Background: LPCAT3 is involved in liver PC remodeling.
Results: LPCAT3 knockdown in the liver significantly accumulates LysoPC, which promotes VLDL production by enhancing liver MTP expression.
Conclusion: Liver LPCAT3 modulates VLDL production.
Significance: LPCAT3-mediated PC remodeling in the liver has an important impact on the production of VLDL, a well known atherogenic particle.
Keywords: Enzyme Inactivation, Lipoprotein Secretion, Liver, Lysophospholipid, Phosphatidylcholine, shRNA, Microsomal Triglyceride Transfer Protein, Very Low Density Lipoprotein
Abstract
After de novo biosynthesis phospholipids undergo extensive remodeling by the Lands' cycle. Enzymes involved in phospholipid biosynthesis have been studied extensively but not those involved in reacylation of lysophosphopholipids. One key enzyme in the Lands' cycle is fatty acyl-CoA:lysophosphatidylcholine acyltransferase (LPCAT), which utilizes lysophosphatidylcholine (LysoPC) and fatty acyl-CoA to produce various phosphatidylcholine (PC) species. Four isoforms of LPCAT have been identified. In this study we found that LPCAT3 is the major hepatic isoform, and its knockdown significantly reduces hepatic LPCAT activity. Moreover, we report that hepatic LPCAT3 knockdown increases certain species of LysoPCs and decreases certain species of PC. A surprising observation was that LPCAT3 knockdown significantly reduces hepatic triglycerides. Despite this, these mice had higher plasma triglyceride and apoB levels. Lipoprotein production studies indicated that reductions in LPCAT3 enhanced assembly and secretion of triglyceride-rich apoB-containing lipoproteins. Furthermore, these mice had higher microsomal triglyceride transfer protein (MTP) mRNA and protein levels. Mechanistic studies in hepatoma cells revealed that LysoPC enhances secretion of apoB but not apoA-I in a concentration-dependent manner. Moreover, LysoPC increased MTP mRNA, protein, and activity. In short, these results indicate that hepatic LPCAT3 modulates VLDL production by regulating LysoPC levels and MTP expression.
Introduction
Dyslipidemia characterized by higher levels of apoB-containing remnant lipoproteins is a major risk factor for atherosclerosis that is still the major cause of mortality in developed countries. Liver produces triglyceride-rich apoB-containing very low density lipoproteins (VLDL) (1, 2). Intravascular remodeling of VLDL produces LDL, the principal atherogenic lipoprotein in human plasma. VLDL assembly begins with the translation of apoB followed by its interaction with the inner leaflet of the endoplasmic reticulum.
Microsomal triglyceride transfer protein (MTP)5 plays a crucial role in the lipidation of this nascent peptide by physically interacting with it and depositing lipids on it, resulting a primordial lipoprotein (3). This process is referred to as “first step of lipidation” of apoB (4–6). The 2nd step of the lipidation, in which the apoB-containing primordial particle fuses with apoB-free/triglyceride-rich lipid droplets, is necessary for VLDL maturation (7–9). Besides MTP, LDL receptors and phospholipid transfer protein may also be involved in hepatic VLDL production (10–12). In addition to various proteins, lipid availability is also crucial for VLDL biogenesis. Abundant TG availability is essential but not sufficient to drive VLDL assembly, as exemplified by studies with hepatic cells treated with n-3 fatty acids (13, 14) or insulin (15), where active TG synthesis does not always enhance VLDL production. Factors other than TG availability must play an important role in facilitating VLDL production. In this regard phosphatidylcholine biosynthesis has been shown to be important for lipoprotein assembly (16). However, very little is known about the role of phospholipid remodeling in VLDL assembly and secretion.
Phosphatidylcholines are first synthesized from glycerol-3-phosphate in the de novo biosynthetic pathway, originally described by Kennedy and Weiss in 1956 (Kennedy pathway) (17) and undergo maturation in the remodeling pathway, as reported by Lands in 1958 (Lands' cycle) (18). The PC remodeling consists of two steps: the deacylation step, which is catalyzed by calcium independent phospholipase A2 (iPLA2) (19), and the reacylation step, which is catalyzed by lysophosphatidylcholine acyltransferases (LPCATs) (20–24).
A potential role of Lands' cycle in VLDL secretion was first suggested by Bar-On et al. (25) who showed that about 50% of VLDL-TG secreted from rat liver might use fatty acids derived from PC remodeling. It was also reported that phospholipid turnover might be associated with VLDL secretion in rat liver cells treated with oleate (26). Furthermore, the assembly of VLDL in rat hepatoma McA-RH7777 cells is inhibited by inhibitors (27). LPCAT activity could also be important in VLDL production by influencing LysoPC levels, which can regulate apoB secretion (28).
Four isoforms of LPCAT have been identified. LPCAT3 encodes a protein of 487 amino acids with a calculated molecular mass of 56 kDa (23). We have previously shown that LPCAT3 is localized to the endoplasmic reticulum and is primarily expressed in metabolic tissues including liver, adipose, pancreas, and small intestine. LPCAT3 is primarily responsible for hepatic LPCAT activity (23). Furthermore, peroxisome proliferator-activated receptor α (PPARα) agonists dose-dependently increased LPCAT3 in liver, implicating a role of LPCAT3 in lipid homeostasis (23).
In this study we investigated the impact of LPCAT3 knockdown on lipoprotein metabolism. We show for the first time that liver LPCAT3 activity directly regulates VLDL production by increasing MTP expression.
EXPERIMENTAL PROCEDURES
Animal and Diet
Male wild type 12 weeks old C57BL/6J mice were fed the rodent chow diet (Purina Laboratory Rodent Chow 5001). All animal procedures were approved by the SUNY Downstate Medical Centre Animal Care and Use Committee.
Lipid Measurements
Fasted blood was collected for lipoprotein isolation and lipid measurement. Plasma total cholesterol, phospholipid, and triglyceride were assayed by enzymatic methods (Wako) (29).
Plasma Apolipoprotein Measurements
Plasma apolipoprotein levels were determined as previously described (30). Briefly, 0.2 μl of plasma was separated by 4–15% SDS gel electrophoresis and immunoblotted with polyclonal antibodies against apoB (U. S. Biochemical Corp.), apoE (Abcam), and apoA-I (Abcam).
Fast Protein Liquid Chromatography (FPLC)
Lipoprotein profiles were obtained by FPLC using a Superose 6B column. A 250-μl aliquot of pooled plasma was loaded onto the column and eluted with FPLC buffer (50 mm Tris, pH 7.4) at a constant flow rate of 0.35 ml/min. An aliquot of 100 μl from each fraction was used for the determination of lipids.
In Vivo VLDL Production Studies
Mice were injected (through femoral vein) with [35S]methionine (10 μCi) to label apoB, [14C]oleic acid (10 μCi) to label triglyceride, and with Poloxamer 407 to block the clearance of VLDL from the circulation. Plasma (150 μl) was collected after 2 h of injection, and VLDL was isolated from the plasma by ultracentrifugation. The same volume of isolated VLDL (25 μl) was loaded on 4–15% gradient gel, and apoB was separated by SDS-PAGE. Incorporation of 35S into ApoB48 and ApoB100 was assessed with a Fuji Bio-Imaging Analyzer (31). Lipids in isolated VLDL were extracted using the Folch method (32) and separated by thin-layer chromatograph (TLC). The corresponding [14C]TG was scratched from the plate. The amount of radioactivity (cpm) in the TG fraction was measured by a liquid scintillation counter.
