Abstract
The infiltration of suppressive myeloid cells into the tumor microenvironment restrains anti-tumor immunity. However, cytokines may alter the function of myeloid lineage cells to support tumor rejection, regulating the balance between pro- and anti-tumor immunity. In this study, it is shown that effector cytokines secreted by adoptively transferred T cells expressing a chimeric antigen receptor (CAR) shape the function of myeloid cells to promote endogenous immunity and tumor destruction. Mice bearing the ovarian ID8 tumor were treated with T cells transduced with a chimeric NKG2D receptor. GM-CSF secreted by the adoptively transferred T cells recruited peripheral F4/80loLy-6C+ myeloid cells to the tumor microenvironment in a CCR2-dependent fashion. T cell IFN-γ and GM-CSF activated local, tumor-associated macrophages, decreased expression of regulatory factors, increased IL-12p40 production, and augmented antigen processing and presentation by host macrophages to antigen-specific T cells. In addition, T cell-derived IFN-γ, but not GM-CSF, induced the production of nitric oxide by F4/80hi macrophages and enhanced their lysis of tumor cells. The ability of CAR T cell therapy to eliminate tumor was moderately impaired when inducible nitric oxide synthase was inhibited, and greatly impaired in the absence of peritoneal macrophages after depletion with clodronate encapsulated liposomes. This study demonstrates that the activation of host macrophages by CAR T cell-derived cytokines transformed the tumor microenvironment from immunosuppressive to immunostimulatory and contributed to inhibition of ovarian tumor growth.
Keywords: chNKG2D, ovarian cancer, adoptive T cell therapy, NKG2D, CD8 T cells
Introduction
The tumor microenvironment is characterized by suppressive leukocytes that restrain anti-tumor immunity and promote tumor growth and survival (1). Tumor-associated macrophages (TAM) comprise a large percentage of the cellular constituents within the tumor milieu. These cells promote tumor growth through secretion of pro-angiogenic cytokines and suppression of anti-tumor immunity (2). Despite their tumor promoting properties, subpopulations of macrophages can support tumor rejection and promote anti-tumor immune responses elicited by cancer immunotherapies (3, 4). The dual function of macrophages is regulated by their immune environment (5). Lymphocytes regulate the immune environment and interact with macrophages to shape their activation, controlling the balance between pro- and anti-tumor immunity (6, 7). Transformation of the tumor milieu, including myeloid cells, supports tumor rejection and enhances immunotherapeutic strategies targeting cancer.
Chimeric antigen receptor (CAR) transduced T cells have been shown to be an effective means to reduce tumor burden and increase survival (8-10). CARs have been developed that recognize several different molecules, including CD19, Her2neu, mesothelin, and NKG2D ligands, and CARs use a variety of signaling motifs to enhance the efficacy of effector T cells (8, 11-14). Because such targeted T cells can mediate a variety of effector responses in addition to direct tumor lysis, CAR T cells have the potential to change the tumor microenvironment, induce host anti-tumor immunity, and lead to long-term tumor-free survival. Many studies have focused on their ability to kill tumor cells and how best to deliver them to tumor-bearing hosts (9). This study investigated the mechanisms of how adoptive T cell therapy altered local tumor myeloid cells to promote tumor destruction and anti-tumor immunity. CAR-bearing T cells engineered to express a chimeric NKG2D (chNKG2D) receptor, which consists of full length NKG2D fused to CD3ζ were used (13, 15, 16). The efficacy of these CAR-bearing T cells involves not only tumor lysis, but cytokine induced changes as well (14, 17, 18). This study demonstrates that one mechanism for CAR-bearing T cell efficacy is through cytokine-induced changes in the tumor microenvironment that cause recruitment and activation of tumor-associated myeloid cells to create an unfavorable milieu for tumor survival.
Materials and Methods
Mice
C57BL/6 and B6-Ly5.2Cr (CD45.1+) were purchased from the National Cancer Institute (Frederick, MD). B6.FVB-Tg(Itgax-DTR/EGFP)57LanJ (ITGAX.DTR), and B6.129S7-Ifngtm1Agt/J (IFN-γ−/−) were purchased from The Jackson Laboratory. B6.129S4-Ccr2tm1Ifc/J (CCR2−/−) mice were provided by Dr. Brent Berwin (Dartmouth Medical School, Lebanon, NH) and GM-CSF-deficient mice on a C57BL/6 background were provided by Dr. Jeff Whitsett (University of Cincinnati, Cincinnati, OH). Mice used in experiments were between 7 to 12 weeks of age. All animal work was performed in the Dartmouth Medical School Animal Facility (Lebanon, NH) in accordance with institutional guidelines.
Generation of CAR expressing T cells
Mouse splenocytes were stimulated with Con A for 18 h (1 μg/ml), retrovirally transduced as previously described, and injected into mice 8 days post-activation (15, 17)
Injection of ID8-GFP cells and treatment of mice with genetically modified T cells
On day 0, ID8-GFP cells (2 × 106) were injected i.p. into mice. The ID8 ovarian carcinoma model recapitulates advanced stages of ovarian carcinoma. ID8 tumor cells express NKG2D ligands (13). Mice were treated with T cells on day +35, as described for each experiment. To inhibit nitric oxide production in vivo, mice were injected with the iNOS selective inhibitor L-nil (3mg/kg) or PBS each day from day 35 to day 41. To deplete macrophage populations, 200 ul of clodronate encapsulated liposomes or control PBS liposomes (gift of Roche Diagnostics, 120 GmbH, Mannheim, Germany) were injected i.p. on day 33 into ID8 tumor-bearing mice, which was two day prior to T cell inoculation. Clodronate liposomes were prepared as previously described (19). The number of solid tumors on the peritoneal wall and the number of GFP+ tumor cells in the peritoneal wash was determined eight weeks (day 56) after ID8 tumor inoculation.
Flow Cytometry and F4/80 Cell Isolation
Cells isolated by peritoneal wash were incubated with anti-CD16/CD32 and mouse γ globulin (Jackson ImmunoResearch) and stained with anti-CD45.1 (clone A20), anti-CD45.2 (clone 104), anti-F4/80 (clone BM8), anti-Ly-6C (clone HK1.4), anti-MHC-II (clone M5/114.15.2), anti-CD86 (clone GL-1), anti-IFNγR (clone MOB-47), anti-CD3e (clone 145-2C11), anti-CD4 (clone GK1.5), anti-CD8b (clone YTS156.7.7), anti-CXCR3 (clone CXCR3-173), anti-CD11c (clone N418), or anti-IL-12p40 (clone C15.6). F4/80+ cell production of ROS was measured by the oxidation-sensitive dye CM-H2DCFDA (Invitrogen). Peritoneal cells were labeled with 10 μM DCFDA for 30 minutes, washed twice, and stained with anti-F4/80 antibody before running flow cytometry. For intracellular staining, 106 peritoneal cells were cultured in complete medium for 24 hours in a 48-well plate. Brefeldin A (10 μg/ml, Sigma Aldrich, St. Louis, MO) and LPS (10 μg/ml) were added to the cultures for the last 5 hours of incubation. Peritoneal cells assayed for IL-6 expression were not stimulated with LPS. Cells were stained with PE or APC-conjugated anti-F4/80, fixed with 1.0% paraformaldehyde, permeabilized with 0.1% saponin, and stained with APC-conjugated anti-IL-12p40, anti-IL-10-PE, anti-IL-6-PE, or anti-NOS2-AlexFluor647 mAbs. Peritoneal F4/80+ cells were isolated from tumor-bearing mice by magnetic bead selection and anti-F4/80 biotin antibody (Miltenyi). The purity of cells after isolation was > 90%.
