Background: Clostridium acetobutylicum synthesizes asparagine (Asn) and Asn-tRNAAsn.
Results: Synthesis of Asn is tRNA-dependent and regulated by a T-box riboswitch that is functional in a Gram-negative environment.
Conclusion: The gene redundancy may be connected to the regulation of Asn tRNA-dependent synthesis.
Significance: This study points to the involvement of aminoacyl-tRNA synthetases beyond their canonical function in Asn-tRNAAsn synthesis.
Keywords: Aminoacyl-tRNA Synthesis, Bacterial Metabolism, Riboswitch, Transcription Regulation, Transfer RNA (tRNA), T-box, Antitermination, tRNA-dependent Synthesis of Asn
Abstract
Analysis of the Gram-positive Clostridium acetobutylicum genome reveals an inexplicable level of redundancy for the genes putatively involved in asparagine (Asn) and Asn-tRNAAsn synthesis. Besides a duplicated set of gatCAB tRNA-dependent amidotransferase genes, there is a triplication of aspartyl-tRNA synthetase genes and a duplication of asparagine synthetase B genes. This genomic landscape leads to the suspicion of the incoherent simultaneous use of the direct and indirect pathways of Asn and Asn-tRNAAsn formation. Through a combination of biochemical and genetic approaches, we show that C. acetobutylicum forms Asn and Asn-tRNAAsn by tRNA-dependent amidation. We demonstrate that an entire transamidation pathway composed of aspartyl-tRNA synthetase and one set of GatCAB genes is organized as an operon under the control of a tRNAAsn-dependent T-box riboswitch. Finally, our results suggest that this exceptional gene redundancy might be interconnected to control tRNA-dependent Asn synthesis, which in turn might be involved in controlling the metabolic switch from acidogenesis to solventogenesis in C. acetobutylicum.
Introduction
Given that ribosome-directed protein synthesis rests upon the supply of 20 species of aminoacyl-tRNAs (aa-tRNAs),4 each organism is in theory expected to encode a complete and unique set of 20 aminoacyl-tRNA synthetases (aaRSs). Each aaRS would be responsible for the attachment of a single amino acid onto its corresponding tRNA. However, only a minority of organisms, especially in prokaryotes, encodes this unique and complete set of aaRSs. The vast majority is either lacking or containing extra aaRSs (1, 2).
In bacteria, the most frequently missing aaRSs are asparaginyl- (AsnRS) and glutaminyl-tRNA synthetases (GlnRS) (3). Their absence is compensated by an alternate two-step route called the transamidation pathway. In this pathway, a mischarged glutamyl-tRNAGln (Glu-tRNAGln) is formed by a non-discriminating glutamyl-tRNA synthetase (GluRS) and subsequently converted by amidation of the attached Glu into glutaminyl-tRNAGln (Gln-tRNAGln) by a tRNA-dependent amidotransferase (AdT). Similarly, asparaginyl-tRNAAsn (Asn-tRNAAsn) is synthesized by AdT-catalyzed amidation of a mischarged Asp-tRNAAsn generated by a non-discriminating aspartyl-tRNA synthetase (AspRS).
All bacterial AdTs isolated so far are composed of three subunits, GatC, GatA, and GatB, that are assembled in a heterotrimeric enzyme called GatCAB (3). They are dually specific, capable of converting both Glu-tRNAGln and Asp-tRNAAsn into Gln-tRNAGln and Asn-tRNAAsn, respectively, at least in vitro (4, 5). In vivo, depending on whether only AsnRS or GlnRS is missing or whether both are absent, a single GatCAB AdT will be required to generate either only one amide aa-tRNA species or both. Note that the GatCAB-mediated transamidation pathway is predominantly used by bacteria to generate the amide aa-tRNA species because of the 1086 bacterial genomes that have been sequenced 90% contain the gatC, -A, and -B genes.
In all bacteria in which amide aa-tRNA formation has been examined, it was found that both pathways, direct charging of tRNA by AsnRS or GlnRS and transamidation by a GatCAB AdT, are mutually exclusive (6) (Fig. 1, A and B). Hence, the use of a GatCAB AdT to generate Gln-tRNAGln precludes that of a GlnRS for the same reaction. Likewise, a GatCAB AdT used to generate Asn-tRNAAsn will exclude the presence of an AsnRS in the organism. However, if asparagine synthetase (AsnA/B), the metabolic enzyme that generates Asn in a tRNA-independent manner, is missing, the GatCAB AdT will be retained together with AsnRS. In this case, the transamidation pathway is necessary for the synthesis of Asn-tRNAAsn under Asn starvation conditions, and AsnRS is more efficient than the AdT when Asn is present in the medium (7).
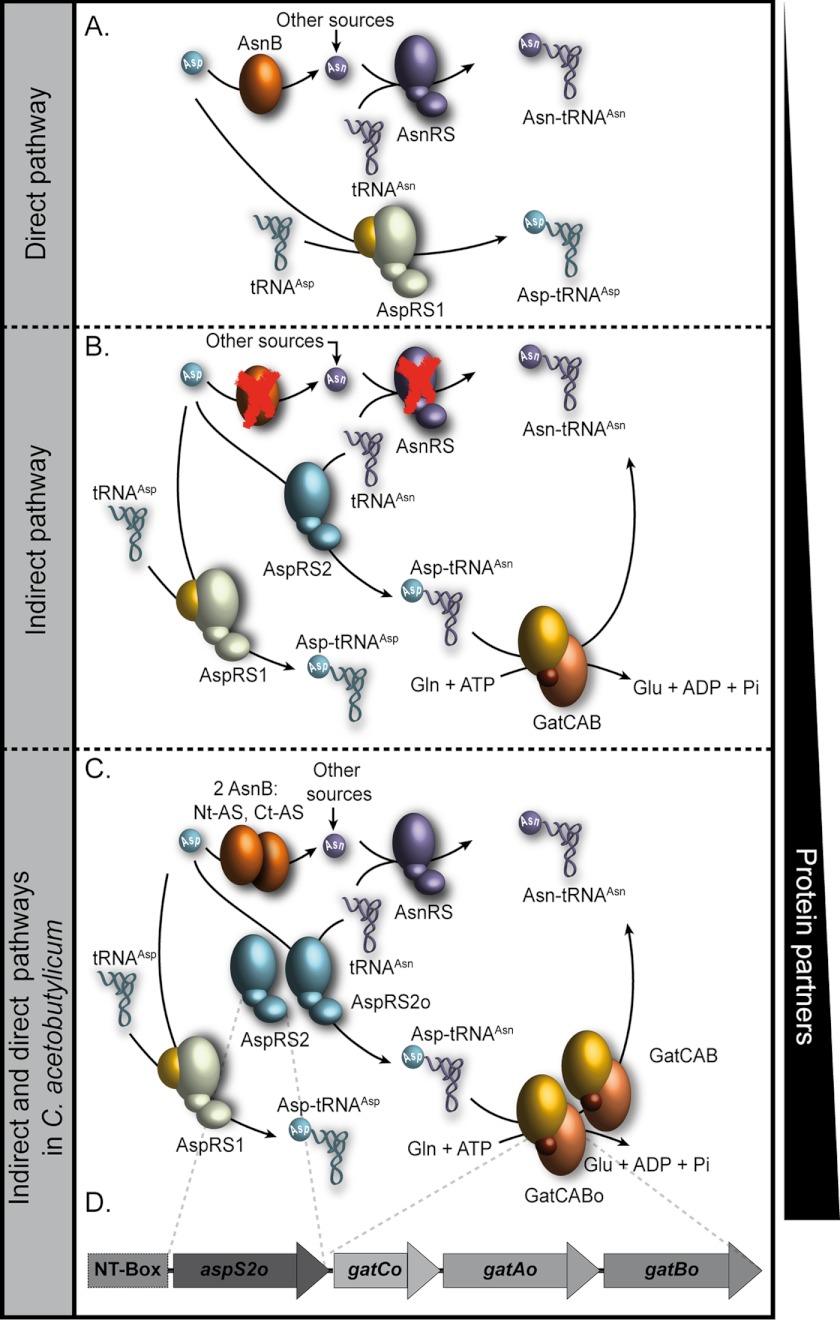
FIGURE 1.
Direct and indirect pathways for Asn and Asn-tRNAAsn synthesis. A, schematic representation of the direct pathway of Asn-tRNAAsn synthesis. AspRS1, bacterial-like aspartyl-tRNA synthetase. B, schematic representation of the indirect pathway that usually compensates for the absence (red cross) of AsnRS or AS. AspRS2, archaeal-like non-discriminating aspartyl-tRNA synthetase. C, increase in the number of protein partners when the indirect pathway is used, especially in the case of Cac. Based on the bacterium gene content, there is triplication in AspRSs (AspRS2o, AspRS2, and AspRS1) and duplication in GatCABs (GatCABo and GatCAB). Cac also encodes for two truncated asparagine synthetases (AsnB), Nt-AS and Ct-AS. D, schematic representation of the operon organization of aspS2ogatCABo regulated at the transcriptional level by the NT-box. The latter is found at the 5′-UTR of the operon. Asn may come from other sources than asparagine synthetase-dependent amidation of Asp.
The presence of extra aaRSs of the same specificity is also widespread among all species of bacteria. The number of copies of the same aaRS rarely exceeds two copies (duplicated aaRS) and has been reported for almost all of the 20 aaRS species (for a review, see Ref. 4). In all cases, the two copies display various degrees of sequence variations and either do not share the same tRNA specificity or are differentially expressed during physiological or environmental changes (8, 9–11). AspRS and GluRS are the most frequently duplicated aaRSs found in bacteria, and duplication of these two aaRS species always correlates with the use of the transamidation pathway to generate one or two amide aa-tRNAs. The rationale for these duplications is that one AspRS or GluRS will charge the cognate tRNA species (tRNAAsp or tRNAGlu, respectively), whereas the other will mischarge the tRNAAsn or tRNAGln species to supply the GatCAB AdT with its mischarged substrate (7, 12).
