Abstract
Serine is generally classified as a nutritionally nonessential (dispensable) amino acid, but metabolically, serine is indispensible and plays an essential role in several cellular processes. Serine is the major source of one-carbon units for methylation reactions that occur via the generation of S-adenosylmethionine. The regulation of serine metabolism in mammalian tissues is thus of critical importance for the control of methyl group transfer. In addition to the well known role of d-serine in the brain, l-serine has recently been implicated in breast cancer and other tumors due in part to the genomic copy number gain for 3-phosphoglycerate dehydrogenase, the enzyme that controls the entry of glycolytic intermediates into the pathway of serine synthesis. Here, we review recent information regarding the synthesis of serine and the regulation of its metabolism and discuss the role played by phosphoenolpyruvate carboxykinase in this process.
Keywords: Glycolysis, Metabolism, Phosphoenolpyruvate Carboxykinase, S-Adenosylmethionine (SAM), Serine
Introduction
The regulation of methyl group transfer is critical in controlling cellular processes, ranging from the synthesis of key metabolic intermediates, such as creatine, phosphatidylcholine, and epinephrine, to the methylation of proteins, DNA, and RNA. Serine, a nutritionally nonessential amino acid, plays a key role in this process by providing one-carbon units to tetrahydrofolate (THF)2 to form N5,N10-methylene-THF and, subsequently, 5-methyl-THF, an intermediate in the methylation of homocysteine to methionine, via homocysteine methyltransferase (methionine synthase) (Fig. 1). This ensures sufficient methionine for optimal functioning of the methionine cycle and for synthesis of S-adenosylmethionine, the key methyl donor in all cells. The regulation of the cellular levels of S-adenosylmethionine in response to metabolic changes and the role of the enzyme glycine N-methyltransferase in this process have been discussed recently in a minireview by Luka et al. (1). This minireview was particularly timely because it details the pioneering work on methyl group transfer of Conrad Wagner and colleagues.
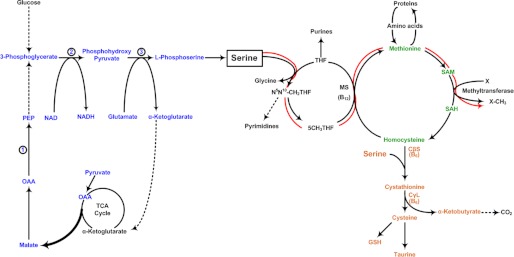
FIGURE 1.
Pathways of serine metabolism in mammals. The pathways for the synthesis and metabolic fate of serine are shown. The intermediates in the pathways shown in blue are involved in the synthesis of serine, either from glucose via glycolysis or from the triose phosphate pool, where carbon is generated from citric acid cycle intermediates. The latter pathway involves PEPCK (step 1), which is a major cataplerotic enzyme in the synthesis of serine. The conversion of 3-phosphoglycerate to phosphohydroxypyruvate is catalyzed by the enzyme 3-phosphoglycerate dehydrogenase (step 2). The final steps in the synthesis of serine involve the transamination of phosphohydroxypyruvate to l-phosphoserine (step 3) and the conversion of l-phosphoserine into serine by phosphoserine phosphatase (step 4). The pathway involved in methyl group transfer is shown by the red arrows, and the transsulfuration pathway is shown in orange. OAA, oxalacetate; PEP, phosphoenolpyruvate; MS, methionine synthase; SAM, S-adenosylmethionine; SAH, S-adenosylhomocysteine; CβS, cystathionine β-synthase; CγL, cystathionine γ-lyase.
Serine is also involved in the ultimate disposal of methionine carbon by condensing with homocysteine to form cystathionine, a reaction that is catalyzed by cystathionine β-synthase. Cystathionine is subsequently split into cysteine and α-ketobutyrate by cystathionine γ-lyase. This cascade of cysteine synthesis has been termed the “transsulfuration pathway” (Fig. 1). Over the past decade, the importance of the two enzymes of this pathway in the generation of hydrogen sulfide has been recognized. In addition, the metabolism of serine has been linked to the growth of breast cancer cells (2). The scope of the metabolism of sulfur-containing compounds in the generation of hydrogen sulfide and the role of the latter in the control of blood pressure and the reduction of ischemia/reperfusion injury have been reviewed in detail elsewhere (3–6). Here, we focus on the key role of serine in methyl group transfer and the factors that regulate its metabolism.
