Background: The role of AMPK and PKCs as effectors of metformin action on glucose uptake (GU) in skeletal muscle cells was investigated.
Results: Genetic loss/silencing of AMPK led to only a small repression in metformin-stimulated GU. Novel/conventional, but not atypical, PKCs support metformin-induced stimulation of GU.
Conclusion: Metformin enhances GU by a mechanism largely independent of AMPK.
Significance: Metformin can act via non-AMPK pathways to promote GU.
Keywords: Glucose Transport, Insulin, Insulin Resistance, Metabolic Regulation, Skeletal Muscle
Abstract
The importance of AMP-activated protein kinase (AMPK) and protein kinase C (PKC) as effectors of metformin (Met) action on glucose uptake (GU) in skeletal muscle cells was investigated. GU in L6 myotubes was stimulated 2-fold following 16 h of Met treatment and acutely enhanced by insulin in an additive fashion. Insulin-stimulated GU was sensitive to PI3K inhibition, whereas that induced by Met was not. Met and its related biguanide, phenformin, stimulated AMPK activation/phosphorylation to a level comparable with that induced by the AMPK activator, 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide (AICAR). However, the increase in GU elicited by AICAR was significantly lower than that induced by either biguanide. Expression of a constitutively active AMPK mimicked the effects of AICAR on GU, whereas a dominant interfering AMPK or shRNA silencing of AMPK prevented AICAR-stimulated GU and Met-induced AMPK signaling but only repressed biguanide-stimulated GU by ∼20%. Consistent with this, analysis of GU in muscle cells from α1−/−/α2−/− AMPK-deficient mice revealed a significant retention of Met-stimulated GU, being reduced by ∼35% compared with that of wild type cells. Atypical PKCs (aPKCs) have been implicated in Met-stimulated GU, and in line with this, Met and phenformin induced activation/phosphorylation of aPKC in L6 myotubes. However, although cellular depletion of aPKC (>90%) led to loss in biguanide-induced aPKC phosphorylation, it had no effect on Met-stimulated GU, whereas inhibitors targeting novel/conventional PKCs caused a significant reduction in biguanide-induced GU. Our findings indicate that although Met activates AMPK, a significant component of Met-stimulated GU in muscle cells is mediated via an AMPK-independent mechanism that involves novel/conventional PKCs.
Introduction
Metformin and phenformin are biguanides that exhibit potent antihyperglycemic and insulin-sensitizing properties. Their ability to regulate blood glucose has largely been attributed to a suppression of hepatic gluconeogenesis and increased glucose uptake in peripheral tissues such as skeletal muscle (1–3). The mechanism underpinning their action in skeletal muscle still remains unclear, although a number of studies have suggested they may act to stimulate glucose uptake independently of insulin (4, 5) or may potentiate insulin-stimulated glucose uptake (6), possibly via effects on insulin binding or proximal components of the insulin signaling cascade (7–9). However, the ability of metformin to enhance insulin binding may be secondary to the effects that the drug has on glucose metabolism, which precede changes in insulin binding by ∼18 h (10). One potential candidate that may mediate the effects of biguanides on glucose utilization in muscle cells is the AMP-activated protein kinase (AMPK),4 widely regarded as a cellular “energy sensor” (11). Work by Halestrap and co-workers and Leverve and co-workers (12, 13) revealed that metformin and phenformin are both capable of inhibiting Complex I of the mitochondrial respiratory chain, which would be expected to reduce the cellular energy status and thereby promote AMPK activation. Inhibition of Complex I may also help explain the propensity of these drugs to promote lactic acidosis, an adverse complication that was particularly associated with phenformin therapy that led to its clinical withdrawal in the 1970s. However, although no longer in clinical use, phenformin remains a widely used research tool for helping to delineate cellular and molecular mechanisms that underpin biguanide action.
AMPK activation in skeletal muscle has been shown to promote an increase in glucose uptake via enhanced expression and translocation of GLUT4 (14–16), whereas in other cell types AMPK activation has been linked to a suppression in gluconeogenic gene expression (17) and hepatic glucose production (18), although this view has recently been challenged (19). The proposition that AMPK may function as a metformin effector is supported by work showing that AMPK phosphorylation (activation) is enhanced in skeletal muscle of type 2 diabetics following a sustained (10 weeks) period of metformin therapy and that this is associated with a reduction in intramuscular ATP (20). The observed loss in muscle ATP is most likely a consequence of the effect that the biguanide has upon mitochondrial oxidation given that recent in vitro work has demonstrated that metformin induces a substantial reduction in cellular oxygen utilization (21), consistent with the inhibitory effect the drug has on Complex I. In addition to a reduction in ATP production, reduced cellular respiration has also been proposed to trigger an increase in mitochondrial reactive nitrogen species that may subsequently promote AMPK activation via a Src/PI3K-dependent mechanism (22). If so, activation of PI3K may promote increased signaling by molecules such as protein kinase B (PKB), which lie downstream of PI3K and have been implicated strongly in the regulation of glucose transport and metabolism (23, 24). Indeed, the finding that metformin induces PKB/Akt phosphorylation in rat cardiomyocytes supports such a possibility (25). More recent work has suggested that metformin inhibits AMP deaminase, which would elevate intracellular AMP and thereby promote AMPK activation (26). It has also been suggested that the metformin-induced increase in AMPK sequentially promotes activation of ERK, phosphoinositide-dependent kinase 1 (PDK1), and atypical PKCs (aPKC) and that activation of this signaling axis is responsible for enhancing muscle glucose transport (27). However, as yet, precisely how activation of aPKCs is mechanistically linked to molecules that have been proposed to lie upstream in this signaling pathway remains unclear. In an attempt to gain further insight as to how biguanides may stimulate an increase in muscle glucose uptake, we have studied the effects of metformin on glucose uptake in cultured skeletal muscle cells. In particular, this work has focused on the effect that these compounds have on components of the insulin signaling cascade, AMPK and PKCs, as putative biguanide effectors regulating glucose uptake in muscle cells.
EXPERIMENTAL PROCEDURES
Materials
α-Minimal essential medium, fetal bovine serum (FBS), and antibiotic/antimycotic solution were from Invitrogen. All other reagent-grade chemicals, insulin, phenformin hydrochloride, 1,1-dimethylbiguanide hydrochloride (metformin), AICAR, d-sorbitol, and 2,4-dinitrophenol were obtained from Sigma. Ro 31.8220, Gö6983, and Gö6976 were from Calbiochem. Wortmannin and LY294002 were obtained from Tocris (Bristol, UK). Antibody against the p85 subunit of PI3K and IRS-1 was purchased from Upstate Biotechnology. Antibodies against PKBα, phospho-PKB Ser473, phospho-GSK3α/βSer-9/21, GSK3, atypical phospho-PKCλζThr-410, AMPKα (recognizing the N-terminal domain of both α1 and α2), phospho-AMPK Thr172, phosphotyrosine, horseradish peroxidase-conjugated anti-rabbit IgG, and anti-mouse IgG were from New England Biolabs (Herts, UK). Horseradish peroxidase-conjugated anti-sheep/goat IgG was obtained from Pierce. Antibodies against PKCλ/ζ were from Santa Cruz Biotechnology (Wiltshire, UK). Antibody against phospho-acetyl-CoA carboxylase Ser79/221 was produced by the Division of Signal Transduction and Therapy (University of Dundee, Scotland, UK). Antibodies targeted against the C-terminal epitope of AMPKα1 and -α2 were a gift from Professor Grahame Hardie (University of Dundee). Protein A-Sepharose beads were purchased from Amersham Biosciences. Complete protein phosphatase inhibitor tablets were purchased from Roche Diagnostics.
Culture of L6 Myotubes and Primary Mouse Skeletal Muscle Cells
L6 muscle cells were cultured to the stage of myotubes as described previously (28), whereas wild type and α1−/−/α2−/− double knock-out primary muscle cells were grown as reported by Lantier et al. (29). Lysates from serum-deprived muscle cells were prepared following incubation with appropriate stimuli (e.g. insulin, AICAR, or biguanides) for times and at concentrations indicated in the figure legends. Following such incubations, muscle cells were washed three times with 0.9% (w/v) ice-cold NaCl and lysed in 200 μl of lysis buffer (50 mm Tris, pH 7.4, 0.27 m sucrose, 1 mm sodium orthovanadate, pH 10, 1 mm EDTA, 1 mm EGTA, 10 mm sodium β-glycerophosphate, 50 mm NaF, 5 mm sodium pyrophosphate, 1% (w/v) Triton X-100, 0.1% (v/v) 2-mercaptoethanol and protease inhibitors mixture (1 tablet per 25 ml)). Cells were scraped off the plates using a rubber policeman and homogenized by passing through a 26-gauge hypodermic needle prior to centrifugation (13,000 × g, 4 °C for 10 min) and stored at −20 °C until use.
Glucose Uptake
L6 myotubes were exposed to metformin, phenformin, insulin, and AICAR for times and at concentrations indicated in the figure legends and were serum-starved 2 h prior to assaying glucose uptake. Cells were washed three times with HBS (140 mm NaCl, 20 mm HEPES, 5 mm KCl, 2.5 mm MgSO4, 1 mm CaCl2, pH 7.4). Glucose uptake was assayed by incubation of 2-deoxy-d-[3H]glucose (1 μCi/ml, 26.2 Ci/mmol) for 10 min as described previously (28, 30). Nonspecific binding was determined by quantitating cell-associated radioactivity in the presence of 10 μm cytochalasin B. Radioactive medium was aspirated prior to washing adherent cells three times with 0.9% ice-cold saline. Cells were subsequently lysed in 50 mm NaOH, and radioactivity was quantitated using a Beckman LS 6000IC scintillation counter. Protein concentration in cell lysates was determined using the Bradford method (31).
Immunoprecipitation and Analysis of AMPK Activity
Following treatment with insulin or biguanides, L6 myotubes were lysed as described above. IRS-1 was immunoprecipitated using an antibody against the C-terminal domain of IRS-1. Immunocomplexes were captured by incubation with protein-A-Sepharose beads and solubilized in Laemmli sample buffer prior to immunoblotting. For analysis of AMPK activity, protein G-Sepharose beads were washed three times in PBS and incubated with anti-AMPKα1/α2 for 1 h at 4 °C on an orbital platform shaker. Bead/antibody mixture was then incubated with 500 μg of L6 cell lysate protein for 2 h at 4 °C before washing. The immunoprecipitates were washed twice with 1 ml of lysis buffer containing 0.5 m NaCl and twice with HEPES assay buffer (50 mm Na-HEPES, pH 7.0, 1 mm DTT, 0.02% Brij-35). AMPK activity toward SAMS peptide (HMRSAMSGLHVKRR) was measured as described previously (32).
Immunoblotting
50 μg of cell lysate protein was subjected to SDS-PAGE on a 10% resolving gel as described previously (28). Separated proteins were transferred onto polyvinylidene fluoride (PVDF) membranes, which were subsequently blocked using Tris-buffered saline (TBS) containing 0.1% (v/v) Tween 20 and 5% (w/v) milk. Membranes were probed with antibodies against PKB, phospho-PKB Ser473, phospho-GSK-3α/β, IRS-1, p85-PI3K, phosphotyrosine, PKCλ/ζ, PKCλ/ζ Thr410, N-terminal AMPKα, phospho-AMPK Thr172, phospho-acetyl-CoA carboxylase Ser79/221, and a composite mixture of antibodies against the C-terminal domains of AMPKα1 and AMPKα2. The membranes were washed three times in TBS, 0.1% (v/v) Tween 20 for 15 min prior to incubation with horseradish peroxidase (HRP), anti-rabbit IgG, HRP anti-mouse IgG, or HRP anti-sheep/goat IgG as deemed appropriate. Protein bands on PVDF were visualized using enhanced chemiluminescence (Pierce) by exposure to Konica Medical Film (Konica Corp., Hohenbrunn, Germany).
