Abstract
Granulysin is expressed as two isoforms by human cytotoxic cells: a single mRNA gives rise to 15 kDa granulysin, a portion of which is cleaved to a 9 kDa protein. Studies with recombinant 9 kDa granulysin have demonstrated its cytolytic and proinflammatory properties, but much less is known about the biologic function of the 15 kDa isoform. Here we show that the subcellular localization and functions of 9 kDa and 15 kDa granulysin are largely distinct. 9 kDa granulysin is confined to cytolytic granules that are directionally released following target cell recognition. In contrast, 15 kDa granulysin is located in distinct granules that lack perforin and granzyme B and that are released by activated cytolytic cells. While recombinant 9 kDa granulysin is cytolytic against a variety of tumors and microbes, recombinant 15 kDa granulysin is not. The 15 kDa isoform is a potent inducer of monocytic differentiation to dendritic cells, but the 9 kDa isoform is not. In vivo, mice expressing granulysin show markedly improved anti-tumor responses, with increased numbers of activated dendritic cells and cytokine-producing T cells. Thus, the distinct functions of granulysin isoforms have major implications for diagnosis and potential new therapies for human disease.
Introduction
Cytotoxic cells protect the body from diseases and infection. CTLs and NK cells use a variety of mechanisms to kill target cells, including release of granules containing lytic molecules, e.g., perforin and granzymes, and expression of cell surface molecules such as FasL that trigger apoptosis when bound to cognate receptors on target cells (1–3). In addition, cytotoxic cells release an assortment of soluble molecules, e.g., IFNγ, TNF, RANTES, that can further modulate the immune response (4). Granulysin was identified in a search for genes expressed “late” (3–5 days) after activation of human CD8+ T cells (5). A single mRNA is translated into 15 kDa granulysin, some of which is processed at both the amino and carboxy termini to a 9 kDa protein. Granulysin is co-expressed in cytolytic granules with perforin and granzymes and is released via receptor-mediated granule exocytosis. Recombinant 9 kDa granulysin is lytic for tumors and microbes, including gram positive and gram negative bacteria, Mycobacteria tuberculosis and Plasmodium falciparum, and acts as a chemoattractant for T lymphocytes, monocytes and other inflammatory cells (6–9). Expression of granulysin has been broadly associated with good outcomes in cancer and infection (10–13). The 15 kDa isoform of granulysin is less well characterized, but it has been implicated as the causative agent in Stevens-Johnson syndrome and toxic epidermal necrolysis (14, 15).
Recently we showed that both recombinant 9 and 15 kDa granulysin induced in vitro chemotaxis and activation of both human and mouse immature dendritic cells (iDCs)3, recruited inflammatory leucocytes including antigen presenting cells in mice, and promoted antigen-specific immune responses upon co-administration with an antigen (16). The ability of granulysin to attract and activate monocyte-derived dendritic cells (DCs) and increase intraperitoneal inflammatory cells in vivo suggests that it may prove to be a clinically useful immune adjuvant. Since mice do not express granulysin or a functional homolog, we generated mice expressing human granulysin as a transgene and showed that these animals are more resistant to tumors (17). CTLs and NK cells from these animals exhibit enhanced cytotoxicity against target cells in vitro and granulysin delivered by cytotoxic cells required perforin for killing via an endoplasmic reticulum stress pathway (18).
In this study we detail the expression, intracellular localization, and function of 9 and 15 kDa granulysin. In PBMCs from normal donors, all CD56+ NK cells, the majority of CD3+CD56+ NKT cells, and some CD8+ effector cells express granulysin. 9 kDa granulysin is localized to cytolytic granules, released upon granule exocytosis and is important in causing target cell death. In contrast, 15 kDa granulysin is contained in different vesicles that are secreted by activated cytolytic cells but recombinant 15 kDa granulysin is not cytolytic. 15 kDa but not 9 kDa granulysin activates monocytes to differentiate into iDCs. Mice expressing granulysin exhibit enhanced anti-tumor responses and increased numbers of activated DCs and T cells. Thus, although 9 kDa granulysin results from proteolytic cleavage of the 15 kDa form, the two molecules play very different roles in immune responses.
Materials and Methods
Cells
Human PBMCs were obtained from healthy donor leukopacs and leukocytes enriched by centrifugation over Ficoll; monocytes were obtained from healthy donors by leukopheresis and elutriation (Transfusion Medicine Department, Clinical Center, National Institutes of Health, Bethesda, MD). Mouse peripheral blood was obtained by cardiac puncture and leukocytes were enriched using Ficoll. Mouse bone marrow monocytes were isolated using the EasySep Mouse Monocyte enrichment kit from Stem Cell Technologies (Vancouver, BC). Immature murine bone marrow derived DCs were obtained from bone marrow cells cultured with recombinant murine GM-CSF (20 ng/ml) (PeproTech, Rocky Hill, NJ) for 8 days.
Granulysin expression
Single cells suspensions of PBMCs were stained with fluorochrome-conjugated Abs specific for CD3, CD4, CD8, CD56, CD27, and CD45RA (BD Biosciences, San Jose, CA), fixed and permeabilized (BD Cytofix/Cytoperm, BD Biosciences), and then stained with rabbit anti-granulysin antiserum or preimmune rabbit serum (5, 18). The flow cytometry data were analyzed with FlowJo analysis software (Tree Star, Ashland, OR). CD8+ T and NK cells were prepared from peripheral blood mononuclear cells using negative selection with magnetic bead purification (Stemcell Technologies) (purity was >95%). For activation, 106 NK cells or 107 CD8+ T cells were cultured in medium with or without 50 ng/ml recombinant human IL-15 (eBioscience, San Diego, CA) for 2 days, and supernatants and pellets were collected following centrifugation. Where indicated, PMA (5 ng/ml) plus ionomycin (500 ng/ml) were added for the last 6 hours to induce degranulation. Western blot analysis was conducted as described (6).
Confocal Microscopy
Freshly isolated or IL-15 activated NK cells or the NK-like cell line NK92 were immobilized on poly (L-lysine)-coated slides, fixed in 2% paraformaldehyde and permeabilized (0.01% saponin and 0.1% Triton X-100). Cells were stained with the following antibodies: rabbit anti-granulysin antiserum (5), monoclonal anti-15 kDa granulysin (clone RF10, MBL International, Woburn, MA), perforin (clone SG9, BD Biosciences), granzyme B (clone GB11, Gene-Tex, Irvine, CA), LAMP-1 (clone H4A3, BD Biosciences), and RANTES (clone VL1, Invitrogen, Carlsbad, CA) and counterstained with fluorochrome conjugated anti-rabbit or anti-mouse antibodies (Molecular Probes, Carlsbad, CA). Images from fixed cells were collected with a Zeiss 510 LSCM, using a 63X, 1.4 NA objective (Carl Zeiss Inc, Thornton NY). Z stacks of complete cells were taken with Nyquist sampling frequency for colocalization analysis. Imaris 7.2.3 (Bitplane, Andor) was used for most image processing and for colocalization analysis. Briefly, one channel was used to produce a surface that included the entire cell to be analyzed and this surface was used to make a channel where all pixels outside the cell were assigned a value of 0. This new channel was used to define a region of interest for the Imaris colocalization analysis. Thresholds were set for each channel by selecting the dimmest puntae to be included in the analysis. Data from 25–40 independent cells were collected for each antibody. Adobe PhotoShop and Illustrator (Adobe Systems Inc, San Jose CA) were used to prepare composite figures.