Adoptive Transfer Experiments
CD45.1+ mice bearing tumors for five weeks were injected i.p. with wtNKG2D or chNKG2D T cells. At five weeks post-tumor inoculation in this murine tumor model, there are both free tumor cells and solid tumors present. One hour prior to T cell treatment, a mixture of bone marrow (5 × 106) and spleen cells (5 × 106) isolated from naïve CD45.2+ C57BL/6 or CCR2−/− mice were injected i.v. into CD45.1+ mice. Three days after T cell treatment, the number of CD45.2+, F4/80+ cells in the peritoneum was assessed to determine myeloid cell recruitment. To assess chNKG2D T cell activation of tumor-associated macrophages, CD45.2+ mice bearing five week ID8 tumors were injected i.p. with peritoneal cells isolated from CD45.1+ mice bearing 5 week ID8 tumors. One hour after transfer of tumor-associated CD45.1+ cells, wtNKG2D or chNKG2D T cell were injected i.p. into CD45.2+ mice. The phenotype of CD45.1+ tumor-associated cells was assessed by flow cytometry 3 days after T cell treatment.
Real-Time Polymerase Chain Reaction
F4/80+ peritoneal cells were isolated and total RNA was extracted using RNA-Easy MiniKit (Qiagen). cDNA was synthesized using random hexamer primers (Fermentas) in accordance with the manufacturer’s protocol. cDNA (5 ng) was used as a template for RT-PCR amplification using SYBR-Green Master Mix (Applied Biosystems) in 25 μl of volume according to the manufacturer’s protocol. Statistics were performed using the ΔCT values.
Cytokine secretion
F4/80+ cells (2 × 105) from tumor-bearing mice were cultured for 24 hours in a 96-well plate in complete medium. Cell-free conditioned media was assayed for IL-6 and IL-10 using multiplex analysis (Millipore) by the DartLab of the Norris Cotton Cancer Center (Lebanon, NH). Nitric oxide (NO) production was measured using Greiss’s reagent for nitrite according to the manufacturer’s protocol (Sigma-Aldrich). Peritoneal cells (106) were cultured in a 48 well plate for 24 hours and cell-free supernatants were assay for IFN-γ by ELISA using mouse DuoSet ELISA kits (R&D Systems).
Antigen Processing and Presentation
Antigen processing assays were performed using peritoneal cells (5 × 105) stained for F4/80 prior to pulsing cells with 50 μg/ml of DQ-OVA at 37°C for 15 minutes. Cells were washed three times in PBS at 4°C and incubated for 15 minutes at 37°C before antigen processing by F4/80+ cells was assessed by flow cytometry. For antigen presentation assays, F4/80+ cells (2 × 105) were isolated from peritoneal wash cells and cultured with CFSE labeled OT-I cells (105) positively selected by magnetic beads for CD8b+ cells. Prior to culture F4/80+ cells were pulsed with 10−10 M OVA257-264 peptide. T cell proliferation was measured after 4 days based on CFSE dilution.
Cytotoxicity Assay
Purified F4/80+ cells were cultured with ID8 tumor cells at an effector to target ratio of 25:1 with, or without, 20 μM of the NOS2 selective inhibitor, L-nil (Cayman Chemical), for 24 hours. Macrophage cytotoxicity was measured by LDH release using the manufacturer’s protocol (ProMega).
Statistical Analysis
Differences between groups were analyzed using a Student t-test or ANOVA. A Wilcoxon 2-group test was used to compare ΔCT values in real-time PCR experiments. Values of p<0.05 were considered significant.
Results
CAR T cell GM-CSF recruits myeloid cells to the tumor site in a CCR2-dependent manner
To determine mechanisms whereby CAR T cells regulated myeloid cell populations in the tumor microenvironment, tumor-bearing mice were injected with CAR T cells and the role of specific cytokines was determined. Adoptive transfer of CAR chNKG2D T cells increased the number of peritoneal cells expressing low levels of F4/80 in the tumor microenvironment three days post-treatment (Fig. 1A). Approximately 40% of the F4/80hi and 50% of the F4/80lo macrophages expressed Ly-6C, a marker of recent monocyte recruitment (Fig. 1B). Note that less than 5% of the F4/80+ cells in the tumor microenvironment expressed the granulocytic, neutrophil marker Ly-6G (data not shown). The low expression of F4/80 can distinguish newly recruited monocytes from F4/80hi resident macrophages (20-22). The increase in F4/80lo myeloid cell numbers may be attributed to increased proliferation of local myeloid cells or the recruitment of peripheral monocytes that differentiate into F4/80+ tissue macrophages. To confirm that the increase in F4/80loLy-6C+ cells following adoptive T cell treatment was due to recruitment of myeloid cells, CD45.1+ bone marrow and spleen cells (two sources of peripheral myeloid cells) were injected i.v. into CD45.2+ tumor-bearing mice. These mice were also injected at the tumor site i.p. with chNKG2D T cells or control wtNKG2D T cells. Treatment with CAR T cells preferentially recruited CD45.1+F4/80loLy-6C+ myeloid cells to the peritoneum within three days following T cell treatment (Fig. 1C). GM-CSF-deficient T cells failed to increase the number of F4/80loLy-6C+ cells compared to GM-CSF sufficient chNKG2D T cells indicating that the secretion of GM-CSF by the transferred T cells, but not IFN-γ, was necessary for the recruitment of F4/80loLy-6C+ cells (Fig. 1D). These data indicate that CAR T cell treatment recruits F4/80loLy-6C+ myeloid cells to the tumor microenvironment through secretion of GM-CSF.
Figure 1. T cell-derived GM-CSF recruits Ly-6C+ myeloid cells to the tumor microenvironment in a CCR2-dependent manner.
ID8 tumor-bearing mice were treated with chNKG2D (hatched), wtNKG2D (filled), chNKG2D IFN-γ KO (open), or chNKG2D GM-CSF KO (gray) T cells on week 5. Three days after T cell treatment (A) the number of F4/80hi and F4/80lo cells in the peritoneum was determined 3 days after T cell treatment (n=4). (***,p<0.001 vs. F4/80lo chNKG2D) (B) Representative flow cytometry plots show the expression of Ly-6C on the F4/80hi and F4/80lo populations after treatment with chNKG2D (solid) or wtNKG2D (dashed) T cells. CD45.2+ tumor-bearing mice were injected i.v. with a 1:1 mixture of CD45.1+ bone marrow and spleen cells 1 hour before i.p. injection with chNKG2D or wtNKG2D T cells. (C) The number of CD45.1+ F4/80hi and F4/80lo cells expressing Ly-6C in the peritoneum was assessed 3 days following T cell treatment (n=4). (***,p<0.001 vs. F4/80loLy-6C+ chNKG2D; *,p<0.05 vs. F4/80hiLy-6C+ chNKG2D) (D) The number of F4/80hi and F4/80lo cells expressing Ly-6C was determined after T cell treatment (***,p<0.001 vs. F4/80loLy-6C+ chNKG2D; **,p<0.01 vs. F4/80hiLy-6C+ chNKG2D) (E) F4/80+ cells were isolated from tumor-bearing mice and the expression of CCL2 and CCR2 was determined by RT-PCR. (***,p<0.001; **,p<0.01 vs. chNKG2D) (F) Tumor-bearing CD45.1+ mice were injected i.v. with CD45.2+ BM and spleen cells from naïve C57BL/6 or CCR2−/− donors 1 hour before i.p. injection with chNKG2D or wtNKG2D T cells. The number of F4/80+CD45.2+ cells recruited to the peritoneum was assessed 3 days after T cell treatment (n=4). (***,p<0.001; vs. C57BL/6 chNKG2D) (†, p<0.05 vs. C57BL/6 wtNKG2D). A, B, C, and F are representative data of at least two independent experiments. D and E are cumulative results of two independent experiments. SD is shown (n=8).