Analysis of Cellular PIP3
The effects of metformin and insulin on cellular PIP3 content was assessed using a sensitive time-resolved fluorescence resonance energy transfer (FRET)-based assay that monitors the displacement of GST-tagged GRP1-pleckstrin homology domain from a sensor complex consisting of Eu Lance® chelate-labeled anti-GST antibody, the GST-tagged GRP1-pleckstrin homology domain, biotinylated-PIP3, and the FRET acceptor, streptavidin allophycocyanin by nonbiotinylated lipid (33). After the appropriate incubation of cells with insulin, metformin or wortmannin, cells were rapidly washed, and cellular material was precipitated by the immediate addition of 0.5 ml of ice-cold 0.5 m trichloroacetic acid (TCA). After standing the cells on ice for 5 min, they were harvested from the plates and the acid precipitate was pelleted by centrifugation. The pellet was washed two times with 1 ml of 5% TCA, 1 mm EDTA. Neutral lipids were extracted from the pellet, and the PIP3 content was determined as described previously using an LJL Analyst plate reader (33). PIP3 abundance was calculated by reference to a standard curve constructed by addition of known amounts of the 3-phosphoinositide to the sensor complex.
Adenoviral Infection
L6 cells at day 4 of differentiation were infected with adenovirus expressing green fluorescent protein as control or an adenovirus construct encoding either a constitutively active (CA-AMPK) or dominant negative form (DN-AMPK) of AMPKs that were kindly provided by David Carling (Hammersmith Hospital, London, UK) and Pascal Ferré (INSERM Unit 671, Paris, France). The CA-AMPK construct encodes residues 1–312 of AMPKα1 mutated on the threonine residue to an aspartic acid (T172D), and the vector also encodes for GFP. The DN-AMPK contains Myc-tagged full-length AMPKα1 mutated at position 172 to an alanine (T172A) as described previously (34). After 48 h, most of the cells expressed the viral constructs and were differentiated into myotubes. Cells were treated with biguanides and AICAR for 16 h and serum-starved for 4 h prior to cell lysis or assay of glucose uptake as described above.
Lentivirus Production and Generation of Stable α1-AMPK Knockdown L6 Cells
The strategy to generate lentiviral shRNA constructs against the α1 subunit of AMPK was as reported previously by us (35, 36) for the type 1 cannabinoid receptor (CB1) and the aPKCλ isoform. Briefly, shRNA sequences were inserted into the pLKO.1-puro lentiviral vector (Sigma). Each hairpin consisted of a 21-nucleotide sense sequence, a short hairpin sequence (CTCGAG), a 21-nucleotide antisense sequence, and five thymidines (a stop signal for RNA polymerase). The oligo sequences used for the rat α1-AMPK subunit are shown in Table 1. A control hairpin sequence that was unrelated to the rat α1-AMPK subunit or to atypical PKCλ was inserted into the control lentiviral vector. Additional nucleotides were added to the ends of the oligos as shown in Table 1, such that annealing of the two complementary oligos resulted in overhangs consistent with those generated by EcoRI and AgeI. Oligos were annealed by mixing 2 μg of each oligo, heating to 94 °C for 10 min, and followed by cooling at a rate 1 °C per min until 21 °C is reached. The final double-stranded DNA sequences were then inserted into pLKO.1-puro at the EcoRI and AgeI sites. Correct insertions of shRNA were confirmed by sequencing. Recombinant lentiviruses were produced by co-transfecting HEK 293T cells with the empty pLKO.1-puro vector, pLKO.1-puro/scramble, or pLKO.1-puro/α1-AMPK plasmids with the envelope vector pCMV VSVg, and the packaging vector pHR CMV8.2 ΔR at ratios of 3:2:2, respectively, according to mass. Infectious lentiviral particles were harvested by collecting the cell culture media 72 h later and filtered through 0.45-μm Minisart cellulose-acetate filters. Infection of L6 myoblasts was carried out by adding 1 ml of crude lentivirus preparation to L6 myoblasts seeded in 6-cm dishes in the presence of 8 μg/ml Polybrene. Medium was replaced with standard growth medium containing 3 μg/ml puromycin 24 h post-transduction, and cells were maintained in this selective medium until fully selected.
TABLE 1.
Oligo sequences used for generation of lentiviral plasmids
Oligo sequences are shown 5′ to 3′. Oligos were composed of 21-bp sense and antisense sequences (shown in boldface type) separated by a hairpin loop sequences (shown underlined). Overhangs were added to each end of the oligos to facilitate cloning into pLKO.1-puro.
| sh1339 oligo 1 | CCGGATGAGTCTACAGCTATACCAACTCGAGTTGGTATAGCTGTAGACTCATTTTTTG |
| sh1339 oligo 2 | AATTCAAAAAATGAGTCTACAGCTATACCAACTCGAGTTGGTATAGCTGTAGACTCAT |
| sh9 oligo 1 | CCGGGAAGCAGAAGCACGACGGGCGCTCGAGCGCCCGTCGTGCTTCTGCTTCTTTTTG |
| sh9 oligo 2 | AATTCAAAAAGAAGCAGAAGCACGACGGGCGCTCGAGCGCCCGTCGTGCTTCTGCTTC |
| Control oligo 1 | CCGGCCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGGTTTTTG |
| Control oligo 2 | AATTCAAAAACCTAAGGTTAAGTCGCCCTCGCTCGAGCGAGGGCGACTTAACCTTAGG |
Statistical Analyses
One-way analysis of variance was used to assess statistical significance. Data analysis was performed using GraphPad Prism software and considered statistically significant at p values < 0.05.
RESULTS
Effects of Metformin on Glucose Uptake Do Not Involve Components of the Insulin Signaling Cascade
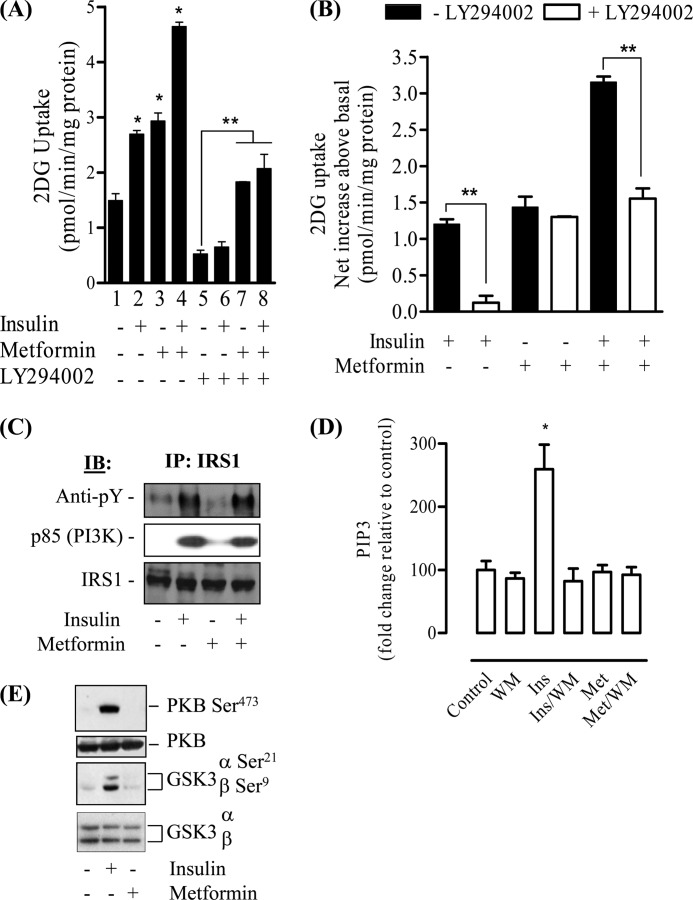
The signaling mechanisms by which metformin imparts beneficial effects upon glucose utilization in skeletal muscle are poorly understood, but previous work in L6 myotubes has established that metformin does not invoke gross changes in the cellular abundance of glucose transporters (37) but does induce their translocation to the plasma membrane (4). In line with these previous studies, we find that exposure of L6 myotubes to 1 mm metformin for 16 h leads to a 2-fold increase in glucose uptake, which was comparable with the increase elicited by an acute (30 min) insulin challenge and was additive when the two stimuli were combined (Fig. 1A). It should be stressed that the period of exposure to metformin used in this study was based on previous work demonstrating that glucose uptake was maximally enhanced in L6 myotubes when challenged with metformin for 16 h (37). As part of this work, we also assayed the effect of LY294002, a PI3K inhibitor, on metformin-stimulated glucose uptake. Consistent with previous work (38), we observed a significant reduction in basal glucose uptake upon inhibition of PI3K (Fig. 1A, compare lanes 1 and 5). Taking account of the effect that the inhibitor has on basal glucose uptake, the net increase in glucose uptake elicited by metformin was unaffected (Fig. 1B). The efficacy of the inhibitor was confirmed by demonstrating that the net increase in glucose uptake by insulin was virtually abolished by LY294002. Moreover, although LY294002 did not inhibit metformin-stimulated glucose uptake, it fully suppressed the additive stimulation in glucose uptake that is seen upon exposure of metformin-treated cells to insulin (Fig. 1, A, compare lanes 3 and 4 with lanes 7 and 8, and B). Although these findings do not support a role for PI3K in metformin-stimulated glucose uptake in L6 myotubes, the biguanide has been reported to induce insulin receptor/IRS phosphorylation in hepatocytes (9), enhance insulin action in cultured C2C12 muscle cells (8), and induce PKB phosphorylation in rat cardiomyocytes (25). Therefore, to further explore whether metformin may induce activation of components involved in proximal insulin signaling, we investigated its effects on the IRS-PI3K-PKB signaling axis. Fig. 1C shows that unlike insulin, which induced tyrosine phosphorylation of IRS-1 (by >2-fold) and association of the p85 PI3K subunit with IRS-1, sustained exposure of skeletal muscle cells to metformin for periods that induce an increase in glucose uptake did not promote in isolation or augment these events when co-incubated with insulin. Consistent with this finding, a sensitive FRET-based assay (33) was unable to detect any metformin-induced changes in PIP3 content (used as an index of PI3K activity), whereas the abundance of this 3-phosphoinositide was elevated by over 2.5-fold in response to insulin, which was fully suppressed by wortmannin (another PI3K inhibitor) (Fig. 1D). Although PKB/Akt has been strongly implicated in the insulin-dependent activation of glucose transport (39), our analysis indicates that phosphorylation of PKB and that of GSK3, a downstream physiological target of PKB, was not observed in L6 muscle cells treated with metformin. In contrast, insulin, which served as a positive control in these experiments, induced phosphorylation of both kinases as expected (Fig. 1E). It is noteworthy that in separate experiments, short term cell exposure to metformin (for periods up to 2 h) failed to elicit any detectable change in PKB phosphorylation status thereby excluding any possibility of a temporal activation of this signaling cascade that may have been overlooked during the more chronic (16 h) metformin incubations that we had employed (data not shown). Collectively, our findings negate the involvement of the IRS-PI3K-PKB signaling axis in the metformin-induced stimulation of glucose uptake in L6 myotubes.
FIGURE 1.
