Abstract
Platinating agents are used in the treatment of many cancers, yet they can induce toxicities and resistance that limit their utility. Using previously published and additional world population panels of diverse ancestry totaling 608 lymphoblastoid cell lines (LCLs), we performed meta-analyses of over 3 million SNPs for both carboplatin- and cisplatin-induced cytotoxicity. The most significant SNP in the carboplatin meta-analysis is located in an intron of NBAS (p = 5.1 × 10−7). The most significant SNP in the cisplatin meta-analysis is upstream of KRT16P2 (p = 5.8 × 10−7). We also show that cisplatin-susceptibility SNPs are enriched for carboplatin-susceptibility SNPs. Most of the variants that associate with platinum-induced cytotoxicity are polymorphic across multiple world populations; therefore, they could be tested in follow-up studies in diverse clinical populations. Seven genes previously implicated in platinating agent response, including BCL2, GSTM1, GSTT1, ERCC2, and ERCC6 were also implicated in our meta-analyses.
Keywords: meta-analysis, pharmacogenomics, cisplatin, carboplatin, cross-population
Introduction
Platinum compounds comprise a class of chemotherapeutic agents that are used worldwide as essential components of many anticancer treatment regimens. In particular, carboplatin and cisplatin are commonly used to treat cancers such as lung, head and neck, colorectal, testicular, ovarian, cervical, and relapsed lymphoma1-6. Intrastrand and interstrand cross-links are thought to be the covalent cytotoxic lesions introduced onto DNA by platinating agents4. Both agents are associated with particular toxicities, predominantly myelosuppression for carboplatin and nephrotoxicity and ototoxicity for cisplatin4,7,8. However, there are currently no reliable means to identify patients at high risk for developing significant platinum-related toxicities, nor means to predict antitumor response. Heterogeneity in sensitivity is consistent with a role for genetic variation in explaining differences in response and toxicity to platinating agents. The similar mechanisms of action of carboplatin and cisplatin could suggest that the same genetic variants might contribute to platinum response. However, different predominant toxicities could mean the two drugs also have independent variants involved in susceptibility to each drug.
Candidate gene investigations of cisplatin-related ototoxicity, focusing on the role of genetic variants in GSTM39, GSTP110, mitochondrial DNA11, or megalin12 were inconclusive, possibly due to the use of single gene approaches and small patient cohorts. Another analysis focused on pathways associated with reactive oxygen species induction, were restricted to glutathione S-transferase (GST) genotypes, and identified variants in GSTP1 and GSTM1 as risk factors for ototoxicity in cisplatin-treated testicular cancer survivors13. Ross et al. tested variants in 220 drug-metabolism genes in children for association with cisplatin-induced hearing loss and found variants in methyltransferase genes (COMT and TPMT) with large 8- to 16-fold risks14. A recent genome-wide association study in non-small cell lung cancer patients receiving platinum treatment identified a SNP in CMKLR1 associated with overall survival, but it was not significant after multiple-testing correction15.
Patient populations of adequate size treated with the same chemotherapeutic dosage regimen are rare, making genome-wide association (GWA) studies of chemotherapeutic response in clinical settings challenging. To avoid confounders such as comorbidities, concomitant medications and diet, LCL models have been developed as useful discovery tools in germline genetic studies of chemotherapeutic susceptibility16-20. Recently, some SNPs associated with chemotherapeutic susceptibility in LCL studies have been replicated in patient populations by associating with phenotypes like tumor response and overall survival, demonstrating the potential utility of this model21,22.
Several GWA studies using LCLs from different population panels of the International HapMap Project23,24 have been performed to find variants and genes associated with platinum cytotoxicity. Previous studies identified variants associated with carboplatin17 and cisplatin18 cytotoxicity that also associated with gene expression in the initial (phase I/II) YRI (Yoruba from Ibadan, Nigeria) and CEU (Northern and Western European ancestry from Utah) HapMap panels. Taking an innovative approach that considered cytotoxicity-associated SNPs in cell lines derived from the population most sensitive to platinating agents (ASN, Japanese from Tokyo and Chinese from Beijing), O’Donnell et al. then identified those that replicated in a combined YRI and CEU population25. Although each of these studies found suggestive variants associated with platinating agent response, the top findings did not always replicate when examined in additional populations.
In this study, our goal was to identify variants that associate with platinating agent-induced cytotoxicity across populations. We believe that, once validated, such cross-population variants could be used to identify individuals who are likely to be sensitive or resistant to carboplatin and/or cisplatin regardless of genetic ancestry. In addition to the population panels mentioned in the studies above, we collected platinating agent cytotoxicity data from the HapMap phase III YRI, CEU, ASW (African ancestry from the Southwestern United States) and CHD (Chinese ancestry from Denver) panels26. Using a meta-analysis approach27,28, we combined the results of GWA studies for carboplatin- or cisplatin-induced cytotoxicity in each of 7 population panels. We identified SNPs associated with each of the two drug phenotypes and an enrichment of carboplatin-associated SNPs in the top cisplatin-associated SNPs. Most of the identified SNPs were common in all 7 panels, but several were specific to a population class. Seven genes previously implicated in platinating response through candidate studies were also implicated in our meta-analyses.
Materials and Methods
Lymphoblastoid Cell Lines
International HapMap Project LCLs from 7 panels were purchased from the Coriell Institute for Medical Research. The panels included 176 genotyped individuals from the Yoruba in Ibadan, Nigeria (YRI1/2 [HAPMAPPT03] and YRI3 [HAPMAPPT04], 83 individuals of African ancestry from the Southwestern United States (ASW [HAPMAPPT07]), 85 individuals of Han Chinese ancestry from Denver, Colorado (CHD [HAPMAPV11]), 90 Japanese from Tokyo and Han Chinese from Beijing (ASN [HAPMAPPT02]), and 174 Utah residents with Northern and Western European ancestry (CEU1/2 [HAPMAPPT01] and CEU3 [HAPMAPPT06]) for which genotype data is available (HapMap r27). Family structure of the panels is indicated in Table 1. Cell lines were maintained in RPMI 1640 (Mediatech, Herndon, VA, USA) supplemented with 15% fetal bovine serum (HyClone Laboratories, Logan, UT, USA) and 1% L-glutamine (Invitrogen, Carlsbad, CA, USA). Cell lines were diluted 3 times per week at a concentration of 3.5 × 105 cells/mL and incubated at 37°C with 5% CO2 and 95% humidity.
