Abstract
THYmocyte differentiation antigen 1 (Thy-1), is a cell surface glycoprotein found on T cells and neurons and is involved in cell-to-cell interactions. In addition, Thy-1 knockouts (KO) are a potential mouse model of restless legs syndrome (RLS) based on clinical observations and the role of dopamine in the disease. In this study, we analyzed the activity and quantity of tyrosine hydroxylase (TH; the rate-limiting enzyme in dopamine production) and determined phosphorylation levels for the enzyme (phospho-serine-40 (pSer-40)). There was no significant difference in the total TH activity and pSer-40 TH levels between Thy-1 KO and control groups; however, TH specific activity was significantly lower (by 26%) in Thy-1 KO mice. This difference is, in part, due to increased TH protein levels in this group (increased by 29%). When analyzed by gender, we determined that Thy-1 KO female mice striata contained less TH specific activity compared to control females (decreased by 41%) and male control or Thy-1 KO animals (decreased by 30%). TH specific activity and pSer-40 TH levels between male Thy-1 KO and control displayed no differences. However, pSer-40 TH was significantly higher in control females (38%) compared to control or Thy-1 KO males. The Thy-1 KO females exhibited significantly lower (28%) pSer-40 TH (normalized to GAPDH or TH) than control females. Indeed, the Thy-1 KO females had 50% of the pSer-40 TH found in controls. Our results suggest a gender effect on TH specific activity, TH protein levels and serine-40 phosphorylation of TH in Thy-1 KO female mice.
Keywords: Thy-1, tyrosine hydroxylase, phosphorylation, gender-specific expression
1. Introduction
Thy-1 (THYmocyte differentiation antigen 1) is a glycoprotein originally associated with T cells (Letarte-Muirhead et al. 1975; Raff 1971). More recently, it has been associated with cell-cell communication and development in the central nervous system. Thy-1 is an abundant neuronal surface glycoprotein and it plays a role in vesicular release of neurotransmitter at the synapse. It is expressed at very high levels on neurons after axonal growth is complete. Moreover, Thy-1 protein is thought to contribute to neuronal plasticity in the grey matter areas of brain (Mayeux-Portas et al. 2000). The highest concentrations of Thy-1 protein in the brain are reported in the striatum and hippocampus (Seki et al. 2002).
Thy-1 is an iron responsive protein that has been shown to be reduced in the dopamine-rich substantia nigra of postmortem restless leg syndrome (RLS) patients (Wang et al. 2004). RLS is a neurological condition characterized by abnormal leg sensations and an uncontrolled urge to move the lower extremities during rest periods. RLS has an estimated prevalence of as high as 10% of the population with a marked female bias (Bjorvatn et al. 2005) in which RLS is observed two-times more frequently in females than males (Berger et al. 2004). In fact, it has been reported that 25–40% of pregnant women can be affected by RLS (Cesnik et al. 2010). Moreover, recent studies have shown that Thy-1 forms a physical interaction with sex hormone-binding globulin (SHBG) (Gnanasekar et al. 2009) and that SHBG is transported into brain cells in a sex hormone specific manner (Caldwell et al. 2007).
Thy-1 knockout dramatically alters dopaminergic systems and may modulate dopamine metabolism (Connor et al. 2008). Tyrosine hydroxylase (TH) catalyzes the rate-limiting and committed step in catecholamine biosynthesis (Levitt et al. 1965). TH requires iron (the ferrous form) as a cofactor and utilizes O2 and biopterin (BH4) as co-substrates and is the subject of extensive transcriptional and post-translational regulation (Dunkley et al. 2004; Kumer and Vrana 1996). Phosphorylation of serine-40 plays a dominant role in the post-translational regulation of the enzyme (Campbell et al. 1986; Haavik et al. 1997; Itagaki et al. 1999; Kleppe et al. 2001; Kumer and Vrana 1996) and increases the activity to a much greater extent than phosphorylation of serine-31 or serine-19 (Dunkley et al. 2004). Among the downstream signaling elements of Thy-1, protein kinase A (cAMP-dependent PKA), protein kinase C (phospholipid-dependent PKC), and calcium/calmodulin-dependent kinase (CaMPK) are prominent (Chen et al. 2007; Haeryfar and Hoskin 2001; Haeryfar and Hoskin 2004; Rege and Hagood 2006b; Yang et al. 2008). These specific systems uniquely converge on the regulation of serine-40 phosphorylation in TH.
Based on these observations, experiments were undertaken to measure the levels of TH activity, TH total protein, and the levels of pSer-40 TH. Findings suggest that there are gender-specific differences in TH regulation in Thy-1 KO mice.
2. Experimental Procedures
This study utilized Thy-1 knockout mice (Nosten-Bertrand et al. 1996) compared with normal animals of the C57BL/6 genetic background. The mice (30g each) were killed by deep anesthesia with sodium pentobarbital (Nembutal) (60 mg/kg i.p.) at about 1 year of age. The brains were quickly removed, and striata were dissected. All procedures involving animals were in accordance with guidelines of the Institutional Animal Care and Utilization Committee of the Pennsylvania State University. Striatal samples were frozen on dry ice and stored at −80°F until analyzed. Samples were pulverized in liquid nitrogen and then homogenized in ten volumes (w/v) buffer [25mM PIPES (pH 6.0), 250mM Sucrose, 100µM EDTA, protease inhibitor cocktail, 1mM DTT, 10µM Fe(NH4)2(SO4)2·6H2O, 0.2% Triton-X-100], sample homogenates were prepared by sonication on ice, and immediately subjected to protein determination, enzyme activity assay and western blot analysis.
2.1. TH Activity Assay and Specific Activity Determination
TH activity was assayed using a radio-enzymatic 3H2O release assay as previously described by Reinhard et al. (Reinhard et al. 1986) and modified by Walker et al. (Walker et al. 1994). Activity values were normalized to total protein present in the homogenized striatal tissue. Protein concentrations were determined by Bradford protein assay (Bradford, Bio-Rad, Hercules, CA), and were expressed as nmol/h/mg. In addition, in selected experiments, TH specific activity was determined by normalization to the amount of immunoreactive TH protein as determined by quantitative western blots (Horellou et al. 1988; Walker et al. 1994). All materials for activity assays were obtained from Sigma (St Louis, MO, USA) with the exception of activated charcoal (Darco G-60; Fisher Scientific, Pittsburgh, PA).
2.2. SDS-PAGE and Western Blot Analysis
The protein signals for detection of TH and pSer-40 TH were determined by SDS-PAGE and western blot analysis. Criterion (4–12% gradient) Tris/Glycine gels were resolved in Tris/Glycine buffer at 200 V, 115 Amp for 50 minutes. The gels were transferred to polyvinylidene fluoride (Immobilon P, Millipore Corporation, Billerica MA.) membrane using a semidry electro-blot apparatus set at 30 V for 1.5h (Owl Scientific, Cambridge, MA, USA). TH was detected by probing with a 1:1500 dilution of affinity-purified mouse anti-TH monoclonal antibody (Sigma). Phospho-serine-40 TH was detected by probing with a 1:1500 dilution of affinity-purified mouse serine-40-specific anti-phospho-TH polyclonal antibody (Sigma, St Louis, MO, USA). A 1:3000 dilution of donkey anti-mouse IgG secondary HRP conjugate (GE Healthcare, Lombard, Illinois) was used for signal detection followed by chemiluminescence (ECL; Amersham, Buckinghamshire, England). The bands were visualized following exposure to x-ray film. All film exposures were made in the linear range of response (data not shown). The x-ray films were scanned and densities were quantified using “ImageQuant TL v2005” software from GE Healthcare (Lombard, Illinois). The quantified intensities were then adjusted to an external standard present on all blots. Western blot signal intensity was normalized to GAPDH (glyceraldehyde-3-phosphate dehydrogenase) as a measure of TH or pSer-40 TH in a sample. In selected analyses, pSer-40 TH signals were normalized to total TH signals as a metric of relative percentage of TH that is phosphorylated.
