Background: TPC1 is an organellar NAADP-activated channel with unknown properties.
Results: TPC1 functions as a pH-, Ca2+-, and voltage-regulated channel. Remarkably, activation of TPC1 by NAADP is dynamically regulated by the membrane potential.
Conclusion: The properties of TPC1 can account for NAADP-evoked organellar Ca2+ oscillations.
Significance: These findings increase understanding of the organellar and receptor-evoked Ca2+ signals.
Keywords: Calcium, Calcium Channels, Calcium Intracellular Release, Calcium Signaling, Calcium Transport, Subcellular Organelles
Abstract
Nicotinic acid adenine dinucleotide phosphate (NAADP) is a potent second messenger that mobilizes Ca2+ from the acidic endolysosomes by activation of the two-pore channels TPC1 and TPC2. The channel properties of human TPC1 have not been studied before, and its cellular function is not known. In the present study, we characterized TPC1 incorporated into lipid bilayers. The native and recombinant TPC1 channels are activated by NAADP. TPC1 activity requires acidic luminal pH and high luminal Ca2+. With Ba2+ as the permeable ion, luminal Ca2+ activates TPC1 with an apparent Km of 180 μm. TPC1 operates in two tightly coupled conductance states of 47 ± 8 and 200 ± 9 picosiemens. Importantly, opening of the large conductance markedly increases the small conductance mean open time. Changes in membrane potential from 0 to −60 mV increased linearly both the small and the large conductances and NPo, indicating that TPC1 is regulated by voltage. Intriguingly, the apparent affinity for activation of TPC1 by its ligand NAADP is not constant. Rather, hyperpolarization increases the apparent affinity of TPC1 for NAADP by 10 nm/mV. The concerted regulation of TPC1 activity by luminal Ca2+ and by membrane potential thus provides a potential mechanism to explain NAADP-induced Ca2+ oscillations. These findings reveal unique properties of TPC1 to explain its role in Ca2+ oscillations and cell function.
Introduction
Ca2+ is critical for the function of intracellular organelles by mediating their biosynthesis, trafficking, fission, and fusion (1, 2). However, Ca2+ homeostasis by intracellular organelles is poorly understood, as outlined in several reviews in a special issue of Cell Calcium (3). A breakthrough was achieved by the identification of the two-pore channels (TPCs),2 TPC1 and TPC2, as the target of the potent second messenger nicotinic acid adenine dinucleotide phosphate (NAADP) (4–6). NAADP was discovered as a Ca2+-mobilizing second messenger in sea urchin eggs (7), where it mobilizes Ca2+ from lysosome-related organelles (8). NAADP is a potent Ca2+-releasing second messenger acting in the nm concentration range, and it releases Ca2+ from stores different from the major intracellular Ca2+ store, the endoplasmic reticulum (7, 9). The function of NAADP has been demonstrated in virtually all cell types examined (9). Ca2+ mobilization by NAADP appears to trigger to Ca2+ release from the endoplasmic reticulum to convert a local to a global Ca2+ signal (5, 10, 11). As such, Ca2+ mobilization by NAADP was linked to several key physiological functions that include regulated exocytosis, fertilization, glucose sensing, and neuronal growth (9).
The identity of the channels activated by NAADP has not been resolved until recently. Initial functional studies suggested that NAADP activates the ryanodine Ca2+ release channels. Later, the TRP channel TRPML1 was suggested as the NAADP receptor (12, 13). However, recently we demonstrated that TRPML1 is unlikely to be the NAADP-activated channel and does not affect the NAADP-mediated Ca2+ release (14). The recent findings from independent groups that NAADP activates the two-pore channels TPC1 and TPC2 established them unequivocally as the channels activated by NAADP to mediate Ca2+ release from internal organelles (4–6). The mammalian TPCs were identified about 10 years before their function was clarified (15). In several cell types examined thus far, the levels of TPC1 are about 10 times higher than TPC2 (4, 6, 16). TPC2 expression appears to be restricted to late endosomes and lysosomes (4, 6, 14). Although some overlap in TPC1 and TPC2 expression is observed and the two expressed channels can be co-immunoprecipitated (14, 18), TPC1 is found in many intracellular organelles free of TPC2 (4, 6, 14). That TPC1 and TPC2 are the NAADP-activated channels was concluded from the finding that overexpression of TPC1 (4) and TPC2 (6, 19, 20) potentiates NAADP-mediated Ca2+ increase. In addition, knockdown of TPCs (4, 6) or overexpression of channel-dead TPC pore mutants, TPC1(L273P) (4), and TPC2(L265P) (21) inhibits endogenous NAADP-mediated Ca2+ release.
The biophysical properties of the TPC channels are known to a very limited extent. Some information is available on the plant TPC1 that somewhat resembles the mammalian TPCs (22, 23). Most information is available on the Arabidopsis AtTPC1, which expresses a single TPC (23, 24). Studies of Ca2+ release in Arabidopsis identified AtTPC1 (25) as a channel that localizes in the vacuole and mediates the slow vacuolar current (26) that regulates germination and stomatal movement (27). AtTPC1 has shorter N and C termini when compared with its mammalian counterpart (25), and it has two Ca2+ binding EF-hands that mediate activation of AtTPC1 by Ca2+ (23) that are not present in the mammalian TPCs. AtTPC1 is also regulated by luminal Ca2+, which shifts the voltage dependence of the channel to more depolarized voltages (28). AtTPC1 (and the mammalian TPCs) have two putative voltage sensors in each of the fourth transmembrane segments S4 and S10 that may mediate the voltage dependence of AtTPC1 (23).
