Background: HIV-1 Tat engages αvβ3, leading to endothelial cell (EC) proangiogenic activation.
Results: Sialic acid (NeuAc)-binding lectins and neuraminidase partially inhibit Tat/αvβ3 interaction and consequent EC proangiogenic activation.
Conclusion: Endothelial αvβ3-associated NeuAc is involved in Tat interaction and consequent EC proangiogenic activation.
Significance: Integrin-associated NeuAc can be considered a target for the development of new treatments for angiogenesis/AIDS-associated pathologies.
Keywords: Angiogenesis, Endothelial Cell, HIV-1, Integrin, Sialic Acid, HIV-1 Tat
Abstract
Sialic acid (NeuAc) is a major anion on endothelial cells (ECs) that regulates different biological processes including angiogenesis. NeuAc is present in the oligosaccharidic portion of integrins, receptors that interact with extracellular matrix components and growth factors regulating cell adhesion, migration, and proliferation. Tat is a cationic polypeptide that, once released by HIV-1+ cells, accumulates in the extracellular matrix, promoting EC adhesion and proangiogenic activation by engaging αvβ3. By using two complementary approaches (NeuAc removal by neuraminidase or its masking by NeuAc-binding lectin from Maackia amurensis, MAA), we investigated the presence of NeuAc on endothelial αvβ3 and its role in Tat interaction, EC adhesion, and proangiogenic activation. αvβ3 immunoprecipitation with biotinylated MAA or Western blot analysis of neuraminidase-treated ECs demonstrated that NeuAc is associated with both the αv and the β3 subunits. Surface plasmon resonance analysis demonstrated that the masking of αvβ3-associated NeuAc by MAA prevents Tat/αvβ3 interaction. MAA and neuraminidase prevent αvβ3-dependent EC adhesion to Tat, the consequent FAK and ERK1/2 phosphorylation, and EC proliferation, migration, and regeneration in a wound-healing assay. Finally, MAA inhibits Tat-induced neovascularization in the ex vivo human artery ring sprouting assay. The inhibitions are specific because the NeuAc-unrelated lectin from Ulex europaeus is ineffective on Tat. Also, MAA and neuraminidase affect only weakly integrin-dependent EC adhesion and proangiogenic activation by fibronectin. In conclusion, NeuAc is associated with endothelial αvβ3 and mediates Tat-dependent EC adhesion and proangiogenic activation. These data point to the possibility to target integrin glycosylation for the treatment of angiogenesis/AIDS-associated pathologies.
Introduction
Polyanionic macromolecules are extremely abundant in the extracellular environment, readily accessible to many proteins for interactions implicated in various biological functions. Among polyanions, sialic acid (NeuAc)-bearing gangliosides and glycoproteins are widely distributed in biological fluids, extracellular matrix, and cell membrane, where they act as receptors for various physiological ligands and for many human viruses, bacteria, and protozoa (1–3).
The term NeuAc encompasses a large family of sugars characterized by a nine-carbon sugar acid common in higher animals and some microorganisms (4). NeuAc is found mainly as a terminal component of glycoprotein and gangliosides, where it regulates various molecular and cellular interactions (5).
NeuAc is the major surface anion on the endothelial cell (EC)2 surface. Accordingly, the lectin from Maackia amurensis (MAA), which specifically binds NeuAc residues attached to galactose through an α(2→3) linkage, binds to ECs of retina, brain, and myocardium (6). NeuAc expression on ECs is regulated during ontogenesis, inflammation (7–9), and possibly neovascularization, as suggested by the observation that the binding of the NeuAc-binding lectin from Limax flavus to ECs increases during angiogenesis in the chick embryo chorioallantoic membrane (8).
NeuAc is involved in different physiological and pathological functions of the endothelium; in its ganglioside- or glycoprotein-associated form, it mediates EC infection by different microorganisms (10) and the transport of HIV-1 or of its proteins across the blood-brain barrier (11, 12). In its ganglioside-associated form, NeuAc takes part in the regulation of neovascularization (13–15). When associated with integrin subunits (including αE (16), α2, (17), α3 (18), α4 (19), α5 and αv (20), β1 (17, 18, 20), β2 (21), and β4 (16, 20)), NeuAc contributes to leukocyte and tumor cell extravasation during inflammation and metastasization, respectively.
