Abstract
Adolescence is a period of radical normative changes and increased risk for substance use, mood disorders, and physical injury. Researchers have proposed that increases in reward sensitivity, i.e., sensitivity of the behavioral approach system (BAS), and/or increases in reactivity to all emotional stimuli (i.e., reward and threat sensitivities) lead to these phenomena. The present study is the first longitudinal investigation of changes in reward (i.e., BAS) sensitivity in 9 to 23-year-olds across a two-year follow-up. We found support for increased reward sensitivity from early to late adolescence and evidence for decline in the early twenties. This decline is combined with a decrease in left nucleus accumbens (Nacc) volume, a key structure for reward processing, from the late teens into the early twenties. Furthermore, we found longitudinal increases in sensitivity to reward to be predicted by individual differences in the Nacc and medial OFC volumes at baseline in this developmental sample. Similarly, increases in sensitivity to threat (i.e., BIS sensitivity) were qualified by sex, with only females experiencing this increase, and predicted by individual differences in lateral OFC volumes at baseline.
Keywords: Adolescence, behavioral approach system (BAS), reward sensitivity
Adolescence is a period of radical social, biological, and psychological changes (Forbes & Dahl, 2010; Casey, Jones, & Hare, 2008; Nelson, Leibenluft, McClure, & Pine, 2005; Steinberg & Morris, 2001), many of which are associated with increased risk-taking behaviors that can have grave consequences (Eaton et al., 2006). Adolescence is also a period of elevated vulnerability for the onset of depressive, anxiety, substance use, and other mental disorders (Kessler et al., 2005). Recent studies have attempted to understand both these normative and psychopathological developmental processes in adolescence from the perspective of increased sensitivity to reward, or behavioral approach system (BAS) sensitivity (e.g., Leen-Feldner, Zvolensky, & Feldner, 2004; Patrick, Blair, & Maggs, 2008).
The present study is the first longitudinal test of changes in reward (i.e., BAS) sensitivity in a large sample of 9 to 23 year-olds over a two-year follow-up. In addition, the present study is the first to examine longitudinal changes in volumes of brain structures that represent key nodes for reward processing, such as medial orbitofrontal cortex (OFC) and nucleus accumbens (Nacc). Although the processing of affectively-salient information is complex and involves numerous brain regions, we selected these structures because of overwhelming evidence that they are involved in responses to reward magnitudes and reward valuation (e.g., Depue and Collins, 1999; Galvan et al., 2006; Kringelbach & Rolls, 2004; Somerville, Hare, & Casey, 2011; but for evidence of their involvement in negative stimuli processing see Faure, Reynolds, Richard, & Berridge, 2008; Levita et al., 2009; Morrison & Salzman, 2009). As a measure of discriminant validity, longitudinal changes in behavioral inhibition system (BIS), or threat sensitivity, are also examined, as well as changes in volumes of brain structures involved in emotional processing, but not specific to reward processing, i.e., amygdala and lateral OFC.
Researchers propose that adolescents experience a normative increase in sensitivity to rewards in the context of immature top-down, inhibitory control (Casey, Jones, & Hare, 2008; Ernst, Pine, & Hardin, 2006; Nelson et al., 2005; Somerville, Jones, & Casey, 2010; Steinberg, 2008). There is preliminary empirical support for this hypothesis from cross-sectional studies, such as stronger effect of monetary incentives (Hardin et al., 2007; Jazbec et al., 2006) and greater positive affect following receipt of monetary reward (Ernst et al., 2005) in adolescents compared to adults. Late adolescents also make more advantageous choices in a decision-making task with variable rewards and risks (i.e., the Iowa Gambling Task, IGT) compared to younger groups (Hooper, Luciana, Conklin, & Yarger, 2004). A recent study using a modified IGT found adolescents to be more sensitive to positive feedback compared to children and adults (Cauffman et al., 2010). Moreover, in adolescence, there is an increase in incentive-driven behaviors with high potential for negative consequences, such as illicit substance/alcohol use and unsafe sex (Eaton et al., 2006).
This reward hypersensitivity during adolescence suggests increased sensitivity of the BAS. The BAS is one of the two behavioral-motivational systems proposed by Gray (1981, 1991, 1994) to guide behavior. The BAS's function is to facilitate approach to rewards and is triggered by incentive cues in reward paradigms and safety cues in active avoidance paradigms (e.g., Depue & Collins, 1999; Gray, 1991; Fowles, 1987). Another behavioral-motivational system, the BIS, is hypothesized to be involved in responses to threat and punishment and to inhibit approach responses in situations of conflict between reward and risk (Gray & McNaughton, 2000). BAS hyper- and hyposensitivity has been implicated in bipolar disorders and unipolar depression, respectively, (e.g., Alloy & Abramson, 2010; Depue & Iacono, 1989; Kasch, Rottenberg, Arnow, & Gotlib, 2002; Pinto-Meza et al., 2006; Urošević, Abramson, Harmon-Jones, & Alloy, 2008), whereas a hypersensitive BIS has been implicated in heightened amygdala activation in response to threat stimuli (e.g., Cools et al., 2004) and in anxiety and depression (e.g., Hundt et al., 2007; Kasch et al., 2002). Thus, extremes on dimensions of BAS and BIS sensitivities are hypothesized to place individuals at risk for psychopathology.
Consistently, in cross-sectional studies, increased BAS sensitivity has been linked to a number of risky behaviors and clinical symptoms during adolescence (see supplementary material for BIS hypersensitivity effects). For example, in a sample of Russian adolescents and young adults, increased BAS sensitivity predicted scores on a composite index of substance use (Knyazev et al., 2004). Similarly, heightened BAS sensitivity predicted alcohol abuse and dysfunctional eating in adolescent females (Loxton & Dawe, 2001), alcohol and tobacco use in undergraduate samples (O'Connor et al., 2009; Patrick, Blair, & Maggs, 2008), greater adolescent delinquent behaviors (Hasking, 2007), increased parental reports of externalizing behaviors (Colder & O'Connor, 2004), and adolescents' excessive computer use that led to functional impairments (e.g., sleep disruption/arguments; Giles & Price, 2008). BAS sensitivity has also predicted greater conflicts with adults and socializing with deviant peers in both adolescent males and females (Knyazev, 2004).
These prior studies of adolescents have assessed sensitivities to reward and punishment (i.e., BAS and BIS sensitivities) using the BIS/BAS scales (Carver & White, 1994), which have substantial empirical support for their construct validity. For example, the BIS/BAS scales correlate with EEG indices of approach and withdrawal affective styles (Harmon-Jones & Allen, 1997; Sutton & Davidson, 1997), as well as predict experimental responses to rewards and punishments (e.g., Carver & White, 1994) and relevant prospective clinical symptoms (e.g., Meyer, Johnson, & Winters, 2001). In a longitudinal twin study, a moderate genetic effect accounted for approximately one third of variance in the BIS/BAS scales (Takashi et al., 2007). Another study found the same four-factor structure of the BIS/BAS scales in large adolescent and adult samples, suggesting that the structure of each system is comparable across development (Cooper, Gomez, & Aucote, 2007). In sum, the BIS/BAS scales are promising, underutilized tool for the assessment of longitudinal, developmental changes in reward and threat sensitivities from adolescence into adulthood.
Additional longitudinal work is needed to assess whether sensitivity to reward actually peaks in adolescence in comparison to other life periods as some researchers have proposed (e.g., Doremus-Fitzwater et al., 2010; Somerville et al., 2010). An alternative explanation would be that BAS sensitivity, and possibly BIS sensitivity, represent stable personality traits across lifetime. Thus, increases in risk-taking and vulnerability to clinical disorders observed in adolescence would be due to environmental changes or to other BAS-irrelevant developmental processes. It could also be that the adolescent trajectory for changes in reward (i.e., BAS) sensitivity differs for individuals who are high versus low on this trait at the onset of adolescence, or for individuals who differ in other important baseline characteristics. This would explain why only a subset of adolescents experiences clinically significant increases in risk-taking, mood disorder symptoms, and substance use. Longitudinal data with assessments of BAS and BIS sensitivities could address these questions but is lacking in the published literature.
Longitudinal data on the structural changes in brain regions relevant for the processing of reward (and threat) during adolescence are also sparse. The Nacc and OFC are key nodes in the brain's reward system (i.e., BAS) and located in frontal and striatal regions that continue to develop during adolescence (Giedd et al., 1999; Sowell et al., 1999). The Nacc has been implicated in translating incentive-reward motivation into behavioral actions (Depue & Collins, 1999), while functioning of the OFC has been separated according to its substructures. The medial OFC and lateral OFC have been implicated in the processing of the expected value of reinforcers and punishers, respectively (Kringelbach & Rolls, 2004; but for different view see Morrison & Salzman, 2009). Thus, the medial OFC is implicated in BAS-relevant behavioral control, whereas the lateral OFC is implicated in BIS-relevant processes and provides discriminative validity test for reward-specific changes during adolescence. Similarly, the amygdala is a key structure for decoding the affective features of both pleasurable and aversive stimuli (e.g., Davis & Whalen, 2001), and as such less likely to be linked to preferential increase in reward sensitivity. These structures (OFC, Nacc, amygdala) represent logical starting places for investigating structural neural development in relation to reward (i.e., BAS) sensitivity.
The majority of studies of healthy adolescents have investigated changes in the functioning of the OFC and Nacc, rather than in their structure. In decision-making paradigms, adolescents, compared to adults, exhibit greater ventral striatal activation (which includes Nacc) after winning money (Ernst et al., 2005) and increased activity in the Nacc during risk-taking (Galvan et al., 2006). There is further evidence that striatal activity in response to unexpected reward feedback (i.e., positive prediction errors; Cohen et al., 2010) and in response to rewarding stimuli (Somerville, Hare, & Casey, 2011) peaks in adolescence. Moreover, adolescence has been linked to decreased ventral striatal activity during reward cue evaluation, but increased ventral striatal activity during anticipation of behavioral responses to rewards (Geier et al., 2010). There is a paucity of longitudinal data assessing structural changes within reward circuitry, and whether structural changes relate to variation in reward sensitivity.
