Abstract
Zinc is an essential metal ion for human growth and development, the disruption of cellular Zn2+ homeostasis being implicated in several major disorders including Alzheimer's disease, diabetes, and cancer. The molecular mechanisms of Zn2+ physiology and pathology are insufficiently understood, however, owing in part to the lack of tools for measuring changes in intracellular Zn2+ concentrations with high spatial and temporal fidelity. To address this critical need, we have synthesized, characterized, and applied an intracellular fluorescent probe for the ratiometric imaging of Zn2+ based on a tautomeric seminaphthofluorescein platform. Zin-naphthopyr 1 (ZNP1) affords single-excitation, dual-emission ratiometric detection of intracellular Zn2+ through Zn2+-controlled switching between fluorescein and naphthofluorescein tautomeric forms. The probe features visible excitation and emission profiles, excellent selectivity responses for Zn2+ over competing Ca2+ and Mg2+ ions at intracellular concentrations, a dissociation constant (Kd) for Zn2+ of <1 nM, and an 18-fold increase in fluorescence emission intensity ratio (λ624/λ528) upon zinc binding. We demonstrate the value of the ZNP1 platform for biological applications by imaging changes in intracellular [Zn2+] in living mammalian cells. Included is the ratiometric detection of endogenous pools of intracellular Zn2+ after NO-induced release of Zn2+ from cellular metalloproteins. We anticipate that ZNP1 and related probes should find utility for interrogating the biology of Zn2+.
Zinc is an indispensable element for sustaining life and is the second-most abundant transition metal in the human body (1, 2). Owing to its unique electronic and structural preferences, Zn2+ plays a central role in regulating cellular metabolism (1, 3). Zn2+ is an essential cofactor in all six classes of enzymes (1, 4) and several families of regulatory proteins, including those that control gene expression (3, 5), DNA repair (6, 7), and apoptosis (8).
The physiological importance of Zn2+ demands that cells exert strict control over the homeostasis of this ubiquitous metal ion (9), and most stores of intracellular Zn2+ are tightly bound and serve as structural and/or catalytic components of metalloprotein scaffolds (1, 2). Nevertheless, histochemical studies reveal that many mammalian organs accumulate pools of labile Zn2+ under normal physiological conditions. Prominent examples include the brain (10), pancreas (11), and prostate (12). In addition, alterations of Zn2+ homeostasis are implicated in a number of significant human disorders; disrupted patterns of intracellular Zn2+ accumulation have been found in patients with Alzheimer's disease (13), diabetes (14), and cancer (15). Despite the far-ranging consequences of Zn2+ homeostasis in human physiology and pathology, however, the mechanistic details surrounding intracellular Zn2+ accumulation, trafficking, and function remain poorly defined even in the simplest single-cell organisms (16).
Fluorescent chemosensors are well suited to meet the need for tools to map the spatial and temporal distribution of ionic Zn2+ pools within living cells. Such reagents have revolutionized the study of calcium in cell biology (17) and hold much promise for enhancing our understanding of zinc in cell biology. Several families of fluorescent Zn2+ probes have been described, including those with peptide- or protein-based scaffolds (18–22). There are also a number of cell-permeable, synthetic chemosensors that induce a change in fluorescence intensity upon Zn2+ binding and do not require microinjection for intracellular use (23–30). Although these and related (31, 32) intensity-based fluorescent probes are of considerable practical value, they cannot provide quantitative information about changes in Zn2+ concentrations, owing to variations in excitation intensity, emission collection efficiency, sample thickness, and artifacts associated with probe concentration and environment. Chemosensors that exhibit a shift in excitation or emission profiles upon Zn2+ complexation can, in principle, provide accurate and quantitative determinations of intracellular [Zn2+] by using ratiometric fluorescence imaging. This method affords simultaneous detection of metal-free (apo) and bound probes using dual-excitation and/or dual-emission measurements. Zn2+-responsive probes based on benzofuran (27, 33) and benzoxazole (34, 35) fluorophores have been used for ratiometric imaging of intracellular Zn2+, but they require UV excitation that can be damaging to cells and cause interfering autofluorescence from native cellular species.
In one approach to this goal we recently reported an esterase-mediated system for ratiometric sensing of Zn2+ that consists of two fluorophores having excitation and emission profiles in the visible region (36). Here, we present the synthesis and properties of a tautomeric, ratiometric probe for Zn2+ and establish its utility for imaging changes in [Zn2+] within living mammalian cells. Included is the detection of endogenous Zn2+ by using a ratiometric chemosensor. The probe Zin-naphthopyr 1 (ZNP1) (Fig. 1), which is based on a hybrid seminaphthofluorescein platform, affords single-excitation, dual-emission ratiometric imaging of intracellular Zn2+ through controllable Zn2+-induced switching between the fluorescein and naphthofluorescein tautomeric forms (Fig. 2). The tautomeric chemosensor features excitation and emission maxima in the visible range, excellent selectivity for Zn2+ over ubiquitous intracellular metal ions such as Na+, K+, Ca2+, and Mg2+, and a dissociation constant (Kd) for Zn2+ of <1 nM. Our results establish the value of the tautomer-based ZNP1 platform for sensing endogenous Zn2+ in biological environments and provide a synthetic and mechanistic basis for the further development of ratiometric probes for investigating the physiology and pathology of intracellular metal ions.