Integrins are widely distributed receptors that interact with extracellular matrix components, growth factors, and microbial proteins regulating adhesion, migration, and proliferation of various normal and transformed cell types (22). Among the various integrins, αvβ3 expressed on the surface of ECs plays a central role in neovascularization (23). Interestingly, NeuAc has been found associated with αvβ3 integrin from melanoma metastatic cell surface (18), but no data are available for αvβ3 from ECs.
HIV-1 Tat is a cationic protein that, once released by HIV-1-infected cells (24), targets ECs, causing a variety of pathological effects that, in turn, lead to different angiogenesis-related AIDS-associated diseases such as Kaposi sarcoma and ocular microangiopathies. Extracellular Tat accumulates in the extracellular matrix where, by binding to endothelial αvβ3, it promotes EC adhesion and proangiogenic activation (25–27). Tat/αvβ3 interaction occurs both via the RGD motif and the basic domain (RKKRRQRRR) of Tat (25). On the basis of what is described above, in this study, we decided to evaluate the presence of NeuAc on integrin αvβ3 expressed at the EC surface and to investigate its role in Tat engagement and consequent biological activities.
EXPERIMENTAL PROCEDURES
Chemicals
Synthetic 86-amino acid Tat was from Xeptagen (Venezia, Italy). The recombinant wild type 86-amino acid form of HIV-1 Tat and its mutants Tat 1e (characterized by the deletion of the amino acid sequence that contains the RGD sequence) and Tat R→A (in which the arginine residues 49, 52, 53, 55, 56, and 57 within the basic domain were mutated to alanine residues) were purified from Escherichia coli as glutathione S-transferase (GST) fusion proteins (28). GST moiety does not interfere with Tat molecular interactions and biological activities (25). Anti-vascular endothelial growth factor receptor-2 (VEGFR2) antibody was gifted by Prof. H. A. Weich, National Research Centre for Biotechnology, Braunschweig, Germany. The heptapeptides GRGDSPK and GRADSPK were from Neosystems Laboratoires, Strasbourg, France, K5NOSH was from Glycores 2000, Milan, Italy, specific αvβ3 antagonist SCH221153 and its inactive analog SCH21668 (27) were from Schering-Plough (Kenilworth, NJ), anti-phospho-FAK antibody was from Santa Cruz Biotechnology (Santa Cruz, CA), anti-phospho-ERK1/2 antibody and anti-phospho-VEGFR2 antibody were from Cell Signaling Technology (Danvers, MA), biotinylated MAA was from Vector Laboratories (Burlingame, CA), streptavidin-Sepharose, 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride, and N-hydroxy-succinimide were from GE Healthcare, anti-paxillin antibody was from Upstate Biotech Millipore (Lake Placid, NY), purified human αvβ3 integrin, anti-αvβ3 LM 609, anti-fascin, anti-αv and anti-β3 antibodies were from Chemicon, Millipore (Billerica, MA), glucosyl ceramide synthase inhibitor d-threo-1-phenyl-2-decanoyl-amino-3-morpholino-1-propanol (PDMP) and d-1-threo-1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol-HCl (PPPP) were from Matreya, LLC (Pleasant Gap, PA), anti-α-tubulin antibody, TRITC-phalloidin, FITC-conjugated anti-mouse IgG, neuraminidase from Clostridium perfringens, MAA, lectin from Ulex europaeus (UEA), poly-l-lysine, fibrinogen, fibronectin (FN), phorbol myristate acetate, 4-6-diamidino-2-phenylindole (DAPI), phenylmethylsulfonyl fluoride (PMSF), amino-n-caproic acid, leupeptin, Na3VO4, and NaF were from Sigma.