Table 1.
Characteristics and mean responses to carboplatin and cisplatin of the HapMap panels included in the meta-analyses.
| YRI1/2 | YRI3 | ASW | CHD | ASN | CEU1/2 | CEU3 | |
|---|---|---|---|---|---|---|---|
| N | 90 | 86 | 83 | 85 | 90 | 90 | 84 |
| family structure |
30 trios | 27 trios, 1 duo, 3 singletons |
10 trios, 20 duos, 13 singletons |
unrelated | unrelated | 30 trios | 22 trios, 5 duos, 8 singletons |
| carboplatin IC501 (μM) |
30.0 (17.4) | 36.8 (14.1) | 20.1 (7.1) | 25.1 (23.7) | 19.7 (8.2) | 24.5 (13.9) | 28.2 (14.0) |
| cisplatin IC501 (μM) |
8.3 (6.5) | 10.2 (6.1) | 4.6 (4.1) | 6.7 (11.5) | 4.5 (3.7) | 7.8 (8.5) | 8.1 (5.6) |
mean (SD)
Cytotoxicity Assays
Cells were treated with carboplatin17 and cisplatin18 as previously described. The concentration required to inhibit 50% of cell growth (IC50) was calculated for each carboplatin- and cisplatin-treated cell line. All IC50 values were either log2- or rank-transformed to normality (rntransform function in the GenABEL R library) before statistical modeling. If the log2-transformed data was not consistent with normality (Shapiro-Wilk test p < 0.05), the phenotype was rank-transformed to normality. The ASW phenotypes were rank-transformed; the phenotypes from the other panels were log2-transformed.
Genome-wide Association Analyses of Individual Panels
GWA studies of carboplatin- and cisplatin-induced cytotoxicity were performed on each of the seven panels individually. Studies of carboplatin- and cisplatin-susceptibility in the YRI1/217,18, CEU1/218, and ASN25 panels have been previously published. To increase genome coverage of YRI3 and CEU3 (HapMap r27), ungenotyped makers were imputed using the BEAGLE software using YRI1/2 and CEU1/2 (HapMap r22) as reference, respectively29. BEAGLE imputes ungenotyped markers for parent-offspring trios by modeling the family structure in the analysis. To measure the accuracy of the imputation at each SNP locus, R2 was calculated as described following 100 imputations of the data29. Imputed SNP genotypes with R2 > 0.80 were carried through the rest of the analysis. For YRI1/2, YRI3, CEU1/2, and CEU3, greater than 2 million SNPs (minor allele frequency (MAF) > 0.05 within the panel, no Mendelian errors and in Hardy-Weinberg equilibrium (p > 0.001)) were tested for association with carboplatin log2(IC50) and cisplatin log2(IC50) using the quantitative trait disequilibrium test (QTDT) total association model30.
To control for population structure in the admixed ASW population (HapMap r27), local ancestry at each genotyped SNP locus was estimated using the HAPMIX software31. Phased genotypes from the YRI1/2 and CEU1/2 populations were used as the two parental populations to estimate the ancestry of the ASW population. For each individual, the algorithm estimated the number of CEU chromosomes (0-2) at each SNP locus. To increase genome coverage of the ASW, ungenotyped makers were imputed using the BEAGLE software29, using both YRI1/2 and CEU1/2 as reference populations. Local ancestry for each imputed SNP was inferred by using the predicted number of CEU chromosomes from nearest genotyped SNP. GWA studies in the ASW population were performed between carboplatin and cisplatin IC50 phenotypes rank-transformed to normality and greater than 2 million SNPs using the quantitative trait disequilibrium test (QTDT) total association model30. Local ancestry (continuous predicted number of CEU chromosomes at each locus) was included as a covariate in the model for each drug.
To increase genome coverage of the CHD (HapMap r27), ungenotyped makers were imputed using the MaCH software with the ASN population (HapMap r27) as reference32. Imputed SNP genotypes with R2 > 0.80 were carried through the rest of the analysis. For CHD and ASN, PLINK software was used to test greater than 2 million SNPs (MAF > 0.05 and in Hardy-Weinberg equilibrium (p > 0.001)) for linear association with carboplatin log2(IC50) and cisplatin log2(IC50)33.
Genomic control lambda (λGC) values34 were calculated for the GWA study of each population panel-drug phenotype combination. Studies with λGC values greater than 1.00 were corrected for residual inflation of the test statistic by dividing the observed test statistic at each SNP by the λGC34 and then the corresponding p-values were carried through the meta-analyses.
Meta-analysis of Seven GWA Studies
To determine which SNPs associate with carboplatin- and cisplatin-induced cytotoxicity across populations, we performed a meta-analysis to combine the results of the individual GWA studies from the 7 panels. We used the software METAL, which combines SNP p-values across studies taking into account a study specific weight (sample size) and direction of effect (positive or negative beta)28. This approach converted the direction of effect and p-value observed in each study into a signed Z-score such that very negative Z-scores indicate a small p-value and an allele associated with lower IC50, whereas large positive Z-scores indicate a small p-value and an allele associated with higher IC50. Z-scores for each SNP were combined across studies in a weighted sum, with weights proportional to the square-root of the sample size for each study28. Q-Q plots of the corresponding p-values are shown in Supplementary Figure S1. Gene region plots of top SNPs were made with LocusZoom35.