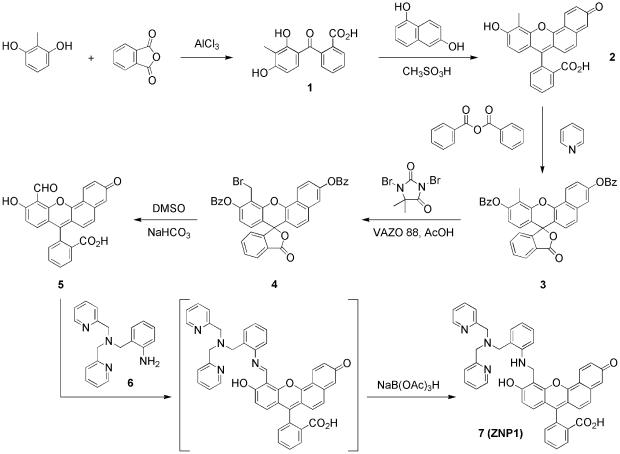
Fig. 1.
Synthesis of ZNP1.
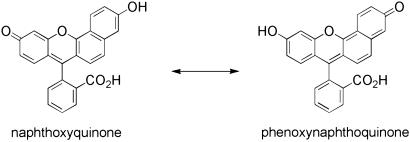
Fig. 2.
The two limiting tautomeric forms of the seminaphthofluorescein fluorophore. The naphthoxyquinone mesomer (Left) has fluorescein-like optical properties, and the phenoxynaphthoquinone mesomer (Right) shares optical characteristics with naphthofluorescein.
Materials and Methods
Synthetic Materials and Methods. Silica gel 60 (70–230 mesh, Merck) and octadecyl-functionalized silica gel (RP18, Aldrich) were used for column chromatography. Analytical thin layer chromatography was performed by using Merck 60 F254 silica gel and Merck RP-18 F254S silica gel (precoated sheets, 0.25 mm thick). Solvents for synthesis were of reagent grade or better and were dried according to standard methods (37). 2-[Bis(2-pyridylmethyl)aminomethyl]aniline (6) was prepared as described (31). All other reagents for synthesis were purchased and used as received. 1H NMR spectra were collected in CDCl3, CD3OD, or d7-dimethylformamide (Cambridge Isotope Laboratories, Cambridge, MA) at 25°C at the Massachusetts Institute of Technology Department of Chemistry Instrumentation Facility (DCIF) on either a Varian Inova 500 or a Varian Mercury 300 spectrometer. All chemical shifts are reported in the standard δ notation of parts per million; positive chemical shifts are to higher frequency from the given reference. High-resolution mass spectral analyses were carried out at the Massachusetts Institute of Technology DCIF.
2′-Carboxy-3-methyl-2,4-dihydroxybenzophenone (1). Under an argon atmosphere, 2-methylresorcinol (10.0 g, 80.6 mmol) and phthalic anhydride (11.2 g, 75.6 mmol) were combined in dry nitrobenzene (250 ml). The mixture was cooled to 0°C, and aluminum(III) chloride (23.5 g, 176 mmol) was added in one portion. The resulting dark olive slurry was allowed to warm to room temperature and stirred for an additional 16 h under argon. The reaction was poured into a vigorously stirring mixture of hexanes (300 ml) and 1 M HCl (1 liter). The precipitate was filtered and recrystallized twice from methanol/water to afford benzophenone 1 as a beige powder (16.0 g, 78% yield). 1H NMR (CD3OD, 500 MHz): δ 8.05 (1 H, dd, J1 = 8.0 Hz, J2 = 1.0 Hz), 7.62 (2 H, dt, J1 = 33.0 Hz, J2 = 9.0 Hz), 7.33 (1 H, d, J = 7.5 Hz), 6.74 (1 H, d, J = 8.5 Hz), 6.20 (1 H, d, J = 8.5 Hz), 2.05 (3 H, s). High-resolution MS (HRMS) (electrospray ionization, ESI) calculated for [M-H]– 271.0601, found 271.0601.
4-Methyl-3,10-dihydroxy-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (2). 2′-Carboxy-3-methyl-2,4-dihydroxybenzophenone (1, 8.16 g, 30.0 mmol) and 1,6-dihydroxynaphthalene (4.81 g, 30.0 mmol) were combined in methanesulfonic acid (120 ml) and sealed in a thick-walled glass tube. The resulting viscous mixture was stirred at 90°C for 24 h. The reaction was poured into ice-cold water (1 liter), and the precipitate was filtered and washed with water (3 × 200 ml). Purification by flash column chromatography (silica gel, 9:1 dichloromethane/methanol) furnished seminaphthofluorescein 2 as a brick red powder (9.40 g, 79% yield). 1H NMR (CD3OD, 500 MHz): δ 8.41 (1 H, d, J = 9.0 Hz), 8.00 (1 H, d, J = 7.0 Hz), 7.69 (2 H, m), 7.27 (1 H, dd, J1 = 8.5 Hz, J2 = 3.5 Hz), 7.23 (1 H, d, J = 10.0 Hz), 7.16 (1 H, d, J = 7.5 Hz), 7.09 (1 H, br s), 6.59 (2 H, m), 6.46 (1 H, d, J = 8.5 Hz), 2.48 (3 H, s). HRMS (ESI) calculated for [M+H]+ 397.1071, found 397.1057.