Surface Plasmon Resonance (SPR) Analysis
A BIAcore X instrument (GE Healthcare) was used. Two different immobilizations were used to study the Tat/αvβ3 interaction. (i) As described previously (25), synthetic Tat (40 μg/ml) was allowed to react with a CM5 sensor chip activated with 50 μl of a mixture of 0.2 m 1-ethyl-3-(3-dimethylaminopropyl)carbodiimide hydrochloride and 0.5 m N-hydroxy-succinimide, leading to the immobilization of 6,470 resonance units (0.35 pmol/mm2) of protein. Similar results were obtained for the immobilization of bovine serum albumin (BSA), used as a negative control and for blank subtraction. Increasing concentrations of integrin αvβ3 in 10 mm Tris, pH 7.8, containing 10 nm Mn2+ were injected over the Tat or BSA surfaces in the absence or in the presence of MAA (250 nm) for 4 min and then washed until dissociation. In parallel experiments, increasing concentrations of integrin αvβ3 in 10 mm Tris, pH 7.8, containing 10 nm Mn2+ were incubated for 1 h with neuraminidase (500 milliunits/ml) before injection. Samples containing αvβ3 to which neuraminidase was added only before injection were used as controls, demonstrating that the presence of the enzyme does not interfere significantly with Tat/αvβ3 interaction. After every run, the sensor chip was regenerated by injection of 2.0 m NaCl in 10 mm HEPES, 3 mm EDTA, 150 mm NaCl, 0.005% surfactant P20. The dissociation constant (Kd) of the Tat/αvβ3 interaction was calculated by the Scatchard plot analysis of the steady-state SPR data. (ii) Anti-GST antibody was immobilized on a CM5 surface using standard amine-coupling chemistry allowing the immobilization of 25,000 resonance units, equal to 0.98 pmol. Then, wild type GST-Tat, GST-Tat 1e, and GST-Tat R→A (120 μg/ml in Tris 10 mm pH 7.8 containing 10 nm Mn2+) were injected over the anti-GST surface at a flow rate of 10 μl/min, allowing the immobilization of about 800 resonance units (equal to about 0.023 pmol) for wild type GST-Tat and GST-Tat R→A, and 1,300 resonance units (equal to 0.037 pmol) for GST-Tat 1e and for the GST moiety alone, used for blank subtraction.
Cell Culture
Transformed fetal bovine aortic endothelial GM7373 cells (obtained from the Human Genetic Mutant Cell Repository, Institute for Medical Research, Camden, NJ) (29), were grown in Dulbecco's modified minimum essential medium (DMEM), 10% fetal calf serum (FCS), and antibiotics (Invitrogen, Paisley, UK). Removal of NeuAc from the cell surface was obtained by a 1-h incubation at 37 °C of cells with phosphate-buffered saline (PBS) containing neuraminidase from C. perfringens (from 125 to 500 milliunits/ml) and used for the various assays described below.
Detection of NeuAc on Integrin αvβ3
GM7373 ECs (1 × 106 cells/sample) were treated with neuraminidase (from 125 to 500 milliunits/ml), washed, scraped in 50 μl of 50 mm Tris-HCl, pH 7.4, containing 150 mm NaCl, 1% Nonidet P-40, 0.25% sodium deoxycholate, 1 mm PMSF, 4 mm amino-n-caproic acid, 10 μg/ml leupeptin, 1 mm Na3VO4, 50 mm NaF (radioimmunoprecipitation modified lysis buffer) and centrifuged (10 min at 12,000 rpm). Cell extracts (30-μg aliquots) were analyzed on nonreducing SDS-6% PAGE followed by Western blot (WB) with anti-αv or anti-β3 antibodies. Human purified αvβ3 (100 ng) was incubated for 1 h at 37 °C with neuraminidase (125 milliunits/ml in PBS) and used as control.
