Abstract
Gaucher disease is a systemic lysosomal storage disorder with a high prevalence among Ashkenazi Jews. It is caused by an inherited deficiency of the lysosomal enzyme glucocerebrosidase. Common signs and symptoms include hepatosplenomegaly, anemia, thrombocytopenia, and skeletal involvement. Oral and dental manifestations are less commonly seen. These manifestations are often asymptomatic, although they may be detected by routine dental x-rays. There are several case reports and a few larger series published describing patients with Gaucher disease who have mandibulo-maxillofacial involvement. This review aims to examine the oral manifestations observed in Gaucher disease and to suggest practical guidelines for dealing with these often worrisome signs. Among the critical issues are the benign nature of Gaucher cell infiltration of the mandible and the critical importance of being prepared for post-procedure bleeding and/or infections. Therefore, it is essential that dental practitioners be aware of the possible oral and dental complications of Gaucher disease, as well as the available treatment modalities.
Keywords: Gaucher disease, Gaucher cells, glucocerebroside, mandible, maxilla, enzyme replacement therapy
Introduction
Gaucher disease, the most common lysosomal storage disorder due to deficiency of the enzyme glucocerebrosidase, results in glucocerebroside accumulation in the cells of monocyte-macrophage system. These abnormally enlarged macrophages, “Gaucher cells”, have a cytoplasm with an engorged, wrinkled tissue paper appearance and displaced round nuclei (Zimran and Elstein, 2010). Many of the disease manifestations result from infiltration of Gaucher cells into organs of the reticulo-endothelial system, particularly the spleen, liver, and bone marrow.
Gaucher disease is inherited as an autosomal recessive disorder. While panethnic, it is most common among the Ashkenazi Jewish population, with a carrier rate of 1:17 and an expected birth frequency of 1:850 (Beutler et al, 1993). Two distinct forms of Gaucher disease are also relatively more common in the Norrbottnian region of Northern Sweden (Svennerholm et al, 1991) and near the Palestinian town of Jenin (Abrahamov et al, 1995). In the general population, the estimated frequency is in the range of 1:50,000 to 1:100,000 (Meikle et al, 1999). Among Ashkenazi Jews, the N370S mutation, which is associated with milder expression of the disease (Zimran and Elstein, 2010) is most common.
Three major types of Gaucher disease are differentiated clinically, based on absence, type 1, or presence and rate of progression of neurological features, types 2 and 3 (Zimran and Elstein, 2010). Key clinical, genetic, and demographic features are summarized in Table 1. While it has been suggested that the varied symptoms fall into a phenotypic continuum (Sidransky, 2004), it is often useful to think of Gaucher disease as three distinct forms to facilitate genetic counseling and management decisions.
Table 1.
Key features of the three types of Gaucher disease (table adapted from Zimran and Elstein, 2010)
| TYPE 1 | TYPE 2 (lethal) | TYPE 3 | |||||
|---|---|---|---|---|---|---|---|
| Sub-type | asymptomatic | symptomatic | neonatal | infantile | 3a | 3b | 3c |
| Common genotype | N370S/N370S or 2 mild mutations | N370S/other or 2 mild mutations | 2 null or recombinant mutations | 1 null and 1 severe mutation | none | L444P/L444P or many others | D409H/D409H |
| Ethnic predilection | Ashkenazi Jews | Ashkenazi Jews | none | none | none | panethnic | Palestinian Arabs, Japanese, Spanish |
| Common presenting features | none | hepato-splenomegaly; hypersplenism; bleeding; bone pains | hydrops fetalis; congenital ichthyosis | abnormal horizontal eye movements; strabismus; opsithotonus; trismus | abnormal horizontal eye movements; myoclonic seizures | abnormal horizontal eye movements; hepatospleno- megaly; growth retardation | abnormal horizontal eye movements; calcification of cardiac valves |
| CNS involvement | rare; patients may develop Parkinson disease |
rare; patients may develop Parkinson disease |
lethal | severe | abnormal horizontal eye movements ; slowly progressive neurologic deterioration | abnormal horizontal eye movements ; gradual cognitive deterioration | abnormal horizontal eye movements brachycephalus |
| Bone involvement | none | variable | not generally documented | not generally documented | mild | moderate to severe; kyphosis (gibbus) | minimal |
There is enormous variability in disease severity of all types of Gaucher disease. Type 1 disease may be asymptomatic, discovered in the course of a population survey (e.g., among Ashkenazi Jews) or incidentally during evaluation of an unrelated hematological disorder. In symptomatic patients, the spleen may be barely palpable or massively enlarged and may produce positional symptoms, such as early satiety or abdominal discomfort. Abdominal pain is rare, and when present may be an indication of a splenic infarction or a sub-capsular hematoma. Hepatomegaly is usually asymptomatic but, in very severe cases (e.g. splenectomized patients), can be associated with liver fibrosis and/or cirrhosis with or without portal hypertension. However, for most patients, liver function tests are within normal limits, unless there is a hepatic co-morbidity such as viral or auto-immune hepatitis.
Bleeding is a common symptom and can manifest as frequent epistaxis, easy bruising, hemorrhaging after surgical/or dental procedures or can be associated with pregnancy or delivery. Bleeding is usually secondary to thrombocytopenia resulting from hypersplenism and/or reduced platelet production, which can be caused by infiltration of the bone marrow by Gaucher cells. Abnormal platelet function (Gillis et al, 1999) or abnormal coagulation and/or fibrinolytic factors (Hollack et al, 1997) are also observed. Fatigue is another common complaint, usually related to anemia, but not invariably. Reduced hemoglobin levels are also primarily due to hypersplenism, but additional causes include iron deficiency (also secondary to bleeding), vitamin B12 deficiency, and autoimmune hemolysis. In children, linear growth retardation is common, regardless of overall disease severity.