Results
Population Panel Characteristics
Carboplatin- and cisplatin-induced cytotoxicity was measured in 608 LCLs from seven HapMap panels. The panels included YRI1/217,18, YRI3, ASW, CHD, ASN25, CEU1/218,36, and CEU3. Percent survival data at several concentrations (0-80 μM carboplatin or 0-20 μM cisplatin) were used to calculate the IC50 for each cell line and drug. Table 1 displays the means and standard deviations of carboplatin and cisplatin IC50 for each panel. For both drugs, the IC50 values are in the range of the plasma platinum concentrations observed in patients after treatment. The mean IC50 values ranged from 19.7-36.8 μM (7.3-13.7 μg/ml) for carboplatin and 4.5-10.2 μM (1.4-3.1 μg/ml) for cisplatin in the LCL panels. In previous pharmacokinetic studies, plasma concentrations of platinum a few hours after administration ranged from 5-20 μg/ml in patients treated with carboplatin37-39 and from 1-10 μg/ml in patients treated with cisplatin38,40.
Meta-analysis reveals SNPs associated with platinating agent cytotoxicity
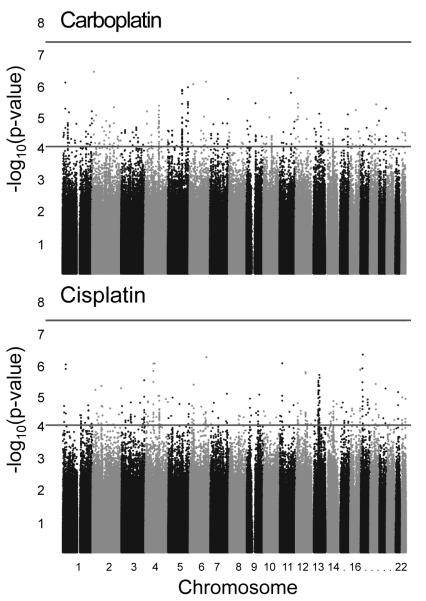
GWA studies of carboplatin- and cisplatin-induced cytotoxicity were performed in each of the seven panels individually. Each study tested 2-2.5 million genotyped or imputed SNPs for association with carboplatin IC50 or cisplatin IC50. To determine which SNPs associate with carboplatin IC50 or cisplatin IC50 across populations, we performed a meta-analysis to combine the results of the individual GWA studies from the 7 panels. Some SNPs are unique to a particular panel or subset of panels and therefore ~3 million SNPs were tested in the meta-analysis. At the suggestive threshold of p < 10−4, 322 SNPs associated with carboplatin IC50 and 334 SNPs with cisplatin IC50 (Figure 1, Supplementary Table S1). Most of the SNPs at this threshold were common (MAF > 0.05) in all 7 HapMap panels (Figure 2).
Figure 1. Meta-analysis results of 7 genome-wide association studies of platinum-induced cytotoxicity.
Each point represents a SNP. Horizontal lines are at the suggestive significance threshold of p = 10−4 and the genome-wide significance threshold of p = 5 × 10−8.
Figure 2. Population class distribution of the top meta-analysis hits.
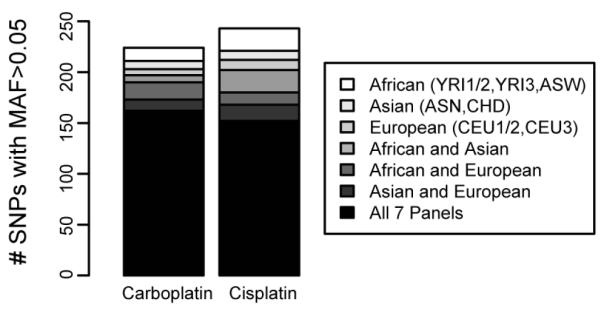
The number of SNPs with a meta p-value < 10−4 that have a MAF > 0.05 in each population panel included in the listed population classes. Most (50.3% for carboplatin and 45.5% for cisplatin) of the top SNPs (p < 10−4) were present in all 7 panels. For the vast majority of these SNPs present in all 7 panels, the direction of effect was the same for either 7/7 or 6/7 panels (98.1% for carboplatin, 96.7% for cisplatin). SNPs not included in this bar chart were present in a subset of panels not included as one of the listed population classes.
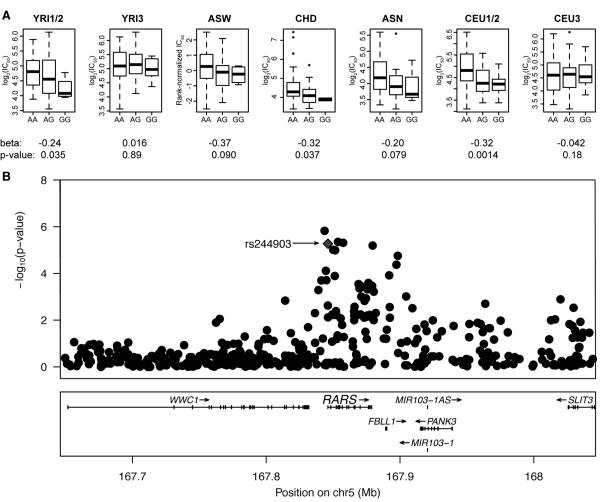
The most significant SNP in the carboplatin IC50 meta-analysis was rs7572081, which is located in an intron of NBAS (Table S1, p = 5.1 × 10−7). The most significant SNP in the cisplatin IC50 meta-analysis was rs7210837 (Table S1, p = 5.8 × 10−7). The most significant missense SNP in the meta-analyses was rs244903, which associated with carboplatin IC50 and is located in the first exon of RARS (p = 5.4 × 10−6, Figure 3).
Figure 3. rs244903 is the most significant missense SNP (p = 5.4 × 10−6) in the metaanalysis.