2.3. Statistical Analysis
The TPH activity and immunoreactive protein determinations were analyzed using GraphPad Prism 5. We analyzed the data for four groups using one-way analysis of variance (ANOVA), followed by the Newman-Keuls Multiple comparison test and for two groups by using a Student's t-test. Statistical significance was associated with values of *p <0.05; **p <0.01, ***p<0.001.
3. Results
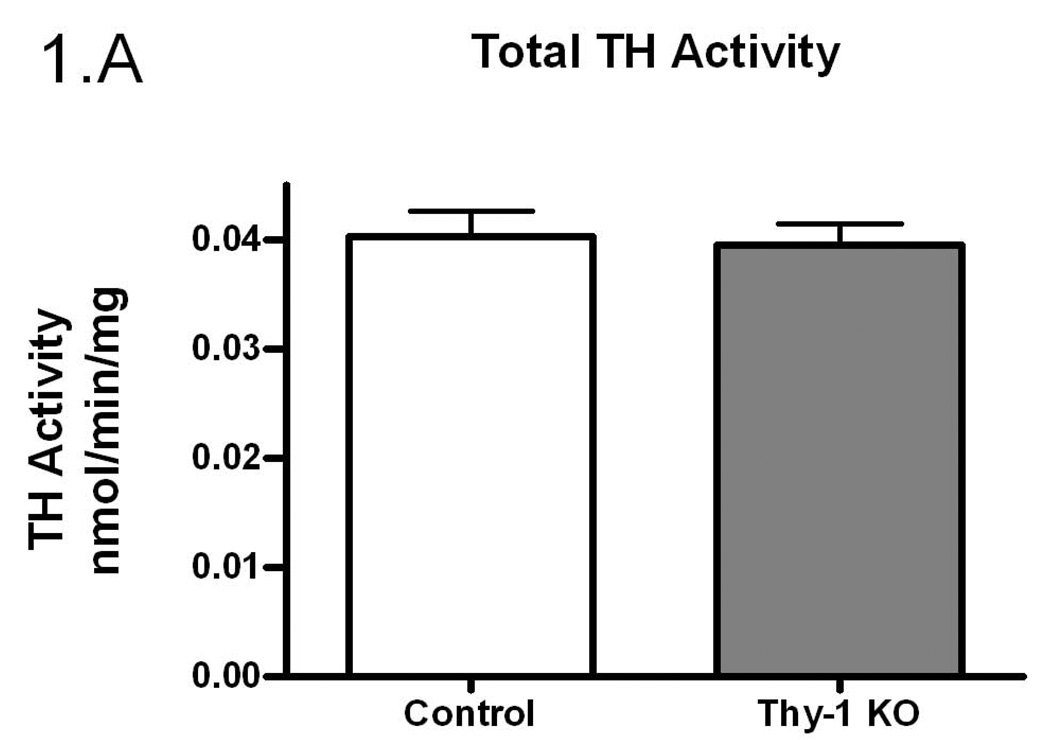
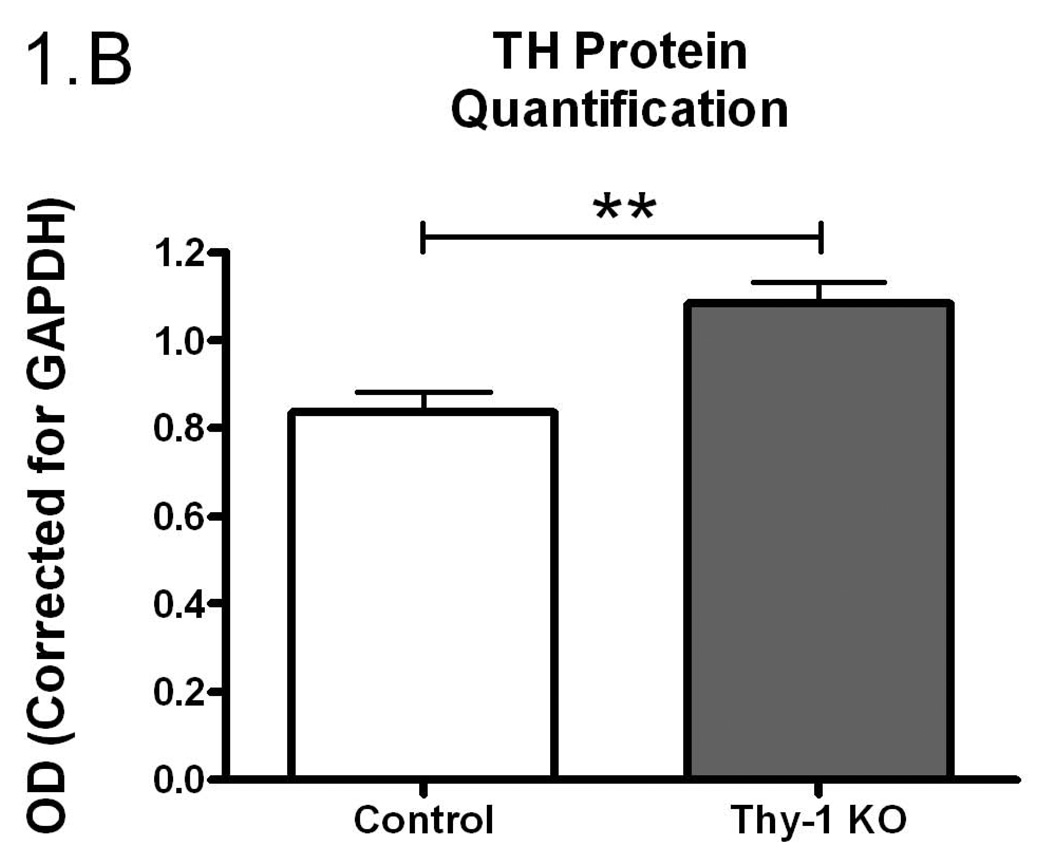
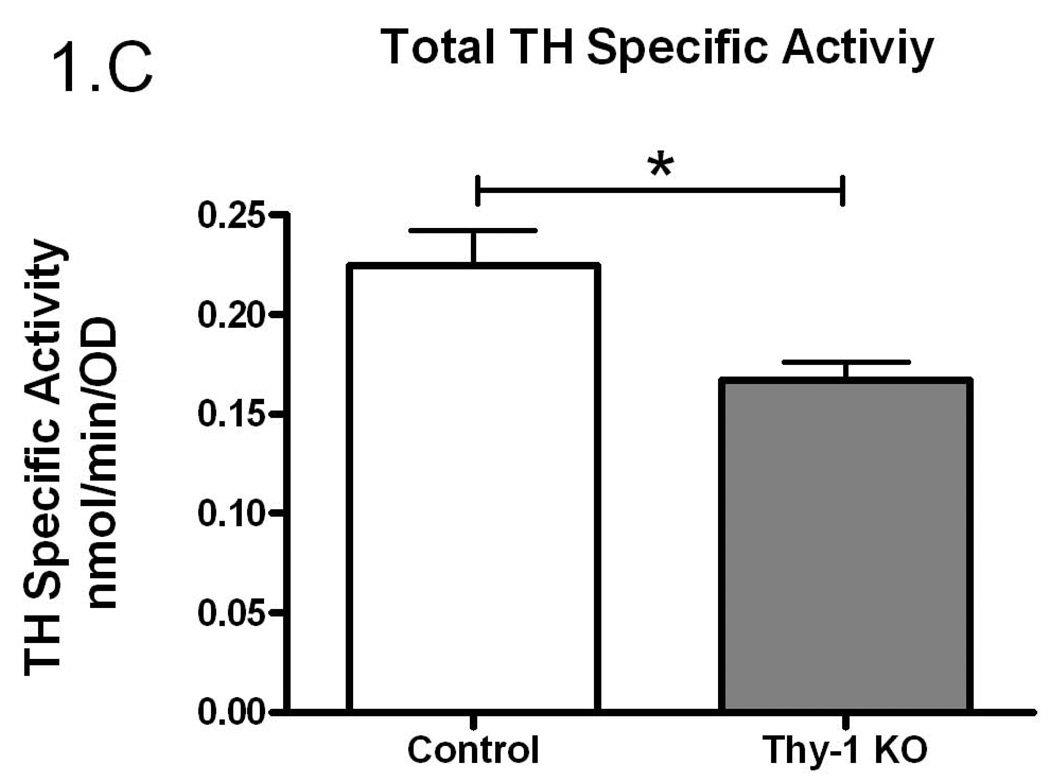
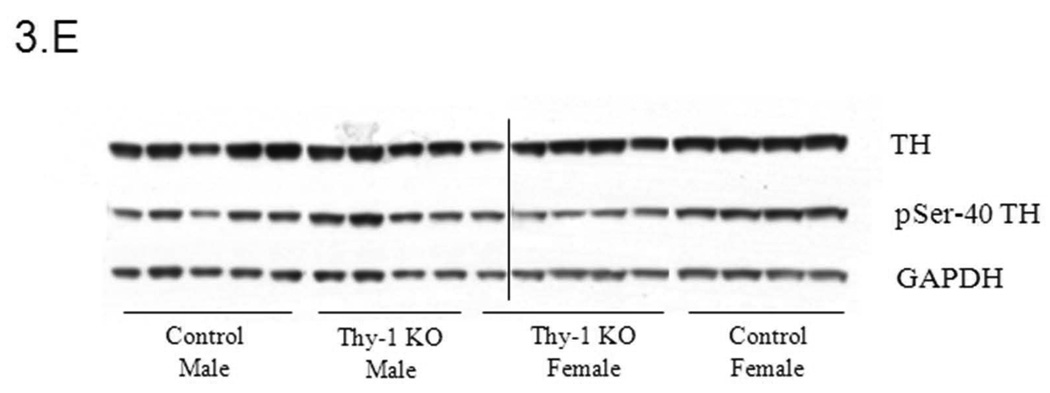
