Background: TCR-induced NF-κB activation is crucial for T cell activation.
Results: PKCδ inhibited TCR-induced NF-κB activation by a mechanism independent of its kinase activity.
Conclusion: PKCδ negatively regulates T cell activation by inhibiting the assembly of CARMA1 signalosome.
Significance: It provides a novel mechanism on negative regulation of T cell activation.
Keywords: Immunology, NF-κB (NF-κB), Protein Kinase C (PKC), Signal Transduction, T Cell, CARMA1, T Cell Activation, Negative Regulation
Abstract
T-cell receptor (TCR)-induced T-cell activation is a critical event in adaptive immune responses. The engagement of TCR complex by antigen along with the activation of the costimulatory receptors trigger a cascade of intracellular signaling, in which caspase recruitment domain-containing membrane-associated guanylate kinase 1 (CARMA1) is a crucial scaffold protein. Upon stimulation, CARMA1 recruits downstream molecules including B-cell CLL/lymphoma 10 (Bcl10), mucosa-associated lymphoid tissue lymphoma translocation gene 1 (MALT1), and TRAF6 to assemble a specific TCR-induced signalosome that triggers NF-κB and JNK activation. In this report, we identified protein kinase Cδ (PKCδ) as a CARMA1-associated protein by a biochemical affinity purification approach. PKCδ interacted with CARMA1 in TCR stimulation-dependent manner in Jurkat T cells. Overexpression of PKCδ inhibited CARMA1-mediated NF-κB activation, whereas knockdown of PKCδ potentiated TCR-triggered NF-κB activation and IL-2 secretion in Jurkat T cells. Reconstitution experiments with PKCδ kinase-dead mutant indicated that the kinase activity of PKCδ was dispensable for its ability to inhibit TCR-triggered NF-κB activation. Furthermore, we found that PKCδ inhibited the interaction between MALT1 and TRAF6, but not the association of CARMA1 with PKCθ, Bcl10, or MALT1. These observations suggest that PKCδ is a negative regulator in T cell activation through inhibiting the assembly of CARMA1 signalosome.
Introduction
TCR-induced activation of T cells plays a central role in adaptive immune responses. Activated T cells undergo clonal expansion, differentiation, and begin to execute their immunological functions either by producing cytokines for regulating immune responses or by killing target cells infected with pathogens directly. The tightly controlled activation of transcription factors of the nuclear factor-κB (NF-κB)4 family plays a crucial role in these processes (1).
TCR-induced activation of NF-κB signaling pathway is initiated from the interaction of TCR with specific antigen in the context of MHC and costimulation of CD28 by its ligands on antigen-presenting cells. Upon stimulation, a cascade of tyrosine phosphorylation of signaling components is successively triggered, which results in the recruitment and activation of a number of signaling proteins, including phosphoinositide-dependent kinase 1 (PDK1) (2). PDK1 phosphorylates PKCθ and recruits it to lipid rafts in the plasma membrane (3). Concurrently PDK1 recruits CARMA1 to lipid rafts. Phosphorylated PKCθ phosphorylates the close-by CARMA1 at specific serine residues, and leads to the conformational changes of CARMA1 from an inactive form to an active one (4–6). Active CARMA1 assembles a CBM complex by associating with Bcl10, which in turn recruits MALT1 (7, 8). The CBM complex subsequently recruits downstream signal proteins, such as TRAF6, caspase 8, and TAK1, to collaboratively activate the inhibitor of κB kinase (IKK) complex. IκB is then phosphorylated and undergoes degradation, which leads to the activation of NF-κB.
Among the CBM signalosome, the scaffold protein CARMA1 is crucial for the recruitment of downstream signal proteins, as well as their plasma membrane localization (9–11). Its activity requires fine tuning to elicit proper activation of NF-κB. Posttranscriptional modifications of CARMA1, such as phosphorylation and ubiquitination, determine the outcome of TCR stimulation. For examples, kinases such as PKCθ, IKKβ, Ca2+/calmodulin-dependent protein kinase II, and hematopoietic progenitor kinase 1 can phosphorylate CARMA1 at different serine residues in the PKC-regulated domain (PRD), and subsequently lead to NF-κB activation (4, 5, 12–14). However, casein kinase 1α phosphorylates CARMA1 at S608, which impairs its ability to activate NF-κB (15). In addition, monoubiquitination of CARMA1 catalyzed by Cbl-b disrupts the formation of CBM complex (16). Although much has been reported, the subtle regulation of CARMA1 signalosome remains not so clear thus far.
To identify potential regulators of CARMA1-mediated NF-κB activation, we performed biochemical affinity purification with CARMA1 as a bait protein, and identified PKCδ as a protein specifically associated with CARMA1. Knockdown of PKCδ accelerated TCR-induced NF-κB activation and IL-2 secretion, suggesting an inhibitory role of PKCδ in TCR-mediated NF-κB activation. Furthermore, we demonstrated that the kinase activity of PKCδ was not required for its inhibitory role. Instead, PKCδ inhibited TCR-induced NF-κB activation by steric hindrance of recruiting TRAF6 to CARMA1 signalosome.
