Abstract
Slamf8 (CD353) is a cell surface receptor that is expressed upon activation of macrophages by interferon-gamma or bacteria. Here we report that a very high Nox2 activity enzyme was found in Slamf8−/− macrophages in response to E.coli or S.aureus, but also to phorbol myristate acetate. The elevated Nox2 activity in Slamf8−/− macrophages was also demonstrated in E.coli or S.aureus phagosomes by using a pH indicator system, and was further confirmed by a reduction of the enzyme activity after transfection of the receptor into Slamf8-deficient primary macrophages or RAW 264.7 cells. Upon exposure to bacteria and/or phorbol myristate acetate, PKC activity in Slamf8−/− macrophages is increased. This results in an enhanced phosphorylation of p40phox, one key component of the Nox2 enzyme complex, which in turn leads to greater Nox2 activity. Taken together, the data show that upon response to inflammation-associated stimuli the inducible receptor Slamf8 negatively regulates inflammatory responses.
Introduction
Several members of the Slam-family [Slamf] of cell surface receptors modulate macrophage functions (1–3). Per example, we recently found that on the surface of macrophages Slamf1 acts as a microbial sensor and modulates two bactericidal processes in the E.coli phagosome: Nox2 (NADPH oxidase) activity and phagosomal maturation (1). Because human and mouse SLAMF8 (BLAME, CD353) are expressed on the surface of myeloid cells (4, 5), we set out to determine whether this receptor would also affect macrophage functions as Slamf1.
We find that mouse Slamf8 is not expressed on the surface of resident peritoneal macrophages, but can be induced by exposure to Interferon γ and bacteria. Using macrophages isolated from Slamf8 deficient (Slamf8−/−) mice or RAW 264.7 macrophages, Slamf8 was found to be a negative regulator of Nox2 activity in bacterial phagosomes. In Slamf8−/− macrophages, in contrast to Slamf8-sufficient cells, bacteria or PMA induce an increased PKC activity resulting in an enhanced phosphorylation of p40phox, which elevates Nox2 activity.
Materials and Methods
Mice
Slamf8−/− [Slamf8ΔE2–4] BALB/c mice were generated, as described in Supplemental Fig. 1A, were generously donated by Drs. Cole and Guttierez-Ramos [Millenium, Inc.]. Slamf8−/− mice develop normally, and spontaneous disease or inflammation was not observed in these mice. In Slamf8−/− mice, T, B cells and macrophages develop similarly as in wt mice (Supplemental Fig. 1). wt BALB/c mice were purchased from The Jackson Laboratory (Bar Harbor, ME); all mice were maintained in a specific pathogen-free facility. The Beth Israel Deaconess Medical Center Institutional Animal Care and Use Committee approved all animal care and experimental procedures.
Cells
F4/80+ CD11b+ Bone Marrow derived, resident peritoneal or Thioglycollate-elicited peritoneal Mφ were obtained, as described (1). Slamf8−/− Mφ and RAW 264.7 macrophages were transfected with a Slamf8-mCherry vector using Amaxa (Lonza Group Ltd, Switzerland).
Real time PCR (Taqman)
Cells RNA was isolated using Trizol reagent (Invitrogen), 100ng RNA was used for Taqman assay, 18S rRNA was used as internal reference gene to normalize the transcripts level, the relative gene mRNA expression level was determined by 2−ΔΔct method.
Nox2 assays
Nox2 assays employing lucigenin (Sigma-Aldrich, St. Louis, MO) were performed after exposure to stimulated heat killed E. coli F18 or S. aureus bacteria (m.o.i. 100) or 1µg/ml PMA using a standard Glomax luminometer (Promega) (1). Phagosomal pH was determined using pHrodo E. coli or S. aureus (m.o.i 15; Invitrogen, Carlsbad, CA), as described (1).
PKC Kinase (PKC) Activity
PKC kinase activity was measured using the ADI-EKS-420A assay kit (Enzo Life Sciences, Plymouth Meeting, PA), as described in the manufacturer's protocol. TGC-elicited Mφ were stimulated with 1µg/ml PMA or heated killed E.coli F18 (m.o.i. 100) for the indicated times and then lysed with 500µl of RIPA buffer. 30µl of lysate per sample was used to quantify PKC activity.