Cytotoxicity of 9 kDa and 15 kDa granulysin
Apoptosis of U937 cells and killing of S. typhimurium were measured as described (7, 8).
Generation and analysis of DCs
Human monocytes were cultured at 2 × 106 cells/ml in RPMI-1640 supplemented with 10% heat-inactivated FBS (Hyclone, Ogden, UT), 2 mM L-glutamine, and 100 U/ml penicillin-streptomycin (complete media). Recombinant 9 and 15 kDa granulysin were prepared as described (5, 19). Monocytes were cultured with 15 kDa granulysin (10 nM) or with recombinant human GM-CSF (10 ng/ml) (BD Biosciences) and recombinant human IL-4 (10 ng/ml) (R & D Systems) for 3–4 days to produce iDCs. Cell surface antigens were assessed by FACS using a panel of fluorochrome conjuated antibodies from BD Biosciences and eBioscience.
Mice
C57BL/6 granulysin transgenic mice (GNLY+/−) (17) were backcrossed to BALB/c mice for >10 generations. Mice were bred at the National Cancer Institute and mice >6 weeks of age were used in all experiments. The Animal Care and Use Committee of the National Cancer Institute approved all animal experiments. GNLY+/− and WT littermates were injected in the right flank with 1.5 × 106 CT26 tumor cells. After 12–14 days, tumors were removed and weighed. Tumor infiltrating lymphocytes and lymphocytes from the draining inguinal and popliteal lymph nodes were analyzed by flow cytometry.
Results
Expression of granulysin by human PBMCs
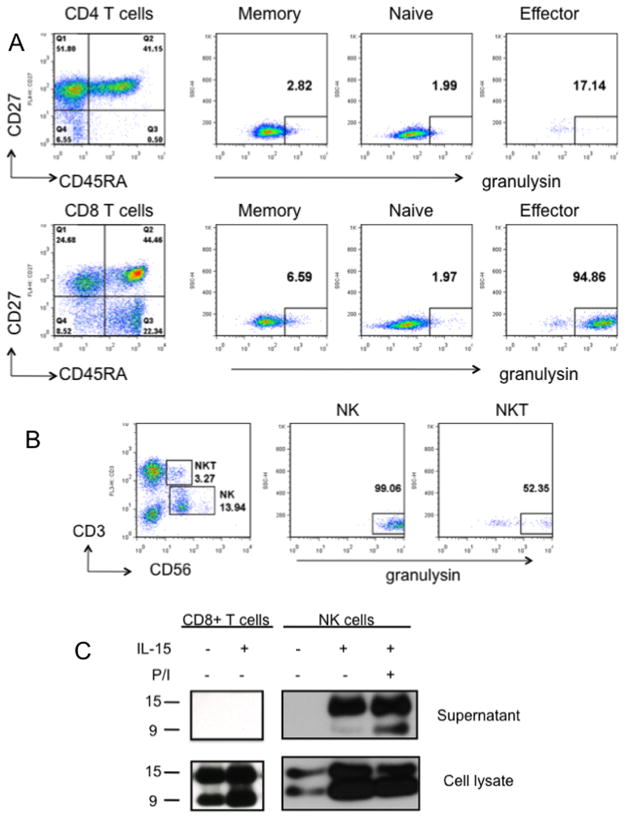
Buffy coats were obtained from 10 healthy donors and granulysin expression was determined by intracellular staining and FACS. PBMCs were stained with various combinations of antibodies to identify CD4+ or CD8+ naïve, effector, and memory cells as well as NK and NKT cells. Cells were then fixed, permeabilized, and stained with rabbit anti-granulysin antiserum or preimmune serum. After gating on lymphocytes, expression of CD27 and CD45RA was used to subdivide CD4+ or CD8+ cells. As previously described, naïve cells are CD27+CD45RA+ (Q2), effector cells are CD27−CD45RA+ (Q1) and memory cells are CD27+CD45RA− (Q3) (20). Table I summarizes the results from all 10 donors, and results from a typical donor are shown in Figure 1A. Granulysin expression is rare in CD4+ or CD8+ memory or naïve T cells. In contrast, 17% of CD4+ and 75% of CD8+ effector T cells express granulysin. Essentially all CD56+ natural killer cells and the majority of CD56+CD3+ natural killer T cells express granulysin (Figure 1B, Table I).
Table I.
Expression of granulysin in freshly isolated PBMCs
| % Granulysin Positivea | Range (% granulysin positive) | |
|---|---|---|
| CD4+ Naïveb T cells | 0.7 | 0 – 3 |
| CD4+ Memoryc T cells | 0.7 | 0 – 3 |
| CD4+ Effectord T cells | 17 | 0 – 39 |
| CD8+ Naïveb T cells | 5 | 0 – 16 |
| CD8+ Memoryc T cells | 7 | 0 – 15 |
| CD8+ Effectord T cells | 75 | 31 – 97 |
| CD56+ | 94 | 87 – 100 |
| CD56+CD3+ | 54 | 35 – 97 |
Mean of 10 donors
CD27+CD45A+
CD27+CD45A−
CD27−CD45RA+
Figure 1.
Granulysin is expressed by a subset of PBMCs. A, PBMCs from normal donors were stained with antibodies specific for either CD4 or CD8 plus CD27, CD45RA and granulysin. After gating on either CD4 or CD8, expression of CD27 and CD45RA was used to divide cells into memory (CD27+CD45RA−), naïve (CD27+CD45RA+) or effector (CD27−CD45RA+) cells. The percent of cells in each subset that stain for granulysin is shown. B, PBMCs were stained with antibodies specific for CD3, CD56 and granulysin. C, 107 CD8+ T cells or 106 NK cells were cultured for 2 days in medium with or without recombinant IL-15 (50 ng/ml). Where indicated, cells were activated with PMA plus ionomycin (P/I) for the final 6 hours of culture. Supernatants and cell pellets were subjected to SDS-PAGE and Western blot was carried out using anti-granulysin antibody. Results are representative of 10 donors (A,B) or 3 donors (C).
To determine which isoforms of granulysin are present in human NK and T cells, CD56+ or CD8+ cells were purified by negative selection using magnetic beads. Cells were then cultured in medium alone or medium supplemented with 50 ng/ml recombinant human IL-15 for 2 days and the supernatants and cell pellets were analyzed for granulysin isoforms by Western blot. Both 15 and 9 kDa granulysin were present in the cell pellets of CD8+ T and NK cells cultured in medium or IL-15 (Figure 1C). IL-15 caused a much greater increase in granulysin levels in NK cells than in CD8+ T cells. CD8+ T cells activated with IL-15 for 2 days did not secrete detectable levels of either 9 kDa or 15 kDa granulysin. NK cells incubated in medium alone did not secrete any granulysin, but NK cells cultured with IL-15 secreted 15 kDa, but not 9 kDa, granulysin (Figure 1C). NK cells cultured with IL-15 and then stimulated with PMA and ionomycin for the final 6 hours of culture secreted both 9 kDa and 15 kDa granulysin, although the amount of 15 kDa granulysin was much higher.