EXPERIMENTAL PROCEDURES
Reagents
The Abs against the following antigens were purchased from the indicated companies: human CD3, human CD28, and mouse IgG1 (BD Biosciences); human IκBα and phospho-IκBα (S32, S36) (Cell Signaling Technology); human PKCδ (Santa Cruz Biotechnology); human β-actin, Flag, and hemagglutinin (HA) epitopes (Sigma-Aldrich). Mouse antisera were raised against recombinant human CARMA1 (aa 1–468), Bcl10 or MALT1 (aa 348–813) respectively. Human IL-2 ELISA kit (Dake Biotechnology), dual-luciferase reporter assay kit (Promega), and Gamma Bind G Plus-Sepharose (GE Healthcare Life Sciences) were purchased from the indicated companies.
Constructs
NF-κB luciferase reporter plasmid was provided by Dr. Gary Johnson. Mammalian expression plasmids for human CARMA1 and its mutants, PKCδ and its mutants, PKCθ, Bcl10, or MALT1, fused with Flag-, HA-, or RFP-epitope, were constructed by standard molecular biology techniques. Double-stranded oligonucleotides for RNA interference corresponding to the target sequences were inserted into pSUPER. Retro vector (Oligoengine) according to protocols recommended by the manufacturer. The target sequence for human PKCδ-siRNA #1 is 5′-AAACACTGGTGCAGAAGAA-3′; for PKCδ-siRNA #3 is 5′-GCAGCAAGTGCAACATCAA-3′.
Protein Purification and Mass Spectrometry Analysis
HEK293 cells (5 × 108) stably transfected with pCTAP-CARMA1 (Stratagene) were collected and the cell lysates were subjected to tandem affinity purification procedures. The purified CARMA1-associated proteins were digested by trypsin in solution. The tryptic peptides were analyzed by HPLC-ESI/MS/MS with a Thermo Finnigan LTQ adapted for nanospray ionization. The tandem spectra were searched against Homo sapiens National Center for Biotechnology Information reference data base using SEQUEST. Results was filtered by Xcorr +1 > 1.9, +2 > 2.2, +3 > 3.5, sp >500, Deltcn >0.1, Rsp < = 5.
Transfection and Reporter Assays
HEK293 cells were transfected with mammalian expression plasmids by standard calcium phosphate precipitation. Jurkat T cells were transfected with siRNAs or expression plasmids by retroviral transduction. For luciferase reporter assays, HEK293 cells (1 × 105) were seeded on 24-well dishes and transfected the next day. In the same experiment, we added empty control plasmid to ensure that each transfection received the same amount of total DNA. To normalize the transfection efficiency, 0.01 μg pRL-TK (Renilla luciferase) reporter plasmid was added to each transfection. Luciferase assays were performed with a dual-specific luciferase assay kit. Firefly luciferase activities were normalized on the basis of Renilla luciferase activities. All reporter assays were repeated for at least three times. Data shown were average values ± S.D. from one representative experiment.
Cell Sorting by FACS
Constructs of MIGR-GFP-PKCδ-WT (wild type) or MIGR-GFP-PKCδ-KD (kinase-dead mutant, K378A) were transfected into PKCδ-knockdown Jurkat T cells (18). Forty-eight hours later, GFP-positive cells were isolated based on fluorescence intensity using the MoFlo XDP Sorter (Beckman Coulter). Approximately 1 × 105 cells of each sample with >95% purity were obtained and cultured for further experiments.
Coimmunoprecipitation and Immunoblot Analysis
For transient transfection and coimmunoprecipitation experiments, HEK293 cells (2 × 106) were transfected for 20 h. The transfected cells were lysed in Nonidet P-40 lysis buffer (20 mm Tris, 150 mm NaCl, 1% Nonidet P-40, 1 mm EDTA, pH 7.5) supplemented with proteases inhibitors (10 μg/ml aprotinin, 10 μg/ml leupeptin, 1 mm phenylmethylsulfonyl fluoride). For each immunoprecipitation, 0.4 ml aliquot of lysates was incubated with 0.5 μg of antibody or control IgG and 25 μl of GammaBind G Plus-Sepharose at 4 °C for 2 h. Sepharose beads were washed three times with 1 ml lysis buffer containing 0.5 m NaCl. The precipitates were analyzed by standard immunoblotting procedures.
For endogenous coimmunoprecipitation experiments, Jurkat T cells (5 × 107) were costimulated by mouse anti-human CD3 (10 μg/ml), mouse anti-human CD28 (10 μg/ml), and 10 μg/ml goat anti-mouse IgG for crosslinking at 37 °C for the indicated times. Cells were then lysed and subjected to coimmunoprecipitation and immunoblot analysis as described above.
ELISA
IL-2 production in the culture medium of Jurkat cells was measured after CD3/CD28 crosslinking for 48 h using a human IL-2 ELISA kit.