When targeted to the plasma membrane, mammalian TPC2 behaved as a channel activated by submicromolar concentrations of NAADP and with a conductance of 100–120 picosiemens in symmetrical Cs+ (21). Incorporation of TPC2 into bilayers resulted in a NAADP-activated channel with a conductance of 300 picosiemens in symmetrical K+ and with 1:3 K+:Ca2+ selectivity. The apparent affinity of the channel to NAADP was regulated by luminal Ca2+ and pH with higher Ca2+ increasing the apparent affinity for NAADP and reduction in luminal pH from 7.2 to 4.8 reducing channel Po, whereas converting the NAADP concentration dependence to a bell-shaped curve (29). On the other hand, recording TPC2 activity using a planar patch clamp system and lysosomes containing TPC2 suggested that TPC2 is active only at acidic luminal pH as found in lysosomes and functions as a highly selective Ca2+ channel (30).
There is no biophysical information on the properties of the mammalian TPC1. In addition, there is almost no information of the physiological function of the TPCs beyond their role as NAADP-activated channels. One study reported that overexpression of sea urchin TPCs in mammalian cells changes endolysosomal morphology (31). To fill these gaps, in the present study, we characterized the channel properties of the mammalian TPC1. Our findings reveal that the mammalian TPC1 functions as a NAADP-activated and voltage-, pH-, and Ca2+-regulated channel. Most importantly, the apparent affinity for activation of TPC1 by NAADP is dynamic and is regulated by the membrane potential. These properties provide a potential mechanism for organellar Ca2+ oscillations that are mediated by dynamic behavior of TPC1-mediated Ca2+ release.
MATERIALS AND METHODS
Constructs
Human TPC1 tagged at the C terminus with GFP in pCS2+ was described previously (4). The channel-dead mutant TPC1(L273P) was generated by site-directed mutagenesis as described (4).
Cell Transfection and Vesicle Preparation
hTPC1-GFP was transfected into HEK293 cell using Lipofectamine 2000 (Invitrogen) reagent. Cells were maintained in DMEM high glucose (Invitrogen), 10% FBS, and 1% penicillin/streptomycin medium. TPC1 expression was verified by monitoring GFP fluorescence after 48 h of incubation. Transfected or nontransfected cells were harvested and homogenized on ice in a Ca2+-free buffer containing (in mm) 200 sucrose, 10 Hepes, 1 EDTA, pH 7.5 with KOH and supplemented with complete protease inhibitor mixture (Roche Applied Science), using a glass Dounce homogenizer and passages through 22- and then 27-gauge needles. The homogenate was spun at 300 × g, and a supernatant was spun at 16,000 × g to collect the endolysosomal fraction. The pellet was resuspended in a storage buffer composed of 10% sucrose, 10 mm MOPS, pH 7.0 with KOH, and the vesicles were aliquoted, snap-frozen, and stored at −80 °C before use in electrophysiological experiments.
TPC1 Knockdown and mRNA Measurements
TPC1 knockdown HEK293 cells were prepared by transfection of hTPC1 siRNA (Thermo Scientific/Dharmacon, J-010710-07-0005) against the hTPC1 sequence CCAUCGAGCUGUAUUUCAU using Lipofectamine 2000 reagent. Cells were incubated with 200 nmol/ml siRNA for 8 h in OPTI-MEM and grown in DMEM. Control cells were transfected with nontargeting siRNA (Thermo Scientific/Dharmacon, D-001810-01-05). The cells were harvested after 52 h either for vesicle isolation as above or for measurements of TPC1 mRNA by quantitative RT-PCR. To compare the relative endogenous mRNA levels for TPC1 and TPC2 in hTPC1 siRNA- and nontargeted siRNA-treated HEK293 cells, total RNA was extracted using a TRIzol reagent (Invitrogen, 15596-018), and 1 μg of each was transcribed into cDNA using an iScript cDNA synthesis kit (Bio-Rad). The quantitative RT-PCR analysis of the TPC1 and TPC2 cDNA levels relative to a GAPDH cDNA level was performed using a StepOnePlus real-time PCR system (Applied Biosystems) and a TaqMan gene expression kit containing primers for GAPDH (Hs02758991_g1), TPC1 (Hs00330542_m1), and TPC2 (Hs01552063_m1) (Applied Biosystems).
Electrophysiological Recordings from Planar Lipid Bilayers and Data Analysis
Planar bilayer lipid membranes were formed with a 3:1 mixture of phosphatidylethanolamine/phosphatidylserine lipids (Avanti Polar Lipids) dissolved in decane on a 200-μm diameter aperture separating cis and trans chambers. The chambers contained the following basic ionic solutions: cis (cytoplasmic) chamber, 102 mm TrisOH, 200 mm HEPES (pH = 7.3, ∼300 mosm); trans (luminal) chamber, 1 mm Ca(OH)2, 49 mm Ba(OH)2, 63 mm TrisOH, 218 mm acetate (pH 5.0, ∼300 mosm), unless otherwise stated. Endolysosomal vesicles were added to the cis chamber followed by continuous stirring for 10–20 min until low level spontaneous single channel activity was observed upon membrane potential shift to −40 or −60 mV relative to the grounded trans chamber. NAADP was added to the cis chamber followed by a 2-min stirring of the solution to stimulate channel activity. When the trans chamber solution pH was 7.3, the solution was buffered with Hepes. Luminal Ca2+ was varied by changing calcium/acetate concentrations in the trans chamber between 0 and 1 mm. Experiments were done at room temperature. Bilayer lipid membranes were voltage-clamped, ion currents were filtered at 500 Hz using a BC-535 amplifier (Warner Instruments), and signals were digitized at 5 kHz and recorded with Digidata 1440A board controlled by the pClamp 10 software (Molecular Devices). Current recording analysis, including mean current, open probabilities (Po), cumulative open probability (NPo), and the current amplitude probability histograms were done using the pClamp 10 module ClampFit. Single channel openings to a small (S) level, and large levels (S+L) were considered as separate events for Po calculation. Nonlinear and linear curve fitting, statistical analysis, and data plots were performed with OriginPro 8 software (OriginLab). All results are presented as mean ± S.E. Channel selectivity was determined from the reversal conditions for I(K+)/I(divalent) using the Goldman-Hodgkin-Katz equation for each ion at the membrane potential E:
 |
where P is permeability, z is charge, and [X]i and [X]o are internal (cis, cytoplasm) and external (trans, lumen) concentrations of the ion X. This yields for the reversal conditions of I(K+) = I(divalent), with only luminal Ca2+and Ba2+ and only cytoplasmic K+ the ratio equation.