Given the proposed link between Thy-1, RLS, and dopamine systems, we undertook experiments to examine TH regulation in Thy-1 KO transgenic mice. In an initial assessment of striatal TH activity, there were no differences between control and Thy-1 KO animals (p=0.59; Figure 1A). However, there were differences in the amount of immunoreactive protein as assessed by western blot analysis (29% increase in the Thy-1 KO; p=0.022; Figure 1B, see Figure 3E for primary western blot data). As a result, dividing enzyme activity by immunoreactive signals illuminates a statistically significant reduction in TH specific activity – a surrogate measure of the individual activity of an enzyme molecule (decreased by 26%, p=0.01; Figure 1C).
Figure 1.
The amount of TH activity (A), TH protein quantification (B) and TH specific activity (C) in the striata of Thy-1 KO mice and controls were measured (as described in Methods). The results shown are the mean of 9 control (4 female; 5 male) and 9 Thy-1 KO (5 female; 4 male) animals. Results were analyzed using a GraphPad Prism5 student t-test. The error bars represent standard error of the mean. Statistical significance * p<0.05; ** p<0.01; *** p<0.001.
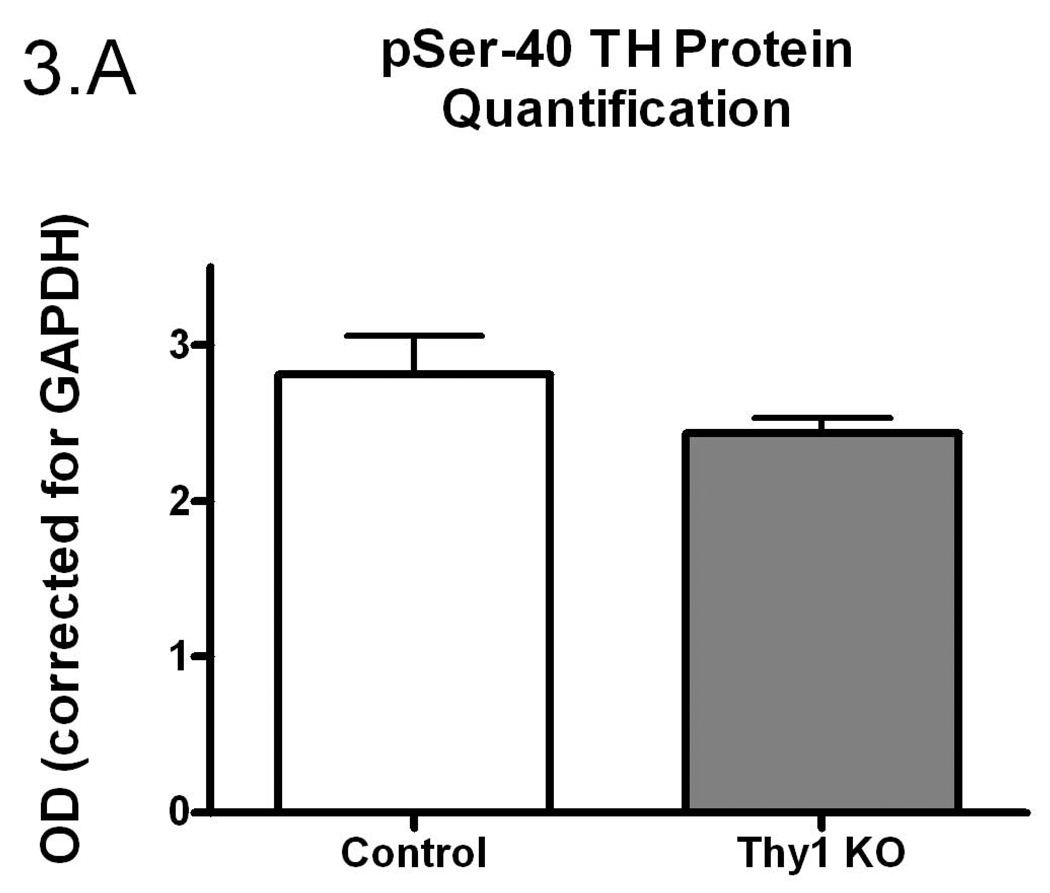
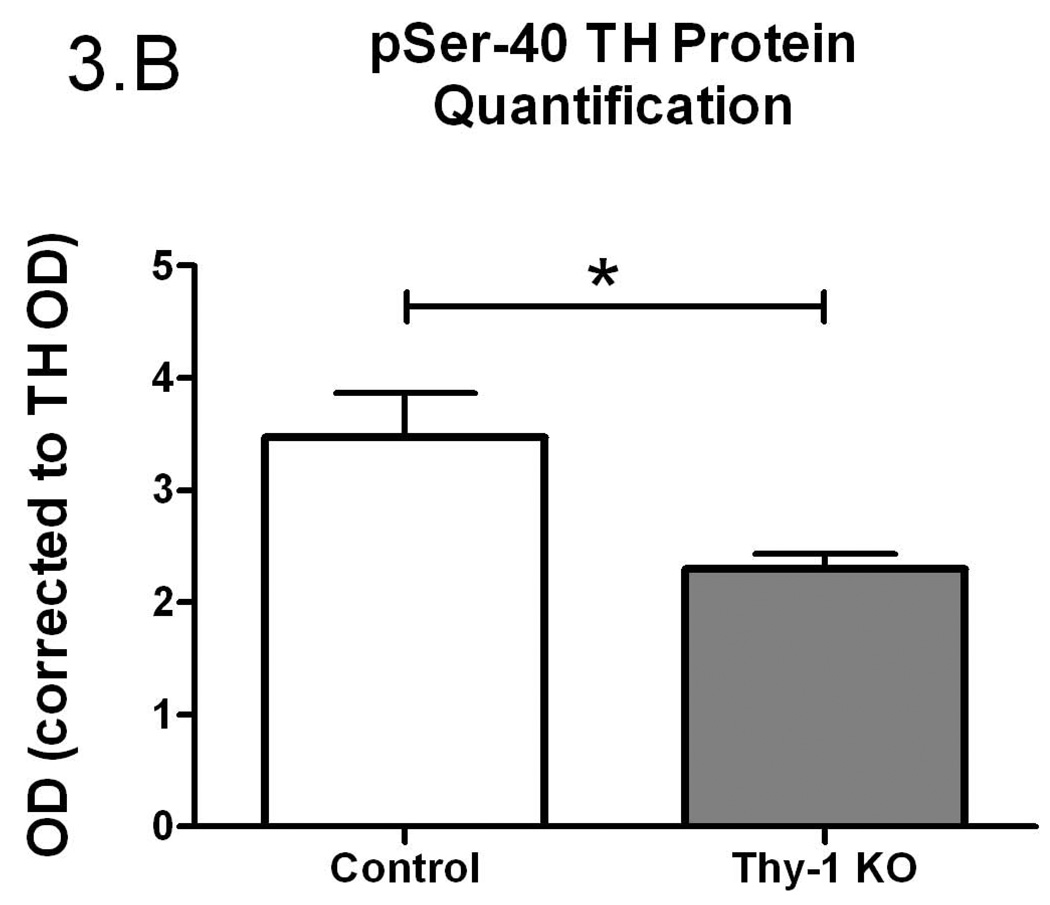
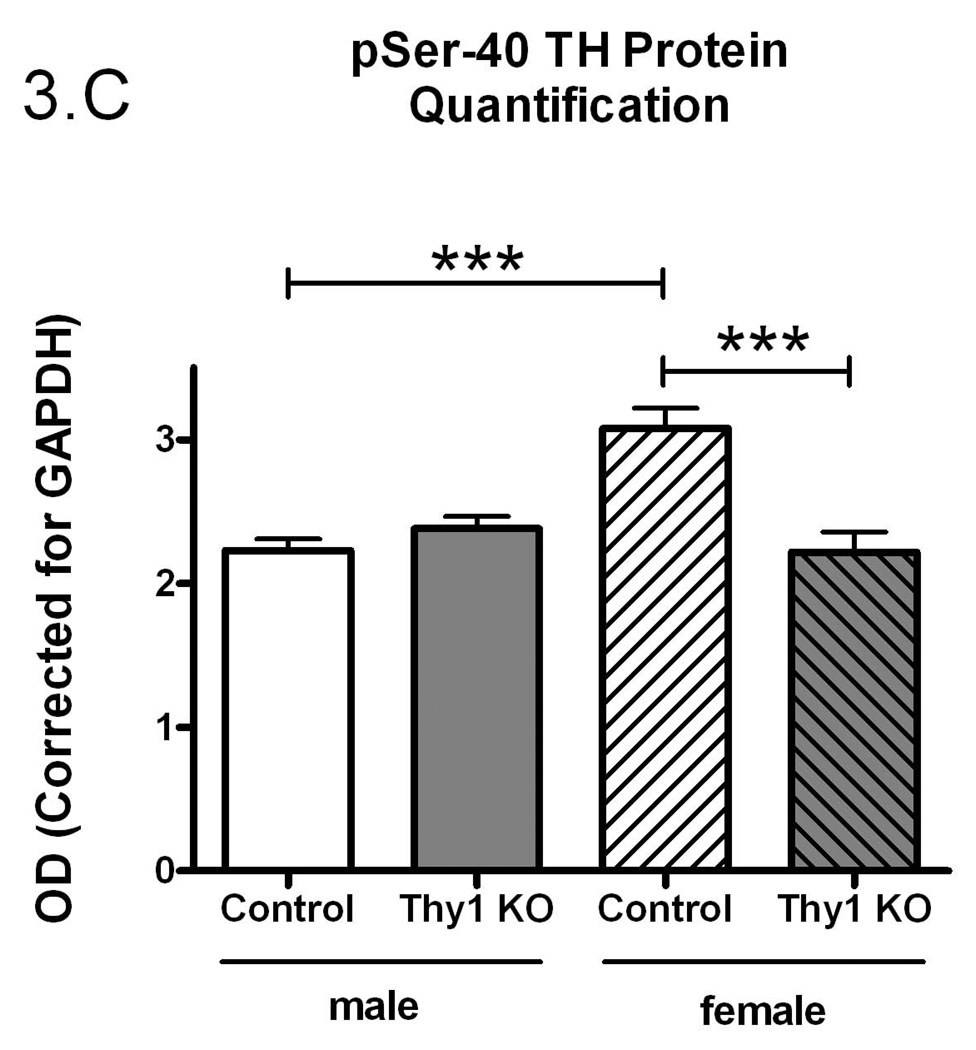
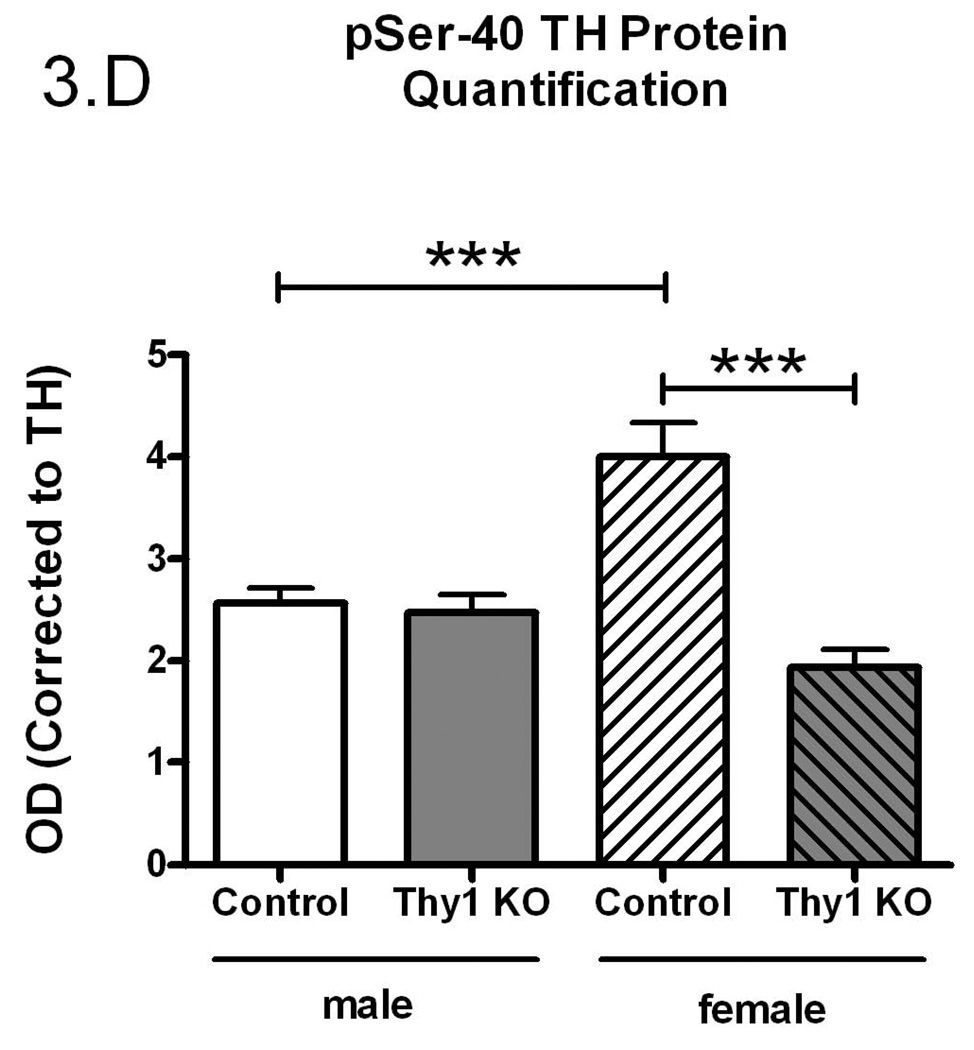
Figure 3.