The present study, with its two-year longitudinal design and a large sample of 9 to 23-year-olds, proposes to address the above-described gaps in the literature (e.g., examining relationship between key neural structures and reward sensitivity in adolescence, as well as investigating longitudinal increase in reward sensitivity during adolescence). Specifically, (1) we will provide a longitudinal test to the hypothesis that reward sensitivity increases in adolescence and also examine specificity of this hypothesis by examining changes in threat sensitivity. (2) We will test whether any observed longitudinal changes in reward and threat sensitivities are qualified by sex. We will also (3) examine longitudinal changes in OFC, Nacc, and amygdala volumes during adolescence, as well as the relationship of developmental changes in the reward-specific structures (i.e., Nacc and medial OFC), and non-reward-specific emotional processing structures (i.e., amygdala and lateral OFC), to changes in self-report measures of reward and threat sensitivities. In all these analyses, we will also examine cross-sectional age effects to further characterize significant developmental changes.
Method
Participants
Participants (n= 184; 83 male, 101 female) without past or current psychopathology participated in the study after providing informed consent and/or assent. Minor participants were recruited by phone from a database maintained by the Institute of Child Development at University of Minnesota, which consists of families that had agreed to be contacted for participation in university studies at the time of their child's birth. Second, postcards were sent out to University of Minnesota civil service (i.e., non-academic) employees directing them to contact our laboratory if they had children within the desired age ranges interested in study participation. Young adults (age 18–23) were recruited through the use of flyers posted throughout campus. In addition, any adult employees who received postcards and were in the desired age range were able to participate.
Study eligibility was determined with a short phone screening and in-person comprehensive clinical interview using the Kiddie - SADS - Present and Lifetime Version (K-SADS-PL; Kaufman, Birmaher, Brent, Rao, & Ryan, 1996). Exclusion criteria included histories of neurological or psychiatric disorders, preterm birth or other birth complications, current or past substance abuse, loss of consciousness, learning disabilities, current or past psychoactive prescription drug use, non-native English speaking, and uncorrected vision or hearing problems. Due to the structural MRI protocol, left-handedness (Oldfield, 1971) and all imaging contraindications (e.g., metallic implants, etc.) also resulted in study exclusion. The study protocol was approved by the local Institutional Review Board.
Participants ranged in age from 9.21 to 23.96 years at the Time 1 (baseline) assessment; 157 participants completed the Time 2 assessment. There were no participant exclusions adopted at Time 2. The Time 2 assessment was completed as close to 2 years after the Time 1 assessment as possible, with the mean length of follow up being 2.12, ± 0.28 years. The sample was predominantly Caucasian (87.4%), with 1.6% of the sample being African American, 1.6% Asian, 4.4% Pacific Islander, and 4.9% self-identified as `other'; the sample's racial/ethnic demographics closely match those of the state of Minnesota in the most recent census (2009 American Community Survey (ACS), published by the U.S. Census Bureau: http://factfinder.census.gov). Participants' socio-economic status was determined by their parental education (66.4% of mothers and 63.4% of fathers completed bachelor's degrees or higher) and average family income (M = 96,411.39, SD = 72,759.72 U.S. dollars), which yielded a sample with a predominantly middle to upper-middle class background. There were no significant differences in demographic information between participants who completed the Time 2 assessment versus those who did not, except that individuals who completed the Time 2 assessment were slightly younger than non-completers (mean age 15.86 (SD = 3.99) and 18.30 (SD = 3.56)). That is, the study's younger cohorts were more likely to be available for the Time 2 assessment. Attrition was largely due to older participants' relocations to other locales.
Procedure
At both time points, participants completed a demographics and diagnostic interview assessment on one day and a set of questionnaires, a neurocognitive battery, psychophysiological testing, and a structural magnetic resonance imaging (MRI) scan on another day. The present study focuses on measures of age, sex, BIS/BAS sensitivities, and OFC, Nacc, and amygdala volumes.
Measures
The BIS/BAS scales
Individual differences in sensitivities to reward and threat were assessed using the BIS/BAS scales (Carver & White, 1994), which are comprised of a 7-item BIS scale (e.g., “I worry about making mistakes,” “I feel worried when I think that I have done poorly at something”) and three BAS subscales: 5-item Reward Responsiveness scale (e.g., “When I get something I want, I feel excited and energized,” “It would excite me to win a contest”), a 4-item Drive scale (e.g., “I go out of my way to get things I want,” “If I see a chance to get something I want, I move on it right away”), and a 4-item Fun Seeking scale (e.g., “I crave excitement and new sensations,” “I will often do things for no other reason than that they might be fun”). The three BAS subscales were also be summed to yield a BAS Total score. Possible scores range from 7 to 28 for BIS, 5 to 20 for BAS Reward Responsiveness, 4 to 16 for BAS Drive and BAS Fun Seeking, and 13 to 52 for the BAS Total.
Magnetic Resonance Imaging (MRI)
Measures of volumes were derived for the following regions of interest (ROIs): left lateral OFC, right lateral OFC, left medial OFC, right medial OFC, left Nacc, right Nacc, left amygdala, and right amygdala, for 149 participants who had MRI data available at both Time 1 and 2 (i.e., 8 participants with behavioral data for Time 1 and 2 did not complete Time 2 MRI scans due to the presence of imaging contraindications, such as orthodontic braces). MRI images were acquired on a 3-Tesla Siemens Trio scanner (Siemens Medical Systems, Erlangen, Germany) at the University of Minnesota's Center for Magnetic Resonance Research. Three-dimensional brain images were obtained with a coronal T1-weighted Magnetization Prepared Rapid Gradient Echo (MPRAGE) sequence (TR = 2530 msec, TE = 3.65 msec, TI = 1100 msec, 240 slices, voxel size =1.0mm × 1.0mm × 1.0mm, flip angle = 7°, FOV = 256 mm).
Estimates of cortical and subcortical volumes for the ROIs were obtained by processing the high-resolution anatomical images in the FreeSurfer v.4.5.0 image analysis suite (http://surfer.nmr.mgh.harvard.edu/). Partway through the Time 2 data collection, the 3 Tesla system was upgraded from a Siemens Trio to a Siemens TIM Trio. In order to minimize variability resulting from this hardware change, including a new gradient set, T1-weighted images were corrected for distortions resulting from gradient nonlinearity (Jovicich, et al., 2006) prior to FreeSurfer processing. After implementing this correction, we followed the standard FreeSurfer processing pipeline for longitudinal data processing (see http://surfer.nmr.mgh.harvard.edu/fswiki/LongitudinalProcessing). Full descriptions of the FreeSurfer processing steps used in the present study have been reported in prior publications by FreeSurfer group (Dale & Sereno, 1993; Dale, Fischl, Sereno, 1999; Fischl et al., 1999; Fischl, Sereno, Dale, 1999; Fischl & Dale, 2000; Fischl, Liu, Dale, 2001; Fischl et al., 2002; Fischl et al., 2004a; Fischl et al., 2004b; Han et al., 2006; Jovicich et al., 2006; Segonne et al., 2004). Briefly, this processing includes: removal of non-brain tissue using a hybrid watershed/surface deformation procedure (Segonne et al., 2004), automated Talairach transformation, segmentation of the subcortical white matter and deep gray matter volumetric structures (Fischl et al., 2002; Fischl et al., 2004a), intensity normalization (Sled, Zijdenbos, Evans, 1998), tessellation of the gray matter/white matter boundary, automated topology correction (Fischl, Liu, Dale, 2001; Segonne et al., 2004), and surface deformation following intensity gradientsto optimally place the gray/white and gray/cerebrospinal fluid borders at the location where the greatest shift in intensity defines the transition to the other tissue class (Dale & Sereno, 1993; Dale, Fischl, Sereno, 1999; Fischl & Dale, 2000). FreeSurfer morphometric procedures exhibit good test-retest reliability across scanner manufacturers and across field strengths (Han et al., 2006). All automatically generated volumes were visually inspected for accuracy, and manual corrections using FreeSurfer tools were carried out as needed. The ROIs used in data analyses were taken from the set of cortical and subcortical structural volumes generated by cortical parcellation (i.e., medial and lateral OFC volumes) and subcortical segmentation (i.e., Nacc and amygdala volumes) procedures in FreeSurfer. Please see Supplemental Materials Figure 1 for examples of ROIs' volumes yielded by these FreeSurfer procedures for a typical participant from the present sample. Volumes of each region were corrected for total brain volume prior to analysis and represented as ratios of ROI volume to total brain volume.
Statistical Approach
Data were analyzed using the Statistical Package for the Social Sciences, version 19.0. To address whether the BIS/BAS sensitivities change over time, whether these changes are qualified by sex and/or age, and whether there are cross-sectional effects of age, repeated measures ANCOVAs were conducted separately for each BIS/BAS subscale. The MRI indices, one per hemisphere, of medial OFC, lateral OFC, Nacc, and amygdala volumes, were analyzed as dependent variables in a series of repeated measures ANCOVAs, which examined effects of continuous covariate age, controlling for scanner upgrade status and sex. Significant age effects were followed up by age group comparisons for ease of interpretation and to be consistent with prior research on reward sensitivity in adolescence (e.g., Galvan et al., 2006; Somerville et al., 2011). In these follow-up analyses, we compared early adolescents (ages 9–12), late adolescents (ages 13–17), vs. young adults (ages 18–23), based on their Time 1 age.
Longitudinal associations between changes in relevant brain volumes and changes in the BIS/BAS scales were explored using hierarchical regression analyses in the whole sample, controlling for effects of sex, age, and scanner upgrade, which is a special case of Analysis of Partial Variance. Partial correlations in Analysis of Partial Variance yield relationships between a predictor variable and outcome variable after regressing out variance due to all other predictor variables in the model. Partial correlation is equivalent to correlating residuals of the predictor and outcome variables, after taking out variance due to predictors entered in Step 1 from both variables. In the present study, partial correlations are equivalent to correlating residual change in the brain volume and residual change in the relevant BIS/BAS subscale, after controlling for sex, age, and scanner upgrade. This approach for the study of change is a special case of the Analysis of Partial Variance (for additional description please see Cohen & Cohen, 1983, pp. 402–422). For an example of an application of this technique to derive conclusions from longitudinal research see Metalsky, Halberstadt, & Abramson, 1987)1.