For immunoprecipitation analysis, GM7373 EC cultures were lysed in radioimmunoprecipitation modified lysis buffer and centrifuged (10 min at 12,000 rpm). Cell extracts (400 μg) were incubated for 1 h at 25 °C with biotinylated MAA (1 μg/sample) and for an additional 16 h at 4 °C with streptavidin-Sepharose (30 μl/sample), centrifuged (1 min at 3,000 rpm), and analyzed on nonreducing SDS-6% PAGE followed by WB with anti-αv or anti-β3 antibodies. Human purified αvβ3 integrin (250 ng) incubated with biotinylated MAA was used as control.
Cell Adhesion Assay
Adhesion assay was performed with GM7373 ECs on polystyrene nontissue culture microtiter plates coated with Tat, with the α5β1-ligand FN, or with the αvβ3 ligand fibrinogen as described (30).
Immunocytochemistry
GM7373 ECs (40,000/cm2) were allowed to adhere to glass coverslips coated with Tat or FN for 4 h in DMEM containing 1% FCS. Cells were then treated with neuraminidase (125 milliunits/ml), washed, fixed, permeabilized, saturated (27), stained for actin by a 30-min incubation at room temperature with 0.9 μg/ml TRITC-phalloidin in PBS containing 3% BSA (PBS/BSA), co-stained for paxillin by a 1-h incubation at room temperature with anti-paxillin antibody (1:800 in PBS/BSA), further incubated for 45 min at room temperature with FITC-conjugated anti-mouse IgG (1:200 in PBS/BSA), and photographed under an Axioplan 2 microscope equipped for epifluorescence (Carl Zeiss, Gottingen, Germany).
FAK, ERK1/2, and VEGFR2 Phosphorylation Analysis
Confluent GM7373 EC cultures were maintained in serum-free DMEM for 16 h, detached, and resuspended in DMEM 1% FCS. Aliquots of 1,000,000 cells were treated with neuraminidase (125 milliunits/ml), maintained in suspension, or allowed to adhere for 30 min to Tat- or FN-coated 35-mm nontissue culture plates. Alternatively, cells preincubated for 60 min with the lectins were allowed to adhere to Tat- or FN-coated 35-mm nontissue culture plates for a further 30 min. At the end of the incubations, cells were lysed in radioimmunoprecipitation modified lysis buffer and centrifuged (10 min at 13,000 rpm). Twenty μg of protein/sample were analyzed on reducing 8% SDS-PAGE followed by WB with anti-phospho-FAK, anti-phospho-ERK1/2, or anti-phospho-VEGFR2 antibodies. Equal loading of the different lanes was confirmed by WB with anti-α-tubulin antibody. The extent of FAK, ERK1/2, or VEGFR2 phosphorylation was quantified by using the Image Pro-Plus analysis system (Media Cybernetics, Silver Spring, MD). Briefly, the autoradiographies for phosphorylated second messengers or tubulin were digitized on a high resolution monitor and stored within the memory of the Pro-Plus analysis system. The integrated densities of the bands were then calculated, and the values of those corresponding to phosphorylated second messengers were normalized to tubulin levels.
EC Proliferation Assay
GM7373 ECs (25,000/well) were seeded onto Tat- or FN-coated polystyrene nontissue culture microtiter plates and incubated for 4 h in DMEM 1% FCS. Then, cells were treated with neuraminidase (125 milliunits/ml) and further incubated for 24 or 48 h in DMEM, 0.4% FCS containing 5-bromo-2-deoxyuridine in the presence of MAA or UEA (62 nm). Incorporation of 5-bromo-2-deoxyuridine was detected with the colorimetric cell proliferation ELISA kit (Roche Applied Science, Mannheim, Germany).
