Abstract
All T cells are dependent on IL-7 for their development and for homeostasis. FoxP3+ T regulatory cells are unique among T cells in that they are dependent on IL-2. Whether such IL-2 dependency is distinct from or in addition to an IL-7 requirement has been a confounding issue, particularly because of the absence of an adequate experimental system to address this question. Here we present a novel in vivo mouse model where IL-2 expression is intact but IL-7 expression was geographically limited to the thymus. Consequently, IL-7 is not available in peripheral tissues. Such mice were generated by introducing a thymocyte-specific IL-7 transgene onto an IL-7-null background. In these mice, T cell development in the thymus, including FoxP3+ Treg cell numbers, was completely restored which correlates with the thymus-specific expression of transgenic IL-7. In peripheral cells, however, IL-7 expression was terminated, which resulted in a general paucity of T cells and a dramatic reduction of FoxP3+ Treg cell numbers. Loss of Treg cells was further accompanied by a significant reduction in FoxP3+ expression levels. These data suggest that peripheral IL-7 is not only necessary for Treg cell survival but also for upregulating FoxP3 expression. Collectively, we assessed the effect of a selective peripheral IL-7 deficiency in the presence of a fully functional thymus, and we document a critical requirement for in vivo IL-7 in T cell maintenance and specifically in FoxP3+ cell homeostasis.
Introduction
Interleukin-7 (IL-7) is an essential and non-redundant cytokine for T cell development in the thymus and also for T cell homeostasis in the periphery. Its importance is exemplified by its absence, which results in T−B+NK− severe combined immunodeficiency (SCID) in humans and in the paucity of both B and T cells in mice (1). IL-7 signaling is mediated through a heterodimeric complex of the IL-7Rα chain and the IL-2 receptor γ-chain, also known as the common γc-chain (γc) (2). Upon IL-7 binding, the IL-7 receptor activates two major downstream signaling pathways, namely the JAK-STAT and PI3K/Akt pathway, which induce expression of anti-apoptotic proteins such as Bcl-2 and Mcl-1 and also upregulate metabolic activities by targeting expression of glucose transporter-1 (3). Thus, IL-7 is a critical cytokine for T cells, chiefly because of its role in providing pro-survival signals.
Regulatory T cells (Treg) are a subset of CD4+ T cells that play a critical role in maintaining self-tolerance. Their importance is manifested by the IPEX (Immune dysregulation, Polyendocrinopathy, Enteropathy, X-linked) syndrome in humans and by the lethal autoimmune lymphoproliferative phenotype of scurfy mice, where in both cases Treg cell generation is impaired (4–7). Expression of the forkhead transcription factor FoxP3 is both necessary and sufficient for CD4+ Treg cell development, and STAT5 has been identified as a major activator of FoxP3+ expression (8–9). Curiously enough, while both IL-7 and IL-2 induce activation of STAT5, it is only the IL-2 receptor-dependent activation of STAT5 that drives the differentiation and maintenance of FoxP3+ cells in vivo (8, 10). As such, IL-2 or IL-2 receptor-deficient mice are severely impaired in Treg cell generation, and IL-2 signaling is uniquely required for Treg cell expansion and homeostasis in vivo (11–12). Why most T cells are dependent on IL-7 but Treg cells are dependent on IL-2, and why IL-7 cannot substitute IL-2 in Treg cell generation, especially in vivo, remain open questions that are important for understanding Treg cell function. A potential explanation for a skewed preference toward IL-2 is the significantly lower IL-7Rα expression level on FoxP3+ Treg cells. FoxP3 directly suppresses IL-7Rα transcription and thus could lower IL-7 sensitivity (13–14). However, IL-7 stimulation of Treg cells still strongly induces STAT5 phosphorylation and upregulates FoxP3 expression in vitro, and as such Treg cells are IL-7 signaling competent (15). Since Treg cells are very likely exposed to the same tonic IL-7 signals as other T cells undergoing homeostasis, a potential role for IL-7 in Treg cells needs to be tested in vivo. Additionally, it is not known whether IL-2 signaling alone is sufficient for Treg cell survival or whether IL-2 only acts in the presence of tonic IL-7 signaling. Consequently, we wished to know if Treg cells are entirely independent of IL-7 or whether Treg cells also require IL-7 as other CD4+ αβ T cells do.
Dissociating IL-7’s role in the thymus from IL-7’s role in peripheral homeostasis in vivo is a daunting task. So far, no experimental system satisfactorily succeeded in removing IL-7 signaling in peripheral tissues only, without also disturbing T cell generation in the thymus. The conventional method of choice had been adoptive transfers of T cells into IL-7-deficient mice (16–17). However, intrinsic problems inevitably linked to such approaches render the read-out of these experiments quite confusing. That is, upon adoptive transfer into a lymphopenic environment, such as into IL-7-deficient mice, donor T cells undergo lymphopenia-induced proliferation (LIP). This in turn induces an activated-memory phenotype in these cells, which does not correspond to a homeostatic, steady state condition (18–19). Additionally, adoptive transfer not only tests the requirement of IL-7 in T cell survival but also its requirement for homeostatic proliferation. As an alternative approach, acute in vivo treatment with neutralizing anti-IL-7 or anti-IL-7Rα antibodies or even conditional deletion of IL-7Rα gene expression using an inducible Cre transgene have been employed with the aim to disable peripheral IL-7 signaling (20–21). But again, all these approaches had the same limitation in that they not only induce a peripheral but also a systemic IL-7 deprivation which affects the thymic output of mature T cells. Thus, the lack of normal thymopoiesis obscures conclusions about the role of IL-7 in peripheral T cell homeostasis. Altogether, these reports indicate that aside the role of in vivo IL-7 in FoxP3+ Treg cells, so far, even the role of in vivo IL-7 in αβ T cell survival has not been firmly demonstrated without the manipulation of donor cells or host mouse.
We considered that an ideal model for the role of IL-7 for peripheral T cell homeostasis would be an in vivo system where IL-7 expression in the thymus is intact but mature thymocytes would then egress into an IL-7-deficient environment. To establish such a system, we crossed mice expressing a proximal lck enhancer driven IL-7 transgene onto an IL7KO background, which we referred to as “K7” mice. We found that K7 mice express high levels of transgenic IL-7 in the thymus but none in peripheral cells. We also found that transgenic IL-7 completely rescued T cell development in the thymus but failed to maintain T cell homeostasis in the periphery as K7 mice had dramatically reduced LN T cell numbers. Thus, K7 mice represent a novel model of peripheral lymphopenia induced by peripheral IL-7 deficiency that can be used to assess IL-7’s effect on T cell survival, homeostasis and function in future studies. Using this model, here we identified a critical role of in vivo IL-7 in αβ T cell homeostasis and we identified IL-7 as a non-redundant cytokine for FoxP3+ Treg cell survival and homeostasis in vivo.