Metabolism of Serine in Vivo and Sources of Serine Carbon
The metabolic importance of serine is underscored by its indispensable role as a major contributor to the one-carbon pool and in the formation of glycine, cysteine, taurine, and phospholipids and of d-serine; the latter plays a critical role as a neuromodulator in the brain. The clinical phenotype and the therapeutic response to exogenous serine in serine deficiency syndromes suggest that the de novo synthesis of serine is critical and that dietary serine is insufficient to meet the demands of whole body serine homeostasis (7). Studies with humans using stable isotopic tracers suggest that virtually all of the methyl groups used for the total body remethylation of homocysteine are derived from serine (8). The pathways that maintain serine flux and serine levels in various tissues are thus of critical importance in sustaining the methylation potential of all cells.
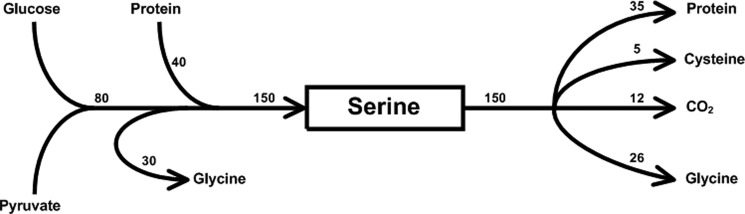
The rate of appearance of serine following an overnight fast in humans is estimated to be ∼150 μmol kg−1 h−1 (9, 10); of this, ∼40 μmol are derived from protein breakdown, and the remaining 110 μmol from de novo synthesis, including 30 μmol of serine that is derived from glycine (9, 11). Thus, 73% of the serine rate of appearance that is determined in fasting humans is the result of serine synthesis; this number is in close approximation to the fraction of serine synthesis from pyruvate (69%) that was measured in our studies using fasted rats (12). The route of disposal of serine is instructive because it illustrates the broad scope of metabolic influence of this amino acid. The synthesis of glycine (and therefore, the generation of one-carbon units) accounts for 26 μmol, of which 5 μmol are involved in the methylation of homocysteine, and 21 μmol are used for thymidylate (dTMP) synthesis (8, 9, 11). Serine is the source of carbon for the synthesis of cysteine (total flux of cysteine, ∼40 μmol kg−1 h−1; contribution of serine, ∼5 μmol kg−1 h−1 (13)). Finally, 35 μmol of serine kg−1 h−1 are converted to protein, and ∼12 μmol kg−1 h−1 are oxidized to CO2 (9). Thus, almost 50% of the disposal of serine in a healthy adult remains unaccounted for. It is of interest that glycine is a significant source of serine, but it is also synthesized from serine, suggesting serine/glycine carbon cycling. The quantitative estimates of serine turnover in healthy adults are presented in Fig. 2.
FIGURE 2.
Quantitative estimates of serine flux in humans. The contribution of precursors to the synthesis of serine and the estimated contribution of serine to its degradation products in human subjects during fasting are presented. The units are μmol kg−1 h−1 (derived from the data in Refs. 8 and 10). Please note that ∼72 μmol of serine disposal kg−1 h−1 are not accounted for in the figure. See the discussion in text.
As noted above, the rate of appearance and the rate of disposal of serine in fasted humans are high (9, 10); the estimated rate of appearance and the rate of de novo synthesis would be even higher if one takes into consideration the dilution of the isotopic tracer in the various extra- and intracellular pools of this amino acid in the body (10, 14). Measurements of arterial-venous concentration gradients in humans and rats show that the kidney is the primary (perhaps the only) site of serine synthesis during fasting (15–17). The release of serine by the kidney and its uptake by other tissues were not altered by the intravenous infusion of amino acids (15). In addition, the release of serine by the rat kidney, as measured by blood flow and arterial-venous difference, does not respond to dietary serine or to the intravenous infusion of serine (16). What then is the source of carbon for the net synthesis of serine in the kidney? On the basis of the stoichiometric relationship between glycine uptake and serine release by the kidney, Lowry et al. (17) showed that glycine accounts for no more than 15% of serine released. Inhibition of the cytosolic form of phosphoenolpyruvate carboxykinase (PEPCK-C) by 3-mercaptopicolinate caused a 55% decrease in serine efflux from the kidney, with a decrease in the influx of glutamine and no significant change in glycine influx into the kidney. In contrast, inhibition of the glycine cleavage system reversed glycine flux into the kidney but had no effect on the release of serine (17). Although plasma glycine and other small peptides cleared by the kidney were suggested by these authors to be the possible precursors of serine, the above data indicate that the generation of phosphoenolpyruvate via PEPCK provides the triose phosphates used for the ultimate synthesis of serine. Using deuterium labeling of the whole body water in the rat, we have recently shown that most of the carbon for de novo synthesis of serine is derived from pyruvate (12). The source of pyruvate in mammalian tissues during fasting is from lactate or is, more importantly, derived from the carbon skeletons of amino acids (Fig. 1). By this scenario, carbon flow for the synthesis of serine is similar to that which occurs in both gluconeogenesis and glyceroneogenesis, i.e. the triose phosphate pool is fed by the phosphoenolpyruvate that is generated by PEPCK from citric acid cycle anions.