EC Membrane Ruffling and Wound-healing Assays
Confluent cultures of GM7373 ECs were allowed to adhere to Tat- or FN-coated 35-mm polystyrene nontissue culture plates, treated with neuraminidase (125 milliunits/ml), wounded with a rubber policeman, and incubated with DMEM, 0.4% FCS for 3 or 48 h for membrane ruffling and wound-healing assay, respectively. Alternatively, neuraminidase-untreated ECs were incubated as described above in the presence of MAA or UEA (62 nm). For membrane ruffling assay, at the end of the 3-h incubation, ECs were stained for nuclei (DAPI), immunostained for fascin (by a 1-h incubation with anti-fascin antibody followed by a further 45-min incubation with TRITC-conjugated anti-mouse IgG antibody), and photographed under an Axioplan 2 microscope equipped for epifluorescence (Carl Zeiss). Then, the number of ruffling-positive cells was counted. For the wound-healing assay, at the end of the 48-h incubation, wounded monolayers were photographed under the inverted microscope. The extent of wound repair (due to both EC migration and proliferation (31)) was evaluated by measuring the area of the wound by computerized image analysis using the Image Pro-Plus analysis system (Media Cybernetics).
MTS Assay
Tat-adherent GM7373 ECs were treated with neuraminidase or lectins as described above and then incubated for 24 or 48 h in DMEM, 0.4% FCS. Then, cell viability was assessed by using the CellTiter 96 AQueous kit from Promega (Madison, WI).
Human Artery Ring Sprouting Assay
This assay was performed as already described (32). Briefly, 1-mm-thick human umbilical artery rings were embedded in fibrin gel and cultured in human EC serum-free medium (Invitrogen) in the absence or in the presence of Tat (5 nm) and the lectins MAA or UEA (62 nm). After 6 days, rings were photographed at 100× magnification using an AxioVert 200M microscope equipped with a 20× objective (LD A PLAN 20×/0.30PH1, Zeiss), and EC sprouts were counted.
RESULTS
NeuAc Is Associated with Endothelial αvβ3 Integrin
To evaluate the presence of NeuAc on glycan(s) linked to αvβ3 of the endothelial surface, ECs were lysed, immunoprecipitated with the NeuAc-binding lectin MAA, and blotted with anti-αv or anti-β3 antibodies. As shown in Fig. 1A, MAA binds to both αv and β3 subunits, similarly to the αv subunit from human placenta integrin. In a second set of experiments, ECs were treated with neuraminidase (500 milliunits/ml), lysed, and analyzed by WB with anti-αv or anti-β3 antibodies. Neuraminidase treatment causes a decrease of the molecular mass of both αv (from 134 to 126 kDa) and β3 (from 70 to 67 kDa). Similar results were obtained with αvβ3 purified from human placenta (Fig. 1B). The effect of neuraminidase is dose-dependent, with a partial removal of NeuAc residues obtained for concentrations of the enzyme lower than 500 milliunits/ml (Fig. 1C). After its enzymatic removal, NeuAc is fully re-exposed on endothelial αvβ3 only after 48 h (Fig. 1D).
FIGURE 1.
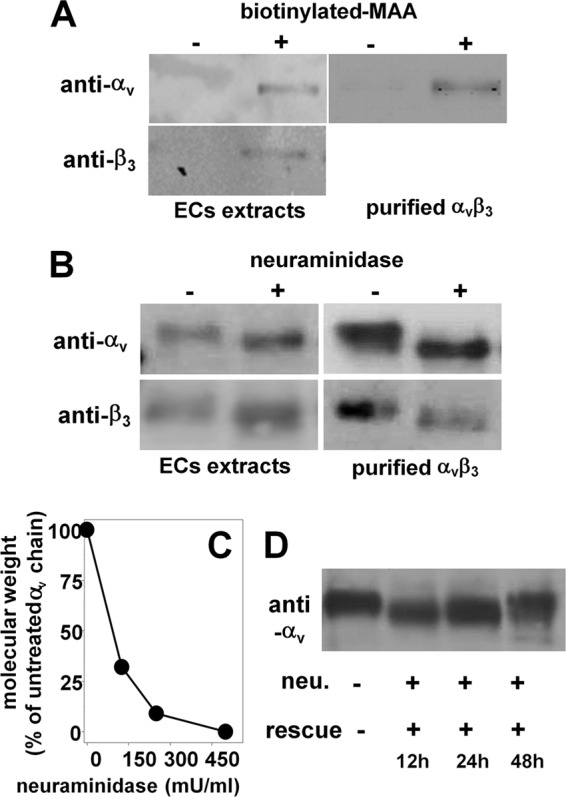
Detection of NeuAc on integrin αvβ3. A, GM7373 ECs or purified human integrin αvβ3 were immunoprecipitated with biotinylated MAA and analyzed in WB with anti-αv or anti-β3 antibodies. B and C, alternatively, they were incubated with 500 milliunits/ml (mU/ml) (B) or with the indicated concentrations (C) of neuraminidase, lysed, and analyzed in WB with anti-αv or anti-β3 antibodies. D, GM7373 ECs were treated with neuraminidase (neu., 500 milliunits/ml), washed, further incubated for the indicated periods of time in the absence of the enzyme (rescue), lysed, and analyzed in WB with anti-αv antibodies. In panels A, B, and D, the data shown are representative of 2–3 other experiments that gave similar results. In C, data are expressed as the percentage of the molecular mass of the αv subunit from neuraminidase-treated ECs with respect to the intact protein from untreated ECs.
