Abstract
Prenatal maternal immune activation has been used to test the neurodevelopmental hypothesis of schizophrenia. Most of the data are in mouse models; far less is available for rats. We previously showed that maternal weight change in response to the immune activator polyinosinic-polycytidylic (Poly IC) in rats differentially affects offspring. Therefore, we treated gravid Harlan Sprague-Dawley rats i.p. on embryonic day 14 with 8 mg/kg of Poly IC or Saline. The Poly IC group was divided into those that lost or gained the least weight, Poly IC (L), versus those that gained the most weight, Poly IC (H), following treatment. The study design controlled for litter size, litter sampling, sex distribution, and test experience. We found no effects of Poly IC on elevated zero-maze, open-field activity, object burying, light-dark test, straight channel swimming, Morris water maze spatial acquisition, reversal, or shift navigation or spatial working or reference memory, or conditioned contextual or cued fear or latent inhibition. The Poly IC (H) group showed a significant decrease in the rate of route-based learning when visible cues unavailable in the Cincinnati water maze and reduced prepulse inhibition of acoustic startle in females, but not males. The Poly IC (L) group exhibited altered responses to acute pharmacological challenges: exaggerated hyperactivity in response to (+)-amphetamine and an attenuated hyperactivity in response to MK-801. This model did not exhibit the cognitive, acoustic startle, or latent inhibition deficits reported in Poly IC-treated rats, but showed changes in response to drugs acting on neurotransmitter systems implicated in the pathophysiology of schizophrenia (dopaminergic hyperfunction and glutamatergic hypofunction).
Keywords: Polyinosinic-polycytidylic acid, Poly IC, (+)-amphetamine-induced locomotor activity, prenatal immune activation, MK-801-induced locomotor activity, Morris water maze, Cincinnati water maze, rat, elevated zero-maze, marble burying, light-dark test, conditioned fear, latent inhibition, autism spectrum disorder
Introduction
Maternal infection during pregnancy is associated with increased risk of neuropsychiatric illness such as autism spectrum disorder (ASD) and schizophrenia in their children (Boksa, 2010;Bronson and Richtand, 2011;Ciaranello and Ciaranello, 1995;Patterson, 2002;Brown and Derkits, 2010). Prenatal influenza infections have been associated with increased risk for the development of schizophrenia (Mednick et al., 1988;Brown et al., 2004a;Brown et al., 2004b) as have prenatal infections with rubella and toxoplasma (Penner and Brown, 2007). More recently a family history of psychotic behavior combined with urinary tract infection during pregnancy has been associated with increased prevalence of schizophrenia in their children (Clarke et al., 2009).
Based on such data, experiments to model prenatal influenza infections during pregnancy have been conducted and shown to induce behavioral abnormalities in the offspring (Shi et al., 2003). Further experiments confirmed and extended these observations (Fatemi et al., 1999;Fatemi et al., 2002;Fatemi et al., 2005;Moreno et al., 2011). To determine if the transplacental effects were attributable to the virus or maternal response to infection, experiments have turned to viral immune activators such as double-stranded synthetic RNA polyinosinic-polycytidylic acid (Poly IC) or lipopolysaccharide (LPS). Injection of Poly IC during gestation induces effects in mouse offspring brain and behavior similar to the effects seen after maternal influenza-induced infection (Ozawa et al., 2006;Shi et al., 2003;Shi et al., 2009;Smith et al., 2007;Cunningham et al., 2007;Bitanihirwe et al., 2010a;Bitanihirwe et al., 2010b;Meyer et al., 2006;Nyffeler et al., 2006). A number of findings using Poly IC have been found in rats as well although differences in strain and other factors have varied making comparisons difficult. (Investigators date the first day of gestation as either embryonic day (E)0 or E1; herein we adjusted all dates to the E0 method.) For example, E14 i.v. Poly IC administered (Zuckerman et al., 2003a;Zuckerman et al., 2003a;Zuckerman and Weiner, 2003;Zuckerman et al., 2003b) was found to reduce latent inhibition, induce exaggerated locomotor activity to (+)-amphetamine and MK-801, reduce T-maze reversal trials-to-criterion, and reduce Morris water maze reversal latency in Wistar rats. E14 i.p. Poly IC (Bronson et al., 2011;Richtand et al., 2011) was found to induce reduced locomotor activity to MK-801 and (+)-amphetamine challenge, E14 i.v. Poly IC (Wolff and Bilkey, 2008;Wolff and Bilkey, 2010;Dickerson et al., 2010;Wolff et al., 2011) reduced prepulse inhibition (PPI) of the acoustic startle response (ASR), and E14 i.v. Poly IC (Yee et al., 2011) decreased PPI and reduced time in open and open entries in the elevated plus maze all in Sprague-Dawley rats. E14 i.v. Poly IC (Howland et al., 2012;Zhang et al., 2011) increased errors on an operant set-shifting task, reduced PPI, reduced locomotor activity to MK-801 before puberty but not after, and reduced novel position but not novel object recognition in Long-Evans rats.
Evidence of prenatal maternal immune activation as a cause of offspring neurochemical, immunohistochemical, neuroinflammatory, and behavioral abnormalities has been recently the subject of several reviews (Shi et al., 2009;Meyer et al., 2009;Meyer et al., 2005;Bronson and Richtand, 2011;Deverman and Patterson, 2009;Boksa, 2010). The convergent evidence is consistent with the hypothesis that maternal proinflammatory cytokine release is one source of transplacental effects on offspring brain development (Smith et al., 2007;Meyer et al., 2007). The evidence in mice that maternal immune activation induces abnormal brain and behavioral development is substantial; the evidence in rats is also extensive but more variable. Not all experiments have taken into account factors in developmental study design, such as litter effects, litter size, and stratification by sex. It is known that over-sampling offspring from a few litters and treating each offspring as if orthogonal can lead to overestimation of significance and overestimate effect size in multiparous species (Holson and Pearce, 1992). For a fuller discussion of this and related issues in prenatal study design, see (Meyer et al., 2005;Meyer et al., 2009) and for cytokine-induced effects on brain development see (Deverman and Patterson, 2009).
The purpose of the present experiment was to assess the effects of prenatal exposure to Poly IC in both male and female Sprague-Dawley offspring using a wider range of behavioral tests than previously undertaken to more fully characterize the effect of Poly IC as has been recommended in other contexts (Crawley, 2007b;Crawley, 2008;Paylor et al., 2006;Vorhees, 1996;Bailey et al., 2006;McIlwain et al., 2001), especially tests assessing cognitive and affective behaviors that have received less attention.
Materials and Methods
Subjects
Harlan Sprague-Dawley rats (Harlan Laboratories, Indianapolis, IN) were bred in-house using experienced sires and nulliparous females (225-250 g upon arrival). Females were acclimated to the vivarium for not less than two weeks prior to cohabitation with males. The vivarium is AAALAC accredited. Females were not staged for estrus prior to pairing, therefore, the day a sperm plug was found was considered E0. Plug-positive females were placed in individual polycarbonate cages (26 x 48 x 20 cm) with sterile woodchip bedding and ad lib NIH-07 diet and filtered water. At the time of conception, females were on average between 90-120 days old. Each cage was provided with a stainless steel enclosure to provide environmental enrichment (Vorhees et al., 2008). Pregnant females were checked twice daily for delivery. The day of parturition was designated postnatal day (P)0. On P1, dams were removed from the cage and offspring counted, sexed, and assigned an arbitrary number. Using a random number table, 4 males and 4 females were retained. All experimental procedures were approved by the Cincinnati Children’s Research Foundation Institutional Animal Care and Use Committee and adhered to NIH and Society for Neuroscience guidelines for the humane care of animals.
Treatment
Polyinosinic-polycytidylic acid (Poly IC) sodium (Sigma-Aldrich #P1530, St. Louis, MO) was dissolved in sterile 0.9% saline at a concentration of 8 mg/ml and 1 ml/kg body weight of solution was administered intraperitoneally for a dose of 8 mg/kg body weight. Forty gravid dams were randomly assigned to two groups: 20 dams to the Saline group and 20 dams to the Poly IC group on E14 (day chosen was based on previous experiments (Bronson et al., 2011;Richtand et al., 2011)). The timing of injection was based upon the work of Zuckerman and colleagues describing outcomes following Poly IC injection on varying gestational dates in rats (Zuckerman et al., 2003a). Because of differences in naming conventions, Zuckerman’s gestational day 15 is defined as E14 in the present experiment, as explained previously. The Poly IC dose was based upon dosage ranges used by others for rat intraperitoneal injection (Fortier et al., 2004b;Gilmore et al., 2005) [range 0.75 to 10 mg/kg]. Maternal body weight was recorded on E14-18 and E20 at the same time each day. On P1, after culling, litters were weighed weekly until the end of the experiment.
Experimental design
Since all litters had 4 males and 4 females, male-female pairs were randomly assigned to different test sequences.
Pair-A
Elevated zero-maze (EZM) on P65
Open-field locomotor activity on P66
Marble burying on P66
Acoustic startle response (ASR) with prepulse inhibition (PPI) on P67
Straight channel swimming on P68
Cincinnati water maze (CWM) beginning on P69
Morris water maze (MWM) matching-to sample (MTS) beginning on P87
Pair-B
Conditioned Fear on P60-62. Tone preexposed subgroup (PE)
Open-field with pharmacological challenge between P63-65
Pair-C
Conditioned Fear on P60-62. Tone non-preexposed subgroup (NPE)
Open-field with pharmacological challenge between P63-65
Pair-D
Light-dark test between P65-68
Straight channel swimming on P69
Standard MWM in four phases: acquisition, reversal, shift (all with hidden platform), and cued (with visible platform) beginning on P70
Housing and weaning
Dams were removed from their litters on P28. On P35, offspring were re-housed in same sex pairs such that from each litter male A and B were housed together, as were female A and B; male C and D were housed together, as were female C and D.