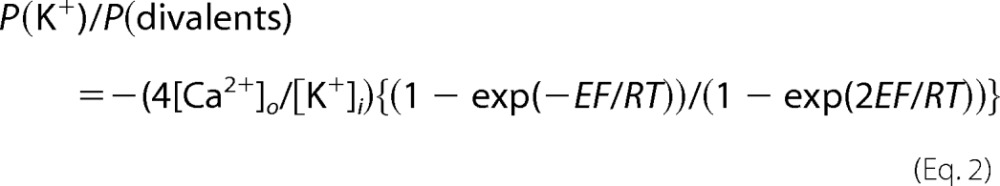 |
RESULTS AND DISCUSSION
Endogenous and Transfected TPC1 Function as NAADP-activated Channels
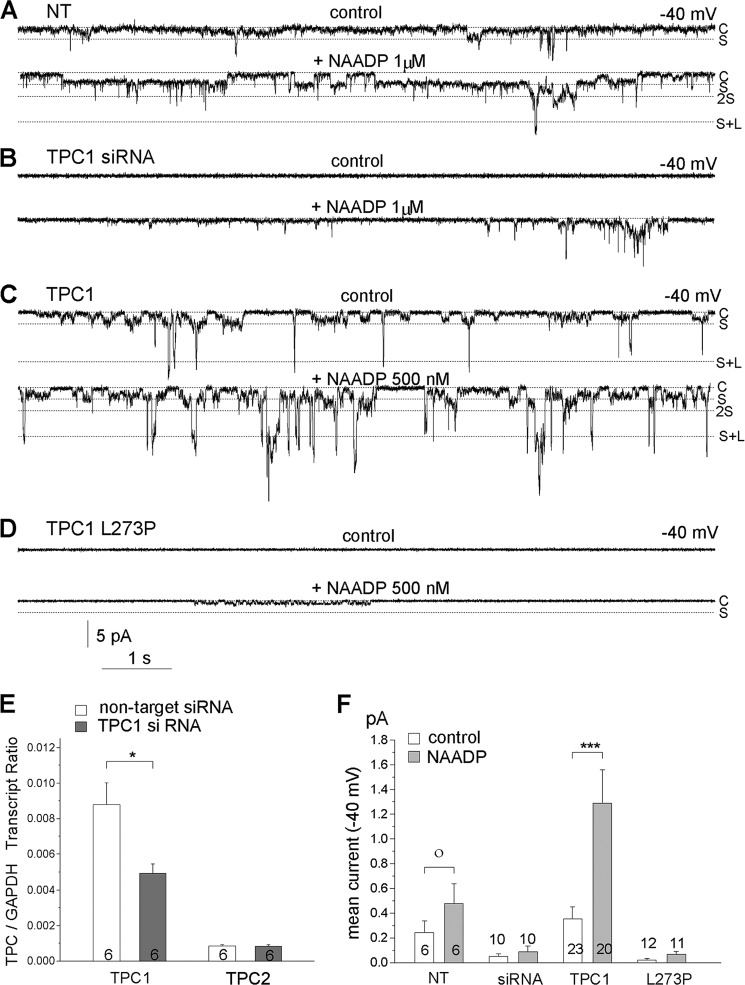
Demonstrating the Ca2+ permeability of the AtTPC1 channel required exposing the luminal side to 50 mm Ca2+ (32). Because high Ca2+ can also partially inhibit the channel (32) and Ba2+ is used to record Ca2+ current to prevent Ca2+-dependent inactivation of Ca2+ channels, in the present study, we used luminal (trans) solutions at a pH of 5.0 containing 49 mm Ba2+ and 1 mm Ca2+ and cytoplasmic (cis) solutions with Tris as the major salt to record TPC1 current. Fig. 1A shows that under these recording conditions and at a holding potential of −40 mV, incorporation of endolysosomes prepared from untransfected cells into bilayers resulted in sparse single channel events. The addition of 1 μm NAADP resulted in increased channel openings with two resolvable single channels conductances of about 2 pA (marked with S for small) and 10 pA (marked with S+L for small + large).
FIGURE 1.
Native and expressed TPC1 operate in two conductance states. A–D, example traces of current recording using microsomes incorporated into bilayers in the absence and presence of 1 μm NAADP and prepared from untransfected (NT) HEK cells (A) and HEK cells treated with TPC1 siRNA (B), transfected with TPC1 (C), and transfected with TPC1(L273P) (D). The closed (C), small (S), and large (L) conductance states are marked by dashed lines. E, quantitative PCR analysis of TPC1 and TPC2 in control HEK 293 cells and cells treated with TPC1 siRNA. * denotes p < 0.05. F, mean current calculated from continuous 60-s recordings before (open columns) and after (gray columns) application of 0.5 or 1 μm NAADP. Results are averages of the number of experiments indicated on the bars. ● denotes p < 0.1, *** denote p < 0.005. Solutions (in mm): luminal, 49 Ba2+, 1 Ca2+, 60 Tris, 218 acetate, pH 5.0; cytoplasmic, 102 Tris, 200 Hepes, pH 7.3; −40 mV cytoplasmic side.
To associate this channel activity with TPC1, we compared the mRNA levels of TPC1 and TPC2. As was found in other cell types (22, 24), the level of TPC1 mRNA was about eight times higher than that of TPC2 in HEK cells (Fig. 1D) (4). Fig. 1E also shows that TPC1-specific siRNA reduced TPC1 mRNA by about 50% without affecting TPC2 mRNA. Importantly, microsomes prepared from cells treated with TPC1 siRNA showed markedly reduced channel activity in response to NAADP (trace in Fig. 1B and summary in Fig. 1F). Finally, transfecting HEK cells with the TPC1(L273P) mutant that failed to elicit Ca2+ release in response to NAADP (4) also failed to show channel activity and almost eliminated the response to NAADP (Fig. 1, D and F). On the other hand, transfecting cells with TPC1 markedly increase channel openings in response to NAADP (Fig. 1, C and F). The channel recorded in microsomes from TPC1-transfected cells showed a Ba2+/K+ selectivity of about 2. Thus, the current with a trans chamber of 1 mm Ca2+, 49 mm Ba2+ and a cis chamber of 100 mm K+ had a reversal potential of −13.9 ± 1.7 mV (n = 9), which translates into a P(K+)/P(divalent) of 2.2.