Materials and Methods
Animals
C57BL/6 (B6) mice were obtained from the Frederick Cancer Research and Development Center, Frederick, MD. IL-7-deficient (IL7KO) and proximal lck-driven IL-7 transgenic (IL7Tg) mice had been previously described (22–23). Transgenic mice expressing IL-7 under the mouse H2-Ea promoter were obtained from The Jackson Laboratory (Bar Harbor, ME). Animal experiments were approved by the NCI Animal Care and Use Committee, and all mice were cared for in accordance with NIH guidelines.
Thymectomy
K7 mice were thymectomized at 3 weeks of age and analyzed 6 weeks after surgery. Mice were anesthetized by i.p. injection of ketamine/xylazine at 0.1ml/10g body weight. For analgesia, buprenorphine 0.5mg/kg of body weight was administered s.c. before surgery. After positioning the mouse in dorsal recumbency, an incision was made from the neck to midway down the chest, and thymus lobes were removed by suction using a small pipette attached to a vacuum container. The chest was closed with absorbable suture, and 0.25% bupivacaine was applied to incision prior to closing the skin. Skin was closed using stainless steel autoclips which were removed after 10 days.
Immunofluorescence and flow cytometry
Single cell suspensions of thymus, spleen or LN were harvested, stained, and analyzed on an LSRII, FACSAria or FACSCalibur (Becton Dickinson). Dead cells were excluded by forward light scatter gating and propidium iodide staining. Data were analyzed using software designed by the Division of Computer Research and Technology at the NIH. Antibodies with the following specificities were used for staining: CD4 (GK1.5 and RM4.5), CD8α (53-6-7), TCRβ (H57-597), IL-2Rα (CD25), IL-2Rγ (4G3), Foxp3 (FJK-16s), CD24 (30-F1), IL-7Rα (A7R34), Qa-2 (69H1-9-9); all from eBiosciences), γδ TCR (GL3), CD44 (IM7), and CD62L (MEL-14), IgG1κ isotype control (B56), Ki-67 (MOPC-21); all from Becton Dickinson. Intracellular staining was done using the FoxP3 staining buffer set according to the manufacturer’s instructions (eBioSciences). CD1d/PBS57 tetramers were from the NIH tetramer facility (Emory University, Atlanta, GA).
Treg cell in vitro suppression assay
CFSE-labeled CD45.1+ CD4+CD25− responder T cells (5 × 104 cells per assay) were incubated in a 96-well round bottom plate with equal numbers of sorted CD4+CD25+ Treg cells from WT or K7 mice. T cell depleted splenocytes (10 × 104 cells per assay) were irradiated at 2,000 rad and used as antigen presenting cells. Cultures were stimulated with soluble anti-CD3ε (1μg/ml) for 3 days. Proliferation of CD45.1+ responder T cells was measured by CFSE dilution.
Quantitative Real-Time PCR
Thymocytes were electronically sorted from WT and K7 thymuses. LNT cells were isolated by depletion of B cells with anti-mouse IgG beads. CD4+ or CD8+ LNT were further purified by depleting LNT with either anti-CD8 or anti-CD4 mAbs. Total RNA was isolated with the RNEasy kit (Qiagen). RNA was reverse transcribed into cDNA by oligo(dT) priming with the QuantiTect Reverse transcription kit (Qiagen). Quantitative RT-PCR (qRT-PCR) was performed with an ABI PRISM 7900HT Sequence Detection System and the QuantiTect SYBR Green detection system (Qiagen) with the following primers: CD5 (Forward: 5 ′-CCAGTGCCTTCCGCTGAGCC-3′; Reverse: 5′-GGCCTTCGTGTCCTGGCACC-3′), CD69 (Forward: 5′-GACATGACGTTTCTGAAGCGATA-3′; Reverse: 5′-AGTCCACAGCGGTAACATTTTTG-3′), IL7 (Forward: 5 ′-CTGATGATCAGCATCGATGAATTGG-3′; Reverse: 5′-GCAGCACGATTTAGAAAAGCAGCTT-3′), and β-actin (Forward: 5′-GAGAGGGAAATCGTGCGTGA-3′; Reverse: 5′-ACATCTGCTGGAAGGTGG-3′). Gene expression values were normalized to those of β-actin in the same sample.
Statistical analysis
Dataare shown as mean ± SEM. The two-tailed Student’s t test was used to calculate P-values. A value of P ≤ 0.05 was considered statistically significant.
Results
FoxP3+ Treg cell generation and homeostasis in IL-7-deficient mice
To confirm the IL7KO phenotype, we first determined total thymocyte numbers in wildtype (WT) and IL7KO mice. As previously reported (23), IL-7KO mice had severely decreased thymocyte numbers but displayed a relatively normal CD4/CD8 thymocyte profile (Fig. 1A and data not shown). IL7KO mice also showed normal proportions of FoxP3+ cells within CD4SP thymocytes (Fig. 1B) but absolute FoxP3+ Treg cell numbers were severely reduced (Fig. 1C). Interestingly, all FoxP3+ cells in the thymus also expressed Helios even in the absence of IL-7 (Fig. 1B) indicating that natural Treg cell generation in the thymus is IL-7-independent (24). Thymic Treg cell numbers, however, appeared to be IL-7-dependent.
Figure 1. Peripheral T lymphopenia and impaired FoxP3+ Treg cell homeostasis in IL7KO mice.
(A) Impaired thymopoiesis in IL7KO mice. Total thymocyte numbers were determined in WT and IL7KO mice. Data show the summary of ten independent experiments.
(B) FoxP3+ Treg cell generation in the absence of in vivo IL-7. CD4SP thymocytes from WT or IL7KO mice were assessed for intracellular FoxP3 and Helios expression by flow cytometry. Data are representative for three independent experiments. Numbers in boxes indicate percentages of gated cells.
(C) Severely reduced Treg cell numbers in IL7KO thymocytes. Total CD4+CD25+FoxP3+ Treg cell numbers were quantified from WT and IL7KO thymuses. Data are the summary of five independent experiments.
(D) Splenic CD4+ T cell numbers remain very low even in aged IL7KO mice. Total CD4+ spleen T cell numbers were determined in WT and IL7KO mice and plotted against the age of each individual mouse.
(E) Impaired FoxP3+ Treg cell homeostasis in IL7KO mice. Absolute numbers of LN CD4+CD25+FoxP3+ Treg cells were determined in WT and IL7KO mice. Data show the summary of five independent experiments with five WT and six IL7KO mice.
Next, to assess T cell homeostasis in IL-7-deficient tissues, we determined CD4+ T cell numbers in WT and IL-7KO spleens. We found that IL7KO mice were peripheral T lymphopenic and that CD4+ T cell numbers remained very low even in older mice (Fig. 1D). More importantly, and in contrast to the study by Mazzucchelli et al. (25), we found that peripheral FoxP3+ Treg cell numbers were drastically reduced in IL7KO mice compared to WT mice (Fig. 1E). These data suggest that IL-7 plays a non-redundant role in peripheral Treg cell homeostasis, but it remains unclear whether IL-7 is essential for Treg cell survival or for homeostatic proliferation induced by a lymphopenic environment.
