Abstract
Aims
To compare prevalence of self-reported comorbid temporomandibular joint muscle disorder (TMJMD)-type, neck, back and joint pains in people with severe headache or migraine; analyze these self-reported pains in the 2000–2005 US National Health Interview Survey (NHIS) by gender and age for Non-Hispanic Whites, Hispanics and Non-Hispanic Blacks (African Americans).
Methods
NHIS data included information on gender, age, race, ethnicity, health status, and common pain types: severe headache or migraine, TMJMD-type, neck, and low back in the last 3 months, as well as prior month joint pains. Analyses included survey prevalence estimation and survey logistic regression to obtain odds ratios and 95% confidence intervals.
Results
189,967 adults, 48% males, 52% females; 73% White, 12% Hispanic, and 11% Black were included. 29,712 (15%) of the entire sample reported severe headache or migraine, 19,228 (64%) had severe headache or migraine with at least one comorbid pain. 10,200 (33%) reported 2 or more comorbid pains, with no gender difference, and with Hispanics (n=1,847 or 32%) and Blacks (n=1,301 or 30%) less likely to report 2 or more comorbid pains than Whites (n=6,747 or 34%) (OR=0.91, p=0.032; OR=0.82, p<0.001, respectively). This group also reported significantly lower ratings of self-rated health (p<0.001). Differences in type of comorbid pain by age patterns were found.
Conclusions
Severe headache or migraine is often associated with other common pains, seldom existing alone. Two or more comorbid pains are common, similarly affecting gender and racial/ethnic groups.
Keywords: comorbid chronic pain, migraine/headaches, neck, back, temporomandibular joint disorders, prevalence, race/ethnicity, gender, age, self-report, sample survey
Introduction
Headaches have been reported to often coexist with other pain disorders (1–5). The transition from recurrent to more debilitating persistent or chronic daily headache (CDH) is in part associated with an increased number of other coexisting body pains (6, 7). Therefore, investigating comorbid body pains in severe headaches along the adult lifespan warrants further attention.
Our earlier research examined the overall prevalence of temporomandibular joint and muscle disorder (TMJMD)-type pain and other common pains, including severe headache or migraine based on gender and race/ethnicity across the adult lifespan, utilizing a very large sample pooled from 6 years of US National Health Interview Survey (NHIS) data (8). We found an overall 3-month period prevalence of severe headache or migraine around 15% – much lower than the previously reported overall prevalence of specific types of headache such as tension type headaches (TTHs) (approximately 38%/year), but higher than migraine (approximately 10–12%/year) (1). The lower overall prevalence from NHIS data represents more severe headache cases as reflected by the NHIS questionnaire (8). Furthermore, the NHIS provides a nationally representative sample of community dwelling US adults (i.e. not care seeking patients), providing a large sample and more unbiased estimates than other studies of more severe headaches compared to previous reports. Since most headache types' severity seems to be strongly associated with coexisting other body pains, it would be compelling to analyze the unique nationally representative NHIS data to investigate the level (i.e. number) and types of coexisting pains among those with severe headache or migraine based on gender and race/ethnicity varying by age.
The aim of this report, therefore, is to investigate the associated comorbid neck, low back, TMJMD-type, and joint pains among self-reported severe headache or migraine sufferers to analyze: 1) the number of comorbid pains; 2) the relationship between severe headache or migraine accompanied by comorbid pains and general health; and 3) specific comorbid pains among those with severe headache or migraine modeled separately by gender and race/ethnicity across the adult lifespan. Our analyses of NHIS data on severe headaches or migraines offer important perspectives of chronic body pain and associated general health along life span.
Materials and Methods
We used a pooled sample of NHIS data, including years 2000–05. In accordance with the UCSF IRB decision tree-process, it was determined that these analyses of NHIS public use data did not consist of clinical research, thus a self-certification process was completed. The NHIS is an ongoing nationwide household survey designed to obtain information on the demographic characteristics, health status, and health care use patterns of the US civilian non-institutionalized population (9). The survey has 3 modules: a basic module; a periodic module; and a topical module. The basic module contains 3 components: the family core, the sample adult core, and the sample child core. The variables utilized for the present analyses were taken from the sample adult core. Data pooled over six years (n=189,967) provide an adequate sample size for detailed analyses by gender and age across the adult lifespan in which we examine racial/ethnic prevalence and differences. The response rates for the sample adult questionnaire ranged from 69% in 2005 to 74% in 2002–2004.
Variable Construction
The sample adult core includes information on sociodemographic characteristics, health conditions and limitations and health care utilization (9).