Effect of metformin on glucose uptake and components of the insulin signaling pathway in L6 myotubes. L6 myotubes were incubated in the absence or presence of metformin (1 mm) for 16 h prior to incubation with insulin (100 nm) and/or PI3K inhibitors (100 nm wortmannin (WM) or 50 μm LY 294002) for the penultimate 30-min period for insulin and 45-min period for PI3K inhibitors. At the end of the incubation period, muscle cells were used for assay of 2-deoxyglucose (2DG) uptake (A). Data from A was used calculate net changes in 2-deoxyglucose uptake so as to take account of any changes in basal uptake induced by the PI3K inhibitor (B). Alternatively, muscle cells were lysed at the end of indicated treatments and used for immunoprecipitation (IP) of IRS-1 and immune pellets subjected to SDS-PAGE and immunoblotting (IB) with antibodies against phosphotyrosine, IRS-1, or p85-PI3K (C), for lipid extraction to measure PIP3 abundance as reported under “Experimental Procedures” (D), or for immunoblotting with antibodies against phospho-PKB Ser473, phospho-GSK3α/β Ser21/9, and native PKB and GSK3 (E). Bar graph results are expressed as a mean ± S.E. for between 3 and 10 independent experiments. The asterisk represents a significant change (p < 0.05) relative to the untreated control, and the double asterisk indicates a significant change (p < 0.05) between the indicated bars. Phosphoblots are representative of three separate experiments.
AMPK Activation Cannot Fully Account for the Insulin-independent Effects of Biguanides on Glucose Uptake in L6 Myotubes
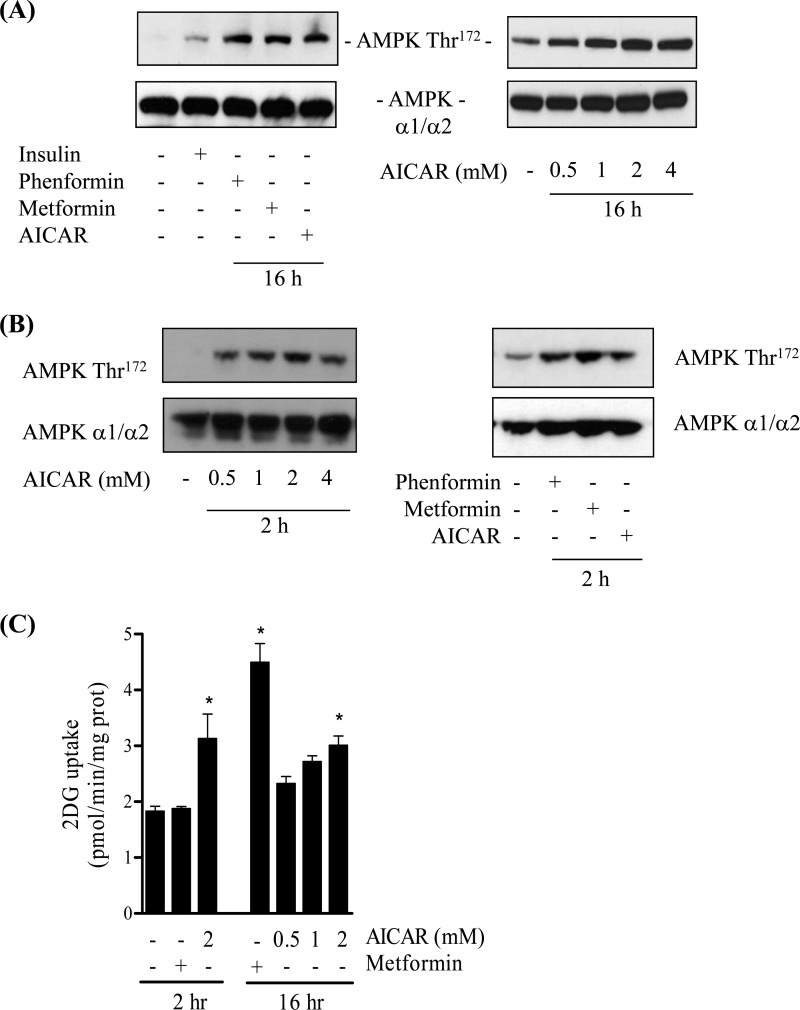
In an attempt to understand the nature of the PI3K-independent pathway that permits metformin to stimulate hexose uptake, we investigated the potential contribution of AMPK. Metformin and its closely related analog phenformin are potent activators of AMPK (40–42), and the kinase has been implicated in the regulation of skeletal muscle glucose uptake in response to both drugs (43). Fig. 2A shows that phosphorylation of the AMPK T-loop residue (Thr172), which correlates closely with kinase activity and thus serves as a marker for AMPK activation (43), was elevated following 16 h of cell incubation with both biguanides and also in response to a maximally effective dose of AICAR (2 mm), a well established AMPK activator. Analysis of AMPK phosphorylation in response to a shorter (2 h) incubation period revealed that all three stimuli also induced a comparable AMPK activation/phosphorylation (Fig. 2B). Intriguingly, although 2 mm AICAR induced a significant increase in glucose uptake after incubation for 2 h, metformin did not induce any detectable increase in glucose uptake during this period (Fig. 2C) despite eliciting a comparable enhancement in AMPK phosphorylation to AICAR (Fig. 2B). Consistent with previous work (4), sustained exposure of muscle cells to metformin (for 16 h) induced a significant increase in glucose uptake, which we find to be at least 2-fold greater than that seen in response to 2- or 16-h incubations with AICAR (Fig. 2C). In separate experiments, very similar observations on glucose uptake were made when metformin was substituted by phenformin (data not shown).
FIGURE 2.
Effects of biguanides and AICAR on AMPK phosphorylation and glucose uptake in L6 myotubes. L6 myotubes were incubated in the absence or presence of metformin (1 mm), phenformin (200 μm), or AICAR (at the indicated concentrations) for 2 or 16 h. In some experiments muscle cells were incubated with insulin (100 nm, 10 min). At the end of these incubations cells were lysed and used for immunoblotting with antibodies against AMPK Thr172 or antibodies detecting both the α1 and α2 subunits of AMPK (A and B). Alternatively, following cell incubation with metformin or AICAR for the times indicated, cells were used for assay of 2DG uptake (C). Blots are representative of at least three separate experiments, and bar graphs show uptake data as means ± S.E. for between 3 and 10 independent experiments. The asterisk represents a significant change (p < 0.05) relative to the untreated control from four separate experiments, each conducted in triplicate.
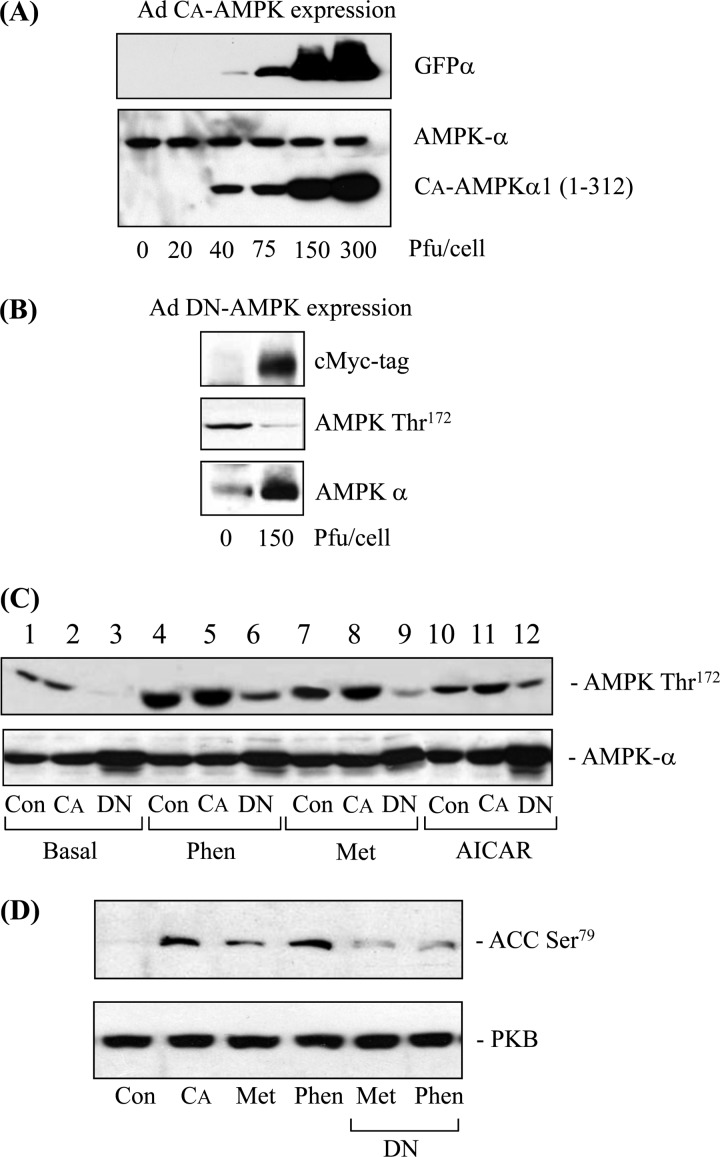
To explore the role of AMPK further, we expressed constitutively active and dominant negative forms of AMPK in L6 muscle cells using an adenoviral delivery system. Fig. 3A shows that exposing muscle cells to an increasing titer of the virus containing the constitutively active (truncated) kinase led to a progressive increase in expression of the active mutant as detected using an antibody to the N-terminal kinase domain. This antibody also detects the endogenous kinase, whose expression was unaltered by viral infection. The viral vector also encodes for GFP, which can be detected using an anti-GFP antibody (Fig. 3A), and by fluorescence microscopy, which revealed more than 90% of myoblasts were infected when exposed to a virus titer of 150 pfu/cell (data not shown). This titer was also used for expressing the full-length dominant interfering kinase whose expression was detected using an antibody against the Myc tag but was also detected as an increase in total AMPK (Fig. 3B). Expressing the dominant interfering mutant (carrying a T172A mutation) resulted in a reduction in basal and stimulus-dependent Thr172 phosphorylation (Fig. 3C) and also blunted the phosphorylation of acetyl-CoA carboxylase-2 (ACC-2), a physiological AMPK target, in response to both metformin and phenformin, as expected (Fig. 3D). The phosphorylation of ACC-2 in cells infected with the α1(1–312) AMPK construct confirms the expression of a constitutively active AMPK in L6 cells (Fig. 3D, 2nd lane).
FIGURE 3.
Effects of expressing constitutively active (CA) or dominant negative (DN) forms of AMPK on biguanide or AICAR-induced AMPK and ACC-2 phosphorylation. A, L6 myoblasts were infected with increasing concentrations of adenovirus encoding the CA form of AMPK. Infected myoblasts were permitted to fully differentiate and lysed 48 h post-infection. Lysates (50 μg of protein) were immunoblotted with antibodies against GFPα and anti-AMPKα (which cross-react with N-terminal domains of both AMPKα1 and AMPKα2). B, L6 myoblasts were infected with 150 pfu/cell of adenovirus expressing a DN form of AMPK. Muscle cells were lysed 48 h post-infection, and lysates (50 μg of protein) were immunoblotted with antibodies against the c-Myc tag, phospho-AMPK Thr172, and AMPK. C and D, L6 cells were infected as described above with 150 pfu/cell of either control adenovirus expressing GFPα (Con), adenovirus encoding the CA-AMPK (CA), or the DN-AMPK (DN). After 48 h, cells were incubated with or without phenformin (Phen) (200 μm), metformin (Met) (1 mm), or AICAR (2 mm) for a further 16 h. Cells were lysed and immunoblotted for antibodies targeting phospho-AMPK Thr172, α-AMPK, phospho-ACC-2 Ser79, or PKB, which was used here as a gel loading control. Immunoblots are representative of two separate experiments.