LPCAT Activity Assay
Acyltransferase activity was determined by measuring the incorporation of radiolabeled acyl-CoAs into phospholipids. The reaction mixture in a total volume of 100 μl contained 75 mm Tris-HCl, pH 7.5, 1 mg/ml fatty acid-free bovine serum albumin, 200 μm lysophosphatidylcholine, 20 μm [1-14C]acyl-CoA, and 5 μg of membrane proteins from hepatocytes or liver. The reaction was started by the addition of the membrane proteins, incubated for 20 min at room temperature, and stopped by adding 1 ml of chloroform/methanol (2:1, v/v). Phospholipids were extracted by the method of Bligh and Dyer (33). The organic phase was air-dried and separated by thin layer chromatography using chloroform/methanol/water (65:25:4, v/v) as the solvent followed by exposure to a Phosphor-Imager screen.
Alternatively, 100 mg of liver tissue was homogenized in 1 ml of homogenization buffer (75 mm Tris-HCl pH 7.5, 1 mg/ml BSA). Nuclear debris was removed by centrifugation at 600 × g, and the supernatant was used as the source of LPCAT3. Each reaction contained 100 μl of homogenization and reaction buffer, 4 μl of 12:0 LysoNBD-PC (Avanti) (0.5 mg/ml), 4 μl of 18:1 (n9) oleoyl coenzyme A (Avanti Polar Lipids) (2.5 mg/ml), and 20 μl of liver lysate (100 μg of protein). Standard 18:1–12:0 NBD-PC was also from Avanti. The reaction tube was kept at room temperature exactly for 10 min, and the reaction was terminated by adding chloroform/methanol (1:2, v/v) and mixing vigorously. The lower organic phase was collected after centrifugation (6000 × g) and dried under nitrogen gas. Lipids were redissolved in 30 μl of chloroform and then were applied to a TLC plate. The running solvent was chloroform/methanol/water (65:25:4, v/v). The fluorescence signal was detected under UV, and the intensity was measured by Image-Pro software.
MTP Activity Assay
Cell and liver microsomal contents were used to measure MTP activity as described (34) using a kit (Chylos, Inc.).
siRNA Transfection
Human LPCAT3 siRNA (200 pmol; Ambion) and control siRNA (silencer negative control 1; Ambion) were transfected using Lipofectamine 2000 (Invitrogen) according to the manufacturer's protocol. After 2 days cells were collected to isolate RNA and to quantify gene expression by reverse transcription and real-time PCR.
LPCAT3 shRNA Adenovirus Preparation
The sequence against mouse LPCAT3 mRNA (ggatccGGCTTAAGGTGTACAGATCTTCAAGAGAGATCTGTACACCTTAAGCCTTTTTTACGCGTgaattc) was cloned into BamHI/EcoRI sites of RNAi ready pSIREN-DNR vector, and the recombinant adenoviruses were generated by Vector BioLabs (Philadelphia, PA). Before in vivo use, both scrambled control adenoviruses and LPCAT3 shRNA were purified through a cesium chloride gradient. C57BL/6 mice (12 weeks) were injected (through leg vein) with 3 × 109 plaque-forming units/mouse of the above adenovirus, and the mice were sacrificed 5 days after injection. Liver tissues were homogenized, and lipids (triglyceride and phosphatidylcholine) were measured by enzymatic assays. Liver LysoPCs were analyzed by LC/MS/MS.
Liver Triglyceride Measurement
Liver tissues (100 mg) were homogenized in 1 ml of PBS, then 200 μl of liver homogenate was mixed with 400 μl of PBS and 850 μl of chloroform/methanol (2:1, v/v) solution, vortexed, and spun at 10,000 rpm for 10 min. Lower organic layer was dried under nitrogen gas. Triglycerides were assayed by enzymatic methods (Wako).
Liver LysoPC and PC Measurement by Infusion-based High Resolution MS
Liver lipid extracts were used for lysoPC and PC measurement. Briefly, samples were homogenized in PBS solution. The homogenized solution was added with internal standard solutions before applying Bligh/Dyer method (33). Data were acquired on a Triple TOF 5600 (AB-Sciex, Concord, ON). The mass spectrometer was operated in TOF mode at a resolution of 35,000, electrospray source voltage of 5500 v on the Turbo V spray interface, declustering potential of 100 V, scanning from 100 to 1200 Da. At this MS resolution, LysoPC and PC do not have any major interference. Of note is that the C13 isotopes of PC species are resolved from the sphingomyelin interferences by ∼60 mDa, making it possible to directly compare the heights of both the PC and sphingomyelin species. Samples were infused at ∼20 μl/min in a solution of mixture of 4:2:1 isopropanol:methanol:chloroform with 10 mm ammonium acetate with a Reliance autosampler (Sparck, Holland, Netherlands) operating in pressurized vessel mode. Analytical samples were spiked with internal standard solutions containing LysoPC and PC (15:0 LysoPC and 14:0/14:0 PC) before extraction. Calibration curves were established by analyzing serial dilutions of multiple LysoPC and PC species in solutions containing internal standards. Most of the analytes reported in this manuscript were quantitated with the curves generated using authentic standards. However, several species were quantitated using their closet analogues. LysoPC 14:0, 16;1, 18:3, and 20:3 levels were inferred from curves using LysoPC 16:0, 18:1, 18:2, and 18;1, respectively. PC 36:3, 36;5, 38:2, 38:3, and 38:5 levels were inferred from the curves of PC 36:4, 36:4, 36:2, 38:4, and 38:4, respectively. Quantitation was accomplished through the use of MultiQuan (AB-Sciex, Concord, ON). A window of ±5 mDa was used to identify the LysoPC and PC from a list of all of the common species. Curves were calculated using 1/× weighting and were applied uniformly.
Exogenous LysoPC Supplementation
Total LysoPC (Avanti Polar Lipids) and 18:1 LysoPC (Avanti Polar Lipids) were dissolved in DMEM containing 0.3% essential fatty acid-free BSA. Huh7 cells were starved in DMEM containing 0.3% essentially fatty acid-free BSA for 3 h, and then Huh7 cells were incubated with different concentration of exogenous total lysoPC or 18:1 LysoPC (0, 25, 50, 100 μm). After 18 h, MTP mRNA, protein, and activity was measured.