Western blotting
TGC-elicited Mφ from wt and Slamf8−/− mice were stimulated with 1µg/ml PMA or E.coli F18 (m.o.i. 100) at the indicated time points. Phospho-p40phox (Thr154) antibody (#4311, Cell Signaling Technology) and p40-phox antibody (sc-30087, San Cruz Biotechnology) were used to detect phosphorylation and protein expression of p40phox by standard Immunoblotting procedures.
Statistical analysis
Statistical significance was determined by Student’s t-test (two tailed distribution with a two sample equal variance). P values less than 0.05 were considered significant.
Results and Discussion
Slamf8 is upregulated in macrophages upon stimulation
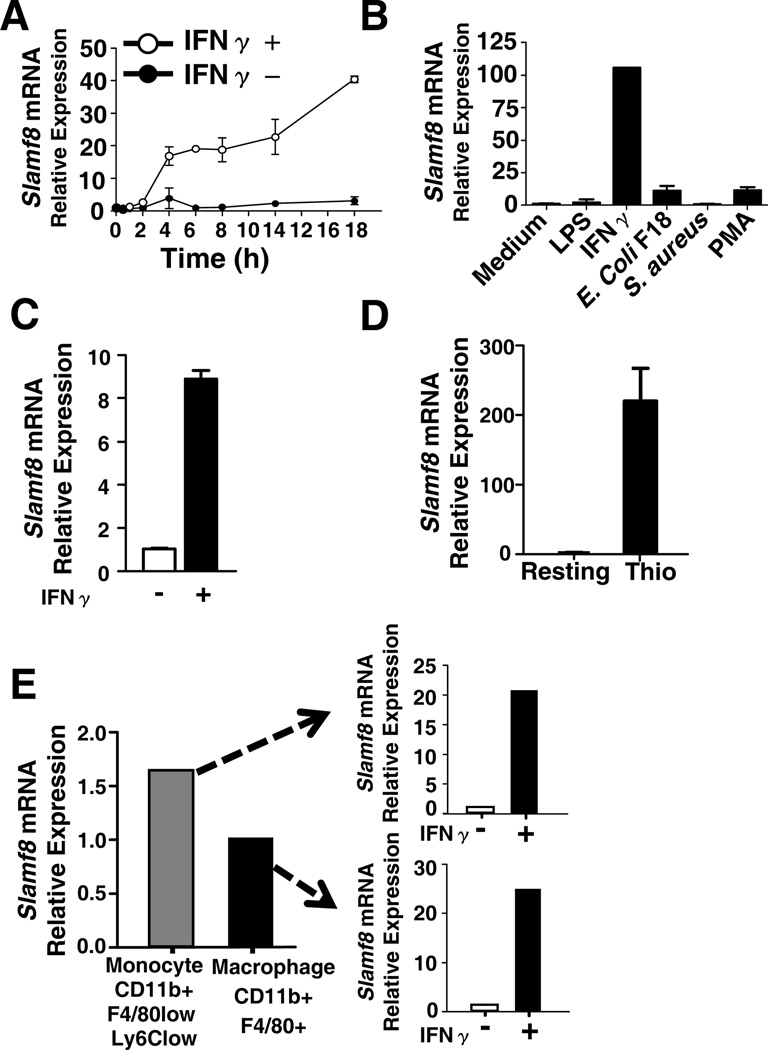
To evaluate whether expression of Slamf8 was induced by inflammatory agents, resident peritoneal macrophages isolated from BALB/c mice were incubated with LPS, IFNγ, E.coli, S.aureus or PMA followed by Taqman analysis. Slamf8 mRNA was virtually undetectable in the resting cells, but increased up to 100 fold after exposure to IFNγ (Fig. 1A & B). E.coli or PMA caused a 10-fold induction of expression, whilst LPS and S.aureus each had a lesser effect (Fig. 1A). IFNγ also significantly induced Slamf8 mRNA expression in bone marrow derived macrophage (Fig. 1C). Slamf8 expression was up to 200 times higher in thioglycolate-elicited macrophages than in resident cells (Fig. 1D). Nonetheless, in response to IFNγ Slamf8 expression was still further up-regulated in cellsorter-purified thioglycolate-elicited monocytes (CD11b+Ly6CLowF4/80Low) and macrophages (CD11b+ F4/80+) (Fig. 1E). This upregulation of Slamf8 in macrophages, particularly by IFNγ could indicate that this receptor functions in late responses to innate stimuli. Thus, Slamf8 expression in macrophages cells depends upon the presence of stimuli associated with bacterial infections.