Common Pain Variables
Adult NHIS participants were asked a series of questions assessing experiences of pains in the past three months. Participants were asked to “report pain that lasted a whole day or more and not to report fleeting or minor aches or pains”. The stem of the question was: “During the past three months, did you have…”: “severe headache or migraine”; “neck pain”; “low back pain”; “facial ache or pain in the jaw muscles or the joint in front of the ear” similar to other studies(10, 11). Persons responding “yes” or “no” to these questions were included in these analyses, excluding those with missing, “don't know” or “refused” responses (less than 1%). The patterns of gender and racial/ethnic characteristics of those excluded from these analyses were similar to the sample as a whole. Thus, these questions assessed 3-month period prevalence of self-reported severe headache or migraine, neck, low back and TMJMD-type pains. Joint pain was assessed (9) with a question: “During the past 30 days have you had any symptoms of pain, aching, or stiffness in or around a joint?” (i.e. 1-month period prevalence). We included joint pain in the pain assessment for 2002–2005, when it was included in the NHIS sample adult questionnaire. Therefore the outcomes of our analyses refer to non-minor pain within the prior 3 months or joint pain within the prior month (9).
While we recognize that respondents reported information about both their racial and ethnic background, we followed the common convention describing the three major racial/ethnic categories available from the National Center for Health Statistics (NCHS) surveillance surveys: Non-Hispanic White (referred to hereafter as White), Non-Hispanic Black (referred to hereafter as Black), and Hispanic adults. We also used the NCHS gender and age variables (9).
We created a summed variable of the number of the 3 comorbid pains: neck pain, low back pain and TMJMD-type pain (range = 0 – 3). We then created a 4 level variable categorizing severe headache or migraine with number of comorbid pains: 1 = severe headache or migraine only; 2 = severe headache or migraine and 1 comorbid pain; 3 = severe headache or migraine and 2 comorbid pains; and 4 = severe headache or migraine and 3 comorbid pains. This variable was used to examine the level of comorbid pain with severe headache or migraine by gender and race/ethnicity. We created variables that assessed the comorbid status of severe headache or migraine with each of the following: neck pain, low back pain, TMJMD-type pain, and joint pain. The variable assessing joint pain was available in the data sets for years 2002–2005 only, thus it was not used in every analysis. Respondents were asked to self-report their general health on a 5-point scale, ranging from excellent to poor and this variable was used to assess the relationship between comorbid pains and health.
Data Analysis
Estimates presented here (prevalence) used sampling weights to reflect national population totals. The weights, provided by NCHS, estimated the inverse of the sampling probability for each respondent, adjusted for nonresponse. Following NCHS technical report (9) recommendations, we divided the weights of 6 years of data by 6, so the results will still pertain to the national population total. For analyses of 4 years of the joint pain estimates, we divided the weights by 4, to reflect population totals. Estimates and test statistics were derived using SAS (version 9.1.3, SAS Institute, Cary, NC) and SUDAAN (version 9, RTI, Research Triangle Park, NC) (12) software that takes into account the complex sample design of the survey, including household and intrafamilial clustering of sample observations. The results are presented in tabular and graphical form.
First, 3-month period prevalence estimates of severe headache or migraine and the 3 comorbid pains for the total sample were presented, for the severe headache or migraine subsample, and within the severe headache or migraine subsample, by gender and race/ethnicity. To distinguish between a lesser and a more severe degree of comorbidity, we dichotomized the comorbidity variable into 0–1 versus 2–3 comorbid pains. The level of significance was 0.05.
Second, the prevalences were presented for the specific comorbid pains – neck pain, low back pain, TMJMD-type pain, and joint pain – for the total sample and by severe headache or migraine status. Logistic regression analyses were conducted to determine odds ratios (ORs) and 95% confidence intervals (CIs) between headache or migraine and each of the 4 pains to determine the significance of differences between those with severe headache or migraine pain and those without; adjusted ORs (aORs) were estimated accounting for age and gender.
Third, separate analyses for males and females were conducted to examine patterns of 2 or more comorbidities with severe headache or migraine pain by age and race/ethnicity to determine the degree of comorbidity with severe headache or migraine pain across the adult lifespan by race/ethnicity. The authors' recent report (8) estimated prevalences for these pains (i.e. TMJMD-type, severe headache or migraine, neck, and low back pains) following the Box-Tidwell regression model approach to test for non-linear age effects and showing linear and quadratic (age2) effects were needed to estimate the race/ethnicity-specific age curves. (Cubic age effects were not statistically significant.) Thus, analyses in this report also used linear and quadratic age effects. Self-reported health between those with 0–1 comorbid pains versus those with 2–3 pains was compared to assess the relationship of more severe comorbid pains with overall health.
Fourth, the racial/ethnic and age patterns of severe headache or migraine pain and each of the specific comorbidities were examined for males and females separately. The interaction between race/ethnicity and age category (<=40, 41–59 and >=60) assessed whether the age differences were consistent among racial/ethnic groups; if the interaction was statistically significant then separate comparisons were made among racial/ethnic groups for each age <=40 and age >=60.
Results
The analyses included 189,967 adults, ages 18 years and older, pooled over six years. Forty-eight percent were males, 52% females; 73% White, 12% Hispanic, 11% Black, and 4% Other. The “Other” group was included in the analyses of overall prevalences, however analyses comparing racial/ethnic groups included only the 3 major racial/ethnic groups because the Other group was heterogeneous and the sample size was small.