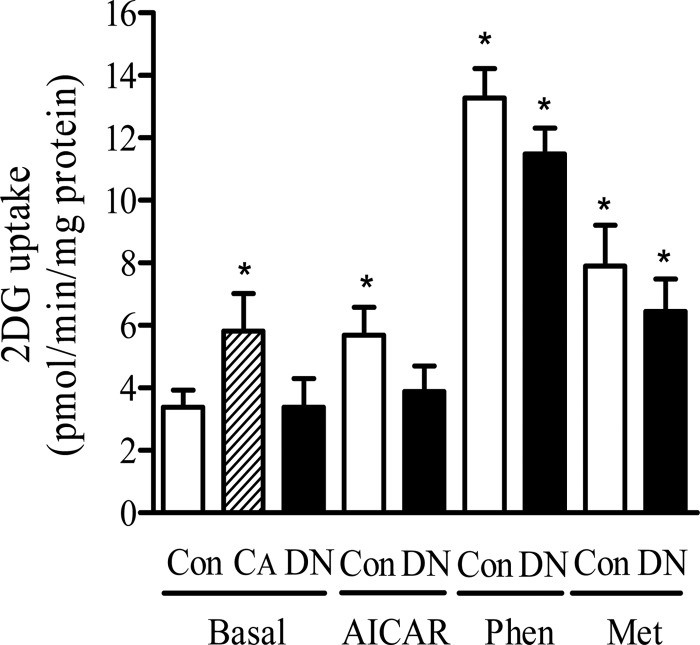
We subsequently tested the effects of expressing the active and dominant negative AMPK mutants on glucose uptake. Fig. 4 shows that expressing the constitutively active AMPK induced an increase in basal glucose uptake by ∼50% (from 3.8 ± 0.5 to 5.8 ± 1.2 pmol/min/mg protein, values are mean ± S.E.). Incubation of muscle cells with 2 mm AICAR for 16 h led to an increase in glucose uptake that was comparable with that seen in cells infected with the constitutively active AMPK. Incubating muscle cells with metformin or phenformin for 16 h stimulated glucose uptake by 2- and ∼3.6-fold, respectively, compared with untreated control cells. In separate experiments, we also assessed the effects of AICAR, metformin, and phenformin on glucose uptake in muscle cells expressing the constitutively active AMPK. We did not observe any significant augmentation in glucose uptake compared with that seen in the presence of each stimulus alone (data not shown). This latter finding implies that any AMPK-mediated increase in glucose uptake that occurs in response to each of these three stimuli was maximal and could not be enhanced further by expression of the constitutively active kinase. Expression of the dominant interfering AMPK did not affect basal glucose uptake but ablated the increase in hexose uptake elicited by AICAR. In contrast, although we consistently observed a modest reduction (∼20%) in biguanide-stimulated glucose uptake in cells expressing the dominant negative AMPK, the residual stimulation caused by each drug remained significant (Fig. 4).
FIGURE 4.
Effects of expressing CA or DN forms of AMPK on glucose uptake. L6 cells were infected with 150 pfu/cell of either the control adenovirus expressing GFPα (Con), the CA-AMPK (CA), or the DN-AMPK (DN). After 48 h infected cells were incubated with or without phenformin (Phen) (200 μm), metformin (Met) (1 mm), or AICAR (2 mm) for 16 h prior to assaying 2 deoxyglucose uptake as described under “Experimental Procedures.” Data represent means ± S.E. from four separate experiments conducted in triplicate. Asterisks indicate significant change from the untreated basal control value (p < 0.05).
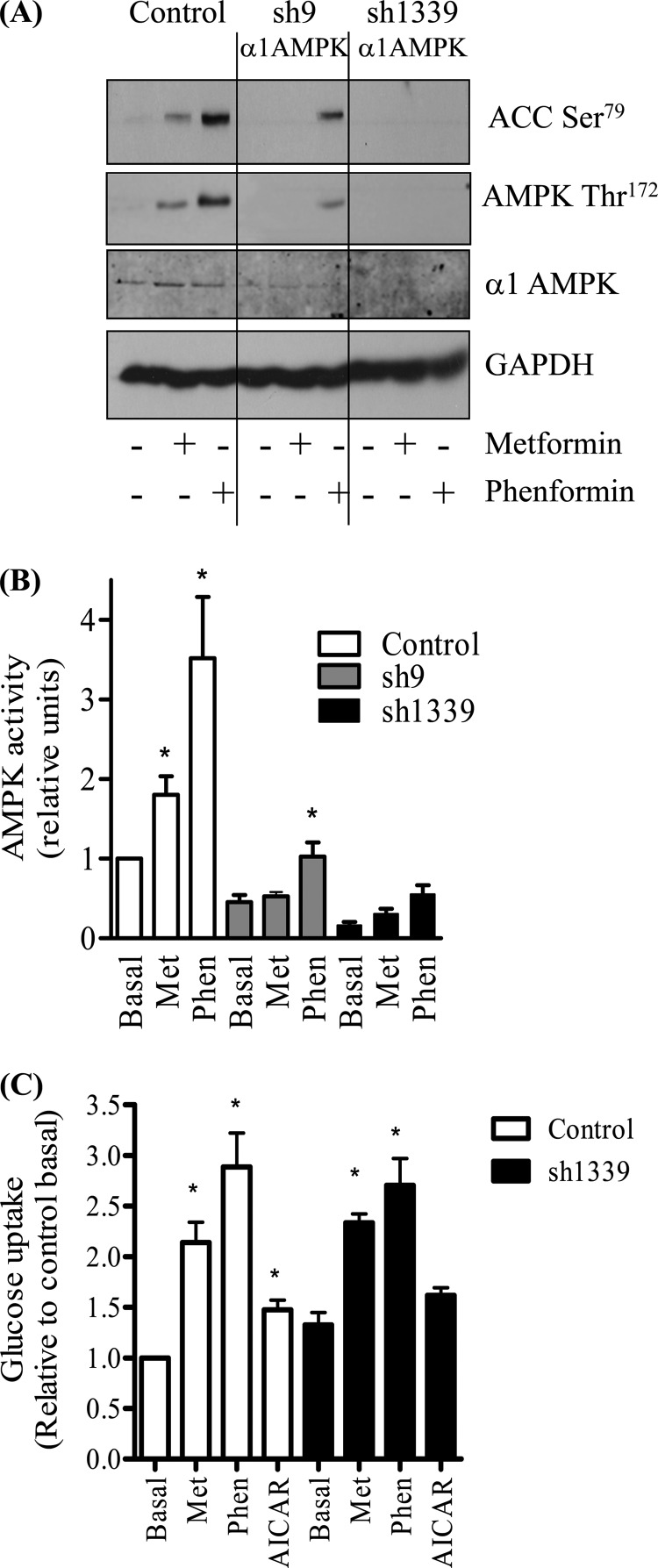
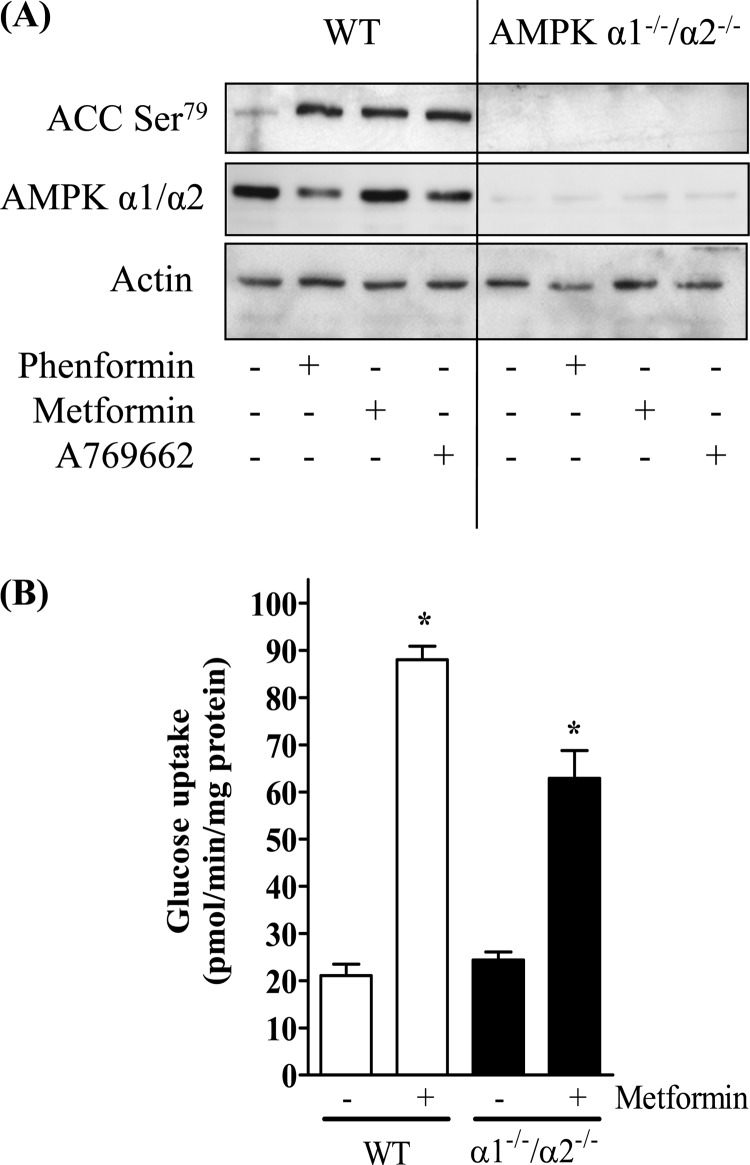
To further explore the extent of AMPK involvement in biguanide-stimulated glucose uptake, we investigated the effects of stably silencing AMPK expression in L6 muscle cells using a lentiviral shRNA strategy. Very recent work has reported that metformin preferentially induces phosphorylation/activation of the α1 subunit of AMPK in L6 muscle cells (44), which is in line with our observation that we were unable to detect any enhancement in AMPK activity in α2-immunoprecipitates prepared from metformin-stimulated cells (data not shown). Consequently, we targeted the α1-AMPK subunit for gene silencing. Two shRNA sequences designed to target α1-AMPK were inserted into a lentiviral knockdown vector and stable L6 cells lines expressing each hairpin established. Compared with muscle cells expressing the lentiviral vector with the control hairpin sequence, those expressing the targeted hairpin sequences (sh9 and sh1339) displayed a marked reduction in α1-AMPK abundance (Fig. 5A). In line with these findings, immunoprecipitable α1-AMPK activity was greatly reduced in sh9 and sh1339 expressing cells, with the effect being more pronounced in the latter, in which the targeted loss of α1-AMPK was greater (Fig. 5B). Subjecting control cells to incubation with metformin or phenformin induced a significant increase α1-AMPK activity (Fig. 5B), which was also validated by the attendant increase in phosphorylation of AMPK on Thr172 and that of ACC-2, which lies downstream of AMPK (Fig. 5A). In contrast, cells in which α1-AMPK had been silenced displayed a substantially muted response to both metformin and phenformin, in terms of acetyl-CoA carboxylase phosphorylation and AMPK activation (Fig. 5, A and B). Given the greater reduction in α1-AMPK expression/activity in the sh1339-L6 cell line, these cells were subsequently propagated to assess the effect of metformin, phenformin, and AICAR on glucose uptake. Fig. 5C shows that whereas glucose uptake was elevated in control cells in response to all three stimuli, the response to both metformin and phenformin was reduced by ∼15 and 27%, respectively, whereas the AICAR-stimulated effect was no longer significant in the α1-AMPK-silenced cells (Fig. 5C). To substantiate these findings further, we monitored the effect of metformin on glucose uptake in primary muscle cultures derived from skeletal muscle of wild type mice or those deficient in both α1- and α2-AMPK subunits. Fig. 6A highlights that although metformin, phenformin, and A769662 (an established AMPK activator) all induced phosphorylation of ACC-2 in wild type cells, this response was not seen in the α1−/−/α2−/− muscle cells consistent with the absence of any catalytic AMPK activity in these cells. However, Fig. 6B shows that when α1−/−/α2−/− muscle cells were subsequently challenged with metformin for 16 h, these cells still exhibited a near 3-fold increase in glucose uptake. The overall stimulation in glucose uptake in α1−/−/α2−/− muscle cells was lower (∼35%) than that observed in muscle cells from wild type mice, which is in line with our other data indicating that biguanide-stimulated glucose uptake has a significant AMPK-independent component.