Reverse Transcription and Real-time PCR
Liver tissues were homogenized, and total RNA was isolated using TRIzol (Invitrogen) according to the manufacturer's instructions. Afterward, cDNA was synthesized from 500 ng of poly(A) RNA utilizing the Invitrogen SuperScriptTM III First-strand Synthesis System for reverse transcriptase-PCR. The TaqMan gene expression assays ordered from Applied Biosystems (Foster City, CA) were Hs00195039_m1 (human LPCAT3) and Mm00520147_m1 (mouse LPCAT3) and Hs_99999905_m1 (human GAPDH) and Mm99999915_g1 (mouse GAPDH). Real-time PCR was performed on a 7900HT Fast Real-time PCR System (Applied Biosystems) using TaqMan Universal PCR Master Mix (Roche Applied Science). Cycle threshold (Ct) values were obtained using Applied Biosystems SDS2.3 software; 18 S RNA was used as a reference gene. Primers used for mouse MTP were 5′- TCTGCTTCCGTTAAAGGTCACA-3′ and 5′-CCTTTGCCCCCATCAAGAA-3′, and primers for human MTP were 5′-ACGGCCATTCCCATTGTG-3′ and 5′-GCCAGAGCTCCGAGAGAGAA-3′. Primers for 18 S RNA were 5′-AGTCCCTTGCCCTTTGTACACA-3′ and 5′-GATCCGAGGGCCTCACTAAAC-3′.
Statistical Analysis
Each experiment was conducted at least three times. Data are typically expressed as the mean ± S.D. Data between two groups were analyzed by the unpaired, two-tailed Student's t test and among multiple groups by analysis of variance followed by the Student-Newman-Keuls (SNK) test.
RESULTS
LPCAT3 Is Major LPCAT in Liver Cells and Plays Role in ApoB Secretion
LPCAT3 has been recently cloned (23). We determined the tissue distribution of mouse LPCAT3 with real-time PCR. The highest level of expression was observed in the liver followed by pancreas, adipose tissues, and small intestine (Fig. 1A). This distribution is similar to that seen in humans (23). To examine the relative contribution of human LPCAT3 to total cellular LPCAT activity, we used siRNA knockdown technology to effectively reduce LPCAT3 mRNA in HepG2 cells (a human hepatoma cell line) to below 15% that of the control levels (Fig. 1B). As indicated by Fig. 1C, the reduced LPCAT3 expression resulted in about 80% reduction in total LPCAT activity compared with the control. We obtained the same results from Huh7 cells, another human hepatoma cell line (23). These results suggested that LPCAT3 is the major enzyme contributing to LPCAT activity in liver cells and is consistent with its highest expression in the human liver (23). Next, we sought to uncover the role of LPCAT3 in lipid metabolism and found that enzyme knockdown significantly increased apoB, but not apoA-I levels as measured by ELISA in the media (Fig. 1, D and E). These studies indicate that normal LPCAT3 levels antagonize apoB secretion.
FIGURE 1.
LPCAT3 is the major LPCAT in liver cells and plays a role in apoB secretion. Panel A, LPCAT3 mRNA in different mouse tissues was measured by real-time PCR using total RNA. Adi, adipose tissue; Mus, muscle; Pan, pancreas; Spl, spleen; SI, small intestine. Panel B, HepG2 cells were transfected with LPCAT3 siRNA and control siRNA, and LPCAT3 mRNA was measured by real-time PCR. Panel C, total LPCAT activity was measured in LPCAT3 knockdown and control HepG2 cells. Panel D, medium apoB was measure by ELISA. Panel E, medium apoA-I was measured by ELISA. Values are the mean ± S.D. n = 5. *, p < 0.01.
Impact of LPCAT3 Knockdown on Hepatic and Plasma Lipid Homeostasis
To study in vivo role of LPCAT3 in the hepatic and plasma lipid metabolism, we prepared adenoviruses expressing-LPCAT3-shRNA (AdV-shLPCAT3) and a control scramble-shRNA (AdV-shControl) and injected both adenoviruses into wild type mice (C57BL/6). The liver is the primary target after peripheral intravenous injection of recombinant adenovirus (35). We found that LPCAT3 mRNA levels were reduced by 83% in the liver but not in the adipose tissue and pancreas of mice transduced with AdV-shLPCAT3 compared with AdV-shControls (Fig. 2A). Also, we found LPCAT3 mRNA is the major LPCAT mRNA expressed in mouse liver, and AdV-shLPCAT3 had no significant effect on LPCAT1, LPCAT2, and LPCT4 mRNA levels (Fig. 2B). LPCAT activity measurements revealed 71% reduction in shRNA-transduced mice (Fig. 2C). These studies indicate that AdV-shLPCAT3 specifically reduces expression of LPCAT3 and significantly diminishes hepatic LPCAT activity.
FIGURE 2.
shRNA-mediated knockdown of LPCAT3 in mice. AdV-shLPCAT3 and AdV-shControl were injected into normal mice (C57BL/6). Panel A, liver, pancreas, and adipose tissue LPCAT3 mRNA was measured by real-time PCR. Panel B, liver LPCAT1–4 mRNAs were measured by real-time PCR. Panel C, liver total LPCAT activity was measured as described under “Experimental Procedures.” Panel D, liver triglyceride levels were measured. Values are the mean ± S.D. n = 6. *, p < 0.05.
We then studied the effects of LPCAT3 knockdown on hepatic LysoPC and PC homeostasis. We used LC/MS/MS to measure liver LysoPC and PC levels. Lipidomics analyses revealed significant changes in subspecies of both LysoPC and PC. We found that LPCAT3 knockdown significantly increases total LysoPC (38.5%, p < 0.01) (Table 1). Moreover, we found that except for LysoPC containing 14:0, 20:3, and 20:4 fatty acyl groups, all other measured LysoPC species were significantly increased. Although total PC levels did not change significantly, analyses of PC subspecies indicated significant reductions in 36:3, 36:4, 38:2, 38:3, and 38:4 (Table 2). These results suggest that LPCAT3-mediated transacylation specifically modulates a subset of LysoPC and PC molecules.
TABLE 1.
Changes in liver LysoPC species after AdV-shLPCAT3 transductions (nmol/mg of liver)
Value are the mean ± S.D. n = 5. NS, not significant.
| LysoPCs | Control-shRNA | LPCAT3-shRNA | p Value | Increase |
|---|---|---|---|---|
| % | ||||
| 14:0 | 0.373 ± 0.039 | 0.393 ± 0.042 | NS | |
| 16:0 | 1.676 ± 0.283 | 2.629 ± 0.547 | <0.01 | 56.8 |
| 16:1 | 0.088 ± 0.025 | 0.128 ± 0.026 | <0.02 | 45.5 |
| 18:0 | 1.082 ± 0.172 | 1.359 ± 0.255 | <0.01 | 25.6 |
| 18:1 | 0.901 ± 0.025 | 1.366 ± 0.065 | <0.001 | 51.6 |
| 18:2 | 1.079 ± 0.178 | 1.622 ± 0.225 | <0.001 | 50.3 |
| 18:3 | 0.086 ± 0.009 | 0.110 ± 0.018 | <0.02 | 27.9 |
| 20:3 | 0.208 ± 0.026 | 0.203 ± 0.026 | NS | |
| 20:4 | 0.668 ± 0.111 | 0.721 ± 0.077 | NS | |
| Total | 6.161 ± 0.919 | 8.531 ± 1.038 | <0.01 | 38.5 |
TABLE 2.