An increased tendency for infections occurs in rare cases, typically among more severely affected or splenectomized patients. Severe pulmonary disease with cyanosis and clubbing occurs in some patients with advanced liver involvement. Direct involvement of the lungs with Gaucher cells has also been observed. Mild pulmonary hypertension as documented by Doppler echocardiography is also observed in some patients (Mistry et al, 2002), especially those who have undergone splenectomy. Pulmonary function tests may show some abnormalities, such as reduced diffusion capacity (Kerem et al, 1996).
The gold standard for the diagnosis of Gaucher disease is the detection of low levels of enzyme activity in peripheral blood cells compared to normal controls samples drawn on the same day. Molecular analyses of genotype, at times, serve as an adjunct in assessing the potential trajectory of disease severity (Zimran, 2011).
There is no cure for Gaucher disease; since its approval by the US Food and Drug Administration (FDA) in 1991 (Barton et al, 1991; Grabowski et al, 1995; Zimran et al, 1995) intravenous infusions of enzyme replacement therapy (ERT) are widely used to treat symptomatic patients. In most patients ERT induces reduction of hepatosplenomegaly, ameliorates hematological abnormalities, and improves bone pain and bone mineral density within 5 years of advent of therapy (Weinreb et al, 2002). Today, there are two FDA approved ERTs that are also widely approved in Europe and other countries: imiglucerase (Cerezyme, Genzyme Corporation, Cambridge MA, USA) and velaglucerase alfa (Vpriv, Shire HGT, Cambridge MA, USA).
Gaucher cell infiltration in the long bones
Involvement of the bones is a common feature of symptomatic Gaucher disease. It is often the most disabling aspect of the disease, placing a heavy burden on quality of life by causing pain, restricting mobility, and interfering with the activities of daily living (Pastores et al, 2000; Stowens et al, 1985). In some surveys, approximately 75% of patients with type 1 Gaucher disease report that they have some degree of bone involvement (Germain, 2004). With recent advances in diagnostic and imaging modalities, it has been observed that 90% of patients with type 1 (Walton-Bowen et al, 2000) or type 3 (chronic neuronopathic) Gaucher disease (Mikosch et al, 2010) have at least one clinical or radiologic manifestation attributable to skeletal involvement, with about 20% exhibiting impaired mobility (Wenstrup et al, 2002). It is important to note that ERT cannot reverse necrotic and lytic changes, and therefore, orthopedic solutions, especially timely joint (hip, shoulder, knee, ankle) replacements, are recommended (Itzchaki et al, 2004).
Skeletal manifestations of clinical relevance include bony infarcts, lytic lesions, cortical thinning, loss of trabecular structure, fractures due to osteopenia, avascular necrosis of both large and small joints, and in some children, height delay (Elstein et al, 1997). The exact mechanisms by which Gaucher cells infiltrate the bone marrow and disrupt the bony architecture, as well as how ERT may impact the bone (Rudzki et al, 2003), are areas of intense interest and research (Goker-Alpan, 2011).
The Mandible in Gaucher disease
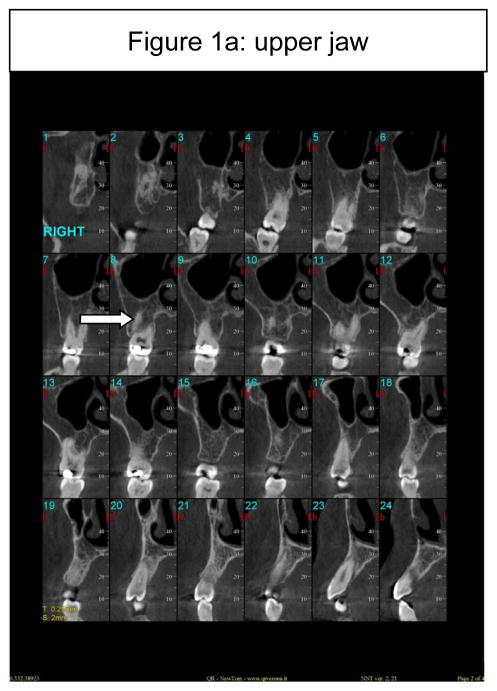
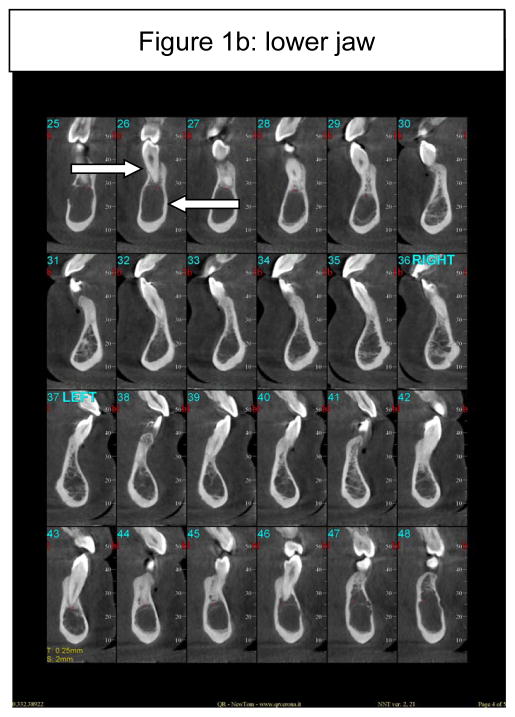
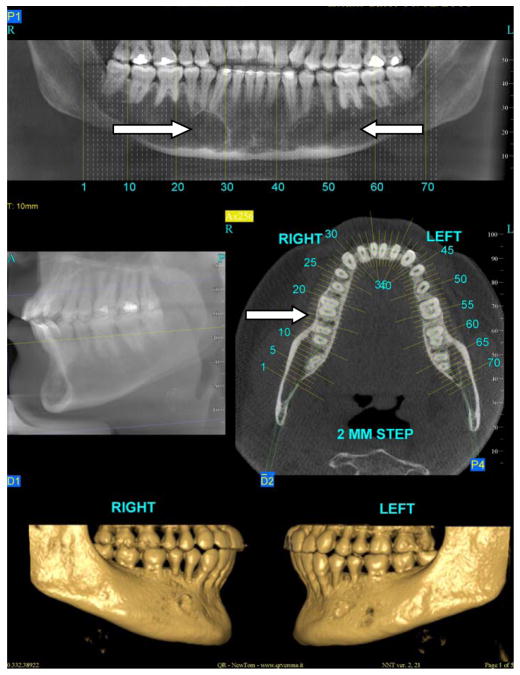
All of the long bones can be considered as potential reservoirs for Gaucher cell infiltration and indeed, virtually all long bones are at risk in Gaucher disease (Wenstrup et al, 2002). The mandible too is a long bone, and unlike the maxilla (Figure 1a), may be a target of bony involvement (Figure 1b). About 100 cases have been published describing patients presenting with some degree of mandibulo-maxillofacial involvement, often noted incidentally on routine dental or panoramic x-rays (Carter et al, 1998). The most common radiographic observation in an affected mandible is the presence of pseudo-cystic or honeycombed radiolucent lesions mainly in the premolar-molar regions (Bender, 1938; Moch, 1953; Spiegel, 1957; Bender, 1959; Weigler et al, 1967; Michanowicz et al, 1967; Bildman et al, 1972; Heasman, 1991; Lustmann et al, 1991; Horwitz et al, 2007). Increased osteopenia and a loss of trabecular architecture within the radiolucent areas are commonly observed. Other radiographic findings include generalized osteoporosis, enlargement and widening of bone marrow spaces, endosteal scalloping, and in some