(A) Boxplots of carboplatin IC50 versus rs244903 genotype, beta terms, and p-values are shown for each population panel. Notice six of seven betas are in the same direction. (B) rs244903 (diamond) is located in the first exon of RARS (arginyl-tRNA synthetase). The DNA change A to G corresponds to the amino acid change isoleucine to valine. The SNP is an eQTL associated with the expression of five genes in YRI43 (p < 10−4). P-values are from the carboplatin IC50 meta-analysis.
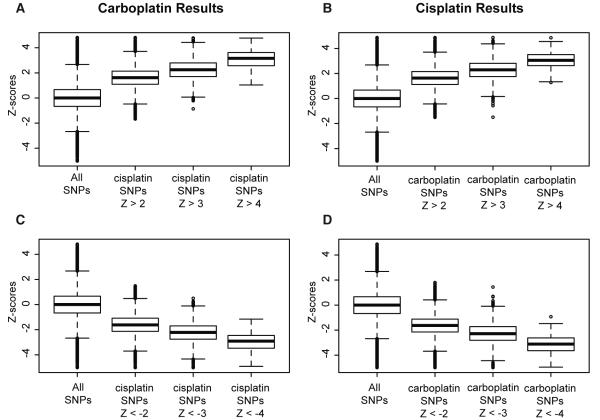
Cisplatin-associated SNPs are enriched for carboplatin-associated SNPs
To compare the top SNP associations for the two drug phenotypes, we examined the Z-score distributions of the top cisplatin IC50 SNPs at various thresholds in the carboplatin IC50 meta-analysis results and vice versa (Figure 4). We verified that the directions of effect of the top SNPs are the same between the two drugs. That is, when the minor allele of a particular SNP is associated with increased resistance to carboplatin, the minor allele is also associated with increased resistance to cisplatin. Therefore, cisplatin-associated SNPs are enriched for carboplatin-associated SNPs and common genetically mediated mechanisms may influence the effect of both chemically related drugs. The Pearson correlation between carboplatin IC50 and cisplatin IC50 varied across HapMap panels (YRI1/2 = 0.84, YRI3 = 0.75, ASW = 0.67, CHD = 0.82, ASN = 0.76, CEU1/2 = 0.58, CEU3 = 0.60).
Figure 4. Meta-analysis Z-score distribution comparison between the two platinating-agents.
The Z-scores for the top cisplatin IC50-associated SNPs at various positive (A) and negative (C) Z-score thresholds were pulled from the overall list of ~3 million SNPs tested for association with carboplatin IC50. The Z-scores for the top carboplatin IC50-associated SNPs at various positive (B) and negative (D) Z-score thresholds were pulled from the overall list of ~3 million SNPs tested for association with cisplatin IC50. The means of the “all SNPs” classes differed from the means of the other classes for both carboplatin and cisplatin (p < 10−16, Student’s t-test).
Genes previously implicated in platinating agent cytotoxicity replicated by meta-analysis
We compiled a list of 41 genes previously implicated in platinating agent response (Supplementary Table S2). This list contained the genes that make up the PharmGKB platinum pathway41, the two methyltransferases found associated with cisplatin-induced hearing loss14, and several genes from a review of platinating agents4. None of the top SNPs (p < 10−4) from both the carboplatin and cisplatin meta-analyses were located within these 41 genes. However, 28 of the 41 genes are expressed in LCLs42 and 7 of these 28 genes were expression targets of top meta-analysis SNP eQTLs43 (Supplementary Table S2).
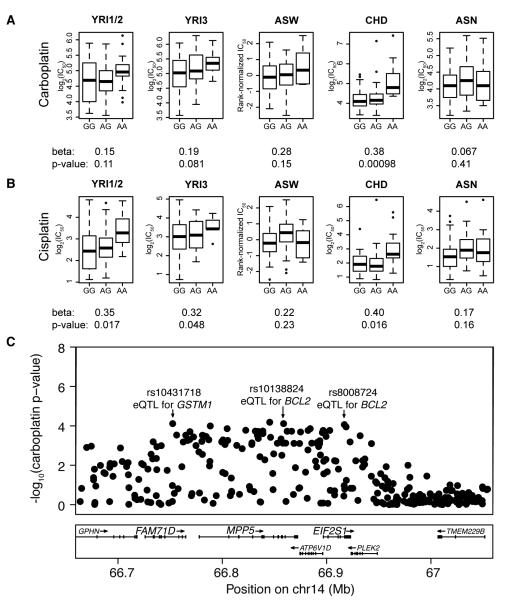
Included in these 7 genes are BCL2 and GSTM1, which are both targeted by YRI1/2 eQTLs from the same region of chromosome 14 that also associate with both carboplatin and cisplatin IC50 (Figure 5). Also included in the previously implicated genes are GSTT1, ERCC6, and ERCC2. rs2191934 is associated with cisplatin IC50 (meta-p = 8.3 × 10−5) and the expression of GSTT1 and ERCC6 (Supplementary Figure S2). rs9527419 is associated with cisplatin IC50 (meta-p = 5.8 × 10−6) and the expression of ERCC2 (Supplementary Figure S3).
Figure 5. 200kb region on chr14 is associated with carboplatin IC50, cisplatin IC50, and the expression of two genes previously implicated in platinum response.
Boxplots of (A) carboplatin (meta-p = 8.2 × 10−5) or (B) cisplatin IC50 (meta-p = 3.6 × 10−5) versus rs10138824 genotype, beta terms, and p-values are shown for each population panel. Only the five panels with MAF > 0.05 were included in the meta-analysis. Notice all betas are in the same direction. (C) rs10138824 is located in an intron of MPP5 (membrane protein, palmitoylated 5) and is associated with the expression of BCL2 (B-cell CLL/lymphoma 2) in YRI43 (p = 10−4). The two other indicated SNPs in the region are also associated with both carboplatin and cisplatin IC50 (p < 10−4). rs10431718 is located in an intron of FAM71D (family with sequence similarity 71, member D) and is associated with the expression of GSTM1 (glutathione S-transferase mu 1) in YRI43 (p = 10−4). rs8008724 is located in an intron of EIF2S1 (eukaryotic translation initiation factor 2, subunit 1 alpha, 35kDa) and is associated with the expression of BCL2 in YRI43 (p = 10−4). P-values are from the carboplatin IC50 meta-analysis.