Confocal microscopy was used to further characterize the subcellular localization of 9 kDa and 15 kDa granulysin in NK cells isolated from PBMCs. We used two antibodies to visualize granulysin: 1) a polyclonal antibody raised against recombinant 9 kDa granulysin that recognizes both the 9 and 15 kDa forms in Western blot (5) and 2) a monoclonal antibody that recognizes an epitope on the 15 kDa but not the 9 kDa isoform (21). Cells were also stained with antibodies specific for perforin, granzyme B, and LAMP-1, proteins located in cytolytic granules. Staining was compared in freshly isolated NK cells (Supplemental Figure 1) and in NK cells activated by a 5 day culture with IL-15 (Figure 2). Both resting and IL-15 activated NK cells stained with the polyclonal and monoclonal anti-granulysin antibodies. Although the polyclonal antibody recognizes both isoforms under denaturing conditions (see Figure 1C), there was minimal overlap in staining with the monoclonal 15 kDa specific antibody, regardless of whether these antibodies were applied simultaneously or sequentially (Figure 2A). Since the 9 and 15 kDa isoforms share a common core that contains the epitopes recognized by the polyclonal antibody, these epitopes must be inaccessible in the native 15 kDa granulysin molecule in vivo. NK cells were then stained with the polyclonal and monoclonal granulysin antibodies as well as a LAMP-1 specific antibody (Figure 2B). Resting NK cells contain very little LAMP-1, and there is little if any colocalization of LAMP-1 with granulysin. IL-15 activated cells express much more LAMP-1, and the majority of it colocalizes with 9 kDa granulysin; minimal colocalization of 15 kDa granulysin and LAMP-1 was observed. Results were similar for perforin and granzyme B: both were expressed at low levels in resting NK cells, expression increased after culture with IL-15, and the 9 kDa, but not the 15 kDa, isoform colocalized with perforin and granzyme B (Figure 2C,D). We performed a similar analysis using the NK-like cell line NK92 (Supplementary Figures 2 and 3). In agreement with our findings in activated NK cells, 9 kDa but not 15 kDa granulysin colocalized with perforin and granzyme B, but not with the chemokine RANTES which has been shown to be secreted in a different type of granule (22). A quantitative summary of these findings is shown in Table II.
Figure 2.
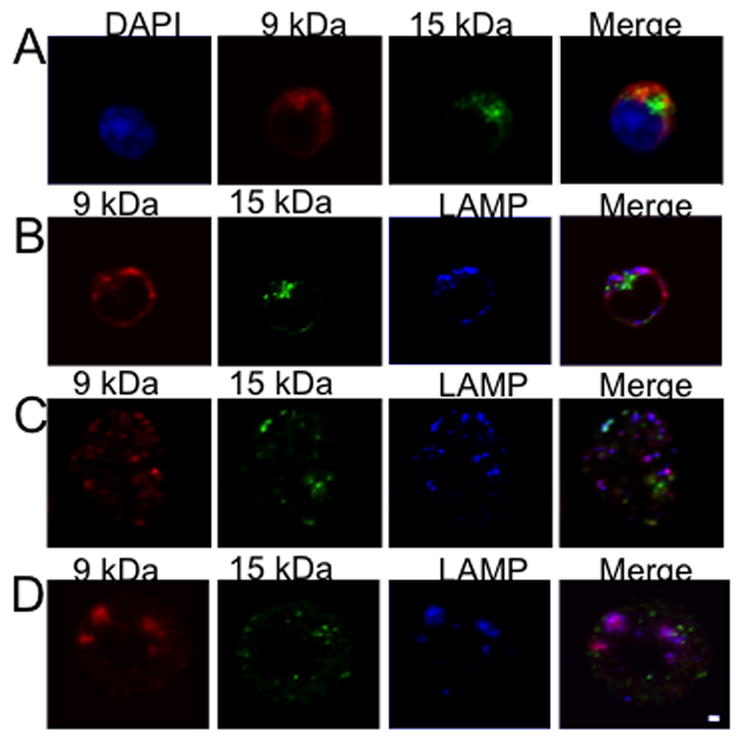
9 kDa granulysin co-localizes with LAMP-1, granzyme B and perforin while 15 kDa granulysin is present in a distinct compartment in human NK cells. A–D, NK cells were isolated from PBMCs activated with IL-15 for 5 days, stained and analyzed by confocal microscopy. Bar indicates 1 μ. Results are representative of 3 similar experiments.
Table II.
Colocalization of granulysin with perforin, granzyme B, LAMP, and RANTES in NK cells
| Resting NK Cells | Activated NK Cells | NK92 cells | ||
|---|---|---|---|---|
| Antibody A | Antibody B | % Antibody A colocalized with Antibody B | % Antibody A colocalized with Antibody B | % Antibody A colocalized with Antibody B |
| 9 kDa Granulysin | 15 kDa Granulysin | 25.55 +/− 4.79 | 12.47 +/− 3.18 | 3.2 +/− 0.2 |
| 9 kDa Granulysin | Perforin | 17.26 +/− 3.28a | 50.86 +/− 3.57d | 69.5 +/− 11.5f |
| 15 kDa Granulysin | Perforin | 6.65 +/− 1.71a | 29.50 +/− 4.98d | 12.65 +/− 7.1f |
| 9 kDa Granulysin | Granzyme B | 32.19 +/− 5.93b | 44.79 +/− 6.71e | 49.0 +/− 17.4g |
| 15 kDa Granulysin | Granzyme B | 7.77 +/− 2.48b | 8.53 +/− 3.05e | 9.35 +/−14.5g |
| 9 kDa Granulysin | LAMP1 | 24.26 +/− 3.21c | 47.62 +/− 7.25 | ND |
| 15 kDa Granulysin | LAMP1 | 11.02 +/− 2.23c | 28.87 +/− 7.9 | ND |
| 9 kDa Granulysin | RANTES | ND | ND | 21.8 +/− 9.5 |
| 15 kDa Granulysin | RANTES | ND | ND | 30.5 +/− 11.7 |
p < 0.01
9 kDa, but not 15 kDa, granulysin is cytolytic while 15 kDa, but not 9 kDa, granulysin activates human monocytes
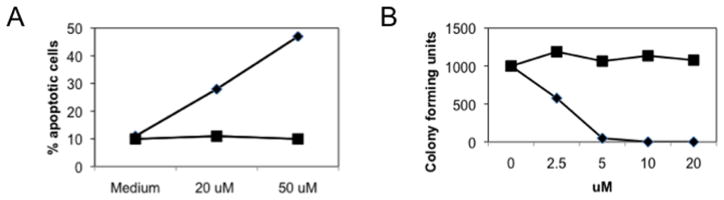
In order to investigate the biologic role of 15 kDa granulysin, it was expressed in insect cells and purified by fast protein liquid chromatography using sequential heparin and cation exchange columns (19). Since recombinant 9 kDa granulysin exhibits lytic activity against a variety of microbes as well as mammalian tumor cells (7, 8), we compared the ability of recombinant 15 kDa and 9 kDa granulysin to kill S. typhimurium and human tumor cells. Recombinant 15 kDa granulysin was not cytolytic at any concentration tested while 9 kDa granulysin was lytic against both targets (Figure 3A,B). Similar results were obtained using other target cells including Jurkat, HUT-78, E. coli and S. aureus (not shown).