Figure 1. Upregulation of expression of Slamf8.
A, Time course of induction of Slamf8 expression by resident pMφ in response to IFNγ (10ng/ml), as judged by Taqman analysis. B, Relative expression of the Slamf8 in resident pMφ upon 12h stimulation with LPS (100ng/ml), IFNγ (10ng/ml), E.coli F18 (m.o.i 100), S.aureus (m.o.i 100) or PMA (1µg/ml). C, Slamf8 expression in bone marrow derived macrophages upon IFNγ (10ng/ml) stimulation. D, Comparison of Slamf8 expression in resting and TGC-elicited peritoneal cells using Taqman analysis. E, Expression of Slamf8 before and after IFNγ (10ng/ml) stimulation in FACS sorted TGC pMφ (CD11b+, F4/80+) and monocytes (CD11b+, Ly6Clow, F4/80low). The data are representative of at least 3 independent experiments.
Slamf8 is a negative regulator of Nox2 activity
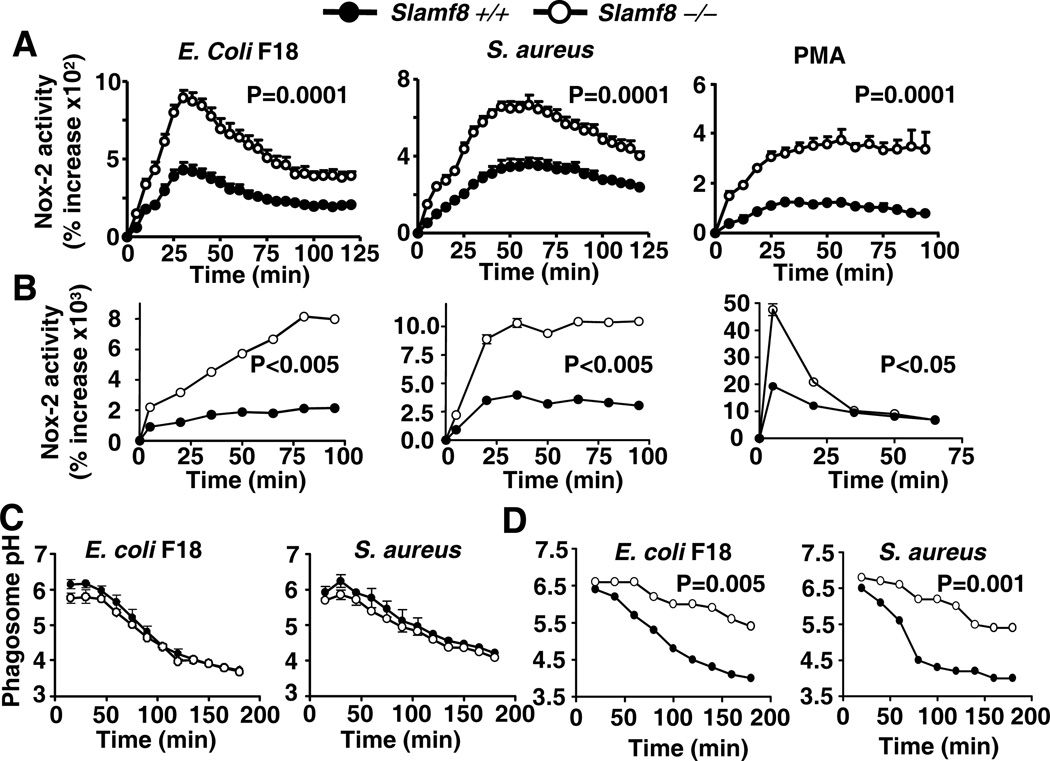
To assess whether Slamf8 would have an effect on macrophage functions, Nox2 (NADPH-oxidase) enzyme activity in Slamf8−/− BALB/c macrophages was tested. Unexpectedly, in response to E.coli and S. aureus a very significantly elevated Nox2 activity was found in Slamf8−/− bone marrow-derived macrophages (Fig. 2A). Surprisingly, this phenotype was also observed in response to the receptor independent PMA stimulus. Importantly, Slamf8−/− thioglycolate-elicited Mφ had a phenotype similar to that of the bone marrow derived macrophages (Fig. 2B), in contrast to resident resting macrophages (not shown).