If one only considers synthesis of amide aa-tRNA species, one would realize the huge variability in the combinations of pathways and enzymes used by the bacterial species. This combinatorial diversity is reflected at the genomic level with an extraordinary variation in the composition of the pool of genes devoted to the synthesis of these two particular aa-tRNAs. However, in Clostridium acetobutylicum, the combination of genes putatively involved in Asn-tRNAAsn synthesis escapes any rationale (Fig. 1C). C. acetobutylicum (Cac) is a spore-forming, Gram-positive, obligate anaerobe with a high A-T base content (72%) (13). Like most Gram-positive bacteria, Cac lacks GlnRS and thus forms Gln-tRNAGln via the transamidation pathway. It is therefore not surprising that the genome encodes a GatCAB AdT. However, the Cac genome reveals the presence of a duplicated set of gatCAB genes in addition to the genes encoding both AsnRS and two truncated asparagine synthetases (Nt-AS and Ct-AS). More surprisingly, the AspRS is triplicated with one copy, AspRS1, resembling bacterial AspRSs and the two other copies, AspRS2 and AspRS2o, being typically of archaeal architecture (8) (Fig. 1C). With respect to Asn-tRNAAsn formation, this gene redundancy looks completely aberrant because the enzymes of both the direct pathway of tRNA asparaginylation (asparagine synthetase and AsnRS) and of two transamidation pathways (two archaeal-like AspRSs and two GatCAB AdTs) seem concomitantly present in this species. One possible explanation for this gene redundancy and pathway duplication is that expression of these enzymes is regulated in response to specific physiological or environmental conditions.
We therefore scanned the 5′- and 3′-untranslated regions (UTRs) flanking the subset of redundant genes involved in Asn-tRNAAsn synthesis, searching for mRNA regulatory elements that might regulate expression of these genes. We found that the genes encoding one of the archaeal-like AspRS2s (aspS2o) and one of the GatCAB AdTs (gatCABo) are potentially organized in an operon (Fig. 1D) under the control of 5′-UTR cis-acting non-coding RNA called T-box. To distinguish the AspRS2 and the GatCAB that are encoded by this operon, we added at the end of each gene name an “o” that stands for “operon” (AspRS2o and GatCABo).
T-box is a cis-acting riboswitch that is predominantly found in Gram-positive bacteria (14, 15). It uses uncharged tRNA as a ligand that binds to the riboswitch and triggers transcription antitermination. However, the inability of charged tRNA to bind the T-box induces transcription termination. T-boxes have been shown to control transcription of a variety of genes encoding aaRSs, amino acid-forming enzymes, or amino acid transporters (15). This riboswitch allows many bacterial species to respond to the changing levels of the corresponding amino acid by adapting the expression of genes transporting or using these amino acids and thus to respond to certain stress signals (16, 17). When suffering from certain nutritional stresses, the ratio of uncharged tRNAs versus charged tRNAs increases, allowing these uncharged tRNAs to bind to T-boxes and act as effector molecules to regulate global gene expression (18, 19). These T-boxes are usually 200–300-nucleotides (nt) long and include a factor-independent (intrinsic) transcription termination signal that adopts a competing antiterminator conformation upon binding of the uncharged tRNA (20, 21), allowing transcriptional read-through of the downstream gene or set of genes. In this case, the increased level of uncharged tRNA serves as a signal, relaying to the transcriptional machinery a deficiency in either aa-tRNA- or amino acid-forming enzymes. So far, it is known that the specificity of the T-box response is dependent on a single codon present in the specifier domain of this riboswitch, which by pairing with the anticodon of the cognate tRNA adapts transcription of genes to the level of a single amino acid intracellular concentration (14, 16).
In the present report, we show the presence in Cac of an operon encoding the enzymes of an entire transamidation pathway (aspS2ogatCABo operon) regulated by a T-box riboswitch. We show that this T-box is functional not only in vitro but also in vivo in a Gram-negative environment. Finally, by a combination of biochemical and genetic approaches including the generation of knock-out strains, we bring answers to the apparent aberrant gene redundancy in Cac Asn-tRNAAsn formation.
EXPERIMENTAL PROCEDURES
Materials
l-Asparagine, l-aspartate, and l-glutamine were from Merck; hydroxyapatite and DEAE-cellulose DE-52 were from Whatman; and Mono-Q columns (Mono-QTM 10/100 GL) were from Amersham Biosciences. All primers were from Sigma, and all enzymes were purchased from Fermentas except restriction enzymes (New England Biolabs) and T7 RNA polymerase, which was prepared as described previously (22). Cac genomic DNA was from ATCC. Plasmid DNA was prepared using the GenEluteTM HP plasmid Maxiprep kit from Sigma-Aldrich. RNA elution was done using Clontech columns (CHROMA SPIN-30). [α-32P]UTP and [γ-32P]ATP were from Hartmann Analytic. Cac cells were broken using FastPrep® from MP Biomedicals. Sonication was performed using VibraCell from Bioblock Scientific. RNA extraction and purification were done using the RNeasy® Mini kit from Qiagen.
Construction of T-box and tRNA Gene Constructs
The NT-box sequence was PCR-amplified using Phusion polymerase, 36.5 nmol (100 ng) of the 4.15 Mb Cac genomic DNA, and 0.1 nmol of sense (NT-box_F) and antisense (NT-box_R) primers (supplemental Table S1). The 425-bp PCR product contains a T7 RNA polymerase promoter sequence (TAATACGACTCACTATA) extended by two G residues fused to the +1 position of the aspS2ogatCABo leader. The 3′-end of the NT-box corresponds to the 46th nucleotide (nt) of the aspS2o open reading frame (ORF). The PCR product was cloned into pUC18 plasmid using the HindIII and BamHI restriction sites flanking the 5′- and 3′-ends, respectively, of the fragment. Cac tRNAAsn(GUU) gene (Cac tRNAAsn) was synthesized by Integrated DNA Technologies, Inc. and was cloned into pIDTSMART plasmid. The tRNAAsn gene was flanked at the 5′-end with a transzyme as described previously (23). Overexpressed Escherichia coli tRNAAsp and Thermus thermophilus tRNAAsn(QUU) (Tth tRNAAsn) were already available (24). The NT-box_placZFT plasmid (25) was constructed for the β-galactosidase activity test and for tRNA-directed antitermination in vitro. The NT-box sequence along with its endogenous promoter was cloned upstream of the lacZ gene using SalI and BamHI restriction sites and transformed into E. coli ER strain (asnA−, asnB−) ordered from the Genetic Stock Center (Yale University, New Haven, CT). The NT-box promoter is typically identical to those of E. coli.
Construction of in Vitro T7 RNA Transcripts
In vitro T7 transcripts of the NT-box were obtained as described previously (26, 27). The NT-box transcript was then resuspended in binding buffer containing 50 mm Tris acetate, pH 7.0, 25 mm calcium acetate, 100 mm ammonium acetate, and 5% (v/v) glycerol prior to binding assays. Transcribed tRNAs and tRNAs overexpressed in E. coli were obtained as described previously (24, 28).
In Vitro tRNA-directed Antitermination Assay
Halted transcription assays were performed using the E. coli RNA polymerase from USB. The reactions were carried out essentially as described previously (29) with some modifications (see supplemental Experimental Procedures).
β-Galactosidase Activity Test
To assay tRNA-dependent antitermination in vivo, the E. coli asparagine auxotroph ER strain was co-transformed with the NT-box_placZFT and the Cac tRNAAsn_pKK223-3 recombinant plasmids. ER strain transformed with the empty (promoterless) placZFT plasmid served as a negative control, and ER strain transformed with the placZFT plasmid in which the β-galactosidase gene was under the control of the bdhB promoter (25) served as a positive control. The growth conditions used for β-galactosidase measurements were as described previously (30), and the spectrofluorometer used was a GloMax® Multi Detection System. All measurements were carried out in triplicate, and all experiments were performed at least twice.
Amidation
The reaction mixture contained 100 mm NaHepes, pH 7.2, 12 mm MgCl2, 10 mm ATP, 2 mm NH4Cl, 2 mm l-Gln, 10 μm l-[14C]Asp (207 mCi/mmol; Amersham Biosciences), and 100 μg of crude extract from E. coli, T. thermophilus, yeast, or Cac. The reaction was conducted at 42 °C as described previously (7), and the labeled products were revealed by scanning with an image plate reader (Fujifilm FLA 5100, Fujifilm Corp.).
tRNA-dependent Transamidation
tRNA-dependent transamidation reactions were performed at 37 °C for 10 min in a 50-μl standard reaction mixture as described previously (5, 7). A 0.6 mm enzyme preparation of Cac GatCABo or of Helicobacter pylori (Hpy) GatCAB was used, and 0.5 mm Cac tRNAAsn transcript or 1.6 μm Hpy_Glu-tRNAGln was added to complete the reaction. 14C-Labeled amino acids were visualized on TLC plates (TLC cellulose plates, 20 × 20 cm2) and revealed by scanning the dried TLC plates with the image plate reader.
Bacterial Growth and Preparation of Protein and RNA Extracts
Cac ATCC 824 cells were grown under batch culture conditions in minimal MES-buffered medium (31) and under strictly anaerobic conditions at 37 °C. When necessary, media were supplemented with asparagine (1 mm), aspartic acid (1 mm), ampicillin (100 μg/ml), chloramphenicol (30 μg/ml), clarithromycin (5 μg/ml), or erythromycin (50 μg/ml). For knock-out strain preparation, Cac ATCC 824 was grown in liquid and solid complex cell growth media (32) when necessary. Thiamphenicol (5 mg/ml) and clarithromycin (5 μg/ml) were added for different mutant selection steps. Genomic DNA from Cac was isolated by a variation of the Marmur procedure (33). E. coli strains were grown on Luria-Bertani medium supplemented when necessary with 200 mg/liter ampicillin. E. coli cell breakage was carried out by sonication, whereas Cac cell breakage was carried out with glass beads using a FastPrep (MP Biomedicals). E. coli transformation, maxi- and minipreparations of double-stranded DNA, DNA manipulations, and agarose-gel electrophoresis were conducted using standard procedures (34). RNA extraction and purification were done by breaking Clostridium cells with the FastPrep and using the RNeasy Mini kit from Qiagen according to the manufacturer's instructions. RNAprotect® Bacteria Reagent from Qiagen was used to protect RNA from degradation after disrupting bacterial cells. For RNA enrichment, we used the MICROBExpressTM kit from Ambion, Inc.