Effect of Protein Restriction on Serine Metabolism
Dietary isocaloric protein restriction or protein malnutrition results in unique changes in methionine and serine metabolism in both humans and animal models. This is especially relevant considering the widespread protein malnourishment in human populations due to dietary patterns (vegetarians), drought, or economic disparities. Ingenbleek et al. (18) observed that protein malnutrition (as evidenced by lower plasma transthyretin levels) in humans was associated with hyperhomocysteinemia. Interestingly, even though the plasma concentration of most essential amino acids was lower in these subjects, the concentration of methionine was in the normal range, suggesting an independent regulation of plasma methionine levels, possibly because of its critical role in cell metabolism. A similar observation was reported by the same investigators to occur in a vegetarian population that had a lower intake of protein and sulfur amino acids (19); this population also had lower levels of cysteine and glutathione. These results were interpreted as being due to an inhibition of the transsulfuration pathway and possibly an increased transmethylation caused by the decrease in protein intake. Limiting rats to 6% protein in their diet for 7–10 days (compared with 24% in control animals) but maintaining caloric intake by pair-feeding the animals caused a marked set of metabolic adaptations (12). Most notable was a doubling of the concentration of serine in the blood and livers of the rats fed the protein-restricted diet, as well as a 50% increase in the de novo synthesis of this amino acid, as measured by isotopic tracer dilution. There was no change in the concentration of serine in the kidneys of the protein-restricted rats. Although the whole body rates of turnover of phenylalanine and methionine (measures of protein turnover) were not different, there was a marked decrease in the concentration of essential amino acids in the skeletal muscle and a decreased expression of genes encoding urea cycle enzymes in the livers of protein-restricted animals. The activity of the enzymes of the transsulfuration pathway was lower in the protein-restricted animals, although the isotopic tracer-measured transsulfuration flux was unchanged. Also of interest was the marked drop in the concentration of taurine in the liver (from 6.5 to 2.5 μmol/g of liver). Taurine is synthesized from cysteine, which itself is derived from serine in the transsulfuration pathway (Fig. 1). In addition to a possible decrease in the rate of its synthesis, the low intracellular levels of taurine may be due to its efflux from cells as an osmotic response. One interpretation of these findings is that isocaloric protein restriction results in increased production of serine in the liver, but not in the kidney, due to an increase in its rate of synthesis. There are a number of studies demonstrating that restricting dietary protein (or removing it completely from the diet) increases the concentration of serine in the blood (20–22). The exact mechanism or the possible signals that cause these responses in the biosynthesis of serine are not known.
Restricting dietary protein for 7–10 days induced the hepatic expression of the genes for several of the enzymes involved in serine synthesis (12, 21). Under normal circumstances, serine is not synthesized in the liver because a key enzyme in the pathway of synthesis of this amino acid, 3-phosphoglycerate dehydrogenase (3-PGDH), is absent or at negligible levels of activity in this organ (23). However, dietary protein restriction caused an 8-fold induction of the mRNA for this enzyme, as well a 3-fold increase in the mRNA for phosphoserine aminotransferase, another enzyme involved in the synthesis of serine from triose phosphates (12, 21). Earlier studies by Mauron et al. (24) reported that increasing the protein content of the diet fed to rats induced the hepatic activity of serine dehydratase, the key enzyme in the degradation of serine, and decreased the expression of 3-PGDH. The effect of dietary protein on the latter enzyme was ascribed to the effect of dietary methionine and cysteine on the levels of the enzyme. Achouri et al. (23) found that feeding rats a protein-free diet (but not starving the animals) induced the appearance of mRNA for 3-PGDH in the liver, and the administration of either methionine or cysteine to the animals reduced the abundance of 3-PGDH mRNA by ∼50% after 8 h. The observed effect was not due to an alteration in transcription of the gene for 3-PGDH, as determined by nuclear run-off assays, but rather to a destabilization of the mRNA for the enzyme, in particular by cysteine. In addition, when added to hepatocytes in culture, glucagon alone inhibited transcription of the gene for 3-PGDH, whereas insulin stimulated gene transcription. These responses are interesting when one considers the source of carbon for serine synthesis via PEPCK because they suggest that in the insulin-induced anabolic states, when serine biosynthesis is stimulated and PEPCK is suppressed, glucose may be the primary source of serine carbon. We conclude from these studies that the level of cysteine in the liver can control the rate of synthesis of serine by altering the stability of the mRNA for the initial enzyme in the synthesis of serine, 3-PGDH. This is especially important because cysteine is made from serine and can act as feedback regulator of its own synthesis by limiting the amount of serine synthesized from triose phosphates, particularly in the liver.