Fifteen percent (15%) of the entire sample reported severe headache or migraine pain in the prior 3 months and of those, 36% had severe headache or migraine without comorbid TMJMD-type, neck, or low back pain (Table 1). Thus, most people (64%) with severe headache or migraine pain also reported one or more comorbid pains. One-third (33%) of those with severe headache or migraine reported 2 or more comorbid pains, which did not differ significantly by gender. Hispanic (32%) and Black (30%) adults were significantly less likely to report 2 or more comorbid pains than White (34%) adults (OR= 0.91, p=0.032; OR= 0.82, p<0.001, respectively). Respondents with 2 or more comorbid pains reported significantly lower ratings of self-rated health (p<0.001).
Table 1.
Prevalence (percent) of Severe Headache or Migraine and Comorbid Pains; Odds Ratios (95% Confidence Intervals) Comparing Headache or Migraine Pain with 2–3 Comorbid Conditions by Sex and Race/Ethnicity
| Severe headache or migraine with number of comorbid pains % | |||||||
|---|---|---|---|---|---|---|---|
| 0 | 1 | 0 or 1 | 2 | 3 | 2 or 3 | Odds Ratio (95% CI) | |
| Total Sample (n=189,967) | 5.5 | 4.7 | 10.2 | 3.8 | 1.3 | 5.1 | |
| Severe headache/migraine pain sample (n=29,712) | 36.1 | 30.7 | 66.8 | 24.7 | 8.6 | 33.3 | |
| Gender | |||||||
| Female | 36.4 | 30.2 | 66.6 | 24.3 | 9.1 | 33.4 | 1.02 (0.96–1.1) |
| Malea | 35.4 | 31.7 | 67.2 | 25.6 | 7.2 | 32.8 | |
| Race/ethnicity | |||||||
| Whitea | 34.9 | 31.0 | 65.9 | 25.3 | 8.8 | 34.1 | |
| Hispanic | 38.4 | 29.5 | 67.9 | 24.0 | 8.1 | 32.1 | 0.91 (0.84–0.99)* |
| Black | 38.9 | 31.3 | 70.1 | 22.4 | 7.5 | 29.9 | 0.82 (0.76–0.90)** |
| Other | 43.1 | 27.2 | 70.4 | 21.1 | 8.5 | 29.6 | |
referent group
p=0.032;
p<0.001
Table 2 presents the 3-month period prevalences of severe headache or migraine pain with specific comorbidities. Among those with severe headache or migraine pain, 16% had TMJMD-type pain in the prior 3 months (quarter), compared to 3% of those without severe headache or migraine (OR=7.0, p< 0.001), 38% had prior quarter neck pain, compared to 11% of those without severe headache or migraine (OR=5.0, p<0.001), 53% had prior quarter low back pain compared to 24% of those without severe headache or migraine (OR=3.6, p<0.001) and 47% of those with prior quarter severe headache or migraine had prior month joint pain, compared to 28% of those without severe headache or migraine (OR=2.3, p<0.001). Each of these differences remained significant in adjusted multivariable analyses when models controlled for gender and age.
Table 2.
Prevalence of TMJMD-type Pain, Low Back Pain, and Joint Pain for Total Sample and by Severe Headache or Migraine Pain Status: Prevalences, Odds Ratios (ORs), Adjusted ORs (aORs), 95% Confidence Intervals (CIs)
| TMJMD-type pain3 | Neck pain3 | Low back pain3 | Joint pain1 | |||||
|---|---|---|---|---|---|---|---|---|
| % | OR/aOR (CI) | % | OR/aOR (CI) | % | OR/aOR (CI) | % | OR/aOR (CI) | |
| Total sample | 4.6 | 14.9 | 28.0 | 30.9 | ||||
| Severe headache or migraine: | ||||||||
| yes | 15.6 | 37.6 | 52.6 | 46.9 | ||||
| noa | 2.6 | 10.8 | 23.5 | 28.0 | ||||
| OR (CI) | 7.0 (6.6–7.5)* | 5.0 (4.8–52.)* | 3.6 (3.5–3.7)* | 2.3 (2.2–2.4)* | ||||
| aOR (CI) | 6.5b (6.1–6.9)* | 5.4b (5.2–5.6)* | 3.9b (3.8–4.1)* | 3.1b (2.9–3.2)* | ||||
referent group;
p≤.001;
aOR adjusted for age and gender
3-month period prevalence
1-month period prevalence
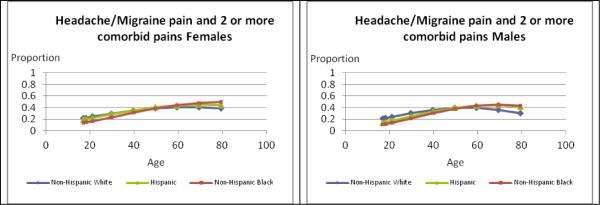
Figure 1 illustrates differences by age and race/ethnicity separately for females and males among those with severe headache or migraine with 2–3 comorbid pains. The race/ethnicity × age group interaction was statistically significant for women and for men (p<0.050). Prevalences tended to increase with age with some variation by race/ethnicity. Comparing prevalences to White females (30.0%), Black females (22.3%) had significantly lower prevalences up to age 40 (p<0.001), and slightly higher ones from age 60 on, but these differences were not significant. The pattern for males differed somewhat, with lower prevalences for Black (19.1%, p<0.001) and Hispanic (23.0%, p=0.001) males than Whites (30.2%) at younger ages, and significantly higher ones for Hispanic (45.0%) versus White (33.0%) males from age 60 on (p=0.017).