Figure 2. Slamf8 is a negative regulator of Nox2 activity in Mφ.
A and B, Bone marrow derived (A) and TGC-elicited (B) Mφ were stimulated with E.coli F18 (m.o.i 100), S.aureus (m.o.i 100) or PMA (1µg/ml) and assessed for Nox2 activity using lucigenin. C and D, Resident peritoneal or TGC-elicited pMφ were examined for phagosomal acidification using E.coli or S.aureus (m.o.i 15) pHrodo bacteria. The data are representative of 3 independent experiments.
To determine whether the increased Nox2 activity in Slamf8−/− macrophages occurs in the bacterial phagosome, we used a fluorescence-based assay based on the principle that conversion of the reactive oxygen species that are produced by Nox2 to HO2 and H2O2 consumes protons, which enter the phagosome via a proton pump. Thus, a defective Nox2 activity results in less proton consumption in the bacterial phagosome and hence the pH is lowered more rapidly (1). We therefore assumed that a “hyperactive” Nox2 enzyme in the bacterial phagosome would result in an increased proton consumption and an impaired acidification. To test this, a pH sensitive dye, pHrodo, coupled to either E.coli or S.aureus was used in conjunction with flow cytometry. Consistent with the Nox2 phenotype, Slamf8−/− resident macrophages had no detectable changes in phagosomal acidification in comparison with wt cells (Fig. 2C). As predicted, Slamf8−/− TGC-elicited Mφ displayed a subnormal phagosomal acidification, as compared to Slamf8+/+ cells (Fig. 2D). We conclude that Slamf8 negatively modulates phagosomal Nox2 activity and acidification in a cargo-independent manner.
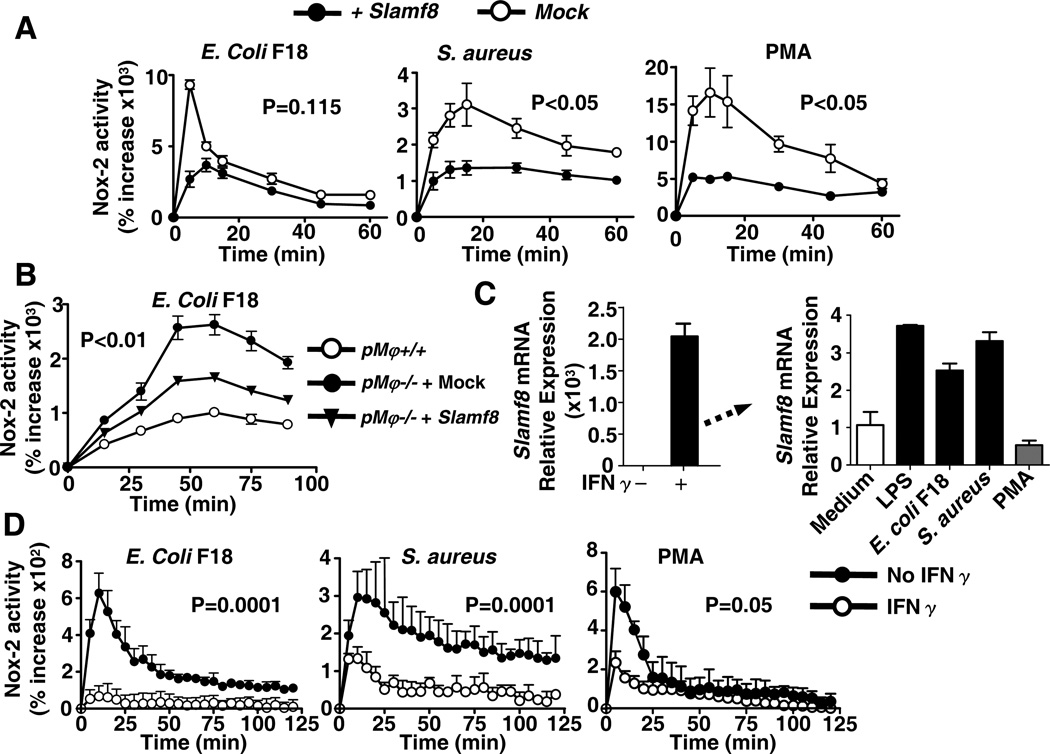
In order to confirm that Slamf8 negatively modulates Nox2 activity, RAW 264.7 macrophages were transfected with Slamf8, which greatly reduced Nox2 activity in response to bacteria and PMA (Fig. 3A). Furthermore, transfection of Slamf8 into Slamf8−/− primary Mφ also resulted in a significant reduction of the production of reactive oxygen species by Nox2 (Fig. 3B).