The combined results in Fig. 1 indicate that the native NAADP-activated, TPC1-like channel can be observed and that both the native and the transfected TPC1 have two conductive states of 47 ± 8 and 200 ± 9 picosiemens. The large conductance state can be seen more frequently in transfected cells. It is not clear whether TPC1 has two independent conductance states, whether the low conductance is a subconductance of the large conductance, or whether the large conductance is simultaneous opening of TPC1 clusters made of 4–5 TPC1 channels. The results below favor functioning of TPC1 in two conductance states.
Luminal pH and Ca2+ Affect TPC1 Function
An important ligand that affects TPC activity is luminal H+ with acidic pH affecting the voltage dependence of AtTPC1 (23) and the activity of human TPC2 (29). We therefore analyzed TPC1 activity at neutral and acidic luminal pH. Fig. 2, A–C, show that at symmetric cytosolic and luminal pH of 7.3, TPC1 expressed in HEK cells is not active in the presence or absence of NAADP. Reducing luminal pH to 5.0 as found in endolysosomes resulted in significant spontaneous activity of TPC1 that was markedly increased by 1 μm NAADP. TPCs are also regulated by luminal Ca2+, with luminal Ca2+ inhibiting AtTPC1 monovalent conductance (25) but increasing the apparent affinity of human TPC2 for activation by NAADP when conducting K+ (29). Conductance of Ba2+ by TPC1 allowed us to measure regulation of the channel by luminal Ca2+ and when conducting a divalent ion. Fig. 2D shows that even in the presence of 50 mm luminal Ba2+, the channel shows no spontaneous or NAADP-dependent activity in the absence of luminal Ca2+. In the presence of 500 nm NAADP and at −40 mV, luminal Ca2+ dose-dependently activated TPC1 with apparent affinity of about 180 μm.
FIGURE 2.
TPC1 is activated by acidic luminal pH and luminal Ca2+. A and B, example activity traces of TPC1 expressed in HEK cells at cytoplasmic pH of 7.3 and luminal pH of 7.3 (A) and 5.0 (B) in the absence (left traces) or presence (right traces) of 1 μm NAADP and at a membrane potential of −60 mV. The indicated fragments are shown at an expanded time scale. C shows the mean ± S.E. of the indicated numbers of experiments with *** denoting p < 0.001. D, for determination of luminal Ca2+ dependence, recording conditions were as in Fig. 1, except that the luminal solution contained the indicated Ca2+ concentrations and TPC1 was activated by 500 nm cytoplasmic NAADP. The numbers next to symbols indicate the numbers of experiments, and the curve was fitted with a Hill equation with an apparent Km of 180 μm.
Regulation by Ca2+ and acidic luminal pH is common to the TPCs, and we reported similar regulation by pH of the other organellar channels, where TRPML3 is regulated by acidic pH (33) and regulation of lysosomal pH by TRPML1 (34, 35). In the case of TRPML3, regulation by acidic pH is mediated by a string of histidines in a luminal loop (33). Whether a similar domain senses pH in the TPCs remains to be determined. Such a regulation likely ensures that Ca2+ release through the TPCs takes place only when the organelles are acidified. A possible advantage for this is that at neutral pH, when the Ca2+ buffering capacity of the organelles is relatively high, the channels are not active to reduce Ca2+ permeability and allow Ca2+ accumulation by the organelles. Acidification of the organelles then increases the free Ca2+ concentration in the organelle lumen by decreasing the Ca2+ buffering capacity to facilitate its release.
Association between Small and Large Conductance States
It is not clear whether the two conductance states of TPC1 represent two different levels of a single channel or a coupled gating of a channel cluster. To address this in part, we analyzed the relationship between the states. As illustrated in Fig. 3A and the expanded example traces in Fig. 3B, the two states appear completely coupled in that the large conductance always initiates from an open small conductance state. Analysis of the current amplitude and amplitude probability identified only multiples of the small conductance and of the coupled conductances (Fig. 3C). Of the total events recorded, only 2/810 events of the large conductance started from an apparent closed state (Fig. 3D). The possible role of the coupling of the two conductances is suggested by the analysis of the open time probability (Fig. 3E) and the mean open time of each state (Fig. 3F). The coupled large conductance has low mean open probability with median open time of 20 ms. The median open time of the small conductance alone was 60 ms, but it was markedly increased to 420 ms by coupling to the large conductance.
FIGURE 3.
Multimodal gating of TPC1. A, representative current recording of TPC1 expressed in HEK cells and obtained from the same bilayer (Vm,cyt = −40 mV) in the absence (control) and presence of 500 nm NAADP illustrating the presence of characteristic small (S) and large (L) open states. B, eight 400-ms fragments collected from the trace in panel A aligned by the time of the initial opening, illustrating appearance of the L conductance after opening of the S conductance. C, amplitude histograms were calculated from the 60-s long current recordings in A. Amplitude combinations of S and L conductances are indicated by arrows above the peaks. D, statistics of single channel openings collected from five different recordings of total duration of ∼14 min elucidating the prevalence of the S conductance and the coupling of S+L openings. E, open time probability histograms for single S conductance and coupled S and L openings analyzed in D. F, cumulative open time probability histogram yields median open times of 62 ms for single S openings, 420 ms for coupled S openings, and 20 ms for coupled L openings.