Results
Relations among the BIS/BAS Scales
Means and standard deviations for the BIS/BAS scales at Time 1 and Time 2 are presented in Table 1. Based on Pearson's r correlations from the Time 1 assessment of the whole sample, the BAS subscales were significantly related to each other: r = .50 for Drive and Fun Seeking, r = .33 for Drive and Reward Responsiveness, r = .27 for Reward Responsiveness and Fun Seeking. In addition, consistent with prior research (e.g., Carver & White, 1994), BAS Reward Responsiveness was positively related to the BIS scale in the whole sample at Time 1 (r = .35). Similar patterns were found for interrelations among the BIS/BAS scales at the Time 2: r = .48 for Drive and Fun Seeking, r = .23 for Drive and Reward Responsiveness, r = .26 for Reward Responsiveness and Fun Seeking, and r = .31 for Reward Responsiveness and BIS. Internal consistencies of the BIS/BAS scales were acceptable and ranged from a Cronbach's alpha of .66 for BIS, .73 for BAS Drive, .59 for BAS Reward Responsiveness, and .61 for BAS Fun Seeking at Time 1 and from .76 for BIS, .76 for BAS Drive, .53 for BAS Reward Responsiveness, and .61 for BAS Fun Seeking at Time 2. Overall these patterns indicate that the BIS/BAS scales are performing as well as in other reported studies in terms of internal consistency and observed patterns of intercorrelation among subscales.
Table 1.
Means and Standard Deviations for BIS/BAS Scales at Time 1 and Time 2 Assessments
| Time 1 | |||||
|---|---|---|---|---|---|
|
| |||||
| Drive | Fun Seeking | Reward Responsiveness | BAS Total | BIS Scale | |
| Early Adolescents (n = 45) | 9.27 (2.61) | 11.44 (1.71) | 16.53 (2.38) | 37.24 (4.95) | 18.61 (3.49) |
| Late Adolescents (n = 72) | 10.47 (2.39) | 11.99 (2.02) | 17.50 (1.69) | 39.96 (5.07) | 19.21 (3.00) |
| Young Adults (n = 67) | 11.21 (1.58) | 12.51 (2.00) | 17.64 (1.61) | 41.36 (3.25) | 20.15 (3.42) |
| Total sample (N=184) | 10.45 (2.30) | 12.04 (1.98) | 17.32 (1.90) | 39.80 (4.71) | 19.41 (3.32) |
|
| |||||
| Time 2 | |||||
|
| |||||
| Early Adolescents (n = 43) | 9.40 (2.46) | 11.59 (1.94) | 17.21 (2.02) | 38.19 (5.11) | 18.58 (3.76) |
| Late Adolescents (n = 61) | 10.56 (2.20) | 11.98 (2.07) | 17.69 (1.89) | 40.23 (4.72) | 19.82 (3.81) |
| Young Adults (n = 53) | 11.02 (2.21) | 11.85 (2.04) | 17.11 (1.53) | 39.98 (3.90) | 20.64 (3.76) |
| Total sample (N=157) | 10.39 (2.36) | 11.83 (2.02) | 17.36 (1.82) | 39.59 (4.63) | 19.76 (3.84) |
Longitudinal Changes in BAS sensitivity and Age and Sex Effects
The BAS scales
In a series of repeated measures ANCOVAs with sex as a between-subject factor, age as a continuous covariate, and the relevant BAS scale as a repeated measures variable with two time points, main effects of time would reflect change, regardless of age, from Time 1 to Time 2. Main effects of age would suggest age effects on the BAS scales irrespective of time. Age by time interactions would indicate differential patterns of change across time for different ages.
As summarized in Table 2, there was a significant increase in BAS Reward Responsiveness scores from Time 1 to Time 2 for the whole sample and a main effect of sex on BAS Reward Responsiveness, with females exhibiting higher scores than males. There were also significant main effects of age on BAS Total and BAS Drive, with follow-up partial correlations (i.e., controlling for gender) revealing positive relationship between age and BAS Total, r = .27, p = .001, and BAS Drive, r = .36, p <.001, scores averaged across two time points. Moreover, there was a significant interaction of time by age for BAS Reward Responsiveness. There were no other significant main effects or interaction effects on the BAS scales. Please see Supplementary Materials for analogous analyses with the BIS scale.
Table 2.
Longitudinal Changes and Effects of Age on the BIS/BAS Scales
| F | df | p | ηp2 | |
|---|---|---|---|---|
| Age Main Effects | ||||
|
| ||||
| BAS Total | 11.94 | (1, 154) | .001 | .072 |
| BAS Drive | 23.95 | (1, 154) | <.001 | .135 |
| BIS | 8.88 | (1, 154) | .003 | .055 |
|
| ||||
| Time Main Effects | ||||
|
| ||||
| BAS Reward Responsiveness | 5.82 | (1, 154) | .017 | .036 |
|
| ||||
| Sex Main Effects | ||||
|
| ||||
| BAS Reward Responsiveness | 12.06 | (1, 154) | .001 | .073 |
| BIS | 17.72 | (1, 154) | <.001 | .103 |
|
| ||||
| Age × Time Interaction Effects | ||||
|
| ||||
| BAS Reward Responsiveness | 5.88 | (1, 154) | .016 | .037 |
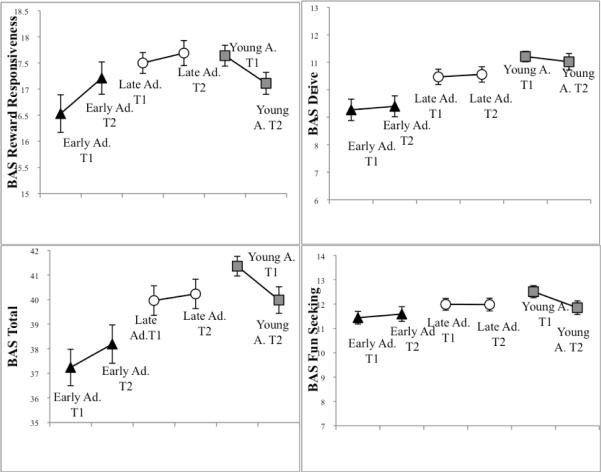
In order to further illuminate age effects and increase their interpretability, ANOVAs with between-subject factors of sex and age groups, i.e., early adolescents, late adolescents, and young adults, were conducted. In follow up for the main effect of age on BAS Total, the ANOVA with Bonferroni-corrected post-hoc analyses yielded lower BAS Total scores for early adolescents as compared to both late adolescents (p = .008) and young adults (p = .003) across the Time 1 and Time 2 assessments; the latter two groups did not differ from each other (see Figure 1). In analogous follow-up for significant age main effect on BAS Drive, Bonferroni-corrected post-hoc analyses yielded lower BAS Drive scores for early adolescents as compared to both late adolescents (p = .005) and young adults (p < .001), who did not differ from each other, as illustrated in Figure 1. Early adolescents are 9–12 years of age at Time 1 and 11 to 14 years of age at Time 2. This patterning suggests that BAS-Drive scores, like the BAS Total, either increase markedly from early adolescence to late adolescence and then persist into young adulthood, or that there are significant age-cohort effects.
Figure 1. Longitudinal changes in BAS scales from Time 1 to Time 2.
Early adolescents are ages 9 to 12 at Time 1 and ages 11–14 at Time 2. Late adolescents are ages 13–17 at Time 1 and ages 15 to 19 at Time 2. Young adults are ages 18 to 23 at Time 1 and ages 20 to 25 at Time 2. This patterning suggests an inverted-U-shaped trajectory for BAS Reward Responsiveness through adolescence.
To follow up on the time by age interaction for BAS Reward Responsiveness, time effects were examined for each age group in separate repeated measures ANOVAs. BAS Reward Responsiveness significantly changed over the two-year follow-up interval only for the young adults, F (1, 52) = 6.35, p = .015, ηp2 = .11. Scores were lower at Time 2 (ages 20–25) versus Time 1 (ages 18–23), indicating that BAS Reward Responsiveness decreases from the late teens to the mid-twenties. To follow up on this interaction further, the age groups' scores were compared at the Time 1 and at the Time 2 assessments separately in univariate ANOVAs with post-hoc tests. At Time 1, early adolescents exhibited lower scores than both late adolescents and young adults (ps ≤ .02). At Time 2, the groups' scores did not differ. Figure 1 illustrates these longitudinal changes in the three age groups for all 3 BAS scales and the BAS Total, highlighting the non-significant trend for increases in BAS Reward Responsiveness for early adolescents (p = .166), relatively stable scores for late adolescents, and a significant decline for young adults, from the Time 1 to the Time 2 assessment.
MRI brain volumes
Next, relevant brain volumes were similarly analyzed, using the same repeated measures approach of a series of ANCOVAs with repeated measures (for the same two time points), in order to assess effects of age and sex on each hemisphere's relevant brain volumes. Scanner-upgrade status was entered as a covariate, age as continuous covariate, and sex as between-subjects factor. In order to follow-up on significant age effects, analogous comparisons of age groups were conducted as with the BAS scales.
As summarized in Table 3, there was a significant main effect of time on the left Nacc with volumes decreasing for the whole sample from Time 1 to Time 2. There were also significant effects of sex on the right Nacc and the right amygdala, with females exhibiting greater volumes than males in both brain regions, and a significant interaction of time by sex on the left amygdala, with females exhibiting greater left amygdala volume than males at Time 2 (p = .017) and no difference at Time 1. In addition, there was a significant main effect of age on the left lateral OFC and the right Nacc, with significant inverse partial correlation (i.e., controlling for sex and scanner upgrade status) between age and left lateral OFC, r = −.17, p = .042, and right Nacc, r = −.18, p = .03, volumes averaged across two time points. Moreover, there was a significant interaction of age by time for the left Nacc. There were no other main effects or interactions on the ROI's volumes.
Table 3.