Finally, the concentration of serine itself can also act as a key regulator of its own synthesis by inhibiting phosphoserine phosphatase, the final and irreversible step in the synthesis of serine (25). Serine blocks the formation of the phosphoenzyme complex, a critical part of the reaction mechanism of the enzyme. In a control analysis of the pathway of serine synthesis, Fell and Snell (26) stressed the importance of the concentration of serine in the regulation of its synthesis. This story is confounded by the observations that serine in rats (16, 17) or amino acids in humans (15) did not decrease the efflux of serine from the kidney.
Serine and the Fetus
The biological significance of the unique metabolism of serine in the uteroplacental unit and the fetus is not understood. It is interesting to note that in late gestation, serine flux in the sheep fetus, as measured by isotopic tracer dilution, is extremely high (∼2700 μmol kg−1 h−1) (27, 28). In addition, most of the serine in the fetal circulation is produced by hepatic synthesis from glycine (28). Although there is a significant uptake of maternal serine by the placenta, it is converted to glycine by the placenta and released into the fetal circulation as glycine in equimolar quantities (28–30). A unique interorgan flux of serine and glycine is evident in the sheep fetus, where glycine is taken up by the fetus from the placenta and converted to serine in the fetal liver. A small quantity of serine from the fetal circulation is also taken up by the placenta and converted to glycine (28). These data suggest a very high methylation demand by the sheep fetus (placenta) in late gestation. Whether such a situation also exists in humans is not known, except the whole body rate of transmethylation is significantly increased late in pregnancy, and there is limited transfer of serine from the mother to the fetus (31).
Serine and Metabolism of Cancer Cells
After being in the shadow of molecular genetics for decades, metabolic research has risen like a phoenix in the current era and has been employed in studies of disorders as seemingly diverse as obesity and cancer. A number of studies have emphasized the key role of the anaplerosis of citric acid cycle intermediates in the metabolism of tumor cells, which have been described as being “addicted” to glutamine (32). Serine now has joined glutamine in a complex story of metabolism and tumor cell growth. A high rate of serine biosynthesis, i.e. an increased activity of 3-PGDH, was reported in human colon carcinoma, rat sarcoma, and rat hepatoma cell lines during the proliferative phase in the pioneering research on serine metabolism by Snell et al. in the 1980s (33–35). Recently, using a novel negative-selection method for identifying cancer targets, Possemato et al. (36) noted that expression of the gene for 3-PGDH was markedly higher in several breast cancer cell lines and in estrogen receptor-negative breast cancers. The mechanism responsible for the increased levels of 3-PGDH has not been investigated in detail, except for the work of Locasale et al. (37), who noted that particularly in melanoma cells, there is an amplification of the pericentromeric region of chromosome 1, where the gene for 3-PGDH resides. Interestingly, suppression of the level of 3-PGDH mRNA using shRNA did not decrease the concentration of serine in breast cancer cells but did lower the levels of α-ketoglutarate. The conversion of 3-phosphopyruvate to 3-phosphoserine involves transamination, a process that generates α-ketoglutarate; the glutamate used in this transamination is proposed to come from glutamine (36). In some breast cancer cell lines studied (but not all cell lines), 50% of the anaplerotic flux of glutamate carbon into the citric acid cycle is derived as a byproduct of the biosynthesis of serine (36). It has been proposed that in certain breast cancer cell lines, cell growth is dependent on serine and that 3-PGDH should be considered as a potential anticancer target.