4-Methyl-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (3). Under an argon atmosphere, 4-methyl-3,10-dihydroxy-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (2, 396 mg, 1.00 mmol) and benzoic anhydride (475 mg, 2.10 mmol) were combined in dry pyridine (10 ml). The resulting dark red mixture was refluxed under argon for 3 h at 140°C, cooled to 75°C, and poured into vigorously stirring cold water (25 ml). The peach-colored precipitate was filtered and washed with water (2 × 100 ml). Purification by flash column chromatography (silica gel, 2:1 hexanes/ethyl acetate) delivered dibenzoate 3 as a lemon yellow solid (500 mg, 83% yield). 1H NMR (CDCl3, 500 MHz): δ 8.64 (1 H, d, J = 9.0 Hz), 8.27 (4 H, m), 8.09 (1 H, d, J = 7.5 Hz), 7.68 (5 H, m), 7.57 (5 H, m), 7.50 (1 H, d, J = 9.0 Hz), 7.21 (1 H, d, J = 7.5 Hz), 6.97 (1 H, d, J = 8.5 Hz), 6.86 (1 H, d, J = 8.5 Hz), 6.82 (1 H, d, J = 8.5 Hz), 2.58 (s, 3H). HRMS (ESI) calculated for [M+H]+ 605.1595, found 605.1611.
4-Bromomethyl-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (4). 4-Methyl-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (3, 4.85 g, 8.02 mmol) and 1,3-dibromo-5,5-dimethylhydantoin (2.58 g, 9.02 mmol) were combined in dry chlorobenzene (175 ml). Acetic acid (100 μl) and 1,1′-azobis(cyclohexanecarbonitrile) (VAZO 88, 108 mg, 0.44 mmol) were added, and the solution was stirred at 50°C for 96 h. Hot water (200 ml) was added to the reaction, and the organic layer was separated, washed with water (2 × 100 ml), and dried over Na2SO4. The solvent was removed by rotary evaporation, and the remaining oil was dissolved in toluene (10 ml). Precipitation with ethanol (75 ml) gives bromomethyl compound 4 as a pale peach powder (4.30 g, 78% yield) that was used without further purification. 1H NMR (CDCl3, 500 MHz): δ 8.75 (1 H, d, J = 9.5 Hz), 8.72 (1 H, d, J = 9.5 Hz), 8.30 (4 H, m), 8.27 (1 H, m), 7.73 (5 H, m), 7.60 (5 H, m), 7.24 (1 H, m), 7.10 (1 H, m), 6.96 (1 H, t, J = 9.5 Hz), 6.86 (1 H, d, J = 9.0 Hz), 4.94 (2 H, m). HRMS (ESI) calculated for [M+H]+ 683.0700, found 683.0692.
4-Carboxaldehyde-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (5). 4-Bromomethyl-3,10-dibenzoatespiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (4, 200 mg, 0.29 mmol) and sodium bicarbonate (200 mg, 2.38 mmol) were combined in dry dimethyl sulfoxide (7 ml), and the mixture was heated under argon for 3 h at 140°C. The reaction was cooled to 80°C and poured into 4 M HCl (35 ml). The resulting precipitate was filtered and washed with water (50 ml). Purification by flash column chromatography (silica gel, 19:1 dichloromethane/methanol) yields aldehyde 5 as a red solid (45 mg, 37% yield). 1H NMR (25:1 CDCl3/CD3OD, 500 MHz): δ 10.84 (1 H, s), 8.81 (2 H, m), 8.45 (1 H, t, J = 7.5 Hz), 8.25 (1 H, d, J = 9.0 Hz), 8.02 (1 H, m), 7.96 (1 H, m), 7.65 (2 H, m), 7.46 (1 H, m), 7.10 (1 H, d, J = 7.5 Hz), 6.88 (1 H, d, J = 9.0 Hz), 6.63 (1 H, d, J = 7.0 Hz). HRMS (ESI) calculated for [M+H]+ 411.0863, found 411.0876.
4-[2-{Bis(2-pyridylmethyl)aminomethyl}-N-methylaniline]-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (ZNP1, 7). 4-Carboxaldehyde-3,10-dibenzoate-spiro[7H-benzo[c]xanthen-7,1′(3′H)-isobenzofuran]-3′-one (5, 52 mg, 0.127 mmol) and 2-[bis(2-pyridylmethyl)-aminomethyl]aniline (6, 40 mg, 0.131 mmol) were combined in a mixture of dry chloroform (7 ml) and dry methanol (2 ml). The wine-colored solution was stirred at room temperature for 24 h and diluted with dry 1,2-dichloroethane (4 ml). Sodium triacetoxyborohydride (41 mg, 0.193 mmol) was added in one portion, the solution color changed to pale orange, and the reaction was stirred for an additional 24 h at room temperature. Removal of the solvent and purification by preparative TLC (octadecyl-functionalized silica gel, methanol) afforded chemosensor 7 as a pale red powder (49 mg, 55% yield). 1H NMR (d7-dimethylformamide, 300 MHz): δ 8.55 (1 H, d, J = 8.5 Hz), 8.35 (1 H, d, J = 10.0 Hz), 8.28 (2 H, d, J = 8.0 Hz), 8.15 (1 H, d, J = 7.0 Hz), 7.75–7.94 (2 H, m), 7.54 (1 H, d, J = 7.5 Hz), 7.46–7.50 (1 H, m), 7.42 (2 H, d, J = 13.0 Hz), 7.35 (2 H, d, J = 12.0 Hz), 7.24 (2 H, dd, J1 = 23.0 Hz, J2 = 14.0 Hz), 6.96–7.19 (5 H, m), 6.83 (1 H, d, J = 14.5 Hz), 6.49–6.71 (2 H, m), 5.60 (1 H, br s), 4.84 (2 H, br s), 3.50–3.76 (6 H, m). HRMS (ESI) calculated for [M+H]+ 699.2602, found 699.2573. The diacetate derivative, ZNP1-Ac, was prepared by reaction of 7 with acetic anhydride/Cs2CO3 in DMF. HRMS (ESI) calculated for [M+H]+ 783.2819, found 783.2799.