Inflammatory monocytes have been shown to preferentially express the chemokine receptor CCR2, which they employ to migrate into inflamed tissue and the ovarian tumor microenvironment (20, 22, 23). Macrophages isolated from the peritoneum of chNKG2D T cell treated mice expressed higher amounts of the chemokine receptor CCR2 and produced more of the CCR2 ligand, CCL2 (Fig. 1E). Tumor-bearing mice treated with chNKG2D CAR T cells increased total CCL2 production by cells within the tumor microenvironment and this was dependent upon adoptive T cell production of GM-CSF but not IFN-γ (18). To determine if the recruitment of myeloid cells following CAR T cell therapy was CCR2-dependent, CD45.2+ bone marrow and spleen cells from C57BL/6 or CCR2−/− mice were injected i.v. into tumor-bearing CD45.1+ mice one hour before i.p. treatment with chNKG2D or wtNKG2D T cells. CCR2-deficient cells had a reduced ability to traffic to the tumor site after chNKG2D T cell treatment (Fig. 1F, CCR2 KO). However, chNKG2D T cell treatment still recruited significantly more CCR2− myeloid cells relative to wtNKG2D T cell treated mice, indicating the recruitment of myeloid cells from the periphery was not solely CCR2-dependent.
CAR T cell IFN-γ is required to upregulate expression of MHC and costimulatory molecules on endogenous macrophages
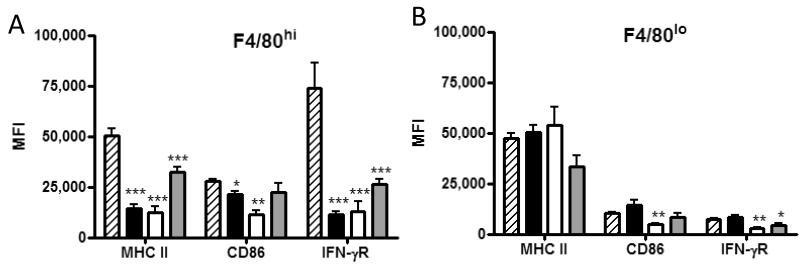
Cytokines secreted by T cells shape macrophage activation upon tissue entry. To determine if cytokines secreted by adoptively transferred T cells regulated macrophage activation, tumor-bearing mice were treated with chNKG2D T cells deficient in IFN-γ or GM-CSF and the expression of cell surface proteins was determined. IFN-γ from the adoptively transferred chNKG2D T cells, but not GM-CSF, was required to upregulate MHC II, CD86, and IFN-γR expression on F4/80hi macrophages following chNKG2D T cell treatment (Fig. 2A). There was a trend that F4/80hi cells isolated from GM-CSF-deficient chNKG2D T cells expressed lower amounts of activation markers compared to F4/80hi cells from mice treated with cytokine-sufficient chNKG2D T cells. Transfer of cytokine-deficient T cells resulted in reduced expression of these activation markers on F4/80lo cells relative to cytokine-sufficient T cells (Fig. 2B). F4/80lo cells represent a population of newly recruited macrophages, and they were similar between chNKG2D and control wtNKG2D T cell treated mice. The mature F4/80hi macrophage population consists of both tumor-associated and newly recruited macrophages that upregulate F4/80 upon maturation. The upregulation of activation markers on F4/80hi macrophages indicated that IFN-γ from CAR T cells activated host macrophages.
Figure 2. T cell-derived IFN-γ is required to activate host macrophages.
ID8 tumor-bearing mice were treated with chNKG2D (hatched), wtNKG2D (filled), chNKG2D IFN-γ KO (open), or chNKG2D GM-CSF KO (gray) T cells at week 5, and peritoneal washes were performed 3 days after T cell treatment. (A and B, top) Representative flow cytometry plots showing the expression of MHC II, CD86, and IFN-γR on F4/80hi and F4/80lo cells from mice treated with chNKG2D (solid) or wtNKG2D (dashed) T cells, or isotype control (gray histogram). (A and B, bottom) The MFI of F4/80hi and F4/80lo cells expressing MHC II, CD86, and IFN-γR was determined after adoptive T cell treatment (n=4). Flow plots and data are representative of 2-4 independent experiments. (***,p<0.001; **,p<0.01; *,p<0.05 vs. chNKG2D).
CAR T cells alter the activation of tumor-associated macrophages present at the tumor site at the time of treatment
Following treatment with CAR-expressing T cells, two populations of endogenous macrophages within the tumor microenvironment are present. Newly recruited macrophages constitute the majority of F4/80+ cells three days post-adoptive therapy. A second population of tumor-associated myeloid cells that differentiate in the tumor milieu prior to T cell treatment have been shown to suppress tumor-specific T cell responses (2). However, their re-education through activating signals has been shown to stimulate adaptive immunity and promote regression of advanced tumors (4, 24). It is controversial as to what extent tumor-associated macrophages are malleable and can be induced to become anti-tumor effector cells. To determine if T cell effector cytokines could activate suppressive tumor-associated macrophages already present within a tumor, five week tumor-bearing CD45.2+ mice were injected locally with peritoneal cells isolated from five week tumor-bearing CD45.1+ mice prior to CAR T cell transfer. The majority of the transferred peritoneal cells from the tumor-bearing CD45.1+ mice expressed F4/80 (data not shown). ChNKG2D T cell treatment activated CD45.1+ tumor-associated macrophages as evidenced by an increase in the expression of MHC II, CD86, and IFN-γR on F4/80hi macrophages (Fig. 3A). Both IFN-γ and GM-CSF secreted by CAR T cells were necessary to activate tumor-associated macrophages. Treatment with chNKG2D T cells had no significant effect on tumor-associated F4/80lo cells (Fig 3B). However, approximately 75% of the transferred tumor-associated macrophages were F4/80hi (data not shown). These data indicate that both newly recruited and tumor-associated suppressive macrophages within a tumor can be activated through a combination of IFN-γ and GM-CSF produced by the CAR-bearing T cells.
Figure 3. Both GM-CSF and IFN-γ secreted by chNKG2D T cells are required to activate tumor-associated macrophages.
Peritoneal cells isolated from CD45.2+ tumor-bearing mice were injected i.p. into CD45.1+ tumor-bearing mice 1 hour before injection of chNKG2D (hatched), wtNKG2D (filled), chNKG2D IFN-γ KO (open), or chNKG2D GM-CSF KO (gray) T cells. Peritoneal washes were performed 3 days after T cell treatment and the MFI of MHC II, CD86, and IFN-γR was determined on CD45.2+F4/80hi (A) and CD45.2+F4/80lo (B) macrophages. Representative results and SD of two independent experiments is shown (n=4). (***,p<0.001; **,p<0.01; *,p<0.05 vs chNKG2D).