Longitudinal Changes and Effects of Age on the OFC Nacc, and Amygdala Volumes
| F | df | p | ηp2 | |
|---|---|---|---|---|
| Age Main Effects | ||||
|
| ||||
| Left lateral OFC | 4.21 | (1, 145) | .042 | .028 |
| Right Nacc | 4.83 | (1, 145) | .030 | .032 |
|
| ||||
| Time Main Effects | ||||
|
| ||||
| Left Nacc | 4.33 | (1, 145) | .039 | .029 |
|
| ||||
| Sex Main Effects | ||||
|
| ||||
| Right Nacc | 19.21 | (1, 145) | <.001 | .117 |
| Right Amygdala | 9.61 | (1, 145) | .002 | .062 |
|
| ||||
| Age × Time Interaction Effects | ||||
|
| ||||
| Left Nacc | 5.59 | (1, 145) | .019 | .037 |
|
| ||||
| Sex × Time Interaction Effects | ||||
|
| ||||
| Left Amygdala | 8.57 | (1, 145) | .004 | .056 |
Note: All volumes were corrected for total brain volume.
In order to further illuminate age effects, repeated measures ANCOVAs with age group and sex as between-subjects factors and scanner upgrade status as a covariate were conducted for the right Nacc and left lateral OFC. There were no significant differences between age groups for the left lateral OFC, but repeated measures ANOVAs with Bonferoni post-hoc comparisons for the right Nacc yielded greater volumes for early adolescents compared to young adults (p = .023).
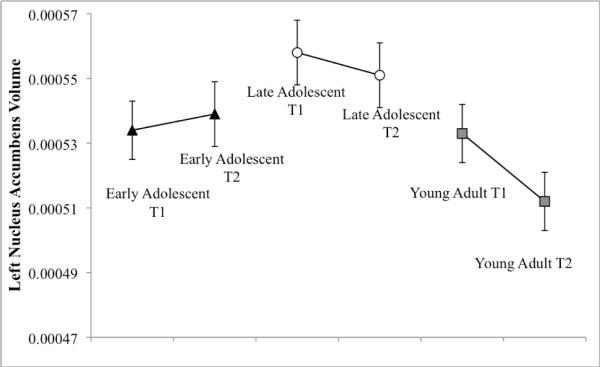
To follow up on the significant time by age interaction on the left Nacc, repeated measures ANCOVAs were run separately for each age group, which detected no significant longitudinal changes during the follow-up within any age group. We further explored this interaction by examining age group differences separately for Time 1 and Time 2. For the Time 2 analysis, scanner-upgrade status was added as a covariate. There was a significant effect of age group on left Nacc volumes at Time 2, F (2, 143) = 4.37, p = .014, ηp2 = .06, but not at Time 1. Bonferroni-corrected post-hoc analyses yielded greater left Nacc volumes for late adolescents compared to young adults (p = .008) at Time 2. Late adolescents are ages 13–17 at Time 1 and ages 15–19 at Time 2, while young adults are ages 18–23 at Time 1 and 20–25 at Time 2. This pattern of results suggests that there is a decrease in the left Nacc volume that occurs in the transition from the late teens to early twenties, but likely over a more protracted time period than the two-year follow-up captured by this study (see Figure 2).
Figure 2. Interaction effect of Age Group X Time on the left Nacc volume corrected for total brain volume.
The pattern of results suggests a decrease in the left Nacc volumes (corrected for total brain volume) from the late teens into the early twenties.
Finally, only for the left Nacc, there was a significant main effect of scanner upgrade status, F (1, 145) = 9.26, p = .003, ηp2 = .06, but no significant interaction effects of scanner upgrade status by age and/or time. Thus, this scanner upgrade effect does not account for the observed age by time interaction.
Associations between Longitudinal Changes in Regional Brain Volumes and Longitudinal Changes in BAS Sensitivity
In order to assess whether individual differences in the ROI's volumes predict individual differences in longitudinal changes in BAS sensitivity, a series of hierarchical regression analyses was conducted. For each BAS scale from Time 2 as the outcome variable, four regressions were ran to predict longitudinal change in the relevant BAS scale as a function of changes in regional brain volumes of the nucleus accumbens, medial OFC, lateral OFC, and amygdala. In Step 1, age, sex, scanner upgrade status, and the relevant BAS scale from Time 1 were entered as predictors. In Step 2, relevant right and left hemisphere volumes at Time 1 were entered as predictors. In Step 3, relevant right and left hemisphere volumes at Time 2 were entered as predictors, to examine the effect of developmental change in each brain region's volumes and the unique contribution of longitudinal change in each hemisphere. The findings from Step 2 were of primary interest given the study's hypotheses.
Change in BAS Total
In regression analyses predicting changes in BAS Total, medial OFC volumes at baseline (i.e., left and right hemispheres at Time 1 combined) significantly predicted the longitudinal increases in BAS Total, change R2 = .03, p = .041, controlling for all Step 1 predictors, overall R2 = .30, p < .001. The left medial OFC volume at Time 1 had a significant unique effect controlling for all Step 1 predictors including right medial OFC volume, partial r = .21, p = .012. There were no significant effects of amygdala, lateral OFC, or Nacc volumes on the longitudinal change in BAS Total.
Change in BAS Drive
In regression analyses predicting changes in BAS Drive, longitudinal increases in Nacc volumes from Time 1 to Time 2 significantly predicted longitudinal increases in BAS Drive scores from Time 1 to Time 2, change R2 = .03, p = .039 (see Table 2 for additional results). There were no significant effects of lateral OFC, medial OFC and amygdala volumes on BAS Drive score changes from Time 1 to Time 2.
Change in BAS Reward Responsiveness
In regression analyses predicting changes in BAS Reward Responsiveness, Nacc volumes at baseline significantly predicted the longitudinal increase in BAS Reward Responsiveness, change R2 = .04, p = .032 (see Table 2 for additional results). There was no significant relationship between longitudinal change in Nacc volumes and longitudinal change in BAS Reward Responsiveness from Time 1 to Time 2. Furthermore, there were no significant effects of amygdala, lateral OFC, or medial OFC volumes on the longitudinal change in BAS Reward Responsiveness.
Change in BAS Fun Seeking
There were no significant effects of Nacc, lateral OFC, medial OFC, and amygdala volumes on the BAS Fun Seeking score changes from Time 1 to Time 2.
Please see Supplementary Material for description of analogous analyses involving the BIS scale.
Discussion
Overall, the present findings support developmental peak in reward sensitivity during adolescence. However, there are important individual differences, such as differences in baseline volumes of relevant brain structures, qualifying these changes in reward sensitivity, as well as changes in threat sensitivity, during this developmental period.
Developmental Changes in Reward Sensitivity during Adolescence
Based on the present longitudinal findings, specific aspects of reward sensitivity, i.e., positive affective responses to rewards as assessed by BAS Reward Responsiveness (Carver & White, 1994), appear to peak in mid- to late adolescence and decline into young adulthood (i.e., into the mid-twenties). These developmental changes in affective responses to rewards appear to be coupled with structural changes in a brain region that is important for the evaluation of salient incentive stimuli, i.e., Nacc. MRI analyses suggest a decrease in left Nacc volumes, which occurs from late adolescence into young adulthood (i.e., between the late teens and the early twenties). This decrease is approximately 8% of the left Nacc volume when calculated from the late adolescents' Time 1 mean to the young adults' Time 2 mean and potentially may be due to the ongoing pruning of synapses in this brain region. This coupling of behavioral and brain structural changes can be visualized by comparing data in Figures 1 and 2. Furthermore, these findings are consistent with functional imaging studies, in which adolescents exhibit greater Nacc activation in response to rewards compared to adults (e.g., Ernst et al., 2005; Galvan et al., 2006; Van Leijenhorst et al., 2010).
Additionally, in cross-sectional analyses, we found early adolescents (i.e., ages 9 to 12) to exhibit lower levels of BAS Drive and the BAS Total as compared to late adolescents (i.e., ages 13 to 17) and young adults (i.e., ages 18 to 23), with no differences between the two latter age groups. This pattern of results suggests a fine-tuned point in time for developmental increases in reward sensitivity, i.e., during the transition from early adolescence (ages 9–12) into late adolescence (ages 13–17). Alternatively, the pattern suggests age-cohort effects.
One interpretation of the present longitudinal and cross-sectional age effects is that different aspects of reward sensitivity show different developmental trajectories during adolescence. The BAS Drive is proposed to measure persistence in pursuit of rewards, the BAS Fun Seeking a mix of desire for novel rewards and impulsive approach to rewards, and the BAS Total, as a composite measure of the three scales, an index of overall reward sensitivity (Carver & White, 1994). Thus, the present study suggests that persistence in pursuit of rewards and overall reward sensitivity remain elevated from the late teens into young adulthood, even while positive affective responses to rewards (i.e., BAS Reward Responsiveness) start to decrease in the early twenties. The multifaceted nature of reward sensitivity is supported by multi-factor solutions in factor analyses of the BAS scales' items (Cooper et al., 2007) and by Carver and White's original formulation of the scales (1994).
The present cross-sectional findings of stability of certain aspects of reward sensitivity (e.g., persistence in reward pursuit) from late adolescence into young adulthood are consistent with prior longitudinal findings of two-year stability in BAS sensitivity during mid-twenties (Takahashi et al., 2007). Interestingly, there is also preliminary cross-sectional evidence for decreases in BAS sensitivity in elderly populations (Jorm et al., 1999). Thus, like positive affective responses to rewards, the overall reward sensitivity and persistence in pursuit of rewards (i.e., BAS Total and BAS Drive) may also show an inverted U-shape in the normative population, but a more protracted one across the lifetime. Future research is needed to assess this hypothesis of a protracted decline in certain aspects of BAS sensitivity in older adulthood, a patterning that would map coherently onto late life changes in dopamine reward-relevant activity (Li & Backman, 2010).
Overall, the present study supports and bolsters prior studies of reward sensitivity during adolescence (e.g., Ernst et al., 2006). Both the present longitudinal and cross-sectional analyses support that reward sensitivity is elevated during adolescence, but with potential different trajectories for different aspects of reward sensitivity during early 20's. The present study is also consistent with the prior cross-sectional findings of a curvilinear pattern for increases in sensation seeking, an overlapping but distinct construct from reward sensitivity, during adolescence (Harden & Tucker-Drob, 2010; Steinberg et al. 2008). Importantly, the present study extends this prior literature by providing longitudinal data on self-reported reward and threat sensitivities' changes and changes in volumes of relevant brain regions.