Behavioral Methods
Pair-A
Elevated zero-maze
The apparatus was an elevated (72 cm above the floor) 10-cm wide circular track 105 cm in diameter made of black Kydex divided equally in 4 quadrants, 2 enclosed by 28 cm high black side walls and 2 open but with 1.3 cm high clear polyethylene curbs to prevent slipping at the edges (Braun et al., 2011). The room was dimly lighted (11.7 lux at the center) and animals were tested as previously described (Braun et al., 2011) for 10 min after being started by being placed in the middle of one of the closed zones. Behavior was monitored via an overhead closed circuit video camera to an observer outside the test room. The observer scored time-in-open, head dips, number of zone crossings, and number of stretch-attend episodes. Testing was done once between P65-68.
Locomotor activity
The apparatus were clear polyethylene test chambers ~40 x 40 cm (Accuscan Instruments, Columbus, OH) with 16 LED-photodetector pairs located along the x and y-axes and spaced every 2.5 cm, and another 16 pairs elevated above the floor to detect rearing. A clear lid with ventilation holes covered each chamber. Rats were tested for 60 min and data captured using VersaMax software in 5 min intervals for horizontal activity (all beam interruptions), distance (successive beam interruptions (cm), rearing frequency, and time spent in the central and peripheral zones.
Marble burying
The test was conducted in a standard housing cage using 18 blue marbles arranged in a symmetrical grid, evenly spaced using a template overlay in three rows of six marbles per row. The cage was filled with woodchip bedding to a standard depth of 5 cm. Rats were placed in the cage for 20 min with a filter top cover. At the end of 20 min, the rat was removed and the number of marbles at least two-thirds covered was counted.
Acoustic startle response (ASR) with prepulse inhibition (PPI)
The apparatus were SR-LAB startle chambers (San Diego Instruments, San Diego, CA). Rats were placed in an acrylic cylinder closed at both ends by sliding doors. The cylinder was mounted to an acrylic plate with four legs which were placed on a stage in which each leg fit into a specific recessed cup. The platform had a force transducer mounted on the underside. The stage was placed inside a sound attenuated test chamber with ventilation fan. The background white noise was 70 dB and the startle signal (SS) was a 120 dB (SPL) white noise burst lasting 20 ms. Prepulses were also white noise bursts of 20 ms duration at sound levels of 73, 75, or 80 dB. Prepulses preceded the startle signal by 100 ms from prepulse (PP) onset to SS onset. Rats were placed in the apparatus and given a 5 min acclimation period with only background noise. Trials were of 5 types: no signal, SS alone, PP-73 + SS, PP-75 + SS, or PP-80 + SS. These were presented in a 5 x 5 Latin square repeated twice for a total of 50 trials. Responses were recorded for 100 ms after SS onset and recorded as voltage change in mV. The dependent measure was the maximum voltage change within the response window.
Straight channel
Testing was in a 15 x 244 x 50 cm straight swimming channel of high-density black polyethylene filled to a depth of 30 cm with a platform submerged 1.5 cm below the surface of the water at one end. Each rat received four consecutive trials. On each trial, the rats were placed at one end facing the wall and timed until they climbed on the platform at the opposite end (maximum time = 2 min/trial). This procedure teaches rats that escape is possible, tests swimming ability, and motivation to escape from the water. Rats not given this procedure prior to the CWM cannot successfully maintain searching long enough to find the exit.
CWM
The CWM, a test of route-based egocentric learning, began the day following straight channel. The maze, as described elsewhere (Vorhees, 1987;Vorhees et al., 1991), consists of nine T-shaped cul-de-sacs that branch from a central circuitous pathway. The width of the channels was 15 cm with walls 51 cm high filled to a depth of 30 cm with water. Water was changed at least twice per week and allowed to equilibrate overnight to room temperature (21 ± 1°C). Testing was conducted in complete darkness using infrared LED emitters and a near infrared camera was placed above the maze and connected to a closed circuit monitor located in an adjacent room where the experimenter recorded performance (Vorhees et al., 2011). Administering the test under infrared light eliminates extramaze cues and prevents animals from using distal cues to navigate. The animals were started facing the wall at the start position and allowed 5 min per trial to find the escape platform with a 5 min intertrial interval if they failed to locate the goal on the first trial of the day within the time limit. Animals were given two trials/day for 18 days. Errors and latency to reach the goal were recorded. An error was scored when an animal digressed from the central pathway into a stem or dead-end arm of one of the T-shaped cul-de-sacs. Repetitive errors within a T were counted individually. Occasionally an animal did not find the escape after multiple trials. When this occurred, some animals discontinued searching and treaded water. This resulted in few errors compared to animals that remained actively searching. To correct for this, error scores for non-searching trials were assigned the number of errors committed by the animal in the experiment that made the most errors + 1.
MWM Matching-to-Sample
The maze was constructed of stainless steel and measured 210 cm in diameter and 51 cm high filled to a depth of 30 cm with room temperature water (21 ± 1°C). The escape platform was 10 cm in diameter and was positioned in one of four ordinal positions halfway between the center and the tank wall and submerged 1.5 cm below the surface. The maze was painted flat black and the platform was made of clear acrylic and was not visible against the black background. Rats were tested for 21 days for 2 trials/day. Every day the start and platform were placed in new positions in a pseudo-random order. The daily trials consisted of 2 trials with the same start and platform positions. Trial-1 was the sample and Trial-2 the matching trial to assess trial-dependent working memory. Data were captured using an overhead camera connected to a PC with AnyMaze tracking software (Stoelting Instruments, Wood Dale, IL). The data analyzed were latency, path length (cm), and cumulative distance from the platform (m) recorded every 0.2 s.
Pair-B
Latent Inhibition
Latent inhibition was assessed using a fear conditioning paradigm following a previously reported method (Smith et al., 2007). On each day, animals were placed in test chambers (Coulbourn Instruments, Allentown, PA) fitted with a speaker and grid floor connected to a scrambled foot shock source, and video camera mounted in the ceiling connected to Coulbourn Freeze Frame software. Test chambers were placed inside larger sound-attenuating enclosures. Pair-B was the PE (pre-exposed) group. On day 1, animals were placed in the test chambers for 10 min during which they were presented with 30 tones (82 dB, 2 kHz, 30 s duration) separated by 30 s inter-stimulus intervals, followed by 3 tone-footshock pairings with the shock occurring during the last 1 s of each of these final tone presentations (0.5 mA scrambled and delivered through the grid floor). Day-2, rats were returned to the test chamber to assess contextual conditioning for 6 min during which immobility intervals of >4 s were scored from replayed data files. Day-3, rats were returned to the test chamber with a different floor texture and tested for 6 min; the first 3 min with no-tone and the last 3 min with tone and scored for immobility intervals of >4 s and the pre- and post-tone immobility were compared.
Locomotor activity with drug challenge
Rats were placed in the locomotor activity apparatus for 30 min with no drug to re-habituate them to the test chambers. They were then briefly removed, injected subcutaneously with 1.0 mg/kg of (+)-amphetamine or 0.3 mg/kg of MK-801 (depending on the subgroup assignment) and placed back in the chambers for an additional 180 min.
Arm-C
Latent Inhibition
This was as described above except that Pair-C was assigned to the NPE group such that they received the same test procedure except that on Day-1 they received no tone exposure prior to the 3 tone-shock pairings.
Locomotor activity with drug challenge
Rats were tested the same as Pair-B with half the animals challenged with 1.0 mg/kg of (+)-amphetamine and half with 0.3 mg/kg of MK-801.
Pair-D
Light/Dark Test
The test was conducted in the locomotor activity chambers described above except that a black acrylic box with lid was inserted that occupied 50% of the floor area (i.e., 20 x 40 cm). Rats were placed in the lighted side and data recorded for 10 min. Data analyzed were dark side entries, latency to first entry to the dark side, and time spent in the light side.
Straight Channel
Straight channel testing for this pair of offspring was conducted the same as above.
MWM
Spatial learning and memory were assessed in the MWM using procedures described previously (Vorhees and Williams, 2006). Testing was performed in the same apparatus as for Pair-A, but with a different procedure. Animals were tested in 4 phases, 3 with hidden platforms and 1 with a marked platform. Phase-1 was acquisition (platform in SW quadrant), Phase-2 was reversal (platform in NE quadrant), and Phase-3 was shift (platform in NW quadrant), and Phase-4 was cued. Briefly, animals received 4 trials per day for 6 days followed by a 30 s probe trial on day-7 with the platform removed for each of the three hidden platform phases. Each phase used a platform of a different size (10, 7, and 5 cm diameter, respectively). Following the three hidden phases, a cued phase was conducted to ensure that the rats could swim normally and see properly to locate the platform with a visible cue above it. For this procedure, the 10 cm platform was used with the addition of a plastic ball mounted on top of a brass rod that protruded 10 cm above the surface of the water to mark the platform’s location. Curtains were closed around the maze to minimize extramaze cues and the animals were given four trials per day for two days with the platform and start positions changed randomly for every trial such that spatial strategies were ineffective to locate the platform. During hidden platform trials, a camera and tracking software were used to map the animals’ performance (AnyMaze, Stoelting Instruments, Wood Dale, IL). On platform trials, latency, cumulative distance, path length, and swim speed were analyzed. For probe trials, platform site crossovers, swim speed, and average distance from the target were analyzed. For the cued phase, latency was recorded manually because the curtains reduced the amount of light reaching the maze and prevented computerized tracking.
Statistical Analysis
Data were analyzed using completely randomized block analyses of variance (ANOVA) mixed linear models (SAS V9.2, Proc Mixed, SAS Institute, Cary, NC). Treatment and sex were fixed factors within blocks (litters), hence, these factors are controlled for litter effects. The Kenward-Roger adjusted degrees of freedom method was used. Interactions were analyzed using the slice-effect ANOVA method at each level of the within-subject factor. A posteriori group comparisons were analyzed using the Hochberg step-up method to control for multiple comparisons. The significance threshold was set at p ≤ 0.05 and data are presented as least square (LS) mean ± LS SEM.