Transgenic IL-7 restores T cell development and FoxP3+ Treg cell numbers in IL-7KO thymocytes
To limit IL-7 deficiency to peripheral tissues, next, we generated a new in vivo model where IL-7 is exclusively expressed in thymocytes. K7 mice are IL-7KO mice that are transgenic for an lck-proximal enhancer driven murine IL-7 cDNA (22). Thymocyte development in K7 mice was completely restored if not enhanced regarding cell numbers compared to WT mice (Fig. 2A). Also, αβ T cell maturation and CD4/CD8 lineage differentiation (Fig. 2B and Suppl. Fig. 1A) as well as γδ T cell development were intact in K7 thymuses (Fig. 2C). Transgenic IL-7 also effectively rescued and even increased NKT cell numbers in K7 thymuses compared to non-transgenic IL-7KO mice (Fig. 2D and Suppl. Fig. 1B). Finally, FoxP3+ Treg cell numbers in K7 mice were recovered to a comparable level of FoxP3+ cell numbers in WT mice (Fig. 2E). Collectively, we document that a thymocyte-specific IL-7 transgene is sufficient to restore development of all major thymocyte populations, including FoxP3+ Treg cells, on a germline IL-7-deficient background.
Figure 2. Thymocyte-specific expression of transgenic IL-7 restores thymopoiesis and Treg cell generation in IL7KO mice.
(A) A proximal lck-enhancer driven IL7Tg restores thymocytes numbers in IL7KO mice. Thymocyte numbers were determined in WT and K7 (IL7KOIL7Tg) mice. Data show the summary of ten independent experiments.
(B) Whole thymocyte profile of K7 mice. Contour plots show CD4/CD8 expression profiles of total (top) and TCRβ+ gated (bottom) WT and K7 thymocytes. Data are representative of ten independent experiments.
(C) Thymic γδ T cell development is restored in K7 mice. γδ T cells were identified by surface TCRδ versus TCRβ staining in WT, IL7KO and K7 mice. Numbers in boxes indicate percentage of TCRδ+ cells in the thymus. Data are representative of four independent experiments.
(D) Transgenic IL-7 enhances thymic NKT cell development in K7 mice. Thymic NKT cell numbers were determined by CD1d-tetramer and TCRβ staining followed by flow cytometric analysis. Data show the summary of two independent experiments with three K7 and three IL7KO mice.
(E) Restoration of Treg cell numbers in K7 thymus. Thymic CD4+CD25+FoxP3+ Treg cell numbers were determined in WT and K7 mice. Data are the result of six independent experiments.
Lck-proximal enhancer driven IL-7 transgene expression is terminated upon positive selection
In K7 mice, the only in vivo sources of IL-7 are thymocytes expressing the IL-7 transgene. To monitor such IL-7 expression, we assessed IL-7 mRNA levels in whole thymocytes and purified LN T cells from K7 mice. Transgenic IL-7 mRNA expression was extinguished in mature LN T cells indicating an absence of IL-7 in peripheral tissues (Fig. 3A). To further pinpoint termination of IL-7 expression to a specific developmental stage, we subdivided whole thymocytes into three populations, pre-selection (I), post-selection (III), and cells undergoing positive selection (II), based on surface CD69 and TCRβ expression (Fig. 3B and Suppl. Fig. 1C). These populations were then sorted from K7 thymocytes and tested for correct purification by assessing CD69 and CD5 expression using quantitative real time PCR (Suppl. Fig. 1D). When tested for IL-7 mRNA expression, we found that IL-7 mRNA expression was terminated in mature thymocytes (Fig. 3B). Specifically, these results showed that IL-7 was highly expressed in CD69−TCRβlow pre-selection thymocytes but that transgenic IL-7 expression was terminated upon TCR signaling at the CD69+ stage.
Figure 3. Peripheral IL-7 deficiency in K7 mice.
(A) Mature peripheral K7 T cells are devoid of IL-7 expression. Whole thymocytes and purified LN T cells from K7 mice were assessed for IL-7 mRNA expression by real time PCR. Data are representative of three independent experiments.
(B) Transgenic IL-7 expression is terminated in post-selection thymocytes. Transgenic IL-7 mRNA expression was monitored during T cell development in the thymus based on CD69 and TCRβ expression levels (left). Population I, pre-selection thymocytes; Population II, TCR signaled thymocytes; Population III, post-selection thymocytes. IL-7 mRNA expression was determined in sorted populations by real time PCR (right). Data are the summary of four independent experiments.
Peripheral T cell lymphopenia in K7 mice
To assess how in vivo IL-7 deprivation affects peripheral T cells, total spleen cells were stained for TCRβ and CD4/CD8 expression in WT and K7 mice. K7 spleen cells contained a much smaller T cell population compared to WT mice with a preferential decrease in CD8+ T cells (Fig. 4A and Suppl. Fig. 2A). These results further translated into severely diminished T cell numbers in K7 lymph nodes and to a skewed CD4/CD8 ratio towards CD4 cells (Fig. 4B and Fig. 4C). CD8 coreceptor expression is a sensitive marker for in vivo IL-7 signaling (26). To examine whether the loss of peripheral T cells is due to absent in vivo IL-7 signaling, next, we quantified surface CD8 levels on K7 CD8 T cells and found significant reduction of surface CD8 levels compared to WT CD8 T cells (Fig. 4D). Importantly, such lower CD8 levels were not developmentally set as newly generated K7 and WT CD8SP thymocytes expressed identical levels of surface CD8 in the thymus (Suppl. Fig. 2B). In addition to CD8 levels, IL-7 signaling is also critical for maintaining anti-apoptotic Bcl-2 expression (3). Consequently, we wished to know whether Bcl-2 levels were reduced in K7 T cells compared to WT cells. Upon intracellular staining for Bcl-2, we found that K7 CD8+ T cells expressed significantly lower Bcl-2 levels than WT cells (Fig. 4E), which was also the case for K7 CD4+ T cells (Suppl. Fig. 2C). Collectively, these results showed that peripheral K7 T cells showed all the indications for absent IL-7 signaling in vivo and these data formally document the importance of peripheral IL-7 in the survival and maintenance of a mature T cell pool. Interestingly, IL-7 receptor levels were much lower in K7 LN T cells than in WT cells (Fig. 4F). This was contrary to our expectations since in vivo IL-7 downregulates IL-7Rα expression (27), and IL-7 signaling is presumably absent in peripheral K7 T cells. Consequently, we wished to know the identity of surviving T cells in K7 mice and thus phenotyped these cells for naïve and activated memory cell markers. In striking contrast to IL-7KO mice, where a large fraction of LN T cells are CD122hiCD44hi activated/memory cells (Fig. 4G), LNT cells in K7 mice were predominantly naïve and expressed significantly reduced percentages of memory T cell markers (Fig. 4G and Suppl. Fig. 2D). Also, intracellular staining for the nuclear proliferation antigen Ki-67 revealed that K7 LNT cells are mostly quiescent non-proliferating cells, suggesting that peripheral lymphopenia fails to induce homeostatic proliferation in the absence of IL-7 (Suppl. Fig. 2E). Based on these results, we consider it likely that most K7 T cells are recent thymic emigrants which got recently signaled by IL-7 in the thymus, and hence are expressing low levels of IL-7Rα. In the absence of peripheral IL-7, these cells survive only a short time without further accumulating or undergoing homeostatic proliferation into activated memory phenotype cells.