Cloning, Expression, and Purification of Cac GatCABo AdT
We designed the Cac gatCABo operon as described previously (35) except that the 3′-end of the gatB gene was extended in-frame by the sequence encoding the V5 epitope and His6. The gatCABo operon was synthesized by Genscript with codon optimization and subcloned into pET20b (Novagen) between the NdeI and XhoI restriction sites. Cells were grown in Luria-Bertani medium at 37 °C until midexponential phase, and expression was then induced by the addition of 0.5 mm IPTG. 10% (w/v) glucose was added for the expression of stress chaperones, and the cells were left to grow at 18 °C with shaking. Purification of the AdT was carried out as described previously (36) with modifications (see supplemental Experimental Procedures).
Reverse Transcription-PCR (RT-PCR) Operon Validation
RT-PCR was performed as described previously (37) with some modifications. cDNA was generated using purified Cac total RNA as a template and the following reverse primers (supplemental Table S1): aspS2oRevRT, gatCoRevRT, gatAoRevRT, and gatARevRT. cDNA synthesis was done in one cycle (1 h at 42 °C, 15 min at 70 °C, and cooling at 4 °C), and then PCR amplification was carried out using the cDNA and the corresponding primers (supplemental Table S1).
Preparation of Cac Knock-out Strains
Knock-out strains for aspS2 (CAC3564), aspS1 (CAC2269), gatBo (CAC2976), and Nt-AS (CAC2243) were prepared using the clostridial ClosTron system (38). Primers were designed using the Targetron Gene Knock-out System kit from Sigma-Aldrich according to the manufacturer's instructions. For every integration site, four primers were used including the exon binding site universal primer. Integration control was performed as described previously (38). All knock-outs were checked for pMTL007 loss using thiamphenicol selection. All those that lost thiamphenicol resistance and gained clarithromycin resistance were selected. All cultures were grown in complex cell growth medium or minimal MES medium at 37 °C under anaerobic conditions.
Electrophoresis Mobility Shift Assay
[γ-32P]ATP was used for 5′-end tRNA labeling, which was performed according to standard protocols. Binding between tRNAs and NT-box was assessed using a PAGE mobility shift assay. The binding assay was performed by mixing 0.35 μm 5′-32P-labeled tRNAs with 0.078–20 μm NT-box. After denaturing and renaturing the two RNAs separately, the NT-box transcript was treated by adding Mg2+ to the binding mixture before tRNA addition. The radiolabeled tRNA was added at constant concentration to an increasing amount of NT-box, and the mixture was left at room temperature for 25–30 min before loading on 6% (v/v) non-denaturing polyacrylamide gel. The gel composition was 6% (v/v) polyacrylamide in Tris borate buffer, 5 mm MgCl2, 50 mm NaCl, and 5% (v/v) glycerol. The shifted complex and free [32P]tRNAs were visualized by scanning with the Fujifilm image plate reader.
Size Exclusion Chromatography Assay
The size exclusion chromatography assay was performed using an analytical size exclusion chromatography column and ÄKTA Purifier HPLC (Amersham Biosciences). The binding assay was performed by mixing different concentrations of tRNAAsn and NT-box. Before binding, Cac and Tth tRNAAsn were denatured for 5 min at 90 °C and allowed to fold for 10 min at room temperature. The NT-box was heated at 70 °C for 10 min and mixed immediately in the electrophoretic mobility shift assay (EMSA) binding buffer with 25 mm Mg2+ and tRNAAsn. The mixture was then incubated at room temperature for 25 min before loading on the column. 20 μl of sample volume containing the Cac tRNAAsn transcript were loaded. The Tth tRNAAsn transcript was loaded in a 40-μl sample volume. All size exclusion chromatographies were performed at 4 °C using the binding buffer.
Analysis of Fermentation Products by GC
The concentrations of the fermentation products acetone, ethanol, butanol, butyrate, acetate, and 3-hydroxybutanone (acetoin) were determined by gas chromatography as described previously (31).
RESULTS AND DISCUSSION
Cac Gene Redundancy for Asn/Asp-tRNA Synthesis
Analysis of the genomic content of Cac shows a high redundancy in genes encoding enzymes involved in Asn- and Asp-tRNA formation (Fig. 1C). In addition, preliminary analysis of the loci encoding these genes suggests that both gatCAB sets of genes may be arranged in an operon except that one operon, aspS2ogatCABo, would also include an additional archaeal-like AspRS2 located upstream of the AdT genes (Fig. 1D). Our first goal was to confirm the operon organization of the two gatCAB genes (gatCABo and gatCAB) and to validate the presence of a bigger operon for containing the aspS2o and the gatCABo.
Existence of gatCAB and aspS2ogatCABo Operon in Cac
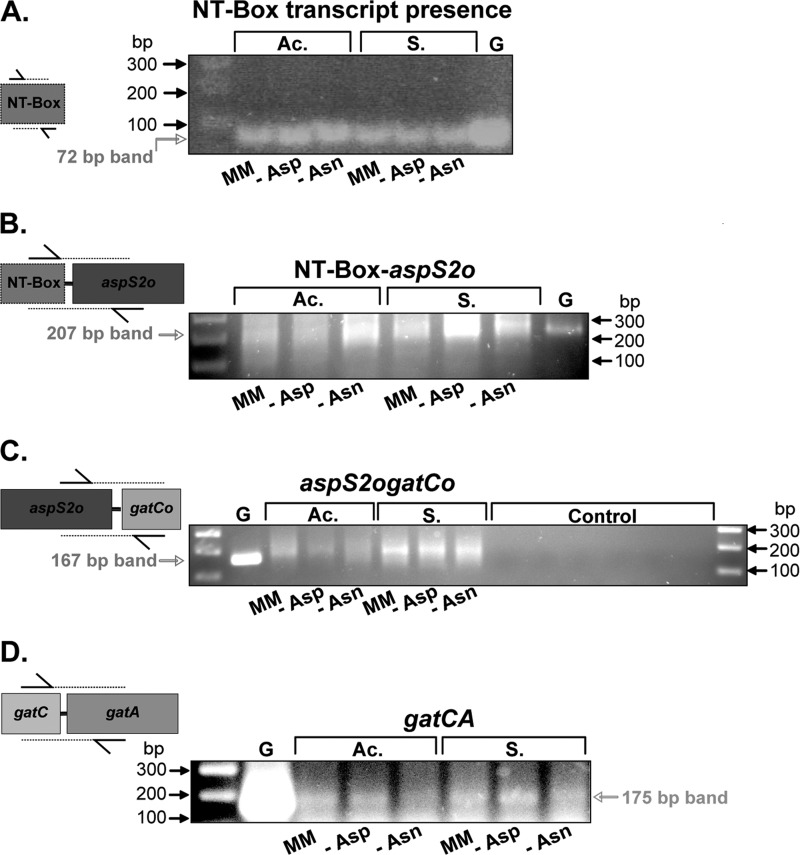
Using RNA extracts from different growth conditions, we were able to amplify by RT-PCR the RNA sequences located between aspS2o and gatCo as well as between gatC and gatA, thereby confirming the presence of an aspS2ogatCABo and a gatCAB operon (Fig. 2). The RT-PCR experiments showed that both operons are transcribed regardless of the metabolic phase used by Cac to process carbohydrates. Indeed, Cac is capable of fermenting a large variety of carbohydrates into acids and solvents. Acids like acetate and butyrate are produced during exponential growth phase (also called the acidogenesis phase), and their accumulation triggers a shift to a solventogenesis phase during which solvents such as butanol are produced (39). Therefore, one possible explanation for this gene redundancy and pathway duplication is that these enzymes are differentially expressed during acidogenesis and solventogenesis.
FIGURE 2.
Verification of operon organization for aspS2ogatCABo and gatCAB genes using RT-PCR. Six RNA preparations corresponding to six growth conditions were used: MM, minimal medium without asparagine and aspartic acid; −Asp, minimal medium in the presence of asparagine and absence of aspartic acid; and −Asn, minimal medium in the presence of aspartic acid and absence of asparagine. For the three conditions, RNAs were extracted from cells grown until acidogenesis (Ac.) or solventogenesis (S.). A, verification of the presence of the NT-box using specific oligonucleotides (see “Experimental Procedures”). The synthesized cDNA was 72 bp. The same band size was obtained when genomic DNA (G) was used as a positive control. B, verification of the presence of the NT-box upstream of the aspS2ogatCABo mRNA. The same conditions were used as in A, and the amplified band size was 207 bp. C, verification of the operon arrangement between aspS2o and gatCABo. The same conditions were used as in A, and the amplified band size was 167 bp. A PCR on RNA preparations was done without reverse transcription to check for DNA contamination (Control). D, verification of the gatCAB operon arrangement. The same conditions were used as in A, and the amplified band size was 175 bp. For B, C, and D, oligonucleotides were chosen to amplify the junction between two cistrons (see schematic representation).
Construction and Analysis of Cac Strains Knocked Out for Glutamine-dependent Amidotransferase (gat) or aspS Genes
To test the aforementioned hypothesis, we engineered Cac knock-out strains for a subset of these redundant genes. Using the ClosTron system based on the genomic integration of a group II intron in the target genes, we successfully targeted four genes: CAC3564, coding for the archaeal-like AspRS2; CAC2269, coding for the bacterial-type AspRS1; CAC2243, coding for the Nt-AS; and CAC2976, coding for the GatBo subunit of the GatCABo AdT (supplemental Fig. S2). Except Nt-AS (Nt-AsnB) mutant strains, all knock-out strains grew on minimal medium in both acidogenesis and solventogenesis (not shown). This observation strongly suggests that the activity of the corresponding enzymes can be compensated by their duplicated homologs and that there are indeed redundant genes of Asn/Asp-tRNA synthesis in Cac. Until now, it was not known why the Nt-AS-encoding gene would be essential. The most obvious possibility is that Nt-AS exhibits an essential function beyond Asn synthesis.