Results
Maternal body weight
We previously observed that maternal body weight gain or loss after Poly IC predicts behavioral outcome (Bronson et al., 2011). Based on the assumption that change in maternal body weight represents the degree of immune reaction and therefore the magnitude of cytokine response reaching the fetal brain, in the present experiment we divided the Poly IC groups into subgroups as a function of maternal weight change following Poly IC on E14 into those that gained the most vs. those that gained the least or lost weight. These maternal body weight groups are shown in Table 1. A repeated measures ANOVA showed no effect of treatment, but a significant effect of day (p<0.0001) and treatment x day (F(12,217) = 6.12, p<0.0001). Pairwise comparisons showed that group differences were significant on E18-20 only.
Table 1.
Maternal body weight (g) during gestation in Saline and Poly IC treated dams with treatment being on E14
| Embryonic day (E) | Saline | Poly IC (H) | Poly IC (L) |
|---|---|---|---|
| N | 20 | 10 | 10 |
| E14 | 312.4 ± 4.2 | 314.6 ± 5.9 | 314.0 ± 5.9 |
| E15 | 321.5 ± 4.2 | 323.7 ± 6.0 | 314.5 ± 5.9 |
| E18 | 362.8 ± 4.2 | 363.5 ± 5.9 | 349.9 ± 5.9* |
| E20 | 396.5 ± 4.2 | 396.2 ± 5.9 | 378.9 ± 5.9* |
Maternal body weight (g) during gestation (LS mean ± SEM)
P<0.05 vs. Saline (one-tailed).
We determined maternal body weight change for each interval because maternal weight gain during gestation can fluctuate. To ensure that the weight change subdivision was reliable we determined the weight difference between body mass on E14 prior to treatment vs. on E15; we repeated this for each subsequent day of gestation, i.e., E16-E14, E17-E14 and rank ordered the differences from lowest to highest for each of the weight gain interval and summed the ranks to obtain an overall rank order and used this to divide the Poly IC group into the Poly IC (H) (i.e., high-gain) and Poly IC (L) (i.e., low-gain) groups which were used in all subsequent analyses. Analysis of body weight gain showed a significant main effect of treatment (F(2,37) = 13.92, p<0.0001), day (p<0.0001) and treatment x day (F(6,109) = 2.23, p<0.05). A posteriori comparisons showed that the Poly IC (L) group gained significantly less weight on all intervals examined from E17 through E20 (all p<0.002) compared with the other groups.
Gestation Length, Litter Size, and Sex ratio. ANOVA of gestation length showed no significant treatment effects (Table 2). Litters were not disturbed on the day of birth (P0). On P1, dams were removed and offspring counted, sexed, and weighed prior to culling. There were no significant effects of treatment on litter number, or the number of males or females within litters (Table 2).
Table 2.
Gestation length and litter characteristics, including litter size and average number of males and females per litter
| Saline | Poly IC (H) | Poly IC (L) | |
|---|---|---|---|
| N | 20 | 10 | 10 |
| Gestation length (day) | 21.2 ± 0.2 | 22.0 ± 0.2 | 22.1 ± 0.2 |
| Litter size (P1)* | 13.7 ± 0.4 | 13.5 ± 0.6 | 12.1 ± 0.6 |
| No. males/litter | 6.8 ± 0.4 | 6.9 ± 0.6 | 5.9 ± 0.6 |
| No. females/litter | 6.9 ± 0.4 | 6.6 ± 0.6 | 6.2 ± 0.6 |
Litter size prior to culling (LS mean ± SEM)
Offspring Body Weight
Prior to culling, offspring were removed from the dam on P1,then sexed and weighed. Body weight analysis on P1 prior to culling showed no significant main effect of treatment, but there were significant effects of sex (F(1,277) = 29.20, p<0.0001) and treatment x sex (F(2,277) = 8.64, p<0.0002). Slice-effect ANOVAs for treatment on each sex showed no significant effect for males or females. Slice-effect ANOVAs for sex on each treatment group showed no sex difference for Saline controls (mean ± SEM; males = 7.3 ± 0.2 vs. females = 7.2 ± 0.2), but significant differences for the Poly IC (H) (males = 7.6 ± 0.2 vs. females = 7.0 ± 02, p<0.0001) and Poly IC (L) groups (males = 7.3 ± 0.2 vs. females = 7.1 ± 0.2, p<0.05).
After culling, offspring were weighed weekly. Analysis of preweaning body weight showed no significant main effect of treatment or treatment x sex interaction, but there was a significant treatment x week effect (F(6,839) = 3.53, p<0.002); no other interactions were significant. Slice-effect ANOVAs on P7, 14, 21, and 28 body weights showed no significant treatment effect on any single week indicating that the body weight effect was small.
Postweaning body weight data were analyzed similarly P35-91. The treatment main effect was not significant. There were significant main effects of sex (p<0.0001) and week (p<0.0001). There were also two significant interactions with treatment: treatment x week (F(16,2165) = 2.45, p<0.001) and treatment x sex x week (F(16,2166) = 1.73, p<0.05). Treatment slice-effect ANOVAs for each sex for each week showed significant treatment effects for males on P84 (p=0.05) and a suggestive trend on P91 (p<0.07). For females, a significant effect of treatment occurred on P91 (p=0.05) with trends on P84 (p<0.08) and P77 (p<0.07). Body weights at selected ages are shown in Table 3.
Table 3.
Offspring body weights at representative ages from weaning to the end of behavioral testing
| Age (P) | Saline | Poly IC (H) | Poly IC (L) | |||
|---|---|---|---|---|---|---|
| M | F | M | F | M | F | |
| 28 | 97.5 ± 1.0 | 88.7 ± 1.0 | 95.1 ± 1.4 | 85.6 ± 1.4 | 95.9 ± 1.4 | 87.5 ± 1.4 |
| 56 | 290.0 ± 3.0 | 201.4 ± 3.0 | 289.0 ± 4.3 | 194.6 ± 4.3 | 290.4 ± 4.3 | 202.4 ± 4.3 |
| 84 | 392.9 ± 3.0 | 255.7 ± 3.0 | 380.4 ± 4.3 | 245.0 ± 4.3 | 392.8 ± 4.3 | 257.5 ± 4.3 |
| 91 | 407.3 ± 3.1 | 265.3 ± 3.0 | 394.9 ± 4.3 | 252.8 ± 4.3 | 403.3 ± 4.3 | 264.4 ± 4.3 |
Offspring body weight (g) (LS mean ± SEM)
Non-significant behavioral outcomes
No significant treatment main effects or treatment-related interactions were found on several tests including elevated zero maze, open-field locomotor activity, marble burying, straight channel swimming, MWM-MTS, conditioned fear (contextual and cued), or the difference between cued with or without prior cue exposure as an index of latent inhibition. Similarly, no treatment-related effects were obtained from the light-dark test or MWM hidden platform trials for acquisition, reversal, or shift, or on probe trials after each of these phases (Table 4).
Table 4.