Discussion
We performed a meta-analysis of the results of GWA studies for carboplatin- and cisplatin-induced cytotoxicity in 7 HapMap panels that included a total of 608 LCLs. We identified 322 SNPs that associate with carboplatin IC50 and 334 SNPs that associate with cisplatin IC50 at the suggestive threshold of p < 10−4. About half of the identified SNPs were common in all 7 panels. By the nature of the meta-analysis, this indicates that the allelic relationship with IC50 was the same in most if not all of the populations. Therefore, if these variants are confirmed in clinical cohorts, they could be used to predict chemotherapeutic response in individuals from most world populations. However, several SNPs were specific to a population class and therefore were not interrogated in as many individuals. For these population-specific SNPs, additional cell lines from the appropriate population are needed to confirm these findings.
We identified an enrichment of carboplatin-associated SNPs in the top cisplatin-associated SNPs. This is somewhat expected given that the phenotypes are correlated. The correlation between carboplatin and cisplatin IC50 within a HapMap panel ranges from 0.58 in CEU1/2 to 0.84 in YRI1/2. Both carboplatin and cisplatin are platinating agents that act through the formation of intrastrand and interstrand DNA cross-links, which result in DNA strand breaks leading to cell death4. Our results support that common genetic mechanisms may influence the effects of both drugs. However, there are also likely unique genetic mechanisms contributing to the different clinical toxicities observed between the two drugs. Although findings in LCL studies have been replicated by associating with patient phenotypes like tumor response and overall survival21,22, studies testing top LCL findings for association with patient toxicity phenotypes are also needed to fully understand the utility of the LCL model.
Several genes connected to top SNPs associated with platinum susceptibility have been implicated in tumorigenesis. For instance, the most significant SNP in the carboplatin IC50 meta-analysis, rs7572081, is located in an intron of NBAS (neuroblastoma amplified sequence). Carboplatin is often used in neuroblastoma treatment regimens44,45 and increased NBAS expression has been associated with poorer outcome in patients greater than 18 months old46. The most significant missense SNP in the meta-analyses was rs244903, which associated with carboplatin IC50 and is located in the first exon of RARS (arginyl-tRNA synthetase). Since RARS is necessary for protein synthesis, it has been recognized as a potential chemotherapeutic drug target47. The RARS SNP is also an eQTL associated with the expression of five genes in YRI1/2, including NOL1 and CCNG243. Reduced expression of NOL1, also known as p120, reduced cell growth in the human breast cancer line MCF-748. CCNG2 is a negative regulator of cell cycle progression and decreased expression of the gene has been observed in oral cancers49.
Seven genes previously implicated in platinating response out of 28 tested were also implicated in our meta-analyses. These genes included BCL2 and GSTM1, which are both targeted by eQTLs located in the same region of chromosome 14 that also associate with both carboplatin and cisplatin IC50. Inhibition of apoptosis by increased BCL2 expression has been shown to lead to cisplatin resistance50. Here, the opposite effect was observed: the eQTL minor alleles associated with decreased BCL2 expression42 and increased IC50. Further studies are necessary to elucidate how BCL2 may be functioning in LCLs in response to platinating agents. GSTM1 is an enzyme that contributes to the detoxification of platinating agents41. Here, alleles associated with increased GSTM1 expression42 were also associated with increased IC50. Correlations have been observed between high levels of the related protein GSTP1 and cisplatin resistance in colon, lung adenocarcinoma, and glioblastoma tumor cell lines; however, results in other studies are inconsistent4.
GSTT1, another glutathione S-transferase, was also implicated in our meta-analysis. rs2191934 associated with cisplatin IC50 and the expression of GSTT1 and ERCC6. rs9527419 associated with cisplatin IC50 and the expression of ERCC2. ERCC2 and ERCC6 are components of the platinum pathway involved in nucleotide excision repair41. Platinating agent DNA adduct repair occurs primarily through nucleotide excision repair4. Here alleles associated with increased expression of ERCC2 and ERCC6 were also associated with cisplatin resistance (increased IC50). Cisplatin resistance is correlated with the increased expression of several nucleotide excision repair genes; in ovarian cancer, XPA and ERCC1 were shown to have increased expression in tumors of patients resistant to platinum treatment51,52. Similarly, a study of gastric cancer patients showed a correlation between cisplatin resistance and ERCC1 levels53. The observation that numerous candidate genes are not replicated here is not necessarily surprising since our unbiased GWA approach might not be expected to identify prior candidate genes, since it makes no assumption that these are the most important. In addition, some of the SNPs within candidate genes have a MAF <0.05 and therefore would not be tested in this model.
One previous study from our laboratory identified six SNPs that contribute to cisplatin-induced cytotoxicity through their effects on the expression of 8 genes in the combined CEU1/2 and YRI1/2 population18. Although not one of our top findings, one of these six SNPs remained associated with cisplatin IC50 when the additional panels were added in the current study (rs2136241, meta-p = 6.9 × 10−4). This SNP was present and the direction of effect was the same in all seven HapMap panels. The association signal for the other five SNPs was lost, due to discordant directions of effect when the additional panels were added. The previous study examined 176 individuals, so power to detect associations was limited. Another study from our group identified cytotoxicity-associated SNPs in cell lines derived from the population most sensitive to platinating agents (ASN, n=90) and showed that 13 of the SNPs also associated with either carboplatin or cisplatin IC50 in a combined CEU1/2 and YRI1/2 population (n=106)25. One of these SNPs, rs6691275, remained associated with carboplatin IC50 in the current study (meta-p = 3.1 × 10−4) and the direction of effect was the same in six of the seven tested HapMap panels. The association signal for the other 12 SNPs was lost, due to discordant directions of effect when the additional panels were added, again likely due to limited power in the initial study. However, the direction of effect was the same in the two panels of Asian ancestry (ASN, CHD) for 10 of the 13 SNPs, indicating the SNPs could associate with cytotoxicity in a population specific manner.