The incredibly tight coupling of the two conductance states and the profound effect of the large conductance on the mean open time of the small conductance favor two conductance states of TPC1 rather than opening of TPC1 clusters. Cluster opening should not show such a tight coupling and should not affect the open time of the small conductance. At present, it is not clear how the two conductances are coupled and how the large conductance so profoundly increases the open time of the small conductance. One possibility is that opening of the large conductance requires ion flow through the channel, and thus, perhaps ions in the conductive pathway may regulate TPC1 opening. The brief flipping of the channel to the large conductance state may induce a channel open conformation that is somewhat more stable than the open conformation of the same small conductance prior to the flip to the large conductance.
TPC1 Is a Voltage-gated Channel
All TPC channels have putative voltage sensors in transmembrane domains 4 and 10, as found in classical voltage-gated channels (23). However, the reports that the mammalian TPC2 is not regulated by voltage were unexpected (21, 29). Therefore, we analyzed the effect of voltage on TPC1 activity. Fig. 4A shows that stepwise changes in the voltage from 0 to −60 mV increased TPC1 open probability. TPC1 activity could be observed even in the absence of NAADP at a membrane potential of −60 mV. NAADP (1 μm) increased TPC1 open probability at all voltages. The traces at the expanded time scale on the right show that the small and large conductances are observed at all voltages.
FIGURE 4.
Voltage dependence of TPC1 function. Currents were recorded using microsomes prepared from HEK cells overexpressing TPC1 and incorporated into bilayers. A, example traces of currents recorded from the same bilayer held sequentially at 0, −20, −40, and −60 mV before and after the addition of 1 μm NAADP. Traces on the right are at expanded time scale corresponding to the numbers in the main traces demonstrating the S/L pattern of channel openings at all membrane potentials tested. Dotted lines represent main levels of S conductance, L conductance, and their combinations. B, amplitude probability histograms for the S conductance obtained from the 60 s traces and at 1 μm NAADP. C, amplitude probability histograms for the coupled L conductance. Arrows in B and C indicate voltage-dependent shift of the S and L conductance states. D, voltage dependence of the S and L conductance states determined from the histogram peaks of 4–14 measurements for each data point. E, voltage dependence of open probabilities for S conductance (Po(S)) and coupled L conductance (Po(L)) and the cumulative open probability NPo calculated from 6–8 experiments for each condition.
Fig. 4B illustrates the relationship between the current amplitude and open probability of the small conductance, clearly showing that the membrane potential increases the TPC1 small conductance open probability. The voltage dependence of the small and large conductance current amplitudes is linear (Fig. 4D) with slope conductances of 47 ± 8 and 200 ± 9 picosiemens, respectively. All points histogram analysis of the relationship between open probability and current amplitude for the coupled large conductance in given in Fig. 4C. Fig. 4E is the summary of the effect of the membrane potential on the open probability of the two conductances and on channel NPo to show that the membrane potential increases TPC1 NPo.
Voltage Dependence of TPC1 Activation by NAADP
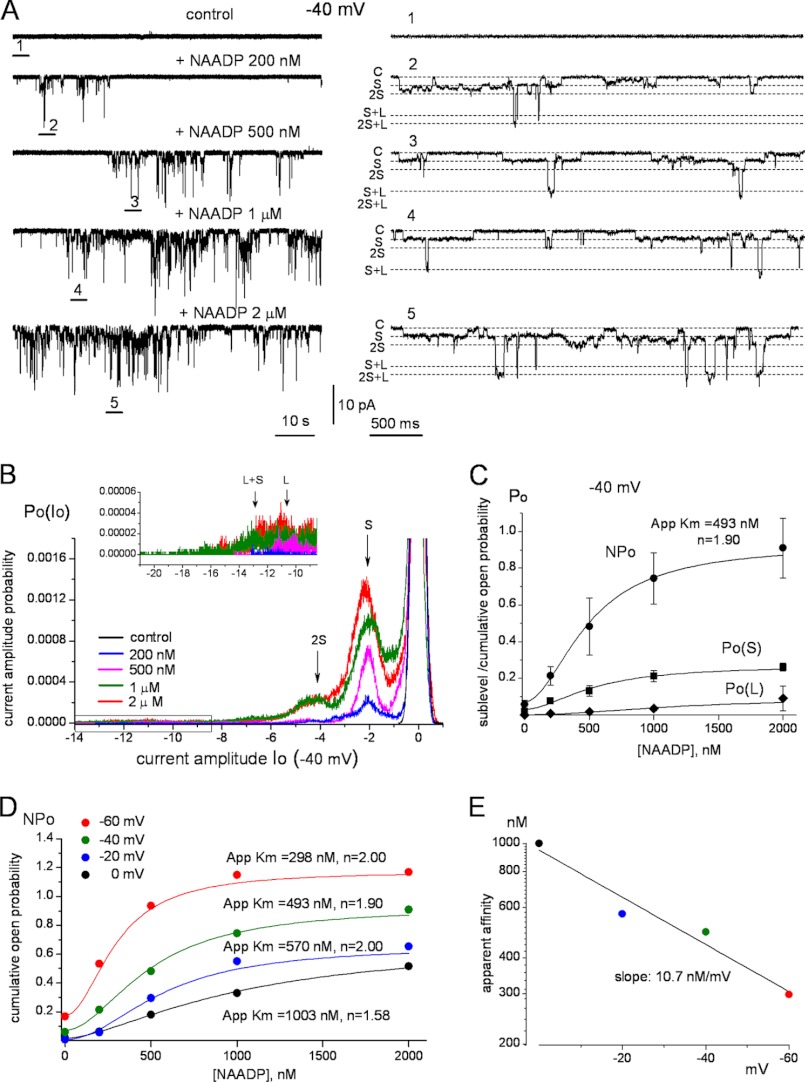
In the next set of experiments, we determined the concentration dependence for activation of TPC1 channel activity by NAADP. The example traces in Fig. 5A show that increasing NAADP up to 2 μm increased TPC1 activity. The dependence of the open probability of each of the conductance states and of NPo at −40 mV on NAADP concentration had an apparent Km of 493 nm and could be fitted best with a Hill coefficient of 1.9 (Fig. 5C). The latter finding suggests that interaction of NAADP is more complex than simple saturation binding to a single site. Most notably, the apparent affinity for NAADP was modulated by the membrane potential (Fig. 5D). Fig. 5E shows the nearly logarithmic dependence of the apparent affinity for NAADP on the membrane potential with increased apparent affinity by about 10 nm for each mV of hyperpolarization. A similar Hill coefficient of close to 2 was measured at all membrane potentials between 0 and −60 mV (Fig. 5D). Thus, voltage modulates the apparent affinity of activation by NAADP.