Spectroscopic Materials and Methods. Millipore water was used to prepare all aqueous solutions, which were passed through 0.2-μm cellulose filters before use. All spectroscopic measurements were performed under simulated physiological conditions using buffer solutions containing 50 mM Hepes, 4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid, and 100 mM KCl adjusted to pH 7.5. A glass electrode (Orion, Boston), calibrated before each use, was used to determine solution pH. Solutions of Zn2+ were prepared from 100-mM stock solutions of ZnCl2 in water. Absorption spectra were recorded on a Hewlett–Packard 8453A diode array spectrophotometer, and fluorescence spectra were recorded on a Photon Technology International (Lawrenceville, NJ) Quanta Master 4 L-format scanning spectrofluorimeter equipped with an LPS-220B 75-W xenon lamp and power supply, A-1010B lamp housing with integrated igniter, switchable 814 photon-counting/analog photomultiplier detection unit, and MD-5020 motor driver. Samples for absorption and emission measurements were contained in 1-cm × 1-cm quartz cuvettes (3.5 ml volume, Starna, Atascadero, CA). The experiments for measuring quantum yields, apparent dissociation constants (Kd), and metal ion selectivities were performed by using standard protocols (24). Quantum yields were determined by reference to fluorescein in 0.1 M NaOH (< = 0.95) (38).
Cell Culture. COS-7 cells were cultured in DMEM (Invitrogen) supplemented with 10% FCS (Invitrogen), glutamine (2 mM), and gentamycin (50 μg/ml, Invitrogen). One day before imaging, cells were passed and plated on 24-mm glass coverslips coated with poly-l-lysine (50 μg/ml) and moved to glass-bottomed live imaging dishes (MatTek, Ashland, MA). Immediately before labeling, cells were washed twice with DMEM, and then the medium was replaced with Zn2+-free Krebs–Ringer buffer. Krebs–Ringer buffer was prepared according to a published method (39). Cells were incubated with ZNP1 (20 μM) for 20 min at 37°C under 5% CO2, and then washed once with Krebs–Ringer media before imaging.
Fluorescence Imaging. Confocal fluorescence imaging experiments were performed with a Zeiss LSM510 laser scanning microscopy system containing an Axiovert 200M inverted fluorescence microscope. The microscope was equipped with an argon ion laser (488-nm excitation) and objective lenses (×100), and scanning was performed by using the META detection system operating in λ mode (Zeiss) with 10.7-nm collection windows. During imaging measurements, cell samples were kept on the microscope stage in a CTI-3700 incubator at 37°C under 5% CO2. Additions of Zn2+ as the pyrithione complex (2-mercaptopyridine N-oxide), N,N,N′,N′-tetra(2-picolyl)ethylenediamine (TPEN), or S-nitrosocysteine (SNOC) to cell samples were performed directly on the microscope stage by bath application to the media. SNOC was prepared immediately before use (40).
Results and Discussion
Design and Synthesis of ZNP1. To develop a Zn2+-responsive chemosensor for ratiometric imaging, we based our probe design on the tautomeric interconversion of two forms of a seminaphthofluorescein (Fig. 2). In this approach, a hybrid fluorophore platform is constructed by combining two chromophore pieces, each having a distinctly different excitation and emission profile. The hybrid fluorophore can attain two limiting forms (tautomers) with corresponding optical characteristics of the two respective chromophore constitutents. Integration of a metal ion receptor into the π system of the hybrid fluorophore platform allows analyte binding to influence the ratio of these electronically different tautomers, triggering a shift in excitation and/or emission profiles.
Drawing design inspiration from known hybrid fluorescein/ rhodamine Ca2+ (41) and naphthofluorescein/naphthorhodamine-based pH probes (42), we chose the seminaphthofluorescein platform for investigation. The two limiting tautomeric forms of the seminaphthofluorescein fluorophore are the naphthoxyquinone mesomer, which has fluorescein-like optical properties, and the phenoxynaphthoquinone mesomer, which shares optical characteristics with naphthofluorescein (Fig. 2). Incorporation ofaZn2+ receptor onto the seminaphthofluorecein platform was expected to afford Zn2+-dependent switching between the fluorescein- and naphthofluorescein-like tautomers. Ratiometric fluorescence imaging can then distinguish the relative amounts of these tautomers induced by changes in [Zn2+].
The synthetic strategy for preparing ZNP1 is outlined in Fig. 1. This convergent approach offers a general method for assembling asymmetric seminaphthofluorescein dyes and a useful monofunctionalized aldehyde building block for attachment to a wide variety of metal ion receptors. Reaction of 2-methylresorcinol with phthalic anhydride in the presence of AlCl3 generates 2′-carboxy-3-methyl-2,4-dihydroxybenzophenone 1 in 78% yield after recrystallization from methanol/water mixtures. Condensation of benzophenone 1 and 1,6-dihydroxynaphthalene in methanesulfonic acid furnishes the desired asymmetric seminaphthofluorescein dye 2 in excellent yield (79%) after column chromatography. Notably, analysis of the crude reaction mixture shows that only trace amounts of symmetric fluorescein and naphthofluorescein side products are formed (<5% each). The installation of benzoate protecting groups proceeds smoothly to afford 3 in 83% yield after column chromatography. Bromination of 3 under mild free radical conditions produces 4 in 78% yield. Product 4 was carried on without purification to the next step. Oxidation and benzoate deprotection are achieved in a one-pot reaction by treatment of bromide 4 with dimethyl sulfoxide and sodium bicarbonate under anhydrous conditions. The resulting aldehyde product 5 is obtained in modest yield (37%) after column chromatography. The preassembled seminaphthofluorescein aldehyde is a convenient synthon for the creation of new chemosensor targets. Schiff-base condensation of 5 and aniline 6 followed by reduction of the imine intermediate with sodium triacetoxyborohydride delivers ZNP1 in 55% yield.