cases, apical root resorption, all putatively due to the density of Gaucher cells in the apical regions (Bender, 1938; Bender, 1959; Bender et al, 1996; Carter et al, 1998). The cortical bone, however, remains intact (Figure 2c). The areas of mandibular radiopacity have also been conjectured to be due to temporary bone regeneration after extraction (Bender, 1959) or an osteosclerotic reaction to the Gaucher cell infiltration (Heasman, 1991). In practical terms, however, it is critically important to appreciate the fact that these cyst-like areas that may completely fill the jaw on x-ray are not ‘empty’ or lacking bone to anchor the teeth. Even when there is evidence of root resorption, the process can be relatively benign and can reverse spontaneously. It is most likely that these cyst-like space-occupying lesions are Gaucher cells that act like ‘petrified’ Gaucher bone. The lower jaw has normal alveolar bone, but below, there seems to be an area of demarcation where the teeth end, the cortical bone appears not to encompass any normal trabecular bone (Figure 1b). This can be appreciated in the panoramic-style view of the mandible (Figure 2, panel a) where the area of translucencies are overt bilaterally. However, one can see bone anchoring the teeth (Figure 2, panel b) and the presence of unaffected cortical bone (Figure 2, panel c). This description of loss of normal trabecularization is comparable to what is seen in other long bones in Gaucher disease. Since the teeth remain anchored, this putative root resorption should be not be treated only on the basis of the apparent areas of translucencies. Biopsies to ascertain the nature of the bony matrix in the mandible, as well as bone grafting or dental extractions because of concern about the abnormal x-ray appearance of the mandibular bony matrix, should be tempered by a second evaluation of the clinical diagnosis in the context of overwhelming Gaucher cell infiltration.
Figure 1.
Figure 1a: Serial presentation of CT findings of the upper jaw and teeth, showing normal appearance of alveolar and cancellous bone (arrow).
Figure 1b: Serial presentation of CT findings of the lower jaw and teeth, showing normal appearance of alveolar bone (upper arrow) but no trabecular bone although the cortical bone is intact (lower arrow); There appears to be a demarcation line where the teeth end and below which the bone is ‘hollow’. There is apparent root resorption on the lower anterior teeth and blunting of the roots on the lower first molars bilaterally.
Figure 2.
panel a: Large areas of translucency in mandible bilaterally (arrows) probably due to Gaucher cell infiltration with cortical bone preserved; panel b right side: horizontal slice showing preservation of bone between teeth (arrow); panel c: view of the right and left lower jaw showing normal cortical bone.
Bildman et al (1972) reported establishing diagnosis of Gaucher disease following a biopsy of mandibular marrow tissue; others have confirmed Gaucher disease based on the presence of Gaucher cells in biopsies from the mandible (Shira et al, 1953; Moch, 1953; Weigler et al, 1967; Sela et al, 1972; Hall et al, 1985; Lustman et al, 1991). However, it should be emphasized that biopsy of the mandible in Gaucher disease is not recommended, unless another condition (e.g., malignancy) is suspected. If Gaucher disease is suspected, an enzyme assay and not a biopsy or a bone marrow sampling is the appropriate means to make the diagnosis.
Dental extractions and other dental surgery
It was hypothesized that debridement of the lesions, and extraction of the teeth would ameliorate the translucent areas in the jaw because of post-manipulation bone regeneration (Moch, 1953; Michanowicz et al, 1967; Hall et al, 1985). This is true in patients with Gaucher disease, as in otherwise healthy individuals, but the existence of Gaucher disease per se is not a reason for curettage or primary extractions. In most cases reported in the literature, post-operative healing was normal without hemorrhagic complications (Weigler et al, 1967; Michanowicz et al, 1967; Bildman et al, 1972; Regenye et al, 1992; Bender et al, 1996; Browne, 1997; Horwitz et al, 2007). Nonetheless, prophylaxis against a bleeding diathesis is recommended even in patients on ERT (see below) in cases of extractions, dental surgery, or dental prophylaxis that may be extensive. Healing of the extraction site or periodontal tissue may be transient, as lesions may re-appear (Bender et al, 1996).