Phospho-serine-40 (pSer-40) TH protein quantification (A); Controls and Thy-1 KO pSer-40 TH protein quantification (corrected for GAPDH) (B); Controls and Thy-1 KO pSer-40 TH protein quantification (corrected to TH) (C); Gender-based pSer-40 TH protein quantification (corrected for GAPDH) Thy-1 KO (5 female; 4 male) and control (4 female; 5 male) mice, (D). Total TH, pSer-40 TH and GAPDH protein levels in striatal samples were determined by quantitative western blots. Results were analyzed by using one-way ANOVA followed by a Newman-Keuls post-hoc test. The error bars represent standard error of the mean. Statistical significance * p<0.05; ** p<0.01; *** p<0.001. Representative primary western immunoblot signals from two gels are provided in panel (E).
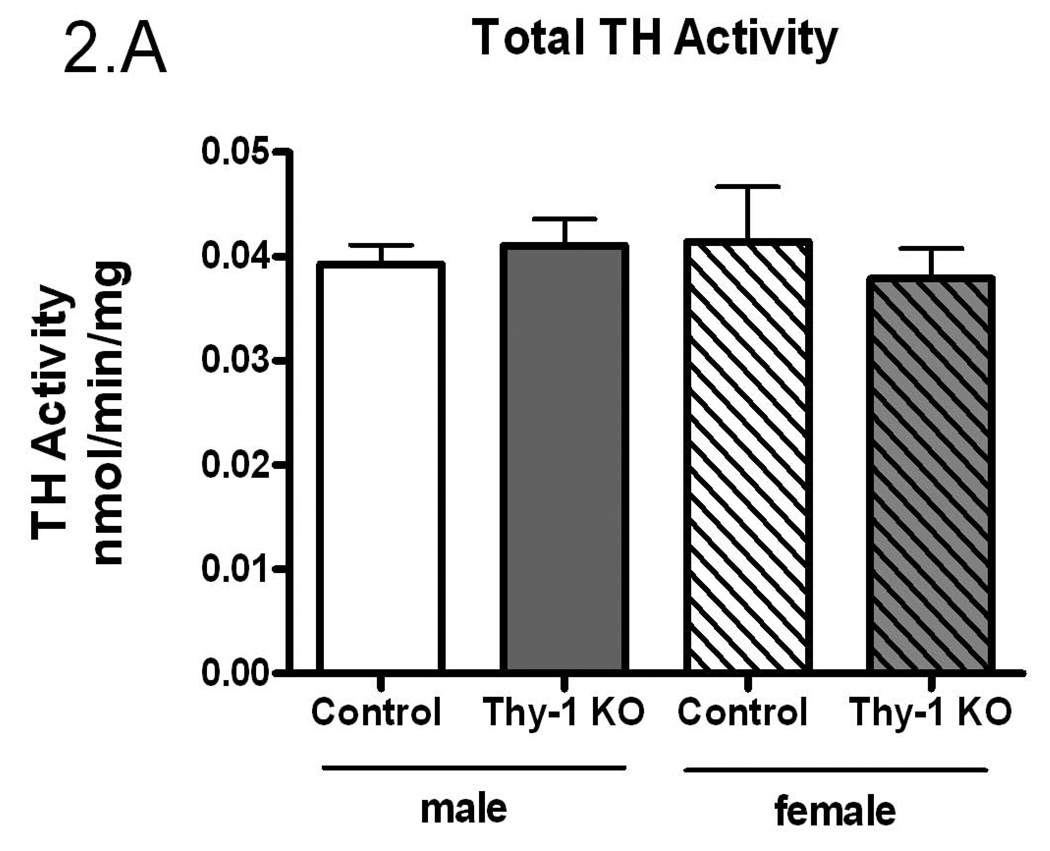
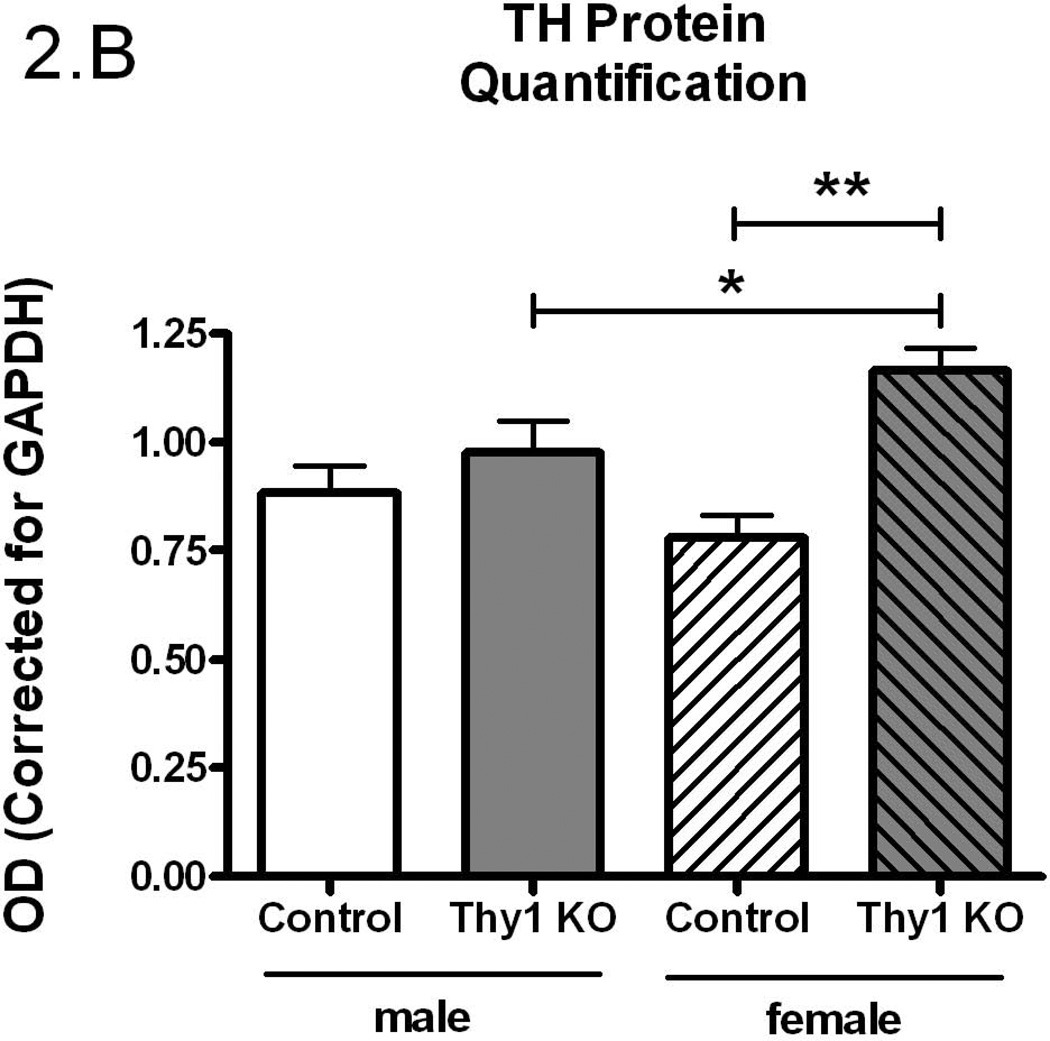
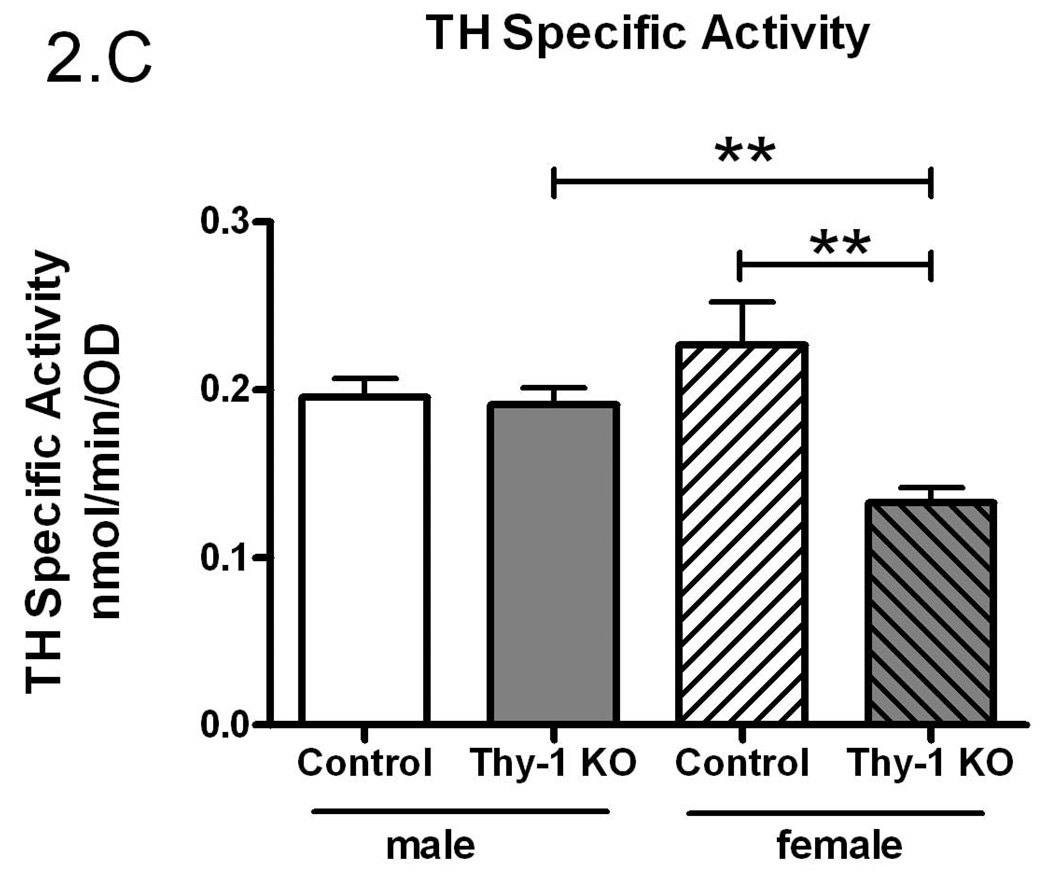
The preceding studies were conducted in a mixed gender design. Given the suggestion that there may be a gender-specific expression of RLS (Garcia-Borreguero et al. 2006), the data were further analyzed by sex. While there were no differences in total TH activity (p=0.84, Figure 2A), there was significantly more striatal immunoreactive TH protein in Thy-1 KO female mice (p=0.003, Figure 2B) that resulted in a significantly lower TH specific activity in females (p=0.003, Figure 2C). Thy-1 KO female mice striata exhibited 41% less TH specific activity compared to control female and 30% less than males of either genotype. Therefore, the entire decrease in TH specific activity in Thy-1 KO animals can be attributed to the total amount of TH protein in female knockout animals.
Figure 2.
