Background: Gαz can block insulin secretion, but no one had looked at its role in glucose intolerance.
Results: Mice that do not express Gαz mice do not develop glucose intolerance when fed a high fat diet.
Conclusion: A Gαz signaling pathway contributes to the pathophysiology of glucose intolerance.
Significance: This is the first work describing the specific role(s) of Gαz in glucose intolerance.
Keywords: Beta cell, Diabetes, G Protein-coupled Receptors (GPCR), G Proteins, Prostaglandins
Abstract
Insufficient plasma insulin levels caused by deficits in both pancreatic β-cell function and mass contribute to the pathogenesis of type 2 diabetes. This loss of insulin-producing capacity is termed β-cell decompensation. Our work is focused on defining the role(s) of guanine nucleotide-binding protein (G protein) signaling pathways in regulating β-cell decompensation. We have previously demonstrated that the α-subunit of the heterotrimeric Gz protein, Gαz, impairs insulin secretion by suppressing production of cAMP. Pancreatic islets from Gαz-null mice also exhibit constitutively increased cAMP production and augmented glucose-stimulated insulin secretion, suggesting that Gαz is a tonic inhibitor of adenylate cyclase, the enzyme responsible for the conversion of ATP to cAMP. In the present study, we show that mice genetically deficient for Gαz are protected from developing glucose intolerance when fed a high fat (45 kcal%) diet. In these mice, a robust increase in β-cell proliferation is correlated with significantly increased β-cell mass. Further, an endogenous Gαz signaling pathway, through circulating prostaglandin E activating the EP3 isoform of the E prostanoid receptor, appears to be up-regulated in insulin-resistant, glucose-intolerant mice. These results, along with those of our previous work, link signaling through Gαz to both major aspects of β-cell decompensation: insufficient β-cell function and mass.
Introduction
Both insulin resistance and β-cell decompensation (i.e. the failure of β-cells to maintain sufficient insulin secretion to properly regulate blood glucose levels, because of β-cell dysfunction and/or loss of β-cell mass) are essential events in the development of type 2 diabetes mellitus (T2DM)2 (1–5). Consistent with this idea, recent genome-wide association studies have shown that the majority of small nucleotide polymorphisms that associate with T2DM susceptibility are linked to β-cell decompensation and not insulin resistance (6).
The regulation of insulin secretion has been studied intensively for more than three decades, yet much is left to learn about this process. We have previously shown that the heterotrimeric Gi protein α-subunit, Gαz, modulates an endogenous signaling pathway that is inhibitory to glucose-stimulated insulin secretion (GSIS) in a rat β-cell-derived cell line (7). The mechanism for Gαz action appears to be a tonic negative regulation of adenylate cyclase, which when lost leads to constitutively increased cyclic AMP production (7, 8). This work was among the first to define a physiologic role for endogenous Gαz, a protein that was first described in 1988 (9, 10) and characterized biochemically in 1990 (11, 12).
The availability of Gαz-null mouse lines provided the opportunity to study the role of this G protein in normal tissue function and in disease pathophysiology. In our initial experiments with young, lean Balb/c mice, Gαz-null mice secreted more insulin in response to glucose and had more rapid glucose clearance (8), as might be predicted from the loss of an inhibitor of GSIS. This effect was cell autonomous, because islets isolated from Gαz-null mice had an increased response to stimulatory glucose (8). The in vivo and in vitro phenotypes of Gαz-null islets fit with the loss of a negative regulator of cAMP production, because cAMP is a ubiquitous second messenger involved in the glucose-dependent potentiation of GSIS. Taken together, these results suggested that deletion or suppression of Gαz might be protective against the development of β-cell dysfunction.
C57Bl/6 mice are susceptible to the development of obesity and metabolic derangements after high fat diet (HFD) feeding, resulting in the development of glucose intolerance (13), whereas Balb/c mice are less susceptible. Because our prior work on Gαz-null mice was performed in the Balb/c background, we utilized the Gαz-null mice in a C57Bl/6 background for the current studies (14). Wild-type and Gαz-null C57Bl/6 mice were challenged with HFD feeding for up to 30 weeks and then phenotyped for insulin and glucose tolerance. HFD-fed Gαz-null mice were completely protected from fasting hyperglycemia and glucose intolerance, even though they were equally insulin resistant as wild-type HFD-fed mice. This impact on glucose tolerance was largely independent of protection of β-cell function but was instead associated with a dramatic and somewhat unexpected impact of Gαz loss on β-cell proliferation, as measured by Ki67 immunofluorescence of fixed pancreas sections. This increased proliferation correlated positively with increased islet volume and β-cell mass. Selective G protein-coupled receptor (GPCR) agonists were utilized to delineate the signaling pathway(s) linked specifically to Gαz. Similar to our previous work with a rat β-cell line (7), in C57Bl/6 islets we confirmed a role for the E prostanoid receptor in transmitting signals to Gαz. Further, we suggest a link between the pathophysiology of glucose intolerance and a constitutively activated prostaglandin E2 signaling pathway in the β-cell, which is relieved in the absence of Gαz protein. The results of this study, along with those of our previous works (7, 8), define the role of Gαz in regulating β-cell function and mass in normal and pathological conditions, providing insight into the mechanisms of β-cell decompensation.
MATERIALS AND METHODS
Mouse Care, Husbandry, and Diet-induced Obesity (DIO) Study
C57Bl/6 mice containing a genomic insertion of a pGKneor cassette 160 base pairs downstream of the translation start site of the Gαz gene (gene symbol: gnaz) were developed by the Ian Hendry lab at Australian National University and shown to be completely deficient in Gαz protein expression as compared with wild-type controls in numerous tissues (15–19). Straws containing frozen sperm from confirmed Gαz-null C57Bl/6 mice were purchased from the Australian National University Phenomics Facility (Canberra, Australia) (14). The C57Bl/6 Gαz-null line was regenerated at Duke University using in vitro fertilization and intrauterine implantation into wild-type C57Bl/6 females (Charles River Laboratories, Wilmington, MA). Adult mice were placed into breeding triads on a 12-h light/dark cycle with ad libitum access to breeder chow (5058 PicoLab Mouse Diet 20; LabDiet, Brentwood, MO).
Gαz-null and wild-type control mice were generated by heterozygous matings to produce littermate pairs. Upon weaning, the male mice were housed five or fewer per cage with ad libitum access to low fat control chow (5053 PicoLab Rodent Diet 20, LabDiet). Before sexual maturity, the mice were placed by pairs of the same genotype into a new cage. At 8 (pilot study) or 11 weeks of age (full study), the chow was changed to a diet containing 45 kcal% fat (D12451; Research Diets, New Brunswick, NJ) or the appropriate low fat control diet (D12450B; Research Diets; 10 kcal% fat). Food was weighed weekly during the pilot study and replaced weekly for both the pilot and full study. Experimental parameters recorded were: initial glucose tolerance, weight change, final insulin tolerance, final glucose tolerance, final adiposity, and final pancreas weight (wet).
Adiposity was determined from dual emission x-ray absorptiometry scanning and/or subgonadal fat pad weight (wet). Dual emission x-ray absorptiometry scans were performed and analyzed on ketamine/xylazine-anesthetized mice using a Lunar PIXImus II and associated software (GE Healthcare Lunar, Madison, WI). Gonadal fat pad weights were either recorded as the sum of both fat pads combined or just a single fat pad; therefore, in each instance, the weights were normalized to those of the mean of the wild-type control diet-fed mice to obtain meaningful comparisons for the whole set. Not all parameters were recorded for all mice. The animals were handled in accordance with the principles and guidelines established by the Duke University Animal Care and Use Committee.
Antibodies and Reagents
Guinea pig anti-insulin (1:500), rabbit anti-glucagon (1:300), mouse anti-Ki67 (1:100), antibody diluent, citrate pH 6 target retrieval solution, Protein Block, and 10× wash buffer were purchased from Dako (Carpinteria, CA). Highly cross-absorbed Alexa Fluor® 488 goat anti-guinea pig IgG, Alexa Fluor® 680 donkey anti-mouse IgG, Alexa Fluor® 568 donkey anti-rabbit IgG, ProLong® Gold antifade reagent with DAPI, and sterile d-glucose in PBS were from Invitrogen. Insulin (Humulin® R) and sterile insulin diluent were from Lilly (Indianapolis, IN). Superfrost® Plus microscope slides were from Fisher Scientific (Hampton, NH), and fluorescence quality 1.5-mm coverslips were from Corning Life Sciences (Lowell, MA). The rat/mouse insulin ELISA kit was from Crystal Chem Inc. (Downers Grove, IL). The prostaglandin E (PGE) metabolite kit was from Cayman Chemical (Ann Arbor, MI). The cAMP Biotrak® enzyme immunoassay kit was from GE Healthcare. CGS-12066A, BW-72386, sulprostone, 3-isobutyl-1-methylxanthine, pertussis toxin from Bordetella pertussis (buffered aqueous glycerol solution), and Cellytic® M cell lysis buffer were from Sigma-Aldrich. Complete® EDTA-free protease inhibitor mixture tablets were from Roche Applied Sciences (Indianapolis, IN). The BCA protein assay was from Thermo Fisher Scientific (Indianapolis, IN).