Table 2 summarizes many of the cases reported in the literature. One riveting report describes the 60-year follow-up of a single patient who also eventually was treated with ERT when it became available (Bender, 1938; Bender, 1959; Bender et al, 1996). Importantly, despite the decades-long history of Gaucher cell impaction in the jaw, the patient’s dental history was marked by multiple extractions. Thus unless there is an associated condition that requires dental extractions, the Gaucher cell infiltration is not an ample justification to extract teeth. Moreover, although in the past, the option of dental implants to replace the missing and extracted teeth was not recommended because of what was considered to be the poor quality of the mandibular bony milieu (Horwitz et al, 2007), this hypothesis may not be valid. In the past, hip, shoulder, knee or ankle replacements were also not offered to patients with Gaucher disease because of the same concerns. However, despite massive Gaucher cell infiltration in the hip, shoulder, knee, or ankle, surgical prostheses are well-tolerated and well-incorporated (Lebel et al, 2001; Lebel et al, 2011). Thus, the issue of implants in Gaucher disease merits further consideration and prospective study.
Table 2.
Summary of published reports of dental manifestations of Gaucher disease
| Author | Age /Sex | Findings | Treatment | SPX | Site | ||||
|---|---|---|---|---|---|---|---|---|---|
| Bender, 1938 | 13/F | Cyst-like radiolucency & loss of trabecular structure in premolar & molar regions; generalized porosity in man region | Extraction of lower 1st molars | yes | man & max | ||||
| Bender, 1959 follow up | Distinct radiolucent areas; further loss of trabecular structure; endosteal bone degeneration & thickness reduction; episodes of spontaneous hemorrhage in region of hard palate | see above | |||||||
| Bender et al, 1996 follow up | Marked improvement in radiolucency with re-formation of trabeculae | Uncomplicated extraction of posterior teeth; ERT (1991) subsequently | |||||||
| Moch et al, 1953 | 39/F | Pseudo-cystic radiolucent areas on both sides of man; alveolar abscess of upper right 2nd premolar | Extraction of abscessed tooth & curettage of peri-apical area (6000 units penicillin qd + 500cc whole blood) | no | man | ||||
| Spiegel 1957 | 19/F | Radiolucency & loss of trabecular structure in premolar & molar region; osteolysis in left premolar region of max; prolonged bleeding after minor surgery | Removal of tissue flap over lower right 3rd molar | no | man & max | ||||
| Michan-owicz et al, 1967 | 21/M | Small & large carious lesions in many of man & max teeth; radiolucency in right 3rd molar and 1st first molar; generalized vacuolation & osteoporosis in man | Extraction of man right 3rd molar, left 1st molar, & max left 2nd molar | yes | man | ||||
| Weigler et al, 1967 | 28/M | Large radiolucent region from 1st premolar to 3rd molar; widening of marrow spaces; loss of lamina dura at root of lower left 2nd premolar & 1st molar | Extraction & currettage of lower left 1st molar | no | man | ||||
| Bildman 1972 | 16/F | Radiolucent areas in body of man | Extraction of upper and lower right molars | no | man | ||||
| Hall et al, 1985 | 47/M | (Gaucher) bony lesion in left man with infarction & secondary infection | Debridement of left man body & ramus; extraction of man 2nd premolar & 1st + 2nd molar; IV & oral penicillin | yes | man | ||||
| Heas-man, 1991 | 40/F | Hemorrhagic lesions in face & lips; areas of radiopacity in premolar & molar regions of man; generalized, edematous gingivitis | yes | man | |||||
| Kara-bulut et al, 1997 | 14/F | Diffuse osteopenia; trabecular loss; coarsening of man & max; opacification of sphenoid & max sinuses due to expansion of medullary spaces | yes | man & max & sphen-oid bones | |||||
| Horwitz et al, 2007 | 47/F | Bilateral cyst-like lesions; severe root resorption in premolar-molar region; enlargement of bone marrow space; loss of trabecular structure; effacement of cortical borders of man canal | Scaling; root planing; access flap surgery; extraction of 3 teeth (2,15,16) | no? | man | ||||
SPX = splenectomy ; man = mandible; max = maxilla; ERT = enzyme replacement therapy; IV = intravenous
The Maxillary Bone in Gaucher disease
Because it is not a long bone, the maxilla is far less often affected in Gaucher disease. In the maxilla (Figure 1a) both the alveolar bone surrounding the tooth and the cancellous bone have a normal appearance. There have been four case reports of maxillary involvement, generally with lesions in the canine-premolar area (Bender, 1938; Spiegel, 1957; Lustman et al, 1991; Karabulut et al, 1997). Opacification of one or more of the maxillary antrums was noted, as well as the presence of Gaucher cells.
Eruption of Teeth
Carter et al (1998) documented a strong correlation between delayed permanent dentition and the presence of mild to moderate bone involvement in younger patients. The delayed eruption of the teeth in virtually all children with Gaucher disease, and the subsequent delayed eruption of the permanent dentition, is apparently parallel to the delayed achievement of peak bone mass, and often, of full adult height in some patients with Gaucher disease. Catch-up growth, both of height and peak bone mineral density, occurs even without benefit of ERT (Dweck et al, 2002), although ERT is credited with reducing the gap earlier (Kaplan et al, 1996).
Oral features
Typically none of the soft tissues in the mouth are sites of Gaucher cell infiltration, and hence are rarely involved except consequent to some other ailment. An example is the putative risk for amyloidosis in Gaucher disease (only five cases have been described in the literature) which is accompanied by oral manifestations (Elstein et al, 2003).
It has been reported that salivary output in patients with Gaucher disease is decreased relative to healthy controls (Dayan et al, 2003). There is currently no explanation for this finding, and since it is not improved in patients receiving ERT, it remains a conundrum.