Figure 1.
Estimates of Prevalences for Severe Headache or Migraine Based on Number of Comorbid Pains for Females and Males
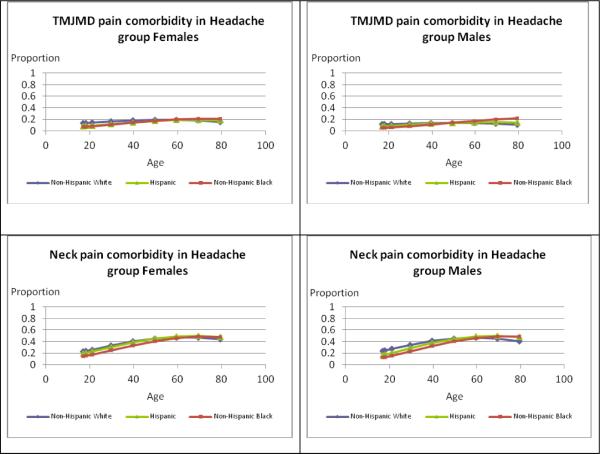
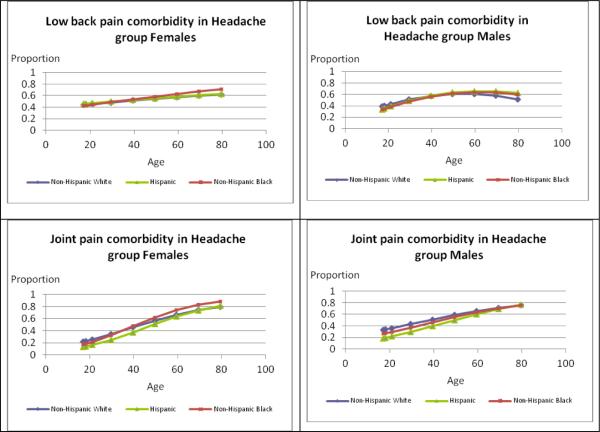
The graphs in Figure 2 illustrate differences by age and race/ethnicity based on severe headache or migraine with specific pain comorbidities for females and males. In each case, the race/ethnicity × age group interaction was statistically significant (p<0.050) except for joint pain comborbidity in men with severe headache or migraine (p=0.088). For females, regardless of race/ethnicity, the pattern of prior quarter TMJMD-type comorbid pain remained fairly constant across the age groups, ranging roughly from 5–17%. There was some variation by race/ethnicity. Comparing rates to Black (10.6%) and Hispanic (11.8%) females, White females (17.0%) had significantly higher rates up to age 40 (p<0.001) and differences at older ages were not significant. This pattern was the same for males with higher prevalences for White (13.0%) versus Black (7.2%, p<0.001) males at younger ages, but no significant differences were seen at older ages. Comorbid neck pain with severe headache or migraine for females increased from younger ages and plateaued around age 60. The pattern of racial/ethnic differences was the same for females and males. Black (23.5% female; 20.8% male) and Hispanic (30.2% female; 26.3% male) adults had significantly lower prevalences than Whites (33.3% females; 34.5% males) up to age 40 (Hispanic females p=0.014, all others p<0.001) with no significant differences at older ages. Approximately 40% of females reported comorbid low back pain at younger ages and the prevalences increased across the age groups. There were no significant racial/ethnic differences for females at younger or older ages. Among males, prevalences plateaued around age 60, with no significant racial/ethnic differences up to age 40, but from age 60 on Hispanic males (66.6%) had significantly higher prevalences than White males (55.3%, p=0.029). For females, comorbid joint pain with severe headache or migraine showed a steep increase across the age groups. Hispanic females (23.3%) had significantly lower prevalences at younger ages compared to White females (33.8%, p<0.001) and there were no significant differences after age 60. For males, comorbid joint pain also increased across the age span but did not differ significantly by race/ethnicity (interaction p=0.088).
Figure 2.