Glucose Tolerance Tests
The mice were fasted for 4–6 h, and intraperitoneal glucose tolerance tests (IP-GTTs) were performed before the administration of the high fat or control diet (11 weeks of age) and after 21–25 weeks on the high fat or control diet (32–36 weeks of age). Glucose readings were taken from tail blood using an Ascensia Breeze 2 meter (Bayer Diabetes Care, Tarrytown, NY) before glucose injection (t = 0) and 25, 60, 120, and 180 min after glucose injection. The glucose readings were averaged within genotypes at each time point, giving the means ± S.E. (n = 17–25 mice for each group). During the IP-GTTs, blood samples were collected at 0 and 10 min into EDTA-coated tubes to generate plasma samples for insulin ELISA conducted as previously described (8). One to three replicates of each plasma sample were performed depending on the total sample volume available; the insulin values for all of the replicates of a single sample were averaged, and the averages for all mice of the same treatment group were used to calculate the means ± S.E.
Plasma PGE Determination
PGE metabolite levels were measured in plasma samples collected at t = 0 of the IP-GTTs using a specific ELISA kit and manufacturer's protocol (Cayman Chemical). Derivatized samples were diluted 1:50 into assay buffer to obtain readings in the linear range of the assay. The samples were run in duplicate, the PGE metabolite values were averaged, and the averages for all mice of the same treatment group were used to calculate the means ± S.E.
Insulin Tolerance Tests
Insulin tolerance tests were performed essentially as described for the IP-GTTs, but instead of injecting glucose, the mice were injected intraperitoneally with 0.75 units/kg regular insulin in sterile insulin diluent. Glucose readings were taken at 0, 30, 60, and 90 min postinjection. Insulin tolerance tests were performed at least 2 weeks before and 2 weeks after the IP-GTTs. The results for each mouse were averaged to give as close a picture of insulin tolerance at the time of the IP-GTT as possible. These averaged glucose readings were averaged within groups at each time point, giving the means ± S.E.
Pancreatic Islet Isolation and Morpohometric Analysis
Mouse pancreatic islets were isolated essentially as previously described (8). To determine islet volume, isolated islets were cultured overnight in RPMI 1640 with 8.4 mm glucose and penicillin/streptomycin to recover from the isolation procedure. Islets were swirled to the middle of the culture dish for imaging, the image calibrated by taking a picture of a ruler, and the diameter of every islet in the culture was measured to calculate the islet volume. To determine islet protein content, islets were cultured overnight as described. The entire islet culture was hand-picked into 1.5-ml microcentrifuge tubes containing 1 ml of sterile PBS in batches of 50–100/tube. The islets were pelleted two times by pulsing to 10,000 × g, washed with 1 ml of PBS, and resuspended in Cellytic® M lysis reagent containing protease inhibitors. The protein content was determined by BCA assay according to the manufacturer's protocol.
GSIS Assays and cAMP Production Assays
GSIS assays and cAMP production assays were performed essentially as previously described (8). With both GSIS and cAMP production, the islets were cultured overnight in RPMI medium containing submaximal stimulatory glucose (8.4 mm for insulin secretion and 11.1 mm for cAMP). On the day of the assays, the islets were washed once with Krebs-Ringer bicarbonate buffer containing 1.7 mm glucose and then preincubated for 45 min in the same buffer before being transferred to the desired stimulatory buffer for 45 min.
cAMP production assays were conducted in the presence of 100 μm 3-isobutyl-1-methylxanthine to block cAMP degradation. The cAMP production for each sample was normalized to its protein content using BCA assay. Insulin secreted into the cAMP stimulation medium was also measured and normalized to protein content. For GSIS assays, the insulin secreted into the medium was normalized to the total insulin content. For each GSIS and cAMP assay, the normalized technical replicates were averaged, and the averages from all experiments were used to calculate the standard error.
Immunofluorescence and Immunohistological Assays
Pancreata were dissected, fixed, and sectioned as previously described, with minor modifications (7). For immunofluorescence experiments, the slides were deparaffinized and subjected to antigen retrieval. The slides were then washed and blocked for 1 h at room temperature. For fluorescence detection, a primary antibody mix (anti-insulin, anti-glucagon, and anti-Ki67) was added to the slide after blocking, covered, and incubated overnight at 4 °C. The slides were washed extensively and then incubated with secondary antibodies at 1:4000 for 1 h at room temperature. The slides were imaged using a Zeiss Axioplan2 fluorescence microscope with a Qimaging Exi camera driven by Openlab software. Each channel was imaged separately, and the final images were overlaid in Photoshop.
For insulin immunostaining, 5-micron sections 100 microns apart were stained using the EnVisionTM diaminobenzidine (DAB) reagents according to manufacturer's protocol (Dako), along with a 30-min room temperature incubation of 1:500 guinea pig anti-insulin primary antibody. The slides were lightly counterstained with hematoxylin, and six to eight independent sections from two or three mice of each group were processed. The slides were imaged using the Qimaging camera on color settings using a 1.25× lens. β-Cell fractional area was calculated by processing the resulting RGB images in open source National Institutes of Health ImageJ/64 software using shading correction and the H DAB color deconvolution vector.
Statistical Analyses
The data were analyzed using GraphPad Prism v. 5 (GraphPad Software Inc., San Diego, CA). A t test or two-way ANOVA was used to determine the p value as indicated in the figure legends. p < 0.05 was considered significant.
RESULTS
Adult Gαz-null HFD-fed Mice Retain Glucose Tolerance
Male wild-type and Gαz-null C57Bl/6 mice were fed either a control diet containing 10% of calories as fat, or a diet composed of 45% of calories as fat (HFD). Male C57Bl/6 mice are known to respond to HFD feeding by becoming obese and insulin-resistant and developing glucose intolerance (8). A pilot DIO study was conducted first with three groups of 8-week-old mice (wild type: control diet, n = 11; wild type: HFD, n = 11; Gαz-null: HFD, n = 12) to determine whether 1) there was a significant difference in food intake and/or weight gain based on genotype and 2) to design appropriate parameters for the full study. The results of this pilot study indicated that both the wild-type and Gαz-null mice became obese when fed on HFD compared with the wild-type control diet groups, with the Gαz-null HFD-fed mice exhibiting a greater initial weight gain that ultimately plateaued at the same level as the wild-type HFD mice (supplemental Fig. S1A). Of note, the Gαz-null HFD mice ate significantly more as normalized to body weight than either of the other two groups at week 1 and tended to eat more until week 6 of the pilot study, at which point there was no difference between the food eaten (kcal/g body weight; supplemental Fig. S1B). This increased food intake seemed to explain the increased initial rate of weight gain of the Gαz-null mice and suggested possible changes to the experimental protocol. Specifically, the Gαz-null mice are known to be runted because of a failure to thrive phenotype, with catch-up growth postweaning (15). We hypothesized that the increased initial food intake of the Gαz-null mice was a compensation for a decreased body weight during a period where the mouse is still developing. Thus, for the full experimental set, 17–25 mice of each genotype were placed on the control or HFD at 11 weeks of age, when the Gαz-null mice are closer in weight to their wild-type littermates and are essentially fully developed (13).
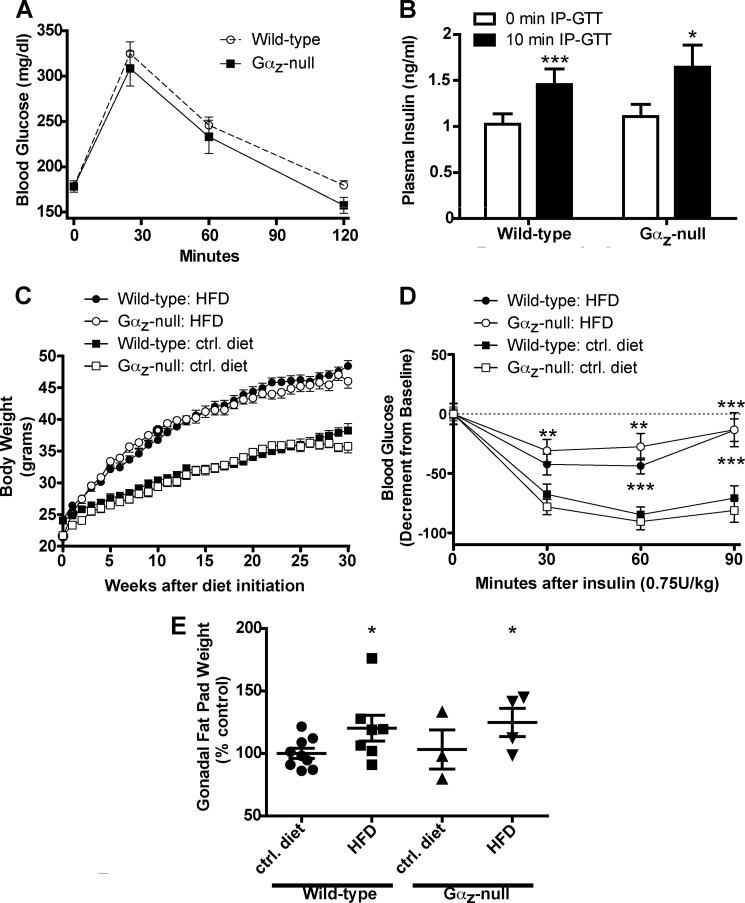
The Gαz-null mice at 11 weeks of age were still somewhat lighter than their wild-type littermates (24.14 ± 0.096 (wild type, n = 49) versus 21.64 ± 0.083 (Gαz-null, n = 34); p < 0.001), although dual emission x-ray absorptiometry scanning revealed no significant differences in their initial body composition (n = 4 mice of each genotype; p = 0.2073 (data not shown and Ref. 8). There were also no significant differences in the glucose tolerance of wild-type and Gαz-null C57Bl/6 mice at a dose of 1 g/kg glucose (Fig. 1A), although the glucose tolerance of the Gαz-null mice trended toward a lower area under the curve, consistent with our previous observations using 2 g/kg glucose in Balb/c mice (8). The lack of difference in glucose tolerance was also reflected in the lack of significant differences between the plasma insulin levels of the wild-type and Gαz-null mice at t = 0 or t = 10 min; both genotypes had similar and significant increases in plasma insulin at t = 10 min (Fig. 1B).