Changes in liver PC species after AdV-shLPCAT3 transductions (nmol/mg of liver)
Values are the mean ± S.D. n = 5. NS, not significant.
| PCs | Control-shRNA | LPCAT3-shRNA | p Value | Decrease |
|---|---|---|---|---|
| % | ||||
| 32:0 | 5.2 ± 0.8 | 4.3 ± 0.7 | NS | |
| 32:1 | 3.5 ± 0.6 | 4.6 ± 1.5 | NS | |
| 34:0 | 5.5 ± 0.5 | 5.9 ± 0.9 | NS | |
| 34:1 | 52.8 ± 5.5 | 58.6 ± 10.2 | NS | |
| 34:2 | 65.2 ± 6.2 | 68.1 ± 7.5 | NS | |
| 34:3 | 3.3 ± 0.3 | 3.5 ± 0.5 | NS | |
| 36:1 | 8.3 ± 0.6 | 8.6 ± 1.2 | NS | |
| 36:2 | 28.7 ± 1.9 | 27.9 ± 2.2 | NS | |
| 36:3 | 21.2 ± 1.5 | 17.3 ± 1.3 | <0.05 | 18.3 |
| 36:4 | 33.4 ± 2.4 | 28.4 ± 2.6 | <0.01 | 15.0 |
| 36:5 | 3.1 ± 0.5 | 3.1 ± 0.6 | NS | |
| 38:2 | 1.4 ± 0.1 | 0.9 ± 0.1 | <0.01 | 36.7 |
| 38:3 | 6.6 ± 0.4 | 4.2 ± 0.5 | <0.01 | 36.3 |
| 38:4 | 18.0 ± 1.3 | 13.1 ± 0.7 | <0.01 | 27.2 |
| 38:5 | 12.6 ± 0.9 | 11.3 ± 0.5 | NS | |
| 38:6 | 36.8 ± 3.0 | 35.5 ± 5.4 | NS | |
| 40:6 | 11.0 ± 0.8 | 10.4 ± 1.0 | NS | |
| Total | 316.6 ± 55.1 | 305.7 ± 39.3 | NS |
We next sought to study the effect of LPCAT3 knockdown on other lipids. LPCAT3 knockdown had no effect on hepatic cholesterol levels (data not shown), but unexpectedly we found that it significantly reduced triglycerides (Fig. 2D). Moreover, we measured triglyceride levels in the plasma of mice transduced with AdV-shLPCAT3 and found that LPCAT3 knockdown significantly increases plasma triglyceride with no significant effect on other lipids (Table 3). FPLC (Fig. 3A), on a Superose 6B column, showed that increases in plasma triglyceride were mainly due to increases in VLDL/LDL (Fig. 3A). In addition, we found that plasma apoB and apoE, but not apoA-I (Fig. 3, B–E) levels, were significantly increased in the LPCAT3 knockdown animals compared with controls. These studies indicated that LPCAT3 deficiency increases plasma triglyceride-rich apoB-containing lipoproteins.
TABLE 3.
Plasma lipid measurements in mice transduced with AdV-shLPCAT3
Values are the mean ± S.D., n = 5.
| Adv | Phospholipids | Cholesterol | Sphingomyelin | TG |
|---|---|---|---|---|
| mg/dl | ||||
| AdV-control shRNA | 188 ± 12 | 96 ± 6 | 27 ± 5 | 66 ± 8 |
| AdV-LPCAT3 shRNA | 193 ± 19 | 99 ± 11 | 30 ± 7 | 103 ± 12a |
a p < 0.01.
FIGURE 3.
LPCAT3-shRNA-mediated knockdown increases plasma triglyceride-rich lipoprotein levels. Panel A, shown is mouse plasma TG distribution on FPLC. Pooled mouse plasma (250 μl) was loaded on a Superose 6 column. Triglyceride was measured in each fraction as described under “Experimental Procedures.” Panel B, plasma apolipoprotein was measurement by Western blot. Panel C, quantitative display of plasma apoB is shown. Panel D, quantitative display of plasma apoA-I is shown. Panel E, quantitative display of plasma apoE is shown. Values are the mean ± S.D. n = 6. *, p < 0.01.
Next, we hypothesized that LPCAT3 deficiency might lead to increased production of triglyceride-rich apoB-containing lipoproteins. To directly examine in vivo VLDL production rates, both knockdown and control mice were injected with [35S]methionine to label apoB, with [14C]oleic acid to label triglyceride, and with Poloxamer 407 to block the clearance of VLDL from the circulation. We collected plasma after 2 h of injection and isolated VLDL from the plasma by ultracentrifugation and observed that [14C]triglyceride production was significantly increased in LPCAT3 knockdown mice compared with control mice (2.9-fold, p < 0.001) (Fig. 4A). 35S-Labeled apoB production levels were also dramatically increased in LPCAT3 knockdown mice compared with controls (3.9-fold, p < 0.0001) (Fig. 4B). These results suggested that reduction in LPCAT3 activity enhances assembly and secretion of triglyceride-rich apoB-containing lipoproteins.
FIGURE 4.
LPCAT3 knockdown increases triglyceride-rich lipoprotein production. Mice were injected simultaneously with [35S]methionine, [14C]oleic acid, and Poloxamer 407. Plasma (150 μl) was collected after 2 h of injection, and VLDL was isolated from the plasma by ultracentrifugation. Lipids in isolated VLDL were extracted using the Folch method and separated by TLC. The corresponding [14C]TG was scratched from the plate. The amount of radioactivity (cpm) in the TG fraction was measured by a liquid scintillation counter. The same volume of isolated VLDL (25 μl) was loaded on 4–15% gradient gel, and apoB was separated by SDS-PAGE. Incorporation of 35S into ApoB48 and ApoB100 was assessed with a Fuji Bio-Imaging Analyzer. Panel A, total secreted [14C]triglyceride. Panel B, secreted [35S]apoB. Values are the mean ± S.D. n = 5. *, p < 0.01.