CAR T cell treatment inhibits tumor-associated macrophage production of regulatory proteins
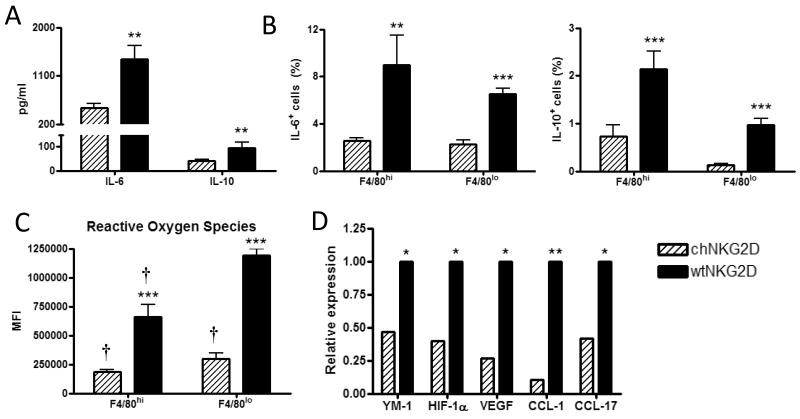
Ovarian cancer cells have been shown to polarize macrophages toward a tumor-associated phenotype (25). To determine the effect of adoptive CAR T cell treatment to modify the suppressive capabilities of tumor-associated regulatory macrophages, macrophage production of suppressive factors was assessed three days after T cell treatment. F4/80hi and F4/80lo macrophages produced lower amounts of IL-10 and IL-6 following treatment with chNKG2D T cells (Fig 4A and 4B). CAR T cell treatment decreased the production of reactive oxygen species (ROS) by F4/80hi and F4/80lo cells (Fig. 4C), which can suppress T cell function through downregulation of CD3ζ (1). In addition to direct suppression of anti-tumor immune responses, tumor-associated macrophages can recruit CCR8+ and CCR4+ T reg cells to potentiate suppression (26, 27). ChNKG2D T cell treatment reduced macrophage expression of the CCR8 and CCR4 ligands, CCL1 and CCL17 (Fig. 4D). Macrophages also expressed less YM-1, HIF-1α, and VEGF, indicating a less alternatively activated phenotype (Fig. 4D) (28). The decrease in expression of suppressive and angiogenic factors by macrophages at the tumor site indicates that chNKG2D T cell treatment activated host macrophages resulting in a reduced ability to suppress immune responses.
Figure 4. ChNKG2D T cell treatment reduces macrophage expression of regulatory factors.
F4/80+ cells were isolated from the peritoneum of tumor-bearing mice 3 days after T cell treatment and cultured in medium for 24 hours. (A) Cell-free supernatant was assayed for IL-10 and IL-6. (B) F4/80hi and F4/80lo peritoneal cells were assessed for IL-6 and IL-10 expression by intracellular flow cytometry. (C) F4/80hi and F4/80lo peritoneal cells were assessed for reactive oxygen species production using a DCFDA dye. (D) The expression of YM-1, HIF-1α, VEGF, CCL1, and CCL17 on purified F4/80+ cells was determined by real-time PCR. Cumulative data and SD of at least two independent experiments is shown. (***,p<0.001; **,p<0.01; *,p<0.05 vs. chNKG2D) († p<0.05 vs. F4/80lo wtNKG2D).
CAR T cells increase antigen presentation capacity of tumor-associated macrophages in a cytokine dependent manner
The ability of tumor-associated macrophages to present antigen and induce a T cell response is often impaired (29, 30). T cell effector cytokines can regulate antigen processing and presentation in the tumor microenvironment and promote endogenous immunity (31, 32). A higher percentage of both F4/80hi and F4/80lo macrophages from chNKG2D T cell treated mice effectively processed antigen as measured by processing of DQ-OVA, a protein which fluoresces upon proteolytic degradation and is widely used as a measure of endosomal protein processing. Both IFN-γ and GM-CSF secretion by adoptively transferred T cells were required to enhance antigen processing by macrophages following CAR T cell treatment (Fig. 5A).
Figure 5. ChNKG2D T cell treatment induces macrophage production of pro-inflammatory cytokines and enhances antigen processing and presentation.
Tumor-bearing mice were treated with chNKG2D (hatched), wtNKG2D (filled), chNKG2D IFN-γ KO (open), or chNKG2D GM-CSF KO (gray) T cells. (A) F4/80+ cells isolated from the peritoneum 3 days after T cell treatment were pulsed with DQ-OVA and the percent of F4/80hi and F4/80lo cells processing antigen was assessed after 15 minutes by flow cytometry. (B) Purified peritoneal F4/80+ cells isolated from naïve (checkered) or tumor-bearing mice treated with chNKG2D (hatched), wtNKG2D (filled), chNKG2D IFN-γ KO (open), or chNKG2D GM-CSF KO (gray) T cells were pulsed with 10−10 M OVA257-264 peptide and cultured with CFSE-labeled OT-I T cells. OT-I cell proliferation was measured by CFSE dilution and flow cytometry after 4 days of culture. (C) Three days after T cell treatment, peritoneal cells were isolated and cultured with BFA for 24 hours. During the last five hours of culture, cells were stimulated with LPS and the percent of F4/80hi and F4/80lo cells producing IL-12p40 was determined by intracellular flow cytometry. Representative data and SD of at least two independent experiments is shown (n=4). (***,p<0.001; **,p<0.01; *,p<0.05 vs. chNKG2D) († p<0.01 vs. wtNKG2D).
To determine the effects of chNKG2D T cell cytokines on the ability of tumor-associated macrophages to stimulate antigen-specific T cells, F4/80+ cells were isolated from the tumor site after T cell injection, pulsed with SIINFEKL peptides, and the proliferation of naïve OT-I T cells was determined. F4/80+ cells isolated from mice treated with cytokine-deficient CAR T cells induced less OT-I T cell proliferation than F4/80+ cells isolated from mice treated with cytokine-sufficient CAR T cells (Fig. 5B). Macrophages from mice treated with IFN-γ or GM-CSF-deficient chNKG2D T cells had an impaired ability to stimulate OT-I proliferation compared to cytokine intact chNKG2D T cells. F4/80+ cells from wtNKG2D and IFN-γ-deficient T cell treated mice induced less OT-I proliferation than F4/80+ cells from naïve mice, indicative of the suppressive nature of these tumor-associated macrophages. These data, together with previous findings showing enhanced antigen presentation in the draining lymph node and tumor site, indicate that CAR-bearing T cells stimulated antigen processing and presentation in a cytokine dependent manner (17, 18).
T cell-derived IFN-γ and GM-CSF induce macrophage production of IL-12 to potentiate IFN-γ secretion and TH1 differentiation (33). However, tumor-associated macrophages demonstrate a reduced ability to produce IL-12 upon stimulation with IFN-γ or LPS (34, 35). Intracellular flow cytometry using an anti-IL-12 antibody showed that both mature F4/80hi and newly recruited F4/80lo macrophages produced more IL-12 after treatment with CAR T cells. The increased IL-12 production by newly recruited F4/80lo cells was dependent on chNKG2D T cell-derived GM-CSF, but not IFN-γ (Fig. 5C). In contrast, both chNKG2D-derived GM-CSF and IFN-γ were required to increase IL-12p40 production by F4/80hi tumor-associated macrophages. Thus, CAR-bearing T cells can promote local macrophages to produce IL-12 and support activation of TH1 T cells and NK cells through their production of IFN-γ and GM-CSF.
CAR T cells activate macrophages within the tumor microenvironment to kill tumor cells through nitric oxide (NO) production
T cell effector cytokines can regulate macrophage cytotoxicity of tumor cells (36, 37). It was previously shown that IFN-γ secreted by chNKG2D T cells, but not GM-CSF, was required to increase host NO production after T cell transfer (18). Following chNKG2D T cell treatment, macrophages were the major source of nitric oxide in the tumor microenvironment and produced significantly more NO than wtNKG2D T cell treated mice (Fig. 6A). F4/80hi cells expressed higher amounts of inducible nitric oxide synthase (iNOS) compared to F4/80lo cells after chNKG2D T cell therapy (Fig. 6B). CAR T cell treatment enhanced macrophage lysis of ID8 tumor cells compared to control CAR T cell treated mice (Fig. 6C). The increased direct tumoricidal activity of macrophages was dependent on secretion of NO because inhibiting iNOS production through the selective inhibitor L-nil impaired their ability to kill ID8 tumor cells in vitro (Fig. 6C). To determine the role of NO on tumor elimination in vivo, tumor-bearing mice were treated with L-nil one hour before CAR T cells, and for the following six days. The ability of CAR T cell therapy to eliminate free tumor cells was significantly, but moderately, impaired when iNOS was inhibited by L-nil at the tumor site (Fig. 6D). However, inhibiting iNOS had no effect on the ability of chNKG2D T cells to inhibit the growth of solid tumors. Thus, treatment with CAR T cells resulted in an increased ability of local macrophages to kill tumor cells, which enhanced the anti-tumor effects of CAR T cell therapy at the tumor site.