FIGURE 1.
Initial glucose tolerance, adiposity, and insulin resistance among mice subjected to the DIO study. A, blood glucose values recorded during IP-GTTs (1 g/kg glucose) of 11-week-old lean wild-type (n = 40) and Gαz-null mice (n = 23) mice. The data were analyzed by two-way repeated measures ANOVA followed by Bonferroni post-test. B, plasma insulin values recorded at t = 0 and t = 10 min during the IP-GTT after 1 g/kg glucose in both the wild-type (n = 23) and Gαz-null (n = 21) mice. The data were analyzed by paired t test for the effect of time within genotype and unpaired t test for the effect of genotype at each time point. C, body weight measurements taken during the course of the DIO experiment (wild type: control diet, n = 25; wild type: HFD, n = 24; Gαz-null: control diet, n = 17; Gαz-null: HFD, n = 17). The data were analyzed by two-way repeated measures ANOVA followed by Bonferroni post-test. D, decrement in blood glucose recorded during intraperitoneal insulin tolerance tests (0.75 units/kg insulin) of mice fed the control diet or HFD (wild type: control diet, n = 21; wild type: HFD, n = 20; Gαz-null: control diet, n = 16; Gαz-null: HFD, n = 14). The data were analyzed by two-way repeated measures ANOVA followed by Bonferroni post-test. E, gonadal fat pad weights recorded from representative mice from each group. The data were analyzed by unpaired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ctrl, control.
After being placed on the appropriate diet at 11 weeks of age, the HFD-fed mice gained significantly more weight than the control diet-fed mice, and the weight gain did not vary by genotype (Fig. 1C). The insulin tolerance of obese HFD-fed mice, as recorded as the average insulin tolerance before and after the IP-GTT, was significantly worse than the control diet-fed mice and did not differ by genotype (Fig. 1D). This confirms that Gαz loss does not induce insulin resistance, which was not surprising based on our previous work (8). Furthermore, the adiposity of the Gαz-null mice did not differ from that of the wild-type mice in either treatment group, although gonadal fat pad mass measurements revealed that the HFD-fed mice had significantly increased adiposity as compared with the appropriate control mice (Fig. 1E).
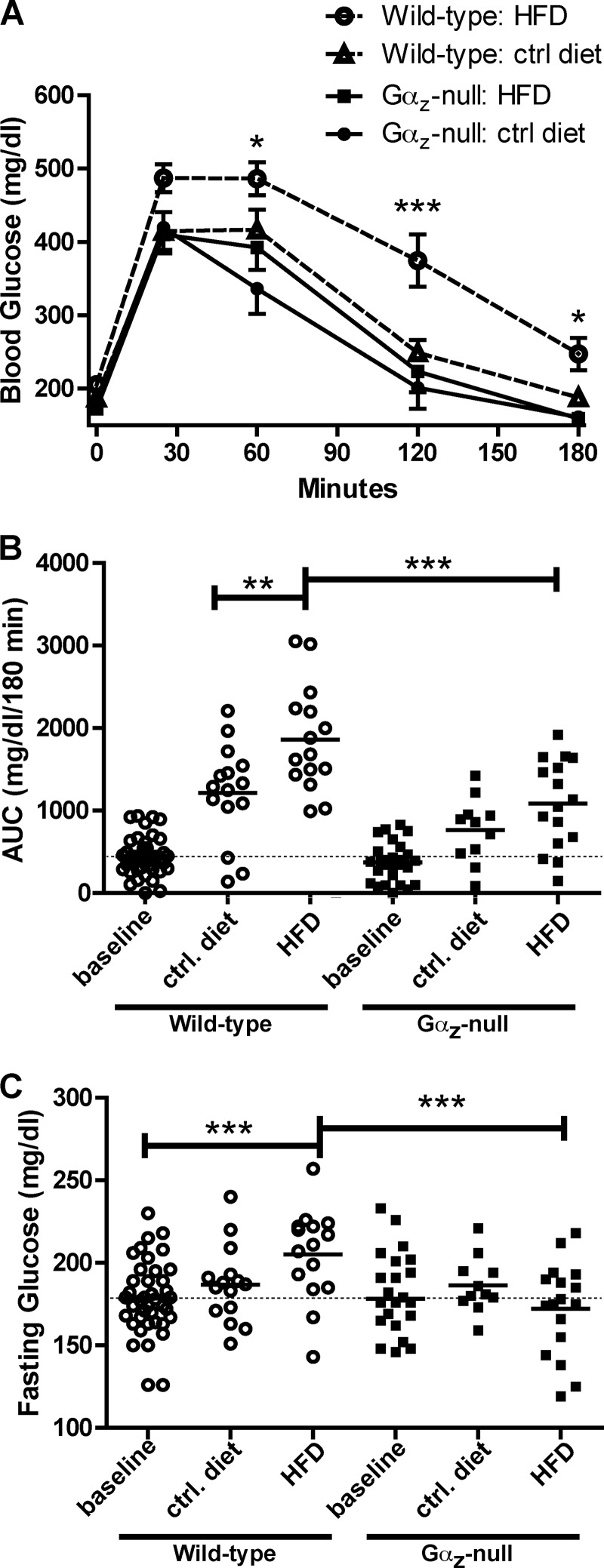
IP-GTTs (1 g/kg glucose) were performed 21–25 weeks after beginning the DIO study, when the mice were 32–36 weeks of age. As expected, HFD-fed wild-type C57Bl/6 mice exhibited impaired glucose clearance compared with wild-type mice fed on the control diet (Fig. 2A). The area under the curve for blood glucose was significantly elevated in HFD-fed wild-type mice versus control diet-fed mice, which in turn was significantly higher than that observed in the 11-week-old mice (Fig. 2B). Furthermore, the fasting glucose level for HFD-fed wild-type mice was significantly higher than that observed at 11 weeks or in the control diet-fed mice (Fig. 2C). Elevated fasting plasma glucose and impaired glucose tolerance are consistent with the development of prediabetes in obese C57Bl/6 mice (20).
FIGURE 2.
Loss of Gαz normalizes glucose tolerance in mice fed the HFD. A, blood glucose values recorded during an IP-GTT (1 g/kg glucose) performed after 21–25 weeks on the HFD or control diet (wild type: control diet, n = 15; wild type: HFD, n = 17; Gαz-null: control diet, n = 12; Gαz-null: HFD, n = 15). The data were analyzed by two-way repeated measures ANOVA followed by Bonferroni post-test. B, area under the curve (AUC) measurements performed on IP-GTT curves at 11 weeks of age (base line; Fig. 1A) or at 21–25 weeks after beginning the DIO study. The data were analyzed by unpaired t test. C, fasting glucose levels taken at t = 0 during the IP-GTTs indicate a better maintenance of base-line glycemia in the Gαz-null mice with HFD feeding. The data were analyzed by unpaired t test. **, p < 0.01; ***, p < 0.001. ctrl, control.