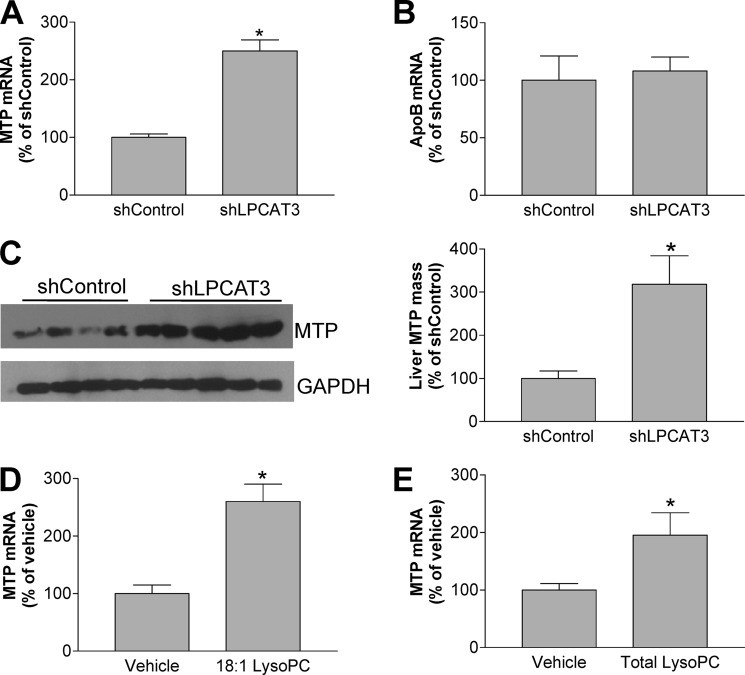
LPCAT3 Knockdown Induces MTP Expression through LysoPC Accumulation
The MTP, an intraluminal protein in the endoplasmic reticulum, is essential for the assembly of triglyceride-rich lipoproteins, including VLDL and chylomicron. We examined the impact of LPCAT3 deficiency on MTP and apoB expression in the liver and found that liver-specific LPCAT3 knockdown significantly increases MTP mRNA (Fig. 5A) and protein (Fig. 5C) with no effect on apoB mRNA (Fig. 5B). The above results indicated that LPCAT3 deficiency could influence VLDL secretion through MTP. More than 10 years ago we showed that LysoPC increased triglyceride-rich particle secretion in HepG2 cells (28), but at that time we did not evaluate the effect of LysoPC on MTP. Therefore, we hypothesized that LPCAT3 knockdown-mediated LysoPC accumulation in hepatocyte may up-regulate MTP expression and promote assembly and secretion of triglyceride-rich apoB particle. We treated Huh7 cells with LPCAT3 siRNA and control siRNA and found that LysoPC levels were significantly increased (18%, p < 0.05, supplemental Fig. 1). To directly examine effect of LysoPC on MTP, we treated Huh7 cells with 50 μm 18:1 LysoPC or total LysoPC (LysoPC mixture from egg) and then measured MTP mRNA by real-time PCR. We found that both LysoPC treatments significantly increased MTP mRNA (2.1- and 1.9-fold, p < 0.001, respectively) (Fig. 5, D and E). We also treated Huh7 cells with LPCAT3 siRNA and control siRNA and found that LysoPC levels were significantly increased (supplemental Fig. 1).
FIGURE 5.
LPCAT3 knockdown increases MTP mRNA and protein levels. Liver MTP and apoB mRNA was measured by real-time PCR, and MTP mass was measured as described under “Experimental Procedures.” Panel A, real-time PCR for MTP. Panel B, real-time for apoB. Panel C, liver MTP Western blot. Huh7 cells were treated with 50 μm 18:1 LysoPC or total LysoPC, and real-time PCR was formed for MTP. Panel D, 18:1 LysoPC treatment. Panel E, total LysoPC treatment. Values are the mean ± S.D. n = 5. *, p < 0.001.
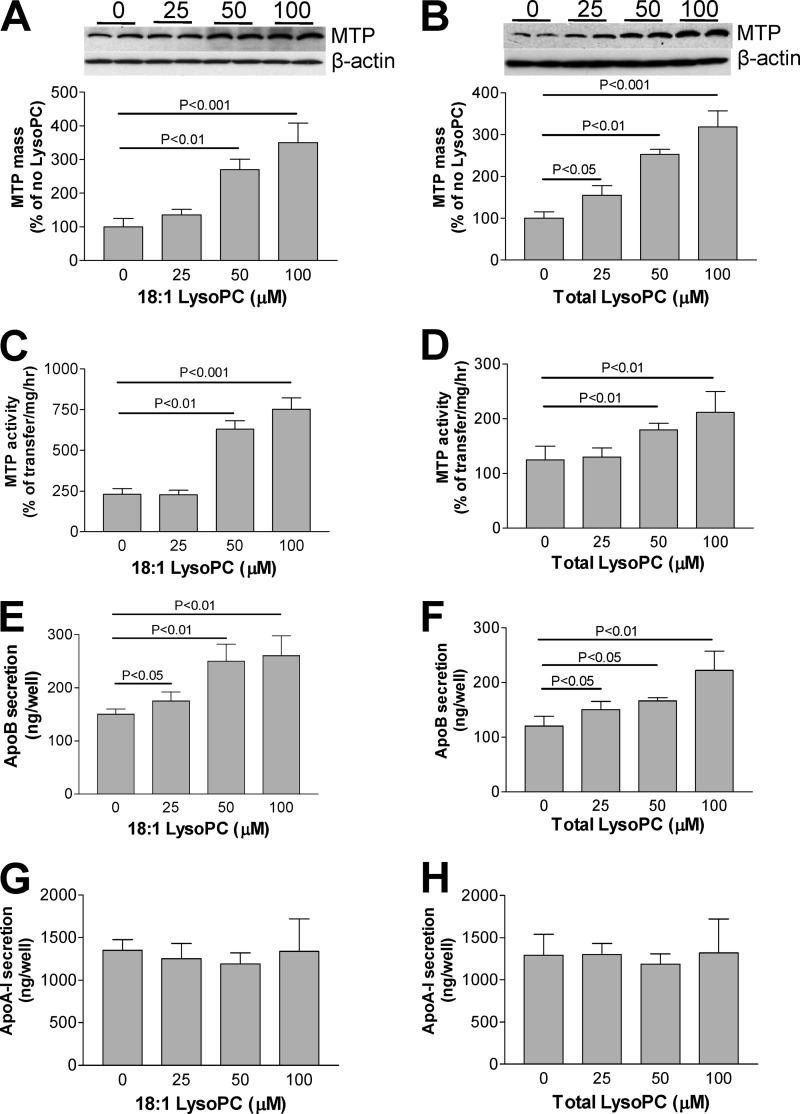
Next, we treated Huh7 cells with different concentrations of 18:1 LysoPC or egg LysoPC and then measured MTP mass by Western blot and found that both 18:1 LysoPC and egg LysoPC increased MTP mass in a dose-dependent manner (Fig. 6, A and B). This is also true for MTP activity (Fig. 6, C and D). Under the same conditions apoB (Fig. 6, E and F) but not apoA-I (Fig. 6, G and H) secretion was also increased in a dose-dependent manner. Therefore, we suggested that LPCAT3 knockdown-mediated LysoPC accumulation promotes MTP expression and VLDL production.
FIGURE 6.
The effect of exogenous total LysoPC and 18:1 LysoPC on MTP. Huh7 cells were incubated with exogenous 0, 25, 50, 100 μm concentrations of total LysoPC or 18:1 LysoPC for 18 h, and cellular, MTP mass, and MTP activity were measured as described under “Experimental Procedures.” Panel A, shown is a MTP Western blot after treatment with 18:1 LysoPC. Panel B, shown is a MTP Western blot after treatment with total LysoPC. Panel C, shown is cellular MTP activity after treatment with 18:1 LysoPC. Panel D, shown is cellular MTP activity after treatment with total LysoPC. Panel E, shown are medium apoB levels after treatment with 18:1 LysoPC. Panel F, shown are medium apoB levels after treatment with total lysoPC. Panel G, shown are medium apoA-I levels after treatment with 18:1 LysoPC. Panel H, shown are medium apoA-I levels after treatment with total LysoPC. Values are the mean ± S.D. n = 5. *, p < 0.05.
DISCUSSION
In this study we demonstrated (based on mRNA measurement) that, as in humans, LPCAT3 is the major LPCAT in the mouse liver (Fig. 2B). Furthermore, we show that liver-specific LPCAT3 knockdown can significantly 1) increase certain species of LysoPC and total LysoPC levels and decrease certain species of PC but not total PC levels, 2) decrease hepatic triglyceride levels, and 3) increase plasma triglyceride and apoB levels. More importantly, LPCAT3 knockdown-mediated LysoPC accumulation in hepatocyte up-regulates MTP expression and promotes triglyceride-rich apoB-containing particle secretion.