Figure 4. Absent IL-7 signaling results in peripheral T lymphopenia in K7 mice.
(A) Spleen cell analysis of K7 mice. Total splenocytes from WT and K7 mice were assessed for TCRβ and CD4/CD8 expression. Data are representative of ten independent experiments.
(B) Peripheral T lymphopenia in K7 mice. LN T cell numbers were quantified in WT, IL7KO and K7 mice. Data are the summary from ten independent experiments.
(C) Increased CD4/CD8 ratio in K7 LN T cells. CD4 versus CD8 ratio of LN T cells were determined in WT and K7 mice. Data are summary of ten independent experiments.
(D) Reduced surface CD8 levels on K7 CD8 LN T cells. Surface CD8α expression was determined on WT and K7 LN T cells. Data are representative of ten independent experiments,
(E) K7 LN T cells express low levels of Bcl-2. Intracellular Bcl-2 expression was determined in WT and K7 CD8 LN T cells. Data are the summary of three independent experiments.
(F) IL-7Rα expression on CD4 and CD8 T cells of WT and K7 mice. Relative expression of surface IL-7Rα expression was assessed by mean fluorescence intensities on WT and K7 T cells. Data are the summary of three independent experiments.
(G) Peripheral IL-7-deficiency results in significantly decreased memory phenotype CD8 T cells. LN CD8 T cells were stained for CD122 and CD44 expression (left). Percentages of memory phenotype-like cells were determined in multiple experiments (right). Data show the result of ten independent experiments.
Peripheral T cell survival in thymectomized K7 mice
To determine the contribution of thymic output on peripheral K7 T cell numbers, next, we thymectomized K7 mice and analyzed their peripheral T cell compartment after six weeks of procedure (Suppl. Fig. 3A). Compared to sham operated control K7 mice, thymectomized K7 mice showed a dramatic decrease in peripheral T cell percentages with equal reduction in both CD4 and CD8 T cells and a significant loss in absolute LN T cell numbers (Fig. 5A and Fig. 5B). Notably, the vast majority of remaining CD4 T cells was composed of CD62LloCD44hi memory phenotype cells (Fig. 5C). This was also the case for the CD8 T cell compartment, which similarly lost naïve T cells but accumulated CD122hiCD44hi memory phenotype cells (Fig. 5D). Such changes in the composition and size of the peripheral T cell pool were also observed in spleens of thymectomized K7 mice (Suppl. Fig. 3B and 3C), which was similarly accompanied with a specific reduction in naïve T cell and concomitant increase in memory T cell percentages (Suppl. Fig. 3D and 3E). In sum, these results indicate that the naïve T cell pool in K7 mice is primarily maintained by thymic output, and that survival and homeostasis of naïve T cells are critically dependent on peripheral IL-7. Thus, peripheral T cells in K7 mice represent a unique opportunity to examine the role of IL-7 in the maintenance and homeostasis of mature T cells.
Figure 5. Thymic output is the major source of peripheral T cells in K7 mice.
(A) LN cell analysis of thymectomized K7 mice. Lymph node cells from sham operated versus thymectomized K7 mice were assessed for TCRβ and CD4 versus CD8 expression. Data are representative of three independent experiments.
(B) Thymic output is necessary to maintain peripheral T cells in K7 mice. TCRβ+ LN T cell numbers were determined in sham operated and thymectomized K7 mice. Data show the summary of three independent experiments.
(C) Accumulation of CD4+ memory phenotype cells in thymectomized K7 mice. LN CD4 T cells were assessed for CD62L and CD44 expression. Numbers indicate percentage of CD62LloCD44hi memory phenotype-like cells. Data are representative of three independent experiments.
(D) Accumulation of CD8+ memory phenotype cells in thymectomized K7 mice. LN CD8 T cells were assessed for CD122 and CD44 expression. Numbers indicate percentage of CD122hiCD44hi memory phenotype-like cells. Data are representative of three independent experiments.
Impaired FoxP3+ Treg cell homeostasis in the absence of peripheral IL-7
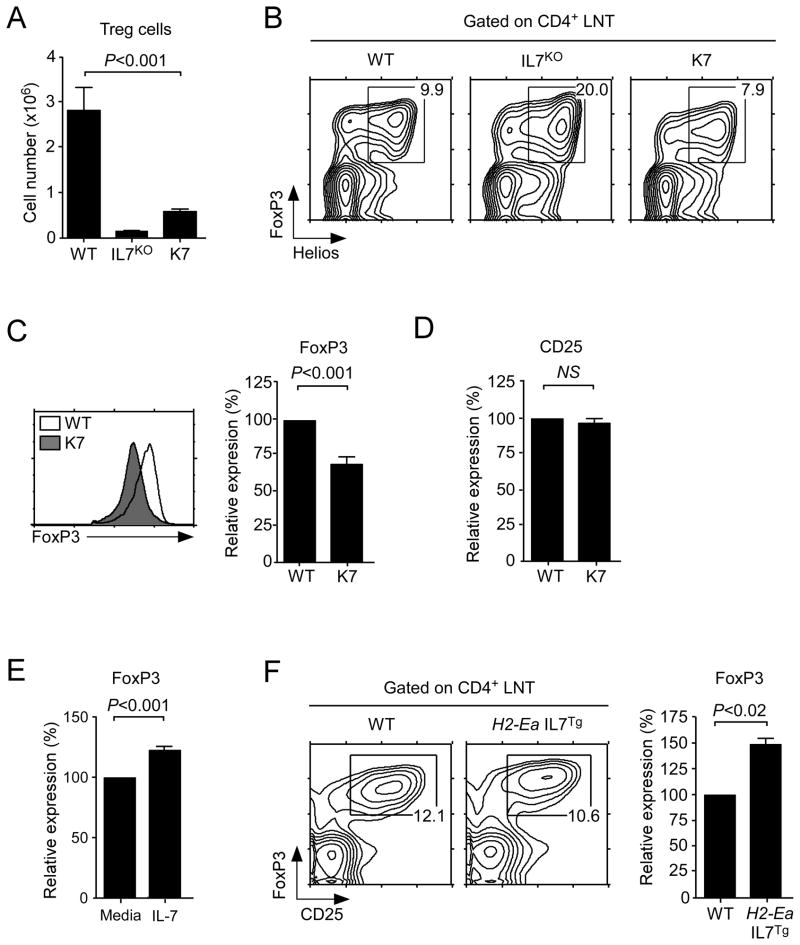
Using this new in vivo model of peripheral IL-7 deficiency, next we assessed the role of IL-7 in FoxP3+ Treg cell homeostasis. Surprisingly, despite normal numbers of Treg cells in the thymus (Fig. 2E), we found a dramatic reduction of FoxP3+ Treg cell numbers in K7 lymph nodes (Fig. 6A). Proportionally, however, CD4+CD25+FoxP3+ Treg cell percentages among K7 CD4 T cells was largely unaffected, and most FoxP3+ cells were also Helios+ indicating that these surviving cells were thymus derived nTreg cells (Fig. 6B). These data further support the idea that the majority of peripheral K7 T cells, including Treg cells, are recent thymic emigrants. Strikingly, in the absence of peripheral IL-7, FoxP3 levels in K7 Treg cells were significantly reduced compared to WT Treg cells (Fig. 6C). CD25 expression on the same cells, however, was