RESULTS
Identification of PKCδ as a CARMA1-associated Protein
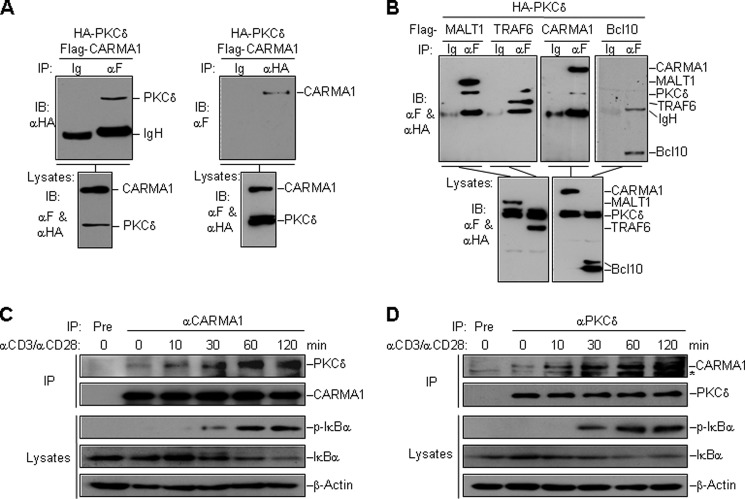
CARMA1 plays a central role in signaling to NF-κB activation by TCR or BCR engagement. For a better understanding of signaling events in this pathway, we purified CARMA1-associated proteins by a tandem affinity purification system, and identified the eluted proteins by a shotgun mass spectrometry analysis method. By comparing with other unrelated purifications using the same method, we identified several candidates, including PKCδ, as specific CARMA1-associated proteins. Coimmunoprecipitation results confirmed the interaction between ectopic PKCδ and CARMA1 in HEK293 cells (Fig. 1A). Besides, PKCδ also interacted with MALT1 and TRAF6, but not Bcl10 (Fig. 1B).
FIGURE 1.
Identification of PKCδ as a CARMA1-associated protein. A, interaction of overexpressed HA-PKCδ and Flag-CARMA1. Expression plasmids encoding HA-PKCδ (10 μg) and Flag-CARMA1 (4 μg) were transfected into HEK293 cells. Lysates were immunoprecipitated with antibodies to Flag (left panel), HA (right panel), or control IgG, and then analyzed by immunoblotting with an antibody to HA (upper left panel) or Flag (upper right panel). Expression of the transfected proteins were analyzed by immunoblotting with antibodies to Flag and HA (lower panel). B, PKCδ interacts with MALT1 and TRAF6 but not Bcl10. HEK293 cells were transfected with the indicated plasmids. Coimmunoprecipitation and immunoblot analysis were performed with the indicated antibodies. C and D, CD3/CD28 costimulation induces the association between PKCδ and CARMA1. Jurkat T cells were stimulated by CD3/CD28 crosslinking for the indicated time. Lysates were immunoprecipitated with an antibody to CARMA1 (C) or PKCδ (D). The precipitates and lysates were analyzed by immunoblotting with the indicated antibodies. Ig, control mouse IgG; αF, anti-Flag; Pre, preimmune serum; *, nonspecific band.
To determine whether CARMA1 and PKCδ interact in untransfected cells, we stimulated Jurkat T cells by crosslinking CD3 and CD28 with antibodies, and performed endogenous coimmunoprecipitation. The results showed that endogenous PKCδ was recruited to CARMA1 after CD3/CD28 costimulation (Fig. 1, C and D). Immunofluorescent confocal microscopy also indicated that a population of PKCδ was translocated to the plasma membrane upon TCR stimulation (supplemental Fig. S1).
PKCδ Negatively Regulates TCR-induced Activation of NF-κB
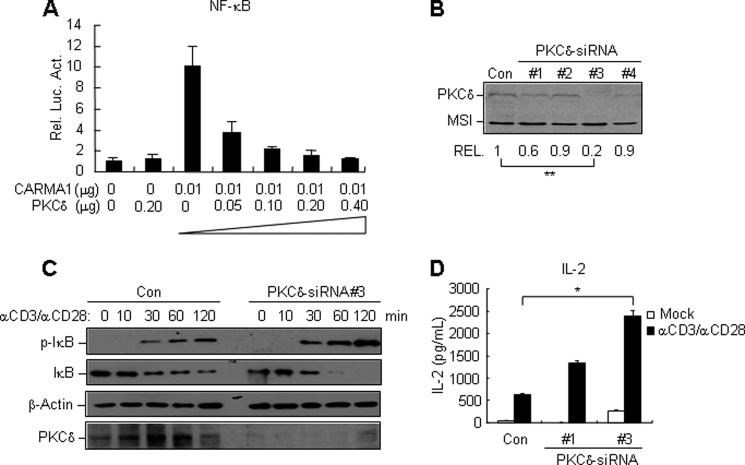
To address whether PKCδ is involved in CARMA1-dependent NF-κB activation, we performed NF-κB reporter assays. Overexpression of PKCδ alone had no marked effect on the basal level of NF-κB activation. However, when cotransfected with CARMA1, PKCδ inhibited CARMA1-induced NF-κB activation in a dose-dependent manner (Fig. 2A).
FIGURE 2.