One of the key findings of this study is that LPCAT3 is a major LPCAT isoforms in the liver. We found that when LPCAT3 was effectively knocked down (Fig. 2A), the total hepatic LPCAT activity was reduced by more than 70% (Fig. 2C). This activity reduction causes induction in total LysoPC (Table 1), reduction in certain species of PC (Table 2), and reduction in triglyceride (Fig. 2D). Notably, with reduced LPCAT3 expression, there was more than a 50% increase in LysoPC with 16:0, 18:1, and 18:2 fatty acyl groups and more than a 25% increase in LysoPC with 18:0 and 18:3 fatty acyl groups, whereas other species that contain 14:0, 20:3, and 20:4 fatty acyl groups had no significant changes (Table 1), indicating that the influence of LPCAT3 reduction in the liver is specific to certain LysoPC species. These changes signify an important role of LPCAT3 activity in phospholipid as well as triglyceride metabolism.
LPCAT3 has LPCAT activities with all tested fatty acyl-CoA, including 12:0-CoA, 16:0-CoA, 18:1-CoA, 18:2-CoA, and 20:4-CoA (23). However, we only see certain species of PCs, but not 34:1-PC and 34:2-PC were decreased. Because both PCs compose about 40% of total PCs in the liver (Table 2), PC biosynthesis pathways other than LPCAT3 could influence 34:1-PC and 34:2-PC levels.
Second, we observed that liver LPCAT3 knockdown significantly increased plasma levels of triglycerides and apoB. This phenomenon could be explained by increasing production or decreasing catabolism of apoB-containing particles. Our physiologic studies indicated that LPCAT3 deficiency increases production of triglyceride-rich apoB-containing lipoproteins (Fig. 4, A and B). Our mechanistic studies indicated that increases in LysoPC lead to enhanced expression of MTP (Figs. 5, D and E, and 6, A–D). Thus, increased plasma levels of triglyceride-rich apoB-containing lipoprotein are most likely due to increased hepatic VLDL production.
Third, LPCAT3 knock down reduced hepatic triglycerides. Lower hepatic triglycerides could occur as a result of enhanced beta-oxidation or increased lipoprotein assembly and secretion. We did not see significant changes in the mRNA levels of key genes involved in beta-oxidation AOX, CPT1 and peroxisome proliferator-activated receptor α (data not shown). However, we did see significant increases in the production of apoB-lipoproteins. Therefore, we conclude that reductions in hepatic triglycerides after LPCAT3 knockdown might be a consequence of enhanced lipoprotein production.
LysoPC plays important physiological and pathophysiological roles. Specifically, elevated levels of LysoPC have been linked to the cardiovascular complications associated with diabetes (36), atherosclerosis (37, 38), and hyperlipidemia (39). ApoE KO mice have significantly higher plasma LysoPCs (40, 41), which might mediate the defect in efferocytosis in fat-fed apoE KO mice (40, 41). It has been known that LysoPC can be produced under physiological conditions by PLA2-mediated hydrolysis of phosphatidylcholine (42) or from the hydrolysis of oxidized PC by PAF-acetylhydrolase (43). In addition to these known effects of LysoPC, the current studies uncovered a new role of LysoPC in the regulation of plasma and hepatic triglyceride homeostasis involving regulation of apoB-lipoprotein assembly and secretion.
We previously showed that LysoPC specifically increased apoB secretion without affecting apoA-I secretion in HepG2 cells (28). However, we did not know the mechanism for this effect at that time. The current studies show that LysoPC can promote apoB secretion in Huh-7 cells as well. Furthermore, we show that increases in hepatic LysoPC are associated with increased production of apoB-lipoproteins. Mechanistic studies revealed that LysoPC has no effect on apoB mRNA levels, but it significantly increases MTP mRNA, protein and activity levels, thus, promoting lipoprotein assembly and secretion.
At this time we are unaware of mechanisms involved in the regulation of MTP mRNA by LysoPC. It should be pointed out MTP mRNA levels have been shown to be regulated involving transcriptional (44, 45) as well as post-transcriptional mechanisms (6, 46). Hence, it is possible that LysoPC might increase hepatic MTP gene transcription. Studies are under way to explore how LyosPC regulates MTP expression.
Accumulating evidence suggests that VLDL assembly is accomplished by a “two-step” model (47, 48). The first step is MTP-mediated primordial particle formation. The second step is incorporation of a bulk of lipids, triglyceride, and PC into the particles to form mature VLDL (49). The first step that is mediated by MTP is critical for VLDL assembly, because deficiency of MTP results in markedly reduced secretion and levels of VLDL in human abetalipoproteinemia (4). In this study we for the first time found that 18:1 or total LysoPC can up-regulate MTP expression. It remains to be determined whether regulation of MTP expression has LysoPC species specificity.
Another possible mechanism linking LPCAT3 activity and VLDL production is that LPCAT3 could provide proper phosphatidylcholines for apoB-contain particle assembly and then secretion, as it has been estimated that more than 50% of PC for lipoprotein production is acquired from PC remodeling after de novo synthesis (50–52). However, we found that LPCAT3 knockdown significantly decreases certain species of PC, such as 36:3, 36:4, 38:2, 38:3, and 38:4 (Table 2). It is possible that these PC species are preferentially utilized in lipoprotein production or might even be involved in enhanced assembly of apoB-lipoproteins. The regulation of PC metabolism by LPCAT3 and its role in lipoprotein production is open for further investigation. In summary, these studies show that LPCAT3 is the major LPCAT in the liver, and its hepatic activity modulates VLDL production by regulating LysoPC levels and MTP expression.
Acknowledgments
We thank Youyan Zhang, Yang Zhao, Robert J. Konrad at Eli Lilly and Co. for technical support.
This work was supported, in whole or in part, by National Institutes of Health Grant HL093419 (to X.-C. J.). This study was also supported by American Heart Association Grant 10SDG4040054 (to Z. L.).

This article contains supplemental Fig. 1.
- MTP
- microsomal triglyceride transfer protein
- LPCAT3
- Lysophosphatidylcholine acyltransferase 3
- PC
- phosphatidylcholine
- LysoPC
- lysophosphatidylcholine
- AdV
- adenoviruses
- TG
- triglyceride.