Figure 3. Introduction of Slamf8 into cells by transfection or IFNγ stimulation rescues the Nox2-associated phenotype.
A, RAW 264.7 cells were transfected with Slamf8-mCherry or a mock construct, stimulated with E.coli F18 (m.o.i 100), S.aureus (m.o.i 100) or PMA (1µg/ml) and assessed for Nox2 oxidase activity. B, Slamf8−/− Primary Mφ were transfected with Slamf8-mCherry, stimulated with E.coli F18 (m.o.i 100) and assessed for Nox2 activity. C, RAW 264.7 cells incubated for 12 h with IFNγ (10ng/ml) were assessed for Slamf8 expression, as in Fig. 1. D, Nox2 activity in IFNγ stimulated RAW 264.7 cells determined as in Fig. 3A. The data are representative of 3 independent experiments.
As we had found that IFNγ significantly enhances Slamf8 expression in RAW 264.7 Mφ (Fig. 1F and 3C), we tested the dependence of Nox2 activity in these macrophages. Indeed the presence of Slamf8 suppressed Nox2 activity in these cells (Fig. 3D). Taken together these studies demonstrate that Slamf8 is a negative regulator of Nox2 activity in Mφ. This function of Slamf8 therefore contrasts with Slamf1, which is a positive regulator of the same enzyme activity in response to E.coli, but not to S.aureus or PMA (1).
Slamf8 negatively controls phosphorylation of p40 upon induction by E.coli and/or PMA
To elucidate how the Nox2 activity in Slamf8−/− macrophages could be increased by bacteria and PMA, we reasoned that the Protein Kinase C (PKC) activity in these cells might be increased, as this enzyme is known to phosphorylate p40phox (6). To this end, we first determined that the protein expression of the key components of Nox2, namely gp91phox, p22phox, p40phox, p47phox and p67phox, was similar in wt and Slamf8−/− Mφ. Western blotting of wt and Slamf8−/− Mφ lysates showed that the basal expressions of all subunits are comparable (Supplemental Fig. 2A). Furthermore, after LPS, E.coli F18, S.aureus or PMA activation, the mRNA expression level of the membrane bound enzyme components gp91phox and p22phox, was identical in wt and Slamf8−/− Mφ (Supplemental Fig. 2B).