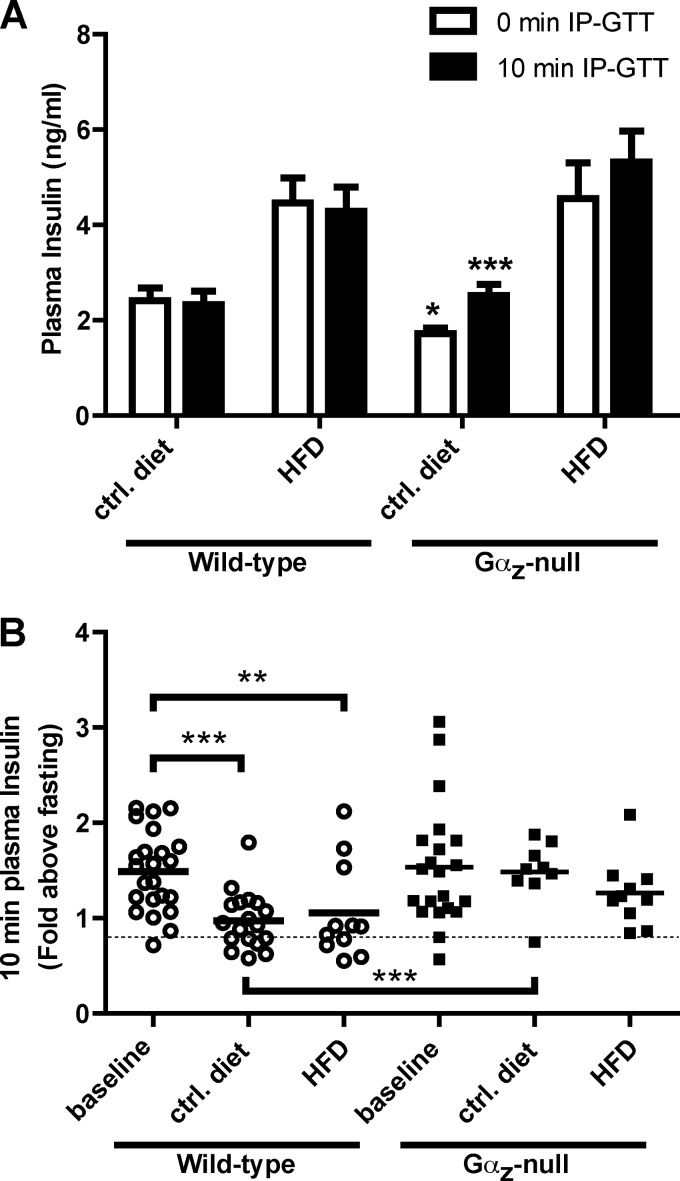
The elevated fasting plasma insulin levels as recorded during the IP-GTT are consistent with the development of insulin resistance in both the HFD-fed wild-type and Gαz-null groups (Fig. 3A, 0 min IP-GTT). In considering the wild-type mice only, neither the HFD-fed nor control diet-fed groups demonstrated an appreciable increase in plasma insulin after glucose challenge (Fig. 3A, 10 min IP-GTT). This is not unexpected, because the acute insulin response after glucose challenge tends to deteriorate with both age and HFD feeding (13, 21). The acute insulin response can be better represented by normalizing the plasma insulin levels after glucose challenge to the fasting insulin levels for each mouse: in our case, 10-min IP-GTT over 0-min IP-GTT. Again, the acute insulin responses of the wild-type mice were significantly impacted by both age and HFD feeding, but those of the Gαz-null mice were not (Fig. 3B). Interestingly, the Gαz-null mice fed the control diet were the only group that displayed plasma insulin levels, indicative of an absence of both insulin resistance and glucose intolerance (Fig. 3).
FIGURE 3.
Gαz-null mice demonstrate a better maintenance of stimulation of plasma insulin levels, both with age and HFD feeding. A, plasma insulin levels recorded at t = 0 and t = 10 min during the final IP-GTT. The data were analyzed by paired t test to determine the effect of time within a group and by unpaired t test with Welch's correction, which corrects for unequal variances, among treatment groups. B, acute insulin response, which is the plasma insulin after glucose challenge (t = 10 min) as normalized to the plasma insulin levels at t = 0 min. The data were analyzed by unpaired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ctrl, control.
Loss of Gαz in Adult HFD-fed Mice Induces β-Cell Proliferation and Increases β-Cell Mass
To further explore the β-cell function of HFD-fed Gαz-null mice, pancreatic islets were isolated from each group of mice 30 weeks after initiation of the DIO study. In the pilot study, islets were isolated from four mice of each group in two experiments, and their diameters were measured using calibrated stereomicroscopic images. We observed the expected increase in size of wild-type islets from mice fed the HFD, as calculated from measuring the diameters of isolated islets and assuming a spherical volume (V = (1/6)πd3) (supplemental Figs. S1C and S2). Interestingly, a further increase in islet volume was observed in islets from Gαz-null HFD-fed mice. Because the islet volume was directly correlated with protein content, protein content per islet was used as a surrogate measurement for islet size in the full DIO study. Again, the size of islets from HFD-fed Gαz-null mice was significantly increased above the normal C57Bl/6 compensatory mechanisms (Fig. 4A). The insulin content of islets from HFD-fed Gαz-null mice also increased significantly and correlated directly with the increase in islet size (Fig. 4B). In vitro GSIS assays were performed at several glucose concentrations to determine the effect of genotype and diet status on β-cell function (as represented by insulin secreted as a percentage of the total). The Gαz-null HFD islets did not secrete more insulin as a percentage of total insulin content (Fig. 4C). This is in contrast to islets from Gαz-null mice fed the control diet, which did display an improved β-cell function (Fig. 4C), consistent with our previous observations in islets from young, lean Balb/c mice (8). This suggested that Gαz may have two separate roles in the β-cell and that an improved insulin response in Gαz-null HFD-fed mice might be primarily due to expansion of β-cell mass rather than enhanced GSIS from individual β-cells.
FIGURE 4.
Islets from Gαz-null HFD-fed mice are significantly larger than those of wild-type mice, but do not demonstrate increased function. A, islet protein content was used as a surrogate measurement for islet volume in the full DIO study (wild type: control diet, n = 25, wild type: HFD, n = 35; Gαz-null: control diet, n = 8; Gαz-null: HFD, n = 17). The data were analyzed by unpaired t test. B, insulin content of islets isolated from mice from each of the four groups (n = 3 mice for each group). The data were analyzed by unpaired t-test. C, GSIS response of islets isolated from mice from each of the four groups to increasing concentrations of glucose (n = 3 mice for each group). The data were analyzed by two-way repeated measures ANOVA followed by Bonferroni post-test. *, p < 0.05; **, p < 0.01; ***, p < 0.001. ctrl, control.
To explore the phenotype of increased β-cell mass in the Gαz-null HFD-fed mice, β-cell fractional area was measured by analysis of anti-insulin DAB staining of formalin-fixed, paraffin-embedded sections (Fig. 5, A–D). Of note, the average size of islets from Gαz-null control diet mice calculated from the DAB-stained sections was consistent with our estimate of islet size based on total protein (Fig. 4A), suggesting a positive impact of Gαz loss on islet size (data not shown). In quantifying the β-cell fractional area, we observed a trend toward an increase in insulin-positive area in pancreas sections from the two HFD-fed groups as compared with their respective control diet-fed cohorts, consistent with a compensatory response to insulin resistance (Fig. 5E). Interestingly, when the mean fractional area values were used to convert total pancreatic mass to β-cell mass, the Gαz-null HFD-fed mice demonstrated significantly increased β-cell mass as compared with the wild-type HFD-fed mice (Fig. 5F).
FIGURE 5.
Improved glucose tolerance in the HFD-fed Gαz-null mice may be due to increased β-cell mass. A–D, representative sections from anti-insulin stained pancreas sections (brown) counterstained with hematoxylin (blue) that were used to calculate fractional β-cell area. A, wild type, control diet; B, wild type, HFD; C, Gαz-null, control diet; D, Gαz-null, HFD. Three nonconsecutive sections were imaged per pancreas, with two or three pancreata imaged per group. The black scale bars indicate 1 mm (ticks indicate 200 μm). E, β-cell fractional area was calculated from insulin-stained pancreas sections and reported as the percentage of total pancreas area on the slide. Three nonconsecutive sections separated by at least 100 microns were quantified for each pancreas (n = 2–3 mice for each group). F, the HFD-fed Gαz-null mice have significantly increased calculated β-cell mass as compared with the wild-type HFD-fed mice. Pancreas weights from individual mice were transformed using the mean fractional area in A. The data were analyzed by unpaired t test. *, p < 0.05. ctrl, control.
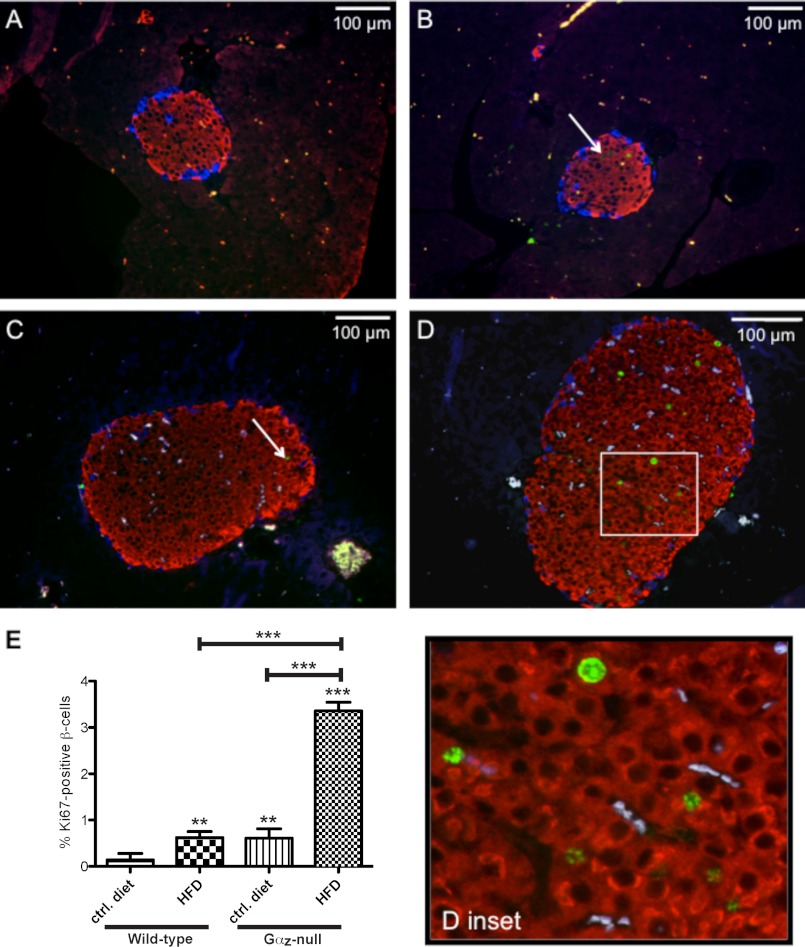
To determine a possible mechanism for increased β-cell mass in the Gαz-null HFD-fed mice, we prepared paraformaldehyde-fixed pancreas sections from each group of mice and used an immunocytochemical assay to measure Ki67, a nuclear marker of active cell replication. In nearly all islets from wild-type animals fed the control diet, no Ki67-positive β-cells were observed (Fig. 6A). Islets from Gαz-null animals fed the control diet or from wild-type animals fed the HFD showed a few Ki67-positive cells per islet (Fig. 6, B and C), whereas islets from Gαz-null HFD-fed mice had a clear increase in nuclear Ki67 immunofluorescence (Fig. 6D and inset). Furthermore, of all of the Ki67-positive islet cells, only five non-β-cells were found (all α-cells, in three different treatment groups: wild-type HFD, Gαz-null control diet, and Gαz-null HFD), suggesting that the increases in islet size were likely driven by the increase in β-cell replication. In quantifying the Ki67-positive β-cell nuclei as a percentage of total β-cell nuclei, HFD feeding of wild-type mice caused a 4-fold increase in Ki67 positive β-cells compared with control diet feeding. Gαz loss in the control diet background also had significant impact on the number of Ki67-positive β-cells, equivalent to the effect of HFD feeding in the wild-type mice. Finally, β-cells from Gαz-null HFD-fed mice demonstrated a 24-fold increase in Ki67 staining, larger than either of the other groups (Fig. 6E).