Figure 3.
15 kDa granulysin is not cytolytic. A, Recombinant 9 kDa (◆) or 15 kDa (■) granulysin was added to U937 tumor cells and apoptosis measured by annexin V and propidium iodide staining. B, Recombinant 9 kDa (◆) or 15 kDa (■) granulysin was added at the indicated concentration to S. typhimurium and colony forming units determined.
We next asked whether either isoform affected freshly isolated human peripheral blood mononuclear cells. Within a few hours, PBMCs treated with the 15 kDa isoform formed aggregates while those treated with the 9 kDa isoform did not (not shown). Cell surface staining and FACS analysis of these cultures revealed that monocytes were responsive to 15 kDa granulysin. Using elutriated CD14+ monocytes, we compared the effects of 15 kDa granulysin, 9 kDa granulysin, and GM-CSF, a well characterized activator of monocytes (23). Within 6 hours, monocytes cultured with 10 nM 15 kDa granulysin, but not with 9 kDa granulysin (tested over a range of 10–10,000 nM) or GM-CSF (10 ng/ml), formed aggregates (Figure 4A). Both 15 kDa granulysin and GM-CSF caused a rapid increase in cell size and upregulation of adhesion molecules including CD11b, CD11c, and CD54, as well as molecules associated with differentiation to iDCs, including CD40, CD80, CD86 and HLA-DR (Figure 4B). 15 kDa granulysin, but not GM-CSF, promoted increased expression of CD83 while GM-CSF, but not 15 kDa granulysin, caused increased expression of CD1a and CD1c.
Figure 4.
15 kDa granulysin activates human monocytes. A, Purified CD14+ monocytes were cultured in medium alone or in medium supplemented with 50 uM 9 kDa granulysin, 10 nM 15 kDa granulysin or 10 ng/ml GM-CSF. Photographs were taken (10X objective) 6 hours later. B, Expression of a panel of cell surface molecules by monocytes cultured with 15 kDa granulysin (10 nM) or GM-CSF (10 ng/ml). Purified monocytes were incubated with medium (grey), 10 nM 15 kDa granulysin (blue) or 10 ng/ml GM-CSF (orange) for 24 hours and then stained with fluorescent antibodies and analyzed by FACS. C, iDC were incubated with 10 nM 15 kDa granulysin (black line) or medium (grey) for an additional 24 hours and then stained with the indicated fluorescent antibodies and analyzed by FACS. Results are representative of 4–6 similar experiments.
Previously we used commercial granulysin to show that 15 kDa granulysin could also promote the differentiation of iDCs to mature DCs (mDCs) (16). We reported that this process required quite high concentrations (2–10 ug/ml) of commercial 15 kDa granulysin. To determine the efficacy of our recombinant 15 kDa granulysin prepared from insect cells on the maturation of iDC, we incubated human monocytes with GM-CSF plus IL-4 for 6 days and then added insect produced 15 kDa granulysin at 10 ng/ml for an additional 24 hours. Cell surface analysis of these cells shows that they exhibit a typical mDC phenotype with low expression of CD14 and high expression of CD40, CD80, CD83, CD86, and HLA-DR (Figure 4C). This observation is in agreement with our previously published finding that our 15 kDa recombinant granulysin is more potent than 15 kDa granulysin obtained from 2 different commercial sources (19).
15 kDa granulysin and GM-CSF activate distinct genes in monocytes
Previously we analyzed the gene expression of monocytes cultured with either 15 kDa granulysin or GM-CSF. We found that GM-CSF altered the expression of genes involved in cell differentiation while 15 kDa granulysin induced expression of proinflammatory cytokines (23). To extend this observation, we selected a subset of immune related genes whose expression was increased by 15 kDa granulysin in the microarray analysis by at least 5 fold at 4 hours and confirmed their expression by real time qPCR (Table III). Monocytes from 5 donors were cultured with medium, 10 nM 15 kDa granulysin or 10 ng/ml GM-CSF and aliquots were removed at 4, 12 and 24 hours. At the 4 hour time point, mRNA for all these genes was increased by 15 kDa granulysin over levels in monocytes cultured in medium alone while at the same time point, only CD274 and CD80 mRNA were slightly upregulated in monocytes cultured with GM-CSF. At 4 and 12 hours, expression of all genes was higher in cells treated with 15 kDa granulysin than in cells treated with GM-CSF. At the 24 hour time point, mRNA levels of all genes except CCL2 and CCL7 were higher in cells treated with15 kDa granulysin than in cells treated with GM-CSF. These data indicate that 15 kDa granulysin affects monocytes differently from GM-CSF, suggesting that 15 kDa granulysin may induce antigen presenting cells that functionally differ from those induced with GM-CSF.
Table III.
Immune related gene expression in monocytes activated with granulysin or GM-CSFa
| 15 kDa Granulysin | GM-CSF | |||||
|---|---|---|---|---|---|---|
| 4 h | 12 h | 24 h | 4 h | 12 h | 24 h | |
| IL-6 | 2802 | 3308 | 940 | 1 | 3 | 2 |
| IL1β | 70 | 206 | 248 | 1 | 6 | 1 |
| CXCL1 | 66 | 131 | 16 | 1 | 2 | 1 |
| CXCL2 | 19 | 81 | 51 | 1 | 3 | 1 |
| CXCL3 | 16 | 83 | 41 | 1 | 1 | 1 |
| CCL2 | 731 | 48 | 8 | 1 | 2 | 43 |
| CCL7 | 79 | 19 | 8 | 1 | 1 | 18 |
| CCL20 | 243 | 375 | 1056 | 1 | 1 | 1 |
| CCL23 | 350 | 1013 | 311 | 2 | 7 | 143 |
| TNFα | 35 | 31 | 6 | 1 | 3 | 3 |
| TNFAIP6 | 62 | 100 | 31 | 2 | 1 | 0 |
| TNFAIP8 | 22 | 5 | 5 | 3 | 1 | 1 |
| ITGB8 | 124 | 220 | 36 | 2 | 1 | 1 |
| TRAF1 | 20 | 21 | 5 | 1 | 1 | 1 |
| TNFRSF4 | 11 | 24 | 2 | 1 | 1 | 1 |
| IL7R | 8 | 23 | 8 | 2 | 2 | 4 |
| CD274 | 319 | 102 | 12 | 11 | 7 | 2 |
Monocytes were cultured for 4, 12, or 24 hours with 15 kDa granulysin (10 nM) or GM-CSF (10 ng/ml) and RNA isolated. Results are expressed as the fold increase relative to the monocytes cultured in medium alone for the indicated time. Results are representative of one of five similar experiments.