Our results show that many genes and variants may be involved in cellular response to platinating agents. Since most of the variants that associate with platinum-induced cytotoxicity are polymorphic across multiple world populations (African, Asian, European), they can be tested in follow-up studies in both LCL and tumor cell line panels from multiple populations, or in diverse clinical populations. Our cell line models allow us to select the most promising SNPs for testing in clinical studies. We plan to clinically validate cytotoxicity-associated variants in a cohort of patients treated with carboplatin or cisplatin to determine their roles in patient response and toxicity.
Supplementary Material
Acknowledgments
This study is supported by NIH/NIGMS Pharmacogenomics of Anticancer Agents grant U01GM61393 (NJC, MED), the University of Chicago Breast Cancer SPORE P50 CA125183 (RSH, NJC, MED), NIH/NIGMS grant K08GM089941 (RSH) and University of Chicago Cancer Center Support Grant P30 CA14599 (RSH, MED). The authors would like to thank the Pharmacogenomics of Anticancer Agents Cell Line Core for assistance in providing and maintaining these cell lines. In addition, the authors thank Marleen Welsh for phenotyping some of the ASW and CEU3 cell lines and Wei Zhang for performing initial imputation analyses in the CHD.
Footnotes
Conflict of interest The authors declare no conflict of interest.
Supplementary information is available at The Pharmacogenomics Journal website.
References
- 1.Borghaei H, Langer CJ, Millenson M, Ruth KJ, Litwin S, Tuttle H, et al. Phase II study of paclitaxel, carboplatin, and cetuximab as first line treatment, for patients with advanced non-small cell lung cancer (NSCLC): results of OPN-017. J Thorac Oncol. 2008;3:1286–1292. doi: 10.1097/JTO.0b013e318189f50e. [DOI] [PubMed] [Google Scholar]
- 2.McWhinney SR, Goldberg RM, McLeod HL. Platinum neurotoxicity pharmacogenetics. Mol Cancer Ther. 2009;8:10–16. doi: 10.1158/1535-7163.MCT-08-0840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Micheletti E, Face B La, Bianchi E, Cagna E, Apostoli P, Ruggeri G, et al. Continuous infusion of carboplatin during conventional radiotherapy treatment in advanced squamous carcinoma of the cervix uteri IIB-IIIB (UICC). A phase I/II and pharmacokinetic study. Am J Clin Oncol. 1997;20:613–620. doi: 10.1097/00000421-199712000-00017. [DOI] [PubMed] [Google Scholar]
- 4.Rabik CA, Dolan ME. Molecular mechanisms of resistance and toxicity associated with platinating agents. Cancer Treat Rev. 2007;33:9–23. doi: 10.1016/j.ctrv.2006.09.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Siddik ZH. Cisplatin: mode of cytotoxic action and molecular basis of resistance. Oncogene. 2003;22:7265–7279. doi: 10.1038/sj.onc.1206933. [DOI] [PubMed] [Google Scholar]
- 6.Wang D, Lippard SJ. Cellular processing of platinum anticancer drugs. Nat Rev Drug Discov. 2005;4:307–320. doi: 10.1038/nrd1691. [DOI] [PubMed] [Google Scholar]
- 7.Celik I, Kars A, Ozyar E, Tekuzman G, Atahan L, Firat D. Major toxicity of cisplatin, fluorouracil, and leucovorin following chemoradiotherapy in patients with nasopharyngeal carcinoma. J Clin Oncol. 1996;14:1043–1044. doi: 10.1200/JCO.1996.14.3.1043. [DOI] [PubMed] [Google Scholar]
- 8.van Glabbeke M, Renard J, Pinedo HM, Cavalli F, Vermorken J, Sessa C, et al. Iproplatin and carboplatin induced toxicities: overview of phase II clinical trial conducted by the EORTC Early Clinical Trials Cooperative Group (ECTG) Eur J Cancer Clin Oncol. 1988;24:255–262. doi: 10.1016/0277-5379(88)90262-3. [DOI] [PubMed] [Google Scholar]
- 9.Peters U, Preisler-Adams S, Hebeisen A, Hahn M, Seifert E, Lanvers C, et al. Glutathione S-transferase genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Drugs. 2000;11:639–643. doi: 10.1097/00001813-200009000-00007. [DOI] [PubMed] [Google Scholar]
- 10.Oldenburg J, Kraggerud SM, Brydoy M, Cvancarova M, Lothe RA, Fossa SD. Association between long-term neuro-toxicities in testicular cancer survivors and polymorphisms in glutathione-s-transferase-P1 and -M1, a retrospective cross sectional study. J Transl Med. 2007;5:70. doi: 10.1186/1479-5876-5-70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Peters U, Preisler-Adams S, Lanvers-Kaminsky C, Jurgens H, Lamprecht-Dinnesen A. Sequence variations of mitochondrial DNA and individual sensitivity to the ototoxic effect of cisplatin. Anticancer Res. 2003;23:1249–1255. [PubMed] [Google Scholar]
- 12.Riedemann L, Lanvers C, Deuster D, Peters U, Boos J, Jurgens H, et al. Megalin genetic polymorphisms and individual sensitivity to the ototoxic effect of cisplatin. Pharmacogenomics J. 2008;8:23–28. doi: 10.1038/sj.tpj.6500455. [DOI] [PubMed] [Google Scholar]
- 13.Oldenburg J, Kraggerud SM, Cvancarova M, Lothe RA, Fossa SD. Cisplatin-induced long-term hearing impairment is associated with specific glutathione s-transferase genotypes in testicular cancer survivors. J Clin Oncol. 2007;25:708–714. doi: 10.1200/JCO.2006.08.9599. [DOI] [PubMed] [Google Scholar]
