectin to leukocyte rolling in vivo. J. Exp. Med. 1995;181:669–675. doi: 10.1084/jem.181.2.669. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Kunkel EJ, Ley K. Distinct phenotype of E-selectin-deficient mice. E-selectin is required for slow leukocyte rolling in vivo. Circ. Res. 1996;79:1196–1204. doi: 10.1161/01.res.79.6.1196. [DOI] [PubMed] [Google Scholar]
- 41.Norman MU, Zbytnuik L, Kubes P. Interferon-gamma limits Th1 lymphocyte adhesion to inflamed endothelium: a nitric oxide regulatory feedback mechanism. Eur. J. Immunol. 2008;38:1368–1380. doi: 10.1002/eji.200737847. [DOI] [PubMed] [Google Scholar]
- 42.Khandoga A, Huettinger S, Khandoga AG, Li H, Butz S, Jauch KW, Vestweber D, Krombach F. Leukocyte transmigration in inflamed liver: A role for endothelial cell-selective adhesion molecule. J. Hepatol. 2009;50:755–765. doi: 10.1016/j.jhep.2008.11.027. [DOI] [PubMed] [Google Scholar]
- 43.Allport JR, Lim YC, Shipley JM, Senior RM, Shapiro SD, Matsuyoshi N, Vestweber D, Luscinskas FW. Neutrophils from MMP-9- or neutrophil elastase-deficient mice show no defect in transendothelial migration under flow in vitro. J. Leukoc. Biol. 2002;71:821–828. [PubMed] [Google Scholar]
- 44.Jung U, Bullard DC, Tedder TF, Ley K. Velocity differences between L- and P-selectin-dependent neutrophil rolling in venules of mouse cremaster muscle in vivo. Am. J. Physiol. 1996;271:H2740–H2747. doi: 10.1152/ajpheart.1996.271.6.H2740. [DOI] [PubMed] [Google Scholar]
- 45.Jung U, Norman KE, Scharffetter-Kochanek K, Beaudet AL, Ley K. Transit time of leukocytes rolling through venules controls cytokine-induced inflammatory cell recruitment in vivo. J. Clin. Invest. 1998;102:1526–1533. doi: 10.1172/JCI119893. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Rot A, von Andrian UH. Chemokines in innate and adaptive host defense: basic chemokinese grammar for immune cells. Annu. Rev. Immunol. 2004;22:891–928. doi: 10.1146/annurev.immunol.22.012703.104543. [DOI] [PubMed] [Google Scholar]
- 47.Yuo A, Kitagawa S, Ohsaka A, Ohta M, Miyazono K, Okabe T, Urabe A, Saito M, Takaku F. Recombinant human granulocyte colony-stimulating factor as an activator of human granulocytes: potentiation of responses triggered by receptor-mediated agonists and stimulation of C3bi receptor expression and adherence. Blood. 1989;74:2144–2149. [PubMed] [Google Scholar]
- 48.Weisbart RH, Kwan L, Golde DW, Gasson JC. Human GM-CSF primes neutrophils for enhanced oxidative metabolism in response to the major physiological chemoattractants. Blood. 1987;69:18–21. [PubMed] [Google Scholar]
- 49.Yong KL. Granulocyte colony-stimulating factor (G-CSF) increases neutrophil migration across vascular endothelium independent of an effect on adhesion: comparison with granulocyte-macrophage colony-stimulating factor (GM-CSF) Br. J. Haematol. 1996;94:40–47. doi: 10.1046/j.1365-2141.1996.d01-1752.x. [DOI] [PubMed] [Google Scholar]
- 50.Muller WA, Luscinskas FW. Assays of transendothelial migration in vitro. Methods Enzymol. 2008;443:155–176. doi: 10.1016/S0076-6879(08)02009-0. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Bevilacqua MP, Pober JS, Mendrick DL, Cotran RS, Gimbrone MA., Jr Identification of an inducible endothelial-leukocyte adhesion molecule. Proc. Natl. Acad. Sci. USA. 1987;84:9238–9242. doi: 10.1073/pnas.84.24.9238. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Ramos CL, Kunkel EJ, Lawrence MB, Jung U, Vestweber D, Bosse R, McIntyre KW, Gillooly KM, Norton CR, Wolitzky BA, Ley K. Differential effect of E-selectin antibodies on neutrophil rolling and recruitment to inflammatory sites. Blood. 1997;89:3009–3018. [PubMed] [Google Scholar]
- 53.Yan HC, Delisser HM, Pilewski JM, Barone KM, Szklut PJ, Chang XJ, Ahern TJ, Langer-Safer P, Albelda SM. Leukocyte recruitment into human skin transplanted onto severe combined immunodeficient mice induced by TNF-alpha is dependent on E-selectin. J. Immunol. 1994;152:3053–3063. [PubMed] [Google Scholar]