Expression of AspRS2 Is Related to Acid and Solvent Production
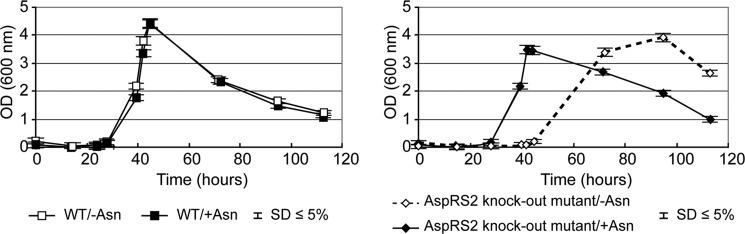
We also grew the knock-out strains on minimal medium in the presence of Asn to check whether Asn down-regulates the expression of some of these genes, consequently rendering the corresponding knock-out strain unable to sustain growth in the presence of this amino acid. In addition, analyses of acid (acetate and butyrate) and solvent (ethanol, acetone, butanol, and 3-hydroxybutanone) production were checked by gas chromatography. The growth (Fig. 3) and level of acid and solvent production of the wild-type (WT) Cac (supplemental Fig. S3) were similar regardless of the presence or absence of Asn. However, the WT strain showed changes in the physiological events when shifting from acidogenesis to solventogenesis after 42 h (Fig. 3 and supplemental Fig. S3) as observed previously (40).
FIGURE 3.
Comparison of growth curves of Cac WT and AspRS2 (CAC3564, aspS2) knock-out strains. The strains were grown on minimal medium with ammonium as the only nitrogen donor and in the presence (+Asn) and absence of Asn (−Asn). Cell density was optically measured at 600 nm. Error bars = mean growth (OD)/time of three independent growth measurement replicates ± S.D. The Standard Deviation (S.D.) was less than 5%.
Analysis of the growth curves and acid and solvent production of the aspS1 knock-out mutants showed a profile similar to that of the WT strain (supplemental Figs. S4 and S5). However, analysis of the growth curve of the aspS2 knock-out mutant showed a significant delay (30 h) of the shift from acidogenesis to solventogenesis (Fig. 3) in the absence of Asn. This growth profile influenced the timing (delay of 30 h) of acid and solvent production, although their concentrations and levels of production remained comparable with that of the WT (supplemental Figs. S3 and S4). Adding Asn to the culture of the aspS2 knock-out mutant allowed the strain to regain the WT growth profile (Fig. 3) as well as the WT acid and solvent production yields (supplemental Fig. S3). These results tend to suggest that there is an Asn-dependent expression of the aspS2 gene and that this regulation is somehow involved in acid and solvent production. The results of these experiments suggest that AspRS2 might be involved in Asn synthesis and that Asn is somehow connected to the switch from acidogenesis to solventogenesis.
Synthesis of Asparagine in Cac Is tRNA-dependent
In all the bacteria examined so far, both pathways, direct charging of Asn onto tRNAAsn by AsnRS and transamidation by a GatCAB AdT, have been shown to be mutually exclusive (6). However, the Cac genome encodes both AsnRS and GatCAB AdTs, suggesting that this organism uses both pathways for Asn synthesis. To clarify this issue, we checked whether Cac was able to generate free Asn.
Two truncated asparagine synthetase B-related ORFs, Nt-AS and Ct-AS, were identified in the Cac genome. Alignment of the Nt-AS and Ct-AS amino acid sequence with that of the E. coli AsnB (supplemental Fig. S6) allowed us to verify the presence and conservation of motifs critical for AS activity. The alignment shows that Nt-AS has conserved the glutamine-binding domain and the AMP-generating domain (41) and might therefore be capable of generating Asn. On the contrary, Ct-AS has only conserved the AMP-generating domain and should therefore be unable to catalyze amidation of Asp into Asn.
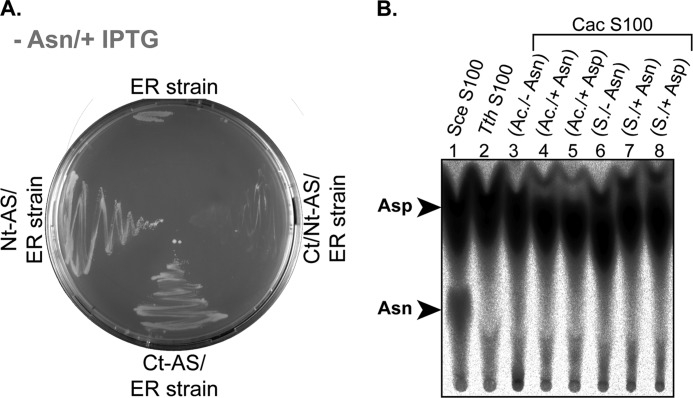
To verify the activity of both Nt-AS and Ct-AS, we first checked their ability to complement, independently or in combination, the E. coli Asn auxotrophic ER strain (asnA−, asnB−) (42). Transformation of the ER strain with the plasmid-borne Cac Nt-AS and Ct-AS was verified by PCR, and expression of the enzymes in the ER recombinant strains was checked by Western blot (not shown). Fig. 4A shows that both the Nt-AS and Ct-AS constructs can complement Asn auxotrophy of the ER strain. However, when the two constructs were co-transformed into the ER strains, they were not able to complement the Asn auxotrophy, suggesting that they are not functional when combined. The discrepancy between the ER complementation assays using Nt-AS and Ct-AS individually or in combination is unquestionably puzzling, and we have no clear answer for it. A possible explanation for this result is that the Ct-AS may regulate the expression or activity of the Nt-AS similarly to what has been described for the nitrogen assimilation control protein (Nac) in E. coli (43). In E. coli, expression of AsnC and AsnA enzymes is repressed by Nac. In this case, Nac directly represses the expression of asnC, whose product is required for the activation of asnA transcription.
FIGURE 4.
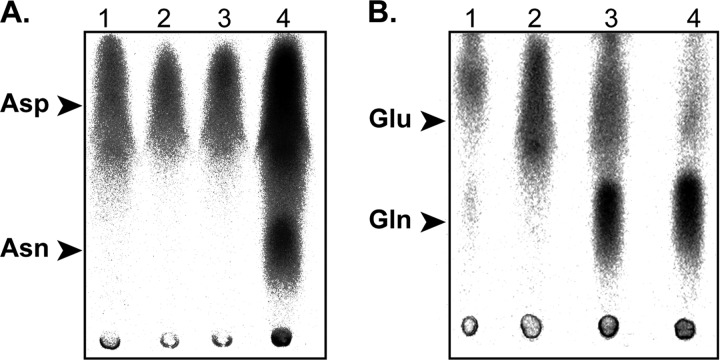
Analysis of Cac Nt-AS and Ct-AS asparagine synthetase activities in vitro and in vivo. A, complementation of the Asn auxotroph E. coli ER strain (asnA−, asnB−) by the Cac Nt-AS_pET15b and the Cac Ct-AS_pET15b constructs. The E. coli ER strain was transformed with either one of the two recombined pET15b vectors or with both recombinant vectors (Ct/Nt-AS_pET15b). Transformants were grown on minimal M9 medium agar plates supplemented with ampicillin and 0.5 mm IPTG in the absence (−) of Asn. B, asparagine synthetase activity assay using Cac protein extracts (S100). Reactions were carried out using a standard amidation mixture (see “Experimental Procedures”) and 100 μg of Sce (lane 1), Tth (lane 2), and Cac (lanes 3–8) S100. Six different Cac S100 extracts were analyzed for their asparagine synthetase activities. Lanes 3–8, S100 extracts taken from cells grown until acidogenesis (Ac.) or solventogenesis (S.) phase. −aa, no amino acids were added for the culture; +Asn, addition of 1 mm Asn; +Asp, addition of 1 mm Asp.
To verify whether Cac is able to generate free Asn by using the conventional pathway catalyzed by AsnB, we checked the capacity of Cac protein extracts to catalyze tRNA-independent Asn formation. Proteins were extracted from Cac cells grown in minimal medium or minimal medium supplemented with either Asp or Asn. Additionally, growth cultures were either stopped during the acidogenesis or the solventogenesis phase. Fig. 4B shows that all extracts were unable to catalyze in vitro amidation of Asp into Asn in conditions in which the asparagine synthetase activity of a Saccharomyces cerevisiae crude extract could be detected.
The results of these experiments confirm that, under conventional physiological states, Cac does not display any detectable tRNA-independent Asn formation activity. This result is in agreement with the absence of complementation of the co-transformed ER strain as well as with the proteomics and transcriptomics results obtained by Janssen and co-workers (44). In their recent work, they showed no evidence for asparagine synthetase expression under these conditions. However, the oddity regarding asparagine synthetase complementation assays has to be further investigated to decipher the real function of both AS proteins. Their individual enzymatic activities as well as their expression profiles must be verified.
No Redundancy in Asn-tRNAAsn Synthesis in Cac
Given that Cac is unable to generate Asn in a tRNA-independent manner, generation of Asn-tRNAAsn is not accomplished by the concomitant use of direct and indirect pathways. However, enzymes of both routes have been kept. It is surprising that AsnRS was retained despite the fact that the organism is unable to synthesize its amino acid substrate. This situation has already been reported for T. thermophilus (7). We therefore hypothesized that, as in the case of Thermus, when Cac can find and import Asn from its environment, AsnRS would catalyze formation of Asn-tRNAAsn probably because of its higher catalytic efficiency. On the other hand, the transamidation pathway has been logically conserved as it is essential when Asn is unavailable. To support this scenario, we checked the capacity of at least one of the GatCAB AdTs (GatCABo) to catalyze in vitro the tRNA-dependent generation of Asn. Fig. 5A shows that the purified GatCABo AdT is able to transamidate Cac Asp-tRNAAsn transcript. This activity is strictly Gln- and tRNA-dependent.
FIGURE 5.
Cac GatCABo AdT catalyzes tRNA-dependent Asn and Gln formation. A, GatCABo tRNA-dependent Asn formation. The transamidation reaction was carried out in the presence of GatCABo and absence of the amide group donor (Gln) (lane 1), tRNAAsn (lane 2), and Deinococcus radiodurans non-discriminating AspRS2 (lane 3). Lane 4, transamidation reaction carried out for 10 min at 37 °C in the presence of GatCABo, D. radiodurans non-discriminating AspRS2, tRNAAsn, and 2 mm Gln. B, GatCABo tRNA-dependent Gln formation. Transamidation reactions were carried out as in A using preformed H. pylori [14C]Glu-tRNAGln and GatCABo (lanes 1 and 3) in the absence (lane 1) or presence (lane 3) of Gln. Lanes 2 and 4, control transamidation reactions without GatCABo (lane 2) or with H. pylori purified GatCAB (lane 4).