From a metabolic view point, the suggestion that the α-ketoglutarate synthesized from serine is critical for tumor cell growth is not easy to understand because there are a number of potential sources of this compound (i.e. it is generated from the transamination of a variety of amino acids and is synthesized directly in the citric acid cycle). As for anaplerosis, the conversion of pyruvate to oxalacetate by pyruvate carboxylase and the transamination of aspartate to form oxalacetate via aspartate aminotransferase coupled with the oxidation of glutamate to α-ketoglutarate or the conversion of propionyl-CoA (generated largely from the breakdown of methionine, isoleucine, or valine) to succinyl Co-A can all contribute to the formation of new citric acid cycle anions. Locasale et al. (37) found that the metabolism of glucose via glycolysis provides the 3-phosphoglycerate for serine biosynthesis, but the actual stoichiometry of this pathway was not investigated, except for the comment that a “substantial portion” of the glucose metabolized by HEK293T cells (a kidney-derived cell line) is converted to serine. Intuitively, one would predict that a major role of serine in tumor cell metabolism would be to generate the methyl groups that are required for cell proliferation and other biological functions (i.e. protein synthesis) that are critical in rapidly dividing cells. This conclusion is confounded by the fact that added serine does not alter cell survival, suggesting a defect in the cellular uptake of serine (36). Other studies have shown a higher expression of the transporter for serine, alanine, cysteine, and threonine (SLC1A4) in highly metastatic breast cancer cells (38). Despite these questions, it is clear that serine is a major amino acid in the overall metabolism of a number of tumor-derived cell lines and is critical for cell growth and proliferation.
Role of Cataplerosis and PEPCK in Serine Synthesis
A key point from the studies reviewed above is the importance of understanding the metabolic pathway that provides 3-phosphoglycerate for the synthesis of serine. This compound can be generated from glucose via glycolysis or from pyruvate via an abbreviated version of gluconeogenesis. Our data from studies in the rat show that pyruvate entry into the gluconeogenic pathway is the major route for the synthesis of serine (12). This pathway would require the active role of the citric acid cycle and both anaplerosis and cataplerosis of the cycle intermediates to provide the carbon for serine synthesis.
The function of the citric acid cycle involves two general processes. This first is its classical role in the oxidation of acetyl-CoA to carbon dioxide with the subsequent generation of NADH and FADH2, which are then reoxidized via the electron transport chain to produce ATP. The second process involves biosynthesis; pathways such as gluconeogenesis, fatty acid synthesis, glyceroneogenesis, and now serine synthesis begin with intermediates from the citric acid cycle. The latter process has two elements that deserve to be stressed. 1) If citric acid cycle anions, such as malate (gluconeogenesis) and citrate (lipogenesis), leave the cycle as part of a biosynthetic pathway, they must be replaced to ensure the continued functioning of the cycle. The replacement of citric acid cycle anions is termed anaplerosis. Quantitatively, the most important anaplerotic reaction in eukaryotes is pyruvate carboxylase, which synthesizes oxalacetate from pyruvate and carbon dioxide in the mitochondria. There are, however, several other anaplerotic reactions, including glutamate dehydrogenase, which generates α-ketoglutarate from glutamate, and aspartate aminotransferase, which makes oxalacetate from aspartate and is anaplerotic when coupled with glutamate dehydrogenase to convert the glutamate formed in transamination to α-ketoglutarate. The cycle is the recipient of many of the carbon skeletons of amino acids as part of their degradation, so citric acid cycle anions are generated from a wide variety of reactions, and 2) they must be removed from the cycle (cataplerosis) to ensure its continued function. Therefore, the citric acid cycle anions must be transported out from the mitochondria to the cytosol of cells and converted to end products such as glucose, fatty acids, glyceride-glycerol, and serine or form pyruvate, which can be decarboxylated to acetyl-CoA in the mitochondria by the pyruvate dehydrogenase complex and subsequently oxidized. The details of the pathway of cataplerosis have been reviewed previously (39, 40).
The carbon skeletons of amino acids enter the citric acid cycle and exit as malate and are subsequently converted to triose phosphates; as mentioned above, PEPCK is a key enzyme in this process. Serine synthesis diverges from gluconeogenesis at the step where 3-phosphoglycerate is converted to 3-phosphohydroxypyruvate by 3-PGDH, a key enzyme in the pathway of serine synthesis from glycolytic/gluconeogenic intermediates. The 3-phosphohydroxypyruvate thus formed is transaminated to 3-phosphoserine by an aminotransferase and then converted to serine by 3-phosphoserine phosphatase. The major route for the breakdown of serine is serine dehydratase, a highly regulated enzyme that responds to a number of signals, including the levels of serine in tissues.