Figure 6. ChNKG2D T cell treatment enhances macrophage cytotoxicity.
Tumor-bearing mice were treated with CAR T cells and F4/80+ cells were purified from the peritoneum three days after T cell treatment. (A) F4/80+ cells (positive fraction), F4/80− cells (negative fraction), or both F4/80+ and F4/80− cells were cultured for 24 hours and cell-free supernatant was assayed for NO production by Greiss’s reagent (n=8). (B) F4/80hi and F4/80lo peritoneal cells were assessed for iNOS expression by intracellular flow cytometry. (***,p<0.001 vs. F4/80hi chNKG2D; *, p<0.05 vs. F4/80lo chNKG2D) (C) F4/80+ cells isolated from T cell treated mice were cultured with ID8 tumor cells in the presence or absence of the iNOS inhibitor, L-nil, to determine the effect of NO blockade on macrophage cytotoxicity of tumor cells (n=8). (D) Tumor-bearing mice were treated with chNKG2D or wtNKG2D T cell on day 35. Mice were injected i.p. with L-nil for one week beginning on the day of T cell treatment. The number of solid tumors on the peritoneal wall and the number of tumor cells in ascites was determined three weeks after T cell transfer. Cumulative results and SD of at least two independent experiments is shown. (***,p<0.001; **,p<0.01; *,p<0.05 vs. chNKG2D + PBS) († p<0.01 vs. wtNKG2D + PBS).
CAR T cell treatment activates macrophages to inhibit ovarian tumor growth and promote endogenous immunity
To determine the role of macrophages in tumor elimination, tumor-bearing mice were injected with clodronate liposomes to deplete host macrophages. Mice treated with clodronate liposomes and CAR T cells had a higher number of solid tumors on the peritoneal wall and more free tumor cells compared to mice treated with CAR T cells and non-depleting PBS liposomes (Fig. 7A). However, mice treated with chNKG2D T cells and clodronate liposome had lower tumor burden than mice that received control CAR T cells. Macrophage depletion by itself had no effect on tumor growth since mice treated with control CAR T cells and clodronate liposomes had similar tumor burdens as mice injected with PBS liposomes. These data indicate that host macrophages are required for complete tumor elimination by CAR T cell therapy.
Figure 7. ChNKG2D T cell treatment requires host macrophages for tumor elimination.
Tumor-bearing mice were treated with clodronate liposomes or PBS liposomes two days before CAR T cell transfer. Tumor burden was assessed three weeks after T cell therapy. (A) The number of solid tumors on the peritoneal wall and the number of free tumor cells in the ascites was determined. (B) Peritoneal cells were cultured for 24 hours and cell-free supernatants were assayed for IFN-γ production. (***,p<0.001; **,p<0.01; vs. chNKG2D + PBS) († p<0.05 vs. wtNKG2D + PBS).
In addition to direct tumor cell killing, macrophages can activate endogenous leukocytes to promote tumor elimination. Host macrophages present antigen and secrete IL-12 to activate lymphocytes. Because host NK and T cells secrete higher amount of IFN-γ at the tumor site following chNKG2D T cell therapy, the effect of macrophage depletion on IFN-γ production was assessed (18). Peritoneal cells isolated from tumor-bearing mice treated with CAR T cells and clodronate liposomes produced lower amounts of IFN-γ compared to mice treated with CAR T cells and PBS liposomes (Fig. 7B). Peritoneal cells produced higher amounts of IFN-γ after CAR T cell treatment and clodronate liposome-mediated macrophage depletion compared to mice treated with control CAR T cells, which demonstrated that CAR T cells led to host lymphocyte activation through other mechanisms as well. These results demonstrate that in addition to direct tumor cell killing, macrophages promote endogenous immunity to eliminate established tumors.
Discussion
The therapeutic success of adoptive T cell therapy is often attributed to direct tumor destruction. However, T cell effector cytokines may have an equally important function in tumor elimination through indirect effects on tumor cells mediated by endogenous leukocytes. T cell effector cytokines alter the tumor microenvironment by shaping leukocyte populations and eliciting endogenous immunity to support CAR T cell-mediated tumor rejection. Treatment with chNKG2D T cells transformed tumor macrophages from immunosuppressive to immunostimulatory through the secretion of specific cytokines. Macrophages following chNKG2D T cell treatment not only promoted anti-tumor immunity, but directly assisted in tumor destruction via nitric oxide. These cytokine-induced changes in myeloid cells were necessary for complete inhibition of ovarian tumor growth.
T cell-derived GM-CSF and IFN-gamma have been shown to tightly correlate with anti-tumor efficacy following adoptive T cell therapy (38). It is hypothesized that these cytokines induce potent host anti-tumor immunity to destroy the tumor (39). We show that IFN-gamma and GM-CSF secreted by CAR-expressing T cells exert unique and synergistic effects on host macrophages which are likely responsible for the increased therapeutic benefit of these cytokines following adoptive T cell therapy. Together, IFN-γ and GM-CSF function in concert to regulate endogenous macrophage recruitment, tumoricidal activity, and activation of an endogenous anti-tumor immune response. Newly recruited F4/80+ cells constitute a large percentage of cellular infiltrate that enters the tumor following chNKG2D T cell-induced inflammation (18). The profile of cell surface markers and production of inflammatory cytokines by macrophages indicates that newly recruited F4/80loLy-6C+ monocytes differentiate into inflammatory macrophages (M1) with anti-tumor functions (22, 40). Few of these cells expressed Ly6G, a molecule associated with granulocytic cells, but they expressed Ly6C, which is associated with monocytic myeloid cells. Ly-6Chi inflammatory monocytes have been shown to have a dependency on the chemokine receptors CCR2 and CCR5 for recruitment to sites of inflammation (20). CCR2-deficieny reduced, but did not completely ablate, the increase in myeloid cell trafficking to the tumor microenvironment following chNKG2D T cell treatment. The partial ablation may be due to recruitment of myeloid cells through another chemokine receptor, such as CCR5, at the same time. It was previously shown that chNKG2D T cell treatment increased the amount of both CCL2 and CCL5 in the tumor microenvironment (18). Treatment with GM-CSF and IFN-γ-deficient T cells demonstrated that GM-CSF was more important than IFN-γ to increase the total chemokine amounts in the peritoneum. Thus, GM-CSF from adoptively transferred CAR T cells induces chemokine production that increases myeloid cell recruitment.
Although IFN-γ was not important for the recruitment of F4/80hiLy-6C+ myeloid cells to the tumor microenvironment, its secretion by adoptively transferred CAR T cells was important in the activation of both newly recruited myeloid cells and local, tumor-associated macrophages. These data support a report showing that IFN-γ reverses the pro-tumoral properties of tumor-associated macrophages, and that their re-education promotes regression of advanced tumors (4). The activation of macrophages through T cell secretion of IFN-γ and GM-CSF may trigger a feedback loop in which these macrophages promote endogenous T cell IFN-γ and GM-CSF secretion to maintain macrophage pro-inflammatory activity. The sustained endogenous IFN-γ response for up to eight weeks post-chNKG2D T cell treatment may be initiated and maintained by these macrophages (17).