15 kDa granulysin is a chemoattractant for murine leukocytes in vivo but does not activate murine monocytes in vitro
To further investigate a role for 15 kDa granulysin in clinical situations, we turned to a mouse model. Because mice do not have a granulysin homologue, we generated mice transgenic for human granulysin (17). To determine the role of secreted 15 kDa granulysin in vivo, we activated splenocytes from WT and GNLY+/− animals with IL-15 for 7 days, isolated NK cells using magnetic beads, and injected 106 WT or GNLY+/− transgenic NK cells into the peritoneum of WT BALB/c mice. Cells were collected 24 hours later and both the absolute number and the variety of cell types present were determined (Figure 5A,B). Activated GNLY+/− NK cells caused the influx of approximately 3 times as many cells as did WT NK cells. However, the percentage of neutrophils, macrophages, T cells, B cells, and NK cells in the infiltrating cells was similar when WT or GNLY+/− transgenic NK cells were injected.
Figure 5.

15 kDa granulysin promotes chemotaxis in vivo of murine leukocytes but does not activate murine monocytes. NK cells from GNLY+/− or WT mice were injected into the peritoneum of WT mice and after 24 hours, infiltrating cells were obtained. A, The total number of cells in the infiltrates was determined. Each point represents one animal. *p < 0.01 B, Infiltrating cells were stained with a panel of antibodies and analyzed by FACS. Bars represent the percent of total cells for the indicated subsets. C, Peripheral blood leukocytes from BALB/c mice were cultured in medium alone or supplemented with GM-CSF (10 ng/ml) or recombinant 15 kDa granulysin (10 nM). After 24 hours, cells were stained for expression of CD11b and CD11c and analyzed by FACS. D, Bone marrow CD11b+Ly-6C− cells from BALB/c mice were cultured for 24 hours with medium (solid grey), 10 nM 15 kDa granulysin (blue line) or 10 ng/ml GM-CSF (blue line) and expression of CD80 and CD86 was determined by FACS. E, Bone marrow cells from BALB/c mice were cultured with GM-CSF (10 ng/ml) plus IL-4 (10 ng/ml) for 8 days and the resulting iDCs were then cultured with medium (solid grey) or 10 nM 15 kDa granulysin (blue line). Expression of CD80, CD86, and CD40 was determined by FACS. Results are representative of 3 similar experiments.
We next asked whether recombinant 15 kDa granulysin activates murine monocytes in vitro. Both peripheral blood and bone marrow were used as a source of monocytes. While GM-CSF induced the differentiation of peripheral blood leukocytes into CD11b+ CD11c+ dendritic-like cells, 15 kDa granulysin did not induce a significant change in the number of CD11b+CD11c+ cells (Figure 5C). Bone marrow is a source of monocyte progenitors, and culture of bone marrow CD11b+Ly-6C− cells with GM-CSF for 8 days caused an increase in cells expressing CD80 and CD86 (Figure 5D). However, culture of bone marrow CD11b+Ly-6C− cells with 15 kDa granulysin for 8 days caused only a slight increase in the number of cells expressing CD80 and CD86. Lastly we tested the ability of 15 kDa granulysin to induce maturation of immature mouse DCs. Bone marrow CD11b+Ly-6C− cells cultured for 8 days with GM-CSF were then incubated for 24 hours with 15 kDa granulysin. 15 kDa granulysin caused a significant increase in the number of cells expressing higher levels of CD80, CD86, and CD40 (Figure 5E), indicating that 15 kDa granulysin can activate immature mouse DCs but not mouse monocytes.
GNLY+/− mice exhibit increased numbers of DCs, activated T cells, and enhanced anti-tumor responses
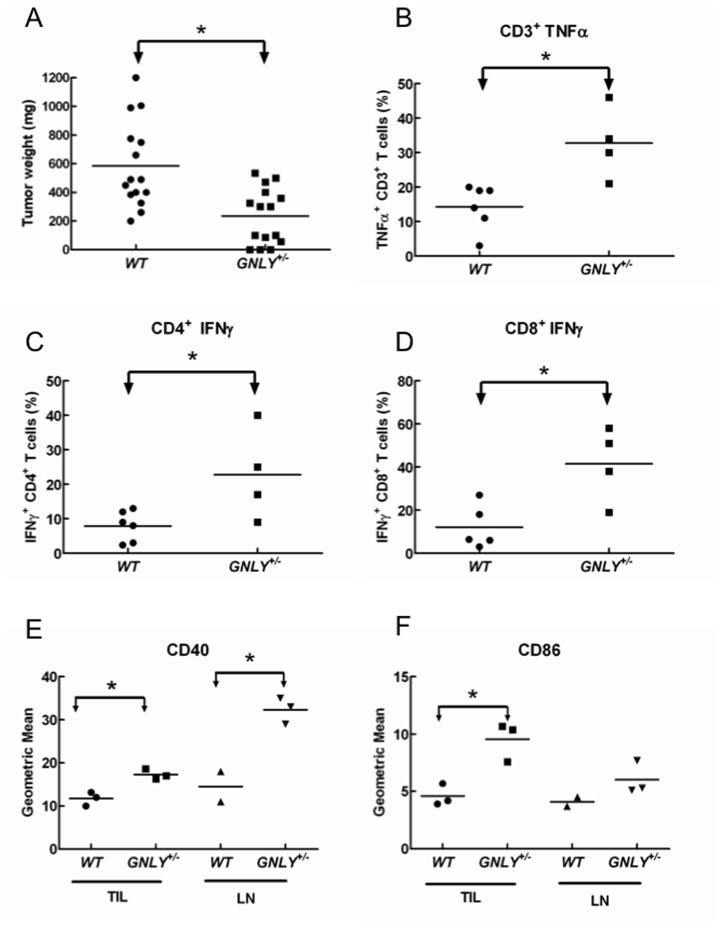
Syngeneic CT26 colon carcinoma cells (24) were injected into the flanks of wild type (WT) and GNLY+/− transgenic mice and 13 days later tumors and draining lymph nodes were removed. The tumors were weighed and tumor-infiltrating lymphocytes (TIL) were prepared. Tumor nodules from GNLY+/− mice were significantly smaller than those from WT mice (Figure 6A). While the number of TIL recovered from the WT mice was consistently higher than from GNLY+/− animals, the percentages of CD3+, CD8+ and DX5+ cells in the TIL were similar from both GNLY+/− and WT mice (not shown). Both 9 and 15 kDa isoforms of granulysin were detected by Western blot in TIL from GNLY+/− mice, with the 9 kDa isoform predominating (not shown). Following stimulation with PMA and ionomycin, IFNγ and TNFα production in lymphocytes isolated from the draining nodes was significantly higher in cells from GNLY+/− mice (Figure 6B–D). Furthermore, antigen-presenting cells from both draining lymph nodes and tumor nodules from GNLY+/− mice expressed higher levels of CD40 and CD86 (Figure 6E,F), indicating that the capacity of T cells to produce granulysin correlates with enhanced costimulatory/coactivating properties of antigen presenting cells in vivo.