not affected, which suggests that IL-7 is specifically acting on FoxP3 (Fig. 6D). To confirm that this is a direct effect of IL-7, we next cultured K7 Treg cells with IL-7 in vitro and assessed their FoxP3 levels the next day. Compared to medium cultured cells, overnight IL-7 treated Treg cells showed significant increase of FoxP3 levels, implicating the absence of in vivo IL-7 as a cause for lower FoxP3 levels in K7 mice (Fig. 6E). Then, to test whether IL-7 can induce FoxP3 expression also in vivo, we assessed FoxP3 levels in H2-Ea IL-7 transgenic mice which overexpress IL-7 in peripheral tissues at high levels. Here we found that FoxP3 levels were significantly increased which further supports the notion of an IL-7-dependent pathway of FoxP3 expression (Fig. 6F). Finally, to assess the functional capability of K7 Treg cells, we tested their activity in a series of in vitrosuppression assays. Surprisingly, despite their initial low levels of FoxP3, K7 Treg cells were excellent suppressors, indistinguishable from their WT counterparts (Suppl. Fig. 4A). But further analysis of in vitro cultured Treg cells revealed that FoxP3 levels in K7 Treg cells had recovered to WT levels, which explains their intact Treg function (Suppl. Fig. 4B and 4C). We consider it likely that accumulation of high levels of IL-2 from responder T cells in vitro would have upregulated FoxP3 in K7 Treg cells and as such has restored any potential functional defects. Collectively, these data demonstrate that peripheral IL-7 is a critical factor for Treg cell survival and, surprisingly, also for upregulating FoxP3 levels under homeostatic conditions.
Figure 6. Impaired FoxP3+ Treg homeostasis in the absence of peripheral IL-7.
(A) Peripheral FoxP3+ Treg cell numbers are drastically reduced in K7 mice. LN CD4+CD25+FoxP3+ Treg cell numbers were assessed in WT (n= 6), IL7KO (n=5), and K7 (n=9) mice.
(B) FoxP3+ cells in K7 mice are Helios positive. CD4+ LNT cells were assessed for both FoxP3 and Helios expression by intracellular staining. Numbers in boxes indicate the percentages of FoxP3+Helios+ natural Treg cells among CD4+ LNT cells. Contour plots are representative of four independent experiments.
(C) FoxP3 levels are attenuated in the absence of peripheral IL-7. Intracellular FoxP3 levels were determined by flow cytometry. Histogram is representative of six independent experiments (left). Mean fluorescence intensities of intracellular FoxP3 levels were determined in WT and K7 LN Treg cells. Graph shows the summary of K7 FoxP3 levels relative to WT levels, which was set to 100 (right). Results are the summary of six independent experiments.
(D) Surface CD25 expression is not affected in K7 Treg cells. Mean fluorescence intensities of surface CD25 levels were determined from WT and K7 LN Treg cells. Graph shows the summary of K7 CD25 levels relative to WT CD25 levels, which was set to 100. Results are the summary of six independent experiments.
(E) IL-7 upregulates FoxP3 expression in K7 Treg cells. K7 Treg cells were stimulated overnight with IL-7 (10 ng/ml) or with medium alone. Next day, FoxP3 levels were determined by intracellular staining, and relative expression was calculated from mean fluorescence intensity. Data are the summary from three independent experiments.
(F) Increased FoxP3 levels in peripheral IL-7 transgenic mice. LN Treg cells from WT or H2-Ea-promoter driven IL-7 transgenic mice (H2-Ea IL7Tg) were assessed for FoxP3 expression. Contour plots are representative of three independent experiments (left). Graph shows the summary of IL-7Tg FoxP3 levels relative to WT levels which was set to 100 (right). Results are the summary of three independent experiments.
Discussion
T cells are strictly dependent on IL-7 for both their thymic development and peripheral homeostasis. However, FoxP3+ Treg cells are unique among T cells in that they are dependent on IL-2. Whether such IL-2 dependency is in addition to an overall in vivo IL-7 requirement, or whether IL-2 itself is sufficient, is unclear. Using a novel in vivo model of peripheral IL-7 deficiency, here we assessed the requirement of IL-7 in T cell survival in general and in Treg cell homeostasis in particular. We found that a thymocyte-specific IL-7 transgene completely restored αβ T cells, γδ T cells and NKT cells development in the thymus when crossed onto an IL-7 null background (K7 mouse). Importantly, CD4+CD25+Foxp3+ Treg cell numbers were also restored in K7 thymuses. However, in the absence of peripheral in vivo IL-7, we found a dramatic decrease in LN T cell and specifically of FoxP3+ Treg cell numbers which was accompanied by a significant decrease in intracellular Foxp3 expression levels. These results document that IL-7 is a critical factor for peripheral Treg cell homeostasis, and also unveil a function of IL-7 in maintaining FoxP3 protein expression in Treg cells.