Gender-based TH activity (A), TH protein quantification (B) and specific activity (C) were measured in the striata of Thy-1 KO (5 female; 4 male) and control (4 female; 5 male) mice. TH and GAPDH protein levels in striatal tissue homogenates were quantified by measuring the optical density of bands detected by western blot. Results were analyzed by using one-way ANOVA followed by a Newman-Keuls post-hoc test. The error bars represent standard error of the mean. Statistical significance * p<0.05; ** p<0.01; *** p<0.001.
There is a rich history demonstrating that TH specific activity is regulated, in large measure, by phosphorylation of TH at serine-40 (Dunkley et al. 2004; Kumer and Vrana 1996). Therefore, using a phospho-specific antibody, we assessed the phosphorylation status (pSer-40) of TH (see Figure 3C for primary data). We found no significant difference in total striatal pSer-40 TH immunoreactivity when normalized to GAPDH (p=0.16, Figure 3A). However, pSer-40 TH protein, normalized to the amount of total immunoreactive TH, paralleled the TH enzyme specific activity (Figure 3B). That is, a smaller percentage of the total TH protein was phosphorylated in the Thy-1 KO and this corresponds to reduced TH specific activity. As with the preceding results, these differences could be ascribed completely to the female genotypes (Figure 3C and 3D).
4. Discussion
Our results show that there is a gender effect on TH specific activity, total TH quantification and pSer-40 TH quantification in Thy-1 KO female mice. Thy-1 is implicated in the coordination of dopaminergic innervation of the striatum during development (Shults and Kimber 1993). Thy-1 expression occurs prior to maturation of dopaminergic terminals and may thus serve to guide outgrowth in the striatum and to suppress further outgrowth from dopaminergic axons (Shults and Kimber 1993). This may contribute, in part, to documented deficits in long-term potentiation and mild learning deficits in the Thy-1 KO (Rege and Hagood 2006a).