Browne (1997) reported a case of involvement of the mandible by Gaucher cells which was also marked by a well-defined area of pigmentation on the internal surface of the buccal mucosa on the right side of the mouth only, in addition to a generalized pigmentation of the face, lips, neck and hands. Although facial discoloration, especially around the mouth, has been reported (Zimran, 2010), in general oral skin pigmentation is rare in Gaucher disease. Petechia have also been reported (Horwitz et al, 2007), but these may be related to a bleeding tendency.
Lymph gland involvement in patients with Gaucher disease, including the submandibular lymph nodes, is rare: lymph gland enlargement is generally due to a secondary process such as transient infection or inflammation. However, enlarged lymph glands at some sites, such as retroperitoneal, are usually densely infiltrated by Gaucher cells, yet they do not seem to be affected by ERT (Hadas-Halpern et al, 2010). There have been reports of aggressive lymphomas involving the parotid gland in two patients (Shvidel et al, 2007) as well as an adenoma of the parotid gland in a single patient (personal observation).
Bleeding tendency
The most important ramification of Gaucher disease in the oral cavity is a tendency to bleeding (Zimran, 2010). The bleeding tendency observed commonly in patients with Gaucher disease can occur as a result of thrombocytopenia because of hypersplenism, alterations in the coagulation cascade (Hollak et al, 1997), or abnormal platelet function (Gillis et al, 1999). Several clotting factor deficiencies have also been described, including factors II, V, IX, X, XI (Horwitz et al, 2007; Hollak et al, 1997; Fischman et al, 2003), but these are co-morbidities that are not directly related to Gaucher disease.
ERT will increase platelet counts and often will improve platelet function abnormalities, so that the tendency to excessive bleeding will be minimized in these patients. Nonetheless, a dentist should be prepared for the possibility of excessive hemorrhage when performing dental procedures that cause bleeding, such as extractions, curettage, and surgeries (Carter et al, 1998; Fischman et al, 2003). In a recent study, Givol et al (2011) noted that the severity of thrombocytopenia did not predict the risk of bleeding; therefore, evaluation of coagulation deficiencies and impaired platelet aggregation is indicated before commencing dental procedures. If necessary, appropriate hematological replacement therapy prior to the procedure including the administration of anti-fibrinolytics (e.g., hexacapron) and/or platelet transfusion after the procedure in high risk patients (Givol et al, 2011) is advisable. Using sutures is also often prudent in these patients.
Infections
There is an increased risk of post-operative oral infections in patients (Moch, 1953; Schubiner et al, 1981), which is an expression of an overall greater risk for infections in Gaucher disease (Aker et al, 1993), especially in patients not treated with ERT (Zimran et al, 1993). This is further enhanced in subjects who have undergone a splenectomy, for whom the usual pre-surgery precautions should be taken. In all cases, it is prudent to ascertain whether antibiotic prophylaxis is necessary for other indications. There have also been reports of early-onset periodontitis in patients with Gaucher disease (Horwitz et al, 2007) which can be effectively managed by proper oral hygiene instructions, scaling and root planing as in other patients. It has also been suggested that increased periodontal disease per se may be a co-morbidity that results from lack of bone density especially in women (Renvert et al, 2011). Although conflicting literature exists (Wactawski-Wende et al, 1996), increased progression of inflammatory destruction is seen in less dense or poorly trabeculated bone. This situation is often ameliorated when bisphosphonates are used (Shinoda et al, 2008).
Bisphosphonate therapy
Various bisphosphonates have been used by patients with Gaucher disease as monotherapy for osteopenia/osteonecrosis or as an adjunct to ERT (Cox et al, 2008). As with other persons on long-term bisphosphonate therapy for non-cancer reasons, the risk of osteonecrosis of the jaw is low (Brock et al, 2011), but carefully following the length of bisphosphonate use s and heightened awareness of this possible complication are recommended.
Oral hygiene
Based on the experience in a large clinic (Fischman et al, 2003) it appears that overall dental-oral health among patients with Gaucher disease is unaffected. In fact, they reported that patients had fewer dental caries and better dental health than obligate carriers of Gaucher disease (based on the Decayed, Missing, and Filled Surfaces index). This unexpected observation may be due to greater awareness of the potential for infections among patients with Gaucher disease and/or better oral hygiene.
Conclusion
In conclusion, we have enumerated some general recommendations for the dentist when evaluating patients with Gaucher disease (Table 3). Although dental involvement is a less common manifestation of Gaucher disease, it is nonetheless imperative for dental practitioners to be aware of this disease, and to be familiar with the possible oral and dental complications that could develop. Many of these recommendations relate to the pathophysiology and progression of Gaucher disease For example, skeletal features of Gaucher disease can be progressive and/or irreversible, even with appropriate treatment with ERT and this may apply to mandibular or maxillary involvement as well. Associated hematologic or coagulation abnormalities may complicate the dental management of patients with Gaucher disease. Appropriate hematologic replacement therapy must always be considered prior to procedures. After dental procedures, comprehensive follow-up sessions and close monitoring for complications are essential for optimal post-operative recovery. Moreover, the importance of proper oral hygiene to maintain good oral health and appropriate periodic evaluations cannot be over-emphasized in patients with Gaucher disease, regardless of the degree of severity or the ongoing disease-specific medical management.
Table 3.
Important consideration in the management of oral and dental manifestation in patients with Gaucher Disease
|
Acknowledgments
This work was supported by the Intramural Research Programs of the National Human Genome Research Institute and the National Institutes of Health. We acknowledge the helpful suggestions of Dr. Grisel Lopez and Dr. Nahid Tayebi. The clinical experience of Dr. Alan Elstein (DDS) is gratefully acknowledged.