FIGURE 6.
Increased β-cell proliferation in Gαz-null islets from mice fed the HFD as compared with their wild-type counterparts. A–D, pancreas sections from wild-type control diet-fed mice (A), Gαz-null control diet-fed mice (B), wild-type HFD-fed mice (C), and Gαz-null HFD-fed mice (D) were incubated with primary antibodies against insulin (red), glucagon (blue), and Ki67 (green). Overlaying the images revealed β-cells that are actively replicating (examples indicated by white arrows). E, quantification of the Ki67-positive β-cells as a percentage of the total β-cells. Three nonconsecutive sections separated by at least 100 microns were quantified for each pancreas. n = 2 mice for each group. The data were analyzed by unpaired t test. **, p < 0.01; ***, p < 0.001. ctrl, control.
The EP3 Isoform of the PGE Receptor Is Coupled Specifically to Gαz to Negatively Regulate β-Cell Function
The finding that Gαz loss alone impacts on β-cell function and mass suggests that, in wild-type mice, tonic activation of a Gαz-coupled receptor increases with both age and HFD feeding and that this signaling pathway is inoperative when Gαz is absent. We showed previously that cAMP production is constitutively increased in islets isolated from Gαz-null Balb/c mice (8). To determine whether this holds true for the C57Bl/6 line studied in this work, we isolated islets from Gαz-null or wild-type C57Bl/6 mice and quantified their constitutive cAMP production. Just as with Balb/c islets, Gαz-null C57Bl/6 islets exhibit significantly increased cAMP production as compared with islets from littermate controls (Fig. 7A, left panel). Furthermore, the amount of insulin secreted into the cAMP stimulation medium directly correlates with the increase in cAMP (Fig. 7A, right panel).
FIGURE 7.
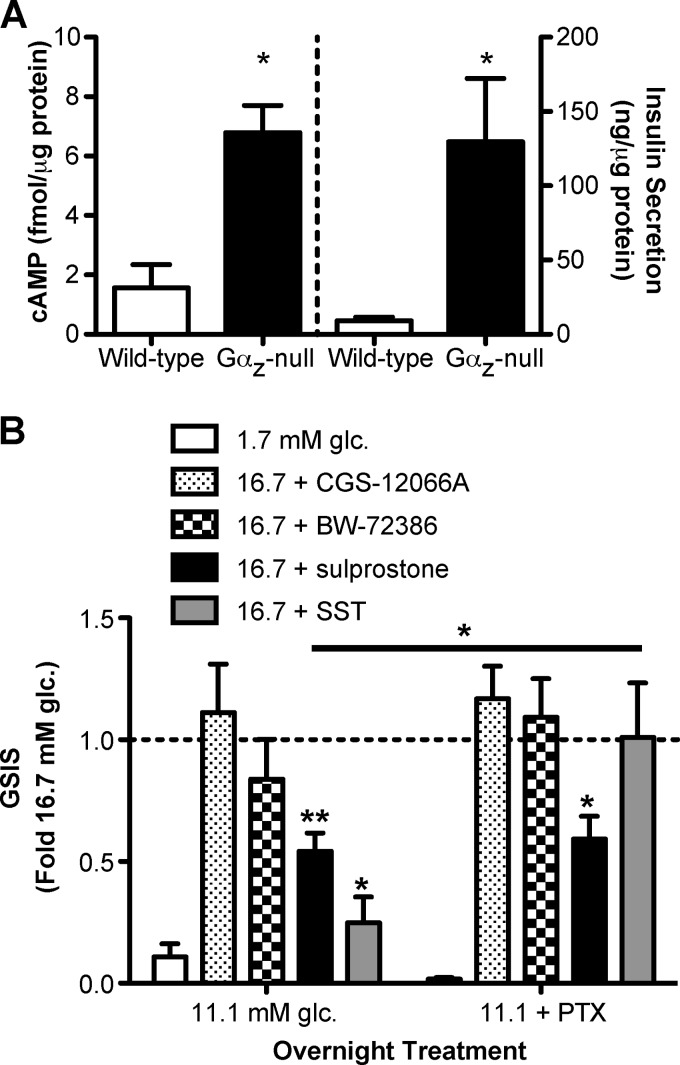
Gαz-null islets produce more cAMP, and the EP3 isoform of the PGE receptor is linked to Gαz to negatively regulate GSIS. A, intracellular cAMP production (left panel) and secreted insulin (right panel) were measured from the same islets samples isolated from wild-type and Gαz-null mice. n = 3 for each group. The data were analyzed by unpaired t test. *, p < 0.05. B, islets were isolated from glucose-intolerant C57Bl/6 ob/ob mice and cultured overnight in medium containing 11.1 mm glucose (glc.) with and without 200 ng/ml pertussis toxin (PTX). In vitro GSIS assays were performed with the addition of the 5HT1 agonist CGS-12066A (100 nm), the 5HT2 agonist BW-723C56 (1 μm), the EP3 agonist sulprostone (10 μm), or SST (2 μg/ml). The data were normalized within each group to the effect of 16.7 mm glucose + Me2SO control. n = 5 for each group, except for SST (n = 3). The data were analyzed by paired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
Certain GPCRs signal through inhibitory G proteins, such as Gαz, to block cAMP production. We have previously shown that the endogenous PGE receptor in a rat β-cell line was exclusively coupled to Gαz to block insulin secretion (7). Of the four isoforms of the PGE receptor, only the EP3 isoform has been shown to couple to inhibitory G proteins. In addition, the GPCR family that has been best linked to regulation of mouse β-cell mass is the serotonin (5HT: 5-hydroxytryptamine) receptor family. Activation of the 5HT2B receptor was found to stimulate β-cell proliferation in pregnant mice, augmenting β-cell mass, while signaling through the 5HT1D receptor restored normal β-cell mass after parturition (22). To explore the role(s) of EP3 and 5HT receptor signaling in islets from an obese, glucose-intolerant mouse model, we isolated islets from 10-week-old C57Bl/6 ob/ob mice and incubated them overnight in medium containing submaximal stimulatory glucose with or without the addition of 200 ng/ml pertussis toxin to inactivate all Gαi subfamily members save Gαz (11). This overnight treatment regimen was shown to completely relieve inhibition of GSIS by somatostatin 28 (SST), which is known to act through other Gαi subfamily members and not Gαz (supplemental Fig. S3). After pertussis toxin treatment, the islets were used in GSIS assays with the selective 5HT1 agonist CGS-12066A, the selective 5HT2 agonist BW-72386, the selective EP3 agonist sulprostone, or SST. Sulprostone and SST both significantly blocked GSIS in the control treated islets, but as expected for a Gαz-coupled receptor, only the sulprostone effect was resistant to PTX (Fig. 7B). Neither CGS-12066A nor BW-72386 had a significant effect on GSIS, regardless of PTX treatment. This suggests that, at least in the moderate β-cell compensation of the C57Bl/6 ob/ob mice, 5HT receptor signaling does not play a role in regulating β-cell function.