Summary of outcomes of behavioral tests on which no significant treatment effects of prenatal Poly IC were found, except for PPI
| Saline | Poly IC (H) | Poly IC (L) | ||||||
|---|---|---|---|---|---|---|---|---|
| N | ||||||||
| Pup No. | Test | Variable | M | F | M | F | M | F |
| A | EZM | N | 18 | 19 | 9 | 10 | 9 | 8 |
| Open time (s) | 85.3 ± 8.3 | 84.3 ± 8.1 | 67.4 ± 11.8 | 88.0 ± 11.2 | 82.0 ± 11.8 | 110.4 ± 12.5 | ||
| Lat. to 1st open (s) | 12.1 ± 1.6 | 9.1 ± 1.6 | 8.4 ± 2.3 | 7.4 ± 2.1 | 5.3 ± 2.3 | 8.6 ± 2.4 | ||
| Head dips | 7.0 ± 1.0 | 8.6 ± 1.0 | 4.8 ± 1.4 | 7.7 ± 1.4 | 6.7 ± 1.4 | 10.4 ± 1.5 | ||
| Open entries | 8.4 ± 0.9 | 12.0 ± 0.9 | 8.0 ± 1.3 | 12.1 ± 1.3 | 9.1 ± 1.3 | 15.2 ± 1.4 | ||
| Open-field | N | 19 | 20 | 10 | 10 | 10 | 10 | |
| Horizontal Activity | 1173.4 ± 125.9 | 874.0 ± 122.6 | 944.2 ± 173.3 | 889.2 ± 173.3 | 904.5 ± 173.3 | 944.2 ± 173.3 | ||
| Central distance (cm) | 142.2 ± 15.2 | 94.5 ± 14.8 | 121.7 ± 20.9 | 102.0 ± 20.9 | 105.1 ± 20.9 | 111.6 ± 20.9 | ||
| Peripheral distance (cm) | 373.8 ± 45.6 | 327.4 ± 44.4 | 339.2 ± 62.8 | 405.8 ± 62.8 | 316.7 ± 62.8 | 341.2 ± 62.8 | ||
| Marble burying | No. 2/3rd buried | 5.5 ± 1.3 | 6.0 ± 1.2 | 3.5 ± 1.8 | 3.8 ± 1.8 | 4.0 ± 1.8 | 3.4 ± 1.8 | |
| ASR | N | 19 | 20 | 11 | 11 | 10 | 10 | |
| Peak amplitude | 309.3 ± 37.7 | 303.7 ± 36.7 | 390.8 ± 52.0 | 221.0 ± 52.0 | 371.0 ± 52.0 | 244.5 ± 52.0 | ||
| PPI | % Inhibition | |||||||
| PP73 | 23.4 ± 6.6 | 6.3 ± 5.6 | 12.9 ± 9.1 | 5.7 ± 7.9 | 20.7 ± 9.6 | 20.8 ± 7.9 | ||
| PP75 | 39.5 ± 6.6 | 25.2 ± 5.6 | 34.5 ± 9.1 | 11.1 ± 7.9 | 29.5 ± 9.6 | 39.6 ± 7.9 | ||
| PP80 | 51.6 ± 6.6 | 40.2 ± 5.6 | 40.5 ± 9.1 | 15.3 ± 7.9* | 52.0 ± 9.1 | 49.3 ± 7.9 | ||
| Straight channel | N | 17 | 18 | 10 | 9 | 10 | 10 | |
| Average latency (s) | 11.3 ± 0.8 | 10.9 ± 0.8 | 12.2 ± 1.0 | 9.2 ± 1.1 | 11.6 ± 1.0 | 11.5 ± 1.0 | ||
| MWM-MTS | N | 17 | 18 | 10 | 10 | 7 | 7 | |
| T1-T2 (s) | 7.3 ± 0.8 | 9.9 ± 0.8 | 8.6 ± 1.1 | 8.4 ± 1.1 | 9.8 ± 1.3 | 8.7 ± 1.3 | ||
| B/C | Cond. Fear | N | 19 | 19 | 10 | 10 | 10 | 10 |
| % Time Freezing: Contextual | NPE | 3.2 ± 2.3 | 2.2 ± 2.3 | 6.9 ±3.2 | 1.0 ± 3.2 | 5.3 ± 3.2 | 0.3 ±3.2 | |
| PE | 5.6 ± 2.3 | 3.7 ± 2.3 | 12.0 ± 3.2 | 1.1 ± 3.2 | 3.1 ± 3.2 | 1.1 ± 3.2 | ||
| Cued | NPE | 48.2 ± 6.5 | 15.7 ± 6.5 | 35.8 ± 9.0 | 26.2 ±9.0 | 37.4 ± 9.0 | 33.7 ± 9.0 | |
| PE | 17.0 ± 6.5 | 7.9 ± 6.5 | 16.4 ± 9.0 | 14.4 ± 9.0 | 10.3 ± 9.0 | 16.8 ± 9.0 | ||
| D | Light-Dark | N | 18 | 19 | 9 | 10 | 9 | 10 |
| Lat. to dark entry (s) | 27.0 ± 7.7 | 29.9 ± 7.5 | 26.2 ± 10.8 | 31.0 ± 10.3 | 17.5 ± 10.8 | 50.6 ± 10.3 | ||
| Light-side entries | 29.4 ± 3.5 | 33.8 ± 3.5 | 44.5 ± 5.0 | 30.8 ± 4.7 | 38.9 ± 5.0 | 33.2 ± 4.7 | ||
| Dark side time (s) | 358.9 ± 26.7 | 292.5 ± 26.1 | 297.3 ± 37.8 | 355.3 ± 35.9 | 373.7 ± 37.8 | 280.5 ± 35.9 | ||
| Straight channel | N | 19 | 17 | 9 | 8 | 9 | 10 | |
| Average latency (s) | 10.7 ± 0.9 | 10.6 ± 0.9 | 12.1 ± 1.3 | 11.9 ± 1.4 | 9.6 ± 1.3 | 11.8 ± 1.2 | ||
| MWM | N | 20 | 19 | 10 | 10 | 9 | 10 | |
| Acq | Path length (m) | 10.1 ± 0.8 | 11.9 ± 0.8 | 10.4 ± 1.1 | 11.1 ± 1.1 | 10.9 ± 1.2 | 11.5 ± 1.1 | |
| Probe | Avg. dist. (m) | 0.62 ± 0.06 | 0.72 ± 0.04 | 0.64 ± 0.05 | 0.72 ± 0.05 | 0.60 ± 0.05 | 0.67 ± 0.05 | |
| Rev | Path length (m) | 8.4 ± 0.9 | 10.8 ± 0.9 | 9.1 ± 1.3 | 9.6 ± 1.3 | 11.1 ± 1.4 | 9.4 ±1.3 | |
| Probe | Avg. dist. (m) | 0.68 ± 0.04 | 0.75 ± 0.04 | 0.72 ± 0.06 | 0.74 ± 0.06 | 0.71 ± 0.06 | 0.72 ± 0.06 | |
| Shift | Path length (m) | 7.3 ± 0.8 | 8.7 ± 0.9 | 6.5 ± 1.2 | 9.8 ± 1.2 | 6.9 ± 1.3 | 7.9 ± 1.2 | |
| Probe | Shift probe | 0.55 ± 0.04 | 0.77 ± 0.04 | 0.61 ± 0.05 | 0.80 ± 0.05 | 0.65 ± 0.06 | 0.71 ± 0.05 | |
| Cued latency (s) | 15.2 ± 2.1 | 21.6 ± 2.1 | 13.9 ± 2.9 | 17.6 ± 2.9 | 21.5 ± 3.1 | 21.7 ± 2.9 | ||
Average group performance (LS mean ± SEM)
P<0.05 compared with Saline within the same sex.
Significant behavioral outcomes
Significant treatment-related effects of Poly IC exposure were obtained on four tests: CWM, ASR/PPI, locomotor activity with (+)amphetamine challenge, and locomotor activity with MK-801 challenge.
ASR/PPI
When the data were analyzed using a group x sex x PP (prepulse intensity level) ANOVA there were no significant main effects of group or sex, but there was for PP (p<0.0001). There were no significant interactions between group and sex, PP, or all three factors. In addition, percent prepulse inhibition was analyzed relative to responses on trials without prepulses also using a group x sex x PP ANOVA. Again, there was a main effect of PP but no group or sex main effects and significant no two-or –three-way interactions. An inspection of the percent inhibition data in Table 4, however, suggested a trend only among females. Therefore, the percent inhibition data were also analyzed by sex. There was no effect of group or group x PP for males, however, for females, both the group main effect (F(2,37) = 3.37, p<0.05) and the group x PP interaction (F(4,74) = 2.62, p<0.05) were significant. Slice-effect ANOVAs for each PP level revealed significant group effects at 75 (p<0.05) and 80 dB (p<0.01) but not at 73 dB PP. Pairwise comparisons between Saline and the Poly IC groups at the 75 dB PP level showed no significant differences despite the significant slice ANOVA. Similar comparisons for the 80 db PP, however, showed a significant Saline compared with Poly IC (H) difference (p<0.05) in which this group showed reduce prepulse inhibition, whereas the Saline compared with Poly IC (L) difference was not significant.
CWM
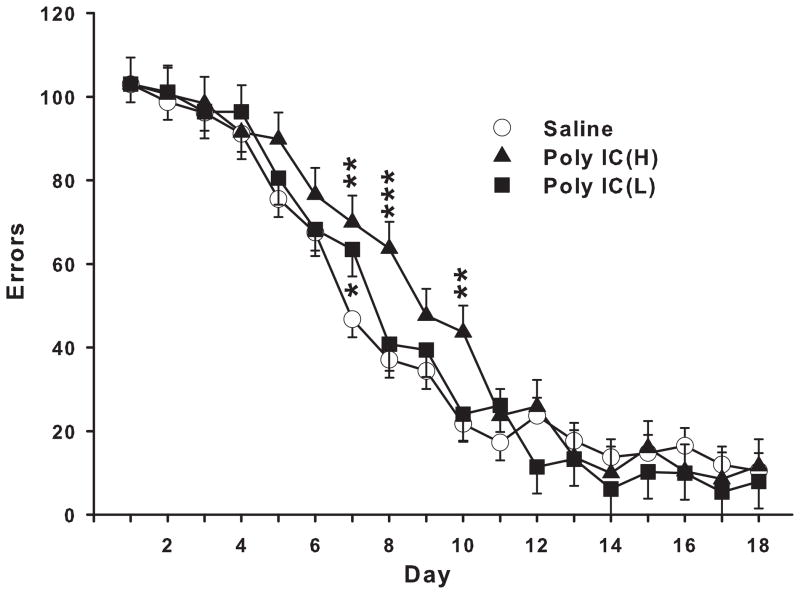
Analysis of errors showed no significant main effect of treatment but a significant treatment x day interaction (F(34,836) = 1.55, p<0.05). Slice-effect ANOVAs on each day showed significant treatment effects on days 7, 8, and 10 (p<0.01-0.02). A posteriori Hochberg pairwise comparisons showed that both the Poly IC (H) and (L) groups committed more errors on day-7, whereas only the Poly IC (H) group committed more errors on days 8 and 10 compared with the control group (Fig. 1).
Fig. 1. Cincinnati water maze.
Number of errors averaged across 2 trials/day for 18 days (mean ± SEM). An error was any head and shoulder entry into the stem of a T-shaped cul-de-sac or cul-de-sac arm. Groups sizes: Total (M/F): Saline = 31 (15/16); Poly IC (H) = 14 (7/7); Poly IC (L) = 14 (7/7). *P<0.05, **P<0.01, ***P<0.001 vs. Saline.