- 54.Zarbock A, Lowell CA, Ley K. Spleen tyrosine kinase Syk is necessary for E-selectin-induced alpha(L)beta(2) integrin-mediated rolling on intercellular adhesion molecule-1. Immunity. 2007;26:773–783. doi: 10.1016/j.immuni.2007.04.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Smith ML, Olson TS, Ley K. CXCR2- and E-selectin-induced neutrophil arrest during inflammation in vivo. J. Exp. Med. 2004;200:935–939. doi: 10.1084/jem.20040424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56.Sun D, Novotny M, Bulek K, Liu C, Li X, Hamilton T. Treatment with IL-17 prolongs the half-life of chemokine CXCL1 mRNA via the adaptor TRAF5 and the splicing-regulatory factor SF2 (ASF) Nat. Immunol. 2011;12:853–860. doi: 10.1038/ni.2081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Datta S, Novotny M, Pavicic PG, Jr, Zhao C, Herjan T, Hartupee J, Hamilton T. IL-17 regulates CXCL1 mRNA stability via an AUUUA/tristetraprolin-independent sequence. J. Immunol. 2010;184:1484–1491. doi: 10.4049/jimmunol.0902423. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58.Henness S, van Thoor E, Ge Q, Armour CL, Hughes JM, Ammit AJ. IL-17A acts via p38 MAPK to increase stability of TNF-alpha-induced IL-8 mRNA in human ASM. Am. J. Physiol-Lung C. 2006;290:L1283–L1290. doi: 10.1152/ajplung.00367.2005. [DOI] [PubMed] [Google Scholar]
- 59.Faour WH, Mancini A, He QW, Di Battista JA. T-cell-derived interleukin-17 regulates the level and stability of cyclooxygenase-2 (COX-2) mRNA through restricted activation of the p38 mitogen-activated protein kinase cascade: role of distal sequences in the 3'-untranslated region of COX-2 mRNA. J. Biol. Chem. 2003;278:26897–26907. doi: 10.1074/jbc.M212790200. [DOI] [PubMed] [Google Scholar]
- 60.Martin B, Hirota K, Cua DJ, Stockinger B, Veldhoen M. Interleukin-17-producing gammadelta T cells selectively expand in response to pathogen products and environmental signals. Immunity. 2009;31:321–330. doi: 10.1016/j.immuni.2009.06.020. [DOI] [PubMed] [Google Scholar]
- 61.Sutton CE, Lalor SJ, Sweeney CM, Brereton CF, Lavelle EC, Mills KH. Interleukin-1 and IL-23 induce innate IL-17 production from gammadelta T cells, amplifying Th17 responses and autoimmunity. Immunity. 2009;31:331–341. doi: 10.1016/j.immuni.2009.08.001. [DOI] [PubMed] [Google Scholar]
- 62.Alcaide P, Maganto-Garcia E, Newton G, Travers R, Croce KJ, Bu DX, Luscinskas FW, Lichtman AH. Difference in Th1 and th17 lymphocyte adhesion to endothelium. J. Immunol. 2012;188:1421–1430. doi: 10.4049/jimmunol.1101647. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 63.Jovanovic DV, Di Battista JA, Martel-Pelletier J, Jolicoeur FC, He Y, Zhang M, Mineau F, Pelletier JP. IL-17 stimulates the production and expression of proinflammatory cytokines, IL-beta and TNF-alpha, by human macrophages. J. Immunol. 1998;160:3513–3521. [PubMed] [Google Scholar]
- 64.Pelletier M, Maggi L, Micheletti A, Lazzeri E, Tamassia N, Costantini C, Cosmi L, Lunardi C, Annunziato F, Romagnani S, Cassatella MA. Evidence for a cross-talk between human neutrophils and Th17 cells. Blood. 2010;115:335–343. doi: 10.1182/blood-2009-04-216085. [DOI] [PubMed] [Google Scholar]
- 65.Hot A, Lenief V, Miossec P. Combination of IL-17 and TNFα induces a pro-inflammatory, pro-coagulant and pro-thrombotic phenotype in human endothelial cells. Ann. Rheum. Dis. 2012 doi: 10.1136/annrheumdis-2011-200468. [DOI] [PubMed] [Google Scholar]
- 66.Soehnlein O. Multiple roles for neutrophils in atherosclerosis. Circ. Res. 2012;110:875–888. doi: 10.1161/CIRCRESAHA.111.257535. [DOI] [PubMed] [Google Scholar]
- 67.Libby P, Ridker PM, Hansson GK. Inflammation in atherosclerosis: from pathophysiology to practice. J. Am. Coll. Cardiol. 2009;54:2129–2138. doi: 10.1016/j.jacc.2009.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Solomon DH, Karlson EW, Rimm EB, Cannuscio CC, Mandl LA, Manson JE, Stampfer MJ, Curhan GC. Cardiovascular morbidity and mortality in women diagnosed with rheumatoid arthritis. Circulation. 2003;107:1303–1307. doi: 10.1161/01.cir.0000054612.26458.b2. [DOI] [PubMed] [Google Scholar]
- 69.Solomon DH, Goodson NJ, Katz JN, Weinblatt ME, Avorn J, Setoguchi S, Canning C, Schneeweiss S. Patterns of cardiovascular risk in rheumatoid arthritis. Ann. Rheum. Dis. 2006;65:1608–1612. doi: 10.1136/ard.2005.050377. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Lee MS, Finch W, Weisz G, Kirtane AJ. Cardiac allograft vasculopathy. Rev. Cardiovasc. Med. 2011;12:143–152. [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.