Figure 6.
GNLY+/− mice exhibit enhanced resistance to tumors, numbers of antigen presenting cells, and T cell responses. WT or GNLY+/− mice were injected with CT26 tumor cells in the left flank and the tumor and draining lymph nodes were removed after 12–14 days. A, Weight of excised tumors. Each point represents one animal. B, Tumor infiltrating lymphocytes were stimulated in vitro with PMA/ionomycin and intracellular expression of TNFα and IFNγ measured by flow cytometry. C, GNLY+/− animals have increased numbers of CD40+ and CD86+ cells in the tumor and draining lymph nodes. *p<0.01. Results are representative of 3 similar experiments.
Discussion
Granulysin was originally identified in a search for genes expressed “late” (3–5 days) after T cell activation (25). Two granulysin isoforms, 9 and 15 kDa, were detected and subsequent studies showed that the 9 kDa isoform resulted from posttranslational processing of 15 kDa granulysin (5, 6). Recombinant 9 kDa granulysin has been well characterized: it causes apoptosis of tumor cells, kills many pathogens, and activates iDCs (7, 8, 16). However, the function of 15 kDa granulysin is less well understood. In this report we compare the expression, intracellular localization, and function of 9 and 15 kDa granulysin. Both isoforms are found in CD8+ effector T cells, NK cells, and NKT cells in freshly isolated PBMCs but only the 15 kDa isoform is detected in supernatants from IL-15 activated cytotoxic cells. 9 and 15 kDa granulysin are located in different compartments in cytolytic cells: the 9 kDa isoform colocalizes with perforin, granzyme B, and LAMP-1 while the 15 kDa form does not. In contrast to the 9 kDa isoform, recombinant 15 kDa granulysin is not cytotoxic. Moreover, 15 kDa granulysin, but not 9 kDa, granulysin, activates human monocytes to differentiate into DCs. 15 kDa granulysin is a potent attractant of immune cells in vivo, and transgenic mice expressing granulysin show enhanced ability to reject tumors.
Granulysin is expressed by essentially all NK cells, a majority of NKT and CD8+ effector T cells, but by few CD4+ effector cells in PBMCs from healthy donors. Granulysin expression in CD4+ naïve and memory T cells is negligible and quite low in CD8+ naïve and memory T cells. These findings confirm and extend those of Ogawa et al. who reported granulysin expression in CD56+ cells as well as in an unidentified portion of CD3+ cells (21). Both the 9 and 15 kDa isoforms of granulysin were present in purified NK or CD8+ T cells, but only the 15 kDa isoform was detected in supernatants of NK cells activated for 48 hours with IL-15. No granulysin was detected in the supernatants from purified CD8+ T cells cultured for 48 hours with IL-15, suggesting that activated NK cells are the major source of serum granulysin in vivo. Surprisingly, we were unable to show by confocal microscopy that the polyclonal antibody raised against recombinant 9 kDa granulysin recognizes the 15 kDa isoform in cells. Possible explanations for this observation are that the 15 kDa molecule adopts a tertiary conformation that masks the 9 kDa epitopes or that 15 kDa granulysin is associated in vivo with molecules that block access to the 9 kDa epitopes.
We were unable to detect any cytotoxicity of the recombinant 15 kDa granulysin against a variety of tumor and bacterial targets in side by side comparison with cytolytic 9 kDa recombinant granulysin. In contrast, Chung et al. reported that 15 kDa granulysin causes keratinocyte death (14). These authors used a recombinant 15 kDa granulysin containing a 6 histidine tag which may explain our different findings. As detailed by Finn et al., commercially available 15 kDa granulysin preparations contain recombinant tags that may affect function (19). The preparation from R and D Systems contains a 10-histidine tag at the C-terminus while the Novus Biologicals material includes an intact GST tag at the N-terminus. We showed that our untagged insect produced 15 kDa granulysin behaves differently from the commercially available tagged forms in a monocyte activation bioassay (19). Furthermore, we show here that our recombinant 15 kDa granulysin is more potent than commercial 15 kDa granulysin for inducing the maturation of iDC. Our 15 kDa granulysin can be used at 10 nM to induce iDC to become mDC while Tewary et al. reported that they needed approximately 100 times as much commercial recombinant 15 kDa granulysin (16). NK cells, like monocytes, are recruited to sites of inflammation or tissue damage. A number of groups have reported that activated NK cells induce maturation of GM-CSF derived iDC (26–28) or activate resting monocytes (29–31). Zhang et al. found that IL-15 activated CD3−CD56bright NK cells triggered CD14+ monocytes to differentiate into TH1 promoting DCs. This process required direct contact of monocytes with NK cells and was mediated by GM-CSF and CD154 (29). Our findings suggest that release of 15 kDa granulysin represents yet another mechanism whereby NK cells can activate monocytes.
While 15 kDa granulysin activates human monocytes, it has little if any effect on mouse monocytes. In contrast, 15 kDa granulysin activates both human and murine iDCs (16). In humans, approximately 95% of blood monocytes are CD14++CD16− and 5% are CD14+CD16−. In mice, monocytes express CD115 (macrophage colony-stimulating factor receptor), CD11b, F4/80, and they can be further subdivided into Ly-6C+CCR2+CD11c− and Ly-6ClowCCR2−CD11c+ populations. Ingersoll et al. recently used microarray gene profiling to investigate the similarities between human and mouse monocyte subsets (32). They found that expression of many genes is similar in monocyte subsets of both species, but that there are a number of genes whose expression patterns are not conserved. We showed previously that toll-like receptor 4 is required for activation of murine and human iDC by granulysin (16). Since mouse monocytes express TLR4 (33) but are not activated by granulysin, we hypothesize that granulysin activates monocytes in a manner analogous to LPS (34, 35). LPS, in the presence of lipopolysaccharide-binding protein, binds to CD14, a glycosylphosphatidylinolsitol–anchored protein that is incapable of initiating transmembrane signaling. LPS is then transferred to MD2, which is associated with TLR4, and TLR4 transduces an activating signal. We hypothesize that 15 kDa granulysin binds to a receptor expressed by human but not mouse monocytes that subsequently interacts with TLR4 to activate monocytes.
DCs are potent antigen presenting cells that initiate primary T cell responses by presenting antigenic peptides in association with major histocompatibility complex molecules. Because DCs are rare in peripheral blood, clinical protocols have been devised for the in vitro expansion of these potent antigen presenting cells from bone marrow or peripheral blood monocytes. The most widely used protocols involve culturing peripheral blood monocytes with GM-CSF and other agents, such as IL-1, IFNγ, or LPS, to mature DCs to augment immune responses in vivo in humans (24, 36, 37). The majority of more than 100 Investigational New Drug applications using this approach are aimed at tumor therapy (38), but similar principles apply for vaccines against infectious agents and perhaps for therapies to alleviate autoimmunity. Despite the popularity of this approach, results have been mixed, with less than 10% overall clinical success rate. DCs generated from monocytes in vitro using 15 kDa granulysin differ in some ways from DCs generated with GM-CSF (23). In general, 15 kDa granulysin induced pathways related to costimulation of T cell activation and Th1 development. In contrast, GM-CSF down-regulated cytokine production, lymphocyte mediated immunity and humoral immune responses at late time points.