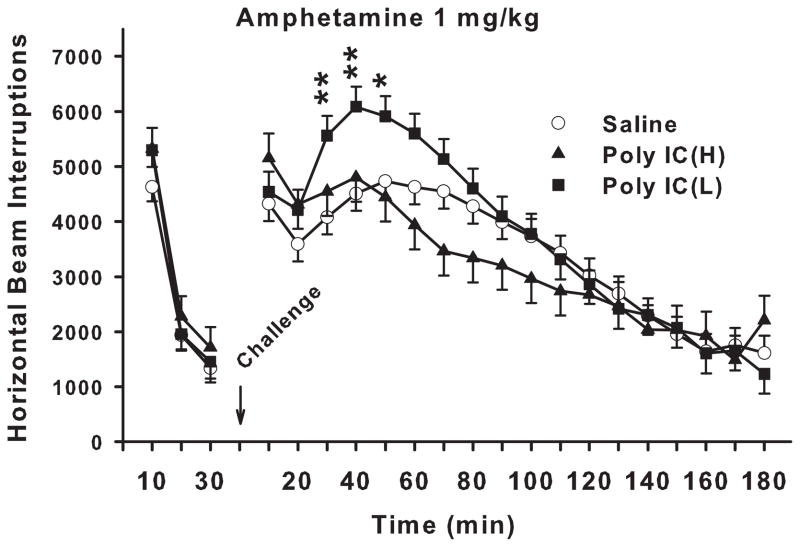
Locomotor activity with (+)-amphetamine challenge
One set of offspring from each litter was re-habituated to the locomotor activity chambers for 30 min prior to subcutaneous injection with 1.0 mg/kg of (+)-amphetamine and then tested for another 180 min (Fig. 2). There were no significant treatment or treatment-related interactions during the pre-drug test period. Post-drug, there was no treatment main effect but there was a significant treatment x interval interaction (F(34,1120) = 1.70, p<0.01). Slice-effect ANOVAs on each interval showed significant treatment effects on intervals 3-7 (30-70 min; p<0.01-0.02). A posteriori Hochberg pairwise group comparisons showed that the Poly IC (L) group exhibited significantly exaggerated hyperactivity in response to (+)-amphetamine compared with Saline controls 30-50 min post-treatment. This was not seen in the Poly IC (H) group; in fact this group showed a non-significant trend toward an attenuated response to (+)-amphetamine. There was also a main effect of sex (p<0.001) and interval (p<0.0001), and an interaction of sex x interval (p<0.02) but no treatment x sex or treatment x sex x interval effects (Fs<1).
Fig. 2. Automated open-field.
Locomotor activity before and after d-amphetamine: Total number of photobeam interruptions (mean ± SEM) per 10 min interval for 30 min prior to drug treatment and 180 min post-treatment with 1.0 mg/kg s.c. (+)-amphetamine. Groups sizes: Total (M/F): Saline = 32 (16/16); Poly IC (H) = 24 (12/12); Poly IC (L) = 16 (8/8). *P<0.05; **P<0.01 vs. Saline.
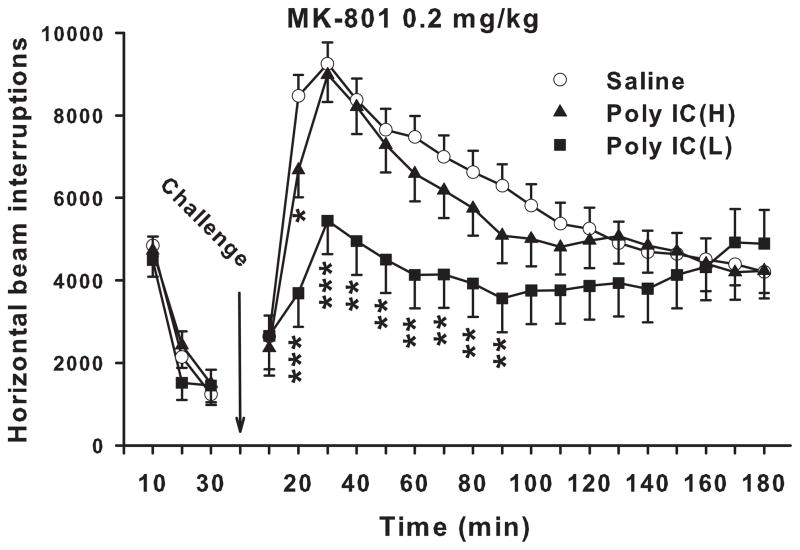
Locomotor activity with MK-801 challenge
A second set of offspring from each litter was re-habituated to the locomotor activity chambers for 30 min prior to subcutaneous injection with 0.2 mg/kg of MK-801 and tested for another 180 min (Fig. 3). There was no significant treatment main effect but there was a treatment x interval interaction during the pre-drug re-habituation test period (F(4,116) = 3.52, p<0.01), however, slice-effect ANOVAs showed no significant treatment effect on any of the pre-challenge intervals (p=.24–.80). Although the nature of the interaction was not revealed by the slice-effect ANOVAs, what is clear is that not even minor trends were observed during the final test interval prior to drug challenge. Post-challenge, the treatment main effect showed a non-significant trend (F(2,18.2) = 3.10, p<0.07) whereas the treatment x interval interaction was significant (F34,1245) = 3.63, p<0.0001). Slice-effect ANOVAs on each interval showed significant treatment effects on intervals 2-9 (20-90 min; p<0.0001-0.02). A posteriori Hochberg pairwise comparisons at each interval showed that the Poly IC (L) group exhibited significantly attenuated hyperactivity in response to MK-801-induced stimulation compared with Saline controls during multiple intervals. By contrast, Poly IC (H) group showed a reduction in MK-801-induced hyperactivity at only the first (10 min) interval compared with Saline controls. There was also a main effect of sex (p<0.001) and interval (p<0.0001) and an interaction of sex x interval (p<0.0001) but no treatment x sex or treatment x sex x interval interaction (Fs<1).
Fig. 3. Automated open-field.
Locomotor activity before and after MK-801: Total number of photobeam interruptions (mean ± SEM) per 10 min interval for 30 min prior to drug treatment and 180 min post-treatment with 0.2 mg/kg s.c. MK-801. Groups sizes: Total (M/F): Saline = 40 (20/20); Poly IC (H) = 24 (12/12); Poly IC (L) = 16 (8/8). *P<0.05; **P<0.01; ***P<0.001; vs. Saline.
Dichotomizing the Poly IC group into high and low gainers is only one way of testing for treatment effects. Another way is to analyze using maternal body weight as a continuous variable against outcome. Among the approaches that could be used is analysis of covariance (ANCOVA) in which outcome would be tested with maternal body weight as the covariate. We tested this using maternal body weight on E20 as the covariate for locomotor response after (+)-amphetamine and MK-801 challenge. For (+)-amphetamine, the same interaction that was significant for the three group analysis reported above was again significant, i.e., the treatment x interval interaction (F17,1156) = 1.69, p<0.04). Slice-effect ANOVAs on each interval showed a pattern similar to that shown in Fig. 2, except that several of the significant intervals in Fig. 2 fell short of significance in this analysis (p-values between p<0.05 and p<0.10 rather than all being p<0.05). A similar ANCOVA was performed on the MK-801 challenge data with a similar outcome, i.e., the treatment x interval interaction remained significant (F(17,1258) = 4.39, p<0.0001). In this case, slice-ANOVAs showed the same intervals as significant as in the three group ANOVA.
Discussion
There is a rapidly growing literature on maternal immune activation in response to agents such as Poly IC and LPS on offspring brain development, neurotransmitters, receptors, and behavior, especially behaviors of relevance to schizophrenia and/or ASD. Many of these studies have been done in mice. While there is a somewhat smaller literature in rats there is a related literature on prenatal LPS in rats, e.g., (Romero et al., 2007;Harvey and Boksa, 2011;Nouel et al., 2011;Cui et al., 2009;Cui et al., 2011;Ashdown et al., 2006;Fortier et al., 2007;Fortier et al., 2004a) supporting the connection between prenatal immune activation and adverse effects on brain development in the progeny.
A number of studies have examined effects of Poly IC in rats (Dickerson et al., 2010;Fortier et al., 2007;Gilmore et al., 2005;Howland et al., 2012;Oh-Nishi et al., 2010;Wolff and Bilkey, 2008;Wolff and Bilkey, 2010;Zhang et al., 2011;Zuckerman et al., 2003a;Zuckerman and Weiner, 2003;Zuckerman and Weiner, 2005). Recently, one of us evaluated E14 i.p. treatment with Poly IC (8 mg/kg) in Sprague-Dawley rats and showed changes in locomotor behavior in the presence of drug challenges (Bronson et al., 2011;Richtand et al., 2011). One of the characteristics seen in these experiments was that the response of offspring to drug challenges was influenced by the maternal response to Poly IC as reflected by maternal body weight changes (reduced gain or transient weight loss) shortly after treatment. Litters for which the dam showed a larger weight change 24 h after treatment showed significantly different responses compared with litters from dams that showed less body weight change. Moreover, in the first of these experiments (Bronson et al., 2011), maternal starting weight significantly predicted body weight change in response to prenatal Poly IC which in turn predicted behavioral response in the progeny to drug challenge. However, one study found that maternal weight change in response to prenatal Poly IC injection was not associated with differences in prepulse inhibition of acoustic startle (Wolff and Bilkey, 2010). In the present study, we reexamined this relationship in groups stratified by maternal weight change in response to Poly IC.
Accordingly, we divided Poly IC-treated dams into those that lost or gained the least weight after treatment compared with those that gained the most weight after treatment. That resulted in 10 dams assigned to the least body weight change after treatment, Poly IC (L) group, and 10 dams assigned to the most body weight change after treatment, Poly IC (H) group. The subdivision was based on the median net weight gain and as such has the advantage of being determined by a rule and hence is unbiased. Our objective was to assess the effect of Poly IC on a wide range of behaviors beyond what Bronson et al. (2011) had done; in effect, to use a test battery as has been recommended for the characterization of rodent models of various disorders (Crawley, 1999;Crawley, 2000;Crawley, 2007b;Crawley, 2007a;Crawley, 2008;Bailey et al., 2006;Branchi et al., 2003;MacQueen et al., 2001;Vorhees, 1996;Vorhees, 1997;Paylor et al., 2006;McIlwain et al., 2001). This did not reveal effects on anxiety-related indices such as elevated zero maze, open-field central zone activity, light-dark box changes, or object burying. Nor were exploratory behavior or habituation altered in the open-field. However, we did see reduced prepulse inhibition the Poly IC (H), but no the Poly IC (L) group in females, but not in males, similar to what has been reported previously (albeit not stratified by maternal weight gain) in mice (Smith et al., 2007) and rats treated prenatally with Poly IC (Zhang et al., 2011;Howland et al., 2012;Yee et al., 2011;Dickerson et al., 2010;Wolff and Bilkey, 2008;Wolff and Bilkey, 2010). In these other experiments where reduced PPI were found, Poly IC was given i.v., whereas we administered it i.p. and our effect was limited to females and only by analyzing females separately, not in an overall group x sex x PP ANOVA. Based on this, it appears that the PPI deficit is influenced by the route of administration. We did not find significant changes in fear conditioning in which contextual and cued fear to a tone were tested, nor in the difference between subgroups preexposed to the tone compared with those not preexposed to the tone as a test of latent inhibition; this effect has been reported to be affected after prenatal Poly IC but in a model in which it is given i.v. (Smith et al., 2007).