PKCδ down-regulates TCR-induced activation of NF-κB. A, PKCδ dose-dependently inhibits CARMA1-induced NF-κB activation in reporter assays. HEK293 cells were transfected with the indicated mammalian expression plasmids and an NF-κB reporter plasmid for 18 h, luciferase assays were performed 16 h after transfection. B, effects of PKCδ-siRNAs on the expression of PKCδ. The PKCδ-siRNA or control siRNA (green fluorescent protein- siRNA) plasmids, together with Flag-PKCδ and Flag-MSI constructs, were transfected into HEK293 cells. Twenty-four hours after transfection, cell lysates were analyzed by immunoblotting with an anti-Flag antibody. The PKCδ bands from three independent experiments were quantitated using the Bio-Rad Quantity One Program and normalized by levels of the control protein MSI. The average levels of PKCδ are shown at the bottom of the blot. **, p < 0.01, n = 3. C, knockdown of PKCδ potentiates TCR-induced activation of NF-κB. Jurkat T cells stably transfected with PKCδ-siRNA #3 or control siRNA were stimulated by CD3/CD28 crosslinking for the indicated times. The cell lysates were analyzed by immunoblotting with the indicated antibodies. D, knockdown of PKCδ enhances IL-2 secretion upon CD3/CD28 costimulation. Jurkat T cells stably transfected with PKCδ-siRNA #1, #3, or control siRNA were stimulated with medium (mock) or CD3/CD28 crosslinking for 48 h. The culture medium was collected and subjected to ELISA. The average levels of IL-2 from three independent experiments are shown as mean ± S.D., n = 3. *, p < 0.05.
To evaluate the role of PKCδ in TCR-induced NF-κB signaling, we used RNAi to knockdown PKCδ in human Jurkat T cells. We made four PKCδ siRNA constructs, and determined their effects on knockdown of PKCδ. As shown in Fig. 2B, the #3 PKCδ-siRNA construct could inhibit the expression of transiently transfected PKCδ to 20% of the control sample (p < 0.01). The #1 siRNA constructs could reduce PKCδ level to 60% of the control sample, whereas #2 and #4 siRNA plasmid had minimal effects. We thus chose #3 and #1 siRNA constructs for further experiments.
We generated stable Jurkat T cells expressing PKCδ-siRNAs by retroviral transduction. Comparing with the controls, PKCδ-knockdown Jurkat T cells exhibited enhanced phosphorylation and degradation of IκB upon the stimulation of CD3/CD28 crosslinking (Fig. 2C). Consistently, knockdown of PKCδ potentiated the production of IL-2, which is a pro-inflammatory cytokine induced by NF-κB activation (Fig. 2D). The extent of IL-2 enhancement was correlated with the efficiency of PKCδ knockdown (Fig. 2D). These evidences suggest that PKCδ itself plays a negative role in TCR-induced activation of NF-κB.
The Kinase Activity of PKCδ Is Not Required for Its Inhibitory Role in TCR-induced NF-κB Activation
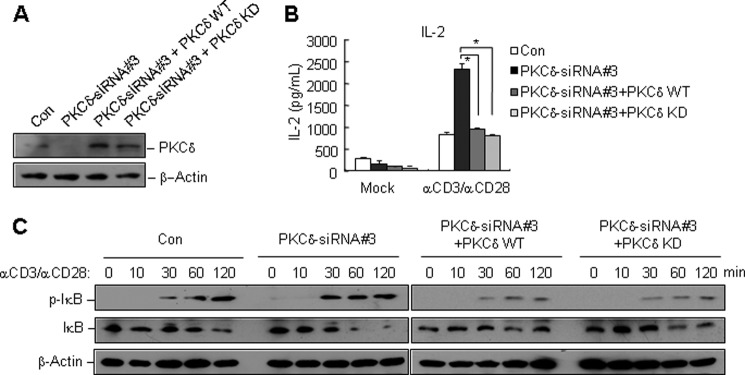
It has been reported that upon TCR ligation, PKCδ is phosphorylated and regulates granule exocytosis in a kinase-dependent manner (17). Therefore, we tested whether the kinase activity of PKCδ was required for TCR-induced NF-κB activation. We reconstituted PKCδ-knockdown Jurkat T cells with PKCδ-wild type (WT) or PKCδ-kinase dead (KD) mutant (18). The reconstitutions were confirmed by immunoblotting (Fig. 3A). The reconstituted cells, PKCδ-knockdown cells and controls were stimulated by CD3/CD28 crosslinking, and then the culture medium of each sample was collected to measure IL-2 production, while the cell lysates were subjected to immunoblots to detect NF-κB activation. The results showed that the ectopic expression of both WT and KD PKCδ reversed the promotion of IL-2 production and IκB phosphorylation and degradation in PKCδ-knockdown cells (Fig. 3, B and C), indicating that the kinase activity was dispensable for the inhibitory role of PKCδ in TCR-induced NF-κB activation.
FIGURE 3.
The kinase activity of PKCδ is dispensable for its inhibitory role in TCR-induced activation of NF-κB. A, PKCδ-knockdown Jurkat T cells were transfected with MIGR-GFP-PKCδ-WT (wild type) or MIGR-GFP-PKCδ-KD (kinase-dead) mutant by retroviral transduction. Seventy-two hours later, reconstituted cells were sorted by FACS. The efficiency of reconstitution was examined by immunoblotting with an antibody to PKCδ or β-actin. B and C, PKCδ-knockdown Jurkat T cells, reconstituted Jurkat T cells with PKCδ WT or KD mutants, and control cell lines were stimulated by CD3/CD28 crosslinking. The culture medium was collected and subjected to ELISA for measuring the production of IL-2 (B). The cell lysates were applied to immunoblots with indicated antibodies (C).