The role of intrathymic IL-7 is manifold. Among others, IL-7 signaling is required for the survival of immature DN thymocytes, opening up the TCRγ-chain locus during γδ T cell development, and also for lineage specification of CD8 cytolytic T cells (28–32). The significance of IL-7 in T cell biology is evident by the failure to generate meaningful T cell numbers in the absence of intrathymic IL-7 signaling. However, such an essential role seems not to include the generation of the FoxP3+ Treg cell subset (33). In fact, the role of IL-7 on intrathymic FoxP3+ Treg cell differentiation has been considered to be negligible or secondary to other γc-cytokines, and only to have any visible effects in context of an IL-2 deficiency (34). Moreover, IL-7’s role in the maintenance of peripheral FoxP3+ Treg cells has been proposed to be dispensable as suggested in the original report by de Latour (33) and recently again by Mazzucchelli et al. (25). According to these studies, Treg cell numbers and phenotypes were unaffected in IL-7-deficient mice compared to WT mice (25, 33), and acute treatment with neutralizing anti-IL-7Rα antibodies further suggested that peripheral Treg cells did not require IL-7R signaling for their accumulation and maintenance in vivo (20). On the other hand, there is also a concurrent view that proposes a vital role for IL-7 in Treg homeostasis over both intrinsic and extrinsic mechanisms. In the latter case, for example, immunoregulatory dendritic cells were found to express IL-7 and provide survival signals to prevent Treg cell apoptosis (35). In agreement to such notion, here we report that, at least in our hands, IL-7-deficient mice were severely lymphopenic not only for αβT cells but also for peripheral Fox3P3+ Treg cells. Furthermore, by assessing K7 mice, which are selectively deficient in peripheral IL-7, we demonstrate that in vivo IL-7 is indeed a critical factor for maintaining Treg cell numbers.
Importantly, and in contrast to other approaches, the K7 mouse system specifically targets IL-7 deficiency to peripheral cells, without affecting the development and thymic output of Treg cells. Thus, absent peripheral IL-7 in the presence of a normal thymic Treg cell output provides a clear cut answer for IL-7’s requirement specifically in peripheral Treg cell survival. Another important aspect of peripheral IL-7 biology in K7 mice was shown by its role in regulating FoxP3 levels. Previous studies have documented that attenuation of FoxP3 expression, such as a 5 to 10-fold reduction of normal FoxP3 protein levels, resulted in a progressive lymphoproliferative disease (36). Along this line, the significant reduction of FoxP3 but not of surface CD25 levels in K7 mouse Treg cells suggests a hitherto unacknowledged role of IL-7 in maintaining the functional fitness of the Treg cells. Initial attempts to address the functionality of K7 Treg cells failed to provide us with a conclusive answer, mainly due to limitations of the in vitro assays, but we are currently considering alternative ways to address this question in vivo. Since previous observations have already placed FoxP3 expression downstream of IL-2 and STAT5 (15), our current observations further establish a role of IL-7 as a homeostatic cytokine in maintaining FoxP3 expression in vivo. In this regard, regulation and fine tuning of FoxP3 levels by IL-7 and other γc-cytokines could be the molecular basis of the plasticity and promiscuity of FoxP3+ T cells as observed for both Treg cells and non-Treg CD4+ T cells (37).
While IL-7 is a critical cytokine for T cells, curiously enough, IL-7 is not produced by T cells themselves. The source of in vivo IL-7 has been elusive for a long time, but recently a flurry of reports has identified CD45neg thymic stromal cells and also a diverse population of other non-T cells in peripheral organs as the major producers of IL-7 (38). Interestingly, while IL-7 is a soluble factor, it has been proposed that IL-7 would be actually presented to T cells in trans by binding to the extracellular matrix of stromal cells or as a heterodimer with the hepatocyte growth factor-β (39–40). Whether such a trans-presentation is necessary for effective IL-7 signaling and whether transgenic IL-7 expressed in cis is sufficient to drive T cell development in the thymus was not known. The complete restoration of T cell development in K7 mice, however, strongly suggests that thymocyte-specific expression of IL-7 is sufficient for T cell development and differentiation in the thymus. Thus, IL-7 produced by thymocytes in cis can replace stromal cell derived IL-7 in all aspects of thymocyte development, including γδT cells, NKT cell and CD8SP thymocyte differentiation.
In addition to its role in T cell development, IL-7 is also critical for maintaining T cells. Such a notion has been first reported in the seminal studies of Lefrancois and colleagues and Surh and colleagues where adoptive T cell transfer into an IL-7-deficient environment revealed an essential and non-redundant role of IL-7 in T cell homeostasis (16–17). In these experiments, adoptively transferred T cells underwent lymphopenia-induced homeostatic proliferation in all γc-cytokine-deficient mice except for IL-7-deficient mice. Importantly, however, adoptive transfer experiments failed to clarify IL-7’s role under steady-state conditions and in the absence of lymphopenia-induced proliferation. Consequently, since then, a variety of different approaches have been employed to further assess this issue by testing acute and conditional depletion of IL-7 signaling in vivo (20–21). Nonetheless, a major problem in depleting IL-7 signaling using systemic anti-IL-7 or anti-IL-7Rα treatment is that it also neutralizes intrathymic IL-7 signaling. In such case, estimating peripheral T cell numbers must not only take account of peripheral IL-7 deficiency but also of impaired thymic output. For example, conditional deletion of IL-7Rα in T cells supported the notion that IL-7 signaling is critical for peripheral T cell survival (21). Accordingly, only after three days of acute IL-7Rα deletion, peripheral T cell numbers were already reduced to one third in vivo (21). A limitation in interpreting this study is the accumulation of T cells that have escaped IL-7Rα deletion and are IL-7 signaling competent. Moreover, without information on thymocyte development and thymic output, it is difficult to delineate the precise peripheral effect of IL-7R deletion from a systemic IL-7 signaling deficiency. In this regard, the K7 mouse is a powerful tool to assess IL-7’s role in peripheral T cell maintenance, and it was to our satisfaction that K7 mice were indeed severely impaired in maintaining the size of the peripheral T cell pool. More importantly, thymectomized K7 mice revealed that virtually all of the peripheral naïve T cells are recent thymic emigrants, whose total numbers were absolutely dependent on thymic output. This is in agreement with a recent assessment of T cell dynamics and turnover in vivo, which identified thymus output as the exclusive mechanism to supplement naïve T cells in adult mice (41). Thus, the dramatically reduced peripheral T cell numbers in K7 mice, and the even more severely reduced naïve T cell numbers in thymectomized K7 mice directly demonstrate the requirement of IL-7 in peripheral T cell survival under steady state conditions.
Collectively, these results provide direct in vivo evidence of peripheral IL-7 requirement for the survival and maintenance of T cells, and particularly of FoxP3+ Treg cells. These data also provide a clear answer to an IL-7 requirement for FoxP3+ cell survival in vivo for which adequate models have not been available. Whether peripheral IL-7 also plays additional roles beyond its involvement in maintaining T cell numbers by controlling primary or recall T cell immune responses or by affecting functions of other immune cell populations can be now tested using this new in vivo model of K7 mice.
Supplementary Material
Acknowledgments
We thank Drs. A. Singer and F. Van Laethem for insightful discussions and critical review of the manuscript. We also thank E. Kuznetsova for thymectomy, and S. Sharrow, A. Adams, and L. Granger for expert flow cytometry.
This study was supported by the Intramural Research Program of the US National Institutes of Health, the National Cancer Institute, and the Center for Cancer Research.