- 14.Ross CJ, Katzov-Eckert H, Dube MP, Brooks B, Rassekh SR, Barhdadi A, et al. Genetic variants in TPMT and COMT are associated with hearing loss in children receiving cisplatin chemotherapy. Nat Genet. 2009;41:1345–1349. doi: 10.1038/ng.478. [DOI] [PubMed] [Google Scholar]
- 15.Wu X, Ye Y, Rosell R, Amos CI, Stewart DJ, Hildebrandt MA, et al. Genome-Wide Association Study of Survival in Non-Small Cell Lung Cancer Patients Receiving Platinum-Based Chemotherapy. J Natl Cancer Inst. 2011 doi: 10.1093/jnci/djr075. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Hartford CM, Duan S, Delaney SM, Mi S, Kistner EO, Lamba JK, et al. Population-specific genetic variants important in susceptibility to cytarabine arabinoside cytotoxicity. Blood. 2009;113:2145–2153. doi: 10.1182/blood-2008-05-154302. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang RS, Duan S, Kistner EO, Hartford CM, Dolan ME. Genetic variants associated with carboplatin-induced cytotoxicity in cell lines derived from Africans. Mol Cancer Ther. 2008;7:3038–3046. doi: 10.1158/1535-7163.MCT-08-0248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Huang RS, Duan S, Shukla SJ, Kistner EO, Clark TA, Chen TX, et al. Identification of genetic variants contributing to cisplatin-induced cytotoxicity by use of a genomewide approach. Am J Hum Genet. 2007;81:427–437. doi: 10.1086/519850. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Li L, Fridley BL, Kalari K, Jenkins G, Batzler A, Weinshilboum RM, et al. Gemcitabine and arabinosylcytosin pharmacogenomics: genome-wide association and drug response biomarkers. PLoS One. 2009;4:e7765. doi: 10.1371/journal.pone.0007765. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Watters JW, Kraja A, Meucci MA, Province MA, McLeod HL. Genome-wide discovery of loci influencing chemotherapy cytotoxicity. Proc Natl Acad Sci U S A. 2004;101:11809–11814. doi: 10.1073/pnas.0404580101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Niu N, Schaid DJ, Fridley BL, Kalari K, Li L, Jenkins G, et al. Oral Session III-B (OIII-B) Molecular Targets and Genetics in Oncology 9:30 am - 10:30 am. Clin Pharmacol Ther. 2011;89:S66–S68. [Google Scholar]
- 22.Ziliak D, O’Donnell PH, Im HK, Gamazon ER, Chen P, Delaney S, et al. Germline polymorphisms discovered via a cell-based, genome-wide approach predict platinum response in head and neck cancers. Transl Res. 2011;157:265–272. doi: 10.1016/j.trsl.2011.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Altshuler D, Brooks LD, Chakravarti A, Collins FS, Daly MJ, Donnelly P. A haplotype map of the human genome. Nature. 2005;437:1299–1320. doi: 10.1038/nature04226. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Frazer KA, Ballinger DG, Cox DR, Hinds DA, Stuve LL, Gibbs RA, et al. A second generation human haplotype map of over 3.1 million SNPs. Nature. 2007;449:851–861. doi: 10.1038/nature06258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.O’Donnell PH, Gamazon E, Zhang W, Stark AL, Kistner-Griffin EO, Huang R Stephanie, et al. Population differences in platinum toxicity as a means to identify novel genetic susceptibility variants. Pharmacogenet Genomics. 2010;20:327–337. doi: 10.1097/FPC.0b013e3283396c4e. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Altshuler DM, Gibbs RA, Peltonen L, Dermitzakis E, Schaffner SF, Yu F, et al. Integrating common and rare genetic variation in diverse human populations. Nature. 2010;467:52–58. doi: 10.1038/nature09298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.de Bakker PI, Ferreira MA, Jia X, Neale BM, Raychaudhuri S, Voight BF. Practical aspects of imputation-driven meta-analysis of genome-wide association studies. Hum Mol Genet. 2008;17:R122–128. doi: 10.1093/hmg/ddn288. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Willer CJ, Li Y, Abecasis GR. METAL: fast and efficient meta-analysis of genomewide association scans. Bioinformatics. 2010;26:2190–2191. doi: 10.1093/bioinformatics/btq340. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Browning BL, Browning SR. A unified approach to genotype imputation and haplotype-phase inference for large data sets of trios and unrelated individuals. Am J Hum Genet. 2009;84:210–223. doi: 10.1016/j.ajhg.2009.01.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Abecasis GR, Cookson WO, Cardon LR. Pedigree tests of transmission disequilibrium. Eur J Hum Genet. 2000;8:545–551. doi: 10.1038/sj.ejhg.5200494. [DOI] [PubMed] [Google Scholar]
- 31.Price AL, Tandon A, Patterson N, Barnes KC, Rafaels N, Ruczinski I, et al. Sensitive detection of chromosomal segments of distinct ancestry in admixed populations. PLoS Genet. 2009;5:e1000519. doi: 10.1371/journal.pgen.1000519. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Li Y, Willer CJ, Ding J, Scheet P, Abecasis GR. MaCH: using sequence and genotype data to estimate haplotypes and unobserved genotypes. Genet Epidemiol. 2010;34:816–834. doi: 10.1002/gepi.20533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Purcell S, Neale B, Todd-Brown K, Thomas L, Ferreira MA, Bender D, et al. PLINK: a tool set for whole-genome association and population-based linkage analyses. Am J Hum Genet. 2007;81:559–575. doi: 10.1086/519795. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Devlin B, Roeder K. Genomic control for association studies. Biometrics. 1999;55:997–1004. doi: 10.1111/j.0006-341x.1999.00997.x. [DOI] [PubMed] [Google Scholar]