FIGURE 5.
Regulation of TPC1 NAADP dependence by membrane potential. Currents were recorded using microsomes prepared from HEK cells expressing TPC1 and incorporated into bilayers. A, sample traces of currents recorded from the same bilayer at −40 mV by gradually increasing NAADP to the indicated concentrations. Traces on the right are at expanded time scale corresponding to the numbers in the main traces demonstrating the S/L pattern of channel openings over the entire range of NAADP concentrations tested. B, amplitude probability histograms obtained from the traces in A. The inset shows a blown-up of the portion labeled by the black rectangle for the coupled L peaks. C, dependence of TPC1 open probability on NAADP concentration for the S conductance (Po(S)) and coupled L conductance (Po(L)) openings and the cumulative NPo at Vm,cyt = −40 mV. Results are mean ± S.E. of 3–11 experiments for each condition and fitted with Hill functions. D, the NAADP dependence of NPo at Vm,cyt of 0, −20, −40, and −60 mV obtained from 3–12 experiments for each point. Fitting parameters are shown next to the traces. E, voltage dependence of the apparent affinity for NAADP obtained for panel D.
Recent studies suggest that NAADP does not appear to interact directly with the TPCs. Rather the NAADP receptor may be a subunit of a channel complex comprising the TPCs (36, 37). Although a Hill coefficient of 2 for NAADP activation reported here (Fig. 5) fits the data best, additional functional studies and in particular detailed binding studies are needed to resolve whether this reflects interaction with multiple sites within the TPC1 complex and/or binding of NAADP to one monomer affecting the binding to the second monomer. Additionally, we did not observe inactivation of TPC1 by μm concentrations of NAADP as reported in native cells (38, 39). This suggests either that the inactivation site is of lower affinity than that of TPC2 (29) or that it may reside on a component of the complex that is lost in the reconstituted bilayer system.
Conclusions
In many cells, TPC1 appears to be expressed at much higher levels than TPC2 (5, 10) and thus is likely the major mediator of the NAADP-evoked Ca2+ release. Despite this, nothing is known about the properties and physiological function of this channel. The present studies provide extensive characterization of TPC1 function and report that TPC1 is a voltage-, Ca2+-, and pH-regulated Ca2+-permeable channel. The unique properties reported here include a requirement for luminal Ca2+ for channel activation by NAADP, operation of the channel at two conductances, and regulation of the apparent affinity for NAADP by the organellar membrane potential. These properties are different from the plant TPC1 homologue as AtTPC1 NPo is actually inhibited by both luminal pH and luminal Ca2+ (40).
Of the TPC1 properties, of particular interest is the finding of regulation of the apparent Km for NAADP by membrane potential. Such a dynamic regulation of the activity of NAADP could generate organellar Ca2+ oscillations. NAADP-evoked Ca2+ signals in vivo are often oscillatory (14, 39, 41), as exemplified by those in the well studied pancreatic acinar cell (11). The combination of regulation of TPC1 activity by organellar Ca2+ (Fig. 2) and by the membrane potential (Fig. 5) provides a potential mechanism for organellar Ca2+ oscillations. A reliable measurement of organellar membrane potential in live cells reported recently suggests that the lysosomal membrane potential is about 20 mV lumen-positive (42). This is in the same range of that estimated from Cl− distribution in endosomes (43). Further, dissipation of the lysosomal pH gradient resulted in hyperpolarization of the lysosomal membrane potential (42). Thus, at the resting organellar membrane potential, the apparent affinity for activation of TPC1 by NAADP is low, and the channel is kept in a close state. Filling the organelles with Ca2+ would generate a more negative organellar membrane potential to increase the affinity for NAADP. Generation of NAADP in response to receptor stimulation activates TPC1 to cause Ca2+ release and perhaps depolarization of the organellar membrane potential by counterion flow required for charge compensation. This is likely Cl− efflux through ClC7 (44) and/or K+ influx (17), both of which result in depolarization of the organellar membrane potential. The depolarization and reduction in organellar Ca2+, in turn, reduce the TPC1 apparent affinity for NAADP to terminate Ca2+ release. This initiates refilling the organelles with Ca2+ and repolarization of the membrane potential to restore responsiveness of TPC1 to NAADP and evoke the next Ca2+ spike. Such a mechanism is equivalent to Ca2+ oscillations driven by Ca2+-dependent changes in the apparent affinity for inositol 1,4,5-trisphosphate (1). Mutations in TPC1 that affect its regulation by Ca2+ by the membrane potential should be helpful in testing the operation of such a mechanism.
This work was supported, in whole or in part, by an intramural National Institutes of Health grant (to S. M.) through the NIDCR/DIR Z1A-DE000735 and extramural Grants HD058577 and ES01678 (to K. K.). This work was also supported by Biotechnology and Biological Sciences Research Council Grant BB/G013721/1 (to S. P.).
- TPC
- two-pore channel
- hTPC1
- human TPC1
- NAADP
- nicotinic acid adenine dinucleotide phosphate
- TRP
- transient receptor potential
- S conductance
- small conductance state
- L conductance
- large conductance state.