Conclusions
For a nonessential amino acid, serine plays an indispensable role in metabolism. Normally synthesized almost entirely in the kidney, a limitation in the availability of protein causes a marked induction of serine synthesis in the liver. This involves the transcription of the gene for 3-PGDH, a protein that is virtually undetectable in the adult rat liver when dietary protein is consumed at normal levels (i.e. 20% of dietary calories). There is surprisingly little information on the details of the regulation of transcription of the gene for 3-PGDH in the liver considering its importance in metabolism; control of gene expression in the brain has been more extensively studied, presumably due to the importance of d-serine as a neurotransmitter and the associated clinical syndrome caused by a d-serine deficiency. In this minireview, we have also stressed the importance of PEPCK in serine synthesis. Once considered solely a gluconeogenic enzyme, the metabolic role of PEPCK has greatly expanded over the years. We can now add a pathway (perhaps we could call it “serinoneogenesis”) that, like glyceroneogenesis, is essentially an abbreviated version of gluconeogenesis that generates triose phosphate for biosynthesis (of serine). This may help explain the long-standing conundrum of the metabolic role of PEPCK in tissues that do not make glucose or are not involved in triglyceride synthesis. In our view, PEPCK should be classified as a cataplerotic enzyme; the metabolic fate of the carbon after it exits the citric acid cycle to become a triose phosphate (i.e. the conversion of oxalacetate to phosphoenolpyruvate) depends on the tissue and the metabolic status of the organism. Serine represents another example of a seemingly simple compound that has been underrated over the years. Serine synthesis is indeed indispensable, and serine metabolism is critical for survival.
Acknowledgment
We thank Manoa Hui for help in the preparation of this manuscript.
This work was supported, in whole or in part, by National Institutes of Health Grant DK079937 (to S. C. K.). This work was also supported by Ellison Foundation Senior Scholar Award AG-SS-2420-10 (to R. W. H.).
- THF
- tetrahydrofolate
- PEPCK
- phosphoenolpyruvate carboxykinase
- 3-PGDH
- 3-phosphoglycerate dehydrogenase.
REFERENCES
- 1. Luka Z., Mudd S. H., Wagner C. (2009) Glycine N-methyltransferase and regulation of S-adenosylmethionine levels. J. Biol. Chem. 284, 22507–22511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. DeBerardinis R. J. (2011) Serine metabolism: some tumors take the road less traveled. Cell Metab. 14, 285–286 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Whiteman M., Le Trionnaire S., Chopra M., Fox B., Whatmore J. (2011) Emerging role of hydrogen sulfide in health and disease: critical appraisal of biomarkers and pharmacological tools. Clin. Sci. 121, 459–488 [DOI] [PubMed] [Google Scholar]
- 4. Dominy J. E., Stipanuk M. H. (2004) New roles for cysteine and transsulfuration enzymes: production of H2S, a neuromodulator and smooth muscle relaxant. Nutr. Rev. 62, 348–353 [DOI] [PubMed] [Google Scholar]
- 5. Olson K. R. (2011) The therapeutic potential of hydrogen sulfide: separating hype from hope. Am. J. Physiol. Regul. Integr. Comp. Physiol. 301, R297–R312 [DOI] [PubMed] [Google Scholar]
- 6. Lavu M., Bhushan S., Lefer D. J. (2011) Hydrogen sulfide-mediated cardioprotection: mechanisms and therapeutic potential. Clin. Sci. 120, 219–229 [DOI] [PubMed] [Google Scholar]
- 7. Tabatabaie L., Klomp L. W., Berger R., de Koning T. J. (2010) l-Serine synthesis in the central nervous system: a review on serine deficiency disorders. Mol. Genet. Metab. 99, 256–262 [DOI] [PubMed] [Google Scholar]
- 8. Davis S. R., Stacpoole P. W., Williamson J., Kick L. S., Quinlivan E. P., Coats B. S., Shane B., Bailey L. B., Gregory J. F., 3rd (2004) Tracer-derived total and folate-dependent homocysteine remethylation and synthesis rates in humans indicate that serine is the main one-carbon donor. Am. J. Physiol. Endocrinol. Metab. 286, E272–E279 [DOI] [PubMed] [Google Scholar]