FIGURE 5.
Effects of α1-AMPK gene silencing on biguanide and AICAR-induced stimulation of glucose uptake in L6 myotubes. Two different stable L6 cell lines containing shRNA targeting the α1-AMPK subunit were established (sh9 and ah1339). Control cells transfected with a nonspecific hairpin and those expressing the sh9 and sh1339 hairpins were incubated in the absence or presence of metformin (1 mm, 16 h) or phenformin (200 μm, 16 h). Lysates were prepared for Western blotting using antibodies targeting phospho-AMPK Thr172, α1-AMPK, phospho-ACC-2 Ser79, or GAPDH, which was used as a gel loading control (A). Immunoblots shown are representative of three separate experiments. B, cells treated as in A were lysed, and immunoprecipitable α1-AMPK activity was assayed toward a substrate peptide. C, control cells and those transfected with the sh1339 hairpin were incubated with metformin (Met) and phenformin (Phen) as described in A and also with AICAR (2 mm, 16 h) prior to assaying 2DG uptake. Bar values (B and C) represent means ± S.E. from at least three separate experiments, and asterisks indicate significant change from the untreated basal value (p < 0.05).
FIGURE 6.
Effects of metformin on glucose uptake in wild type and α1−/−/α2−/− null primary mouse skeletal muscle cultures. Wild type and α1−/−/α2−/− primary muscle cells were cultured in the absence or presence of phenformin (200 μm, 16 h), metformin (1 mm, 16 h), or A769662 (300 μm, 30 min), and at the end of these incubations were lysed and immunoblotted using a pan-α1/α2-AMPK antibody, phospho-ACC-2 Ser79 antibody or one detecting actin, which was used as a gel loading control (A). Immunoblots are representative of two separate experiments. Alternatively, wild type and α1−/−/α2−/− primary muscle cells were cultured in the absence or presence of 1 mm metformin for 16 h prior to assaying 2DG uptake (B). Bar values represent fold changes in uptake expressed as means ± S.E. from between 6 and 9 experimental determinations. Asterisks indicate significant change from the respective untreated basal value (p < 0.05).
Role of PKCs in the Stimulatory Effect of Biguanides on Glucose Uptake in L6 Myotubes
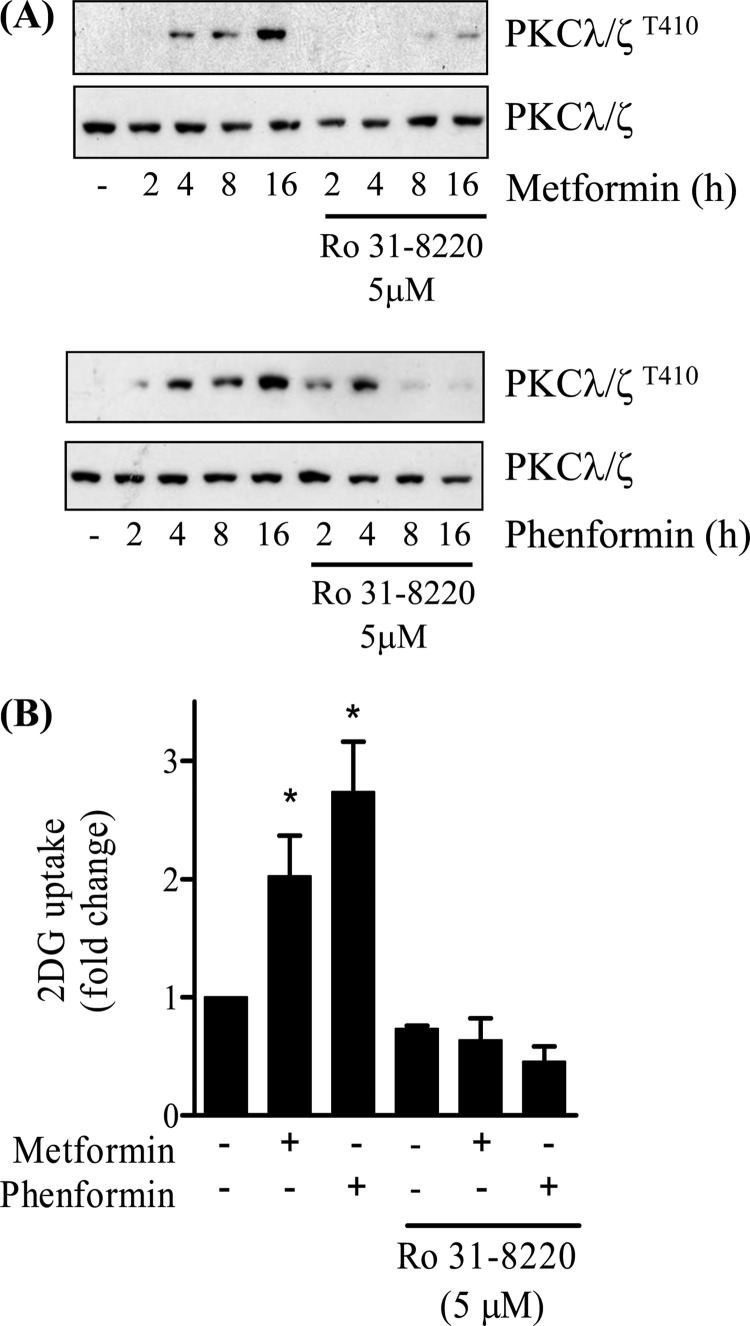
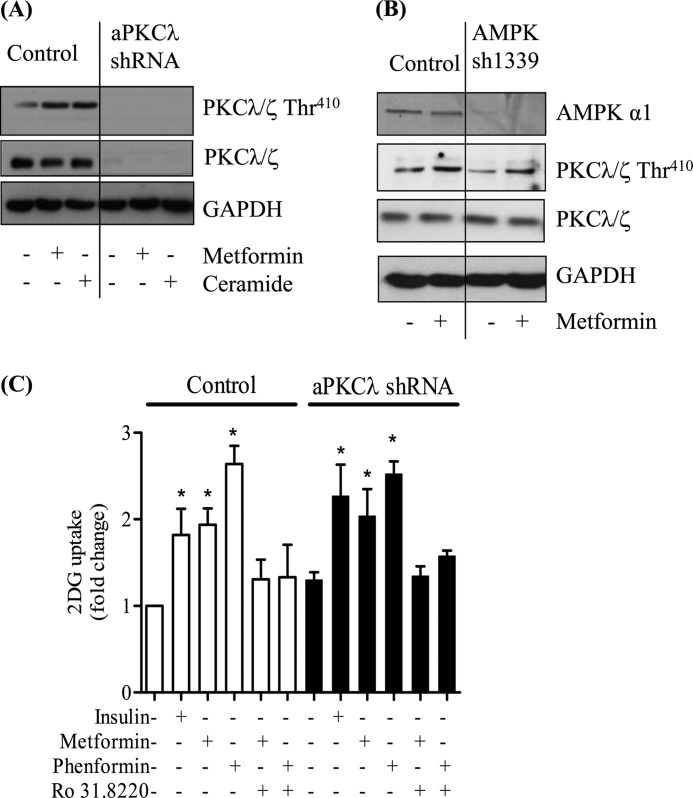
Evidence exists in the literature showing that metformin induces activation of atypical PKCs (PKCλ/ζ) in skeletal and cardiac muscle (45, 46). Indeed, it has recently been suggested that this activation may lie downstream of AMPK and that it supports the stimulatory effect of the biguanide on glucose uptake (27). However, there is also evidence to suggest that metformin may induce PKC activation independently of AMPK (47), and consequently, we sought to establish whether atypical PKCs represent bona fide intermediates in the biguanide-mediated stimulation of glucose uptake in L6 myotubes. Fig. 7A shows that both metformin and phenformin induce a time-dependent activation of aPKCλ/ζ based on phosphorylation of the Thr410 residue that is located within the kinase activation loop. This biguanide-stimulated phosphorylation was severely blunted in myotubes that had been preincubated with Ro 31.8220, an inhibitor known to suppress atypical PKC isoforms when used at micromolar concentrations (48, 49). We subsequently assessed whether this inhibitor antagonizes the biguanide-induced increase in glucose uptake. Fig. 7B shows that consistent with the loss of PKCλ/ζ activation observed when L6 myotubes were preincubated with 5 μm Ro 31.8220, the ability of both biguanides to increase glucose uptake was reduced significantly by the presence of the inhibitor. The data presented in Fig. 7 provides prima face evidence that aPKCs may act as effectors of biguanide action on glucose uptake. However, to test this proposition further, we assessed the effects of both metformin and phenformin upon glucose uptake in muscle cells in which aPKC expression had been silenced. We recently reported that L6 myotubes used in our laboratory only express aPKCλ and that muscle cells harboring a stable loss in expression of this isoform exhibit an increase in insulin sensitivity (36). Fig. 8A shows that muscle cells that have been transfected with a nonspecific control shRNA display enhanced phosphorylation of aPKCλ in response to metformin or following exposure to ceramide, a potent aPKC activator (50). In contrast, muscle cells depleted of aPKCλ do not exhibit this stimulus-induced phosphorylation. If this biguanide-induced activation of aPKC lies downstream of AMPK as has been recently suggested (27), then we would expect it also to be reduced in cells in which AMPK activity had been significantly depleted. However, Fig. 8B shows that L6 cells with a stable reduction in AMPK expression/activity (Fig. 5) retain their ability to induce phosphorylation of aPKCλ (by 42%) in response to metformin, which is similar to that observed in control cells (46%). Analysis of glucose uptake indicates that, in line with the data shown in Fig. 7B, muscle cells transfected with the control shRNA exhibit biguanide-stimulated glucose uptake that is Ro 31.8220-sensitive (Fig. 8C). However, somewhat surprisingly, we also find that muscle cells depleted of aPKCλ not only remain responsive to metformin and phenformin but that biguanide-stimulated glucose uptake remains sensitive to Ro 31.8220 (Fig. 8C).
FIGURE 7.
Effects of metformin and phenformin on phosphorylation of atypical PKCs and glucose uptake in L6 myotubes. L6 myotubes were incubated with either metformin (1 mm) or phenformin (200 μm) for the times indicated in the absence or presence of 5 μm Ro 31.8220 (PKC inhibitor). Muscle cells were then lysed for immunoblotting with a phospho-specific antibody directed against PKC λ/ζThr-410 or pan-anti-PKCλ/ζ antibody (A). Alternatively, following cell incubation with biguanides and/or the PKC inhibitor cells were used for assay of glucose uptake (B). Blots are representative of two separate experiments, whereas uptakes are from between 3 and 5 experiments each conducted in triplicate. Bars are mean ± S.E. Asterisks signify a significant change from the untreated control.
FIGURE 8.