REFERENCES
- 1. Berridge M. J., Bootman M. D., Roderick H. L. (2003) Calcium signaling: dynamics, homeostasis, and remodeling. Nat. Rev. Mol. Cell Biol. 4, 517–529 [DOI] [PubMed] [Google Scholar]
- 2. Kiselyov K., Yamaguchi S., Lyons C. W., Muallem S. (2010) Aberrant Ca2+ handling in lysosomal storage disorders. Cell Calcium 47, 103–111 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3. Patel S., Muallem S. (2011) Acidic Ca2+ stores come to the fore. Cell Calcium 50, 109–112 [DOI] [PubMed] [Google Scholar]
- 4. Brailoiu E., Churamani D., Cai X., Schrlau M. G., Brailoiu G. C., Gao X., Hooper R., Boulware M. J., Dun N. J., Marchant J. S., Patel S. (2009) Essential requirement for two-pore channel 1 in NAADP-mediated calcium signaling. J. Cell Biol. 186, 201–209 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Patel S., Marchant J. S., Brailoiu E. (2010) Two-pore channels: regulation by NAADP and customized roles in triggering calcium signals. Cell Calcium 47, 480–490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Calcraft P. J., Ruas M., Pan Z., Cheng X., Arredouani A., Hao X., Tang J., Rietdorf K., Teboul L., Chuang K. T., Lin P., Xiao R., Wang C., Zhu Y., Lin Y., Wyatt C. N., Parrington J., Ma J., Evans A. M., Galione A., Zhu M. X. (2009) NAADP mobilizes calcium from acidic organelles through two-pore channels. Nature 459, 596–600 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7. Lee H. C., Aarhus R. (1995) A derivative of NADP mobilizes calcium stores insensitive to inositol trisphosphate and cyclic ADP-ribose. J. Biol. Chem. 270, 2152–2157 [DOI] [PubMed] [Google Scholar]
- 8. Churchill G. C., Okada Y., Thomas J. M., Genazzani A. A., Patel S., Galione A. (2002) NAADP mobilizes Ca2+ from reserve granules, lysosome-related organelles, in sea urchin eggs. Cell 111, 703–708 [DOI] [PubMed] [Google Scholar]
- 9. Guse A. H., Lee H. C. (2008) NAADP: a universal Ca2+ trigger. Sci. Signal. 1, re10. [DOI] [PubMed] [Google Scholar]
- 10. Zhu M. X., Evans A. M., Ma J., Parrington J., Galione A. (2010) Two-pore channels for integrative Ca2+ signaling. Commun. Integr. Biol. 3, 12–17 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Petersen O. H. (2004) Local and global Ca2+ signals: physiology and pathophysiology. Biol. Res. 37, 661–664 [DOI] [PubMed] [Google Scholar]
- 12. Zhang F., Li P. L. (2007) Reconstitution and characterization of a nicotinic acid adenine dinucleotide phosphate (NAADP)-sensitive Ca2+ release channel from liver lysosomes of rats. J. Biol. Chem. 282, 25259–25269 [DOI] [PubMed] [Google Scholar]
- 13. Zhang F., Jin S., Yi F., Li P. L. (2009) TRP-ML1 functions as a lysosomal NAADP-sensitive Ca2+ release channel in coronary arterial myocytes. J. Cell. Mol. Med. 13, 3174–3185 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Yamaguchi S., Jha A., Li Q., Soyombo A. A., Dickinson G. D., Churamani D., Brailoiu E., Patel S., Muallem S. (2011) Transient receptor potential mucolipin 1 (TRPML1) and two-pore channels are functionally independent organellar ion channels. J. Biol. Chem. 286, 22934–22942 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ishibashi K., Suzuki M., Imai M. (2000) Molecular cloning of a novel form (two-repeat) protein related to voltage-gated sodium and calcium channels. Biochem. Biophys. Res. Commun. 270, 370–376 [DOI] [PubMed] [Google Scholar]
- 16. Cai X., Patel S. (2010) Degeneration of an intracellular ion channel in the primate lineage by relaxation of selective constraints. Mol. Biol. Evol. 27, 2352–2359 [DOI] [PubMed] [Google Scholar]
- 17. Steinberg B. E., Huynh K. K., Brodovitch A., Jabs S., Stauber T., Jentsch T. J., Grinstein S. (2010) A cation counterflux supports lysosomal acidification. J. Cell Biol. 189, 1171–1186 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Rietdorf K., Funnell T. M., Ruas M., Heinemann J., Parrington J., Galione A. (2011) Two-pore channels form homo- and heterodimers. J. Biol. Chem. 286, 37058–37062 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Zong X., Schieder M., Cuny H., Fenske S., Gruner C., Rötzer K., Griesbeck O., Harz H., Biel M., Wahl-Schott C. (2009) The two-pore channel TPCN2 mediates NAADP-dependent Ca2+ release from lysosomal stores. Pflugers Arch. 458, 891–899 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Brailoiu E., Hooper R., Cai X., Brailoiu G. C., Keebler M. V., Dun N. J., Marchant J. S., Patel S. (2010) An ancestral deuterostome family of two-pore channels mediates nicotinic acid adenine dinucleotide phosphate-dependent calcium release from acidic organelles. J. Biol. Chem. 285, 2897–2901 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Brailoiu E., Rahman T., Churamani D., Prole D. L., Brailoiu G. C., Hooper R., Taylor C. W., Patel S. (2010) An NAADP-gated two-pore channel targeted to the plasma membrane uncouples triggering from amplifying Ca2+ signals. J. Biol. Chem. 285, 38511–38516 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Zhu M. X., Ma J., Parrington J., Galione A., Evans A. M. (2010) TPCs: endolysosomal channels for Ca2+ mobilization from acidic organelles triggered by NAADP. FEBS letters 584, 1966–1974 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23. Hedrich R., Marten I. (2011) TPC1-SV channels gain shape. Mol. Plant 4, 428–441 [DOI] [PubMed] [Google Scholar]