Our results indicate that, while total TH activity in Thy-1 KO mice remains constant, both the total levels and specific activity of the TH protein are altered. While TH recovery following dissection could be possible source of the difference between TH quantity and TH activity levels in Control and Thy-1 KO samples, we note consistency in GAPDH and total TH (with the exception of female Thy-1 animals; see Figure 3E for representative primary data). While levels of TH protein are increased in Thy-1 KO samples, the specific activity is decreased, signifying a decrease in the activity of each individual TH molecule. This effect can be attributed to a gender-specific (female) change in expression of TH protein and specific activity. Indeed, a number of studies report gender-specific expression of TH in various brain regions of the rat (Abreu et al. 1988; Gomes-da-Silva et al. 2000; Ovtscharoff et al. 1992; Thanky et al. 2002). The change in specific activity is further explained upon examination of levels of phosphorylated TH (pSer-40). Phosphorylation of TH at serine-40 has been shown to increase levels of activity (Dunkley et al. 2004; Haycock 1990; Kumer and Vrana 1996). Levels of pSer-40 TH in control females are significantly increased compared with males but, in female Thy-1 KO mice, phospho-TH levels are significantly lower than control animals. This would account for the observed decrease in specific activity. It is noteworthy that these differences were not detected using less sensitive immuno-dot blots in an earlier report (Connor et al. 2008). Moreover, this is clearly not the entire story. Specifically, there are two additional important TH phosphorylation sites (serine-19 and serine-31) that contribute to TH regulation. These sites were not assessed in the present study, primarily because Thy-1 signaling would appear to converge on serine-40 (PKA, PKC, CaMPKII). It is interesting that while pSer-40 and TH specific activity are linked in females (Figures 2C and 3C), males fail to exhibit statistically-significant reductions in TH specific activity, even though their pSer-40 levels are lower than female controls. It seems likely that other sites (ser-19 and/or ser-31) are therefore involved in male TH regulation.
Thy-1 expression persists in the adult brain and is thought to stabilize synapses and modulate kinase activity through the phosphorylation of a number of protein targets (Jeng et al. 1998). Additionally, Thy-1 has been shown to be a component of many types of secretory vesicles, plays a regulatory role in vesicular release of neurotransmitters at the synapse (Jeng et al. 1998), and is involved in trafficking of proteins, including neurotransmitter receptors, to the membrane (Sudhof 1995; Takamori et al. 2000). Several factors are involved in regulating the activity of TH (Kumer and Vrana 1996). In our previous report on the Thy-1 KO (Connor et al. 2008), we observed a dramatic increase in total striatal dopamine in the KO which would be predicted to decrease enzyme phosphorylation and activity (as rev. in (Kumer and Vrana 1996)). On the basis of these observations, we hypothesize that Thy-1 plays a significant role in the dopaminergic system in the brain which, when dysregulated, causes alterations in dopamine synthesis and signaling. The possibility that Thy-1 contributes to gender specific serine-40 TH phosphorylation, and therefore specific activity, could help explain its role in dopamine signaling, and may point to mechanisms of gender-bias in RLS.
Acknowledgments
The authors are grateful to Dr. Melinda Lull and Dr. Kara Kuntz for critical editing of the manuscript. This work was supported by R01GM-38931 (KV) and PA Department of Health (JC).
REFERENCES
- Abreu P, Hernandez G, Calzadilla CH, Alonso R. Reproductive hormones control striatal tyrosine hydroxylase activity in the male rat. Neurosci Lett. 1988;95(1–3):213–217. doi: 10.1016/0304-3940(88)90659-3. [DOI] [PubMed] [Google Scholar]
- Berger K, Luedemann J, Trenkwalder C, John U, Kessler C. Sex and the risk of restless legs syndrome in the general population. Arch Intern Med. 2004;164(2):196–202. doi: 10.1001/archinte.164.2.196. [DOI] [PubMed] [Google Scholar]
- Bjorvatn B, Leissner L, Ulfberg J, Gyring J, Karlsborg M, Regeur L, Skeidsvoll H, Nordhus IH, Pallesen S. Prevalence, severity and risk factors of restless legs syndrome in the general adult population in two Scandinavian countries. Sleep Med. 2005;6(4):307–312. doi: 10.1016/j.sleep.2005.03.008. [DOI] [PubMed] [Google Scholar]
- Caldwell JD, Shapiro RA, Jirikowski GF, Suleman F. Internalization of sex hormone-binding globulin into neurons and brain cells in vitro and in vivo. Neuroendocrinology. 2007;86(2):84–93. doi: 10.1159/000107072. [DOI] [PubMed] [Google Scholar]
- Campbell DG, Hardie DG, Vulliet PR. Identification of four phosphorylation sites in the N-terminal region of tyrosine hydroxylase. J Biol Chem. 1986;261(23):10489–10492. [PubMed] [Google Scholar]
- Cesnik E, Casetta I, Turri M, Govoni V, Granieri E, Strambi LF, Manconi M. Transient RLS during pregnancy is a risk factor for the chronic idiopathic form. Neurology. 2010;75(23):2117–2120. doi: 10.1212/WNL.0b013e318200d779. [DOI] [PubMed] [Google Scholar]
- Chen CH, Chen YJ, Jeng CJ, Yang SH, Tung PY, Wang SM. Role of PKA in the anti-Thy-1 antibody-induced neurite outgrowth of dorsal root ganglionic neurons. J Cell Biochem. 2007;101(3):566–575. doi: 10.1002/jcb.21217. [DOI] [PubMed] [Google Scholar]
- Connor JR, Wang XS, Neely EB, Ponnuru P, Morita H, Beard J. Comparative study of the influence of Thy1 deficiency and dietary iron deficiency on dopaminergic profiles in the mouse striatum. J Neurosci Res. 2008;86(14):3194–3202. doi: 10.1002/jnr.21758. [DOI] [PubMed] [Google Scholar]
- Dunkley PR, Bobrovskaya L, Graham ME, von Nagy-Felsobuki EI, Dickson PW. Tyrosine hydroxylase phosphorylation: regulation and consequences. J Neurochem. 2004;91(5):1025–1043. doi: 10.1111/j.1471-4159.2004.02797.x. [DOI] [PubMed] [Google Scholar]
- Garcia-Borreguero D, Egatz R, Winkelmann J, Berger K. Epidemiology of restless legs syndrome: the current status. Sleep Med Rev. 2006;10(3):153–167. doi: 10.1016/j.smrv.2006.01.001. [DOI] [PubMed] [Google Scholar]
- Gnanasekar M, Suleman FG, Ramaswamy K, Caldwell JD. Identification of sex hormone binding globulin-interacting proteins in the brain using phage display screening. Int J Mol Med. 2009;24(4):421–426. doi: 10.3892/ijmm_00000248. [DOI] [PubMed] [Google Scholar]
- Gomes-da-Silva J, Perez-Rosado A, de Miguel R, Fernandez-Ruiz J, Silva MC, Tavares MA. Neonatal methamphetamine in the rat: evidence for gender-specific differences upon tyrosine hydroxylase enzyme in the dopaminergic nigrostriatal system. Ann N Y Acad Sci. 2000;914:431–438. doi: 10.1111/j.1749-6632.2000.tb05217.x. [DOI] [PubMed] [Google Scholar]