Effects of metformin and phenformin on phosphorylation of atypical PKCs and glucose uptake in L6 myotubes. Control cells transfected with a nonspecific hairpin or those transfected with shRNA yielding a stable loss in (A) aPKCλ (36) or (B) α1-AMPK expression were incubated with metformin (1 mm, 16 h) or where indicated with C2-ceramide (100 μm, 2 h). Cells were lysed and immunoblotted with a phospho-specific antibody directed against PKC λ/ζThr-410, a pan-anti-PKCλ/ζ antibody, or one detecting GAPDH, which was used here as a gel loading control. Immunoblots are representative of two separate experiments. C, control cells or those in which aPKCλ expression had been substantially depleted were incubated with metformin (1 mm, 16 h), phenformin (200 μm, 16 h), or where indicated with insulin (100 nm, 30 min). In some experiments, Ro 31.8220 (5 μm) was co-incubated with either metformin or phenformin. At the end of these incubation periods, cells were used for assay glucose uptake. Bar values are mean ± S.E. from three separate experiments. Asterisks signify a significant change from the untreated control bar.
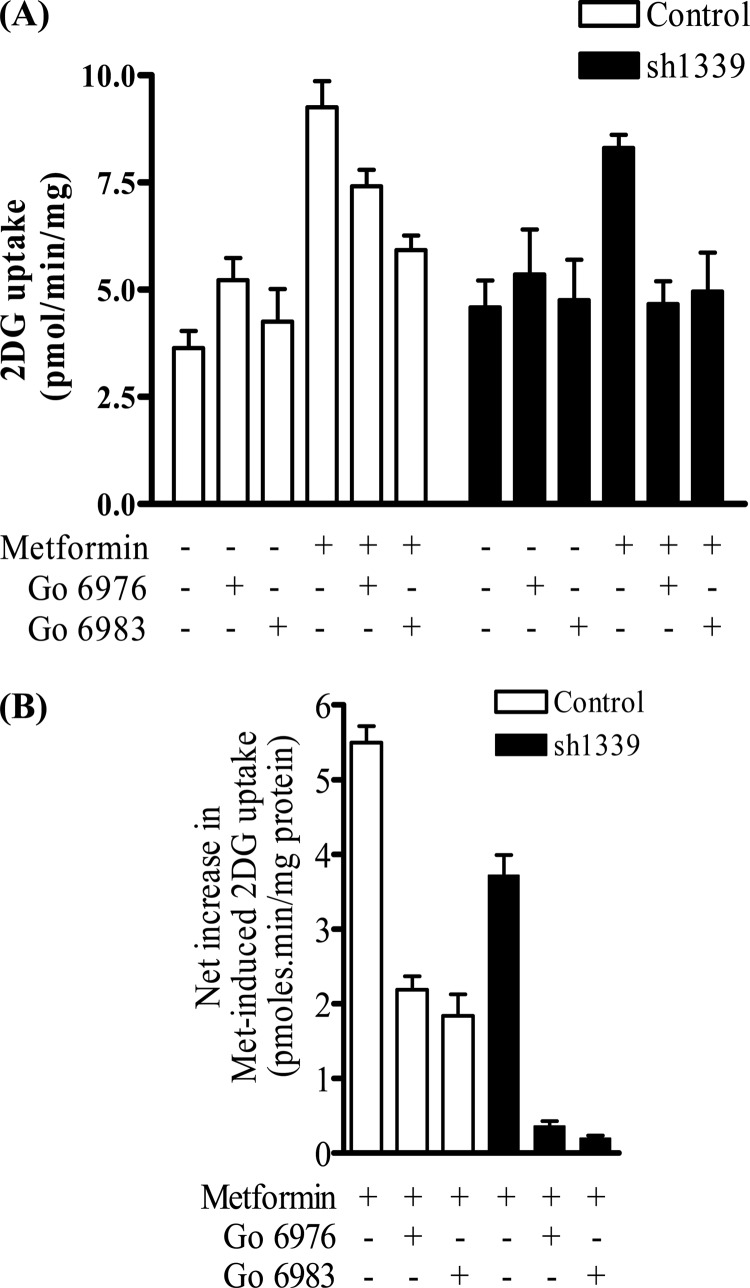
One explanation for why Ro 31.8220 suppresses biguanide-stimulated glucose uptake in cells depleted of aPKCλ is the possibility that the drug, when used at micromolar concentrations, not only targets aPKCs but also inhibits the novel and conventional PKC isoforms (48, 49). To test whether these PKC isoforms might participate in mediating the stimulatory effects of metformin, we tested the effect of the novel and conventional PKC inhibitor Gö6983, and Gö6976, an inhibitor that preferentially targets conventional PKCs, in muscle cells expressing the control hairpin sequence and those in which α1-AMPK had been stably silenced (sh1339). Fig. 9A shows that Gö6976 induces a modest but significant increase in basal glucose uptake, which was not seen in response to cell treatment Gö6983. In the absence of these inhibitors, metformin induced a 2.4-fold increase in glucose uptake (Fig. 9A). To take account of the change in basal uptake caused by Gö6976 and for greater clarity, the net increase in metformin-stimulated glucose uptake was calculated in the absence and presence of these two inhibitors. Fig. 9B shows that Gö6976 and Gö6983 reduced biguanide-stimulated glucose uptake by 60 and 66%, respectively, in the control cells. Consistent with our earlier data, loss of α1-AMPK reduced metformin-stimulated glucose uptake by 32%. Interestingly, the residual metformin-stimulated glucose uptake, which we suggest is AMPK-independent, was virtually abrogated in response to incubation of muscle cells with Gö6976 and Gö6983 (Fig. 9B).
FIGURE 9.
Effect of novel and conventional PKC inhibitors on glucose uptake in control and α1-AMPK silenced L6 myotubes. Control cells transfected with a nonspecific hairpin and those expressing the sh1339 hairpin that targets the α1-AMPK subunit were incubated in the absence or presence of metformin (1 mm, 16 h) and/or with either 2 μm Gö6976 or 2 μm Gö6983 prior to assay of 2DG uptake (A). Data from A was used to calculate net changes in 2DG uptake so as to take account of any changes in basal 2DG uptake induced by the PKC inhibitors (B). Data represent means ± S.E. for between 3 and 7 experimental determinations. Asterisks indicate significant change between the indicated bars (p < 0.05).
DISCUSSION
Metformin exerts a potent glucoregulatory effect that is thought to stem primarily from its metabolic action in the liver, where it serves to suppress numerous processes that contribute to the gluconeogenic drive while also enhancing responsiveness toward insulin in this tissue (51). However, although the liver is considered as the principal site of metformin action, the biguanide also exerts insulin-sensitizing/insulin-like effects in a number of other tissues, including skeletal muscle and adipose tissue (51). Because skeletal muscle constitutes nearly 45% of lean body mass and accounts for ∼80% of insulin-stimulated glucose uptake, the potential importance of metformin action in this tissue should not be discounted. Skeletal muscle expresses OCT1 and OCT3 (52), and these will help facilitate metformin uptake into this tissue, although the mechanisms by which the biguanide exerts its insulin-like effect in muscle cells remains poorly defined. Numerous reports in the literature suggest that metformin can stimulate components of the insulin signaling cascade or potentiate the effects of the hormone on early signaling events (8, 9, 25, 45, 53). In isolated rat cardiomyocytes, an 18-h incubation of cells with metformin induces a significant phosphorylation of PKB via a PI3K-dependent mechanism (25), whereas in aortic endothelial cells, the drug stimulates Src-associated PI3K activity (22). However, the studies presented here in skeletal muscle cells, indicate that the stimulatory effect of metformin on glucose uptake is unlikely to involve activation of PI3K-directed signaling given that we could not detect any enhancement in IRS/PI3K association, PIP3 synthesis, or indeed activation of PKB/Akt, a downstream PI3K target. Moreover, the finding that insulin augments the biguanide-stimulated glucose uptake in a fully additive but PI3K-dependent manner further strengthens the idea that proximal signaling events involved in initiating metformin action are distinct from those involved in insulin signaling.
In liver, metformin contributes significantly toward the suppression of hepatic gluconeogenesis and glucose output (3). Although the biguanide activates AMPK in liver (3), recent work has suggested that inhibition of hepatic gluconeogenesis occurs in the absence of this kinase (19). Nonetheless, based on numerous reports showing that metformin activates AMPK, there is a growing assumption that the metabolic effects of the biguanide in other tissues, such as skeletal muscle, are likely to be mediated primarily via AMPK. However, a direct assessment of whether these biguanides stimulate muscle glucose uptake under circumstances where, for example, (i) AMPK activation is lost or substantially reduced as, for example, in muscle of animals lacking the upstream-activating kinase LKB-1 (54), (ii) a dominant interfering AMPK mutant is being expressed (55), or (iii) animals are deficient in one of the two catalytic AMPK α subunits (56) is still lacking given that the focus of such studies has invariably been to establish the role played by AMPK with respect to AICAR- or contraction-stimulated glucose transport. Although we are aware of one study in which a 3-h incubation of isolated rat muscle with a relatively high metformin concentration was shown to enhance AMPK activity and induce a very modest increase in glucose uptake (57), no direct assessment was made to establish whether the increase in glucose uptake was AMPK-dependent.
Given that a substantial component of the biguanide-stimulated glucose uptake in our studies was PI3K-independent, we explored the role played by AMPK in metformin-stimulated glucose uptake. Our data indicate that although metformin activates AMPK in muscle cells, this appears to make a relatively small contribution toward the overall increase in glucose uptake elicited by the drug. This proposition is based on four separate lines of evidence. First, although AMPK phosphorylation/activation is induced to comparable levels in response to metformin and AICAR within 2 h, only AICAR stimulates glucose uptake at this time point. This may reflect differential targeting of different subcellular pools of AMPK, in which only that activated by AICAR subsequently stimulates glucose uptake. Second, although 16 h of cell incubation with metformin, phenformin, and AICAR induces comparable T-loop phosphorylation/activation of AMPK, the increase in glucose uptake in response to both biguanides was far greater than that seen with AICAR. This latter observation suggests that the stimulatory effects of both biguanides on glucose uptake may require a certain threshold accumulation within muscle cells and only becomes evident upon sustained exposure to the drugs. Third, expression of a dominant interfering AMPK mutant suppresses AMPK-directed signaling (i.e. ACC-2 phosphorylation) in response to both metformin and phenformin, but its ability to inhibit glucose uptake in response to both drugs was marginal. In contrast, expressing the dominant negative AMPK completely suppressed the increase in glucose uptake induced by AICAR treatment. Fourth, muscle cells in which AMPK had been silenced did not exhibit any increase in AICAR-stimulated glucose uptake but still retained a significant stimulatory response to both metformin and phenformin. Finally, muscle cells generated from mice deficient in both catalytic AMPK subunits (α1−/−/α2−/−) retained a significant capacity to up-regulate glucose uptake following metformin incubation. Collectively, these findings imply that the majority of the biguanide-induced increase in glucose uptake in skeletal muscle cells occurs via an AMPK-independent mechanism. Importantly, although our data indicate that there is a small AMPK-dependent component underpinning the biguanide effect on glucose uptake, our findings also highlight the need for caution when using either metformin or phenformin to study the consequences of AMPK activation, a caveat that has already been highlighted by others investigating regulation of hepatic glucose phosphorylation using different AMPK activators (58).