Spectroscopic Properties and Optical Responses to Zn2+. ZNP1 was evaluated at physiological ionic strength and pH [50 mM Hepes (4-(2-hydroxyethyl)-1-piperazineethanesulfonic acid), 100 mM KCl, pH 7.5] in the presence of EDTA to scavenge adventitious metal ions. The seminaphthofluorescein probe exhibits two absorption bands in the visible region centered at 503 nm (ε = 7.2 × 103 M–1 cm–1) and 539 nm (ε = 6.7 × 103 M–1 cm–1). Upon excitation at 499 nm, two attendant emission bands of comparable intensity are observed with maxima at 528 and 604 nm (Fig. 3). The quantum yield for apo ZNP1 is 0.02. The absorption and emission profiles of ZNP1 reveal that the apo chemosensor has major electronic contributions from both naphthoxyquinone and phenoxynaphthoquinone tautomeric forms.
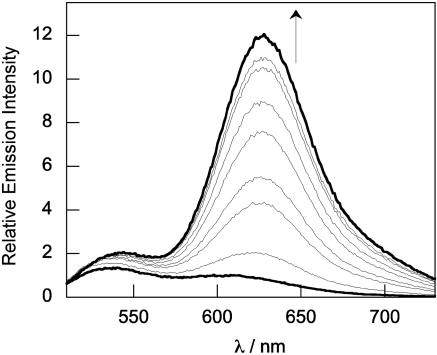
Fig. 3.
Ratiometric fluorescence spectroscopic response of 20 μM ZNP1 to buffered Zn2+ solutions. Spectra were acquired in 50 mM Hepes/100 mM KCl, pH 7.5. Excitation was provided at 499 nm. The standard EDTA/Ca2+/Zn2+ dual-metal buffer system was used to deliver controlled concentrations of buffered free Zn2+ (23). The spectra shown are for free Zn2+ buffered at 0, 0.17, 0.42, 0.79, 1.3, 2.1, 3.4, 5.6, and 10.2 nM. The fluorescence responses occur immediately upon mixing.
Coordination of Zn2+ to ZNP1 leads to striking changes in the optical and electronic properties of the seminaphthofluorescein probe. The observed changes in absorption and emission spectra occur up to a 1:1 [Zn2+]/[ZNP1] ratio, indicating the formation of a 1:1 complex. Upon addition of Zn2+, the visible absorption profile of the ZNP1 chemosensor red shifts to a single broad peak centered at 547 nm (ε = 2.2 × 104 M–1 cm–1). Excitation at 499 nm produces a fluorescence spectrum featuring one dominant emission band centered at 624 nm with a minor component centered at 545 nm (Fig. 3). The quantum yield for the Zn2+–ZNP1 complex is 0.05. Taken together, these data establish that Zn2+-bound ZNP1 resides primarily in the phenoxynaphthoquinone tautomer, and that ZNP1 provides an effective platform for single-excitation, dual-emission ratiometric sensing of Zn2+ through controllable Zn2+-induced switching between the fluorescein- and naphthofluorescein-like tautomers. The ratio of naphthofluorescein- to fluorescein-like emission intensities (λ624/λ528) upon 499-nm excitation varies from 0.4 in the absence of Zn2+ to 7.1 in the presence of Zn2+, an 18-fold increase.
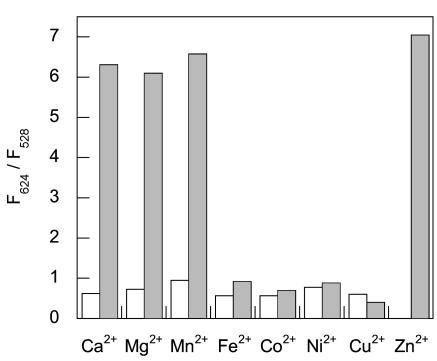
The ratiometric emission response of ZNP1 is Zn2+ selective. Fig. 4 displays the fluorescence response of a 10-μM solution of ZNP1 in the presence of various divalent metal ions. The emission profiles of apo or Zn2+-bound ZNP1 are unperturbed in the presence of 2 mM Ca2+ or Mg2+, indicating excellent selectivities for Zn2+ over these biologically competing alkaline earth cations. Other first-row transition metal ions including Cu2+, Ni2+, Co2+, Fe2+, and Mn2+ at 10-fold excess over probe produce no discernable change in emission ratios. Of these transition-metal ions, only the sample containing Mn2+ affords a ratiometric fluorescence response upon the subsequent addition of 100 μM Zn2+.
Fig. 4.
Ratiometric fluorescence spectroscopic responses of ZNP1 to various metal ions. Bars represent the ratio of fluorescence intensities collected at 624 and 528 nm (F624/F528). All spectra were acquired in 50 mM Hepes/100 mM KCl, pH 7.5. Empty bars represent the addition of an excess of the appropriate metal ion (2 mM for Ca2+ and Mg2+/100 μM for all other metal ions) to a 10-μM solution of ZNP1. Filled bars represent the subsequent addition of 100μM Zn2+ to the solution. Excitation was provided at 499 nm.