We extensively tested for spatial learning and memory in the MWM using two sets of animals given different procedures. In the MWM-MTS procedure, we tested trial-dependent learning using new platform and start positions every day, precluding prior recall of locations as a means of finding the goal. We assessed whether there were differences in savings between trial 1 and 2 each day and found no differences as a function of Poly IC exposure. We also tested groups in the reference memory version of the MWM with variable start and fixed platform positions and found no group difference on any index of learning (latency, path length, or cumulative distance) and no differences in swim speed. Moreover, the absence of spatial learning differences was seen not only on acquisition trials, but also when the platform was smaller and moved to the opposite quadrant (reversal), or was when moved a second time to an adjacent quadrant (shift). After each one of these phases, we tested reference memory on a probe trial given 24 h after the last training trial. We found no group differences on the probe trial following acquisition, reversal, or shift on measures of crossovers, average distance to the platform site, or percent time or distance in the target quadrant. Two other prenatal immune activation rat studies tested offspring in the MWM (Zuckerman and Weiner, 2005;Samuelsson et al., 2006). As in the current study, no effects of Poly IC exposure was found on acquisition learning or the probe trial after acquisition. However, during reversal trials, Poly IC exposed offspring had reduced latency to find the platform on the first block of 2 trials but not during the second block of 2 trials (Zuckerman and Weiner, 2005). We found no impairment of reversal or shift effects of Poly IC and no evidence of better than control performance. Of note, Zuckerman and Weiner (2005) gave Poly IC i.v. The other study administered 9 μg/kg IL-6 i.p. on E7, 9, 11 or E15, 17, 19 to Wistar rats and tested the adult offspring in a MWM and found impaired latency to find the goal in both IL-6 exposed offspring (Samuelsson et al., 2006). While we did not a similar impairment in the MWM, Samuelsson et al. (2006) administered IL-6 rather than Poly IC and gave it not once, but 3 times separated by 48 h which may have resulted in a larger effect than we induced.
On the other hand, we found an increase in errors in a maze that assess route-based egocentric navigation, i.e. in the CWM, in which no distal cues were available by testing the animals in the absence of visible light (under infrared lighting); this effect was seen in the Poly IC (H) rather than in the Poly IC (L) group. Various regions of the brain are implicated in egocentric learning, including neostriatum and dopamine, therefore, further studies of dopamine and dopamine receptors in this region should be examined in future experiments after prenatal Poly IC.
The most striking findings were those in which offspring were challenged with either (+)-amphetamine or MK-801. When challenged with (+)-amphetamine, all groups showed the characteristic pattern of drug-induced hyperactivity that lasted for approximately 2 h. However, the Poly IC (L) group showed an exaggerated hyperactivity at the peak of this response that occurred approximately 40 min post-drug. This was not seen in the Poly IC (H) group. The Poly IC (L) effect of higher than control hyperactivity in response to an indirect dopaminergic agonist, is consistent with what others have reported in both rats (Zuckerman et al., 2003a) and mice (Meyer et al., 2010); see also review (Meyer and Feldon, 2009), and is also consistent with the hypothesis that changes in striatal dopaminergic reactivity are present in schizophrenia, accounting, in part, for the efficacy of dopaminergic receptor antagonist antipsychotic medications to reduce symptoms. However this effect differs from what one of us found previously (Bronson et al., 2011). In Bronson et al. (2011), offspring were challenged with (+)-amphetamine at P90 in a residential activity monitor with no prior testing history, whereas herein we tested offspring after shock-induced fear conditioning at P63-65 in a non-residential activity monitoring system. Moreover, the two systems measured activity differently. Bronson et al. recorded zone crossings whereas we recorded all photobeam interruptions regardless of spacing with the arena.
More compelling than the Poly IC-related response to (+)-amphetamine, was the effect observed in animals challenged with the NMDA antagonist MK-801. MK-801 at 0.2 mg/kg elicited the typical hyperactivity characteristic of this drug. The effect at this dose lasted approximately 2.5 h in the Saline and Poly IC (H) groups, however, in the Poly IC (L) group this hyperactivity response was attenuated, lasting approximately 1.5 h post-challenge. After 1.5 h, this group’s locomotor activity level converged with that of the other groups. Under-response to MK-801 is consistent with the glutamatergic hypofunction hypothesis of schizophrenia and is consistent with the locomotor response to MK-801 previously observed in pre-pubertal males and females and young adult females. We have also previously observed decreased locomotor response to MK-801 treatment following prenatal immune activation (Bronson et al., 2011). In contrast, several studies in adult rats (Zuckerman and Weiner, 2005;Howland et al., 2012) and at least one study in mice (Meyer et al., 2010) report the opposite effect: over-response to MK-801 challenge. On the other hand, an increased response to another NMDA antagonist (ketamine) in mice prenatally exposed to Poly IC has been reported on ASR/PPI (Shi et al., 2003). Interestingly, the MK-801 studies that showed different patterns than we found gave Poly IC i.v. injection rather than i.p.. In fact, most of the mouse and rat models of prenatal Poly IC treatment have used the i.v. route; unfortunately, there are no experiments in which route of administration has been directly compared to determine if this is a critical factor in determining outcome.
We also analyzed the challenge data using maternal body weight as a covariate by analysis of covariance and found the same effect for (+)-amphetamine and MK-801, suggesting that the locomotor response to these drugs is not accounted for by Poly IC-induced maternal body weight changes. Had maternal body weight and drug response in the offspring covaried, one would have predicted that controlling for one would nullify the other. The fact that this did not occur suggests a significant degree of non-overlap of partitioned treatment variance. While this use of ANCOVA is not ideal because this method assumes that the covariate is orthogonal to the dependent variable, it is nonetheless useful in cases where the covariate is suspected of accounting for the outcome. When this is not found, it supports an inference of independent causation. Hence, this strengthens the view that Poly IC has effects on dopaminergic and glutamatergic signaling not related to sickness responses in dams.
It is not clear why we did not obtain some of the effects reported in other studies of prenatal Poly IC exposure, such as a deficit in latent inhibition (see (Boksa, 2010)). There have also been reported reductions in novel object recognition, which we did not test, and other effects (Boksa, 2010); see also (Richtand et al., 2012) and (Richtand et al, 2012, in press). Such differences may be species-dependent inasmuch as most of the learning and memory changes have been reported in mice. Aside from the two rat MWM studies noted above (Zuckerman and Weiner, 2003;Samuelsson et al., 2006), one study found improved learning in a water T-maze during reversal after prenatal Poly IC (Zuckerman and Weiner, 2005). In light of these several experimental and outcome differences, more replication across laboratories and species using similar treatment methods may help resolve some of these different findings.
Acknowledgments
The authors thank Mary Moran for data analyses using SAS. This research was supported by NIH research grants DA021394 (CVV), ES015689 (MTW), MH083192-01 (NMR), and T32 ES007051 predoctoral (AAB) and postdoctoral (DLG) training grant support, and by the Department of Veterans Affairs Medical Research Service.
Footnotes
All other authors report: None.
Authors Conflict of Interest Statement:
Neil M. Richtand discloses the following relationships: Consultant to Bristol-Meyers Squibb, Gerson Lehrman Group, Sunovion Pharmaceuticals Inc./Sepracor. Speaker’s Bureau: Bristol-Meyers Squibb, Otsuka America Pharmaceutical, Schering-Plough Corporation/Merck, Novartis Pharmaceuticals, Sunovion Pharmaceuticals Inc./Sepracor, and Grant/Research Support: Ortho-McNeil Janssen Scientific Affairs, LLC; AstraZeneca Pharmaceuticals.