References
- Abrahamov A, Elstein D, Gross-Tsur V, et al. Gaucher’s disease variant characterized by progressive calcification of heart valves and unique genotype. Lancet. 1995;346:1000–1003. doi: 10.1016/s0140-6736(95)91688-1. [DOI] [PubMed] [Google Scholar]
- Aker M, Zimran A, Abrahamov A, Horowitz M, Matzner Y. Abnormal neutrophil chemotaxis in Gaucher disease. Br J Haematol. 1993;83:187–191. doi: 10.1111/j.1365-2141.1993.tb08270.x. [DOI] [PubMed] [Google Scholar]
- Barton NW, Brady RO, Dambrosia JM, et al. Replacement therapy for inherited enzyme deficiency--macrophage-targeted glucocerebrosidase for Gaucher’s disease. N Engl J Med. 1991;324:1464–1470. doi: 10.1056/NEJM199105233242104. [DOI] [PubMed] [Google Scholar]
- Bender IB. Dental Observations in Gaucher’s Disease. J Dent Res. 1938;17:359. [Google Scholar]
- Bender IB. Dental observations in Gaucher’s disease; a twenty-year follow-up. Oral Surg Oral Med Oral Pathol. 1959;12:546–561. doi: 10.1016/0030-4220(59)90157-4. [DOI] [PubMed] [Google Scholar]
- Bender IB, Bender AL. Dental observations in Gaucher’s disease: review of the literature and two case reports with 13- and 60-year follow-ups. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1996;82:650–659. doi: 10.1016/s1079-2104(96)80440-9. [DOI] [PubMed] [Google Scholar]
- Beutler E, Nguyen NJ, Henneberger MW, et al. Gaucher disease: Gene frequencies in the Ashkenazi Jewish population. Am J Hum Genet. 1993;52:85–91. [PMC free article] [PubMed] [Google Scholar]
- Bildman B, Martinez M, Jr, Robinson LH. Gaucher’s disease discovered by mandibular biopsy: report of case. J Oral Surg. 1972;30:510–512. [PubMed] [Google Scholar]
- Brock G, Barker K, Butterworth CJ, Rogers S. Practical considerations for treatment of patients taking bisphosphonate medications: an update. Dent Update. 2011;38:313–324. doi: 10.12968/denu.2011.38.5.313. [DOI] [PubMed] [Google Scholar]
- Browne WG. Oral pigmentation and root resorption in Gaucher’s disease. J Oral Surg. 1977;35:153–155. [PubMed] [Google Scholar]
- Carter LC, Fischman SL, Mann J, et al. The nature and extent of jaw involvement in Gaucher disease: observations in a series of 28 patients. Oral Surg Oral Med Oral Pathol Oral Radiol Endod. 1998;85:233–239. doi: 10.1016/s1079-2104(98)90432-2. [DOI] [PubMed] [Google Scholar]
- Cox TM, Aerts JM, Belmatoug N, et al. Management of non-neuronopathic Gaucher disease with special reference to pregnancy, splenectomy, bisphosphonate therapy, use of biomarkers and bone disease monitoring. J Inherit Metab Dis. 2008;31:319–336. doi: 10.1007/s10545-008-0779-z. [DOI] [PubMed] [Google Scholar]
- Dayan B, Elstein D, Zimran A, Nesher G. Decreased salivary output in patients with Gaucher disease. QJM. 2003;96(1):53–56. doi: 10.1093/qjmed/hcg006. [DOI] [PubMed] [Google Scholar]
- Dweck A, Abrahamov A, Hadas-Halpern I, Bdolach-Avram T, Zimran A, Elstein D. Type I Gaucher disease in children with and without enzyme therapy. Pediatr Hematol Oncol. 2002;19:389–397. doi: 10.1080/08880010290097143. [DOI] [PubMed] [Google Scholar]
- Elstein D, Itzchaki M, Mankin HJ. Skeletal involvement in Gaucher’s disease. Baillieres Clin Haematol. 1997;10:793–816. doi: 10.1016/s0950-3536(97)80041-8. [DOI] [PubMed] [Google Scholar]
- Elstein D, Rosenmann E, Reinus C, Paz J, Altarescu G, Zimran A. Amyloidosis and gastric bleeding in a patient with Gaucher disease. J Clin Gastroenterol. 2003;37(3):234–237. doi: 10.1097/00004836-200309000-00009. [DOI] [PubMed] [Google Scholar]
- Fischman SL, Elstein D, Sgan-Cohen H, Mann J, Zimran A. Dental profile of patients with Gaucher disease. BMC Oral Health. 2003;23:3–4. doi: 10.1186/1472-6831-3-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Germain DP. Gaucher.s disease: a paradigm for interventional genetics. Clin Genet. 2004;65:77–86. doi: 10.1111/j.0009-9163.2004.00217.x. [DOI] [PubMed] [Google Scholar]
- Gillis S, Hyam E, Abrahamov A, Elstein D, Zimran A. Platelet function abnormalities in Gaucher disease patients. Amer J Hematol. 1999;61:103–106. doi: 10.1002/(sici)1096-8652(199906)61:2<103::aid-ajh5>3.0.co;2-v. [DOI] [PubMed] [Google Scholar]
- Givol N, Goldstein G, Peleg O, et al. Thrombocytopenia and bleeding in dental procedures of patients with Gaucher disease. Haemophilia. 2011 doi: 10.1111/j.1365–2516.2011.02540.x. [DOI] [PubMed] [Google Scholar]
- Goker-Alpan O. Therapeutic approaches to bone pathology in Gaucher disease: Past, present and future. Mol Gen Metab. 2011 doi: 10.1016/j.ymgme.2011.08.004. [DOI] [PubMed] [Google Scholar]