REFERENCES
- 1. Goldstein J. L., Hazzard W. R., Schrott H. G., Bierman E. L., Motulsky A. G. (1973) Hyperlipidemia in coronary heart disease. I. Lipid levels in 500 survivors of myocardial infarction. J. Clin. Invest. 52, 1533–1543 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Brunzell J. D., Albers J. J., Chait A., Grundy S. M., Groszek E., McDonald G. B. (1983) Plasma lipoproteins in familial combined hyperlipidemia and monogenic familial hypertriglyceridemia. J. Lipid Res. 24, 147–155 [PubMed] [Google Scholar]
- 3. Hussain M. M., Shi J., Dreizen P. (2003) Microsomal triglyceride transfer protein and its role in apoB-lipoprotein assembly. J. Lipid Res. 44, 22–32 [DOI] [PubMed] [Google Scholar]
- 4. Wetterau J. R., Aggerbeck L. P., Bouma M. E., Eisenberg C., Munck A., Hermier M., Schmitz J., Gay G., Rader D. J., Gregg R. E. (1992) Absence of microsomal triglyceride transfer protein in individuals with abetalipoproteinemia. Science 258, 999–1001 [DOI] [PubMed] [Google Scholar]
- 5. Shoulders C. C., Brett D. J., Bayliss J. D., Narcisi T. M., Jarmuz A., Grantham T. T., Leoni P. R., Bhattacharya S., Pease R. J., Cullen P. M. (1993) Abetalipoproteinemia is caused by defects of the gene encoding the 97-kDa subunit of a microsomal triglyceride transfer protein. Hum. Mol. Genet. 2, 2109–2116 [DOI] [PubMed] [Google Scholar]
- 6. Hussain M. M., Rava P., Pan X., Dai K., Dougan S. K., Iqbal J., Lazare F., Khatun I. (2008) Microsomal triglyceride transfer protein in plasma and cellular lipid metabolism. Curr. Opin. Lipidol 19, 277–284 [DOI] [PubMed] [Google Scholar]
- 7. Hamilton R. L., Wong J. S., Cham C. M., Nielsen L. B., Young S. G. (1998) Chylomicron-sized lipid particles are formed in the setting of apolipoprotein B deficiency. J. Lipid Res. 39, 1543–1557 [PubMed] [Google Scholar]
- 8. Young S. G. (1990) Recent progress in understanding apolipoprotein B. Circulation 82, 1574–1594 [DOI] [PubMed] [Google Scholar]
- 9. Ginsberg H. N. (1997) Role of lipid synthesis, chaperone proteins, and proteasomes in the assembly and secretion of apoprotein B-containing lipoproteins from cultured liver cells. Clin. Exp. Pharmacol. Physiol. 24, A29–A32 [DOI] [PubMed] [Google Scholar]
- 10. Twisk J., Gillian-Daniel D. L., Tebon A., Wang L., Barrett P. H., Attie A. D. (2000) The role of the LDL receptor in apolipoprotein B secretion. J. Clin. Invest. 105, 521–532 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Jiang X. C., Qin S., Qiao C., Kawano K., Lin M., Skold A., Xiao X., Tall A. R. (2001) Apolipoprotein B secretion and atherosclerosis are decreased in mice with phospholipid-transfer protein deficiency. Nat. Med. 7, 847–852 [DOI] [PubMed] [Google Scholar]
- 12. Jiang X. C., Li Z., Liu R., Yang X. P., Pan M., Lagrost L., Fisher E. A., Williams K. J. (2005) Phospholipid transfer protein deficiency impairs apolipoprotein-B secretion from hepatocytes by stimulating a proteolytic pathway through a relative deficiency of vitamin E and an increase in intracellular oxidants. J. Biol. Chem. 280, 18336–18340 [DOI] [PubMed] [Google Scholar]
- 13. Lang C. A., Davis R. A. (1990) Fish oil fatty acids impair VLDL assembly and/or secretion by cultured rat hepatocytes. J. Lipid Res. 31, 2079–2086 [PubMed] [Google Scholar]
- 14. Wang H., Chen X., Fisher E. A. (1993) N-3 fatty acids stimulate intracellular degradation of apoprotein B in rat hepatocytes. J. Clin. Invest. 91, 1380–1389 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Sparks J. D., Sparks C. E. (1990) Insulin modulation of hepatic synthesis and secretion of apolipoprotein B by rat hepatocytes. J. Biol. Chem. 265, 8854–8862 [PubMed] [Google Scholar]
- 16. Yao Z. M., Vance D. E. (1988) The active synthesis of phosphatidylcholine is required for very low density lipoprotein secretion from rat hepatocytes. J. Biol. Chem. 263, 2998–3004 [PubMed] [Google Scholar]
- 17. Kennedy E. P., Weiss S. B. (1956) The function of cytidine coenzymes in the biosynthesis of phospholipides. J. Biol. Chem. 222, 193–214 [PubMed] [Google Scholar]
- 18. Lands W. E. (1958) Metabolism of glycerolipides. A comparison of lecithin and triglyceride synthesis. J. Biol. Chem. 231, 883–888 [PubMed] [Google Scholar]
- 19. Ma Z., Wang X., Nowatzke W., Ramanadham S., Turk J. (1999) Human pancreatic islets express mRNA species encoding two distinct catalytically active isoforms of group VI phospholipase A2 (iPLA2) that arise from an exon-skipping mechanism of alternative splicing of the transcript from the iPLA2 gene on chromosome 22q13.1. J. Biol. Chem. 274, 9607–9616 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chen X., Hyatt B. A., Mucenski M. L., Mason R. J., Shannon J. M. (2006) Identification and characterization of a lysophosphatidylcholine acyltransferase in alveolar type II cells. Proc. Natl. Acad. Sci. U.S.A. 103, 11724–11729 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Nakanishi H., Shindou H., Hishikawa D., Harayama T., Ogasawara R., Suwabe A., Taguchi R., Shimizu T. (2006) Cloning and characterization of mouse lung-type acyl-CoA:lysophosphatidylcholine acyltransferase 1 (LPCAT1). Expression in alveolar type II cells and possible involvement in surfactant production. J. Biol. Chem. 281, 20140–20147 [DOI] [PubMed] [Google Scholar]
- 22. Shindou H., Hishikawa D., Nakanishi H., Harayama T., Ishii S., Taguchi R., Shimizu T. (2007) A single enzyme catalyzes both platelet-activating factor production and membrane biogenesis of inflammatory cells. Cloning and characterization of acetyl-CoA:LYSO-PAF acetyltransferase. J. Biol. Chem. 282, 6532–6539 [DOI] [PubMed] [Google Scholar]
- 23. Zhao Y., Chen Y. Q., Bonacci T. M., Bredt D. S., Li S., Bensch W. R., Moller D. E., Kowala M., Konrad R. J., Cao G. (2008) Identification and characterization of a major liver lysophosphatidylcholine acyltransferase. J. Biol. Chem. 283, 8258–8265 [DOI] [PubMed] [Google Scholar]
- 24. Hishikawa D., Shindou H., Kobayashi S., Nakanishi H., Taguchi R., Shimizu T. (2008) Discovery of a lysophospholipid acyltransferase family essential for membrane asymmetry and diversity. Proc. Natl. Acad. Sci. U.S.A. 105, 2830–2835 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Bar-On H., Roheim P. S., Stein O., Stein Y. (1971) Contribution of floating fat triglyceride and of lecithin toward formation of secretory triglyceride in perfused rat liver. Biochim. Biophys. Acta 248, 1–11 [DOI] [PubMed] [Google Scholar]