Individual Differences in Adolescent Changes in Reward and Threat Sensitivities
In the present longitudinal analyses, there are important individual differences qualifying developmental changes in reward sensitivity during adolescence. Individuals with greater Nacc volumes at baseline experienced more drastic increases in BAS Reward Responsiveness, whereas individuals with greater medial OFC volumes at baseline experienced more drastic increases in the BAS Total, over the two-year follow-up. Similarly, individuals with greater longitudinal increases in Nacc volumes had greater concurrent increases in BAS Drive, i.e., in persistence of reward pursuit. Importantly, in additional analyses not reported here, there were no significant interaction effects of age and baseline Nacc and medial OFC volumes on reward sensitivity, suggesting that these effects of individual differences in baseline reward-relevant brain volumes hold across the full age range of the sample. There were also no significant effects of the individual differences in non-reward specific volumes of the lateral OFC and amygdala on reward sensitivity changes.
This is an important and novel identification of individual differences in brain structures involved in reward processing that predict different rates of reward sensitivity increases during adolescence. Greater Nacc and medial OFC volumes at baseline potentially may reflect greater density of synapses in these brain regions during a sensitive developmental period, a period with increasing exposure to environmental rewards (e.g., entering high school, starting first dating relationships). Alternatively, individual differences in the Nacc and medial OFC volumes may reflect different patterns of pruning, or a combination of pruning and increased exposure to environmental rewards. In either case, increased Nacc and medial OFC volumes seem to be a biomarker for individuals especially sensitive to developmental or environmental perturbations to the reward system (i.e., BAS). Future studies need to investigate the exact mechanism mediating this relationship between greater baseline Nacc and medial OFC volumes and more marked increases in reward sensitivity during adolescence. Additional research is also needed to further characterize the subset of adolescents who may exhibit especially heightened risk for increases in reward reactivity and consequent reckless pursuit of rewards, i.e., the subgroup at the greatest need for early interventions.
Sex Differences in Adolescent Reward and Threat Sensitivities
Another important set of findings is related to sex differences in reward and threat sensitivities during adolescence, i.e., identifying sex as another qualifier of adolescent changes in emotional reactivity. Specifically, we found females (ages 9 to 23) to exhibit higher reactivity to threat and reward (i.e., BIS and BAS Reward Responsiveness scales) compared to their male peers. Females also exhibited greater volumes of the right Nacc and amygdala (corrected for the total brain volume), key structures implicated in reward and threat information processing, compared to males. Furthermore, early adolescent females exhibited lower reactivity to threat compared to late adolescent and young adult females, with no difference between the two latter groups. In contrast, early adolescent, late adolescent, and young adult males did not differ in their sensitivities to threat. In other words, whereas sensitivity to reward peaks in adolescence for males and females, it appears that only females experience similar increases in sensitivity to threat. Again, we cannot rule out potential age-cohort effects given that these were cross-sectional findings.
This pattern of sex differences partially replicates prior findings of greater threat sensitivity in females (e.g., Knyazev, 2004; Vermeersch et al., 2009) and extends prior studies by suggesting a more specific time interval for the emergence of sex differences in threat sensitivity, i.e., the transition from early adolescence to late adolescence. However, unlike some prior studies, we largely did not find greater BAS sensitivity in males compared to females. It should be noted that one other study has also failed to find this sex difference in reward sensitivity during adolescence (Giles & Price, 2008). Similar to the present findings, adult women have been found to have greater BAS Reward Responsiveness compared to adult men (Jorm et al., 1999). This suggests that sex differences in sensitivity to reward may differ at different developmental stages, but additional research is necessary to fully address these inconsistencies in findings.
In addition, our findings of increased amygdala volume in adolescent females versus males contradict some prior studies of sex dimorphic longitudinal changes in amygdala volume with an increase for males and decrease for females (e.g., Giedd et al., 2006). However, when controlling for total brain volume, cross-sectional analyses have only revealed sex differences in striatum volumes during adolescence and not in amygdala volumes (e.g., Giedd et al., 1997). Clearly, additional studies in sex differences in brain regional volumes during adolescence, controlling for the total brain volume, are needed to address these discrepancies.
The overall pattern of the present sex difference findings implies greater emotional reactivity for females compared to males, and increasing sensitivity to threat for females and not males, during transition from early adolescents to young adulthood. This is especially important in light of evidence that sex differences (i.e., greater risk for women vs. men) in rates of mood disorders emerge around age 13 (e.g., Hankin & Abramson, 2001). Both BAS and BIS hypersensitivities have been linked to greater risks for mood and anxiety disorders, respectively (e.g., Depue & Iacono, 1989; Fowles, 1987; Gray, 1991, 1994; Pinto-Meza et al., 2006; Urošević, Abramson, Harmon-Jones, & Alloy, 2008). Thus, increases in BIS and BAS sensitivities may be one of the mechanisms leading to increased risk for mood disorders in adolescent females compared to adolescent males.
Limitations
The present study is not without limitations. The study enrolled only healthy individuals who were without psychopathology at baseline. This has allowed us to draw conclusions about normative development and normative risk for psychopathology during adolescence. However, replications in clinical populations are needed before generalizations of the present findings to patient populations can be made. Given the present sample's predominantly Caucasian and middle to upper-middle socio-economic background, replications in samples of different ethnic and socio-economic background are also needed to establish greater generalizability of the present findings.
The longitudinal aspect of the study relied on only two time points over a two-year interval. There was also an MR scanner upgrade that occurred in the midst of the second assessment. However, use of specialized preprocessing to account for differences in image distortion before and after the upgrade, use of a unique longitudinal processing procedure to minimize methodological variance, and the use of scanner upgrade as a covariate in all relevant analyses, ensure that the observed brain volume effects are above and beyond effects explained by the scanner upgrade. Still, additional waves of assessment would allow change over time to be more strongly addressed. Another potential limitation is the use of automatized process for subcortical segmentation and cortical parcellation; however, FreeSurfer's outputs were visually inspected and manually edited when necessary and these automatized procedures have been found to be just as reliable as manual procedures in prior studies (e.g., Fischl et al., 2002).
Summary and Future Directions
The present study provides the first longitudinal study's support for developmental increases in reward sensitivity from early adolescence into late adolescence. It also suggests that reward sensitivity is not a uniform construct with a single developmental trajectory during this critical period. Thus, aspects of reward sensitivity, such as persistence in pursuit of rewards, remain stable and elevated from late adolescence into young adulthood (in comparison to early adolescence). However, based on longitudinal analyses, positive affective responses to rewards seem to peak in late adolescence with a subsequent decline in early adulthood. This latter pattern of results was further bolstered by developmental findings of brain volume decrease from late adolescence into young adulthood in the structures relevant for reward, i.e., Nacc. Moreover, in the present study, individuals with greater baseline Nacc and medial OFC volumes exhibited more marked longitudinal increases in reward sensitivity, whereas females and individuals with greater baseline lateral OFC volumes exhibited longitudinal increases in sensitivity to threat. Overall, the present study supports the theory that adolescence is a unique time period in the human lifespan for reward-seeking behavior (Wahlstrom, Collins, White & Luciana, 2010).
Our data also suggest that some of these developmental BAS-relevant processes (e.g., as assessed by BAS Drive) may be protracted over longer intervals than two years. Additional longitudinal research with longer follow-up periods and with samples spanning wider age ranges is necessary in order to examine the full range of changes in reward sensitivity during transition from adolescence into adulthood. Finally, longitudinal studies also need to further explore individual and sex differences in developmental increases in reward (i.e., BAS) sensitivity and threat (i.e., BIS) sensitivity, in order to characterize adolescents at the highest risk for psychopathology.
Supplementary Material
Table 4.
Hierarchical Regressions for Nucleus Accumbens Volumes Predicting Longitudinal Increases in BAS Drive and BAS Reward Responsiveness Scales
| BAS Drive | ||||||
|---|---|---|---|---|---|---|
|
| ||||||
| Model | R2 | F | P | Partial r | t | p |
| Step 1 | 0.26 | 12.36 | <.001 | |||
| Age | .16 | 1.92 | ns | |||
| Sex | .03 | .37 | ns | |||
| Scanner upgrade | −.01 | −.16 | ns | |||
| BAS Drive T1 | .44 | 5.74 | <.001 | |||
|
| ||||||
| Step 2 | 0.26 | 8.26 | <.001 | |||
| Left Nacc T1 | −.04 | −.41 | ns | |||
| Right Nacc T1 | −.04 | −.50 | ns | |||
|
| ||||||
| Step 3 | 0.30 | 7.23 | <.001 | |||
| Left Nacc T2 | .13 | 1.53 | ns | |||
| Right Nacc T2 | .16 | 1.85 | ns | |||
| BAS Reward Responsiveness | ||||||
|---|---|---|---|---|---|---|
| Step 1 | 0.14 | 5.68 | <.001 | |||
| Age | −.10 | −.1.14 | ns | |||
| Sex | .22 | 2.63 | .009 | |||
| Scanner upgrade | .10 | 1.09 | ns | |||
| BAS RR T1 | .27 | 3.26 | .001 | |||
|
| ||||||
| Step 2 | 0.18 | 5.11 | <.001 | |||
| Left Nacc T1 | .06 | .71 | ns | |||
| Right Nacc T1 | .19 | 2.24 | .026 | |||
|
| ||||||
| Step 3 | 0.18 | 3.80 | <.001 | |||
| Left Nacc T2 | −.01 | −.16 | ns | |||
| Right Nacc T2 | −.04 | −.42 | ns | |||
Note: All Nacc volumes were corrected for total brain volume. Outcome variables are indices for BAS Drive and BAS Reward Responsiveness scales at Time 2. BAS RR = BAS Reward Responsiveness scale; T1 = Time 1; T2 = Time 2.