- Grabowski GA, Barton NM, Pastores G, et al. Enzyme therapy in Gaucher disease type 1: comparative efficacy of mannose-terminated glucocerebrosidase from natural and recombinant sources. Ann Int Med. 1995;122:33–39. doi: 10.7326/0003-4819-122-1-199501010-00005. [DOI] [PubMed] [Google Scholar]
- Hadas-Halpern I, Deeb M, Abrahamov A, Zimran A, Elstein D. Gaucher disease: spectrum of sonographic findings in the liver. J Ultrasound Med. 2010;29(5):727–733. doi: 10.7863/jum.2010.29.5.727. [DOI] [PubMed] [Google Scholar]
- Hall MB, Brown RW, Baughman RA. Gaucher’s disease affecting the mandible. J Oral Maxillofac Surg. 1985;43:210–213. doi: 10.1016/0278-2391(85)90162-4. [DOI] [PubMed] [Google Scholar]
- Heasman PA. Mandibular lesions in Gaucher disease. Oral Surg Oral Med Oral Pathol. 1991;72:506. doi: 10.1016/0030-4220(91)90569-x. [DOI] [PubMed] [Google Scholar]
- Hermann G, Pastores GM, Abdelwahab IF, Lorberboym AM. Gaucher disease: assessment of skeletal involvement and therapeutic responses to enzyme replacement. Skeletal Radiol. 1997;26:687–696. doi: 10.1007/s002560050313. [DOI] [PubMed] [Google Scholar]
- Hollak CE, Levi M, Berends F, Aerts JM, van Oers MH. Coagulation abnormalities in type 1 Gaucher disease are due to low-grade activation and can be partly restored by enzyme supplementation therapy. Br J Haematol. 1997;96:470–476. doi: 10.1046/j.1365-2141.1997.d01-2076.x. [DOI] [PubMed] [Google Scholar]
- Horwitz J, Hirsh I, Machtei EE. Oral aspects of Gaucher’s disease: a literature review and case report. J Periodontol. 2007;78:783–788. doi: 10.1902/jop.2007.060341. [DOI] [PubMed] [Google Scholar]
- Itzchaki M, Lebel E, Dweck A, et al. Orthopedic considerations in Gaucher disease since the advent of enzyme replacement therapy. Acta Orthop Scand. 2004;75:641–653. doi: 10.1080/00016470410004003. [DOI] [PubMed] [Google Scholar]
- Kaplan P, Mazur A, Manor O, et al. Acceleration of retarded growth in children with Gaucher disease after treatment with alglucerase. J Pediatr. 1996;129:149–153. doi: 10.1016/s0022-3476(96)70203-2. [DOI] [PubMed] [Google Scholar]
- Karabulut N, Ahmetoglu A, Ariyürek M, Erol C, Gürakan F. Obliteration of maxillary and sphenoid sinuses in Gaucher’s disease. Br J Radiol. 1997;70:533–535. doi: 10.1259/bjr.70.833.9227238. [DOI] [PubMed] [Google Scholar]
- Kerem E, Elstein D, Abrahamov A, et al. Pulmonary function abnormalities in type I Gaucher disease. Eur Respir J. 1996;9:340–345. doi: 10.1183/09031936.96.09020340. [DOI] [PubMed] [Google Scholar]
- Lebel E, Ioscovich A, Itzchaki M, Zimran A, Elstein D. Hip arthroplasty in patients with Gaucher disease. Blood Cells Mol Dis. 2011;46:60–65. doi: 10.1016/j.bcmd.2010.09.001. [DOI] [PubMed] [Google Scholar]
- Lebel E, Itzchaki M, Hadas-Halpern I, Zimran A, Elstein D. Outcome of total hip arthroplasty in patients with Gaucher disease. J Arthroplasty. 2001;16:7–12. doi: 10.1054/arth.2001.19162. [DOI] [PubMed] [Google Scholar]
- Lustmann J, Ben-Yehuda D, Somer M, Ulmansky M. Gaucher’s disease affecting the mandible and maxilla. Report of a case. Int J Oral Maxillofac Surg. 1991;20:7–8. doi: 10.1016/s0901-5027(05)80685-x. [DOI] [PubMed] [Google Scholar]
- Meikle PJ, Hopwood JJ, Clague AE, Carey WF. Prevalence of lysosomal storage disorders. JAMA. 1999;281(3):249–254. doi: 10.1001/jama.281.3.249. [DOI] [PubMed] [Google Scholar]
- Michanowicz AE, Michanowicz JP, Stein GM. Gaucher’s disease: Report of a case. Oral Surg Oral Med Oral Pathol. 1967;23:36–42. doi: 10.1016/0030-4220(67)90481-1. [DOI] [PubMed] [Google Scholar]
- Mikosch P, Hughes D. An overview on bone manifestations in Gaucher disease. Wien Med Wochenschr. 2010;160:609–624. doi: 10.1007/s10354-010-0841-y. [DOI] [PubMed] [Google Scholar]
- Mistry PK, Sirrs S, Chan A, et al. Pulmonary hypertension in type I Gaucher’s disease: Genetic and epigenetic determinants of phenotype and response to therapy. Mol Genet Metab. 2002;77:91–98. doi: 10.1016/s1096-7192(02)00122-1. [DOI] [PubMed] [Google Scholar]
- Moch WS. Gaucher’s disease with mandibular bone lesions. Oral Surg Oral Med Oral Pathol. 1953;6:1250–1254. doi: 10.1016/0030-4220(53)90019-x. [DOI] [PubMed] [Google Scholar]
- Pastores GM, Patel MJ, Firooznia H. Bone and joint complications related to Gaucher disease. Curr Rheumatol Rep. 2000;2:175–180. doi: 10.1007/s11926-000-0059-x. [DOI] [PubMed] [Google Scholar]
- Regenye GR, Huberman BA, Itkin AB. Gaucher’s disease: case report of mandibular trauma. Oral Surg Oral Med Oral Pathol. 1992;73:23–26. doi: 10.1016/0030-4220(92)90148-j. [DOI] [PubMed] [Google Scholar]