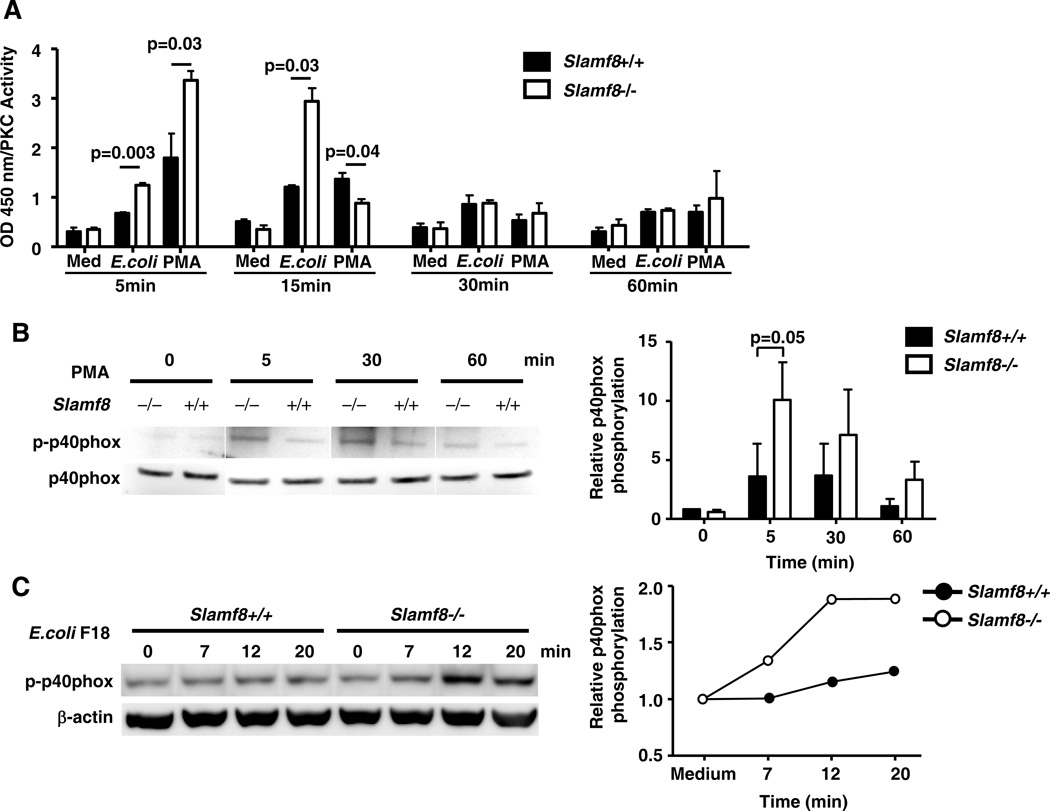
The isoforms of the serine/threonine kinase PKC in mouse macrophages, which are regulated by PMA, are PKC–β, –δ –ε and –η. PKC–δ is known to phosphorylate p40phox on Threonine 154 and Serine 315 (7), Phospho-p40phox stabilizes the assembled Nox2 enzyme complex (8). We first determined the kinetics of overall PKC activity in response to various stimuli in wt and Slamf8−/− Mφ (Fig. 4A). The PKC activity reached a peak at 5 min after PMA stimulation and was significantly higher in Slamf8−/− macrophages than wt cells, which correlates with the Nox2 activity (Fig 2). Similarly, upon exposure to E.coli the enzyme activation was higher in Slamf8−/− than in Slamf8+/+ macrophages, but peaked at 15 min. (Fig. 4A). Consistently with enhanced PKC activity in Slamf8−/− macrophages, the level of p40phox phosphorylation was significantly higher in Slamf8−/− macrophages and phosphorylation in response to PMA preceded that of E.coli (Fig. 4B and 4C).
Figure 4. PKC activity and phosphorylation of p40phox in Slamf8−/− and Slamf8+/+ macrophages upon stimulation with PMA and E.coli.
A, PKC kinase activity in Mφ after stimulation with 1µg/ml PMA and E.coli F18 (m.o.i 100). The data are representative of 2 independent experiments, each consisting of three Slamf8+/+ and Slamf8−/− mice. B and C, Phosphorylated p40phox was determined by Western Blotting in response to 1µg/ml PMA (B) or E.coi F18 (m.o.i 100) (C). Relative p40phox phosphorylation level was normalized to total p40phox (B) based on 3 independent experiments or to β-actin (C), which is representative of 3 independent experiments.
These data indicated Slamf8 negatively controls PKC activity in macrophages, which is known to phosphorylate p40phox, one key component of Nox2 in macrophages. Therefore, in the absence of Slamf8, Nox2 activity increases. Further biochemical and cell biology experiments are required to understand how Slamf8 is coupled to the PKC activated by bacteria and PMA in macrophages and phagosomes in particular.
Taken together, the outcomes of these studies show that Slamf8 is a novel negative regulator of Nox2 activity in Mφ. As Slamf8 expression is increased in monocytes and Mφ upon IFNγ stimulation and/or exposure to bacteria, the data suggest that Slamf8 signaling could become operational to dampen an ongoing innate immune response. This notion is supported by the previous observation that the degree of Mφ activation has a differential effect on phagosome activity (10–12). Because Slamf8 is a negative regulator Nox2 activity in Mφ, Slamf8-based therapeutic strategies could be designed to mitigate inflammatory conditions.