Acknowledgments
This manuscript was supported by National Institute of Mental Health Grant T32 MH 017069 to Snežana Urošević and National Institute on Drug Abuse Grant R01 DA 017843 to Monica Luciana. Thanks to the Center for Neurobehavioral Development and to the Center for Magnetic Resonance Research for resources and support of the presented research.
Footnotes
Publisher's Disclaimer: The following manuscript is the final accepted manuscript. It has not been subjected to the final copyediting, fact-checking, and proofreading required for formal publication. It is not the definitive, publisher-authenticated version. The American Psychological Association and its Council of Editors disclaim any responsibility or liabilities for errors or omissions of this manuscript version, any version derived from this manuscript by NIH, or other third parties. The published version is available at www.apa.org/pubs/journals/dev
There were two additional statistical approaches to examining a change in predictor variable predicting a change in outcome variable that we considered: correlation of difference scores and correlation of unstandardized residuals as change scores (i.e., regressing Time 2 on Time 1 and saving unstandardized residuals). Both of these approaches yielded similar patterns of results as reported with Analyses of Partial Variance. However, reliabilities for difference scores were unacceptably low (e.g., .35 for difference in BIS scores) leading us to reject this approach. The correlation of change residuals was acceptable, but less stringent and sophisticated given that Analyses of Partial Variance provided the correlation of change residuals with additional predictors controlled for, such as sex and age.
References
- Alloy LB, Abramson LY. The role of the behavioral approach system (BAS) in bipolar spectrum disorders. Current Directions in Psychological Science. 2010;19:189–194. doi: 10.1177/0963721410370292. doi:10.1177/0963721410370292. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carver CS, White TL. Behavioral inhibition, behavioral activation, and affective responses to impending reward and punishment: The BIS/BAS scales. Journal of Personality and Social Psychology. 1994;67:319–333. doi:10.1037/00223514.67.2.319. [Google Scholar]
- Casey BJ, Jones RM, Hare TA. The adolescent brain. Annals of New York Academy of Sciences. 2008;1124:111–126. doi: 10.1196/annals.1440.010. doi:10.1196/annals.1440.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cauffman E, Shulman EP, Steinberg L, Claus E, Banich MT, Graham SJ, Woolard J. Age differences in affective decision making as indexed by performance on the Iowa Gambling Task. Developmental Psychology. 2010;46:193–207. doi: 10.1037/a0016128. doi:10.1037/a0016128. [DOI] [PubMed] [Google Scholar]
- Cohen J, Cohen P. Applied multiple regression/correlation analysis for the behavioral sciences. 2nd ed. Erlbaum; Hillsdale, NJ: 1983. [Google Scholar]
- Cohen JR, Asarnow RF, Sabb FW, Bilder RM, Bookheimer SY, Knowlton BJ, Poldrack RA. A unique adolescent response to reward prediction errors. Nature Neuroscience. 2010;13:669–671. doi: 10.1038/nn.2558. doi:10.1038/nn.2558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Colder CR, O'Connor RM. Gray's reinforcement sensitivity model and child psychopathology: Laboratory and questionnaire assessment of the BAS and BIS. Journal of Abnormal Child Psychology. 2004;32:435–451. doi: 10.1023/b:jacp.0000030296.54122.b6. doi:10.1023/B:JACP.0000030296.54122.b6. [DOI] [PubMed] [Google Scholar]
- Cools R, Calder AJ, Lawrence AD, Clark L, Bullmore E, Robbins TW. Individual differences in threat sensitivity predict serotonergic modulation of amygdala response to fearful faces. Psychopharmacology. 2004;180:670–679. doi: 10.1007/s00213-005-2215-5. doi:10.1007/s00213-005-2215-5. [DOI] [PubMed] [Google Scholar]
- Cooper A, Gomez R, Aucote H. The Behavioral Inhibition System and Behavioural Approach System (BIS/BAS) Scales: Measurement and structural invariance across adults and adolescents. Personality and Individual Differences. 2007;43:295–305. doi:10.1016/j.paid.2006.11.023. [Google Scholar]
- Dale AM, Sereno MI. Improved localization of cortical activity by combining EEG and MEG with MRI cortical surface reconstruction: A linear approach. Journal of Cognitive Neuroscience. 1993;5:162–176. doi: 10.1162/jocn.1993.5.2.162. doi:10.1162/jocn.1993.5.2.162. [DOI] [PubMed] [Google Scholar]
- Dale AM, Fischl B, Sereno MI. Cortical surface-based analysis. I.segmentation and surface reconstruction. NeuroImage. 1999;9:179–194. doi: 10.1006/nimg.1998.0395. doi:10.1006/nimg.1998.0395. [DOI] [PubMed] [Google Scholar]
- Davis M, Whalen PJ. The amygdala: Vigilance and emotion. Molecular Psychiatry. 2001;6:13–34. doi: 10.1038/sj.mp.4000812. doi:10.1038/sj.mp.4000812. [DOI] [PubMed] [Google Scholar]
- Depue RA, Collins PF. Neurobiology of the structure of personality: Dopamine, facilitation of incentive motivation, and extraversion. Behavioral and Brain Sciences. 1999;22:491–569. doi: 10.1017/s0140525x99002046. doi:10.1017/S0140525X99002046. [DOI] [PubMed] [Google Scholar]
- Depue RA, Iacono WG. Neurobehavioral aspects of affective disorders. Annual Review of Psychology. 1989;40:457–492. doi: 10.1146/annurev.ps.40.020189.002325. doi:10.1146/annurev.ps.40.020189.002325. [DOI] [PubMed] [Google Scholar]
- Desikan RS, Segonne F, Fischl B, Quinn BT, Dickerson BC, Blacker D, Buckner RL, Dale AM, Maguire RP, Hyman BT. An automated labeling system for subdividing the human cerebral cortex on MRI scans into gyral based regions of interest. NeuroImage. 2006;31:968–980. doi: 10.1016/j.neuroimage.2006.01.021. doi:10.1016/j.neuroimage.2006.01.021. [DOI] [PubMed] [Google Scholar]
- Doremus-Fitzwater TL, Varlinskaya EI, Spear LP. Motivational systems in adolescence: Possible implications for age differences in substance abuse and other risk-taking behaviors. Brain and Cognition. 2010;72:114–123. doi: 10.1016/j.bandc.2009.08.008. doi:10.1016/j.bandc.2009.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eaton DK, Kann L, Kinchen S, Ross J, Hawkins J, Harris WA, Lowry R, McManus T, Chyen D, Shanklin S, Lim C, Grunbaum JA, Wechsler H. Youth Risk Behavioral Surveillance—United States, 2005. Surveillance Summaries. 2006;55/SS-5:1–108. Retrieved from http://www.cdc.gov/mmwr/pdf/ss/ss5505.pdf. [PubMed] [Google Scholar]
- Ernst M, Nelson EE, Jazbec S, McClure EB, Monk CS, Leibenluft E, Blair J, Pine DS. Amygdala and nucleus accumbens in responses to receipt and omission of gains in adults and adolescents. NeuroImage. 2005;25:1279–1291. doi: 10.1016/j.neuroimage.2004.12.038. doi:10.1016/j.neuroimage.2004.12.038. [DOI] [PubMed] [Google Scholar]
- Ernst M, Pine DS, Hardin M. Triadic model of the neurobiology of motivated behavior in adolescence. Psychological Medicine. 2006;36:299–312. doi: 10.1017/S0033291705005891. doi:10.1017/S0033291705005891. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Faure A, Reynolds SM, Richard JM, Berridge KC. Mesolimbic dopamine in desire and dread: Enabling motivation to be generated by localized glutamate disruptions in nucleus accumbens. The Journal of Neuroscience. 2008;28:7184–7192. doi: 10.1523/JNEUROSCI.4961-07.2008. doi:10.1523/JNEUROSCI.4961-07.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Dale AM. Measuring the thickness of the human cerebral cortex from magnetic resonance images. Proceedings of the National Academy of Sciences of the United States of America. 2000;97:11050–11055. doi: 10.1073/pnas.200033797. doi:10.1073/pnas.200033797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Liu A, Dale AM. Automated manifold surgery: Constructing geometrically accurate and topologically correct models of the human cerebral cortex. IEEE Transactions on Medical Imaging. 2001;20:70–80. doi: 10.1109/42.906426. doi:10.1109/42.906426. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Dale AM. Cortical surface-based analysis. II: Inflation, flattening, and a surface-based coordinate system. NeuroImage. 1999;9:195–207. doi: 10.1006/nimg.1998.0396. doi:10.1006/nimg.1998.0396. [DOI] [PubMed] [Google Scholar]
- Fischl B, Sereno MI, Tootell RB, Dale AM. High-resolution intersubject averaging and a coordinate system for the cortical surface. Human Brain Mapping. 1999;8:272–284. doi: 10.1002/(SICI)1097-0193(1999)8:4<272::AID-HBM10>3.0.CO;2-4. doi:10.1002/(SICI)1097-0193(1999)8:4<272∷AIDHBM10>3.0.CO;2–4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fischl B, Salat DH, van der Kouwe AJ, Makris N, Segonne F, Quinn BT, Dale AM. Sequence-independent segmentation of magnetic resonance images. NeuroImage. 2004a;23(Suppl 1):S69–84. doi: 10.1016/j.neuroimage.2004.07.016. doi:10.1016/j.neuroimage.2004.07.016. [DOI] [PubMed] [Google Scholar]
- Fischl B, van der Kouwe A, Destrieux C, Halgren E, Segonne F, Salat DH, Busa E, Seidman LJ, Goldstein J, Kennedy D, et al. Automatically parcellating the human cerebral cortex. Cerebral Cortex. 2004b;14:11–22. doi: 10.1093/cercor/bhg087. doi:10.1093/cercor/bhg087. [DOI] [PubMed] [Google Scholar]