References
- Ashdown H, Dumont Y, Ng M, Poole S, Boksa P, Luheshi GN. The role of cytokines in mediating effects of prenatal infection on the fetus: implications for schizophrenia. Mol Psychiatry. 2006;11:47–55. doi: 10.1038/sj.mp.4001748. [DOI] [PubMed] [Google Scholar]
- Bailey KR, Rustay NR, Crawley JN. Behavioral phenotyping of transgenic and knockout mice: practical concerns and potential pitfalls. ILAR J. 2006;47:124–131. doi: 10.1093/ilar.47.2.124. [DOI] [PubMed] [Google Scholar]
- Bitanihirwe BK, Peleg-Raibstein D, Mouttet F, Feldon J, Meyer U. Late prenatal immune activation in mice leads to behavioral and neurochemical abnormalities relevant to the negative symptoms of schizophrenia. Neuropsychopharmacology. 2010a;35:2462–2478. doi: 10.1038/npp.2010.129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bitanihirwe BK, Weber L, Feldon J, Meyer U. Cognitive impairment following prenatal immune challenge in mice correlates with prefrontal cortical AKT1 deficiency. Int J Neuropsychopharmacol. 2010b;13:981–996. doi: 10.1017/S1461145710000192. [DOI] [PubMed] [Google Scholar]
- Boksa P. Effects of prenatal infection on brain development and behavior: a review of findings from animal models. Brain Behav Immun. 2010;24:881–897. doi: 10.1016/j.bbi.2010.03.005. [DOI] [PubMed] [Google Scholar]
- Branchi I, Bichler Z, Berger-Sweeney J, Ricceri L. Animal models of mental retardation: from gene to cognitive function. Neurosci Biobehav Rev. 2003;27:141–153. doi: 10.1016/s0149-7634(03)00016-2. [DOI] [PubMed] [Google Scholar]
- Braun AA, Skelton MR, Vorhees CV, Williams MT. Comparison of the elevated plus and elevated zero mazes in treated and untreated male Sprague-Dawley rats: effects of anxiolytic and anxiogenic agents. Pharmacol Biochem Behav. 2011;97:406–415. doi: 10.1016/j.pbb.2010.09.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Ahlbrand R, Horn PS, Kern JR, Richtand NM. Individual differences in maternal response to immune challenge predict offspring behavior: contribution of environmental factors. Behav Brain Res. 2011;220:55–64. doi: 10.1016/j.bbr.2010.12.040. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bronson SL, Richtand NM. Handbook of Schizophrenia Spectrum Disorders. I. New York: Springer Science-Business Media B.V; 2011. Developmental consequences of prenatal maternal immune activation; pp. 1–25. [Google Scholar]
- Brown AS, Begg MD, Gravenstein S, Schaefer CA, Wyatt RJ, Bresnahan M, Babulas VP, Susser ES. Serologic evidence of prenatal influenza in the etiology of schizophrenia. Arch Gen Psychiatry. 2004a;61:774–780. doi: 10.1001/archpsyc.61.8.774. [DOI] [PubMed] [Google Scholar]
- Brown AS, Derkits EJ. Prenatal infection and schizophrenia: a review of epidemiologic and translational studies. Am J Psychiatry. 2010;167:261–280. doi: 10.1176/appi.ajp.2009.09030361. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown AS, Hooton J, Schaefer CA, Zhang H, Petkova E, Babulas V, Perrin M, Gorman JM, Susser ES. Elevated maternal interleukin-8 levels and risk of schizophrenia in adult offspring. Am J Psychiatry. 2004b;161:889–895. doi: 10.1176/appi.ajp.161.5.889. [DOI] [PubMed] [Google Scholar]
- Ciaranello AL, Ciaranello RD. The neurobiology of infantile autism. Annu Rev Neurosci. 1995;18:101–128. doi: 10.1146/annurev.ne.18.030195.000533. [DOI] [PubMed] [Google Scholar]
- Clarke MC, Tanskanen A, Huttunen M, Whittaker JC, Cannon M. Evidence for an interaction between familial liability and prenatal exposure to infection in the causation of schizophrenia. Am J Psychiatry. 2009;166:1025–1030. doi: 10.1176/appi.ajp.2009.08010031. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice: experimental design and evaluation of general health, sensory functions, motor abilities, and specific behavioral tests. Brain Res. 1999;835:18–26. doi: 10.1016/s0006-8993(98)01258-x. [DOI] [PubMed] [Google Scholar]
- Crawley JN. Behavioral phenotyping of transgenic and knockout mice. New York: Wiley-Liss; 2000. What’s wrong with my mouse? [Google Scholar]
- Crawley JN. Mouse behavioral assays relevant to the symptoms of autism. Brain Pathol. 2007a;17:448–459. doi: 10.1111/j.1750-3639.2007.00096.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Crawley JN. What’s Wrong with My Mouse: Behavioral Phenotyping of Transgenic and Knockout Mice. Hoboken, NJ: John Wiley & Sons; 2007b. [Google Scholar]
- Crawley JN. Behavioral phenotyping strategies for mutant mice. Neuron. 2008;57:809–818. doi: 10.1016/j.neuron.2008.03.001. [DOI] [PubMed] [Google Scholar]
- Cui K, Ashdown H, Luheshi GN, Boksa P. Effects of prenatal immune activation on hippocampal neurogenesis in the rat. Schizophr Res. 2009;113:288–297. doi: 10.1016/j.schres.2009.05.003. [DOI] [PubMed] [Google Scholar]
- Cui K, Luheshi GN, Boksa P. Effects of endogenous glucocorticoid secretion on the interleukin-6 response to bacterial endotoxin in pregnant and non-pregnant rats. J Endocrinol. 2011;209:95–103. doi: 10.1530/JOE-10-0436. [DOI] [PubMed] [Google Scholar]
- Cunningham C, Campion S, Teeling J, Felton L, Perry VH. The sickness behaviour and CNS inflammatory mediator profile induced by systemic challenge of mice with synthetic double-stranded RNA (poly I:C) Brain Behav Immun. 2007;21:490–502. doi: 10.1016/j.bbi.2006.12.007. [DOI] [PubMed] [Google Scholar]
- Deverman BE, Patterson PH. Cytokines and CNS development. Neuron. 2009;64:61–78. doi: 10.1016/j.neuron.2009.09.002. [DOI] [PubMed] [Google Scholar]
- Dickerson DD, Wolff AR, Bilkey DK. Abnormal long-range neural synchrony in a maternal immune activation animal model of schizophrenia. J Neurosci. 2010;30:12424–12431. doi: 10.1523/JNEUROSCI.3046-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Earle J, Kanodia R, Kist D, Emamian ES, Patterson PH, Shi L, Sidwell R. Prenatal viral infection leads to pyramidal cell atrophy and macrocephaly in adulthood: implications for genesis of autism and schizophrenia. Cell Mol Neurobiol. 2002;22:25–33. doi: 10.1023/A:1015337611258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fatemi SH, Emamian ES, Kist D, Sidwell RW, Nakajima K, Akhter P, Shier A, Sheikh S, Bailey K. Defective corticogenesis and reduction in Reelin immunoreactivity in cortex and hippocampus of prenatally infected neonatal mice. Mol Psychiatry. 1999;4:145–154. doi: 10.1038/sj.mp.4000520. [DOI] [PubMed] [Google Scholar]
- Fatemi SH, Pearce DA, Brooks AI, Sidwell RW. Prenatal viral infection in mouse causes differential expression of genes in brains of mouse progeny: a potential animal model for schizophrenia and autism. Synapse. 2005;57:91–99. doi: 10.1002/syn.20162. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Joober R, Luheshi GN, Boksa P. Maternal exposure to bacterial endotoxin during pregnancy enhances amphetamine-induced locomotion and startle responses in adult rat offspring. J Psychiatr Res. 2004a;38:335–345. doi: 10.1016/j.jpsychires.2003.10.001. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Kent S, Ashdown H, Poole S, Boksa P, Luheshi GN. The viral mimic, polyinosinic:polycytidylic acid, induces fever in rats via an interleukin-1-dependent mechanism. Am J Physiol Regul Integr Comp Physiol. 2004b;287:R759–R766. doi: 10.1152/ajpregu.00293.2004. [DOI] [PubMed] [Google Scholar]
- Fortier ME, Luheshi GN, Boksa P. Effects of prenatal infection on prepulse inhibition in the rat depend on the nature of the infectious agent and the stage of pregnancy. Behav Brain Res. 2007;181:270–277. doi: 10.1016/j.bbr.2007.04.016. [DOI] [PubMed] [Google Scholar]
- Gilmore JH, Jarskog LF, Vadlamudi S. Maternal poly I:C exposure during pregnancy regulates TNF alpha, BDNF, and NGF expression in neonatal brain and the maternal-fetal unit of the rat. J Neuroimmunol. 2005;159:106–112. doi: 10.1016/j.jneuroim.2004.10.008. [DOI] [PubMed] [Google Scholar]
- Harvey L, Boksa P. A stereological comparison of GAD67 and reelin expression in the hippocampal stratum oriens of offspring from two mouse models of maternal inflammation during pregnancy. Neuropharmacology. 2011;62:1767–1776. doi: 10.1016/j.neuropharm.2011.11.022. [DOI] [PubMed] [Google Scholar]
- Holson RR, Pearce B. Principles and pitfalls in the analysis of prenatal treatment effects in multiparous species. Neurotoxicol Teratol. 1992;14:221–228. doi: 10.1016/0892-0362(92)90020-b. [DOI] [PubMed] [Google Scholar]
- Howland JG, Cazakoff BN, Zhang Y. Altered object-in-place recognition memory, prepulse inhibition, and locomotor activity in the offspring of rats exposed to a viral mimetic during pregnancy. Neuroscience. 2012;201:184–198. doi: 10.1016/j.neuroscience.2011.11.011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MacQueen GM, Ramakrishnan K, Croll SD, Siuciak JA, Yu G, Young LT, Fahnestock M. Performance of heterozygous brain-derived neurotrophic factor knockout mice on behavioral analogues of anxiety, nociception, and depression. Behav Neurosci. 2001;115:1145–1153. doi: 10.1037//0735-7044.115.5.1145. [DOI] [PubMed] [Google Scholar]
- McIlwain KL, Merriweather MY, Yuva-Paylor LA, Paylor R. The use of behavioral test batteries: effects of training history. Physiol Behav. 2001;73:705–717. doi: 10.1016/s0031-9384(01)00528-5. [DOI] [PubMed] [Google Scholar]