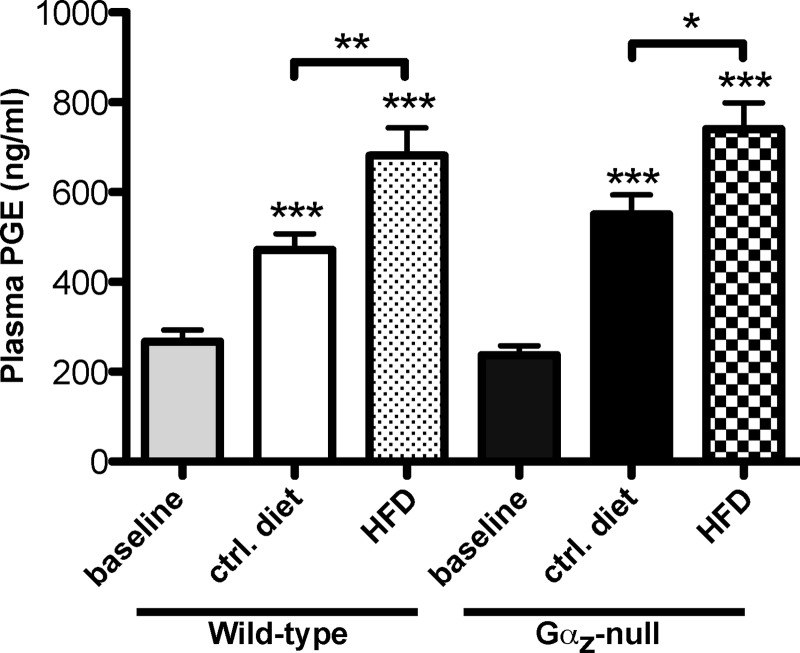
Finally, to explore potential up-regulation of the EP3 signaling pathway in our DIO study, we determined the plasma PGE concentrations before and after control- or HFD feeding. We found that PGE levels were significantly elevated from base line (11-weeks-old) with both age and HFD feeding, regardless of genotype, with mice being fed the HFD having the highest levels of all the groups (Fig. 8). These results, combined with those from Fig. 7, support our hypothesis that tonic activation of a Gαz-coupled receptor by an endogenous ligand limits β-cell compensation in response to aging and nutritional stress and that in the absence of Gαz, this inhibitory pathway is broken.
FIGURE 8.
Increased plasma PGE levels in C57Bl/6 mice with age and HFD supports tonic activation of a Gαz-coupled receptor. Plasma PGE metabolite levels were recorded from plasma samples collected after a 4-h fast, at t = 0 of the IP-GTTs. n = 6 mice per group. The data were analyzed by unpaired t test. *, p < 0.05; **, p < 0.01; ***, p < 0.001.
DISCUSSION
The Centers for Disease Control and Prevention have published sobering statistics for the impact of T2DM on the United States population (2011 National Diabetes Fact Sheet). Currently, 25.8 million people are estimated to have T2DM (8.3% of the total United States population). Furthermore, 1.9 million T2DM cases were newly diagnosed in 2010. The number of T2DM cases in the 10–19 year age group now approximately equals that of type 1 diabetes mellitus (T1DM), and the youngest members of this age group have an overall lifetime risk of developing T2DM of 30–50%.
T2DM susceptibility genes emanating from genome-wide association studies are almost exclusively related to β-cell function and maintenance of mass rather than insulin resistance (6). In light of this, many T2DM treatments under development aim to improve β-cell function and not simply insulin sensitivity (4, 23–25). In this regard, our results with C57Bl/6 Gαz-null mice are quite intriguing, because the C57Bl/6 mouse line has been suggested to be resistant to diabetes because of their β-cell compensatory response (20, 26). Our data suggest there is untapped potential for β-cell expansion and mass even in an animal that has a strong intrinsic compensatory response and that this increase in mass can substitute for a decrease in function.
Our previous studies of mice deficient in Gαz demonstrated constitutively increased cAMP production, leading to enhanced GSIS (8). Interestingly, this difference in in vitro GSIS response was phenocopied in the older C57Bl/6 mice fed the control diet, but not in the Gαz-null mice fed the HFD. This indicates that the loss of Gαz is unable to preserve normal glucose responsiveness in an environment of overnutrition that includes increased plasma levels of glucose, insulin, free fatty acids, triglycerides, cholesterol, oxidative stressors, and/or inflammatory cytokines (3, 27–30). Long term HFD feeding in C57Bl/6 mice causes functional uncoupling of Ca2+ channels from the secretory granules (31); this effect is mimicked by chronic exposure of isolated mouse and human islets to high levels of palmitate (32). To the extent that this constitutes a major mechanism of impaired insulin secretion in response to overnutrition, loss of Gαz, which acts through cAMP potentiation of GSIS (7, 8), would not be expected to repair β-cell dysfunction elicited by uncoupling of the Ca2+ signal from the secretory response.
Rather than affecting function, the dramatic impact of Gαz loss on β-cell replication and β-cell mass appears to be sufficient to protect these mice from developing glucose intolerance. With the role that cAMP has been shown to play in β-cell growth, survival, and/or proliferation (33–43), it is possible that the increased cAMP production observed in Gαz-null islets (Ref. 8 and Fig. 7A) is responsible for the increase in β-cell replication and mass. Interestingly, it appears that augmented cAMP production is a general feature of Gαz loss in tissues where Gαz has been shown to have a functional role. Specifically, platelets from Gαz-null C57Bl/6 mice also display increased cAMP levels, correlating with inhibited platelet aggregation responses to ADP and epinephrine (19).
Gαz has been conclusively linked to five GPCRs in vivo: the Mu opioid, D2 dopamine, E prostanoid, serotonin-1A (5HT1A), and α2A-adrenergic (7, 17, 18, 44, 45). The α2A-adrenoreceptor specifically couples to Gαz in platelets (19, 46). We have previously shown that in a β-cell line, Gαz is specifically coupled to an E prostanoid receptor, and not the α2A-aderenoreceptor, to block cAMP accumulation and subsequent GSIS (7). In the current study, we show that this signaling pathway is also functional in primary islets and that the specific E prostanoid isoform is EP3 (Fig. 7B).
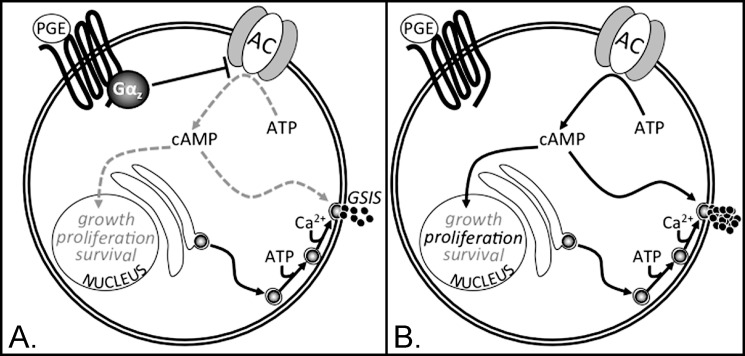
Increased plasma PGE metabolite levels have been found to correlate with diabetic status (47). In our HFD model of insulin resistance and glucose intolerance, plasma PGE levels are increased both with age and HFD feeding, with the latter having the most significant effect (Fig. 8). The pathway from PGEs → EP3 → Gαz → adenylate cyclase leads to decreased cAMP production, which would negatively impact on β-cell compensation. We hypothesize that the absence of Gαz breaks this endogenous signaling pathway, which may simply be a negative consequence of the insulin resistant and/or diabetic condition (see model in Fig. 9).
FIGURE 9.
Model for Gαz as a tonic negative regulator of β-cell adenylate cyclase in conditions of high circulating E prostanoids (PGEs). A, when PGE levels are high, such as with aging and/or after the extended DIO study performed in this work, the PGE receptor activates Gαz, which negatively regulates adenylate cyclase, decreasing the production of cAMP from ATP. cAMP has dual roles in promoting both GSIS and processes involved in the maintenance of β-cell mass (e.g. cell growth, proliferation, and/or survival). Therefore, the positive impact of cAMP on these processes is blocked when the E prostanoid receptor activates Gαz. B, when Gαz is lost, there is no inhibitory G protein coupled to the E prostanoid receptor. Therefore, the tonic inhibition on adenylate cyclase is lost, allowing for increased cAMP production, stimulation of GSIS, and stimulation of proliferation.
Although the hypothesized pathway from circulating PGE to cAMP modulation fits very well with the phenotypic data from Gαz-null mice, there are other possible explanations for the resistance of Gαz-null mice to HFD-induced glucose intolerance. One such explanation comes from the observation that 5HT1A serotonin receptor is coupled to Gαz in the hippocampus to negatively regulate serotonin signaling, consistent with the finding that Gαz-null mice have an enhanced response to serotonin (18). Recently, the 5HT2B receptor has been shown to act downstream of lactogen signaling to promote β-cell proliferation in pregnant mice, whereas 5HT1D receptor signaling reduces β-cell mass after partuition (22). We explored the roles of these families of serotonin receptors in islets from glucose-intolerant C57Bl/6 mice (Fig. 7B). Neither 5HT1 nor 5HT2 agonism had an impact on GSIS from islets harvested from glucose-intolerant C57Bl/6 mice, either in the presence or absence of Gαi subfamily inhibition. Caveats to this study are that serotonin signaling may impact only on β-cell mass and not function, or serotonin receptors may only play roles at certain time points during the β-cell compensatory response to insulin resistance. These questions will be important to research in future works.