PKCδ Is Associated with the MAGUK Region of CARMA1, and Has No Marked Effect on the Interaction between CARMA1 and PKCθ
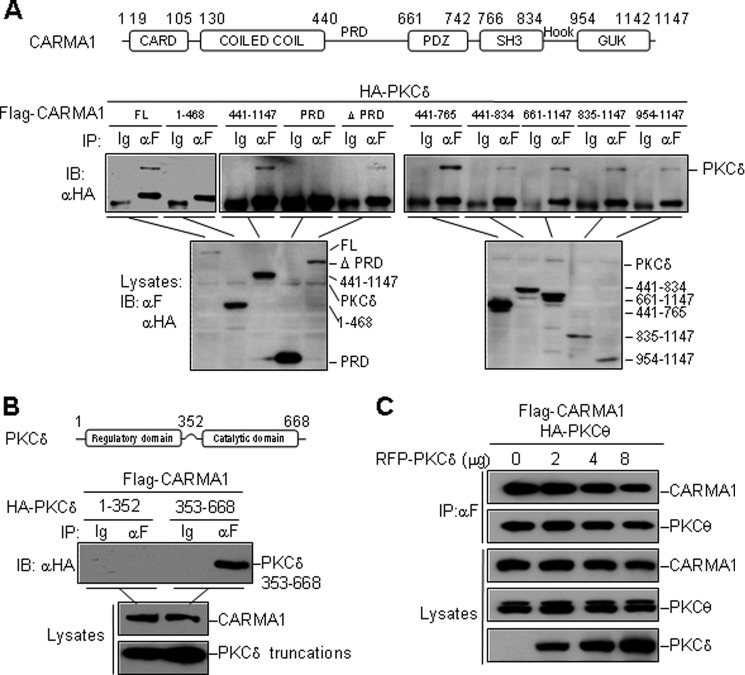
The N terminus of CARMA1 contains a CARD domain and a coiled-coil domain. The C-terminal region contains a MAGUK-homology structure, which is composed of postsynaptic density 95/dislarge/zona occludens 1 (PDZ), Src homology 3 (SH3), and guanylate kinase (GUK) domains (10). PKCθ, a novel PKC isoform, associates with the PRD region of CARMA1, which links the coiled-coil domain and PDZ domain. PKCθ phosphorylates CARMA1 at Ser-552 and Ser-645 in PRD, which is essential for TCR-induced NF-κB activation (4, 5, 7). PKCδ, as another novel PKC isoform, shares 85% in catalytic domain and 62.5% in overall protein sequence homology with PKCθ, thus it may interfere with the interaction between CARMA1 and PKCθ through competitive binding. However, domain-mapping experiments suggested that PKCδ interacted with all the domains consisted in the MAGUK region of CARMA1 but not PRD region (Fig. 4A). CARMA1 associated with the catalytic domain (aa 353–668) of PKCδ, but not the regulatory domain (aa 1–352) (Fig. 4B). As an unexpected result, PKCδ had no marked effect on the CARMA1-PKCθ interaction, determined by competitive coimmunoprecipitation assays (Fig. 4C).
FIGURE 4.
PKCδ interacts with MAGUK region of CARMA1 and has no marked effect on CARMA1-PKCθ interaction. A, mapping of the minimal PKCδ-interaction region in CARMA1. Transfection, immunoprecipitation and immunoblot assays were performed as described before. B, CARMA1 interacts with the catalytic domain of PKCδ (aa 353–668). C, PKCδ has no marked effect on CARMA1-PKCθ interaction. Flag-CARMA1 (4 μg), HA-PKCθ (8 μg), and RFP-PKCδ (0–8 μg as indicated) were transfected into HEK293 cells. Lysates were immunoprecipitated with an anti-Flag antibody and then analyzed by immunoblotting with the indicated antibodies (upper panel). Expression of the transfected proteins were detected by immunoblotting with antibodies to Flag and HA (lower panel).
PKCδ Inhibits the Interaction between MALT1 and TRAF6
We next investigated whether PKCδ acted through inhibiting the assembly of CARMA1 signalosome. The competitive coimmunoprecipitation experiments showed that PKCδ had no marked effect on the interactions between CARMA1-Bcl10, CARMA1-MALT1, or Bcl10-MALT1 (Fig. 5A). In contrast, PKCδ inhibited the association between MALT1 and TRAF6 in a dose-dependent manner (Fig. 5A).
FIGURE 5.