References
- 1.Fry TJ, Mackall CL. The many faces of IL-7: from lymphopoiesis to peripheral T cell maintenance. J Immunol. 2005;174:6571–6576. doi: 10.4049/jimmunol.174.11.6571. [DOI] [PubMed] [Google Scholar]
- 2.Rochman Y, Spolski R, Leonard WJ. New insights into the regulation of T cells by gamma(c) family cytokines. Nat Rev Immunol. 2009;9:480–490. doi: 10.1038/nri2580. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Jiang Q, Li WQ, Aiello FB, Mazzucchelli R, Asefa B, Khaled AR, Durum SK. Cell biology of IL-7, a key lymphotrophin. Cytokine Growth Factor Rev. 2005;16:513–533. doi: 10.1016/j.cytogfr.2005.05.004. [DOI] [PubMed] [Google Scholar]
- 4.Bennett CL, Christie J, Ramsdell F, Brunkow ME, Ferguson PJ, Whitesell L, Kelly TE, Saulsbury FT, Chance PF, Ochs HD. The immune dysregulation, polyendocrinopathy, enteropathy, X-linked syndrome (IPEX) is caused by mutations of FOXP3. Nat Genet. 2001;27:20–21. doi: 10.1038/83713. [DOI] [PubMed] [Google Scholar]
- 5.Brunkow ME, Jeffery EW, Hjerrild KA, Paeper B, Clark LB, Yasayko SA, Wilkinson JE, Galas D, Ziegler SF, Ramsdell F. Disruption of a new forkhead/winged-helix protein, scurfin, results in the fatal lymphoproliferative disorder of the scurfy mouse. Nat Genet. 2001;27:68–73. doi: 10.1038/83784. [DOI] [PubMed] [Google Scholar]
- 6.Chatila TA, Blaeser F, Ho N, Lederman HM, Voulgaropoulos C, Helms C, Bowcock AM. JM2, encoding a fork head-related protein, is mutated in X-linked autoimmunity-allergic disregulation syndrome. J Clin Invest. 2000;106:R75–81. doi: 10.1172/JCI11679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Wildin RS, Ramsdell F, Peake J, Faravelli F, Casanova JL, Buist N, Levy-Lahad E, Mazzella M, Goulet O, Perroni L, Bricarelli FD, Byrne G, McEuen M, Proll S, Appleby M, Brunkow ME. X-linked neonatal diabetes mellitus, enteropathy and endocrinopathy syndrome is the human equivalent of mouse scurfy. Nat Genet. 2001;27:18–20. doi: 10.1038/83707. [DOI] [PubMed] [Google Scholar]
- 8.Burchill MA, Yang J, Vogtenhuber C, Blazar BR, Farrar MA. IL-2 receptor beta-dependent STAT5 activation is required for the development of Foxp3+ regulatory T cells. J Immunol. 2007;178:280–290. doi: 10.4049/jimmunol.178.1.280. [DOI] [PubMed] [Google Scholar]
- 9.Yao Z, Kanno Y, Kerenyi M, Stephens G, Durant L, Watford WT, Laurence A, Robinson GW, Shevach EM, Moriggl R, Hennighausen L, Wu C, O’Shea JJ. Nonredundant roles for Stat5a/b in directly regulating Foxp3. Blood. 2007;109:4368–4375. doi: 10.1182/blood-2006-11-055756. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Chen Q, Kim YC, Laurence A, Punkosdy GA, Shevach EM. IL-2 controls the stability of Foxp3 expression in TGF-beta-induced Foxp3+ T cells in vivo. J Immunol. 2011;186:6329–6337. doi: 10.4049/jimmunol.1100061. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Bayer AL, Yu A, Malek TR. Function of the IL-2R for thymic and peripheral CD4+CD25+ Foxp3+ T regulatory cells. J Immunol. 2007;178:4062–4071. doi: 10.4049/jimmunol.178.7.4062. [DOI] [PubMed] [Google Scholar]
- 12.Fontenot JD, Rasmussen JP, Gavin MA, Rudensky AY. A function for interleukin 2 in Foxp3-expressing regulatory T cells. Nat Immunol. 2005;6:1142–1151. doi: 10.1038/ni1263. [DOI] [PubMed] [Google Scholar]
- 13.Liu W, Putnam AL, Xu-Yu Z, Szot GL, Lee MR, Zhu S, Gottlieb PA, Kapranov P, Gingeras TR, Fazekas de St Groth B, Clayberger C, Soper DM, Ziegler SF, Bluestone JA. CD127 expression inversely correlates with FoxP3 and suppressive function of human CD4+ T reg cells. J Exp Med. 2006;203:1701–1711. doi: 10.1084/jem.20060772. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Seddiki N, Santner-Nanan B, Martinson J, Zaunders J, Sasson S, Landay A, Solomon M, Selby W, Alexander SI, Nanan R, Kelleher A, Fazekas de St Groth B. Expression of interleukin (IL)-2 and IL-7 receptors discriminates between human regulatory and activated T cells. J Exp Med. 2006;203:1693–1700. doi: 10.1084/jem.20060468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Wuest TY, Willette-Brown J, Durum SK, Hurwitz AA. The influence of IL-2 family cytokines on activation and function of naturally occurring regulatory T cells. J Leukoc Biol. 2008;84:973–980. doi: 10.1189/jlb.1107778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Schluns KS, Kieper WC, Jameson SC, Lefrancois L. Interleukin-7 mediates the homeostasis of naive and memory CD8 T cells in vivo. Nat Immunol. 2000;1:426–432. doi: 10.1038/80868. [DOI] [PubMed] [Google Scholar]
- 17.Tan JT, Dudl E, LeRoy E, Murray R, Sprent J, Weinberg KI, Surh CD. IL-7 is critical for homeostatic proliferation and survival of naive T cells. Proc Natl Acad Sci U S A. 2001;98:8732–8737. doi: 10.1073/pnas.161126098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Cho BK, V, Rao P, Ge Q, Eisen HN, Chen J. Homeostasis-stimulated proliferation drives naive T cells to differentiate directly into memory T cells. J Exp Med. 2000;192:549–556. doi: 10.1084/jem.192.4.549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Sprent J, Surh CD. Normal T cell homeostasis: the conversion of naive cells into memory-phenotype cells. Nat Immunol. 2011;12:478–484. doi: 10.1038/ni.2018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Chougnet CA, Tripathi P, Lages CS, Raynor J, Sholl A, Fink P, Plas DR, Hildeman DA. A major role for Bim in regulatory T cell homeostasis. J Immunol. 2011;186:156–163. doi: 10.4049/jimmunol.1001505. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Jacobs SR, Michalek RD, Rathmell JC. IL-7 is essential for homeostatic control of T cell metabolism in vivo. J Immunol. 2010;184:3461–3469. doi: 10.4049/jimmunol.0902593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.El Kassar N, Lucas PJ, Klug DB, Zamisch M, Merchant M, Bare CV, Choudhury B, Sharrow SO, Richie E, Mackall CL, Gress RE. A dose effect of IL-7 on thymocyte development. Blood. 2004;104:1419–1427. doi: 10.1182/blood-2004-01-0201. [DOI] [PubMed] [Google Scholar]