Finally, the binding affinity of ZNP1 for Zn2+ was characterized by using a dual-metal single-ligand buffer system (23). Varying the total Zn2+ concentrations between 0 and 1 mM in the presence of constant concentrations of Ca2+ (2 mM) and EDTA (1 mM) delivers controlled concentrations of buffered free Zn2+ between 0 and 25 nM. ZNP1 responds to nanomolar concentrations of free ionic Zn2+, and binding of Zn2+ to the probe was monitored by measuring the ratio of fluorescence intensities collected at 624 and 528 nm. This analysis was performed in triplicate by using different preparations of Ca2+/ Zn2+/EDTA buffers to determine an apparent Kd value of 0.55 ± 0.1 nM for the fluorescence-responsive 1:1 Zn2+–ZNP1 complex.
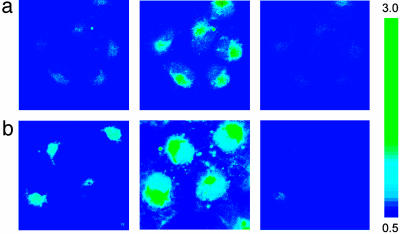
Ratiometric Fluorescence Imaging of Intracellular Zn2+ in Live Mammalian Cultures. Initial experiments established that ZNP1 is impermeable to cell membranes. We therefore prepared the nonfluorescent diacetate derivative of ZNP1, ZNP1-Ac, anticipating that this more lipophilic derivative would permeate the cell and be transformed to fluorescent ZNP1 by the action of intracellular esterases. Incubation of COS-7 cells with 20 μM ZNP1-Ac for 20 min at 37°C results in intracellular staining by ZNP1 as determined from scanning confocal fluorescence microscopy measurements on live samples. Ratiometric fluorescence imaging of ZNP1-stained cells is readily performed by using the META detection system operating in λ mode with optical windows centered at 612 and 526 nm. The ratio of fluorescence intensities at 612 and 526 nm for ZNP1-loaded COS-7 cells reveals that these mammalian cells contain low levels of available ionic Zn2+ (Fig. 5a). Prompt increases in the ratio of cytosolic fluorescence intensities collected at 612 and 526 nm are observed upon the addition of exogenous Zn2+ (50 μM) carried by the ionophore pyrithione (2-mercaptopyridine N-oxide, Fig. 5a). Treatment of the cells with the membrane-permeable metal ion chelator TPEN (100 μM) reverses the fluorescent ratio enhancements to baseline levels (Fig. 5a). These experiments indicate that ZNP1 is an effective ratiometric chemosensor for biological samples that can monitor changes in intracellular [Zn2+] reversibly.
Fig. 5.
Ratio confocal fluorescence imaging in COS-7 cells using the Zeiss LSM510 META system operating in the λ mode. Fluorescence was collected in 10.7-nm optical windows centered at 612 and 526 nm. Pseudocolor figures depict the ratio of fluorescence intensities at these two emission wavelengths. (a) Ratio confocal fluorescence images of live COS-7 cells labeled with ZNP1. Incubation of cells with 20 μM ZNP1-Ac for 20 min at 37°C(Left), ZNP1-stained cells loaded with 50 μM Zn(pyrithione)2 for 5 min (Center), and reversal of the cytosolic ratio enhancements with 100 μM TPEN (Right). Confocal images were taken from a middle optical section (vertical dimension) of the cell samples. (b) Confocal fluorescence images of NO-triggered release of endogenous Zn2+ in live COS-7 cells. Incubation of cells with 20 μM ZNP1-Ac for 20 min at 37°C (Left), ZNP1-stained cells treated with 10 mM SNOC (Center) for 1 h, and reversal of the observed ratio increases with 2 mM TPEN (Right). Confocal images were taken from a middle optical section (vertical dimension) of the cell samples. (Magnification: ×100.)
The successful use of ZNP1 for monitoring changes in intracellular Zn2+ concentrations by using ratiometric fluorescence imaging led us to apply this probe for detecting endogenous pools of intracellular Zn2+. To achieve this goal we took advantage of the role of NO, a key contributor to intracellular Zn2+ homeostasis (40, 43). NO is an important and versatile signaling molecule with far-ranging physiological and pathological functions (44). Relevant cellular targets of NO include transition metal ions, and cysteine thiol residues at structural and/or catalytic sites of proteins react with NO to form S-nitrosothiols (40, 45). In the case of zinc-dependent metalloproteins, formation of SNOC adducts labilizes Zn2+ from the polypeptide scaffold (40, 46). In particular, NO induces Zn2+ release from metallothionein (43, 47, 48) and inhibits the DNA-binding activity of the dizinc(II)-dependent transcription factor LAC9 (49). To test whether ZNP1 can detect the intracellular release of Zn2+ triggered by NO, we treated ZNP1-stained COS-7 cells (Fig. 5b) with the endogenous NO donor SNOC (10 mM). Ratiometric fluorescence imaging using the META detection system operating in λ mode clearly establishes a rise in intracellular [Zn2+] through an increase in the ratios of fluorescence intensity collected at 612 and 526 nm (Fig. 5b). The observed ratio enhancements are reversed by TPEN treatment (2 mM, Fig. 5b), indicating that the NO-induced signals are attributable to labilized ionic Zn2+, as observed (46). Notably, these studies represent the use of a ratiometric fluorescence probe for the detection of endogenous Zn2+ in cells.