NeuAc Is Involved in αvβ3/Tat Interaction
Tat binds to αvβ3 (25). The contribution of the RGD motif and of the basic domain to αvβ3 binding and cell-adhesive capacity was here characterized by two experimental approaches. First, the mutants GST-Tat 1e (in which the RGD sequence has been deleted) and GST-Tat R→A (in which the arginine residues of the basic domain have been substituted with alanine residues) were evaluated for their αvβ3 binding capacity in SPR. As shown in Fig. 2A and Table 1, the two mutants retain the capacity to bind to αvβ3, although decreased in respect to wild type GST-Tat. Second, a cell adhesion assay was performed in the presence of the peptide GRGDSPK (which competes with the RGD motif of Tat for the binding to αvβ3) or in the presence of the K5 derivative K5NOSH (which inhibits EC adhesion to Tat (33) by binding to the basic domain of the transactivating factor). As shown in Fig. 2B, when assayed at the doses of 12 μm (GRGDSPK) and 75 nm (K5NOSH), the two compounds slightly inhibit EC adhesion to Tat, but when assayed together, they completely inhibit the same process. Taken together, these data indicate that both the RGD and the basic domain of Tat contribute to its cell-adhesive capacity, the presence of one of the two domains being enough to ensure a partial αvβ3 binding and cell-adhesive capacity to Tat.
FIGURE 2.
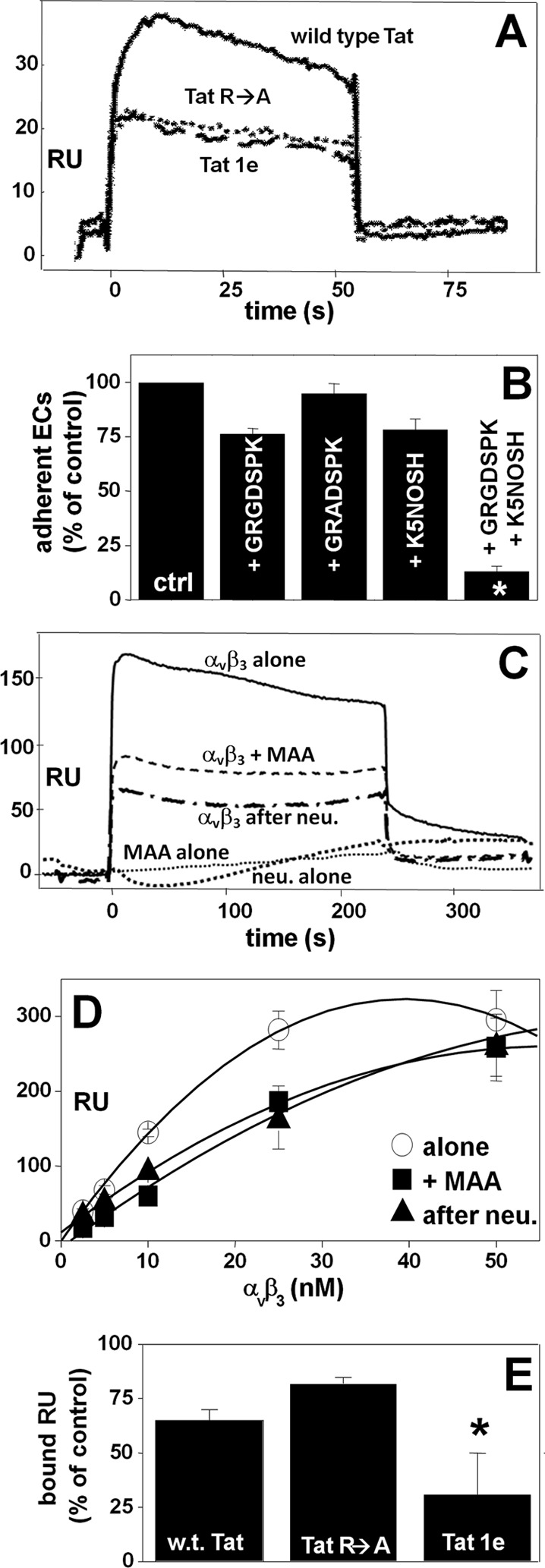
Role of αvβ3-associated NeuAc in Tat interaction. A, overlay of blank-subtracted sensorgrams showing the binding of αvβ3 (10 nm) to sensor chip-immobilized wild type GST-Tat or its mutants GST-Tat 1e or GST-Tat R→A. B, GM7373 ECs were allowed to adhere to Tat in the absence (ctrl) or in the presence of the indicated inhibitors. C, overlay of blank-subtracted sensorgrams showing the binding of native or neuraminidase-treated (neu.) αvβ3 (12.5 nm) to sensor chip-immobilized synthetic Tat in the absence or in the presence of MAA (250 nm). The sensorgrams generated by injecting MAA (250 nm) or neuraminidase (500 