Finally, our results may have important implications for the T1DM field. T1DM occurs when immune-mediated pancreatic β-cell destruction leads to nearly absolute insulin deficiency (48, 49). Most patients with newly diagnosed T1DM still have the capacity to secrete insulin in amounts corresponding to 20–30% of those of nondiabetics (50). Meanwhile, only ∼10% of pancreatic islet transplant patients remain completely insulin independent at 5 years after (51). The residual β-cell function observed in both early T1DM and in pancreatic islet transplant patients indicates the presence of a pool of potentially expandable β-cells. The link between the loss of β-cell mass in both T1DM and late stage T2DM has provided a rationale for testing T2DM treatments that act through cAMP generation in rodent models of T1DM and in human T1DM patients and pancreatic islet transplant recipients. These studies show prolonged survival, improved glycemia, and maintenance of graft function for a longer duration (52–54). Therapeutic strategies that increase islet cell cAMP production, such as those that based on the Gαz pathway described in this manuscript, could be an important tool in T1DM therapy.
In conclusion, Gαz loss is protective against the development of age-related β-cell dysfunction and HFD-induced glucose intolerance in mice, the former via effects on β-cell function and proliferation, and the latter working predominantly through enhanced β-cell proliferation leading to increased β-cell mass. Taken together, our work has helped to define the role of Gαz in normal and pathophysiological pathways in the β-cell and lent credence to the further study of this pathway in additional models of diabetes mellitus.
Acknowledgments
We thank the former and present members of the Kimple, Casey, and Newgard labs, in particular Everett McCook and Missy Infante, for expert technical assistance, and Patrick Fueger, Sam Stephens, Hans Hohmeier, and Jeff Tessem for productive discussions. Finally, we thank Alan Attie at University of Wisconsin-Madison for providing the environment and encouragement to complete this study.
This work was supported, in whole or in part, by National Institutes of Health Grants DK080845 (to M. E. K.), DK042583 (to C. B. N.), and DK076488 (to P. J. C.).

This article contains supplemental Figs. S1–S3.
- T2DM
- type 2 diabetes mellitus
- T1DM
- type 1 diabetes mellitus
- GSIS
- glucose-stimulated insulin secretion
- HFD
- high fat diet
- GPCR
- G protein-coupled receptor
- DIO
- diet-induced obesity
- PGE
- prostaglandin E
- IP-GTT
- intraperitoneal glucose tolerance test
- ANOVA
- analysis of variance
- SST
- somatostatin 28
- G protein
- Guanine nucleotide binding protein
- DAB
- 3,3′-diaminobenzidine.
REFERENCES
- 1. Kahn S. E. (2003) The relative contributions of insulin resistance and β-cell dysfunction to the pathophysiology of Type 2 diabetes. Diabetologia 46, 3–19 [DOI] [PubMed] [Google Scholar]
- 2. Bergman R. N., Finegood D. T., Kahn S. E. (2002) The evolution of β-cell dysfunction and insulin resistance in type 2 diabetes. Eur. J. Clin. Invest. 32, (Suppl. 3) 35–45 [DOI] [PubMed] [Google Scholar]
- 3. Greenberg A. S., McDaniel M. L. (2002) Identifying the links between obesity, insulin resistance and beta-cell function. Potential role of adipocyte-derived cytokines in the pathogenesis of type 2 diabetes. Eur. J. Clin. Invest. 32, (Suppl. 3) 24–34 [DOI] [PubMed] [Google Scholar]
- 4. Gerich J. E. (2002) Redefining the clinical management of type 2 diabetes. Matching therapy to pathophysiology. Eur. J. Clin. Invest. 32, (Suppl. 3) 46–53 [DOI] [PubMed] [Google Scholar]
- 5. Muoio D. M., Newgard C. B. (2008) Mechanisms of disease. Molecular and metabolic mechanisms of insulin resistance and β-cell failure in type 2 diabetes. Nat. Rev. Mol. Cell Biol. 9, 193–205 [DOI] [PubMed] [Google Scholar]
- 6. Billings L. K., Florez J. C. (2010) The genetics of type 2 diabetes. What have we learned from GWAS? Ann. N.Y. Acad. Sci. 1212, 59–77 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Kimple M. E., Nixon A. B., Kelly P., Bailey C. L., Young K. H., Fields T. A., Casey P. J. (2005) A role for Gz in pancreatic islet β-cell biology. J. Biol. Chem. 280, 31708–31713 [DOI] [PubMed] [Google Scholar]
- 8. Kimple M. E., Joseph J. W., Bailey C. L., Fueger P. T., Hendry I. A., Newgard C. B., Casey P. J. (2008) Gαz negatively regulates insulin secretion and glucose clearance. J. Biol. Chem. 283, 4560–4567 [DOI] [PubMed] [Google Scholar]
- 9. Fong H. K., Yoshimoto K. K., Eversole-Cire P., Simon M. I. (1988) Identification of a GTP-binding protein α subunit that lacks an apparent ADP-ribosylation site for pertussis toxin. Proc. Natl. Acad. Sci. U.S.A. 85, 3066–3070 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Matsuoka M., Itoh H., Kozasa T., Kaziro Y. (1988) Sequence analysis of cDNA and genomic DNA for a putative pertussis toxin-insensitive guanine nucleotide-binding regulatory protein α subunit. Proc. Natl. Acad. Sci. U.S.A. 85, 5384–5388 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Casey P. J., Fong H. K., Simon M. I., Gilman A. G. (1990) Gz, a guanine nucleotide-binding protein with unique biochemical properties. J. Biol. Chem. 265, 2383–2390 [PubMed] [Google Scholar]
- 12. Hinton D. R., Blanks J. C., Fong H. K., Casey P. J., Hildebrandt E., Simons M. I. (1990) Novel localization of a G protein, Gz-α, in neurons of brain and retina. J. Neurosci. 10, 2763–2770 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Winzell M. S., Magnusson C., Ahrén B. (2007) Temporal and dietary fat content-dependent islet adaptation to high-fat feeding-induced glucose intolerance in mice. Metab. Clin. Exp. 56, 122–128 [DOI] [PubMed] [Google Scholar]
- 14. Kelleher K. L., Matthaei K. I., Hendry I. A. (2001) Targeted disruption of the mouse Gz-α gene. A role for Gz in platelet function? Thromb. Haemost. 85, 529–532 [PubMed] [Google Scholar]
- 15. Hendry I. A., Kelleher K. L., Bartlett S. E., Leck K. J., Reynolds A. J., Heydon K., Mellick A., Megirian D., Matthaei K. I. (2000) Hypertolerance to morphine in G(z α)-deficient mice. Brain Res. 870, 10–19 [DOI] [PubMed] [Google Scholar]
- 16. Leck K. J., Bartlett S. E., Smith M. T., Megirian D., Holgate J., Powell K. L., Matthaei K. I., Hendry I. A. (2004) Deletion of guanine nucleotide binding protein α z subunit in mice induces a gene dose dependent tolerance to morphine. Neuropharmacology 46, 836–846 [DOI] [PubMed] [Google Scholar]
- 17. Leck K. J., Blaha C. D., Matthaei K. I., Forster G. L., Holgate J., Hendry I. A. (2006) Gz proteins are functionally coupled to dopamine D2-like receptors in vivo. Neuropharmacology 51, 597–605 [DOI] [PubMed] [Google Scholar]
- 18. Oleskevich S., Leck K. J., Matthaei K., Hendry I. A. (2005) Enhanced serotonin response in the hippocampus of Gαz protein knock-out mice. Neuroreport 16, 921–925 [DOI] [PubMed] [Google Scholar]
- 19. Yang J., Wu J., Jiang H., Mortensen R., Austin S., Manning D. R., Woulfe D., Brass L. F. (2002) Signaling through Gi family members in platelets. Redundancy and specificity in the regulation of adenylyl cyclase and other effectors. J. Biol. Chem. 277, 46035–46042 [DOI] [PubMed] [Google Scholar]
- 20. Clee S. M., Attie A. D. (2007) The genetic landscape of type 2 diabetes in mice. Endocr. Rev. 28, 48–83 [DOI] [PubMed] [Google Scholar]
- 21. Winzell M. S., Ahren B. (2004) The high-fat diet-fed mouse. A model for studying mechanisms and treatment of impaired glucose tolerance and type 2 diabetes. Diabetes 53, (Suppl. 3) S215–S219 [DOI] [PubMed] [Google Scholar]
- 22. Kim H., Toyofuku Y., Lynn F. C., Chak E., Uchida T., Mizukami H., Fujitani Y., Kawamori R., Miyatsuka T., Kosaka Y., Yang K., Honig G., van der Hart M., Kishimoto N., Wang J., Yagihashi S., Tecott L. H., Watada H., German M. S. (2010) Serotonin regulates pancreatic β cell mass during pregnancy. Nat. Med. 16, 804–808 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Furman B., Pyne N. (2006) Modulation of cyclic nucleotides and cyclic nucleotide phosphodiesterases in pancreatic islet β-cells and intestinal L-cells as targets for treating diabetes mellitus. Curr. Opin. Investig. Drugs 7, 898–905 [PubMed] [Google Scholar]