- Mednick SA, Machon RA, Huttunen MO, Bonett D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch Gen Psychiatry. 1988;45:189–192. doi: 10.1001/archpsyc.1988.01800260109013. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J. Prenatal exposure to infection: a primary mechanism for abnormal dopaminergic development in schizophrenia. Psychopharmacology (Berl) 2009;206:587–602. doi: 10.1007/s00213-009-1504-9. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Fatemi SH. In-vivo rodent models for the experimental investigation of prenatal immune activation effects in neurodevelopmental brain disorders. Neurosci Biobehav Rev. 2009;33:1061–1079. doi: 10.1016/j.neubiorev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- Meyer U, Feldon J, Schedlowski M, Yee BK. Towards an immuno-precipitated neurodevelopmental animal model of schizophrenia. Neurosci Biobehav Rev. 2005;29:913–947. doi: 10.1016/j.neubiorev.2004.10.012. [DOI] [PubMed] [Google Scholar]
- Meyer U, Nyffeler M, Engler A, Urwyler A, Schedlowski M, Knuesel I, Yee BK, Feldon J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J Neurosci. 2006;26:4752–4762. doi: 10.1523/JNEUROSCI.0099-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Spoerri E, Yee BK, Schwarz MJ, Feldon J. Evaluating early preventive antipsychotic and antidepressant drug treatment in an infection-based neurodevelopmental mouse model of schizophrenia. Schizophr Bull. 2010;36:607–623. doi: 10.1093/schbul/sbn131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meyer U, Yee BK, Feldon J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: the earlier the worse? Neuroscientist. 2007;13:241–256. doi: 10.1177/1073858406296401. [DOI] [PubMed] [Google Scholar]
- Moreno JL, Kurita M, Holloway T, Lopez J, Cadagan R, Martinez-Sobrido L, Garcia-Sastre A, Gonzalez-Maeso J. Maternal influenza viral infection causes schizophrenia-like alterations of 5-HTA and mGlu receptors in the adult offspring. J Neurosci. 2011;31:1863–1872. doi: 10.1523/JNEUROSCI.4230-10.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nouel D, Burt M, Zhang Y, Harvey L, Boksa P. Prenatal exposure to bacterial endotoxin reduces the number of GAD67- and reelin-immunoreactive neurons in the hippocampus of rat offspring. Eur Neuropsychopharmacol. 2011;22:300–307. doi: 10.1016/j.euroneuro.2011.08.001. [DOI] [PubMed] [Google Scholar]
- Nyffeler M, Meyer U, Yee BK, Feldon J, Knuesel I. Maternal immune activation during pregnancy increases limbic GABAA receptor immunoreactivity in the adult offspring: implications for schizophrenia. Neuroscience. 2006;143:51–62. doi: 10.1016/j.neuroscience.2006.07.029. [DOI] [PubMed] [Google Scholar]
- Oh-Nishi A, Obayashi S, Sugihara I, Minamimoto T, Suhara T. Maternal immune activation by polyriboinosinic-polyribocytidilic acid injection produces synaptic dysfunction but not neuronal loss in the hippocampus of juvenile rat offspring. Brain Res. 2010;1363:170–179. doi: 10.1016/j.brainres.2010.09.054. [DOI] [PubMed] [Google Scholar]
- Ozawa K, Hashimoto K, Kishimoto T, Shimizu E, Ishikura H, Iyo M. Immune activation during pregnancy in mice leads to dopaminergic hyperfunction and cognitive impairment in the offspring: a neurodevelopmental animal model of schizophrenia. Biol Psychiatry. 2006;59:546–554. doi: 10.1016/j.biopsych.2005.07.031. [DOI] [PubMed] [Google Scholar]
- Patterson PH. Maternal infection: window on neuroimmune interactions in fetal brain development and mental illness. Curr Opin Neurobiol. 2002;12:115–118. doi: 10.1016/s0959-4388(02)00299-4. [DOI] [PubMed] [Google Scholar]
- Paylor R, Spencer CM, Yuva-Paylor LA, Pieke-Dahl S. The use of behavioral test batteries, II: effect of test interval. Physiol Behav. 2006;87:95–102. doi: 10.1016/j.physbeh.2005.09.002. [DOI] [PubMed] [Google Scholar]
- Penner JD, Brown AS. Prenatal infectious and nutritional factors and risk of adult schizophrenia. Expert Rev Neurother. 2007;7:797–805. doi: 10.1586/14737175.7.7.797. [DOI] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn P, Stanford K, Bronson SL, McNamara RK. Effects of risperidone and paliperidone pre-treatment on locomotor response following prenatal immune activation. J Psychiatr Res. 2011;220:55–64. doi: 10.1016/j.jpsychires.2011.02.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richtand NM, Ahlbrand R, Horn PS, Chamber B, Davis J, Benoit S. Effect of prenatal immune activation and paeri-adolescent stress on amphetamine-induced conditioned place preference in the rat. Psychopharmacology. 2012 doi: 10.1007/s00213-012-2646-8. e-Pub ahead of print:1-12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romero E, Ali C, Molina-Holgado E, Castellano B, Guaza C, Borrell J. Neurobehavioral and immunological consequences of prenatal immune activation in rats. Influence of antipsychotics. Neuropsychopharmacology. 2007;32:1791–1804. doi: 10.1038/sj.npp.1301292. [DOI] [PubMed] [Google Scholar]
- Samuelsson AM, Jennische E, Hansson HA, Holmang A. Prenatal exposure to interleukin-6 results in inflammatory neurodegeneration in hippocampus with NMDA/GABA(A) dysregulation and impaired spatial learning. Am J Physiol Regul Integr Comp Physiol. 2006;290:R1345–R1356. doi: 10.1152/ajpregu.00268.2005. [DOI] [PubMed] [Google Scholar]
- Shi L, Fatemi SH, Sidwell RW, Patterson PH. Maternal influenza infection causes marked behavioral and pharmacological changes in the offspring. J Neurosci. 2003;23:297–302. doi: 10.1523/JNEUROSCI.23-01-00297.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi L, Smith SE, Malkova N, Tse D, Su Y, Patterson PH. Activation of the maternal immune system alters cerebellar development in the offspring. Brain Behav Immun. 2009;23:116–123. doi: 10.1016/j.bbi.2008.07.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Smith SE, Li J, Garbett K, Mirnics K, Patterson PH. Maternal immune activation alters fetal brain development through interleukin-6. J Neurosci. 2007;27:10695–10702. doi: 10.1523/JNEUROSCI.2178-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV. Maze learning in rats: A comparison of performance in two water mazes in progeny prenatally exposed to different doses of phenytoin. Neurotoxicol Teratol. 1987;9:235–241. doi: 10.1016/0892-0362(87)90008-0. [DOI] [PubMed] [Google Scholar]
- Vorhees CV. Design considerations in the use of behavioral test batteries for the detection of CNS dysfunction in laboratory animals. Ment Retard Dev Dis Res Rev. 1996;2:227–233. [Google Scholar]
- Vorhees CV. Methods for detecting long-term CNS dysfunction after prenatal exposure to neurotoxins. Drug Chem Toxicol. 1997;20:387–399. doi: 10.3109/01480549709003895. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, He E, Skelton MR, Graham DL, Schaefer TL, Grace CE, Braun AA, Amos-Kroohs R, Williams MT. Comparison of (+)-methamphetamine, +/−-methylenedioxymethamphetamine, (+)-amphetamine and +/−-fenfluramine in rats on egocentric learning in the Cincinnati water maze. Synapse. 2011;65:368–378. doi: 10.1002/syn.20854. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Herring NR, Schaefer TL, Grace CE, Skelton MR, Johnson HL, Williams MT. Effects of neonatal (+)-methamphetamine on path integration and spatial learning in rats: effects of dose and rearing conditions. Int J Dev Neurosci. 2008;26:599–610. doi: 10.1016/j.ijdevneu.2008.04.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vorhees CV, Weisenburger WP, Acuff-Smith KD, Minck DR. An analysis of factors influencing complex water maze learning in rats: Effects of task complexity, path order and escape assistance on performance following prenatal exposure to phenytoin. Neurotoxicol Teratol. 1991;13:213–222. doi: 10.1016/0892-0362(91)90013-m. [DOI] [PubMed] [Google Scholar]
- Vorhees CV, Williams MT. Morris water maze: procedures for assessing spatial and related forms of learning and memory. Nat Protocols. 2006;1:848–858. doi: 10.1038/nprot.2006.116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav Brain Res. 2008;190:156–159. doi: 10.1016/j.bbr.2008.02.021. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Bilkey DK. The maternal immune activation (MIA) model of schizophrenia produces pre-pulse inhibition (PPI) deficits in both juvenile and adult rats but these effects are not associated with maternal weight loss. Behav Brain Res. 2010;213:323–327. doi: 10.1016/j.bbr.2010.05.008. [DOI] [PubMed] [Google Scholar]
- Wolff AR, Cheyne KR, Bilkey DK. Behavioural deficits associated with maternal immune activation in the rat model of schizophrenia. Behav Brain Res. 2011;225:382–387. doi: 10.1016/j.bbr.2011.07.033. [DOI] [PubMed] [Google Scholar]
- Yee N, Ribic A, de Roo CC, Fuchs E. Differential effects of maternal immune activation and juvenile stress on anxiety-like behaviour and physiology in adult rats: no evidence for the “double-hit hypothesis”. Behav Brain Res. 2011;224:180–188. doi: 10.1016/j.bbr.2011.05.040. [DOI] [PubMed] [Google Scholar]
- Zhang Y, Cazakoff BN, Thai CA, Howland JG. Prenatal exposure to a viral mimetic alters behavioural flexibility in male, but not female, rats. Neuropharmacology. 2011 doi: 10.1016/j.neuropharm.2011.02.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zuckerman L, Rehavi M, Nachman R, Weiner I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: a novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology. 2003a;28:1778–1789. doi: 10.1038/sj.npp.1300248. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Rimmerman N, Weiner I. Latent inhibition in 35-day-old rats is not an “adult” latent inhibition: implications for neurodevelopmental models of schizophrenia. Psychopharmacology (Berl) 2003b;169:298–307. doi: 10.1007/s00213-003-1460-8. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Post-pubertal emergence of disrupted latent inhibition following prenatal immune activation. Psychopharmacology (Berl) 2003;169:308–313. doi: 10.1007/s00213-003-1461-7. [DOI] [PubMed] [Google Scholar]
- Zuckerman L, Weiner I. Maternal immune activation leads to behavioral and pharmacological changes in the adult offspring. J Psychiatr Res. 2005;39:311–323. doi: 10.1016/j.jpsychires.2004.08.008. [DOI] [PubMed] [Google Scholar]