The requirement of IFN-gamma, but not GM-CSF, to activate F4/80loLy-6C+ macrophages may be explained by differences in cytokine activation of signaling pathways and transcription factors that regulate monocyte to macrophage maturation and activation (41-43). STAT1 has been shown to function as a central regulator of inflammatory macrophage activation. Because IFN-gamma potently induces STAT1 activation during maturation, it may be critical in the activation of F4/80loLy-6C+ macrophages recruited to the peritoneum. GM-CSF secreted by CAR-expressing T cells may not be an important signal in F4/80loLy-6C+ macrophage activation because it only weakly induces STAT1 activation during macrophage maturation (44).
The increase in IL-12p40 production following treatment with chNKG2D T cells may contribute to the IFN-γ response by stimulating NK cell production of IFN-γ and inducing a TH1 response to promote endogenous anti-tumor immunity (33). The increased expression of IL-12 following chNKG2D T cell treatment was dependent on their production of both IFN-γ and GM-CSF. However, GM-CSF-deficiency had a greater impact on IL-12p40 production compared to T cell-derived IFN-γ. The greater dependence on GM-CSF may be attributed to the cytokines preference for priming IL-12p40 production, whereas IFN-γ favors IL-12p35 production over p40 (45-47). Despite its preference for IL-12p35, IFN-γ has been shown to prime the IL-12p40 gene promoter, which may be the reason for the sub-optimal IL-12p40 response elicited by IFN-γ-deficient CAR T cells (48). Both chNKG2D T cell-derived IFN-γ and GM-CSF were required to activate endogenous macrophages and enhance stimulation of antigen-specific T cell proliferation. The enhanced antigen processing and presenting abilities of macrophages following chNKG2D T cell therapy in concert with increased IL-12p40 may be responsible for the increase in endogenous T cell activation and trafficking of antigen-specific CD8+ T cells to the tumor site (17, 18).
In addition to upregulating IL-12, chNKG2D T cell therapy increased macrophage production of NO. NO may prevent tumor metastasis and synergize with IL-12 to enhance NK cell IFN-γ secretion and cytotoxicity (49, 50). The enhanced NO-mediated macrophage cytotoxicity of tumor cells following CAR T cell therapy is consistent with previous data showing that macrophages lyse ID8 tumor cells through secretion of NO (4, 51). It was previously shown that adoptive T cell-derived IFN-γ, but not GM-CSF, was responsible for the increase in NO production (18). Thus, macrophage NO cytotoxicity against ID8 tumor cells is IFN-γ-dependent.
The impaired ability of CAR T cell therapy to eliminate tumors in the absence of macrophages (clodronate liposomes) demonstrates the importance of host myeloid cells in tumor destruction. Although macrophages were necessary for complete tumor elimination, chNKG2D T cell treatment significantly inhibited tumor growth in the absence of macrophages. The lower tumor burden seen in mice treated with clodronate liposomes and CAR T cells may be attributed to chNKG2D T cell killing of tumor cells through perforin and granzymes (17). CAR T cell therapy may activate macrophages to enhance host immunity against tumor antigens that are freed upon tumor cell lysis. The inability of CAR T cell therapy to initiate host immunity in the absence of macrophages likely contributes to the impaired ability of CAR T cell therapy to eliminate tumor. Because host NK cells and T cells secrete higher amounts of IFN-γ after chNKG2D T cell treatment, the lower amount of IFN-γ produced at the tumor site in the absence of host macrophages corroborates the role of these local macrophages in initiating host immunity (18). In addition, the greater effect of macrophage depletion on tumor elimination compared to iNOS inhibition indicates additional roles for macrophages in tumor elimination independent of NO-mediated killing.
The reduction in macrophage secretion of IL-10 following treatment with chNKG2D T cells may be due to negative regulation of IL-10 production by IFN-γ (52). The decrease in the total amount of IL-10 in the tumor microenvironment is likely a combination of reduced macrophage secretion of IL-10 and lysis of NKG2D ligand-expressing Foxp3+ T reg cells capable of producing IL-10 (18). Limiting IL-10 production likely enhances tumor-specific CTL responses and inhibit tumor growth (53, 54). In addition to reduced IL-10 secretion, macrophages produced less IL-6 following treatment with chNKG2D T cells (Fig. 5A). In ovarian cancer, TH17 cytokines have been shown to contribute to tumor pathogenesis (55, 56). The reduction in global and macrophage-derived IL-6 may prevent the induction of a TH17 response and enhance the differentiation and maturation of APCs (18, 57).
The data from this study demonstrate that effector cytokines secreted by adoptively transferred CAR T cells induced changes in myeloid cell recruitment and function to transform the tumor microenvironment and inhibit ovarian tumor growth. The results demonstrate both the unique and redundant effects of T cell derived IFN-γ and GM-CSF on tumor-associated myeloid cells, and how these cytokines shape subsequent endogenous immunity in a tumor-bearing host. The mechanism by which adoptively transferred T cell-derived effector cytokines shape endogenous immunity via effects on local myeloid cells should be considered more broadly in optimizing adoptive T cell therapies for the treatment of cancer.
Acknowledgements
The authors thank DartLab for helpful assistance in Luminex analysis (Norris Cotton Cancer Center, Lebanon, NH), Dr. Mitsuhiro Iyori for technical assistance and advice, Dr. Brent Berwin for thoughtful suggestions on the manuscript, and the NIH Biological Resource Branch for providing recombinant human IL-2.
Financial Support: This work was supported by grants from the National Institutes of Health (CA130911, T32 AI007363). The views in this paper reflect the authors’ opinions and do not necessarily reflect the opinions of the National Institutes of Health.