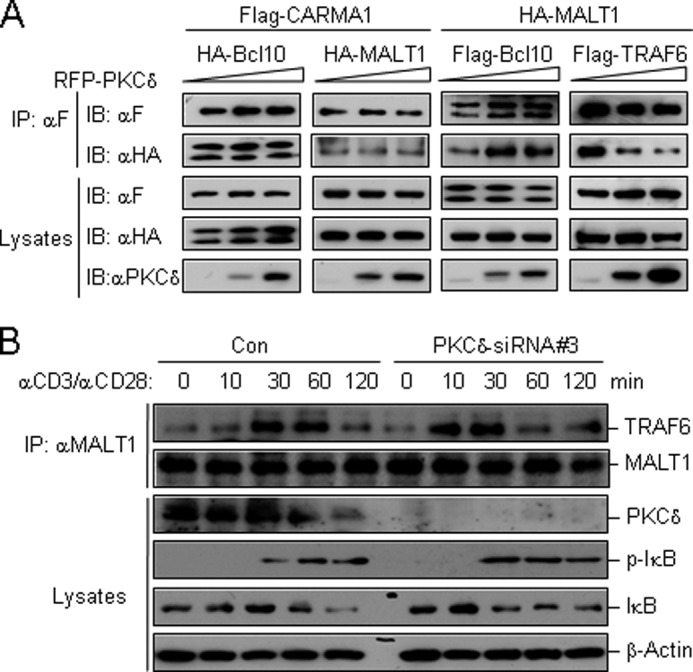
PKCδ inhibits the association of MALT1 with TRAF6. A, PKCδ disrupts the interaction of MALT1-TRAF6, but not CARMA1-Bcl10, CARMA1-MALT1, and Bcl10-MALT1. The expression plasmids were cotransfected in HEK293 cells as indicated. Immunoprecipitation and immunoblots were performed using the indicated antibodies. B, knockdown of PKCδ by siRNA accelerates the recruitment of TRAF6 to MALT1 upon CD3/CD28 costimulation. PKCδ-knockdown Jurkat T cells and controls (5 × 107 each) were stimulated by CD3/CD28 crosslinking for the indicated time. Cell lysates were subjected to immunoprecipitation with an antibody to MALT1. Immunoprecipitates and lysates were immunoblotted using the indicated antibodies.
We further performed endogenous coimmunoprecipitation experiments on PKCδ-knockdown Jurkat T cells or control cells to confirm the effect of PKCδ on MALT1-TRAF6 interaction. The results showed that TRAF6 in controls was associated with MALT1 moderately after CD3/CD28 crosslinking, peaked at 30 min, and became weaker after 60 min. While in PKCδ-knockdown cells, obviously more TRAF6 was associated with MALT1 after CD3/CD28 crosslinking. The association peaked at 10 min, and became weaker after 30 min (Fig. 5B). These results suggested that PKCδ inhibited the assembly of CARMA1 signalosome by interfering the association between MALT1 and TRAF6.
DISCUSSION
In this study, we investigated the role of PKCδ in NF-κB activation in T cells and reported several observations. First, PKCδ participates in the negative control of TCR-induced NF-κB activation. Second, the kinase activity of PKCδ is dispensable for the negative regulation. Third, PKCδ is recruited to CARMA1 upon TCR stimulation, and inhibits the subsequent recruitment of TRAF6 by MALT1.
T lymphocytes express multiple PKC isoforms, among which PKCθ is considered as the most important isoform participating in T cell activation (19). It is essential for CARMA1 phosphorylation and further NF-κB activation. It also phosphorylates RapGEF2 and enhances T cell adhesion (20). Although PKCθ was reported to be the only PKC isoform translocated to immunological synapse after TCR stimulation (21–23), more evidences suggest that other PKCs, such as PKCα, also translocate to the plasma membrane upon TCR stimulation and possibly participate in T cell activation (24). Besides, PKCζ and PKCι are PKCθ-associated PKCs in T cells, and possibly assist in PKCθ-mediated signaling (25). In contrast to these PKCs, PKCδ is reported to serve a negative role in TCR-induced IL-2 cytokine production and T cell proliferation (26). However, the mechanism of this negative regulation is not clear so far.
By identifying the CARMA1-associated proteins, we found that PKCδ dynamically interacted with CARMA1 and was translocated to the plasma membrane in a TCR stimulation-dependent manner. It has been shown that following TCR ligation, PKCδ is rapidly phosphorylated at Thr-505 on the activation loop (17, 27). This phosphorylation leads to the activation of PKCδ, which regulates lytic granule exocytosis in CTLs in a kinase-dependent manner (17). In this study, however, we found that the kinase activity of PKCδ was not required for its regulation of TCR-induced NF-κB activation, because the promotion of either IκB phosphorylation or IL-2 production in PKCδ-knockdown Jurkat T cells could be reversed by reconstitution with kinase-dead mutant of PKCδ, similar to wild-type PKCδ. These surprising findings suggest that negative regulation of TCR-signaling by PKCδ may be mediated through steric hindrance of the assembly of CARMA1 signalosome.
Although PKCδ and PKCθ share high protein sequence homology, they are associated with different region of CARMA1: PKCδ interacts with the MAGUK region, and PKCθ interacts with the PRD region. These findings indicated that PKCδ did not function through competitive binding to CARMA1 with PKCθ, which was confirmed by our competitive binding assays. Upon TCR stimulation, phosphorylated CARMA1 forms a CBM complex with Bcl10 and MALT1. Then MALT1 directly recruits TRAF6 and TAK1 into the CARMA1 signalosome (28, 29). TRAF6 functions as an E3 ligase to catalyze the K63-linked polyubiquitination of MALT1, Bcl10, and IKKγ, and thus promotes signaling to NF-κB activation (28, 30). Our results showed that PKCδ had no apparent effect on the association of CARMA1 with PKCθ, Bcl10, or MALT1. Instead, PKCδ inhibited the interaction between MALT1 and TRAF6 in a dose-dependent manner, whereas knockdown of PKCδ enhanced the TCR-induced recruitment of endogenous TRAF6 to MALT1. These results provide an explanation for the inhibitory role of PKCδ in TCR-induced NF-κB activation: PKCδ is recruited to CARMA1 signalosome upon TCR stimulation and inhibits the recruitment of essential downstream signal components, such as TRAF6.