Granulysin is a member of the alarmin family, a group of structurally diverse endogenous mediators of innate immunity (39). Alarmins share several features: they are rapidly released in response to infection of tissue damage; they have both chemotactic and activating effects on the immune system; and they exhibit potent in vivo immunoactivating activity. Granulysin is expressed by both NK cells and CD8+ T cells, positioning it as a link between innate and adaptive immunity. It is important in this regard to clearly separate the expression and release of granulysin isoforms. 15 kDa granulysin is released by activated cytoxic cells while 9 kDa granulysin is packaged in cytolytic granules and requires receptor-mediated granule exocytosis for release. Thus, release of the 9 kDa cytolytic isoform is tightly regulated while release of the 15 kDa proinflammatory isoform is constitutive by activated NK cells.
Supplementary Material
Acknowledgments
This work was supported by the Intramural Research Program of the National Institutes of Health (NIH), National Cancer Institute, and Center for Cancer Research.
Footnotes
Abbreviations: DC, dendritic cell; iDC, immature dendritic cell; mDC, mature dendritic cell; TIL, tumor infiltrating lymphocytes; WT, wild type
Disclosures: AMK and CC hold patents on granulysin. The remaining authors have no competing interests.
References
- 1.Orange JS. Formation and function of the lytic NK-cell immunological synapse. Nat Rev Immunol. 2008;8:713–725. doi: 10.1038/nri2381. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Siegel RM, Chan FK, Chun HJ, Lenardo MJ. The multifaceted role of Fas signaling in immune cell homeostasis and autoimmunity. Nat Immunol. 2000;1:469–474. doi: 10.1038/82712. [DOI] [PubMed] [Google Scholar]
- 3.de Saint Basile G, Menasche G, Fischer A. Molecular mechanisms of biogenesis and exocytosis of cytotoxic granules. Nat Rev Immunol. 2010;10:568–579. doi: 10.1038/nri2803. [DOI] [PubMed] [Google Scholar]
- 4.Lodoen MB, Lanier LL. Natural killer cells as an initial defense against pathogens. Curr Opin Immunol. 2006;18:391–398. doi: 10.1016/j.coi.2006.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Pena SV, Hanson DA, Carr BA, Goralski TJ, Krensky AM. Processing, subcellular localization, and function of 519 (granulysin), a human late T cell activation molecule with homology to small, lytic, granule proteins. J Immunol. 1997;158:2680–2688. [PubMed] [Google Scholar]
- 6.Hanson DA, Kaspar AA, Poulain FR, Krensky AM. Biosynthesis of granulysin, a novel cytolytic molecule. Mol Immunol. 1999;36:413–422. doi: 10.1016/s0161-5890(99)00063-2. [DOI] [PubMed] [Google Scholar]
- 7.Kaspar AA, Okada S, Kumar J, Poulain FR, Drouvalakis KA, Kelekar A, Hanson DA, Kluck RM, Hitoshi Y, Johnson DE, Froelich CJ, Thompson CB, Newmeyer DD, Anel A, Clayberger C, Krensky AM. A distinct pathway of cell-mediated apoptosis initiated by granulysin. J Immunol. 2001;167:350–356. doi: 10.4049/jimmunol.167.1.350. [DOI] [PubMed] [Google Scholar]
- 8.Stenger S, Hanson DA, Teitelbaum R, Dewan P, Niazi KR, Froelich CJ, Ganz T, Thoma-Uszynski S, Melian A, Bogdan C, Porcelli SA, Bloom BR, Krensky AM, Modlin RL. An antimicrobial activity of cytolytic T cells mediated by granulysin. Science. 1998;282:121–125. doi: 10.1126/science.282.5386.121. [DOI] [PubMed] [Google Scholar]
- 9.Costa G, Loizon S, Guenot M, Mocan I, Halary F, de Saint-Basile G, Pitard V, Dechanet-Merville J, Moreau JF, Troye-Blomberg M, Mercereau-Puijalon O, Behr C. Control of Plasmodium falciparum erythrocytic cycle: gamma-delta T cells target the red blood cell-invasive merozoites. Blood. 2011;118:6952–6962. doi: 10.1182/blood-2011-08-376111. [DOI] [PubMed] [Google Scholar]
- 10.Kishi A, Takamori Y, Ogawa K, Takano S, Tomita S, Tanigawa M, Niman M, Kishida T, Fujita S. Differential expression of granulysin and perforin by NK cells in cancer patients and correlation of impaired granulysin expression with progression of cancer. Cancer Immunol Immunother. 2002;50:604–614. doi: 10.1007/s002620100228. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Nagasawa M, Ogawa K, Imashuku S, Mizutani S. Serum granulysin is elevated in patients with hemophagocytic lymphohistiocytosis. Int J Hematol. 2007;86:470–473. doi: 10.1007/BF02984011. [DOI] [PubMed] [Google Scholar]
- 12.Pages F, Berger A, Camus M, Sanchez-Cabo F, Costes A, Molidor R, Mlecnik B, Kirilovsky A, Nilsson M, Damotte D, Meatchi T, Bruneval P, Cugnenc PH, Trajanoski Z, Fridman WH, Galon J. Effector memory T cells, early metastasis, and survival in colorectal cancer. N Engl J Med. 2005;353:2654–2666. doi: 10.1056/NEJMoa051424. [DOI] [PubMed] [Google Scholar]
- 13.Saigusa S, Ichikura T, Tsujimoto H, Sugasawa H, Majima T, Kawarabayashi N, Chochi K, Ono S, Kinoshita M, Seki S, Ogawa K, Mochizuki H. Serum granulysin level as a novel prognostic marker in patients with gastric carcinoma. J Gastroenterol Hepatol. 2007;22:1322–1327. doi: 10.1111/j.1440-1746.2006.04796.x. [DOI] [PubMed] [Google Scholar]
- 14.Chung WH, Hung SI, Yang JY, Su SC, Huang SP, Wei CY, Chin SW, Chiou CC, Chu SC, Ho HC, Yang CH, Lu CF, Wu JY, Liao YD, Chen YT. Granulysin is a key mediator for disseminated keratinocyte death in Stevens-Johnson syndrome and toxic epidermal necrolysis. Nat Med. 2008;14:1343–1350. doi: 10.1038/nm.1884. [DOI] [PubMed] [Google Scholar]
- 15.Abe R, Yoshioka N, Murata J, Fujita Y, Shimizu H. Granulysin as a marker for early diagnosis of the Stevens-Johnson syndrome. Ann Intern Med. 2009;151:514–515. doi: 10.7326/0003-4819-151-7-200910060-00016. [DOI] [PubMed] [Google Scholar]