GatCABo AdT Is Able to Generate Both Asn-tRNAAsn and Gln-tRNAGln
Because Cac possesses two GatCAB AdTs (GatCABo and GatCAB), one possible explanation for this duplication is that one AdT would be restricted to Asn-tRNAAsn synthesis and the other would be restricted to Gln-tRNAGln formation. However, this would be really surprising because all bacterial GatCAB AdTs have been shown to be dually specific (36). Fig. 5B confirms using pure heterologous H. pylori Glu-tRNAGln that the GatCABo AdT is indeed dually specific and able to form Gln-tRNAGln. Note that none of the studied bacterial GatCAB AdTs exhibited a species specificity for amide tRNAs because all tRNAAsn and tRNAGln display the same tRNA identity elements for the GatCAB AdTs including Cac tRNAs (36). This result additionally shows that the absence of GlnRS in Cac can be compensated by GatCAB-mediated formation of Gln-tRNAGln.
However, this line of evidence further supports the presence of redundant AdTs in Cac, especially when considering the close structural relationship that can be deduced from the phylogeny of both GatCAB GatB subunits (supplemental Fig. S7). We analyzed the phylogeny of GatB because this subunit is restricted to AdTs. The analysis showed that both GatBo and GatB are in the same bacterial clade and are not clustered with the archaeal GatB of the monospecific archaeal GatCAB AdTs (45). As a result, both GatCABs, GatCABo and GatCAB, will very likely display the same activities and specificities. This observation is further supported by our knock-out strain analysis showing that the loss of the GatBo subunit is not lethal. The only explanation for this is the complementation by the remaining GatB subunit (Fig. 1C). However, further investigations are needed to verify whether both AdTs have the exact same substrate specificities or whether they are identically expressed and distributed along the two physiological states.
Regulation of aspS2ogatCABo Transcription by tRNAAsn-dependent T-box Riboswitch
The presence of the two GatCAB AdTs would particularly make sense if, for example, one GatCAB is preferentially expressed during acidogenesis, whereas the other is prevalently used during solventogenesis. From the growth analysis of the knock-out strain we generated, we already knew that removal of GatCABo activity by deletion of GatBo can be compensated by the GatCAB AdT. However, this result does not preclude a preferential use of one GatCAB AdT over the other in a metabolic phase-dependent manner. We therefore screened the 5′- and 3′-UTRs flanking the aspS2ogatCABo and gatCAB operons for mRNA regulatory elements that might regulate their expression and found a putative T-box located in the 5′-UTR of the aspS2ogatCABo operon. RT-PCR amplification of the RNA sequence located between the T-box and aspS2o confirmed the presence of a T-box in the 5′-UTR of the aspS2ogatCABo transcript (Fig. 2, A and B).
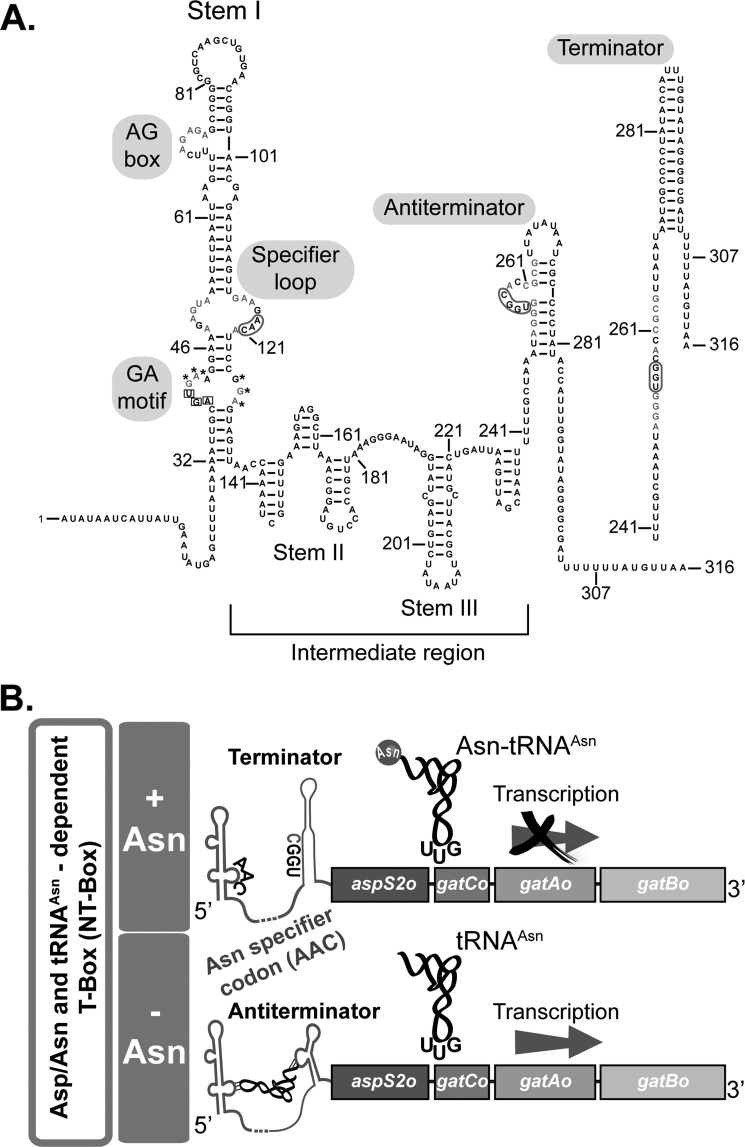
Fig. 6A shows the model of the secondary structure of the Cac aspS2ogatCABo leader sequence that displays the T-box we reconstructed using Mfold (46), the known structures of T-boxes, and the Rfam T-box alignment (47). All idiosyncratic T-box structural and sequence motifs could be found. The presence of an “AAC” Asn codon in the stem I specifier loop suggests that the tRNA ligand of this T-box is tRNAAsn. As a consequence, we named this T-box NT-box (N for asparagine). The sequence also shows stem II and stem III, which both form the unconserved intermediate region of the T-box. The riboswitch ends with a 14-nt-long conserved T-box sequence or domain able to adopt the mutually exclusive terminator or antiterminator conformations in response to tRNA binding. Base pairing between the tRNAAsn and the NT-box is done as follows. In the 5′-end, the NT-box 119AAC121 nucleotides, located in the specifier loop, base pair with the tRNAAsn anticodon triplet 34GUU36. In the 3′-end, the 255UGGC259 NT-box antiterminator bulge base pairs with the tRNAAsn 73GCCA76 3′-end (Fig. 6A). Based on previous studies on T-boxes, the NT-box may respond to Asn starvation or supply. In principle, upon Asn starvation or limitation, tRNAAsn is mainly uncharged and therefore capable to interact with the nascent leader RNA, stabilizing the antiterminator conformation, thereby allowing transcription of aspS2ogatCABo (Fig. 6B). Because this operon encodes both enzymes necessary to the tRNA-dependent formation of Asn and Asn-tRNAAsn, the transcriptional read-through would allow Asn formation and utilization. When the physiological levels of Asn are restored, tRNAAsn is probably mainly charged with Asn and therefore unable to stabilize the antiterminator structure of the T-box domain. This domain, by adopting the more stable terminator structure, may prevent the transcription of the aspS2ogatCABo operon (Fig. 6B).
FIGURE 6.
A T-box is located in the 5′-UTR of aspS2ogatCABo. A, model of the secondary structure of Cac aspS2ogatCABo T-box (NT-box). The sequence shown comprises the full-length T-box from the transcription start site (+1) through stem I, containing the well conserved GA motif and AG box, as well as the specifier loop, which displays the AAC Asn codon sequence. The sequence also shows stem II and stem III, which both form the non-conserved intermediate region of the T-box. The sequence ends with the antiterminator region including the 14-nt conserved T-box sequence. The alternate and more stable terminator structure is shown next to the antiterminator. Nucleotides marked with asterisks form a predicted kink-turn (J. A. Cruz, personal communication). B, schematic description of the putative mechanism of regulation mediated by the NT-box antitermination system in response to Asn starvation or supply.
To validate this regulatory mechanism and to confirm that the NT-box is functional, we designed experiments that aimed at confirming the capacity of purified in vitro transcribed NT-box to specifically recruit and bind Cac tRNAAsn transcript. Formation of the NT-box·Cac tRNAAsn duplex was assayed using both EMSA and size exclusion chromatography.
EMSA Analysis
So far, EMSA studies that analyzed T-box·tRNA complex formation were only performed using truncated T-box transcripts forming either the specifier stem-loop or the T-box domain (19, 48). However, the structural probing studies that analyzed the conformational switch in response to tRNA binding were done using full-length T-boxes (49, 50).
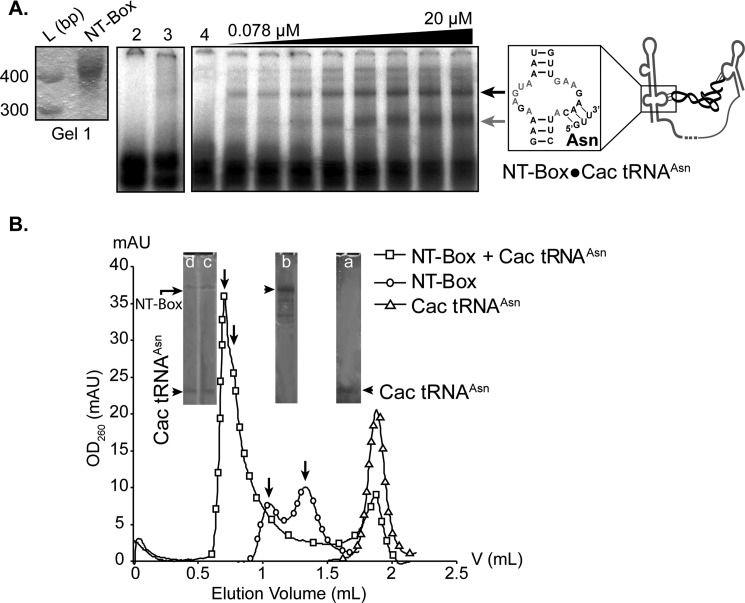
Fig. 7A shows formation of the NT-box·Cac tRNAAsn duplex in which the entire 400-nt-long NT-box transcript was used. The absence of duplex formation using Cac tRNAAsp(GUC) (Cac tRNAAsp) transcript (Fig. 7A, lane 2) confirmed the strict tRNAAsn ligand specificity of this T-box. The tRNA specificity exhibited by the NT-box was remarkably high because the absence of one of the seven base pairs involved in T-box·tRNA duplex formation hindered tRNA binding and/or antitermination. The presence of C36 in tRNAAsp (supplemental Fig. S8) instead of U36, which is found in tRNAAsn, prevented base pairing with NT-box A119. Other residues from the T-box and the tRNA are likely to interact, and some may also be important for specificity either directly or indirectly.