- 9. Kalhan S. C., Gruca L. L., Parimi P. S., O'Brien A., Dierker L., Burkett E. (2003) Serine metabolism in human pregnancy. Am. J. Physiol. Endocrinol. Metab. 284, E733–E740 [DOI] [PubMed] [Google Scholar]
- 10. Gregory J. F., 3rd, Cuskelly G. J., Shane B., Toth J. P., Baumgartner T. G., Stacpoole P. W. (2000) Primed, constant infusion with [2H3]serine allows in vivo kinetic measurement of serine turnover, homocysteine remethylation, and transsulfuration processes in human one-carbon metabolism. Am. J. Clin. Nutr. 72, 1535–1541 [DOI] [PubMed] [Google Scholar]
- 11. Dasarathy S., Kasumov T., Edmison J. M., Gruca L. L., Bennett C., Duenas C., Marczewski S., McCullough A. J., Hanson R. W., Kalhan S. C. (2009) Glycine and urea kinetics in nonalcoholic steatohepatitis in human: effect of intralipid infusion. Am. J. Physiol. Gastrointest. Liver Physiol. 297, G567–G575 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Kalhan S. C., Uppal S. O., Moorman J. L., Bennett C., Gruca L. L., Parimi P. S., Dasarathy S., Serre D., Hanson R. W. (2011) Metabolic and genomic response to dietary isocaloric protein restriction in the rat. J. Biol. Chem. 286, 5266–5277 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Hiramatsu T., Fukagawa N. K., Marchini J. S., Cortiella J., Yu Y. M., Chapman T. E., Young V. R. (1994) Methionine and cysteine kinetics at different intakes of cystine in healthy adult men. Am. J. Clin. Nutr. 60, 525–533 [DOI] [PubMed] [Google Scholar]
- 14. Cuskelly G. J., Stacpoole P. W., Williamson J., Baumgartner T. G., Gregory J. F., 3rd (2001) Deficiencies of folate and vitamin B6 exert distinct effects on homocysteine, serine, and methionine kinetics. Am. J. Physiol. Endocrinol. Metab. 281, E1182–E1190 [DOI] [PubMed] [Google Scholar]
- 15. Brundin T., Wahren J. (1994) Renal oxygen consumption, thermogenesis, and amino acid utilization during intravenous infusion of amino acids in man. Am. J. Physiol. 267, E648–E655 [DOI] [PubMed] [Google Scholar]
- 16. Brosnan J. T., Hall B. (1989) Renal serine production in vivo: effects of dietary manipulation of serine status. Can. J. Physiol. Pharmacol. 67, 1058–1061 [DOI] [PubMed] [Google Scholar]
- 17. Lowry M., Hall D. E., Hall M. S., Brosnan J. T. (1987) Renal metabolism of amino acids in vivo: studies on serine and glycine fluxes. Am. J. Physiol. 252, F304–F309 [DOI] [PubMed] [Google Scholar]
- 18. Ingenbleek Y., Hardillier E., Jung L. (2002) Subclinical protein malnutrition is a determinant of hyperhomocysteinemia. Nutrition 18, 40–46 [DOI] [PubMed] [Google Scholar]
- 19. Ingenbleek Y., McCully K. S. (2012) Vegetarianism produces subclinical malnutrition, hyperhomocysteinemia, and atherogenesis. Nutrition 28, 148–153 [DOI] [PubMed] [Google Scholar]
- 20. Filho J. C., Hazel S. J., Anderstam B., Bergström J., Lewitt M., Hall K. (1999) Effect of protein intake on plasma and erythrocyte free amino acids and serum IGF-I and IGFBP-1 levels in rats. Am. J. Physiol. 277, E693–E701 [DOI] [PubMed] [Google Scholar]
- 21. Antflick J. E., Baker G. B., Hampson D. R. (2010) The effects of a low protein diet on amino acids and enzymes in the serine synthesis pathway in mice. Amino Acids 39, 145–153 [DOI] [PubMed] [Google Scholar]
- 22. Nagao K., Bannai M., Seki S., Mori M., Takahashi M. (2009) Adaptational modification of serine and threonine metabolism in the liver to essential amino acid deficiency in rats. Amino Acids 36, 555–562 [DOI] [PubMed] [Google Scholar]
- 23. Achouri Y., Robbi M., Van Schaftingen E. (1999) Role of cysteine in the dietary control of the expression of 3-phosphoglycerate dehydrogenase in rat liver. Biochem. J. 344, 15–21 [PMC free article] [PubMed] [Google Scholar]
- 24. Mauron J., Mottu F., Spohr G. (1973) Reciprocal induction and repression of serine dehydratase and phosphoglycerate dehydrogenase by proteins and dietary essential amino acids in rat liver. Eur. J. Biochem. 32, 331–342 [DOI] [PubMed] [Google Scholar]