- 23.von Freeden-Jeffry U, Vieira P, Lucian LA, McNeil T, Burdach SE, Murray R. Lymphopenia in interleukin (IL)-7 gene-deleted mice identifies IL-7 as a nonredundant cytokine. J Exp Med. 1995;181:1519–1526. doi: 10.1084/jem.181.4.1519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Thornton AM, Korty PE, Tran DQ, Wohlfert EA, Murray PE, Belkaid Y, Shevach EM. Expression of Helios, an Ikaros transcription factor family member, differentiates thymic-derived from peripherally induced Foxp3+ T regulatory cells. J Immunol. 2010;184:3433–3441. doi: 10.4049/jimmunol.0904028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Mazzucchelli R, Hixon JA, Spolski R, Chen X, Li WQ, Hall VL, Willette-Brown J, Hurwitz AA, Leonard WJ, Durum SK. Development of regulatory T cells requires IL-7Ralpha stimulation by IL-7 or TSLP. Blood. 2008;112:3283–3292. doi: 10.1182/blood-2008-02-137414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Park JH, Adoro S, Lucas PJ, Sarafova SD, Alag AS, Doan LL, Erman B, Liu X, Ellmeier W, Bosselut R, Feigenbaum L, Singer A. ‘Coreceptor tuning’: cytokine signals transcriptionally tailor CD8 coreceptor expression to the self-specificity of the TCR. Nat Immunol. 2007;8:1049–1059. doi: 10.1038/ni1512. [DOI] [PubMed] [Google Scholar]
- 27.Park JH, Yu Q, Erman B, Appelbaum JS, Montoya-Durango D, Grimes HL, Singer A. Suppression of IL7Ralpha transcription by IL-7 and other prosurvival cytokines: a novel mechanism for maximizing IL-7-dependent T cell survival. Immunity. 2004;21:289–302. doi: 10.1016/j.immuni.2004.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Bhatia SK, Tygrett LT, Grabstein KH, Waldschmidt TJ. The effect of in vivo IL-7 deprivation on T cell maturation. J Exp Med. 1995;181:1399–1409. doi: 10.1084/jem.181.4.1399. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Durum SK, Candeias S, Nakajima H, Leonard WJ, Baird AM, Berg LJ, Muegge K. Interleukin 7 receptor control of T cell receptor gamma gene rearrangement: role of receptor-associated chains and locus accessibility. J Exp Med. 1998;188:2233–2241. doi: 10.1084/jem.188.12.2233. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Grabstein KH, Waldschmidt TJ, Finkelman FD, Hess BW, Alpert AR, Boiani NE, Namen AE, Morrissey PJ. Inhibition of murine B and T lymphopoiesis in vivo by an anti-interleukin 7 monoclonal antibody. J Exp Med. 1993;178:257–264. doi: 10.1084/jem.178.1.257. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Park JH, Adoro S, Guinter T, Erman B, Alag AS, Catalfamo M, Kimura MY, Cui Y, Lucas PJ, Gress RE, Kubo M, Hennighausen L, Feigenbaum L, Singer A. Signaling by intrathymic cytokines, not T cell antigen receptors, specifies CD8 lineage choice and promotes the differentiation of cytotoxic-lineage T cells. Nat Immunol. 2010;11:257–264. doi: 10.1038/ni.1840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Ye SK, Maki K, Kitamura T, Sunaga S, Akashi K, Domen J, Weissman IL, Honjo T, Ikuta K. Induction of germline transcription in the TCRgamma locus by Stat5: implications for accessibility control by the IL-7 receptor. Immunity. 1999;11:213–223. doi: 10.1016/s1074-7613(00)80096-5. [DOI] [PubMed] [Google Scholar]
- 33.Peffault de Latour R, Dujardin HC, Mishellany F, Burlen-Defranoux O, Zuber J, Marques R, Di Santo J, Cumano A, Vieira P, Bandeira A. Ontogeny, function, and peripheral homeostasis of regulatory T cells in the absence of interleukin-7. Blood. 2006;108:2300–2306. doi: 10.1182/blood-2006-04-017947. [DOI] [PubMed] [Google Scholar]
- 34.Bayer AL, Lee JY, de la Barrera A, Surh CD, Malek TR. A function for IL-7R for CD4+CD25+Foxp3+ T regulatory cells. J Immunol. 2008;181:225–234. doi: 10.4049/jimmunol.181.1.225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Harnaha J, Machen J, Wright M, Lakomy R, Styche A, Trucco M, Makaroun S, Giannoukakis N. Interleukin-7 is a survival factor for CD4+ CD25+ T-cells and is expressed by diabetes-suppressive dendritic cells. Diabetes. 2006;55:158–170. [PubMed] [Google Scholar]
- 36.Wan YY, Flavell RA. Regulatory T-cell functions are subverted and converted owing to attenuated Foxp3 expression. Nature. 2007;445:766–770. doi: 10.1038/nature05479. [DOI] [PubMed] [Google Scholar]
- 37.Miyao T, Floess S, Setoguchi R, Luche H, Fehling HJ, Waldmann H, Huehn J, Hori S. Plasticity of foxp3(+) T cells reflects promiscuous foxp3 expression in conventional T cells but not reprogramming of regulatory T cells. Immunity. 2012;36:262–275. doi: 10.1016/j.immuni.2011.12.012. [DOI] [PubMed] [Google Scholar]
- 38.Kim GY, Hong C, Park JH. Seeing is believing: illuminating the source of in vivo interleukin-7. Immune Netw. 2011;11:1–10. doi: 10.4110/in.2011.11.1.1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Banwell CM, Partington KM, Jenkinson EJ, Anderson G. Studies on the role of IL-7 presentation by mesenchymal fibroblasts during early thymocyte development. Eur J Immunol. 2000;30:2125–2129. doi: 10.1002/1521-4141(2000)30:8<2125::AID-IMMU2125>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 40.Lai L, Goldschneider I. Cutting edge: Identification of a hybrid cytokine consisting of IL-7 and the beta-chain of the hepatocyte growth factor/scatter factor. J Immunol. 2001;167:3550–3554. doi: 10.4049/jimmunol.167.7.3550. [DOI] [PubMed] [Google Scholar]
- 41.den Braber I, Mugwagwa T, Vrisekoop N, Westera L, Mogling R, de Boer AB, Willems N, Schrijver EHR, Spierenburg G, Gaiser K, Mul E, Otto SA, Ruiter AFC, Ackermans MT, Miedema F, Borghans JAM, de Boer RJ, Tesselaar K. Maintenance of Peripheral Naive T Cells Is Sustained by Thymus Output in Mice but Not Humans. Immunity. 2012;36:288–297. doi: 10.1016/j.immuni.2012.02.006. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.