- Fischl B, Salat DH, Busa E, Albert M, Dieterich M, Haselgrove C, van der Kouwe A, Killiany R, Kennedy D, Klaveness S. Whole brain segmentation: Automated labeling of neuroanatomical structures in the human brain. Neuron. 2002;33:341–355. doi: 10.1016/s0896-6273(02)00569-x. doi:10.1016/S0896-6273(02)00569-X. [DOI] [PubMed] [Google Scholar]
- Forbes EE, Dahl RE. Pubertal development and behavior: Hormonal activation of social and motivational tendencies. Brain and Cognition. 2010;72:66–72. doi: 10.1016/j.bandc.2009.10.007. doi:10.1016/j.bandc.2009.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fowles DC. Application of a behavioral theory of motivation to the concepts of anxiety and impulsivity. Journal of Research in Personality. 1987;21:417–435. doi:10.1016/0092-6566(87)90030-4. [Google Scholar]
- Galvan A, Hare TA, Parra CE, Penn J, Voss H, Glover G, Casey BJ. Earlier development of the accumbens relative to orbitofrontal cortex might underlie risk-taking behaviors in adolescents. The Journal of Neuroscience. 2006;26:6885–6892. doi: 10.1523/JNEUROSCI.1062-06.2006. doi:10.1523/JNEUROSCI.1062-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Geier CF, Terwilliger R, Teslovich T, Velanova K, Luna B. Immaturities in reward processing and its influence on inhibitory control in adolescence. Cerebral Cortex. 2010;20:1613–1629. doi: 10.1093/cercor/bhp225. doi:10.1093/cercor/bhp225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giedd JN, Blumenthal J, Jeffries NO, Castellanos FX, Liu H, Zijdenbos A, Paus T, Evans AC, Rapoport JL. Brain development during childhood and adolescence: A longitudinal MRI study. Nature Neuroscience. 1999;2:861–863. doi: 10.1038/13158. doi:10.1038/13158. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Castellanos FX, Rajapakse JC, Vaituzis AC, Rapoport JL. Sexual dimorphism of the developing human brain. Progress in Neuro-Psychopharmacology & Biological Psychiatry. 1997;21:1185–1201. doi: 10.1016/s0278-5846(97)00158-9. doi:10.1016/S0278-5846(97)00158-9. [DOI] [PubMed] [Google Scholar]
- Giedd JN, Clasen LS, Lenroot R, Greenstein D, Wallace GL, Ordaz S, Molloy EA, Blumenthal JD, Tossell JW, Stayer C, Samango-Sprouse CA, Shen D, Davatzikos C, Merke D, Chrousos GP. Puberty-related influences on brain development. Mollecular and Cellular Endocrinology. 2006;254–255:154–162. doi: 10.1016/j.mce.2006.04.016. doi:10.1016/j.mce.2006.04.016. [DOI] [PubMed] [Google Scholar]
- Giles G, Price IR. Adolescent computer use: Approach, avoidance, and parental control. Australian Journal of Psychology. 2008;60:63–71. doi:10.1080/00049530701829896. [Google Scholar]
- Gray JA. Neural systems, emotion and personality. In: Madden J IV, editor. Neurobiology of learning, emotion and affect. Raven Press; New York, NY: 1991. pp. 273–306. [Google Scholar]
- Gray JA. Three fundamental emotion systems. In: Ekman P, Davidson RJ, editors. The Nature of Emotion: Fundamental Questions. Oxford University Press; New York, NY: 1994. pp. 243–247. [Google Scholar]
- Gray JA, McNaughton N. The neuropsychology of anxiety. Oxford University Press; Oxford, England: 2000. [Google Scholar]
- Hankin BL, Abramson LY. Development of gender differences in depression: An elaborated cognitive vulnerability-transactional stress theory. Psychological Bulletin. 2001;127:773–796. doi: 10.1037/0033-2909.127.6.773. doi:10.1037/0033-2909.127.6.773. [DOI] [PubMed] [Google Scholar]
- Hankin BL, Abramson LY, Moffitt TE, McGee R, Silva PA, Angell KE. Development of depression from preadolescence to young adulthood: Emerging gender differences in a 10-year longitudinal study. Journal of Abnormal Psychology. 1998;107:128–140. doi: 10.1037//0021-843x.107.1.128. doi:10.1037/0021-843X.107.1.128. [DOI] [PubMed] [Google Scholar]
- Harden KP, Tucker-Drob EM. Individual differences in the development of sensation seeking and impulsivity during adolescence: Further evidence for a dual systems model. Developmental Psychology. 2010;47:739–746. doi: 10.1037/a0023279. doi:10.1037/a0023279. [DOI] [PubMed] [Google Scholar]
- Hardin MG, Schroth E, Pine DS, Ernst M. Incentive-related modulation of cognitive control in healthy, anxious, and depressed adolescents: Development and psychopathology related differences. Journal of Child Psychology and Psychiatry. 2007;48:446–454. doi: 10.1111/j.1469-7610.2006.01722.x. doi:10.1111/j.1469-7610.2006.01722.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harmon-Jones E. Anger and the behavioral approach system. Personality and Individual Differences. 2003;35:995–1005. doi:10.1016/S0191-8869(02)00313-6. [Google Scholar]
- Harmon-Jones E, Allen JJB. Behavioral activation sensitivity and resting frontal EEG asymmetry: Covariation of putative indicators related to risk for mood disorders. Journal of Abnormal Psychology. 1997;106:159–163. doi: 10.1037//0021-843x.106.1.159. doi:10.1037/0021-843X.106.1.159. [DOI] [PubMed] [Google Scholar]
- Hasking PA. Reinforcement sensitivity, coping, and delinquent behaviour in adolescents. Journal of Adolescence. 2007;30:739–749. doi: 10.1016/j.adolescence.2006.11.006. doi:10.1016/j.adolescence.2006.11.006. [DOI] [PubMed] [Google Scholar]
- Han X, Jovicich J, Salat D, van der Kouwe A, Quinn B, Czanner S, Busa E, Pacheco J, Albert M, Killiany R, et al. Reliability of MRI-derived measurements of human cerebral cortical thickness: The effects of field strength, scanner upgrade and manufacturer. NeuroImage. 2006;32:180–194. doi: 10.1016/j.neuroimage.2006.02.051. doi:10.1016/j.neuroimage.2006.02.051. [DOI] [PubMed] [Google Scholar]
- Henderson HA, Wachs TD. Temperament theory and the study of cognition-emotion interactions across development. Developmental Review. 2007;27:396–427. doi:10.1016/j.dr.2007.06.004. [Google Scholar]
- Hooper CJ, Luciana M, Conklin HM, Yarger RS. Adolescents' performance on the Iowa gambling task: Implications for the development of decision making and ventromedial prefrontal cortex. Developmental Psychology. 2004;40:1148–1158. doi: 10.1037/0012-1649.40.6.1148. doi:10.1037/0012-1649.40.6.1148. [DOI] [PubMed] [Google Scholar]
- Hundt NE, Nelson-Gray RO, Kimbrel NA, Mitchell JT, Kwapil TR. The interaction of reinforcement sensitivity and life events in the prediction of anhedonic depression and mixed anxiety-depression symptoms. Personality and Individual Differences. 2007;43:1001–1012. doi:10.1016/j.paid.2007.02.021. [Google Scholar]
- Jazbec S, Hardin MG, Schroth E, McClure E, Pine DS, Ernst M. Age-related influence of contingencies on a saccade task. Experimental Brain Research. 2006;174:754–762. doi: 10.1007/s00221-006-0520-9. doi:10.1007/s00221-006-0520-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jorm AF, Christensen H, Henderson AS, Jacomb PA, Korten AE, Rodgers B. Using the BIS/BAS scales to measure behavioural inhibition and behavioural activation: Factor structure, validity and norms in a large community sample. Personality and Individual Differences. 1999;26:49–58. doi:10.1016/S0191-8869(98)00143-3. [Google Scholar]
- Jovicich J, Czanner S, Greve D, Haley E, van der Kouwe A, Gollub R, Kennedy D, Schmitt F, Brown G, Macfall J, et al. Reliability in multi-site structural MRI studies: Effects of gradient non-linearity correction on phantom and human data. NeuroImage. 2006;30:436–443. doi: 10.1016/j.neuroimage.2005.09.046. doi:10.1016/j.neuroimage.2005.09.046. [DOI] [PubMed] [Google Scholar]
- Kasch KL, Rottenberg J, Arnow BA, Gotlib IH. Behavioral activation and inhibition systems and the severity and course of depression. Journal of Abnormal Psychology. 2002;111:589–597. doi: 10.1037//0021-843x.111.4.589. doi:10.1037/0021-843X.111.4.589. [DOI] [PubMed] [Google Scholar]
- Kaufman J, Birmaher B, Brent D, Rao U, Ryan N. Kiddie-SADS-Present and Lifetime (K-SADS-PL) Version 1.0. University of Pittsburgh School of Medicine, Western Psychiatric Institute and Clinics; Pittsburgh, PA: 1996. Retrieved from http://www.wpic.pitt.edu/research/AssessmentTools/ChildAdolescent/ksadspl.pdf. [Google Scholar]
- Kessler RC, Berglund P, Demler O, Jin R, Merikangas KR, Walters EE. Lifetime prevalence and age-of-onset distributions of DSM-IV disorders in the National Comorbidity Survey Replication. Archives of General Psychiatry. 2005;62:593–602. doi: 10.1001/archpsyc.62.6.593. doi:10.1001/archpsyc.62.6.593. [DOI] [PubMed] [Google Scholar]
- Knyazev GG. Behavioural activation as predictor of substance use: Mediating and moderating role of attitudes and social relationships. Drug and Alcohol Dependence. 2004;75:309–321. doi: 10.1016/j.drugalcdep.2004.03.007. doi:10.1016/j.drugalcdep.2004.03.007. [DOI] [PubMed] [Google Scholar]
- Knyazev GG, Slobodskaya HR, Kharchenko II, Wilson GD. Personality and substance use in Russian youths: The predictive and moderating role of behavioural activation and gender. Personality and Individual Differences. 2004;37:827–843. doi:10.1016/j.paid.2003.10.010. [Google Scholar]
- Kringelbach ML, Rolls ET. The functional neuroanatomy of the human orbitofrontal cortex: Evidence from neuroimaging and neuropsychology. Progress in Neurobiology. 2004;72:341–372. doi: 10.1016/j.pneurobio.2004.03.006. doi:10.1016/j.pneurobio.2004.03.006. [DOI] [PubMed] [Google Scholar]