- 35.Pruim RJ, Welch RP, Sanna S, Teslovich TM, Chines PS, Gliedt TP, et al. LocusZoom: regional visualization of genome-wide association scan results. Bioinformatics. 2010;26:2336–2337. doi: 10.1093/bioinformatics/btq419. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Huang RS, Kistner EO, Bleibel WK, Shukla SJ, Dolan ME. Effect of population and gender on chemotherapeutic agent-induced cytotoxicity. Mol Cancer Ther. 2007;6:31–36. doi: 10.1158/1535-7163.MCT-06-0591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Harland SJ, Newell DR, Siddik ZH, Chadwick R, Calvert AH, Harrap KR. Pharmacokinetics of cis-diammine-1,1-cyclobutane dicarboxylate platinum(II) in patients with normal and impaired renal function. Cancer Res. 1984;44:1693–1697. [PubMed] [Google Scholar]
- 38.Lerza R, Vannozzi MO, Tolino G, Viale M, Bottino GB, Bogliolo G, et al. Carboplatin and cisplatin pharmacokinetics after intrapleural combination treatment in patients with malignant pleural effusion. Ann Oncol. 1997;8:385–391. doi: 10.1023/a:1008203100410. [DOI] [PubMed] [Google Scholar]
- 39.Oguri S, Sakakibara T, Mase H, Shimizu T, Ishikawa K, Kimura K, et al. Clinical pharmacokinetics of carboplatin. J Clin Pharmacol. 1988;28:208–215. doi: 10.1002/j.1552-4604.1988.tb03134.x. [DOI] [PubMed] [Google Scholar]
- 40.Peng B, English MW, Boddy AV, Price L, Wyllie R, Pearson AD, et al. Cisplatin pharmacokinetics in children with cancer. Eur J Cancer. 1997;33:1823–1828. doi: 10.1016/s0959-8049(97)00341-9. [DOI] [PubMed] [Google Scholar]
- 41.Marsh S, McLeod H, Dolan E, Shukla SJ, Rabik CA, Gong L, et al. Platinum pathway. Pharmacogenet Genomics. 2009;19:563–564. doi: 10.1097/FPC.0b013e32832e0ed7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Duan S, Huang RS, Zhang W, Bleibel WK, Roe CA, Clark TA, et al. Genetic architecture of transcript-level variation in humans. Am J Hum Genet. 2008;82:1101–1113. doi: 10.1016/j.ajhg.2008.03.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Gamazon ER, Zhang W, Konkashbaev A, Duan S, Kistner EO, Nicolae DL, et al. SCAN: SNP and copy number annotation. Bioinformatics. 2010;26:259–262. doi: 10.1093/bioinformatics/btp644. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Kushner BH, Kramer K, Modak S, Cheung NK. High-dose carboplatin-irinotecan-temozolomide: treatment option for neuroblastoma resistant to topotecan. Pediatr Blood Cancer. 2011;56:403–408. doi: 10.1002/pbc.22855. [DOI] [PubMed] [Google Scholar]
- 45.Veal GJ, Cole M, Errington J, Pearson AD, Gerrard M, Whyman G, et al. Pharmacokinetics of carboplatin and etoposide in infant neuroblastoma patients. Cancer Chemother Pharmacol. 2010;65:1057–1066. doi: 10.1007/s00280-009-1111-9. [DOI] [PubMed] [Google Scholar]
- 46.Kaneko S, Ohira M, Nakamura Y, Isogai E, Nakagawara A, Kaneko M. Relationship of DDX1 and NAG gene amplification/overexpression to the prognosis of patients with MYCN-amplified neuroblastoma. J Cancer Res Clin Oncol. 2007;133:185–192. doi: 10.1007/s00432-006-0156-y. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47.Bence AK, Crooks PA. The mechanism of L-canavanine cytotoxicity: arginyl tRNA synthetase as a novel target for anticancer drug discovery. J Enzyme Inhib Med Chem. 2003;18:383–394. doi: 10.1080/1475636031000152277. [DOI] [PubMed] [Google Scholar]
- 48.Saijo Y, Perlaky L, Valdez BC, Busch RK, Henning D, Zhang WW, et al. The effect of antisense p120 construct on p120 expression and cell proliferation in human breast cancer MCF-7 cells. Cancer Lett. 1993;68:95–104. doi: 10.1016/0304-3835(93)90134-u. [DOI] [PubMed] [Google Scholar]
- 49.Kim Y, Shintani S, Kohno Y, Zhang R, Wong DT. Cyclin G2 dysregulation in human oral cancer. Cancer Res. 2004;64:8980–8986. doi: 10.1158/0008-5472.CAN-04-1926. [DOI] [PubMed] [Google Scholar]
- 50.Eliopoulos AG, Kerr DJ, Herod J, Hodgkins L, Krajewski S, Reed JC, et al. The control of apoptosis and drug resistance in ovarian cancer: influence of p53 and Bcl-2. Oncogene. 1995;11:1217–1228. [PubMed] [Google Scholar]
- 51.Dabholkar M, Vionnet J, Bostick-Bruton F, Yu JJ, Reed E. Messenger RNA levels of XPAC and ERCC1 in ovarian cancer tissue correlate with response to platinum-based chemotherapy. J Clin Invest. 1994;94:703–708. doi: 10.1172/JCI117388. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52.Li Q, Yu JJ, Mu C, Yunmbam MK, Slavsky D, Cross CL, et al. Association between the level of ERCC-1 expression and the repair of cisplatin-induced DNA damage in human ovarian cancer cells. Anticancer Res. 2000;20:645–652. [PubMed] [Google Scholar]
- 53.Metzger R, Leichman CG, Danenberg KD, Danenberg PV, Lenz HJ, Hayashi K, et al. ERCC1 mRNA levels complement thymidylate synthase mRNA levels in predicting response and survival for gastric cancer patients receiving combination cisplatin and fluorouracil chemotherapy. J Clin Oncol. 1998;16:309–316. doi: 10.1200/JCO.1998.16.1.309. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.