Estimates of Prevalences for Types of Comorbid Pain for Females and Males
Discussion
Our study offers new information on the prevalence of self-reported severe headache or migraine with other comorbid body pains modeled by age based on gender, and race/ethnicity. Two unique findings relate to the significant differences in the prevalence pattern of severe headache or migraine with comorbid pains compared to the overall prevalence (i.e. regardless of comorbidity) (Tables 1, 2), and the differences in prevalence patterns across lifespan based on number and type of comorbid pain by gender and race/ethnicity (Figs. 1, 2). The results also confirm previous reports on overall prevalence patterns of headaches based mostly on gender.
The lower overall 3-month prevalence for severe headache or migraine of 15% previously reported by our group (8) from NHIS data could be explained by: including only severe forms of headache or migraine regardless of clinical diagnoses – and differences in time prevalence (i.e. 3 month instead of 12 month or lifetime prevalence) compared to most previous studies (1). Therefore, as reflected by the NHIS questionnaire, the NHIS data represent more severe headache cases, (mostly TTH and migraine) since the other types of headaches, not excluded, are of much lower prevalence (1).
The high percent (64%) of people with severe headache or migraine who had one or more common comorbid pains may actually be even higher, since other common chronic pains, such as fibromyalgia and abdominal pain (4,13), were not included in the NHIS questionnaire. Therefore, we can conclude that regardless of clinical diagnosis, most severe headaches or migraines seldom exist alone, being often associated with other body pains. Furthermore, our results demonstrated that severe headache or migraine with 2 or more comorbid pains differed greatly from the previously reported overall prevalence pattern of severe headache or migraine (i.e. regardless of comorbidity) based on gender, race and age. Both genders are equally affected by severe headache or migraine with 2 or more comorbid pains with Whites more affected than minorities (Table 1) compared to previously reported overall gender difference and overall lack of racial/ethnic differences (1, 8, 13). Furthermore, increased number of comorbid pains was significantly associated with lower level of perceived general health. Therefore, the coexistence of other pains changes the profile of severe headache or migraine and their impact on general health. Importantly, both genders and all 3 racial/ethnic groups seem to be similarly affected by the number of comorbid pains and their impact on general health across lifespan. Regardless of gender or race/ethnicity, severe headache or migraine prevalence for those with 2–3 comorbid pains increased with age by more than double (Fig. 1), compared to the overall headache prevalence reported to decrease with age (1, 8). Differences in comorbidity of severe headache or migraine with specific types of comorbid pain were also noted. The highest comorbidity was with low back pain, followed by joint pain (Table 2). This highlights to clinicians the importance of a comprehensive evaluation when patients seek help for severe headache or migraine pain. High comorbidity with musculoskeletal pains including neck and back pains were reported previously (2,3). Neck pain was highly associated with migraine attacks, being more frequent than other specific migraine symptoms, such as nausea, and also more frequent in more severe cases (14). Central sensitization was considered to play a role in this referred pain (14). The comorbidity with TMJMD-type pain is also not surprising. The 2 conditions share a common trigeminal projection as well as common anatomical sites (i.e. temporal region), thus generating possible methodological problems related to classification, which has been raised recently (15, 16). Classification implications must be addressed more carefully by both fields (i.e. headaches (17) and TMDs (15)). Among our unique findings were also those related to gender and race/ethnicity differences between the overall prevalence of each pain (i.e. TMJMDs, joint pain, etc., regardless of comorbidity) (8) and the prevalence of the same pain when comorbid with severe headaches across the lifespan (Figure 2). For example, gender- and race/ethnicity-specific prevalence patterns of TMJMD-type pain with severe headache or migraine across the lifespan differed greatly from the overall pattern of each condition demonstrating much less change (i.e. from around 10% at the young age to around 15% at 80) regardless of gender and race/ethnicity (Figure 2). Both severe headache or migraine and TMJMD-type pain, as previously reported (8), vary greatly with age increasing to about 40 years of age but decreasing thereafter. The coexistence of joint pain in those with severe headache or migraine, on the other hand, demonstrated a very different profile from those with TMJMD-type pain (from around 10% at young age to 80% at old age) (Figure 2). These findings reflect not only on the differences in natural history along lifespan of each specific comorbid pain, but also shed new light on the unique relationship of each with severe headache or migraine and the impact they have on each other across lifespan.
The cross-sectional NHIS data limit the conclusions regarding natural history and specific mechanisms responsible for the coexistence of these pains with severe headache or migraine. The most accepted hypotheses regarding the mechanisms involved in the coexistence chronic pains are those related to the plastic changes within the peripheral and central neural networks mediating nociception. These changes, called sensitization, are considered responsible for duration, level and location of pain (18). Sensitization at the level of the trigeminal complex (19) may be involved in chronic headaches, including migraines (20, 21), TMJMD-type pain (21–24), neck pain (21, 25, 26), and their coexistence. Allodynia, a clinical correlate of sensitization (20), often present in these comorbid pains (6, 7, 22), has been associated with longer headache duration, diminished response to preemptive migraine treatments (i.e. increased severity), and increased number of comorbid pains (4, 27–34). Our data support these findings by showing that the prevalence of severe headache or migraine coexisting with 2–3 other pains increases with age. (Figure. 1) Such findings raise provocative questions such as does analgesic overuse for joint, back and neck pain increase the chance to develop more severe forms of headaches such as medication overuse headaches (MOH). Developing MOH was described as related to preexisting headaches, especially migraines or TTH, perhaps from central sensitization (35–37). Targeted studies should address such important clinical issues.