Concluding Remarks
We have described the synthesis, properties, and biological applications of ZNP1, a type of tautomeric probe for ratiometric fluorescence imaging of intracellular Zn2+. The seminaphthofluorescein-based ZNP1 allows for single-excitation, dualemission ratio detection of Zn2+ through Zn2+-triggered switching between fluorescein- and naphthof luorescein-like tautomers. ZNP1 exhibits an excellent selectivity response for Zn2+ over biologically competing alkali and alkaline earth metal ions, and its long-wavelength visible excitation and emission profiles minimize cell and tissue damage while avoiding interfering autofluorescence from native cellular species. Moreover, we have demonstrated the biological value of this probe by measuring changes in intracellular [Zn2+] in living mammalian cells. In particular, we have achieved the detection of endogenous stores of intracellular Zn2+ by using a ratiometric fluorescence chemosensor by exploiting the NO-triggered release of protein-bound Zn2+. Finally, we highlight the convergent synthetic approach of the preassembled seminaphthofluorescein building block, which should allow facile access to a number of promising future candidates for ratiometric fluorescence sensing.
Acknowledgments
This work was supported by National Institute of General Medical Sciences Grant GM 65519 (to S.J.L.). C.J.C. acknowledges the Jane Coffin Childs Foundation for a postdoctoral fellowship. E.M.N. thanks the U.S. Department of Defense for a National Defense Science and Engineering Graduate Fellowship. M.S. is an Investigator at the Howard Hughes Medical Institute. The Massachusetts Institute of Technology Department of Chemistry Instrument Facility is funded by the National Science Foundation (Grants CHE-9808061, CHE-9808063, and DBI-9729592).
Abbreviations: ZNP1, Zin-naphthopyr 1; HRMS, high-resolution MS; ESI, electrospray ionization; TPEN, N,N,N′,N′-tetra(2-picolyl)ethylenediamine; SNOC, S-nitrosocysteine.
References
- 1.Vallee, B. L. & Falchuk, K. H. (1993) Physiol. Rev. 73, 79–118. [DOI] [PubMed] [Google Scholar]
- 2.Lippard, S. J. & Berg, J. M. (1994) Principles of Bioinorganic Chemistry (University Science Books, Mill Valley, CA).
- 3.Berg, J. M. & Shi, Y. (1996) Science 271, 1081–1085. [DOI] [PubMed] [Google Scholar]
- 4.Coleman, J. E. (1998) Curr. Opin. Chem. Biol. 2, 222–234. [DOI] [PubMed] [Google Scholar]
- 5.O'Halloran, T. V. (1993) Science 261, 715–724. [DOI] [PubMed] [Google Scholar]
- 6.Ho, E. & Ames, B. N. (2002) Proc. Natl. Acad. Sci. USA 99, 16770–16775. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Daiyasu, H., Osaka, K., Ishino, Y. & Toh, H. (2001) FEBS Lett. 503, 1–6. [DOI] [PubMed] [Google Scholar]
- 8.Truong-Tran, A. Q., Carter, J., Ruffin, R. E. & Zalewski, P. D. (2001) BioMetals 14, 315–330. [DOI] [PubMed] [Google Scholar]
- 9.Finney, L. A. & O'Halloran, T. V. (2003) Science 300, 931–936. [DOI] [PubMed] [Google Scholar]
- 10.Frederickson, C. J. (1989) Int. Rev. Neurobiol. 31, 145–238. [DOI] [PubMed] [Google Scholar]
- 11.Zalewski, P. D., Millard, S. H., Forbes, I. J., Kapaniris, O., Slavotinek, A., Betts, W. H., Ward, A. D., Lincoln, S. F. & Mahadevan, I. (1994) J. Histochem. Cytochem. 42, 877–884. [DOI] [PubMed] [Google Scholar]
- 12.Sorenson, M. B., Stoltenberg, M., Juhl, S., Danscher, G. & Ernst, E. (1997) Prostate 31, 125–130. [DOI] [PubMed] [Google Scholar]
- 13.Suh, S. W., Jensen, K. B., Jensen, M. S., Silva, D. S., Kesslak, P. J., Danscher, G. & Frederickson, C. J. (2000) Brain Res. 852, 274–278. [DOI] [PubMed] [Google Scholar]
- 14.Chausmer, A. B. (1998) J. Am. Coll. Nutr. 17, 109–115. [DOI] [PubMed] [Google Scholar]
- 15.Henshall, S. M., Afar, D. E. H., Rasiah, K. K., Horvath, L. G., Gish, K., Caras, I., Ramakrishnan, V., Wong, M., Jeffry, U., Kench, J. G., et al. (2003) Oncogene 22, 6005–6012. [DOI] [PubMed] [Google Scholar]
- 16.MacDiarmid, C. W., Milanick, M. A. & Eide, D. J. (2003) J. Biol. Chem. 278, 15065–15072. [DOI] [PubMed] [Google Scholar]
- 17.Tsien, R. W. & Tsien, R. Y. (1990) Annu. Rev. Cell Biol. 6, 715–760. [DOI] [PubMed] [Google Scholar]
- 18.Godwin, H. A. & Berg, J. M. (1996) J. Am. Chem. Soc. 118, 6514–6515. [Google Scholar]