- 25. Collet J. F., Stroobant V., Van Schaftingen E. (1999) Mechanistic studies of phosphoserine phosphatase, an enzyme related to P-type ATPases. J. Biol. Chem. 274, 33985–33990 [DOI] [PubMed] [Google Scholar]
- 26. Fell D. A., Snell K. (1988) Control analysis of mammalian serine biosynthesis. Feedback inhibition on the final step. Biochem. J. 256, 97–101 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Cetin I., Fennessey P. V., Quick A. N., Jr., Marconi A. M., Meschia G., Battaglia F. C., Sparks J. W. (1991) Glycine turnover and oxidation and hepatic serine synthesis from glycine in fetal lambs. Am. J. Physiol. 260, E371–E378 [DOI] [PubMed] [Google Scholar]
- 28. Cetin I., Fennessey P. V., Sparks J. W., Meschia G., Battaglia F. C. (1992) Fetal serine fluxes across fetal liver, hind limb, and placenta in late gestation. Am. J. Physiol. 263, E786–E793 [DOI] [PubMed] [Google Scholar]
- 29. Cetin I., Marconi A. M., Bozzetti P., Sereni L. P., Corbetta C., Pardi G., Battaglia F. C. (1988) Umbilical amino acid concentrations in appropriate and small for gestational age infants: a biochemical difference present in utero. Am. J. Obstet. Gynecol. 158, 120–126 [DOI] [PubMed] [Google Scholar]
- 30. Chung M., Teng C., Timmerman M., Meschia G., Battaglia F. C. (1998) Production and utilization of amino acids by ovine placenta in vivo. Am. J. Physiol. 274, E13–E22 [DOI] [PubMed] [Google Scholar]
- 31. Dasarathy J., Gruca L. L., Bennett C., Parimi P. S., Duenas C., Marczewski S., Fierro J. L., Kalhan S. C. (2010) Methionine metabolism in human pregnancy. Am. J. Clin. Nutr. 91, 357–365 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Wise D. R., Thompson C. B. (2010) Glutamine addiction: a new therapeutic target in cancer. Trends Biochem. Sci. 35, 427–433 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Snell K., Natsumeda Y., Weber G. (1987) The modulation of serine metabolism in hepatoma 3924A during different phases of cellular proliferation in culture. Biochem. J. 245, 609–612 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Snell K., Weber G. (1986) Enzymatic imbalance in serine metabolism in rat hepatomas. Biochem. J. 233, 617–620 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Snell K. (1984) Enzymes of serine metabolism in normal, developing, and neoplastic rat tissues. Adv. Enzyme. Regul. 22, 325–400 [DOI] [PubMed] [Google Scholar]
- 36. Possemato R., Marks K. M., Shaul Y. D., Pacold M. E., Kim D., Birsoy K., Sethumadhavan S., Woo H. K., Jang H. G., Jha A. K., Chen W. W., Barrett F. G., Stransky N., Tsun Z. Y., Cowley G. S., Barretina J., Kalaany N. Y., Hsu P. P., Ottina K., Chan A. M., Yuan B., Garraway L. A., Root D. E., Mino-Kenudson M., Brachtel E. F., Driggers E. M., Sabatini D. M. (2011) Functional genomics reveal that the serine synthesis pathway is essential in breast cancer. Nature 476, 346–350 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Locasale J. W., Grassian A. R., Melman T., Lyssiotis C. A., Mattaini K. R., Bass A. J., Heffron G., Metallo C. M., Muranen T., Sharfi H., Sasaki A. T., Anastasiou D., Mullarky E., Vokes N. I., Sasaki M., Beroukhim R., Stephanopoulos G., Ligon A. H., Meyerson M., Richardson A. L., Chin L., Wagner G., Asara J. M., Brugge J. S., Cantley L. C., Vander Heiden M. G. (2011) Phosphoglycerate dehydrogenase diverts glycolytic flux and contributes to oncogenesis. Nat. Genet. 43, 869–874 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Pollari S., Käkönen S. M., Edgren H., Wolf M., Kohonen P., Sara H., Guise T., Nees M., Kallioniemi O. (2011) Enhanced serine production by bone metastatic breast cancer cells stimulates osteoclastogenesis. Breast Cancer Res. Treat. 125, 421–430 [DOI] [PubMed] [Google Scholar]
- 39. Owen O. E., Kalhan S. C., Hanson R. W. (2002) The key role of anaplerosis and cataplerosis for citric acid cycle function. J. Biol. Chem. 277, 30409–30412 [DOI] [PubMed] [Google Scholar]
- 40. Yang J., Kalhan S. C., Hanson R. W. (2009) What is the metabolic role of phosphoenolpyruvate carboxykinase? J. Biol. Chem. 284, 27025–27029 [DOI] [PMC free article] [PubMed] [Google Scholar]