References
- 1.Gabrilovich DI, Nagaraj S. Myeloid-derived suppressor cells as regulators of the immune system. Nat Rev Immunol. 2009;9:162–174. doi: 10.1038/nri2506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mantovani A, Sozzani S, Locati M, Allavena P, Sica A. Macrophage polarization: tumor-associated macrophages as a paradigm for polarized M2 mononuclear phagocytes. Trends Immunol. 2002;23:549–555. doi: 10.1016/s1471-4906(02)02302-5. [DOI] [PubMed] [Google Scholar]
- 3.Ostrand-Rosenberg S. Immune surveillance: a balance between protumor and antitumor immunity. Curr Opin Genet Dev. 2008;18:11–18. doi: 10.1016/j.gde.2007.12.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Hagemann T, Lawrence T, McNeish I, Charles KA, Kulbe H, Thompson RG, Robinson SC, Balkwill FR. “Re-educating” tumor-associated macrophages by targeting NF-kappaB. J Exp Med. 2008;205:1261–1268. doi: 10.1084/jem.20080108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Mosser DM, Edwards JP. Exploring the full spectrum of macrophage activation. Nat Rev Immunol. 2008;8:958–969. doi: 10.1038/nri2448. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.DeNardo DG, Andreu P, Coussens LM. Interactions between lymphocytes and myeloid cells regulate pro-versus anti-tumor immunity. Cancer Metastasis Rev. 29:309–316. doi: 10.1007/s10555-010-9223-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Biswas SK, Mantovani A. Macrophage plasticity and interaction with lymphocyte subsets: cancer as a paradigm. Nat Immunol. 11:889–896. doi: 10.1038/ni.1937. [DOI] [PubMed] [Google Scholar]
- 8.Sadelain M, Brentjens R, Riviere I. The promise and potential pitfalls of chimeric antigen receptors. Curr Opin Immunol. 2009;21:215–223. doi: 10.1016/j.coi.2009.02.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Sadelain M, Riviere I, Brentjens R. Targeting tumours with genetically enhanced T lymphocytes. Nat Rev Cancer. 2003;3:35–45. doi: 10.1038/nrc971. [DOI] [PubMed] [Google Scholar]
- 10.Barber A, Zhang T, Sentman CL. Immunotherapy with chimeric NKG2D receptors leads to long-term tumor-free survival and development of host antitumor immunity in murine ovarian cancer. J Immunol. 2008;180:72–78. doi: 10.4049/jimmunol.180.1.72. [DOI] [PubMed] [Google Scholar]
- 11.Brentjens RJ, Latouche JB, Santos E, Marti F, Gong MC, Lyddane C, King PD, Larson S, Weiss M, Riviere I, Sadelain M. Eradication of systemic B-cell tumors by genetically targeted human T lymphocytes co-stimulated by CD80 and interleukin-15. Nat Med. 2003;9:279–286. doi: 10.1038/nm827. [DOI] [PubMed] [Google Scholar]
- 12.Ahmed N, Ratnayake M, Savoldo B, Perlaky L, Dotti G, Wels WS, Bhattacharjee MB, Gilbertson RJ, Shine HD, Weiss HL, Rooney CM, Heslop HE, Gottschalk S. Regression of experimental medulloblastoma following transfer of HER2-specific T cells. Cancer Res. 2007;67:5957–5964. doi: 10.1158/0008-5472.CAN-06-4309. [DOI] [PubMed] [Google Scholar]
- 13.Barber A, Zhang T, DeMars LR, Conejo-Garcia J, Roby KF, Sentman CL. Chimeric NKG2D receptor-bearing T cells as immunotherapy for ovarian cancer. Cancer Res. 2007;67:5003–5008. doi: 10.1158/0008-5472.CAN-06-4047. [DOI] [PubMed] [Google Scholar]
- 14.Zhang T, Barber A, Sentman CL. Chimeric NKG2D modified T cells inhibit systemic T-cell lymphoma growth in a manner involving multiple cytokines and cytotoxic pathways. Cancer Res. 2007;67:11029–11036. doi: 10.1158/0008-5472.CAN-07-2251. [DOI] [PubMed] [Google Scholar]
- 15.Zhang T, Lemoi BA, Sentman CL. Chimeric NK-receptor-bearing T cells mediate antitumor immunotherapy. Blood. 2005;106:1544–1551. doi: 10.1182/blood-2004-11-4365. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang T, Barber A, Sentman CL. Generation of antitumor responses by genetic modification of primary human T cells with a chimeric NKG2D receptor. Cancer Res. 2006;66:5927–5933. doi: 10.1158/0008-5472.CAN-06-0130. [DOI] [PubMed] [Google Scholar]
- 17.Barber A, Sentman CL. Chimeric NKG2D T cells require both T cell- and host-derived cytokine secretion and perforin expression to increase tumor antigen presentation and systemic immunity. J Immunol. 2009;183:2365–2372. doi: 10.4049/jimmunol.0900721. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Barber A, Rynda A, Sentman CL. Chimeric NKG2D expressing T cells eliminate immunosuppression and activate immunity within the ovarian tumor microenvironment. J Immunol. 2009;183:6939–6947. doi: 10.4049/jimmunol.0902000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Van Rooijen N, Sanders A. Liposome mediated depletion of macrophages: mechanism of action, preparation of liposomes and applications. J Immunol Methods. 1994;174:83–93. doi: 10.1016/0022-1759(94)90012-4. [DOI] [PubMed] [Google Scholar]
- 20.Tacke F, Alvarez D, Kaplan TJ, Jakubzick C, Spanbroek R, Llodra J, Garin A, Liu J, Mack M, van Rooijen N, Lira SA, Habenicht AJ, Randolph GJ. Monocyte subsets differentially employ CCR2, CCR5, and CX3CR1 to accumulate within atherosclerotic plaques. J Clin Invest. 2007;117:185–194. doi: 10.1172/JCI28549. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Nibbering PH, Leijh PC, van Furth R. Quantitative immunocytochemical characterization of mononuclear phagocytes. II. Monocytes and tissue macrophages. Immunology. 1987;62:171–176. [PMC free article] [PubMed] [Google Scholar]
- 22.Geissmann F, Manz MG, Jung S, Sieweke MH, Merad M, Ley K. Development of monocytes, macrophages, and dendritic cells. Science. 327:656–661. doi: 10.1126/science.1178331. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Hart KM, Bak SP, Alonso A, Berwin B. Phenotypic and functional delineation of murine CX(3)CR1 monocyte-derived cells in ovarian cancer. Neoplasia. 2009;11:564–573. doi: 10.1593/neo.09228. 561 p following 573. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Scarlett UK, Cubillos-Ruiz JR, Nesbeth YC, Martinez DG, Engle X, Gewirtz AT, Ahonen CL, Conejo-Garcia JR. In situ stimulation of CD40 and Toll-like receptor 3 transforms ovarian cancer-infiltrating dendritic cells from immunosuppressive to immunostimulatory cells. Cancer Res. 2009;69:7329–7337. doi: 10.1158/0008-5472.CAN-09-0835. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Hagemann T, Wilson J, Burke F, Kulbe H, Li NF, Pluddemann A, Charles K, Gordon S, Balkwill FR. Ovarian cancer cells polarize macrophages toward a tumor-associated phenotype. J Immunol. 2006;76:5023–5032. doi: 10.4049/jimmunol.176.8.5023. [DOI] [PubMed] [Google Scholar]
- 26.Iellem A, Mariani M, Lang R, Recalde H, Panina-Bordignon P, Sinigaglia F, D’Ambrosio D. Unique chemotactic response profile and specific expression of chemokine receptors CCR4 and CCR8 by CD4(+)CD25(+) regulatory T cells. J Exp Med. 2001;194:847–853. doi: 10.1084/jem.194.6.847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Curiel TJ, Coukos G, Zou L, Alvarez X, Cheng P, Mottram P, Evdemon-Hogan M, Conejo-Garcia JR, Zhang L, Burow M, Zhu Y, Wei S, Kryczek I, Daniel B, Gordon A, Myers L, Lackner A, Disis ML, Knutson KL, Chen L, Zou W. Specific recruitment of regulatory T cells in ovarian carcinoma fosters immune privilege and predicts reduced survival. Nat Med. 2004;10:942–949. doi: 10.1038/nm1093. [DOI] [PubMed] [Google Scholar]