PKCδ-deficient mice were reported to be susceptible to autoimmune disease, indicating that PKCδ plays a regulatory role in immune systems. Although the development of immune organs in PKCδ-deficient mice appears to be normal, cultured PKCδ-deficient lymphocytes, both B and T cells, display enhanced proliferation in response to various mitogenic stimuli (26, 31). Since signal transduction from antigen receptors in both B and T cells to NF-κB shares the same downstream signaling events through CARMA1-Bcl10-MALT1 complex, our results might also explain the enhanced proliferation of PKCδ-deficient B cells. Taken together, our findings suggest that PKCδ negatively regulates TCR-induced NF-κB activation and IL-2 production by inhibiting the assembly of CARMA1 signalosome.
Acknowledgments
We thank Prof. Youjia Cao in Nankai University and Prof. Yongsheng Chang in Institute of Basic Medical Sciences in Peking Union Medical College for providing Jurkat T cells and phenix cells. We thank Dr. Fuquan Yang and Peng Xue from the Institute of Biophysics at Chinese Academy of Sciences and Dr. Ying Li from our laboratory for help with mass spectrometry.
This work was supported by grants from the National Natural Science Foundation of China (30700417, 30972719, and 30921001), and the Ministry of Science and Technology of China (2012CB910201).

This article contains supplemental Fig. S1.
- NF-κB
- nuclear factor-κB
- TCR
- T-cell receptor
- IKK
- inhibitor of κB kinase
- CARMA
- caspase recruitment domain-containing membrane-associated guanylate kinase
- PDK
- phosphoinositide-dependent kinase.
REFERENCES
- 1. Jun J. E., Goodnow C. C. (2003) Scaffolding of antigen receptors for immunogenic versus tolerogenic signaling. Nat. Immunol. 4, 1057–1064 [DOI] [PubMed] [Google Scholar]
- 2. van Oers N. S., Chen Z. J. (2005) Cell biology. Kinasing and clipping down the NF-κB trail. Science 308, 65–66 [DOI] [PubMed] [Google Scholar]
- 3. Lee K. Y., D'Acquisto F., Hayden M. S., Shim J. H., Ghosh S. (2005) PDK1 nucleates T cell receptor-induced signaling complex for NF-κB activation. Science 308, 114–118 [DOI] [PubMed] [Google Scholar]
- 4. Matsumoto R., Wang D., Blonska M., Li H., Kobayashi M., Pappu B., Chen Y., Wang D., Lin X. (2005) Phosphorylation of CARMA1 plays a critical role in T cell receptor-mediated NF-κB activation. Immunity 23, 575–585 [DOI] [PubMed] [Google Scholar]
- 5. Sommer K., Guo B., Pomerantz J. L., Bandaranayake A. D., Moreno-García M. E., Ovechkina Y. L., Rawlings D. J. (2005) Phosphorylation of the CARMA1 linker controls NF-κB activation. Immunity 23, 561–574 [DOI] [PubMed] [Google Scholar]
- 6. Rueda D., Thome M. (2005) Phosphorylation of CARMA1: the link(er) to NF-κB activation. Immunity 23, 551–553 [DOI] [PubMed] [Google Scholar]
- 7. Wang D., Matsumoto R., You Y., Che T., Lin X. Y., Gaffen S. L., Lin X. (2004) CD3/CD28 costimulation-induced NF-κB activation is mediated by recruitment of protein kinase C-θ, Bcl10, and IκB kinase β to the immunological synapse through CARMA1. Mol. Cell Biol. 24, 164–171 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lucas P. C., Yonezumi M., Inohara N., McAllister-Lucas L. M., Abazeed M. E., Chen F. F., Yamaoka S., Seto M., Nunez G. (2001) Bcl10 and MALT1, independent targets of chromosomal translocation in malt lymphoma, cooperate in a novel NF-κB signaling pathway. J. Biol. Chem. 276, 19012–19019 [DOI] [PubMed] [Google Scholar]
- 9. Thome M. (2004) CARMA1, BCL-10, and MALT1 in lymphocyte development and activation. Nat. Rev. Immunol. 4, 348–359 [DOI] [PubMed] [Google Scholar]
- 10. Rawlings D. J., Sommer K., Moreno-García M. E. (2006) The CARMA1 signalosome links the signaling machinery of adaptive and innate immunity in lymphocytes. Nat. Rev. Immunol. 6, 799–812 [DOI] [PubMed] [Google Scholar]