- 24. Triplitt C. L. (2007) New technologies and therapies in the management of diabetes. Am. J. Manag. Care 13, S47–S54 [PubMed] [Google Scholar]
- 25. Jellinger P. S. (2011) Focus on incretin-based therapies. Targeting the core defects of type 2 diabetes. Postgrad. Med. 123, 53–65 [DOI] [PubMed] [Google Scholar]
- 26. Clee S. M., Nadler S. T., Attie A. D. (2005) Genetic and genomic studies of the BTBR ob/ob mouse model of type 2 diabetes. Am. J. Ther. 12, 491–498 [DOI] [PubMed] [Google Scholar]
- 27. Hsu C. C., Yen H. F., Yin M. C., Tsai C. M., Hsieh C. H. (2004) Five cysteine-containing compounds delay diabetic deterioration in Balb/cA mice. J. Nutr. 134, 3245–3249 [DOI] [PubMed] [Google Scholar]
- 28. Keren P., George J., Shaish A., Levkovitz H., Janakovic Z., Afek A., Goldberg I., Kopolovic J., Keren G., Harats D. (2000) Effect of hyperglycemia and hyperlipidemia on atherosclerosis in LDL receptor-deficient mice. Establishment of a combined model and association with heat shock protein 65 immunity. Diabetes 49, 1064–1069 [DOI] [PubMed] [Google Scholar]
- 29. Lingohr M. K., Buettner R., Rhodes C. J. (2002) Pancreatic β-cell growth and survival. A role in obesity-linked type 2 diabetes? Trends Mol. Med. 8, 375–384 [DOI] [PubMed] [Google Scholar]
- 30. Nonogaki K., Iguchi A. (1997) Stress, acute hyperglycemia, and hyperlipidemia role of the autonomic nervous system and cytokines. Trends Endocrinol. Metab. 8, 192–197 [DOI] [PubMed] [Google Scholar]
- 31. Collins S. C., Hoppa M. B., Walker J. N., Amisten S., Abdulkader F., Bengtsson M., Fearnside J., Ramracheya R., Toye A. A., Zhang Q., Clark A., Gauguier D., Rorsman P. (2010) Progression of diet-induced diabetes in C57BL6J mice involves functional dissociation of Ca2+ channels from secretory vesicles. Diabetes 59, 1192–1201 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Hoppa M. B., Collins S., Ramracheya R., Hodson L., Amisten S., Zhang Q., Johnson P., Ashcroft F. M., Rorsman P. (2009) Chronic palmitate exposure inhibits insulin secretion by dissociation of Ca2+ channels from secretory granules. Cell Metab. 10, 455–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Shibasaki T., Takahashi H., Miki T., Sunaga Y., Matsumura K., Yamanaka M., Zhang C., Tamamoto A., Satoh T., Miyazaki J., Seino S. (2007) Essential role of Epac2/Rap1 signaling in regulation of insulin granule dynamics by cAMP. Proc. Natl. Acad. Sci. U.S.A. 104, 19333–19338 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Li Y., Hansotia T., Yusta B., Ris F., Halban P. A., Drucker D. J. (2003) Glucagon-like peptide-1 receptor signaling modulates β cell apoptosis. J. Biol. Chem. 278, 471–478 [DOI] [PubMed] [Google Scholar]
- 35. Drucker D. J. (2003) Glucagon-like peptides. Regulators of cell proliferation, differentiation, and apoptosis. Mol. Endocrinol. 17, 161–171 [DOI] [PubMed] [Google Scholar]
- 36. Aronoff D. M., Canetti C., Serezani C. H., Luo M., Peters-Golden M. (2005) Cutting edge. Macrophage inhibition by cyclic AMP (cAMP). Differential roles of protein kinase A and exchange protein directly activated by cAMP-1. J. Immunol. 174, 595–599 [DOI] [PubMed] [Google Scholar]
- 37. Xu G., Stoffers D. A., Habener J. F., Bonner-Weir S. (1999) Exendin-4 stimulates both β-cell replication and neogenesis, resulting in increased β-cell mass and improved glucose tolerance in diabetic rats. Diabetes 48, 2270–2276 [DOI] [PubMed] [Google Scholar]
- 38. Wang Q., Brubaker P. L. (2002) Glucagon-like peptide-1 treatment delays the onset of diabetes in 8 week-old db/db mice. Diabetologia 45, 1263–1273 [DOI] [PubMed] [Google Scholar]
- 39. Sturis J., Gotfredsen C. F., Romer J., Rolin B., Ribel U., Brand C. L., Wilken M., Wassermann K., Deacon C. F., Carr R. D., Knudsen L. B. (2003) GLP-1 derivative liraglutide in rats with beta-cell deficiencies. Influence of metabolic state on β-cell mass dynamics. Br. J. Pharmacol. 140, 123–132 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Perfetti R., Zhou J., Doyle M. E., Egan J. M. (2000) Glucagon-like peptide-1 induces cell proliferation and pancreatic-duodenum homeobox-1 expression and increases endocrine cell mass in the pancreas of old, glucose-intolerant rats. Endocrinology 141, 4600–4605 [DOI] [PubMed] [Google Scholar]
- 41. Gedulin B. R., Nikoulina S. E., Smith P. A., Gedulin G., Nielsen L. L., Baron A. D., Parkes D. G., Young A. A. (2005) Exenatide (exendin-4) improves insulin sensitivity and β-cell mass in insulin-resistant obese fa/fa Zucker rats independent of glycemia and body weight. Endocrinology 146, 2069–2076 [DOI] [PubMed] [Google Scholar]
- 42. Farilla L., Hui H., Bertolotto C., Kang E., Bulotta A., Di Mario U., Perfetti R. (2002) Glucagon-like peptide-1 promotes islet cell growth and inhibits apoptosis in Zucker diabetic rats. Endocrinology 143, 4397–4408 [DOI] [PubMed] [Google Scholar]
- 43. Mu J., Petrov A., Eiermann G. J., Woods J., Zhou Y. P., Li Z., Zycband E., Feng Y., Zhu L., Roy R. S., Howard A. D., Li C., Thornberry N. A., Zhang B. B. (2009) Inhibition of DPP-4 with sitagliptin improves glycemic control and restores islet cell mass and function in a rodent model of type 2 diabetes. Eur. J. Pharmacol. 623, 148–154 [DOI] [PubMed] [Google Scholar]
- 44. Garzon J., Martinez-Pena Y., Sanchez-Blazquez P. (1997) Gx/z is regulated by mu but not delta opioid receptors in the stimulation of the low Km GTPase activity in mouse periaqueductal grey matter. Eur. J. Neurosci. 9, 1194–1200 [DOI] [PubMed] [Google Scholar]
- 45. Meng J., Casey P. J. (2002) Activation of Gz attenuates Rap1-mediated differentiation of PC12 cells. J. Biol. Chem. 277, 43417–43424 [DOI] [PubMed] [Google Scholar]
- 46. Yang J., Wu J., Kowalska M. A., Dalvi A., Prevost N., O'Brien P. J., Manning D., Poncz M., Lucki I., Blendy J. A., Brass L. F. (2000) Loss of signaling through the G protein, Gz, results in abnormal platelet activation and altered responses to psychoactive drugs. Proc. Natl. Acad. Sci. U.S.A. 97, 9984–9989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Axelrod L., Shulman G. I., Blackshear P. J., Bornstein W., Roussell A. M., Aoki T. T. (1986) Plasma level of 13,14-dihydro-15-keto-PGE2 in patients with diabetic ketoacidosis and in normal fasting subjects. Diabetes 35, 1004–1010 [DOI] [PubMed] [Google Scholar]
- 48. Mathis D., Vence L., Benoist C. (2001) β-Cell death during progression to diabetes. Nature 414, 792–798 [DOI] [PubMed] [Google Scholar]
- 49. Devendra D., Liu E., Eisenbarth G. S. (2004) Type 1 diabetes. Recent developments. BMJ 328, 750–754 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Madsbad S., Krarup T., Regeur L., Faber O. K., Binder C. (1980) Insulin secretory reserve in insulin dependent patients at time of diagnosis and the first 180 days of insulin treatment. Acta Endocrinol. 95, 359–363 [DOI] [PubMed] [Google Scholar]
- 51. Ryan E. A., Paty B. W., Senior P. A., Bigam D., Alfadhli E., Kneteman N. M., Lakey J. R., Shapiro A. M. (2005) Five-year follow-up after clinical islet transplantation. Diabetes 54, 2060–2069 [DOI] [PubMed] [Google Scholar]
- 52. Yanay O., Moralejo D., Kernan K., Brzezinski M., Fuller J. M., Barton R. W., Lernmark A., Osborne W. R. (2010) Prolonged survival and improved glycemia in BioBreeding diabetic rats after early sustained exposure to glucagon-like peptide 1. J. Gene Med. 12, 538–544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53. Kielgast U., Holst J. J., Madsbad S. (2009) Treatment of type 1 diabetic patients with glucagon-like peptide-1 (GLP-1) and GLP-1R agonists. Curr. Diabetes Rev. 5, 266–275 [DOI] [PubMed] [Google Scholar]
- 54. Faradji R. N., Froud T., Messinger S., Monroy K., Pileggi A., Mineo D., Tharavanij T., Mendez A. J., Ricordi C., Alejandro R. (2009) Long-term metabolic and hormonal effects of exenatide on islet transplant recipients with allograft dysfunction. Cell Transplant. 18, 1247–1259 [DOI] [PubMed] [Google Scholar]