- Haavik J, Almas B, Flatmark T. Generation of reactive oxygen species by tyrosine hydroxylase: a possible contribution to the degeneration of dopaminergic neurons? J Neurochem. 1997;68(1):328–332. doi: 10.1046/j.1471-4159.1997.68010328.x. [DOI] [PubMed] [Google Scholar]
- Haeryfar SM, Hoskin DW. Selective pharmacological inhibitors reveal differences between Thy-1- and T cell receptor-mediated signal transduction in mouse T lymphocytes. Int Immunopharmacol. 2001;1(4):689–698. doi: 10.1016/s1567-5769(01)00002-9. [DOI] [PubMed] [Google Scholar]
- Haeryfar SM, Hoskin DW. Thy-1: more than a mouse pan-T cell marker. J Immunol. 2004;173(6):3581–3588. doi: 10.4049/jimmunol.173.6.3581. [DOI] [PubMed] [Google Scholar]
- Haycock JW. Phosphorylation of tyrosine hydroxylase in situ at serine 8, 19, 31, and 40. J Biol Chem. 1990;265(20):11682–11691. [PubMed] [Google Scholar]
- Horellou P, Le Bourdelles B, Clot-Humbert J, Guibert B, Leviel V, Mallet J. Multiple human tyrosine hydroxylase enzymes, generated through alternative splicing, have different specific activities in Xenopus oocytes. J Neurochem. 1988;51(2):652–655. doi: 10.1111/j.1471-4159.1988.tb01088.x. [DOI] [PubMed] [Google Scholar]
- Itagaki C, Isobe T, Taoka M, Natsume T, Nomura N, Horigome T, Omata S, Ichinose H, Nagatsu T, Greene LA, Ichimura T. Stimulus-coupled interaction of tyrosine hydroxylase with 14-3-3 proteins. Biochemistry. 1999;38(47):15673–15680. doi: 10.1021/bi9914255. [DOI] [PubMed] [Google Scholar]
- Jeng CJ, McCarroll SA, Martin TF, Floor E, Adams J, Krantz D, Butz S, Edwards R, Schweitzer ES. Thy-1 is a component common to multiple populations of synaptic vesicles. J Cell Biol. 1998;140(3):685–698. doi: 10.1083/jcb.140.3.685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kleppe R, Toska K, Haavik J. Interaction of phosphorylated tyrosine hydroxylase with 14-3-3 proteins: evidence for a phosphoserine 40-dependent association. J Neurochem. 2001;77(4):1097–1107. doi: 10.1046/j.1471-4159.2001.00318.x. [DOI] [PubMed] [Google Scholar]
- Kumer SC, Vrana KE. Intricate regulation of tyrosine hydroxylase activity and gene expression. J Neurochem. 1996;67(2):443–462. doi: 10.1046/j.1471-4159.1996.67020443.x. [DOI] [PubMed] [Google Scholar]
- Letarte-Muirhead M, Barclay AN, Williams AF. Purification of the Thy-1 molecule, a major cell-surface glycoprotein of rat thymocytes. Biochem J. 1975;151(3):685–697. doi: 10.1042/bj1510685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Levitt M, Spector S, Sjoerdsma A, Udenfriend S. Elucidation of the Rate-Limiting Step in Norepinephrine Biosynthesis in the Perfused Guinea-Pig Heart. J Pharmacol Exp Ther. 1965;148:1–8. [PubMed] [Google Scholar]
- Mayeux-Portas V, File SE, Stewart CL, Morris RJ. Mice lacking the cell adhesion molecule Thy-1 fail to use socially transmitted cues to direct their choice of food. Curr Biol. 2000;10(2):68–75. doi: 10.1016/s0960-9822(99)00278-x. [DOI] [PubMed] [Google Scholar]
- Nosten-Bertrand M, Errington ML, Murphy KP, Tokugawa Y, Barboni E, Kozlova E, Michalovich D, Morris RG, Silver J, Stewart CL, Bliss TV, Morris RJ. Normal spatial learning despite regional inhibition of LTP in mice lacking Thy-1. Nature. 1996;379(6568):826–829. doi: 10.1038/379826a0. [DOI] [PubMed] [Google Scholar]
- Ovtscharoff W, Eusterschulte B, Zienecker R, Reisert I, Pilgrim C. Sex differences in densities of dopaminergic fibers and GABAergic neurons in the prenatal rat striatum. J Comp Neurol. 1992;323(2):299–304. doi: 10.1002/cne.903230212. [DOI] [PubMed] [Google Scholar]
- Raff MC. Surface antigenic markers for distinguishing T and B lymphocytes in mice. Transplant Rev. 1971;6:52–80. doi: 10.1111/j.1600-065x.1971.tb00459.x. [DOI] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1 as a regulator of cell-cell and cell-matrix interactions in axon regeneration, apoptosis, adhesion, migration, cancer, and fibrosis. FASEB J. 2006a;20(8):1045–1054. doi: 10.1096/fj.05-5460rev. [DOI] [PubMed] [Google Scholar]
- Rege TA, Hagood JS. Thy-1, a versatile modulator of signaling affecting cellular adhesion, proliferation, survival, and cytokine/growth factor responses. Biochim Biophys Acta. 2006b;1763(10):991–999. doi: 10.1016/j.bbamcr.2006.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reinhard JF, Jr, Smith GK, Nichol CA. A rapid and sensitive assay for tyrosine-3-monooxygenase based upon the release of 3H2O and adsorption of [3H]-tyrosine by charcoal. Life Sci. 1986;39(23):2185–2189. doi: 10.1016/0024-3205(86)90395-4. [DOI] [PubMed] [Google Scholar]
- Seki M, Nawa H, Morioka T, Fukuchi T, Oite T, Abe H, Takei N. Establishment of a novel enzyme-linked immunosorbent assay for Thy-1; quantitative assessment of neuronal degeneration. Neurosci Lett. 2002;329(2):185–188. doi: 10.1016/s0304-3940(02)00654-7. [DOI] [PubMed] [Google Scholar]
- Shults CW, Kimber TA. Thy-1 immunoreactivity distinguishes patches/striosomes from matrix in the early postnatal striatum of the rat. Brain Res Dev Brain Res. 1993;75(1):136–140. doi: 10.1016/0165-3806(93)90073-j. [DOI] [PubMed] [Google Scholar]
- Sudhof TC. The synaptic vesicle cycle: a cascade of protein-protein interactions. Nature. 1995;375(6533):645–653. doi: 10.1038/375645a0. [DOI] [PubMed] [Google Scholar]
- Takamori S, Riedel D, Jahn R. Immunoisolation of GABA-specific synaptic vesicles defines a functionally distinct subset of synaptic vesicles. J Neurosci. 2000;20(13):4904–4911. doi: 10.1523/JNEUROSCI.20-13-04904.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thanky NR, Son JH, Herbison AE. Sex differences in the regulation of tyrosine hydroxylase gene transcription by estrogen in the locus coeruleus of TH9-LacZ transgenic mice. Brain Res Mol Brain Res. 2002;104(2):220–226. doi: 10.1016/s0169-328x(02)00383-2. [DOI] [PubMed] [Google Scholar]
- Walker SJ, Liu X, Roskoski R, Vrana KE. Catalytic core of rat tyrosine hydroxylase: terminal deletion analysis of bacterially expressed enzyme. Biochim Biophys Acta. 1994;1206(1):113–119. doi: 10.1016/0167-4838(94)90079-5. [DOI] [PubMed] [Google Scholar]
- Wang X, Wiesinger J, Beard J, Felt B, Menzies S, Earley C, Allen R, Connor J. Thy1 expression in the brain is affected by iron and is decreased in Restless Legs Syndrome. J Neurol Sci. 2004;220(1–2):59–66. doi: 10.1016/j.jns.2004.02.004. [DOI] [PubMed] [Google Scholar]
- Yang SH, Chen YJ, Tung PY, Lai WL, Chen Y, Jeng CJ, Wang SM. Anti-Thy-1 antibody-induced neurite outgrowth in cultured dorsal root ganglionic neurons is mediated by the c-Src-MEK signaling pathway. J Cell Biochem. 2008;103(1):67–77. doi: 10.1002/jcb.21387. [DOI] [PubMed] [Google Scholar]