- 11. Blonska M., Lin X. (2009) CARMA1-mediated NF-κB and JNK activation in lymphocytes. Immunol. Rev. 228, 199–211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Shinohara H., Maeda S., Watarai H., Kurosaki T. (2007) IκB kinase β-induced phosphorylation of CARMA1 contributes to CARMA1 Bcl10 MALT1 complex formation in B cells. J. Exp. Med. 204, 3285–3293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Ishiguro K., Green T., Rapley J., Wachtel H., Giallourakis C., Landry A., Cao Z., Lu N., Takafumi A., Goto H., Daly M. J., Xavier R. J. (2006) Ca2+/calmodulin-dependent protein kinase II is a modulator of CARMA1-mediated NF-κB activation. Mol. Cell Biol. 26, 5497–5508 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Brenner D., Brechmann M., Röhling S., Tapernoux M., Mock T., Winter D., Lehmann W. D., Kiefer F., Thome M., Krammer P. H., Arnold R. (2009) Phosphorylation of CARMA1 by HPK1 is critical for NF-κB activation in T cells. Proc. Natl. Acad. Sci. U.S.A. 106, 14508–14513 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Bidère N., Ngo V. N., Lee J., Collins C., Zheng L., Wan F., Davis R. E., Lenz G., Anderson D. E., Arnoult D., Vazquez A., Sakai K., Zhang J., Meng Z., Veenstra T. D., Staudt L. M., Lenardo M. J. (2009) Casein kinase 1α governs antigen-receptor-induced NF-κB activation and human lymphoma cell survival. Nature 458, 92–96 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kojo S., Elly C., Harada Y., Langdon W. Y., Kronenberg M., Liu Y. C. (2009) Mechanisms of NKT cell anergy induction involve Cbl-b-promoted monoubiquitination of CARMA1. Proc. Natl. Acad. Sci. U.S.A. 106, 17847–17851 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Ma J. S., Haydar T. F., Radoja S. (2008) Protein kinase Cδ localizes to secretory lysosomes in CD8+ CTL and directly mediates TCR signals leading to granule exocytosis-mediated cytotoxicity. J. Immunol. 181, 4716–4722 [DOI] [PubMed] [Google Scholar]
- 18. D'Costa A. M., Denning M. F. (2005) A caspase-resistant mutant of PKC-δ protects keratinocytes from UV-induced apoptosis. Cell Death Differ. 12, 224–232 [DOI] [PubMed] [Google Scholar]
- 19. Spitaler M., Cantrell D. A. (2004) Protein kinase C and beyond. Nat. Immunol. 5, 785–790 [DOI] [PubMed] [Google Scholar]
- 20. Letschka T., Kollmann V., Pfeifhofer-Obermair C., Lutz-Nicoladoni C., Obermair G. J., Fresser F., Leitges M., Hermann-Kleiter N., Kaminski S., Baier G. (2008) PKC-theta selectively controls the adhesion-stimulating molecule Rap1. Blood 112, 4617–4627 [DOI] [PubMed] [Google Scholar]
- 21. Monks C. R., Kupfer H., Tamir I., Barlow A., Kupfer A. (1997) Selective modulation of protein kinase C-θ during T-cell activation. Nature 385, 83–86 [DOI] [PubMed] [Google Scholar]
- 22. Monks C. R., Freiberg B. A., Kupfer H., Sciaky N., Kupfer A. (1998) Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 395, 82–86 [DOI] [PubMed] [Google Scholar]
- 23. Szamel M., Appel A., Schwinzer R., Resch K. (1998) Different protein kinase C isoenzymes regulate IL-2 receptor expression or IL-2 synthesis in human lymphocytes stimulated via the TCR. J. Immunol. 160, 2207–2214 [PubMed] [Google Scholar]
- 24. Trushin S. A., Pennington K. N., Carmona E. M., Asin S., Savoy D. N., Billadeau D. D., Paya C. V. (2003) Protein kinase Cα (PKCα) acts upstream of PKCθ to activate IκB kinase and NF-κB in T lymphocytes. Mol. Cell Biol. 23, 7068–7081 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Gruber T., Fresser F., Jenny M., Uberall F., Leitges M., Baier G. (2008) PKCθ cooperates with atypical PKCζ and PKCι in NF-κB transactivation of T lymphocytes. Mol. Immunol. 45, 117–126 [DOI] [PubMed] [Google Scholar]
- 26. Gruber T., Barsig J., Pfeifhofer C., Ghaffari-Tabrizi N., Tinhofer I., Leitges M., Baier G. (2005) PKCδ is involved in signal attenuation in CD3+ T cells. Immunol. Lett 96, 291–293 [DOI] [PubMed] [Google Scholar]
- 27. Gorelik G., Fang J. Y., Wu A., Sawalha A. H., Richardson B. (2007) Impaired T cell protein kinase C δ activation decreases ERK pathway signaling in idiopathic and hydralazine-induced lupus. J. Immunol. 179, 5553–5563 [DOI] [PubMed] [Google Scholar]
- 28. Oeckinghaus A., Wegener E., Welteke V., Ferch U., Arslan S. C., Ruland J., Scheidereit C., Krappmann D. (2007) Malt1 ubiquitination triggers NF-κB signaling upon T-cell activation. EMBO J. 26, 4634–4645 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Ruland J., Duncan G. S., Wakeham A., Mak T. W. (2003) Differential requirement for Malt1 in T and B cell antigen receptor signaling. Immunity 19, 749–758 [DOI] [PubMed] [Google Scholar]
- 30. Sun L., Deng L., Ea C. K., Xia Z. P., Chen Z. J. (2004) The TRAF6 ubiquitin ligase and TAK1 kinase mediate IKK activation by BCL10 and MALT1 in T lymphocytes. Mol. Cell 14, 289–301 [DOI] [PubMed] [Google Scholar]
- 31. Miyamoto A., Nakayama K., Imaki H., Hirose S., Jiang Y., Abe M., Tsukiyama T., Nagahama H., Ohno S., Hatakeyama S., Nakayama K. I. (2002) Increased proliferation of B cells and auto-immunity in mice lacking protein kinase Cδ. Nature 416, 865–869 [DOI] [PubMed] [Google Scholar]