Previous work in L6 myotubes has shown that metformin does not instigate changes in GLUT1 or GLUT4 protein expression (37) and that the increase in glucose uptake elicited by the drug is associated with an elevation in the plasma membrane abundance of glucose transporters (4). Similar findings have also recently been reported using isolated rat cardiomyocytes in which it has been suggested that metformin increases glucose transport by reducing GLUT4 endocytosis via an AMPK-dependent mechanism (25). However, based on the arguments presented above, it seems unlikely that changes in cell surface recycling kinetics of glucose transporters can be wholly accounted for by AMPK in L6 muscle cells. It is plausible that metformin may signal via other pathways regulating carrier trafficking and/or recycling, but in separate experiments using a battery of kinase inhibitors that target the ERK, p38 MAPK, JNK, and mammalian target of rapamycin pathways, we have found no evidence to suggest that these act as effectors of biguanide action on glucose transport in L6 muscle cells (data not shown). Intriguingly, however, as part of these kinase inhibitor studies, we did find that Ro 31.8220, a widely used PKC inhibitor, suppressed biguanide-stimulated glucose uptake. This observation is compatible with a recent study that implicated a major role for aPKCs in metformin-stimulated glucose uptake in muscle cells, and it was further strengthened by our own observation that both metformin and phenformin stimulate aPKC in L6 myotube muscle cells in an Ro 31.8220-sensitive manner. However, our data indicate that although both metformin and phenformin activate aPKC in L6 myotubes, aPKC activation appears to be dispensable with respect to stimulation of glucose uptake, given that it is retained in muscle cells stably depleted of aPKC.
Evidence exists in the literature showing that in other cell types metformin can promote PKC activation independently of AMPK (47, 59). We therefore hypothesized that the suppressive effect of Ro 31.8220 on metformin-stimulated glucose uptake may reflect its inhibition of other PKC family members involved in the biguanide effect. A role for calcium-dependent PKCs in the activation of glucose uptake in L6 muscle cells in response to stimuli, such as dinitrophenol, which uncouple mitochondrial function and elevate cytosolic calcium, has been suggested (60). Metformin also impairs mitochondrial function via its inhibitory effect on Complex I (40, 41) and, intriguingly, calcium appears to play a permissive role in the metformin-mediated stimulation of muscle glucose uptake (4). It is unclear how increases in cytosolic calcium might be instigated, but metformin has been reported to promote depolarization of liver cells both in vivo and in vitro (61) that may contribute to enhanced calcium entry. These observations may be of relevance given that in L6 muscle cells membrane depolarization has been shown to elevate intracellular calcium and promote a gain in surface GLUT4 by reducing endocytosis via a mechanism that is AMPK-independent but involves activation of conventional PKCs (62). Our finding that selective inhibitors of novel and conventional PKCs exert a suppressive effect on metformin-stimulated glucose uptake is consistent with these studies. Establishing whether metformin can depolarize muscle cells and if this is mechanistically linked to its stimulatory effect upon glucose uptake via activation of select PKC isoforms (of the novel and/or conventional PKC family) represent an important investigative goal of future studies.
This work was supported in part by the Canadian Diabetes Association, Canadian Institutes for Health Research, Integrated Project Contract LSHM-CT-20004-005272 from the European Commission, Medical Research Council, Biotechnology and Biological Sciences Research Council, Wellcome Trust Programme Grant 081195, Diabetes Research and Wellness Foundation, and Diabetes UK.
- AMPK
- AMP-activated protein kinase
- aPKC
- atypical PKC
- 2DG
- 2-deoxyglucose
- PKB
- protein kinase B
- CA-AMPK
- constitutively active AMPK
- DN-AMPK
- dominant negative AMPK
- oligo
- oligonucleotide
- PIP3
- phosphatidylinositol 3,4,5-trisphosphate
- AICAR
- 5-amino-1-β-d-ribofuranosyl-imidazole-4-carboxamide
- IRS
- insulin receptor substrate.
REFERENCES
- 1. Stumvoll M., Nurjhan N., Perriello G., Dailey G., Gerich J. E. (1995) Metabolic effects of metformin in non-insulin-dependent diabetes mellitus. N. Engl. J. Med. 333, 550–554 [DOI] [PubMed] [Google Scholar]
- 2. Hundal R. S., Krssak M., Dufour S., Laurent D., Lebon V., Chandramouli V., Inzucchi S. E., Schumann W. C., Petersen K. F., Landau B. R., Shulman G. I. (2000) Mechanism by which metformin reduces glucose production in type 2 diabetes. Diabetes 49, 2063–2069 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Shaw R. J., Lamia K. A., Vasquez D., Koo S. H., Bardeesy N., Depinho R. A., Montminy M., Cantley L. C. (2005) The kinase LKB1 mediates glucose homeostasis in liver and therapeutic effects of metformin. Science 310, 1642–1646 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Hundal H. S., Ramlal T., Reyes R., Leiter L. A., Klip A. (1992) Cellular mechanism of metformin action involves glucose transporter translocation from an intracellular pool to the plasma membrane in L6 muscle cells. Endocrinology 131, 1165–1173 [DOI] [PubMed] [Google Scholar]
- 5. Thomas C. R., Turner S. L., Jefferson W. H., Bailey C. J. (1998) Prevention of dexamethasone-induced insulin resistance by metformin. Biochem. Pharmacol. 56, 1145–1150 [DOI] [PubMed] [Google Scholar]
- 6. Borst S. E., Snellen H. G., Lai H. L. (2000) Metformin treatment enhances insulin-stimulated glucose transport in skeletal muscle of Sprague-Dawley rats. Life Sci. 67, 165–174 [DOI] [PubMed] [Google Scholar]
- 7. Dominguez L. J., Davidoff A. J., Srinivas P. R., Standley P. R., Walsh M. F., Sowers J. R. (1996) Effects of metformin on tyrosine kinase activity, glucose transport, and intracellular calcium in rat vascular smooth muscle. Endocrinology 137, 113–121 [DOI] [PubMed] [Google Scholar]
- 8. Kumar N., Dey C. S. (2002) Metformin enhances insulin signaling in insulin-dependent and -independent pathways in insulin-resistant muscle cells. Br. J. Pharmacol. 137, 329–336 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Gunton J. E., Delhanty P. J., Takahashi S., Baxter R. C. (2003) Metformin rapidly increases insulin receptor activation in human liver and signals preferentially through insulin-receptor substrate-2. J. Clin. Endocrinol. Metab. 88, 1323–1332 [DOI] [PubMed] [Google Scholar]
- 10. Fantus I. G., Brosseau R. (1986) Mechanism of action of metformin. Insulin receptor and postreceptor effects in vitro and in vivo. J. Clin. Endocrinol. Metab. 63, 898–905 [DOI] [PubMed] [Google Scholar]
- 11. Hardie D. G. (2011) AMP-activated protein kinase. An energy sensor that regulates all aspects of cell function. Genes Dev. 25, 1895–1908 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Owen M. R., Doran E., Halestrap A. P. (2000) Evidence that metformin exerts its anti-diabetic effects through inhibition of complex 1 of the mitochondrial respiratory chain. Biochem. J. 348, 607–614 [PMC free article] [PubMed] [Google Scholar]
- 13. El-Mir M. Y., Nogueira V., Fontaine E., Avéret N., Rigoulet M., Leverve X. (2000) Dimethylbiguanide inhibits cell respiration via an indirect effect targeted on the respiratory chain complex I. J. Biol. Chem. 275, 223–228 [DOI] [PubMed] [Google Scholar]
- 14. Zheng D., MacLean P. S., Pohnert S. C., Knight J. B., Olson A. L., Winder W. W., Dohm G. L. (2001) Regulation of muscle GLUT-4 transcription by AMP-activated protein kinase. J. Appl. Physiol. 91, 1073–1083 [DOI] [PubMed] [Google Scholar]
- 15. Kurth-Kraczek E. J., Hirshman M. F., Goodyear L. J., Winder W. W. (1999) 5′-AMP-activated protein kinase activation causes GLUT4 translocation in skeletal muscle. Diabetes 48, 1667–1671 [DOI] [PubMed] [Google Scholar]
- 16. Buhl E. S., Jessen N., Schmitz O., Pedersen S. B., Pedersen O., Holman G. D., Lund S. (2001) Chronic treatment with 5-aminoimidazole-4-carboxamide-1-β-d-ribofuranoside increases insulin-stimulated glucose uptake and GLUT4 translocation in rat skeletal muscles in a fiber type-specific manner. Diabetes 50, 12–17 [DOI] [PubMed] [Google Scholar]
- 17. Lochhead P. A., Salt I. P., Walker K. S., Hardie D. G., Sutherland C. (2000) 5-Aminoimidazole-4-carboxamide riboside mimics the effects of insulin on the expression of the two key gluconeogenic genes PEPCK and glucose 6-phosphatase. Diabetes 49, 896–903 [DOI] [PubMed] [Google Scholar]
- 18. Bergeron R., Russell R. R., 3rd, Young L. H., Ren J. M., Marcucci M., Lee A., Shulman G. I. (1999) Effect of AMPK activation on muscle glucose metabolism in conscious rats. Am. J. Physiol. 276, E938–E944 [DOI] [PubMed] [Google Scholar]
- 19. Foretz M., Hébrard S., Leclerc J., Zarrinpashneh E., Soty M., Mithieux G., Sakamoto K., Andreelli F., Viollet B. (2010) Metformin inhibits hepatic gluconeogenesis in mice independently of the LKB1/AMPK pathway via a decrease in hepatic energy state. J. Clin. Invest. 120, 2355–2369 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Musi N., Hirshman M. F., Nygren J., Svanfeldt M., Bavenholm P., Rooyackers O., Zhou G., Williamson J. M., Ljunqvist O., Efendic S., Moller D. E., Thorell A., Goodyear L. J. (2002) Metformin increases AMP-activated protein kinase activity in skeletal muscle of subjects with type 2 diabetes. Diabetes 51, 2074–2081 [DOI] [PubMed] [Google Scholar]
- 21. Hawley S. A., Ross F. A., Chevtzoff C., Green K. A., Evans A., Fogarty S., Towler M. C., Brown L. J., Ogunbayo O. A., Evans A. M., Hardie D. G. (2010) Use of cells expressing γ subunit variants to identify diverse mechanisms of AMPK activation. Cell Metab. 11, 554–565 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zou M. H., Kirkpatrick S. S., Davis B. J., Nelson J. S., Wiles W. G., 4th, Schlattner U., Neumann D., Brownlee M., Freeman M. B., Goldman M. H. (2004) Activation of the AMP-activated protein kinase by the anti-diabetic drug metformin in vivo. Role of mitochondrial reactive nitrogen species. J. Biol. Chem. 279, 43940–43951 [DOI] [PubMed] [Google Scholar]
- 23. Hajduch E., Litherland G. J., Hundal H. S. (2001) Protein kinase B (PKB/Akt). A key regulator of glucose transport? FEBS Lett. 492, 199–203 [DOI] [PubMed] [Google Scholar]
- 24. Whiteman E. L., Cho H., Birnbaum M. J. (2002) Role of Akt/protein kinase B in metabolism. Trends Endocrinol. Metab. 13, 444–451 [DOI] [PubMed] [Google Scholar]
- 25. Yang J., Holman G. D. (2006) Long term metformin treatment stimulates cardiomyocyte glucose transport through an AMP-activated protein kinase-dependent reduction in GLUT4 endocytosis. Endocrinology 147, 2728–2736 [DOI] [PubMed] [Google Scholar]