Supplementary Material
Acknowledgments
We thank Glenn E Brown and Michael B Yaffe [MIT] for help with the initial Nox2 analyses. We are grateful to Jacky Flipse for helpful discussions and Drs. Chunyan Ma and Marton Keszei for a critical review of the manuscript.
This work was supported by grants from the NIH (AI-15066 to CT) and the Ministry of Education and Science of Spain (SAF2006-08529; SAF2007-62562 to AA). SB and XR were recipients of a fellowship from the Crohn’s and Colitis Foundation of America.
Abbreviations used in this paper
- Slam
signaling lymphocyte activation molecule
- PMA
phorbol-12-myristate-13-acetate
- Mφ
Macrophages
- TGC
Thyoglycollate
- M.o.i
multiplicity of infection
References
- 1.Berger SB, Romero X, Ma C, Wang G, Faubion WA, Liao G, Compeer E, Keszei M, Rameh L, Wang N, et al. SLAM is a microbial sensor that regulates bacterial phagosome functions in macrophages. Nat. Immunol. 2010;11:920–927. doi: 10.1038/ni.1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Calpe S, Wang N, Romero X, Berger SB, Lanyi A, Engel P, Terhorst C. The SLAM and SAP gene families control innate and adaptive immune responses. Adv. Immunol. 2008;97:177–250. doi: 10.1016/S0065-2776(08)00004-7. [DOI] [PubMed] [Google Scholar]
- 3.Cannons JL, Tangye SG, Schwartzberg PL. SLAM family receptors and SAP adaptors in immunity. Annu. Rev. Immunol. 2011;29:665–705. doi: 10.1146/annurev-immunol-030409-101302. [DOI] [PubMed] [Google Scholar]
- 4.Kingsbury GA, Feeney LA, Nong Y, Calandra SA, Murphy CJ, Corcoran JM, Wang Y, Prabhu Das MR, Busfield SJ, Fraser CC, et al. Cloning, expression, and function of BLAME, a novel member of the CD2 family. J. Immunol. 2001;166:5675–5680. doi: 10.4049/jimmunol.166.9.5675. [DOI] [PubMed] [Google Scholar]
- 5.Heng TS, Painter MW C. Immunological Genome Project. The Immunological Genome Project: networks of gene expression in immune cells. Nat. Immunol. 2008;9:1091–1094. doi: 10.1038/ni1008-1091. [DOI] [PubMed] [Google Scholar]
- 6.Bertram A, Ley K. Protein kinase C isoforms in neutrophil adhesion and activation. Arch. Immunol. Ther. Exp. (Warsz) 2011;59:79–87. doi: 10.1007/s00005-011-0112-7. [DOI] [PubMed] [Google Scholar]
- 7.Bouin AP, Grandvaux N, Vignais PV, Fuchs A. p40(phox) is phosphorylated on threonine 154 and serine 315 during activation of the phagocyte NADPH oxidase. Implication of a protein kinase c-type kinase in the phosphorylation process. J. Biolo. Chem. 1998;273:30097–30103. doi: 10.1074/jbc.273.46.30097. [DOI] [PubMed] [Google Scholar]
- 8.Bedard K, Krause KH. The NOX family of ROS-generating NADPH oxidases: physiology and pathophysiology. Physiol. Rev. 2007;87:245–313. doi: 10.1152/physrev.00044.2005. [DOI] [PubMed] [Google Scholar]
- 9.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 2010;327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Yates RM, Hermetter A, Taylor GA, Russell DG. Macrophage activation downregulates the degradative capacity of the phagosome. Traffic. 2007;8:241–250. doi: 10.1111/j.1600-0854.2006.00528.x. [DOI] [PubMed] [Google Scholar]
- 11.Rybicka JM, Balce DR, Khan MF, Krohn RM, Yates RM. NADPH oxidase activity controls phagosomal proteolysis in macrophages through modulation of the lumenal redox environment of phagosomes. Proc. Natl. Acad. Sci. USA. 2010;107:10496–10501. doi: 10.1073/pnas.0914867107. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.