- Kuperberg GR, Broome MR, McGuire PK, David AS, Eddy M, Ozawa F, Goff D, West WC, Williams SC, van der Kouwe AJ, et al. Regionally localized thinning of the cerebral cortex in schizophrenia. Archives of General Psychiatry. 2003;60:878–888. doi: 10.1001/archpsyc.60.9.878. doi:10.1001/archpsyc.60.9.878. [DOI] [PubMed] [Google Scholar]
- Lamm C, Zelazo PD, Lewis MD. Neural correlates of cognitive control in childhood and adolescence: Disentangling the contributions of age and executive function. Neuropsychologia. 2006;44:2139–2148. doi: 10.1016/j.neuropsychologia.2005.10.013. doi:10.1016/j.neuropsychologia.2005.10.013. [DOI] [PubMed] [Google Scholar]
- Leen-Feldner EW, Zvolensky MJ, Feldner MT, Lejues CW. Behavioral inhibition: Relation to negative emotion regulation and reactivity. Personality and Individual Differences. 2004;36:1235–1247. doi:10.1016/S0191-8869(02)00113-7. [Google Scholar]
- Leen-Feldner EW, Zvolensky MJ, Feldner MT. Behavioral inhibition sensitivity and emotional response suppression: A laboratory test among adolescents in a fear-relevant paradigm. Journal of Clinical Child and Adolescent Psychology. 2004;33:783–791. doi: 10.1207/s15374424jccp3304_13. doi:10.1207/s15374424jccp3304_13. [DOI] [PubMed] [Google Scholar]
- Levita L, Hare TA, Voss HU, Glover G, Ballon DJ, Casey BJ. The bivalent side of the nucleus accumbens. Neuroimage. 2009;44:1178–1187. doi: 10.1016/j.neuroimage.2008.09.039. doi:10.1016/j.neuroimage.2008.09.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S, Backman L. Dopaminergic modulation of cognition across the life span. Neuroscience and Biobehavioral Reviews. 2010;34:625–630. doi: 10.1016/j.neubiorev.2010.02.003. doi:10.1016/j.neubiorev.2010.02.003. [DOI] [PubMed] [Google Scholar]
- Loxton NJ, Dawe S. Alcohol abuse and dysfunctional eating in adolescent girls: The influence of individual differences in sensitivity to reward and punishment. International Journal of Eating Disorders. 2001;29:455–462. doi: 10.1002/eat.1042. doi:10.1002/eat.1042. [DOI] [PubMed] [Google Scholar]
- Metalsky GI, Halberstadt LJ, Abramson LY. Vulnerability to depressive mood reactions: Toward a more powerful test of the diathesis-stress and causal mediation components of the reformulated theory of depression. Journal of Personality and Social Psychology. 1987;52:386–393. doi: 10.1037//0022-3514.52.2.386. doi:10.1037/0022-3514.52.2.386. [DOI] [PubMed] [Google Scholar]
- Meyer B, Johnson SL, Winters R. Responsiveness to threat and incentive in bipolar disorder: Relations of the BIS/BAS scales with symptoms. Journal of Psychopathology and Behavioral Assessment. 2001;23:133–143. doi: 10.1023/A:1010929402770. doi:10.1023/A:1010929402770. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison SE, Salzman CD. The convergence of information about rewarding and aversive stimuli in single neurons. The Journal of Neuroscience. 2009;29:11471–11483. doi: 10.1523/JNEUROSCI.1815-09.2009. doi:10.1523/JNEUROSCI.1815-09.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muetzel RL, Collins PF, Mueller BA, Schissel AM, Lim KO, Luciana M. The development of corpus callosum microstructure and associations with bimanual task performance in healthy adolescence. NeuroImage. 2008;39:1918–1925. doi: 10.1016/j.neuroimage.2007.10.018. doi:10.1016/j.neuroimage.2007.10.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nelson EE, Leibenluft E, McClure EB, Pine DS. The social re-orientation of adolescence: A neuroscience perspective on the process and its relation to psychopathology. Psychological Medicine. 2005;35:163–174. doi: 10.1017/s0033291704003915. doi:10.1017/S0033291704003915. [DOI] [PubMed] [Google Scholar]
- O'Connor RM, Stewart SH, Watt MC. Distinguishing BAS risk for university students' drinking, smoking, and gambling behaviors. Personality and Individual Differences. 2009;46:514–519. doi:10.1016/j.paid.2008.12.002. [Google Scholar]
- Oldfield RC. The assessment and analysis of handedness: The Edinburgh inventory. Neuropsychologia. 1971;9:97–113. doi: 10.1016/0028-3932(71)90067-4. doi:10.1016/0028-3932(71)90067-4. [DOI] [PubMed] [Google Scholar]
- Olson EA, Collins PF, Hooper CJ, Muetzel R, Lim KO, Luciana M. White matter integrity predicts delay discounting behavior in 9- to 23-year-olds: A diffusion tensor imaging study. Journal of Cognitive Neuroscience. 2009;21:1406–1421. doi: 10.1162/jocn.2009.21107. doi:10.1162/jocn.2009.21107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Patrick ME, Blair C, Maggs JL. Executive function, approach sensitivity, and emotional decision making as influences on risk behaviors in young adults. Journal of Clinical and Experimental Neuropsychology. 2008;30:449–462. doi: 10.1080/13803390701523109. doi:10.1080/13803390701523109. [DOI] [PubMed] [Google Scholar]
- Pinto-Meza A, Caseras X, Soler J, Puigdemont D, Perez V, Torrubia R. Behavioural inhibition and behavioural activation systems in current and recovered major depression participants. Personality and Individual Differences. 2006;40:215–226. doi:10.1016/j.paid.2005.06.021. [Google Scholar]
- Rosas HD, Liu AK, Hersch S, Glessner M, Ferrante RJ, Salat DH, van der Kouwe A, Jenkins BG, Dale AM, Fischl B. Regional and progressive thinning of the cortical ribbon in Huntington's disease. Neurology. 2002;58:695–701. doi: 10.1212/wnl.58.5.695. Retrieved from http://www.aan.com/go/elibrary/journal. [DOI] [PubMed] [Google Scholar]
- Salat DH, Buckner RL, Snyder AZ, Greve DN, Desikan RS, Busa E, Morris JC, Dale AM, Fischl B. Thinning of the cerebral cortex in aging. Cerebral Cortex. 2004;14:721–730. doi: 10.1093/cercor/bhh032. doi:10.1093/cercor/bhh032. [DOI] [PubMed] [Google Scholar]
- Segonne F, Dale AM, Busa E, Glessner M, Salat D, Hahn HK, Fischl B. A hybrid approach to the skull stripping problem in MRI. NeuroImage. 2004;22:1060–1075. doi: 10.1016/j.neuroimage.2004.03.032. doi:10.1016/j.neuroimage.2004.03.032. [DOI] [PubMed] [Google Scholar]
- Sled JG, Zijdenbos AP, Evans AC. A nonparametric method for automatic correction of intensity nonuniformity in MRI data. IEEE Transactions on Medical Imaging. 1998;17:87–97. doi: 10.1109/42.668698. doi:10.1109/42.668698. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Jones RM, Casey BJ. A time of change: Behavioral and neural correlates of adolescent sensitivity to appetitive and aversive environmental cues. Brain and Cognition. 2010;72:124–133. doi: 10.1016/j.bandc.2009.07.003. doi:10.1016/j.bandc.2009.07.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of Cognitive Neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. doi:10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sowell ER, Thompson PM, Holmes CJ, Jernigan TL, Toga AW. In vivo evidence for post-adolescent brain maturation in frontal and striatal regions. Nature Neuroscience. 1999;2:859–861. doi: 10.1038/13154. doi:10.1038/13154. [DOI] [PubMed] [Google Scholar]
- Steinberg L. A social neuroscience perspective on adolescent risk-taking. Developmental Review. 2008;28:78–106. doi: 10.1016/j.dr.2007.08.002. doi:10.1016/j.dr.2007.08.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Steinberg L, Albert D, Cauffman E, Banich M, Graham S, Woolard J. Age differences in sensation seeking and impulsivity as indexed by behavior and self-report: Evidence for a dual systems model. Developmental Psychology. 2008;44:1764–1778. doi: 10.1037/a0012955. doi:10.1037/a0012955. [DOI] [PubMed] [Google Scholar]
- Steinberg L, Morris AS. Adolescent development. Annual Review of Psychology. 2001;52:83–110. doi: 10.1146/annurev.psych.52.1.83. doi:10.1146/annurev.psych.52.1.83. [DOI] [PubMed] [Google Scholar]
- Sutton SK, Davidson RJ. Prefrontal brain asymmetry: A biological substrate of the behavioral approach and behavioral inhibition systems. Psychological Science. 1997;8:204–210. doi:10.1111/j.1467-9280.1997.tb00413.x. [Google Scholar]
- Takahashi Y, Yamagata S, Kijima N, Shigemasu K, Ono Y, Ando J. Continuity and change in behavioral inhibition and activation systems: A longitudinal behavioral genetic study. Personality and Individual Differences. 2007;43:1616–1625. doi:10.1016/j.paid.2007.04.030. [Google Scholar]
- Urošević S, Abramson LY, Harmon-Jones E, Alloy LB. Dysregulation of the behavioral approach system (BAS) in bipolar spectrum disorders: Review of theory and evidence. Clinical Psychology Review. 2008;28:1188–1205. doi: 10.1016/j.cpr.2008.04.004. doi:10.1016/j.cpr.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Van Leijenhorst L, Zanolie K, Van Meel CS, Westenberg PM, Rombouts SARB, Crone EA. What motivates the adolescent? Brain regions mediating reward sensitivity across adolescence. Cerebral Cortex. 2010;20:61–69. doi: 10.1093/cercor/bhp078. doi:10.1093/cercor/bhp078. [DOI] [PubMed] [Google Scholar]
- Wahlstrom D, Collins P, White T, Luciana M. Developmental changes in dopamine neurotransmission in adolescence: Behavioral implications and issues in assessment. Brain and Cognition. 2010;72:146–159. doi: 10.1016/j.bandc.2009.10.013. doi:10.1016/j.bandc.2009.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.