Based on our longitudinal studies of young women (13) we reported that the onset of headaches usually preceded those of TMJMD, neck, back, and abdominal pains. Although, overall no racial difference in prevalence of severe headaches was found (13), at young ages (i.e.20–30 years old) a statistically significant racial difference emerged: young White women were more affected than young Black women. Young White women were also more affected by TMJMDs (twice as much) than young Black women (8, 13). These findings suggest that headaches may predispose people to other types of pain, explaining in part the racial differences in young women's chronic pains, such as TMJMDs. At young adult ages, Whites seem to be more affected by comorbid pains (i.e. TMJMD, head, and neck pains) than minorities, but pain seems to increase with age in minorities, especially Hispanics (i.e. back and joint pain, Figure 1). These age differences may also explain the apparent inconsistency in racial/ethnic differences reported previously for headaches as being higher in White adolescents (38) as well as adults (1, 39) compared to the lack of overall differences in severe headache or migraine nationally (8). Previous reports showed that even though Blacks overall report fewer headaches, those affected generally reported higher pain levels compared to Whites (38). Therefore, based on such findings the overall lack of racial/ethnic differences would be expected for the severe headaches or migraines.
The complex relationship between age and headaches, including migraine, requires more sophisticated analyses as previously acknowledged. To model the relationship between age and prevalence a mixture of two Normal (Gaussian) distributions was recently used (40). We also modeled the complex relationship between age and 3-month period prevalence, but used polynomial regression, finding significant nonlinearity in which a quadratic component explained the relationship, while higher order components (e.g. cubic to test the lack of adequate fit of quadratic) were not statistically significant (8). Thus, based on 6 years of NHIS data our results showed the overall prevalence of severe headaches or migraines in females increased and peaked (once) around 30–40s and decreased thereafter (8). This prevalence pattern differs from the one mentioned above for “migraine” on 1 year NHIS data which reported 2 peaks (40). Methodological differences related to the number of subjects (1 year vs. 6 years) and types of analyses based on age could account for the differences in reported results. However, both that study (40) and ours (8) utilized the NHIS with the actual questionnaire wording pertaining to “severe headache or migraine”, not only “migraine” as previously reported (40).
This study had various limitations. Cross-sectional studies, compared to longitudinal studies, have inherent limitations especially while investigating age effects. The results could be due to a cohort effect, rather than a true age or growth effect. Furthermore, pain questionnaires do not differentiate among different severe headache types partially due to different clinical/scientific groups adding items to the NHIS at different times, as well as, space and time constraints resulting in the wording differing from existing ICH criteria. Additionally, we have considered TMJMD-type pain and neck pain as potential comorbid pains accompanying severe headache or migraine; however, it is possible that these pains could be part of the severe headache or migraine experience and thus not separate comorbid phenomena. Despite these issues, the limitations are far outweighed by the benefits of investigating common comorbidities in a large nationally representative sample and analyzing the relationships of age with gender and race/ethnicity differences. The results shed new light on the prevalence and relationship of different common pains in people with prior quarter severe headache or migraine along the lifespan. Future longitudinal studies targeting more specific questions regarding headaches with other pain progression should explore the complex mechanisms involved in chronic human pain.
In conclusion our results offer a new glimpse into the complexity of human pain supporting existing evidence regarding high comorbidity of the severe headaches with other common pains and providing new evidence regarding their age related differences based on gender and race/ethnicity. These findings have clinical implications for general health as well as research. Although at specific ages, racial/ethnic and gender differences may exist in the prevalence patterns, overall both genders, regardless of race/ethnicity, are almost equally affected by the coexistence of these pains.