- 28.Carmeliet P, Dor Y, Herbert JM, Fukumura D, Brusselmans K, Dewerchin M, Neeman M, Bono F, Abramovitch R, Maxwell P, Koch CJ, Ratcliffe P, Moons L, Jain RK, Collen D, Keshert E. Role of HIF-1alpha in hypoxia-mediated apoptosis, cell proliferation and tumour angiogenesis. Nature. 1998;394:485–490. doi: 10.1038/28867. [DOI] [PubMed] [Google Scholar]
- 29.Gabrilovich D. Mechanisms and functional significance of tumour-induced dendritic-cell defects. Nat Rev Immunol. 2004;4:941–952. doi: 10.1038/nri1498. [DOI] [PubMed] [Google Scholar]
- 30.Watson GA, Lopez DM. Aberrant antigen presentation by macrophages from tumor-bearing mice is involved in the down-regulation of their T cell responses. J Immunol. 1995;155:3124–3134. [PubMed] [Google Scholar]
- 31.Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Hamilton JA. Colony-stimulating factors in inflammation and autoimmunity. Nat Rev Immunol. 2008;8:533–544. doi: 10.1038/nri2356. [DOI] [PubMed] [Google Scholar]
- 33.Trinchieri G. Interleukin-12 and the regulation of innate resistance and adaptive immunity. Nat Rev Immunol. 2003;3:133–146. doi: 10.1038/nri1001. [DOI] [PubMed] [Google Scholar]
- 34.Sica A, Saccani A, Bottazzi B, Polentarutti N, Vecchi A, van Damme J, Mantovani A. Autocrine production of IL-10 mediates defective IL-12 production and NF-kappa B activation in tumor-associated macrophages. J Immunol. 2000;164:762–767. doi: 10.4049/jimmunol.164.2.762. [DOI] [PubMed] [Google Scholar]
- 35.Sica A, Larghi P, Mancino A, Rubino L, Porta C, Totaro MG, Rimoldi M, Biswas SK, Allavena P, Mantovani A. Macrophage polarization in tumour progression. Semin Cancer Biol. 2008;18:349–355. doi: 10.1016/j.semcancer.2008.03.004. [DOI] [PubMed] [Google Scholar]
- 36.Grabstein KH, Urdal DL, Tushinski RJ, Mochizuki DY, Price VL, Cantrell MA, Gillis S, Conlon PJ. Induction of macrophage tumoricidal activity by granulocyte-macrophage colony-stimulating factor. Science. 1986;232:506–508. doi: 10.1126/science.3083507. [DOI] [PubMed] [Google Scholar]
- 37.Spitalny GL, Havell EA. Monoclonal antibody to murine gamma interferon inhibits lymphokine-induced antiviral and macrophage tumoricidal activities. J Exp Med. 1984;159:1560–1565. doi: 10.1084/jem.159.5.1560. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Aruga A, Shu S, Chang AE. Tumor-specific granulocyte/macrophage colony-stimulating factor and interferon gamma secretion is associated with in vivo therapeutic efficacy of activated tumor-draining lymph node cells. Cancer Immunol Immunother. 1995;41:317–324. doi: 10.1007/BF01517220. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Nagoshi M, Goedegebuure PS, Burger UL, Sadanaga N, Chang MP, Eberlein TJ. Successful adoptive cellular immunotherapy is dependent on induction of a host immune response triggered by cytokine (IFN-gamma and granulocyte/macrophage colony-stimulating factor) producing donor tumor-infiltrating lymphocytes. J Immunol. 1998;160:334–344. [PubMed] [Google Scholar]
- 40.Martinez FO, Helming L, Gordon S. Alternative activation of macrophages: an immunologic functional perspective. Annu Rev Immunol. 2009;27:451–483. doi: 10.1146/annurev.immunol.021908.132532. [DOI] [PubMed] [Google Scholar]
- 41.Hagemann T, Biswas SK, Lawrence T, Sica A, Lewis CE. Regulation of macrophage function in tumors: the multifaceted role of NF-kappaB. Blood. 2009;113:3139–3146. doi: 10.1182/blood-2008-12-172825. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Ohmori Y, Hamilton TA. Requirement for STAT1 in LPS-induced gene expression in macrophages. J Leukoc Biol. 2001;69:598–604. [PubMed] [Google Scholar]
- 43.Vakkila J, Demarco RA, Lotze MT. Coordinate NF-kappaB and STAT1 activation promotes development of myeloid type 1 dendritic cells. Scand J Immunol. 2008;67:260–269. doi: 10.1111/j.1365-3083.2007.02068.x. [DOI] [PubMed] [Google Scholar]
- 44.Coccia EM, Del Russo N, Stellacci E, Testa U, Marziali G, Battistini A. STAT1 activation during monocyte to macrophage maturation: role of adhesion molecules. Int Immunol. 1999;11:1075–1083. doi: 10.1093/intimm/11.7.1075. [DOI] [PubMed] [Google Scholar]
- 45.Trinchieri G. Interleukin-12: a cytokine at the interface of inflammation and immunity. Adv Immunol. 1998;70:83–243. doi: 10.1016/s0065-2776(08)60387-9. [DOI] [PubMed] [Google Scholar]
- 46.Hayes MP, Wang J, Norcross MA. Regulation of interleukin-12 expression in human monocytes: selective priming by interferon-gamma of lipopolysaccharide-inducible p35 and p40 genes. Blood. 1995;86:646–650. [PubMed] [Google Scholar]
- 47.Sweet MJ, Campbell CC, Sester DP, Xu D, McDonald RC, Stacey KJ, Hume DA, Liew FY. Colony-stimulating factor-1 suppresses responses to CpG DNA and expression of toll-like receptor 9 but enhances responses to lipopolysaccharide in murine macrophages. J Immunol. 2002;168:392–399. doi: 10.4049/jimmunol.168.1.392. [DOI] [PubMed] [Google Scholar]
- 48.Ma X, Chow JM, Gri G, Carra G, Gerosa F, Wolf SF, Dzialo R, Trinchieri G. The interleukin 12 p40 gene promoter is primed by interferon gamma in monocytic cells. J Exp Med. 1996;183:147–157. doi: 10.1084/jem.183.1.147. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Diefenbach A, Schindler H, Rollinghoff M, Yokoyama WM, Bogdan C. Requirement for type 2 NO synthase for IL-12 signaling in innate immunity. Science. 1999;284:951–955. doi: 10.1126/science.284.5416.951. [DOI] [PubMed] [Google Scholar]
- 50.Weiss JM, Ridnour LA, Back T, Hussain SP, He P, Maciag AE, Keefer LK, Murphy WJ, Harris CC, Wink DA, Wiltrout RH. Macrophage-dependent nitric oxide expression regulates tumor cell detachment and metastasis after IL-2/anti-CD40 immunotherapy. J Exp Med. 207:2455–2467. doi: 10.1084/jem.20100670. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51.Miguel R. D. Vicetti, Cherpes TL, Watson LJ, McKenna KC. CTL induction of tumoricidal nitric oxide production by intratumoral macrophages is critical for tumor elimination. J Immunol. 185:6706–6718. doi: 10.4049/jimmunol.0903411. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Hu X, Paik PK, Chen J, Yarilina A, Kockeritz L, Lu TT, Woodgett JR, Ivashkiv LB. IFN-gamma suppresses IL-10 production and synergizes with TLR2 by regulating GSK3 and CREB/AP-1 proteins. Immunity. 2006;24:563–574. doi: 10.1016/j.immuni.2006.02.014. [DOI] [PubMed] [Google Scholar]
- 53.Curiel TJ. Tregs and rethinking cancer immunotherapy. J Clin Invest. 2007;117:1167–1174. doi: 10.1172/JCI31202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54.Saraiva M, O’Garra A. The regulation of IL-10 production by immune cells. Nat Rev Immunol. 10:170–181. doi: 10.1038/nri2711. [DOI] [PubMed] [Google Scholar]
- 55.Langowski JL, Zhang X, Wu L, Mattson JD, Chen T, Smith K, Basham B, McClanahan T, Kastelein RA, Oft M. IL-23 promotes tumour incidence and growth. Nature. 2006;442:461–465. doi: 10.1038/nature04808. [DOI] [PubMed] [Google Scholar]
- 56.Kryczek I, Wei S, Zou L, Altuwaijri S, Szeliga W, Kolls J, Chang A, Zou W. Cutting edge: Th17 and regulatory T cell dynamics and the regulation by IL-2 in the tumor microenvironment. J Immunol. 2007;178:6730–6733. doi: 10.4049/jimmunol.178.11.6730. [DOI] [PubMed] [Google Scholar]
- 57.Menetrier-Caux C, Montmain G, Dieu MC, Bain C, Favrot MC, Caux C, Blay JY. Inhibition of the differentiation of dendritic cells from CD34(+) progenitors by tumor cells: role of interleukin-6 and macrophage colony-stimulating factor. Blood. 1998;92:4778–4791. [PubMed] [Google Scholar]