- 26. Wiggins D., Gibbons G. F. (1996) Origin of hepatic very low density lipoprotein triacylglycerol. The contribution of cellular phospholipid. Biochem. J. 320, 673–679 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Tran K., Sun F., Cui Z., Thorne-Tjomsland G., St Germain C., Lapierre L. R., McLeod R. S., Jamieson J. C., Yao Z. (2006) Attenuated secretion of very low density lipoproteins from McA-RH7777 cells treated with eicosapentaenoic acid is associated with impaired utilization of triacylglycerol synthesized via phospholipid remodeling. Biochim. Biophys. Acta 1761, 463–473 [DOI] [PubMed] [Google Scholar]
- 28. Zhou Z., Luchoomun J., Bakillah A., Hussain M. M. (1998) Lysophosphatidylcholine increases apolipoprotein B secretion by enhancing lipid synthesis and decreasing its intracellular degradation in HepG2 cells. Biochim. Biophys. Acta 1391, 13–24 [DOI] [PubMed] [Google Scholar]
- 29. Warnick G. R., Benderson J., Albers J. J. (1982) Dextran sulfate-Mg2+ precipitation procedure for quantitation of high density lipoprotein cholesterol. Clin. Chem. 28, 1379–1388 [PubMed] [Google Scholar]
- 30. Rohlmann A., Gotthardt M., Hammer R. E., Herz J. (1998) Inducible inactivation of hepatic LRP gene by cre-mediated recombination confirms role of LRP in clearance of chylomicron remnants. J. Clin. Invest. 101, 689–695 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Aalto-Setälä K., Fisher E. A., Chen X., Chajek-Shaul T., Hayek T., Zechner R., Walsh A., Ramakrishnan R., Ginsberg H. N., Breslow J. L. (1992) Mechanism of hypertriglyceridemia in human apolipoprotein (apo) CIII transgenic mice. Diminished very low density lipoprotein fractional catabolic rate associated with increased apoCIII and reduced apoE on the particles. J. Clin. Invest. 90, 1889–1900 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Folch J., Lees M., Sloane Stanley G. H. (1957) A simple method for the isolation and purification of total lipides from animal tissues. J. Biol. Chem. 226, 497–509 [PubMed] [Google Scholar]
- 33. Bligh E. G., Dyer W. J. (1959) A rapid method of total lipid extraction and purification. Can J. Biochem. Physiol. 37, 911–917 [DOI] [PubMed] [Google Scholar]
- 34. Athar H., Iqbal J., Jiang X. C., Hussain M. M. (2004) A simple, rapid, and sensitive fluorescence assay for microsomal triglyceride transfer protein. J. Lipid Res. 45, 764–772 [DOI] [PubMed] [Google Scholar]
- 35. Herz J., Gerard R. D. (1993) Adenovirus-mediated transfer of low density lipoprotein receptor gene acutely accelerates cholesterol clearance in normal mice. Proc. Natl. Acad. Sci. U.S.A. 90, 2812–2816 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Shi A. H., Yoshinari M., Wakisaka M., Iwase M., Fujishima M. (1999) Lysophosphatidylcholine molecular species in low density lipoprotein of type 2 diabetes. Horm. Metab. Res. 31, 283–286 [DOI] [PubMed] [Google Scholar]
- 37. Portman O. W., Alexander M. (1969) Lysophosphatidylcholine concentrations and metabolism in aortic intima plus inner media. Effect of nutritionally induced atherosclerosis. J. Lipid Res. 10, 158–165 [PubMed] [Google Scholar]
- 38. Thorp E., Tabas I. (2009) Mechanisms and consequences of efferocytosis in advanced atherosclerosis. J. Leukoc. Biol. 86, 1089–1095 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Zhang B., Fan P., Shimoji E., Itabe H., Miura S., Uehara Y., Matsunaga A., Saku K. (2006) Modulating effects of cholesterol feeding and simvastatin treatment on platelet-activating factor acetylhydrolase activity and lysophosphatidylcholine concentration. Atherosclerosis 186, 291–301 [DOI] [PubMed] [Google Scholar]
- 40. Aprahamian T., Rifkin I., Bonegio R., Hugel B., Freyssinet J. M., Sato K., Castellot J. J., Jr., Walsh K. (2004) Impaired clearance of apoptotic cells promotes synergy between atherogenesis and autoimmune disease. J. Exp. Med. 199, 1121–1131 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Peter C., Waibel M., Radu C. G., Yang L. V., Witte O. N., Schulze-Osthoff K., Wesselborg S., Lauber K. (2008) Migration to apoptotic “find-me” signals is mediated via the phagocyte receptor G2A. J. Biol. Chem. 283, 5296–5305 [DOI] [PubMed] [Google Scholar]
- 42. Parthasarathy S., Barnett J. (1990) Phospholipase A2 activity of low density lipoprotein. Evidence for an intrinsic phospholipase A2 activity of apoprotein B-100. Proc. Natl. Acad. Sci. U.S.A. 87, 9741–9745 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Tew D. G., Southan C., Rice S. Q., Lawrence M. P., Li H., Boyd H. F., Moores K., Gloger I. S., Macphee C. H. (1996) Purification, properties, sequencing, and cloning of a lipoprotein-associated, serine-dependent phospholipase involved in the oxidative modification of low density lipoproteins. Arterioscler. Thromb. Vasc. Biol. 16, 591–599 [DOI] [PubMed] [Google Scholar]
- 44. Hussain M. M., Nijstad N., Franceschini L. (2011) Regulation of microsomal triglyceride transfer protein. Clin. Lipidol. 6, 293–303 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Pan X., Zhang Y., Wang L., Hussain M. M. (2010) Diurnal regulation of MTP and plasma triglyceride by CLOCK is mediated by SHP. Cell Metab. 12, 174–186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Iqbal J., Dai K., Seimon T., Jungreis R., Oyadomari M., Kuriakose G., Ron D., Tabas I., Hussain M. M. (2008) IRE1β inhibits chylomicron production by selectively degrading MTP mRNA. Cell Metab. 7, 445–455 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Borén J., Rustaeus S., Olofsson S. O. (1994) Studies on the assembly of apolipoprotein B-100- and B-48-containing very low density lipoproteins in McA-RH7777 cells. J. Biol. Chem. 269, 25879–25888 [PubMed] [Google Scholar]
- 48. Swift L. L. (1995) Assembly of very low density lipoproteins in rat liver. A study of nascent particles recovered from the rough endoplasmic reticulum. J. Lipid Res. 36, 395–406 [PubMed] [Google Scholar]
- 49. Wang Y., McLeod R. S., Yao Z. (1997) Normal activity of microsomal triglyceride transfer protein is required for the oleate-induced secretion of very low density lipoproteins containing apolipoprotein B from McA-RH7777 cells. J. Biol. Chem. 272, 12272–12278 [DOI] [PubMed] [Google Scholar]
- 50. Lands W. E. (1960) Metabolism of glycerolipids. 2. The enzymatic acylation of lysolecithin. J. Biol. Chem. 235, 2233–2237 [PubMed] [Google Scholar]
- 51. MacDonald J. I., Sprecher H. (1991) Phospholipid fatty acid remodeling in mammalian cells. Biochim. Biophys. Acta 1084, 105–121 [DOI] [PubMed] [Google Scholar]
- 52. Choy P. C., Skrzypczak M., Lee D., Jay F. T. (1997) Acyl-GPC and alkenyl/alkyl-GPC:acyl-CoA acyltransferases. Biochim. Biophys. Acta 1348, 124–133 [DOI] [PubMed] [Google Scholar]