- 26. Ouyang J., Parakhia R. A., Ochs R. S. (2011) Metformin activates AMP kinase through inhibition of AMP deaminase. J. Biol. Chem. 286, 1–11 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Sajan M. P., Bandyopadhyay G., Miura A., Standaert M. L., Nimal S., Longnus S. L., Van Obberghen E., Hainault I., Foufelle F., Kahn R., Braun U., Leitges M., Farese R. V. (2010) AICAR and metformin, but not exercise, increase muscle glucose transport through AMPK-, ERK-, and PDK1-dependent activation of atypical PKC. Am. J. Physiol. Endocrinol. Metab. 298, E179–E192 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Hajduch E., Alessi D. R., Hemmings B. A., Hundal H. S. (1998) Constitutive activation of protein kinase Bα by membrane targeting promotes glucose and system A amino acid transport, protein synthesis, and inactivation of glycogen synthase kinase 3 in L6 muscle cells. Diabetes 47, 1006–1013 [DOI] [PubMed] [Google Scholar]
- 29. Lantier L., Mounier R., Leclerc J., Pende M., Foretz M., Viollet B. (2010) Coordinated maintenance of muscle cell size control by AMP-activated protein kinase. FASEB J. 24, 3555–3561 [DOI] [PubMed] [Google Scholar]
- 30. Blair A. S., Hajduch E., Litherland G. J., Hundal H. S. (1999) Regulation of glucose transport and glycogen synthesis in L6 muscle cells during oxidative stress. Evidence for cross-talk between the insulin and SAPK2/p38 mitogen-activated protein kinase signaling pathways. J. Biol. Chem. 274, 36293–36299 [DOI] [PubMed] [Google Scholar]
- 31. Bradford M. M. (1976) A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 72, 248–254 [DOI] [PubMed] [Google Scholar]
- 32. Hardie D. G., Salt I. P., Davies S. P. (2000) Analysis of the role of the AMP-activated protein kinase in the response to cellular stress. Methods Mol. Biol. 99, 63–74 [DOI] [PubMed] [Google Scholar]
- 33. Gray A., Olsson H., Batty I. H., Priganica L., Peter Downes C. (2003) Nonradioactive methods for the assay of phosphoinositide 3-kinases and phosphoinositide phosphatases and selective detection of signaling lipids in cell and tissue extracts. Anal. Biochem. 313, 234–245 [DOI] [PubMed] [Google Scholar]
- 34. Woods A., Azzout-Marniche D., Foretz M., Stein S. C., Lemarchand P., Ferré P., Foufelle F., Carling D. (2000) Characterization of the role of AMP-activated protein kinase in the regulation of glucose-activated gene expression using constitutively active and dominant negative forms of the kinase. Mol. Cell. Biol. 20, 6704–6711 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35. Lipina C., Stretton C., Hastings S., Hundal J. S., Mackie K., Irving A. J., Hundal H. S. (2010) Regulation of MAPK-directed mitogenic and protein kinase B-mediated signaling by cannabinoid receptor type 1 in skeletal muscle cells. Diabetes 59, 375–385 [DOI] [PMC free article] [PubMed] [Google Scholar] [Retracted]
- 36. Stretton C., Evans A., Hundal H. S. (2010) Cellular depletion of atypical PKCλ is associated with enhanced insulin sensitivity and glucose uptake in L6 rat skeletal muscle cells. Am. J. Physiol. Endocrinol. Metab. 299, E402–E412 [DOI] [PubMed] [Google Scholar]
- 37. Klip A., Gumà A., Ramlal T., Bilan P. J., Lam L., Leiter L. A. (1992) Stimulation of hexose transport by metformin in L6 muscle cells in culture. Endocrinology 130, 2535–2544 [DOI] [PubMed] [Google Scholar]
- 38. Tsakiridis T., McDowell H. E., Walker T., Downes C. P., Hundal H. S., Vranic M., Klip A. (1995) Multiple roles of phosphatidylinositol 3-kinase in regulation of glucose transport, amino acid transport, and glucose transporters in L6 skeletal muscle cells. Endocrinology 136, 4315–4322 [DOI] [PubMed] [Google Scholar]
- 39. Green C. J., Göransson O., Kular G. S., Leslie N. R., Gray A., Alessi D. R., Sakamoto K., Hundal H. S. (2008) Use of Akt inhibitor and a drug-resistant mutant validates a critical role for protein kinase B/Akt in the insulin-dependent regulation of glucose and system A amino acid uptake. J. Biol. Chem. 283, 27653–27667 [DOI] [PubMed] [Google Scholar]
- 40. Fryer L. G., Parbu-Patel A., Carling D. (2002) The anti-diabetic drugs rosiglitazone and metformin stimulate AMP-activated protein kinase through distinct signaling pathways. J. Biol. Chem. 277, 25226–25232 [DOI] [PubMed] [Google Scholar]
- 41. Hawley S. A., Gadalla A. E., Olsen G. S., Hardie D. G. (2002) The antidiabetic drug metformin activates the AMP-activated protein kinase cascade via an adenine nucleotide-independent mechanism. Diabetes 51, 2420–2425 [DOI] [PubMed] [Google Scholar]
- 42. Pilon G., Dallaire P., Marette A. (2004) Inhibition of inducible nitric-oxide synthase by activators of AMP-activated protein kinase. A new mechanism of action of insulin-sensitizing drugs. J. Biol. Chem. 279, 20767–20774 [DOI] [PubMed] [Google Scholar]
- 43. Hardie D. G., Ross F. A., Hawley S. A. (2012) AMPK: a nutrient and energy sensor that maintains energy homeostasis. Nat. Rev. Mol. Cell Biol. 22, 251–262 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Bogachus L. D., Turcotte L. P. (2010) Genetic down-regulation of AMPK-α isoforms uncovers the mechanism by which metformin decreases FA uptake and oxidation in skeletal muscle cells. Am. J. Physiol. Cell Physiol. 299, C1549–C1561 [DOI] [PubMed] [Google Scholar]
- 45. Longnus S. L., Ségalen C., Giudicelli J., Sajan M. P., Farese R. V., Van Obberghen E. (2005) Insulin signaling downstream of protein kinase B is potentiated by 5′-AMP-activated protein kinase in rat hearts in vivo. Diabetologia 48, 2591–2601 [DOI] [PubMed] [Google Scholar]
- 46. Luna V., Casauban L., Sajan M. P., Gomez-Daspet J., Powe J. L., Miura A., Rivas J., Standaert M. L., Farese R. V. (2006) Metformin improves atypical protein kinase C activation by insulin and phosphatidylinositol-3,4,5-(PO4)3 in muscle of diabetic subjects. Diabetologia 49, 375–382 [DOI] [PubMed] [Google Scholar]
- 47. Saeedi R., Parsons H. L., Wambolt R. B., Paulson K., Sharma V., Dyck J. R., Brownsey R. W., Allard M. F. (2008) Metabolic actions of metformin in the heart can occur by AMPK-independent mechanisms. Am. J. Physiol. Heart Circ. Physiol. 294, H2497–H2506 [DOI] [PubMed] [Google Scholar]
- 48. Standaert M. L., Galloway L., Karnam P., Bandyopadhyay G., Moscat J., Farese R. V. (1997) Protein kinase Cζ as a downstream effector of phosphatidylinositol 3-kinase during insulin stimulation in rat adipocytes. Potential role in glucose transport. J. Biol. Chem. 272, 30075–30082 [DOI] [PubMed] [Google Scholar]
- 49. Alessi D. R. (1997) The protein kinase C inhibitors Ro 318220 and GF 109203X are equally potent inhibitors of MAPKAP kinase-1β (Rsk-2) and p70 S6 kinase. FEBS Lett. 402, 121–123 [DOI] [PubMed] [Google Scholar]
- 50. Powell D. J., Hajduch E., Kular G., Hundal H. S. (2003) Ceramide disables 3-phosphoinositide binding to the pleckstrin homology domain of protein kinase B (PKB)/Akt by a PKCζ-dependent mechanism. Mol. Cell. Biol. 23, 7794–7808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Diamanti-Kandarakis E., Christakou C. D., Kandaraki E., Economou F. N. (2010) Metformin. An old medication of new fashion. Evolving new molecular mechanisms and clinical implications in polycystic ovary syndrome. Eur. J. Endocrinol. 162, 193–212 [DOI] [PubMed] [Google Scholar]
- 52. Chen L., Pawlikowski B., Schlessinger A., More S. S., Stryke D., Johns S. J., Portman M. A., Chen E., Ferrin T. E., Sali A., Giacomini K. M. (2010) Role of organic cation transporter 3 (SLC22A3) and its missense variants in the pharmacologic action of metformin. Pharmacogenet. Genomics 20, 687–699 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Holland W., Morrison T., Chang Y., Wiernsperger N., Stith B. J. (2004) Metformin (glucophage) inhibits tyrosine phosphatase activity to stimulate the insulin receptor tyrosine kinase. Biochem. Pharmacol. 67, 2081–2091 [DOI] [PubMed] [Google Scholar]
- 54. Sakamoto K., McCarthy A., Smith D., Green K. A., Grahame Hardie D., Ashworth A., Alessi D. R. (2005) Deficiency of LKB1 in skeletal muscle prevents AMPK activation and glucose uptake during contraction. EMBO J. 24, 1810–1820 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55. Mu J., Brozinick J. T., Jr., Valladares O., Bucan M., Birnbaum M. J. (2001) A role for AMP-activated protein kinase in contraction- and hypoxia-regulated glucose transport in skeletal muscle. Mol. Cell 7, 1085–1094 [DOI] [PubMed] [Google Scholar]
- 56. Jørgensen S. B., Viollet B., Andreelli F., Frøsig C., Birk J. B., Schjerling P., Vaulont S., Richter E. A., Wojtaszewski J. F. (2004) Knockout of the α2 but not α1,5′-AMP-activated protein kinase isoform abolishes 5-aminoimidazole-4-carboxamide-1-β-4-ribofuranoside but not contraction-induced glucose uptake in skeletal muscle. J. Biol. Chem. 279, 1070–1079 [DOI] [PubMed] [Google Scholar]
- 57. Zhou G., Myers R., Li Y., Chen Y., Shen X., Fenyk-Melody J., Wu M., Ventre J., Doebber T., Fujii N., Musi N., Hirshman M. F., Goodyear L. J., Moller D. E. (2001) Role of AMP-activated protein kinase in mechanism of metformin action. J. Clin. Invest. 108, 1167–1174 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Guigas B., Bertrand L., Taleux N., Foretz M., Wiernsperger N., Vertommen D., Andreelli F., Viollet B., Hue L. (2006) 5-Aminoimidazole-4-carboxamide-1-β-d-ribofuranoside and metformin inhibit hepatic glucose phosphorylation by an AMP-activated protein kinase-independent effect on glucokinase translocation. Diabetes 55, 865–874 [DOI] [PubMed] [Google Scholar]
- 59. Hwang Y. P., Jeong H. G. (2010) Metformin blocks migration and invasion of tumor cells by inhibition of matrix metalloproteinase-9 activation through a calcium and protein kinase Cα-dependent pathway. Phorbol 12-myristate 13-acetate-induced/extracellular signal-regulated kinase/activator protein-1. Br. J. Pharmacol. 160, 1195–1211 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60. Patel N., Khayat Z. A., Ruderman N. B., Klip A. (2001) Dissociation of 5′-AMP-activated protein kinase activation and glucose uptake stimulation by mitochondrial uncoupling and hyperosmolar stress: differential sensitivities to intracellular Ca2+ and protein kinase C inhibition. Biochem. Biophys. Res. Commun. 285, 1066–1070 [DOI] [PubMed] [Google Scholar]
- 61. Lutz T. A., Estermann A., Haag S., Scharrer E. (2001) Depolarization of the liver cell membrane by metformin. Biochim. Biophys. Acta 1513, 176–184 [DOI] [PubMed] [Google Scholar]
- 62. Wijesekara N., Tung A., Thong F., Klip A. (2006) Muscle cell depolarization induces a gain in surface GLUT4 via reduced endocytosis independently of AMPK. Am. J. Physiol. Endocrinol. Metab. 290, E1276–E1286 [DOI] [PubMed] [Google Scholar]