- 24. Patel S., Ramakrishnan L., Rahman T., Hamdoun A., Marchant J. S., Taylor C. W., Brailoiu E. (2011) The endolysosomal system as an NAADP-sensitive acidic Ca2+ store: role for the two-pore channels. Cell Calcium 50, 157–167 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Furuichi T., Cunningham K. W., Muto S. (2001) A putative two-pore channel AtTPC1 mediates Ca2+ flux in Arabidopsis leaf cells. Plant Cell Physiol. 42, 900–905 [DOI] [PubMed] [Google Scholar]
- 26. Peiter E., Maathuis F. J., Mills L. N., Knight H., Pelloux J., Hetherington A. M., Sanders D. (2005) The vacuolar Ca2+-activated channel TPC1 regulates germination and stomatal movement. Nature 434, 404–408 [DOI] [PubMed] [Google Scholar]
- 27. Pottosin I. I., Schönknecht G. (2007) Vacuolar calcium channels. J. Exp. Bot. 58, 1559–1569 [DOI] [PubMed] [Google Scholar]
- 28. Pottosin I. I., Martínez-Estévez M., Dobrovinskaya O. R., Muñiz J., Schönknecht G. (2004) Mechanism of luminal Ca2+ and Mg2+ action on the vacuolar slowly activating channels. Planta 219, 1057–1070 [DOI] [PubMed] [Google Scholar]
- 29. Pitt S. J., Funnell T. M., Sitsapesan M., Venturi E., Rietdorf K., Ruas M., Ganesan A., Gosain R., Churchill G. C., Zhu M. X., Parrington J., Galione A., Sitsapesan R. (2010) TPC2 is a novel NAADP-sensitive Ca2+ release channel, operating as a dual sensor of luminal pH and Ca2+. J. Biol. Chem. 285, 35039–35046 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Schieder M., Rötzer K., Brüggemann A., Biel M., Wahl-Schott C. A. (2010) Characterization of two-pore channel 2 (TPCN2)-mediated Ca2+ currents in isolated lysosomes. J. Biol. Chem. 285, 21219–21222 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Ruas M., Rietdorf K., Arredouani A., Davis L. C., Lloyd-Evans E., Koegel H., Funnell T. M., Morgan A. J., Ward J. A., Watanabe K., Cheng X., Churchill G. C., Zhu M. X., Platt F. M., Wessel G. M., Parrington J., Galione A. (2010) Purified TPC isoforms form NAADP receptors with distinct roles for Ca2+ signaling and endolysosomal trafficking. Current biology 20, 703–709 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Ward J. M., Schroeder J. I. (1994) Calcium-activated K+ channels and calcium-induced calcium release by slow vacuolar ion channels in guard cell vacuoles implicated in the control of stomatal closure. Plant Cell 6, 669–683 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Kim H. J., Li Q., Tjon-Kon-Sang S., So I., Kiselyov K., Soyombo A. A., Muallem S. (2008) A novel mode of TRPML3 regulation by extracytosolic pH absent in the varitint-waddler phenotype. EMBO J. 27, 1197–1205 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Soyombo A. A., Tjon-Kon-Sang S., Rbaibi Y., Bashllari E., Bisceglia J., Muallem S., Kiselyov K. (2006) TRP-ML1 regulates lysosomal pH and acidic lysosomal lipid hydrolytic activity. J. Biol. Chem. 281, 7294–7301 [DOI] [PubMed] [Google Scholar]
- 35. Miedel M. T., Rbaibi Y., Guerriero C. J., Colletti G., Weixel K. M., Weisz O. A., Kiselyov K. (2008) Membrane traffic and turnover in TRP-ML1-deficient cells: a revised model for mucolipidosis type IV pathogenesis. J. Exp. Med. 205, 1477–1490 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36. Lin-Moshier Y., Walseth T. F., Churamani D., Davidson S. M., Slama J. T., Hooper R., Brailoiu E., Patel S., Marchant J. S. (2012) Photoaffinity labeling of nicotinic acid adenine dinucleotide phosphate (NAADP) targets in mammalian cells. J. Biol. Chem. 287, 2296–2307 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37. Walseth T. F., Lin-Moshier Y., Jain P., Ruas M., Parrington J., Galione A., Marchant J. S., Slama J. T. (2012) Photoaffinity labeling of high affinity nicotinic acid adenine dinucleotide phosphate (NAADP)-binding proteins in sea urchin egg. J. Biol. Chem. 287, 2308–2315 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38. Galione A., Ruas M. (2005) NAADP receptors. Cell Calcium 38, 273–280 [DOI] [PubMed] [Google Scholar]
- 39. Cancela J. M., Gerasimenko O. V., Gerasimenko J. V., Tepikin A. V., Petersen O. H. (2000) Two different but converging messenger pathways to intracellular Ca2+ release: the roles of nicotinic acid adenine dinucleotide phosphate, cyclic ADP-ribose, and inositol trisphosphate. EMBO J. 19, 2549–2557 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Beyhl D., Hörtensteiner S., Martinoia E., Farmer E. E., Fromm J., Marten I., Hedrich R. (2009) The fou2 mutation in the major vacuolar cation channel TPC1 confers tolerance to inhibitory luminal calcium. Plant J. 58, 715–723 [DOI] [PubMed] [Google Scholar]
- 41. Churchill G. C., Galione A. (2001) NAADP induces Ca2+ oscillations via a two-pool mechanism by priming IP3- and cADPR-sensitive Ca2+ stores. EMBO J. 20, 2666–2671 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Koivusalo M., Steinberg B. E., Mason D., Grinstein S. (2011) In situ measurement of the electrical potential across the lysosomal membrane using FRET. Traffic 12, 972–982 [DOI] [PubMed] [Google Scholar]
- 43. Sonawane N. D., Thiagarajah J. R., Verkman A. S. (2002) Chloride concentration in endosomes measured using a ratioable fluorescent Cl− indicator: evidence for chloride accumulation during acidification. J. Biol. Chem. 277, 5506–5513 [DOI] [PubMed] [Google Scholar]
- 44. Weinert S., Jabs S., Supanchart C., Schweizer M., Gimber N., Richter M., Rademann J., Stauber T., Kornak U., Jentsch T. J. (2010) Lysosomal pathology and osteopetrosis upon loss of H+-driven lysosomal Cl− accumulation. Science 328, 1401–1403 [DOI] [PubMed] [Google Scholar]