FIGURE 7.
Specific binding of uncharged Cac tRNAAsn transcript to NT-box monitored by EMSA and size exclusion chromatography. A, EMSA. Gel 1 shows the purified (gel-eluted) NT-box transcript used in the experiments. L, ladder. EMSA analysis of the NT-box·Cac tRNAAsn duplex formation (schematized on the right side of the figure) was performed as described under “Experimental Procedures.” Only tRNA was 5′-[32P]-labeled. Lane 2, Cac tRNAAsp transcript; lane 3, Cac tRNAAsp transcript with 20 μm NT-box; lanes 4–12, Cac tRNAAsn transcript in the absence (lane 4) and presence of NT-box transcript (lanes 5–12). Increasing concentrations of the NT-box transcript from 0.078 to 20 μm (lanes 5-12, 0.078, 0.5, 1, 2, 5, 10, 15, and 20 μm, respectively) allowed the determination of a dissociation constant (Kd) for each of the two forms of the NT-box·Cac tRNAAsn duplex indicated by arrows: black arrow, upper duplex form; gray arrow, lower duplex form. B, analysis of the NT-box·Cac tRNAAsn duplex formation by size exclusion chromatography. Elution profiles of Cac tRNAAsn (5 μm) (▵), NT-box transcript (5 μm) (○), and a mixture of Cac tRNAAsn and NT-box (5 μm each) (□) are shown. Insets (a, b, c, and d) represent denaturing PAGE analysis of the transcripts marked by dark arrowheads and present in the peak fractions (▴). Arrows on the elution profiles indicate the presence of two conformations of the NT-box (○) or the NT-box·Cac tRNAAsn duplex (□). mAU, milliabsorbance units.
In the EMSA, we noticed the presence of two main NT-box·Cac tRNAAsn duplexes. The use of increasing concentrations of NT-box allowed the determination of the two dissociation constants (Kd) corresponding to the two forms of NT-box·Cac tRNAAsn duplex. Kd values of 6–8 and 10 μm were determined for the upper (black arrow) and lower duplex forms, respectively Fig. 7A (see supplemental Experimental Procedures for Kd determination). These Kd values are 6–9-fold lower than the Kd value that was previously determined for binding of the Bacillus subtilis antiterminator domain of the tyrS T-box with its cognate tRNATyr(A73U) (Kd = 63 μm) (48). In addition, the NT-box·Cac tRNAAsn duplex starts to form at a minimal concentration of 0.078 μm NT-box.
A dissociation constant of 2–3 μm was determined using the pure in vivo expressed Tth tRNAAsn (supplemental Fig. S9A). Because this tRNAAsn was overexpressed in E. coli and therefore harbors E. coli tRNAAsn post-transcriptional modifications (51), our results suggest that Cac nucleotide modifications might increase the affinity of the NT-box for its cognate tRNAAsn. Note that the effect of the tRNA nucleotide modifications on the affinity for T-boxes has yet to be studied. Nonetheless, altogether, the affinity of NT-box for tRNAAsn is comparable with the affinities measured for aaRS·tRNA duplex formation (52), which is expected because NT-box has to compete with possibly two archaeal AspRSs (AspRS2o and AspRS2) and AsnRS for tRNAAsn binding to trigger transcription of the aspS2ogatCABo operon.
Size Exclusion Chromatography
We further confirmed formation of the NT-box·Cac tRNAAsn duplex using a different approach, namely analytical size exclusion chromatography. This technique was never applied before to study T-box·tRNA duplex formation. Fig. 7B shows that when the NT-box was mixed with Cac tRNAAsn a new elution peak was obtained. This peak accounts for elution of a higher molecular weight particle than that of the NT-box alone or of Cac tRNAAsn. Denaturing PAGE analysis of the RNA species present in this peak confirmed that both the NT-box and Cac tRNAAsn were present in the corresponding fractions (Fig. 7B, lanes c and d). Fig. 7B and supplemental Fig. S9B show that the NT-box·Cac tRNAAsn peak was not completely symmetric, suggesting the presence of two conformations for the duplex probably due to high dynamics in assembly of the RNA·RNA complex.
In addition to the EMSA results, the size exclusion chromatography assay provides new evidence showing that the two conformations of the duplex may be due to the presence of two NT-box conformations (Fig. 7B, NT-box elution profile). The elution profiles of the NT-box and the NT-box·Cac tRNAAsn duplex showed that these two alternative conformations are present regardless of whether Cac tRNAAsn is bound or not to the NT-box (Fig. 7B). It is likely that these conformations are the result of a certain domain in the T-box structure that is not involved in tRNAAsn binding. We hypothesize that the long intermediate region, located between the specifier and T-box domain, can adopt alternative conformations, yielding the two conformers we observed both in our EMSA and size exclusion assays. This is in agreement with previous results reporting the possibility that T-box long intermediate regions might alternatively fold into a pseudoknot structure (16). The presence of two conformers due to alternative conformations of the intermediate region has also been observed in a report describing the structural probing experiments done on the glyQS T-box in B. subtilis. In fact, Yousef and co-workers (50) showed the existence of conformational changes in the intermediate region just upstream of the antiterminator element.
The use of the post-transcriptionally modified Tth tRNAAsn yielded the same elution profile as the transcript. However, when Cac tRNAAsp was used for assaying duplex formation with NT-box, no elution peak relative to a particle of higher molecular weight could be detected, confirming that the NT-box·Cac tRNAAsp is also not detected using size exclusion chromatography (not shown). These experiments not only confirmed the results obtained using EMSA but also validated the use of size exclusion chromatography for analyzing T-box·tRNA complex formation.
Design of in Vivo NT-box Antitermination Assay in Gram-negative Environment
Because the in vitro read-through experiment was not conclusive regarding NT-box antitermination (supplemental Fig. S10), we searched for another approach to demonstrate the capacity of the NT-box to perform amino acid- and tRNA-dependent transcription antitermination.
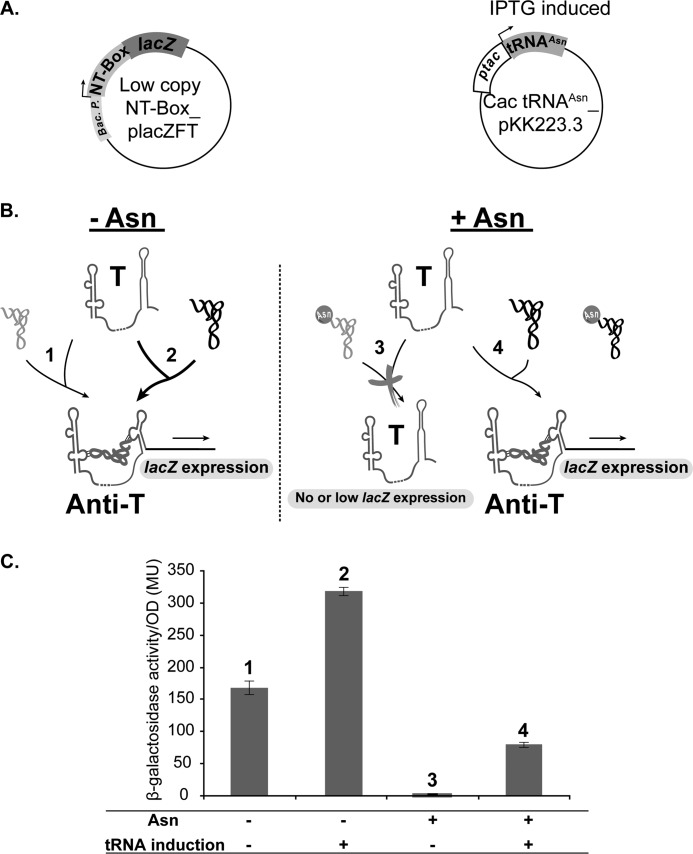
Most of the antitermination experiments have been done using amino acid auxotroph B. subtilis strains genetically modified to encode a single chromosomal copy of a β-galactosidase gene under the control of the studied T-box. This experimental design allows following, in vivo, T-box read-through as a function of amino acid starvation by measuring β-galactosidase activity (18). However, using the same experimental design in Cac was simply not feasible mainly because of technical constraints when working with a strict anaerobe. On the other hand, trying to use this experimental design in a Gram-negative environment has never been reported. We engineered an E. coli plasmid-based system in which the β-galactosidase reporter gene is under the control of the NT-box. Because the NT-box should in principle trigger read-through of the downstream gene in response to Asn starvation, we used the E. coli asparagine auxotroph strain (ER strain). This strain was used to be able to control the precise amounts of the amino acid supplemented into the medium. This plasmid construct is based on a promoterless, low copy plasmid, placZFT, that has already been used to measure promoter strength in Cac (25). In our construct, the lacZ gene is preceded by the NT-box sequence along with its endogenous promoter (Fig. 8A). Results of the in vitro halted complex transcription assay with the E. coli RNA polymerase indicated that the endogenous Cac promoter is well recognized by E. coli RNA polymerase. A pKK223-3 recombinant plasmid containing an IPTG-inducible Cac tRNAAsn gene was also introduced into the strain.
FIGURE 8.