Acknowledgements
Supported in part by US DHHS NIH / NIDCR R03DE018759
Footnotes
Conflict of Interest: The authors report no conflict of interest
References
- 1.Robbins MS, Lipton RB. The epidemiology of primary headache disorders. Semin Neurol. 2010;30:107–119. doi: 10.1055/s-0030-1249220. [DOI] [PubMed] [Google Scholar]
- 2.Scher AI, Bigal ME, Lipton RB. Comorbidity of migraine. Curr Opin Neurol. 2005;18:305–310. doi: 10.1097/01.wco.0000169750.52406.a2. [DOI] [PubMed] [Google Scholar]
- 3.Scher A, Stewart WF, Lipton RB. The comorbidity of headache with other pain syndromes. Headache. 2006;46:1416–1423. doi: 10.1111/j.1526-4610.2006.00584.x. [DOI] [PubMed] [Google Scholar]
- 4.De Tommaso M, Sandaro M, Serpino C, et al. Fibromyalgia comorbidity in primary headaches. Cephalalgia. 2009;29:453–464. doi: 10.1111/j.1468-2982.2008.01754.x. [DOI] [PubMed] [Google Scholar]
- 5.Crystal SC, Robbins MS. Epidemiology of tension-type headache. Curr Pain Headache Rep. 2010;14:449–454. doi: 10.1007/s11916-010-0146-2. [DOI] [PubMed] [Google Scholar]
- 6.Scher AI, Midgette LA, Lipton RB. Risk factors for headache chronification. Headache. 2008;48:16–25. doi: 10.1111/j.1526-4610.2007.00970.x. [DOI] [PubMed] [Google Scholar]
- 7.Goncalves DAG, Bigal ME, Jales LCF, Speciali JG. Headache and symptoms of temporomandibular disorders: An epidemiological study. Headache. 2010;50:231–241. doi: 10.1111/j.1526-4610.2009.01511.x. [DOI] [PubMed] [Google Scholar]
- 8.Plesh O, Adams S, Gansky SA. Racial/ethnic and gender prevalences in reported common pains in a national sample. J Orofacial Pain. 2011;25:10–16. [PMC free article] [PubMed] [Google Scholar]
- 9.National Center for Health Statistics . Data File Documentation, National Health Interview Survey, 2004 (machine readable data file and documentation) National Center for Health Statistics, Centers for Disease Control and Prevention; Hyattsville: 2005. [Accessed 11 March 2010]. National Center for Health Statistics. NHIS Survey Description. http://www.cdc.gov/nchs/data/nhis/srvydesc.pdf. [Google Scholar]
- 10.van Korff M, Dworkin F, LeResche L, Kruger A. An epidemiological comparison of pain complaints. Pain. 1988;32:173–183. doi: 10.1016/0304-3959(88)90066-8. [DOI] [PubMed] [Google Scholar]
- 11.Nilson IM, Drangsholt M. Reliability and validity of self-reported temporomandibular disorder pain in adolescents. J Orofacial Pain. 2006;20:138–144. [PubMed] [Google Scholar]
- 12.Research Triangle Institute . SUDAAN Manual Release 9.0. Research Triangle Institute; Research Triangle Park, NC: 2004. [Google Scholar]
- 13.Plesh O, Crawford PB, Gansky SA. Chronic pain in a biracial population of young women. Pain. 2002;99:515–523. doi: 10.1016/S0304-3959(02)00262-2. [DOI] [PubMed] [Google Scholar]
- 14.Calhoun AH, Sutapa F, Millen C, et al. The prevalence of neck pain in migraine. Headache. 2010;50:1273–1277. doi: 10.1111/j.1526-4610.2009.01608.x. [DOI] [PubMed] [Google Scholar]
- 15.Schiffman E, Truelove EL, Ohrbach R, Anderson GC, et al. The Research Diagnostic Criteria for Temporomandibular Disorders. I: Overview and methodology for assessment of validity. J Orofacial Pain. 2010;24:7–24. [PMC free article] [PubMed] [Google Scholar]
- 16.Anderson GC, John MK, Ohrbach R, Nixdorf D, Schiffman EL. Influence of headache frequency on clinical signs and symptoms of TMD in subjects with temple headache and TMD pain. Pain. 2011;152:765. doi: 10.1016/j.pain.2010.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Headache Classification Subcommitteee of the International Headache Society The International Classification of Headache Disorders: 2nd edition. Cephalalgia. 2004;24(Supl 1):1–60. doi: 10.1111/j.1468-2982.2003.00824.x. [DOI] [PubMed] [Google Scholar]
- 18.Kuner R. Central mechanisms of pathological pain. Nature Med. 2010;16:1258–1266. doi: 10.1038/nm.2231. [DOI] [PubMed] [Google Scholar]
- 19.Sessle BJ. Trigeminal central sensitization. Rev Analg. 2005;8:85–102. [Google Scholar]
- 20.Edelmayer RM, Vanderah TW, Majuta L, Zang ET, et al. Medullary pain facilitating neurons mediate allodynia in headache-related pain. Ann Neurol. 2009;65:184–193. doi: 10.1002/ana.21537. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bevilaqua-Grossi D, Lipton RB, Napchan U, Grosberg B, Ashina S, Bigal ME. Temporomandibular disorders and cutaneous allodynia are associated in individuals with migraine. Cephalalgia. 2010;30:425–432. doi: 10.1111/j.1468-2982.2009.01928.x. [DOI] [PubMed] [Google Scholar]