- Renvert S, Berglund J, Persson RE, Persson GR. Osteoporosis and periodontitis in older subjects participating in the Swedish National Survey on Aging and Care (SNAC-Blekinge) Acta Odontol Scand. 2011;69(4):201–217. doi: 10.3109/00016357.2010.549501. [DOI] [PubMed] [Google Scholar]
- Rudzki Z, Okoń K, Machaczka M, Rucińska M, Papla B, Skotnicki AB. Enzyme replacement therapy reduces Gaucher cell burden but may accelerate osteopenia in patients with type I disease - a histological study. Eur J Haematol. 2003;70:273–281. doi: 10.1034/j.1600-0609.2003.00047.x. [DOI] [PubMed] [Google Scholar]
- Schubiner H, Letourneau M, Murray DL. Pyogenic osteomyelitis versus pseudo-osteomyelitis in Gaucher’s disease. Clin Pediatr. 1981;20:667. doi: 10.1177/000992288102001009. [DOI] [PubMed] [Google Scholar]
- Schwartz MR, Weycer JS, McGavran MH. Gaucher’s disease involving the maxillary sinuses. Arch Otolaryngol Head Neck Surg. 1988;114:203–206. doi: 10.1001/archotol.1988.01860140101032. [DOI] [PubMed] [Google Scholar]
- Sela J, Polliack A, Ulmansky M. Involvement of the mandible in Gaucher’s disease. Report of a case with post-mortem findings. Br J Oral Surg. 1972;9:246–250. doi: 10.1016/s0007-117x(71)80041-0. [DOI] [PubMed] [Google Scholar]
- Shinoda H, Takeyama S, Suzuki K, Murakami S, Yamada S. Pharmacological topics of bone metabolism: a novel bisphosphonate for the treatment of periodontitis. J Pharmacol Sci. 2008;106(4):555–558. doi: 10.1254/jphs.fm0070272. [DOI] [PubMed] [Google Scholar]
- Shira RB. Manifestations of systemic disorders in the facial bones. J Oral Surg (Chic) 1953;11:286–307. [PubMed] [Google Scholar]
- Shvidel L, Sigler E, Shtalrid M, Feldberg E, Berrebi A. Parotid gland involvement, the presenting sign of high grade non-Hodgkin lymphoma in two patients with Gaucher disease and sicca syndrome. J Inherit Metab Dis. 2007;30(5):825. doi: 10.1007/s10545-007-0610-2. [DOI] [PubMed] [Google Scholar]
- Sidransky E. Gaucher disease: complexity in a “simple” disorder. Mol Genet Metab. 2004;83:6–15. doi: 10.1016/j.ymgme.2004.08.015. [DOI] [PubMed] [Google Scholar]
- Spiegel LH. Gaucher’s disease. Oral Surg Oral Med Oral Pathol. 1957;10:158–166. doi: 10.1016/s0030-4220(57)80086-3. [DOI] [PubMed] [Google Scholar]
- Stowens DW, Teitelbaum SL, Kahn AJ, Barranger JA. Skeletal complications of Gaucher disease. Medicine (Baltimore) 1985;64:310–322. doi: 10.1097/00005792-198509000-00003. [DOI] [PubMed] [Google Scholar]
- Svennerholm L, Erikson A, Groth CG, et al. Norrbottnian type of Gaucher disease—Clinical, biochemical and molecular biology aspects: Successful treatment with bone marrow transplantation. Dev Neurosci. 1991;13:345–351. doi: 10.1159/000112184. [DOI] [PubMed] [Google Scholar]
- Wactawski-Wende J, Grossi SG, Trevisan M, Genco RJ, Tezal M, Dunford RG, Ho AW, Hausmann E, Hreshchyshyn MM. The role of osteopenia in oral bone loss and periodontal disease. J Periodontol. 1996;67(10 Suppl):1076–1084. doi: 10.1902/jop.1996.67.10s.1076. [DOI] [PubMed] [Google Scholar]
- Walton-Bowen K, Mantick N. Gaucher Registry Annual Aggregate Data Report. 2000. [Google Scholar]
- Weigler JM, Seldin R, Minkowitz S. Gaucher’s disease involving the mandible: report of case. J Oral Surg. 1967;25:158–163. [PubMed] [Google Scholar]
- Weinreb NJ, Charrow J, Andersson HC, et al. Effectiveness of enzyme replacement therapy in 1028 patients with type 1 Gaucher disease after 2 to 5 years of treatment: a report from the Gaucher Registry. Am J Med. 2002;113:112–119. doi: 10.1016/s0002-9343(02)01150-6. [DOI] [PubMed] [Google Scholar]
- Wenstrup RJ, Roca-Espiau M, Weinreb NJ, Bembi B. Skeletal aspects of Gaucher disease: a review. Br J Radiol. 2002;75(Suppl 1):A2–12. doi: 10.1259/bjr.75.suppl_1.750002. [DOI] [PubMed] [Google Scholar]
- Zimran A. How I treat Gaucher disease. Blood. 2011;118(6):1463–1471. doi: 10.1182/blood-2011-04-308890. [DOI] [PubMed] [Google Scholar]
- Zimran A, Abrahamov A, Aker M, Matzner Y. Correction of neutrophil chemotaxis defect in patients with Gaucher disease by low-dose enzyme replacement therapy. Am J Hematol. 1993;43:69–71. doi: 10.1002/ajh.2830430118. [DOI] [PubMed] [Google Scholar]
- Zimran A, Elstein D. Lipid storage diseases. In: Lichtman MA, Kipps T, Seligsohn U, Kaushansky K, Prchal JT, editors. Williams Hematology. 8. New York: McGraw-Hill; 2010. pp. 1065–1071. [Google Scholar]
- Zimran A, Elstein D, Levy-Lahad E, et al. Replacement therapy with imiglucerase for type 1 Gaucher’s disease. Lancet. 1995;345:1479–1480. doi: 10.1016/s0140-6736(95)91038-7. [DOI] [PubMed] [Google Scholar]