Design and analysis of in vivo NT-box-mediated antitermination assay in Gram-negative E. coli bacterium. A, schematic representation of the in vivo antitermination system in E. coli. The E. coli ER strain was co-transformed with the NT-box_placZFT and Cac tRNAAsn_pKK223-3 recombinant plasmids. “Bac. P.” corresponds to the endogenous NT-box promoter. “ptac” corresponds to the IPTG-inducible promoter controlling Cac tRNAAsn gene expression. B, schematic representation of the NT-box-controlled lacZ expression in E. coli under the four conditions tested (see C) using the two RNAs: Cac NT-Box and tRNAAsn (colored in black). The endogenous E. coli tRNAAsn (colored in light gray) has a “GUU” anticodon sequence. The tRNAAsn colored in dark gray and bound to the NT-box corresponds to the E. coli tRNAAsn and Cac uncharged tRNAAsn, which both can affect antitermination. T, NT-box terminator conformation; Anti-T, NT-box antiterminator conformation. C, in vivo NT-box-mediated antitermination assay. Bars for each graph represent β-galactosidase activity in Miller units (MU) relative to cell density (53). Error bars = Mean of β-galactosidase activity/cell density of three independent activity measurement replicates ± S.D. The effect of Asn presence and tRNA induction were compared by taking 5-ml aliquots from each culture and measuring the β-galactosidase activity after 4 h of culture. The β-galactosidase activity value was calculated using the following equation: 1000 × ((A420 − (1.75 × A560))/(T × V × A595)) where “A420” is the absorbance of the o-nitrophenol product (see “Experimental Procedures”), “A560” is the absorbance of the cell debris pellet, “A595” is the absorbance of bacterial suspension, “T” is the reaction time in minutes, and “V” is the volume in milliliters of the treated cells used to measure β-galactosidase activity.
In Vivo NT-box Antitermination Detection
Fig. 8B shows as expected an increased β-galactosidase activity (measured as Miller units) under Asn starvation (−Asn, −IPTG), showing that NT-box read-through requires uncharged tRNAAsn, which in these conditions is that of E. coli (Fig. 8A). When Asn was added up to 50 μg/ml (+Asn, −IPTG), no detectable β-galactosidase activity could be measured, indicating that Asn-tRNAAsn formation could no longer trigger formation of the antiterminator conformation (Fig. 8, A and B). As expected, we observed a maximum read-through when combining both Asn starvation and Cac tRNAAsn overproduction (Fig. 8B, −Asn, +IPTG). Consequently, both molecules may have a cooperative effect on NT-box antitermination. When Cac tRNAAsn expression was induced, even in the presence of Asn (+Asn, +IPTG) a β-galactosidase activity 2 times smaller than the activity measured without tRNA induction and without Asn (−Asn, −IPTG) was detected (Fig. 8B). This shows that overexpression of tRNA triggers the adoption of the NT-box antiterminator conformation in an amino acid-independent manner. Moreover, it shows that the level of tRNA expression is an important effector of T-box antitermination. This makes sense if one considers that endogenous AsnRS would probably be unable to aminoacylate the non-physiological amounts of tRNAAsn that are produced during the overexpression of the heterologous Cac tRNAAsn. In these conditions, production of Asn-tRNAAsn is not limited by the intracellular concentration of Asn but by AsnRS activity. Therefore, the excess pool of uncharged tRNAAsn will bind to the NT-box even if physiological amounts of Asn-tRNAAsn are formed.
In our in vivo experiment, we observed that the excess pool of the induced uncharged tRNAAsn may be responsible for a “leaky” read-through. It is worth noting that a different sort of leaky read-through has also been noticed in vitro when using the B. subtilis RNA polymerase (50). Therefore, this leaky read-through could have a biological significance. For example, the maintenance of a small amount of the operon mRNA resulting from leaky read-through could be used to give a quick initial response to the need for aminoacyl-tRNA synthesis because it only needs to be translated. Meanwhile, the T-box-controlled accumulation of the operon mRNA would be essential to prolong and amplify the response.
Concluding Remarks
The present report provides compelling evidence that, in contrast to what could be deduced from Cac gene content, the direct and indirect routes to Asn and Asn-tRNAAsn synthesis are not redundant in this organism. Although our biochemical and genetic experiments could not elucidate the reason why Cac encodes extra AspRSs and GatCABs, they suggest a differential preferential use of each copy during the various metabolic phases adopted by this bacterium. More importantly, our results point toward an important and interconnected role of Asn and AspRS triplication for the switch between acidogenesis and solventogenesis as seen by the phenotypic effect of the AspRS2 knock-out. These observations suggest that some of the redundant genes encoding enzymes of Asn/Asp-tRNA formation could participate in Cac homeostasis. However, this does still not explain the relevance of this redundancy.
The presence of an effective T-box tightly regulating the expression of an entire transamidation pathway is another argument in favor of Asn as a potential effector for the metabolic switch. Although we have not yet proved the involvement of the NT-box in the metabolic switch, we think that it is very likely the case. Indeed, AspRS2 is connected to the metabolic switch but also controls the level of tRNAAsn charging, which is crucial for aspS2ogatCABo-mediated formation of Asn.
Considering that the quantity of uncharged tRNAAsn that governs NT-box read-through and Asn production can potentially be controlled by the charging activities of at least three enzymes, AsnRS, AspRS2o, and AspRS2, one can easily predict that AspRS2 duplication reflects their involvement in NT-box regulation and therefore Asn synthesis. Further transcriptomics studies will be needed to decipher the intricate story of Cac Asn and Asn-tRNAAsn synthesis.
Our in vitro studies made use of size exclusion chromatography as a novel method to detect T-box·tRNA complex formation and confirmed the presence of the two NT-box conformers observed in the EMSA. The unstable conformation of the NT-box intermediate region did not hinder tRNAAsn binding. It would be interesting to see whether this intermediate region is essential for NT-box antitermination in vivo. If so, the stabilization of the intermediate region conformation could necessitate the presence of protein cofactors. The latter could also be involved in hindering leaky read-through or promoting transcription antitermination. Finally, our study shows that T-box riboswitches, which are essentially restricted to Gram-positive bacteria, are fully functional in a Gram-negative environment.
Acknowledgment
We thank C. Stathopoulos from the University of Patras for critical reading of the manuscript.
This work was supported in part by the Association pour la Recherche sur le Cancer, the University of Strasbourg, the CNRS, and Agence Nationale de la Recherche Grant ANR-09-BLAN-0091-02.

This article contains supplemental Experimental Procedures, Table S1, and Figs. S2–S10.
- aa
- aminoacyl
- RS
- tRNA synthetase
- Cac
- C. acetobutylicum
- Tth
- T. thermophilus
- AdT
- tRNA-dependent amidotransferase
- AsnB or AS
- asparagine synthetase
- Kd
- dissociation constant
- Ct
- C-terminal
- Nt
- N-terminal
- nt
- nucleotide(s)
- IPTG
- isopropyl 1-thio-β-d-galactopyranoside
- Gat
- Glutamyl-tRNAGln amidotransferase
- Nac
- nitrogen assimilation control protein
- ER
- E. Reich.
REFERENCES
- 1. Sheppard K., Yuan J., Hohn M. J., Jester B., Devine K. M., Söll D. (2008) From one amino acid to another: tRNA-dependent amino acid biosynthesis. Nucleic Acids Res. 36, 1813–1825 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. Becker H. D., Reinbolt J., Kreutzer R., Giegé R., Kern D. (1997) Existence of two distinct aspartyl-tRNA synthetases in Thermus thermophilus. Structural and biochemical properties of the two enzymes. Biochemistry 36, 8785–8797 [DOI] [PubMed] [Google Scholar]
- 3. Curnow A. W., Hong K., Yuan R., Kim S., Martins O., Winkler W., Henkin T. M., Söll D. (1997) Glu-tRNAGln amidotransferase: a novel heterotrimeric enzyme required for correct decoding of glutamine codons during translation. Proc. Natl. Acad. Sci. U.S.A. 94, 11819–11826 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Nureki O., O'Donoghue P., Watanabe N., Ohmori A., Oshikane H., Araiso Y., Sheppard K., Söll D., Ishitani R. (2010) Structure of an archaeal non-discriminating glutamyl-tRNA synthetase: a missing link in the evolution of Gln-tRNAGln formation. Nucleic Acids Res. 38, 7286–7297 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Becker H. D., Min B., Jacobi C., Raczniak G., Pelaschier J., Roy H., Klein S., Kern D., Söll D. (2000) The heterotrimeric Thermus thermophilus Asp-tRNAAsn amidotransferase can also generate Gln-tRNAGln. FEBS Lett. 476, 140–144 [DOI] [PubMed] [Google Scholar]
- 6. Tumbula D. L., Becker H. D., Chang W. Z., Söll D. (2000) Domain-specific recruitment of amide amino acids for protein synthesis. Nature 407, 106–110 [DOI] [PubMed] [Google Scholar]
- 7. Becker H. D., Kern D. (1998) Thermus thermophilus: a link in evolution of the tRNA-dependent amino acid amidation pathways. Proc. Natl. Acad. Sci. U.S.A. 95, 12832–12837 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Becker H. D., Roy H., Moulinier L., Mazauric M. H., Keith G., Kern D. (2000) Thermus thermophilus contains an eubacterial and an archaebacterial aspartyl-tRNA synthetase. Biochemistry 39, 3216–3230 [DOI] [PubMed] [Google Scholar]