- 22.Ayesh EE, Jensen TS, Svensson P. Hypersensitivity to mechanical and intraarticular electrical stimuli in person with painful temporomandibular joints. J Dent Res. 2007;86:1187–1192. doi: 10.1177/154405910708601209. [DOI] [PubMed] [Google Scholar]
- 23.Sessle BJ. Acute and chronic craniofacial pain; brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit Rev Oral Biol Med. 2000;11:57–91. doi: 10.1177/10454411000110010401. [DOI] [PubMed] [Google Scholar]
- 24.Lim PF, Smith S, Bhalang K, Slade GD, Maixner W. Development of temporomandibular disorders is associated with greater bodily pain experience. Clin J Pain. 2010;26:116–120. doi: 10.1097/AJP.0b013e3181c507ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baranauskas G, Nistri A. Sensitization of pain pathways in the spinal cord: Cellular mechanisms. Prog Neurobiol. 1998;54:349–365. doi: 10.1016/s0301-0082(97)00067-1. [DOI] [PubMed] [Google Scholar]
- 26.Lidbeck J. Central Hyperexcitability in chronic musculoskeletal pain. A conceptual breackthrough with multiple clinical applications. Pain Res Mangmt. 2002;7:81–92. doi: 10.1155/2002/310974. [DOI] [PubMed] [Google Scholar]
- 27.Mathew NT, Kailasam J, Seifert T. Clinical recognition of allodynia in migraine. Neurology. 2004;63:848–852. doi: 10.1212/01.wnl.0000137107.27585.f7. [DOI] [PubMed] [Google Scholar]
- 28.Lipton RB, Bigal ME, Ashina S, Burstein R, Silberstein S, Reed ML, et al. Cutaneous allodynia in migraine population. Ann Neurol. 2008;63:148–158. doi: 10.1002/ana.21211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Ashkenazi A, Silberstein S, Jakubowski M, Berstein R. Improved identification of allodynic migraine patients using questionnaire. Cephalalgia. 2007;27:325–329. doi: 10.1111/j.1468-2982.2007.01291.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Tietjen GE, Brandes IL, Peterlin BL, Eloff A, Dafer RM, Stein MR, et al. Allodynia in migraine: association with comorbid pain conditions. Headache. 2009;49:1333–1344. doi: 10.1111/j.1526-4610.2009.01521.x. [DOI] [PubMed] [Google Scholar]
- 31.Centonze V, Bassi A, Cassiano MA, Munno A, Dalfino L, Causarano V. Migraine daily chronic headache and fibromyalgia in the same patient: an evaluative `continuum' of non organic chronic pain? About 100 clinical cases. Neurol Sci. 2004;25:S291–92. doi: 10.1007/s10072-004-0314-4. [DOI] [PubMed] [Google Scholar]
- 32.Staud R, Rodriguez ME. Mechanism of disease: Pain in fibromyalgia syndrome. Nat Clinic Pract Rheum. 2006;2:90–98. doi: 10.1038/ncprheum0091. [DOI] [PubMed] [Google Scholar]
- 33.Burstein R, Cutrer MF, Yarnisky D. The development of cutaneous allodynia during a migraine attack: clinical evidence for the sequential recruitment of spinal and supraspinal nociceptive neurons in migraine. Brain. 2000:1703–07. doi: 10.1093/brain/123.8.1703. [DOI] [PubMed] [Google Scholar]
- 34.Berstein R, Yarnesky D, Goor-Aryeh I, et al. An association between migraine and cutaneous allodinia. Ann Neurol. 2000;47:614–624. [PubMed] [Google Scholar]
- 35.Bahra A, Walsh M, Menon S, Goadsby PJ. Does chronic daily headache arise de novo in association with regular use of analgesics. Headache. 2003;43:179–190. doi: 10.1046/j.1526-4610.2003.03041.x. [DOI] [PubMed] [Google Scholar]
- 36.Geppetti P, Cesaris F, Nicoletti P, Benemei S. Chronic headaches and medication overuse. Intern Emerg Med. 2010;5:S7–9. doi: 10.1007/s11739-010-0461-y. [DOI] [PubMed] [Google Scholar]
- 37.Jonsson P, Hedenrud T, Linde M. Epidemiology of medication overuse headache in the general Swedish population. Cephalalgia. 2011;31:1015–1022. doi: 10.1177/0333102411410082. [DOI] [PubMed] [Google Scholar]
- 38.Rhee H. Racial/ethnic differences in adolescents' physical symptoms. J Pediatr Nurs. 2005;20:153–63. doi: 10.1016/j.pedn.2005.01.003. [DOI] [PubMed] [Google Scholar]
- 39.Stewart WF, Lipton RB, Liberman J. Variation in migraine prevalence by race. Neurol. 1996;47:52–57. doi: 10.1212/wnl.47.1.52. [DOI] [PubMed] [Google Scholar]
- 40.Victor TW, Hu X, Campbell JC, Buse DC, Lipton RB. Migraine prevalence by age and sex in the United States: A life-span study. Cephalalgia. 2011;30:1065–1072. doi: 10.1177/0333102409355601. [DOI] [PubMed] [Google Scholar]