- 16.Tewary P, Yang D, de la Rosa G, Li Y, Finn MW, Krensky AM, Clayberger C, Oppenheim JJ. Granulysin activates antigen-presenting cells through TLR4 and acts as an immune alarmin. Blood. 2010;116:3465–3474. doi: 10.1182/blood-2010-03-273953. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Huang LP, Lyu SC, Clayberger C, Krensky AM. Granulysin-mediated tumor rejection in transgenic mice. J Immunol. 2007;178:77–84. doi: 10.4049/jimmunol.178.1.77. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Saini RV, Wilson C, Finn MW, Wang T, Krensky AM, Clayberger C. Granulysin delivered by cytotoxic cells damages endoplasmic reticulum and activates caspase-7 in target cells. J Immunol. 2011;186:3497–3504. doi: 10.4049/jimmunol.1003409. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Finn MW, Clayberger C, Krensky AM. Expression and purification of 15 kDa granulysin utilizing an insect cell secretion system. Protein Expr Purif. 2011;75:70–74. doi: 10.1016/j.pep.2010.07.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hamann D, Baars PA, Rep MH, Hooibrink B, Kerkhof-Garde SR, Klein MR, van Lier RA. Phenotypic and functional separation of memory and effector human CD8+ T cells. J Exp Med. 1997;186:1407–1418. doi: 10.1084/jem.186.9.1407. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Ogawa K, Takamori Y, Suzuki K, Nagasawa M, Takano S, Kasahara Y, Nakamura Y, Kondo S, Sugamura K, Nakamura M, Nagata K. Granulysin in human serum as a marker of cell-mediated immunity. Eur J Immunol. 2003;33:1925–1933. doi: 10.1002/eji.200323977. [DOI] [PubMed] [Google Scholar]
- 22.Catalfamo M, Karpova T, McNally J, Costes SV, Lockett SJ, Bos E, Peters PJ, Henkart PA. Human CD8+ T cells store RANTES in a unique secretory compartment and release it rapidly after TcR stimulation. Immunity. 2004;20:219–230. doi: 10.1016/s1074-7613(04)00027-5. [DOI] [PubMed] [Google Scholar]
- 23.Castiello L, Stroncek DF, Finn MW, Wang E, Marincola FM, Clayberger C, Krensky AM, Sabatino M. 15 kDa Granulysin versus GM-CSF for monocytes differentiation: analogies and differences at the transcriptome level. J Transl Med. 2011;9:41. doi: 10.1186/1479-5876-9-41. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wu YG, Wu GZ, Wang L, Zhang YY, Li Z, Li DC. Tumor cell lysate-pulsed dendritic cells induce a T cell response against colon cancer in vitro and in vivo. Med Oncol. 2009 doi: 10.1007/s12032-009-9277-x. [DOI] [PubMed] [Google Scholar]
- 25.Jongstra J, Schall TJ, Dyer BJ, Clayberger C, Jorgensen J, Davis MM, Krensky AM. The isolation and sequence of a novel gene from a human functional T cell line. J Exp Med. 1987;165:601–614. doi: 10.1084/jem.165.3.601. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Ferlazzo G, Tsang ML, Moretta L, Melioli G, Steinman RM, Munz C. Human dendritic cells activate resting natural killer (NK) cells and are recognized via the NKp30 receptor by activated NK cells. J Exp Med. 2002;195:343–351. doi: 10.1084/jem.20011149. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Gerosa F, Baldani-Guerra B, Nisii C, Marchesini V, Carra G, Trinchieri G. Reciprocal activating interaction between natural killer cells and dendritic cells. J Exp Med. 2002;195:327–333. doi: 10.1084/jem.20010938. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Piccioli D, Sbrana S, Melandri E, Valiante NM. Contact-dependent stimulation and inhibition of dendritic cells by natural killer cells. J Exp Med. 2002;195:335–341. doi: 10.1084/jem.20010934. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Zhang AL, Colmenero P, Purath U, Teixeira de Matos C, Hueber W, Klareskog L, Tarner IH, Engleman EG, Soderstrom K. Natural killer cells trigger differentiation of monocytes into dendritic cells. Blood. 2007;110:2484–2493. doi: 10.1182/blood-2007-02-076364. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Welte S, Kuttruff S, Waldhauer I, Steinle A. Mutual activation of natural killer cells and monocytes mediated by NKp80-AICL interaction. Nat Immunol. 2006;7:1334–1342. doi: 10.1038/ni1402. [DOI] [PubMed] [Google Scholar]
- 31.Dalbeth N, Gundle R, Davies RJ, Lee YC, McMichael AJ, Callan MF. CD56bright NK cells are enriched at inflammatory sites and can engage with monocytes in a reciprocal program of activation. J Immunol. 2004;173:6418–6426. doi: 10.4049/jimmunol.173.10.6418. [DOI] [PubMed] [Google Scholar]
- 32.Ingersoll MA, Spanbroek R, Lottaz C, Gautier EL, Frankenberger M, Hoffmann R, Lang R, Haniffa M, Collin M, Tacke F, Habenicht AJ, Ziegler-Heitbrock L, Randolph GJ. Comparison of gene expression profiles between human and mouse monocyte subsets. Blood. 2010;115:e10–19. doi: 10.1182/blood-2009-07-235028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Poltorak A, He X, Smirnova I, Liu MY, Van Huffel C, Du X, Birdwell D, Alejos E, Silva M, Galanos C, Freudenberg M, Ricciardi-Castagnoli P, Layton B, Beutler B. Defective LPS signaling in C3H/HeJ and C57BL/10ScCr mice: mutations in Tlr4 gene. Science. 1998;282:2085–2088. doi: 10.1126/science.282.5396.2085. [DOI] [PubMed] [Google Scholar]
- 34.Tobias PS, Soldau K, Gegner JA, Mintz D, Ulevitch RJ. Lipopolysaccharide binding protein-mediated complexation of lipopolysaccharide with soluble CD14. J Biol Chem. 1995;270:10482–10488. doi: 10.1074/jbc.270.18.10482. [DOI] [PubMed] [Google Scholar]
- 35.Shimazu R, Akashi S, Ogata H, Nagai Y, Fukudome K, Miyake K, Kimoto M. MD-2, a molecule that confers lipopolysaccharide responsiveness on Toll-like receptor 4. J Exp Med. 1999;189:1777–1782. doi: 10.1084/jem.189.11.1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Chen X, Chang CH, Goldenberg DM. Novel strategies for improved cancer vaccines. Expert Rev Vaccines. 2009;8:567–576. doi: 10.1586/erv.09.11. [DOI] [PubMed] [Google Scholar]
- 37.Han TH, Jin P, Ren J, Slezak S, Marincola FM, Stroncek DF. Evaluation of 3 clinical dendritic cell maturation protocols containing lipopolysaccharide and interferon-gamma. J Immunother. 2009;32:399–407. doi: 10.1097/CJI.0b013e31819e1773. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Melief CJ. Cancer immunotherapy by dendritic cells. Immunity. 2008;29:372–383. doi: 10.1016/j.immuni.2008.08.004. [DOI] [PubMed] [Google Scholar]
- 39.Oppenheim JJ, Yang D. Alarmins: chemotactic activators of immune responses. Curr Opin Immunol. 2005;17:359–365. doi: 10.1016/j.coi.2005.06.002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.