- 9. Lévêque F., Plateau P., Dessen P., Blanquet S. (1990) Homology of lysS and lysU, the two Escherichia coli genes encoding distinct lysyl-tRNA synthetase species. Nucleic Acids Res. 18, 305–312 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10. Saluta M. V., Hirshfield I. N. (1995) The occurrence of duplicate lysyl-tRNA synthetase gene homologs in Escherichia coli and other procaryotes. J. Bacteriol. 177, 1872–1878 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Skouloubris S., Ribas de Pouplana L., De Reuse H., Hendrickson T. L. (2003) A noncognate aminoacyl-tRNA synthetase that may resolve a missing link in protein evolution. Proc. Natl. Acad. Sci. U.S.A. 100, 11297–11302 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12. Chang K. M., Hendrickson T. L. (2009) Recognition of tRNAGln by Helicobacter pylori GluRS2—a tRNAGln-specific glutamyl-tRNA synthetase. Nucleic Acids Res. 37, 6942–6949 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Nölling J., Breton G., Omelchenko M. V., Makarova K. S., Zeng Q., Gibson R., Lee H. M., Dubois J., Qiu D., Hitti J., Wolf Y. I., Tatusov R. L., Sabathe F., Doucette-Stamm L., Soucaille P., Daly M. J., Bennett G. N., Koonin E. V., Smith D. R. (2001) Genome sequence and comparative analysis of the solvent-producing bacterium Clostridium acetobutylicum. J. Bacteriol. 183, 4823–4838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Grundy F. J., Hodil S. E., Rollins S. M., Henkin T. M. (1997) Specificity of tRNA-mRNA interactions in Bacillus subtilis tyrS antitermination. J. Bacteriol. 179, 2587–2594 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Wels M., Groot Kormelink T., Kleerebezem M., Siezen R. J., Francke C. (2008) An in silico analysis of T-box regulated genes and T-box evolution in prokaryotes, with emphasis on prediction of substrate specificity of transporters. BMC Genomics 9, 330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Gutiérrez-Preciado A., Henkin T. M., Grundy F. J., Yanofsky C., Merino E. (2009) Biochemical features and functional implications of the RNA-based T-box regulatory mechanism. Microbiol. Mol. Biol. Rev. 73, 36–61 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Henkin T. M., Glass B. L., Grundy F. J. (1992) Analysis of the Bacillus subtilis tyrS gene: conservation of a regulatory sequence in multiple tRNA synthetase genes. J. Bacteriol. 174, 1299–1306 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Grundy F. J., Winkler W. C., Henkin T. M. (2002) tRNA-mediated transcription antitermination in vitro: codon-anticodon pairing independent of the ribosome. Proc. Natl. Acad. Sci. U.S.A. 99, 11121–11126 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Nelson A. R., Henkin T. M., Agris P. F. (2006) tRNA regulation of gene expression: interactions of an mRNA 5′-UTR with a regulatory tRNA. RNA 12, 1254–1261 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Henkin T. M., Grundy F. J. (August 19, 2008) U. S. Patent 7,413,856
- 21. Putzer H., Condon C., Brechemier-Baey D., Brito R., Grunberg-Manago M. (2002) Transfer RNA-mediated antitermination in vitro. Nucleic Acids Res. 30, 3026–3033 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Becker H. D., Giegé R., Kern D. (1996) Identity of prokaryotic and eukaryotic tRNAAsp for aminoacylation by aspartyl-tRNA synthetase from Thermus thermophilus. Biochemistry 35, 7447–7458 [DOI] [PubMed] [Google Scholar]
- 23. Fechter P., Rudinger J., Giegé R., Théobald-Dietrich A. (1998) Ribozyme processed tRNA transcripts with unfriendly internal promoter for T7 RNA polymerase: production and activity. FEBS Lett. 436, 99–103 [DOI] [PubMed] [Google Scholar]
- 24. Roy H., Becker H. D., Mazauric M. H., Kern D. (2007) Structural elements defining elongation factor Tu mediated suppression of codon ambiguity. Nucleic Acids Res. 35, 3420–3430 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Feustel L., Nakotte S., Dürre P. (2004) Characterization and development of two reporter gene systems for Clostridium acetobutylicum. Appl. Environ. Microbiol. 70, 798–803 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Milligan J. F., Groebe D. R., Witherell G. W., Uhlenbeck O. C. (1987) Oligoribonucleotide synthesis using T7 RNA polymerase and synthetic DNA templates. Nucleic Acids Res. 15, 8783–8798 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Milligan J. F., Uhlenbeck O. C. (1989) Synthesis of small RNAs using T7 RNA polymerase. Methods Enzymol. 180, 51–62 [DOI] [PubMed] [Google Scholar]
- 28. Bailly M., Blaise M., Roy H., Deniziak M., Lorber B., Birck C., Becker H. D., Kern D. (2008) tRNA-dependent asparagine formation in prokaryotes: characterization, isolation and structural and functional analysis of a ribonucleoprotein particle generating Asn-tRNAAsn. Methods 44, 146–163 [DOI] [PubMed] [Google Scholar]
- 29. McDowell J. C., Roberts J. W., Jin D. J., Gross C. (1994) Determination of intrinsic transcription termination efficiency by RNA polymerase elongation rate. Science 266, 822–825 [DOI] [PubMed] [Google Scholar]
- 30. Henkin T. M. (2009) Analysis of tRNA-directed transcription antitermination in the T box system in vivo. Methods Mol. Biol. 540, 281–290 [DOI] [PubMed] [Google Scholar]
- 31. Thormann K., Feustel L., Lorenz K., Nakotte S., Dürre P. (2002) Control of butanol formation in Clostridium acetobutylicum by transcriptional activation. J. Bacteriol. 184, 1966–1973 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Roos J. W., McLaughlin J. K., Papoutsakis E. T. (1985) The effect of pH on nitrogen supply, cell lysis, and solvent production in fermentations of Clostridium acetobutylicum. Biotechnol. Bioeng. 27, 681–694 [DOI] [PubMed] [Google Scholar]
- 33. Johnson J. L. (1994) in Methods for General and Molecular Bacteriology (Gerhardt P., R. Murray G. E., Wood W. A., Krieg N. R., eds) pp. 655–682, American Society for Microbiology, Washington, D. C. [Google Scholar]
- 34. Sambrook J., Fritsch E. F., Maniatis T. (1989) Molecular Cloning, 2nd Ed., Cold Spring Harbor Laboratory, Cold Spring Harbor, NY [Google Scholar]
- 35. Frechin M., Senger B., Brayé M., Kern D., Martin R. P., Becker H. D. (2009) Yeast mitochondrial Gln-tRNAGln is generated by a GatFAB-mediated transamidation pathway involving Arc1p-controlled subcellular sorting of cytosolic GluRS. Genes Dev. 23, 1119–1130 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Bailly M., Giannouli S., Blaise M., Stathopoulos C., Kern D., Becker H. D. (2006) A single tRNA base pair mediates bacterial tRNA-dependent biosynthesis of asparagine. Nucleic Acids Res. 34, 6083–6094 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. ten Broeke-Smits N. J., Pronk T. E., Jongerius I., Bruning O., Wittink F. R., Breit T. M., van Strijp J. A., Fluit A. C., Boel C. H. (2010) Operon structure of Staphylococcus aureus. Nucleic Acids Res. 38, 3263–3274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Heap J. T., Pennington O. J., Cartman S. T., Carter G. P., Minton N. P. (2007) The ClosTron: a universal gene knock-out system for the genus Clostridium. J. Microbiol. Methods 70, 452–464 [DOI] [PubMed] [Google Scholar]
- 39. Dürre P. (2008) Fermentative butanol production: bulk chemical and biofuel. Ann. N.Y. Acad. Sci. 1125, 353–362 [DOI] [PubMed] [Google Scholar]
- 40. Grupe H., Gottschalk G. (1992) Physiological events in Clostridium acetobutylicum during the shift from acidogenesis to solventogenesis in continuous culture and presentation of a model for shift induction. Appl. Environ. Microbiol. 58, 3896–3902 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Larsen T. M., Boehlein S. K., Schuster S. M., Richards N. G., Thoden J. B., Holden H. M., Rayment I. (1999) Three-dimensional structure of Escherichia coli asparagine synthetase B: a short journey from substrate to product. Biochemistry 38, 16146–16157 [DOI] [PubMed] [Google Scholar]
- 42. Felton J., Michaelis S., Wright A. (1980) Mutations in two unlinked genes are required to produce asparagine auxotrophy in Escherichia coli. J. Bacteriol. 142, 221–228 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Poggio S., Domeinzain C., Osorio A., Camarena L. (2002) The nitrogen assimilation control (Nac) protein represses asnC and asnA transcription in Escherichia coli. FEMS Microbiol. Lett. 206, 151–156 [DOI] [PubMed] [Google Scholar]
- 44. Janssen H., Döring C., Ehrenreich A., Voigt B., Hecker M., Bahl H., Fischer R. J. (2010) A proteomic and transcriptional view of acidogenic and solventogenic steady-state cells of Clostridium acetobutylicum in a chemostat culture. Appl. Microbiol. Biotechnol. 87, 2209–2226 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Sheppard K., Söll D. (2008) On the evolution of the tRNA-dependent amidotransferases, GatCAB and GatDE. J. Mol. Biol. 377, 831–844 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Zuker M. (2003) Mfold web server for nucleic acid folding and hybridization prediction. Nucleic Acids Res. 31, 3406–3415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Griffiths-Jones S., Bateman A., Marshall M., Khanna A., Eddy S. R. (2003) Rfam: an RNA family database. Nucleic Acids Res. 31, 439–441 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Gerdeman M. S., Henkin T. M., Hines J. V. (2002) In vitro structure-function studies of the Bacillus subtilis tyrS mRNA antiterminator: evidence for factor-independent tRNA acceptor stem binding specificity. Nucleic Acids Res. 30, 1065–1072 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49. Luo D., Condon C., Grunberg-Manago M., Putzer H. (1998) In vitro and in vivo secondary structure probing of the thrS leader in Bacillus subtilis. Nucleic Acids Res. 26, 5379–5387 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50. Yousef M. R., Grundy F. J., Henkin T. M. (2005) Structural transitions induced by the interaction between tRNAGly and the Bacillus subtilis glyQS T box leader RNA. J. Mol. Biol. 349, 273–287 [DOI] [PubMed] [Google Scholar]
- 51. Sprinzl M., Vassilenko K. S. (2005) Compilation of tRNA sequences and sequences of tRNA genes. Nucleic Acids Res. 33, D139–D140 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 52. Sauter C., Lorber B., Cavarelli J., Moras D., Giegé R. (2000) The free yeast aspartyl-tRNA synthetase differs from the tRNAAsp-complexed enzyme by structural changes in the catalytic site, hinge region, and anticodon-binding domain. J. Mol. Biol. 299, 1313–1324 [DOI] [PubMed] [Google Scholar]
- 53. Miller J. H. (1972) Experiments in Molecular Genetics, Cold Spring Harbor Laboratory Press, Cold Spring Harbor, NY [Google Scholar]