- 19.Walkup, G. K. & Imperiali, B. (1996) J. Am. Chem. Soc. 118, 3053–3054. [Google Scholar]
- 20.Shults, M. D., Pearce, D. A. & Imperiali, B. (2003) J. Am. Chem. Soc. 125, 10591–10597. [DOI] [PubMed] [Google Scholar]
- 21.Thompson, R. B., Cramer, M. L., Bozym, R. & Fierke, C. A. (2002) J. Biomed. Optics 7, 555–560. [DOI] [PubMed] [Google Scholar]
- 22.Barondeau, D. P., Kassman, C. J., Tainer, J. A. & Getzoff, E. D. (2002) J. Am. Chem. Soc. 124, 3522–3524. [DOI] [PubMed] [Google Scholar]
- 23.Walkup, G. K., Burdette, S. C., Lippard, S. J. & Tsien, R. Y. (2000) J. Am. Chem. Soc. 122, 5644–5645. [DOI] [PubMed] [Google Scholar]
- 24.Burdette, S. C., Walkup, G. K., Spingler, B., Tsien, R. Y. & Lippard, S. J. (2001) J. Am. Chem. Soc. 123, 7831–7841. [DOI] [PubMed] [Google Scholar]
- 25.Burdette, S. C. & Lippard, S. J. (2001) Coord. Chem. Rev. 216–217, 333–361. [Google Scholar]
- 26.Chang, C. J., Nolan, E. M., Jaworski, J., Burdette, S. C., Sheng, M. & Lippard, S. J. (2004) Chem. Biol., in press. [DOI] [PubMed]
- 27.Gee, K. R., Zhou, Z. L., Ton-That, D., Sensi, S. L. & Weiss, J. H. (2002) Cell Calcium 31, 245–251. [DOI] [PubMed] [Google Scholar]
- 28.Hirano, T., Kikuchi, K., Urano, Y. & Nagano, T. (2002) J. Am. Chem. Soc. 124, 6555–6562. [DOI] [PubMed] [Google Scholar]
- 29.Lim, N. C., Yao, L., Freake, H. C. & Brückner, C. (2003) Bioorg. Med. Chem. Lett. 13, 2251–2254. [DOI] [PubMed] [Google Scholar]
- 30.Kimber, M. C., Mahadevan, I. B., Lincoln, S. F., Ward, A. D. & Tiekink, E. R. T. (2000) J. Org. Chem. 65, 8204–8209. [DOI] [PubMed] [Google Scholar]
- 31.Burdette, S. C., Frederickson, C. J., Bu, W. & Lippard, S. J. (2003) J. Am. Chem. Soc. 125, 1778–1787. [DOI] [PubMed] [Google Scholar]
- 32.Gee, K. R., Zhou, Z.-L., Qian, W.-J. & Kennedy, R. (2002) J. Am. Chem. Soc. 124, 776–778. [DOI] [PubMed] [Google Scholar]
- 33.Maruyama, S., Kikuchi, K., Hirano, T., Urano, Y. & Nagano, T. (2002) J. Am. Chem. Soc. 124, 10650–10651. [DOI] [PubMed] [Google Scholar]
- 34.Henary, M. M. & Fahrni, C. J. (2002) J. Phys. Chem. A 106, 5210–5220. [Google Scholar]
- 35.Taki, M., Wolford, J. L. & O'Halloran, T. V. (2004) J. Am. Chem. Soc. , in press. [DOI] [PubMed]
- 36.Woodroofe, C. C. & Lippard, S. J. (2003) J. Am. Chem. Soc. 125, 11458–11459. [DOI] [PubMed] [Google Scholar]
- 37.Pangborn, A. B., Giardello, M. A., Grubbs, R. H., Rosen, R. K. & Timmers, F. J. (1996) Organometallics 15, 1518–1520. [Google Scholar]
- 38.Brannon, J. H. & Magde, D. (1978) J. Phys. Chem. 82, 705–709. [Google Scholar]
- 39.Qian, W.-J., Gee, K. R. & Kennedy, R. T. (2003) Anal. Chem. 75, 3468–3475. [DOI] [PubMed] [Google Scholar]
- 40.Kröncke, K.-D. & Kolb-Bachofen, V. (1999) Methods Enzymol. 301, 126–135. [DOI] [PubMed] [Google Scholar]
- 41.Smith, G. A., Metcalfe, J. C. & Clarke, S. D. (1993) J. Chem. Soc. Perkin Trans. 2, 1195–1204. [Google Scholar]
- 42.Haugland, R. P. (2002) Handbook of Fluorescent Probes and Research Products (Molecular Probes, Eugene, OR), 9th Ed.
- 43.Spahl, D. U., Berendji-Grün, D., Suschek, C. V., Kolb-Bachofen, V. & Kröncke, K.-D. (2003) Proc. Natl. Acad. Sci. USA 100, 13952–13957. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Gryglewski, R. J. & Minuz, P., eds. (2000) Nitric Oxide: Basic Research and Clinical Applications (IOS Press, Amsterdam), NATO Science Series, Vol. 317.
- 45.Gu, Z., Kaul, M., Yan, B., Kridel, S. J., Cui, J., Strongin, A., Smith, J. W., Liddington, R. C. & Lipton, S. A. (2002) Science 297, 1186–1190. [DOI] [PubMed] [Google Scholar]
- 46.Berendji, D., Kolb-Bachofen, V., Meyer, K. L., Grapenthin, O., Weber, H., Wahn, V. & Kröncke, K.-D. (1997) FEBS Lett. 405, 37–41. [DOI] [PubMed] [Google Scholar]
- 47.Chen, Y., Irie, Y., Keung, W. M. & Maret, W. (2002) Biochemistry 41, 8360–8367. [DOI] [PubMed] [Google Scholar]
- 48.Pearce, L. L., Wasserloos, K., Croix, C. M. S., Gandley, R., Levitan, E. S. & Pitt, B. R. (2000) J. Nutr. 130, 1467S–1470S. [DOI] [PubMed] [Google Scholar]
- 49.Kröncke, K.-D., Fehsel, K., Schmidt, T., Zenke, F. T., Dasting, F., Wesener, J. R., Bettermann, H., Breunig, K. D. & Kolb-Bachofen, V. (1994) Biochem. Biophys. Res. Commun. 200, 1105–1110. [DOI] [PubMed] [Google Scholar]