milliunits/ml) on the Tat surface are also shown. D, saturation curves obtained using the values of resonance units (RU) bound at equilibrium from injection of increasing concentrations of native or neuraminidase-treated αvβ3 onto immobilized synthetic Tat in the absence or in the presence of MAA (250 nm). E, αvβ3 (50 nm) was injected onto the sensor chip containing the indicated GST-Tat proteins in the absence or in the presence of MAA (250 nm). In A and C, the sensorgrams shown are representative of three others that gave similar results. In D, each point is the mean ± S.E. of 3 independent injections. In B and E, each point is the mean ± S.E. of 3 independent experiments in duplicate and is expressed as the percentage of ECs adherent to Tat in the absence of any inhibitor or of αvβ3 bound to the sensor chip at the equilibrium in the absence of MAA, respectively (* = p < 0.05, Student's t test).
TABLE 1.
Affinity of the interaction of αvβ3 to the various Tat mutants immobilized to a BIAcore sensor chip
The values of dissociation constant (Kd) reported have been calculated by the Scatchard plot analysis of the steady-state SPR data in the different experimental conditions adopted. For a comparison, the Kd of the Tat/heparin interaction previously calculated by Scatchard plot analysis of the steady-state SPR (33) is also reported.
| Ligand | Analyte | Kd |
|---|---|---|
| nm | ||
| Synthetic Tat | Native αvβ3 | 19.9 |
| αvβ3 after neuraminidase treatment | 157.1 | |
| αvβ3 + MAA | 49.5 | |
| GST-Tat wild type | Native αvβ3 | 40.3 |
| GST-Tat R→A | 102.6 | |
| GST-Tat 1e | 136.2 | |
| GST-Tat wild type | Heparin | 16.0 |
Besides αvβ3, the basic domain also mediates the binding of Tat to the polyanionic heparin/heparan sulfate (28), the two interactions occurring with similar affinities (Table 1). These observations suggest that the basic domain of Tat, besides interacting with the negatively charged sulfated groups of heparin, may as well make contact with the negatively charged NeuAc residues of αvβ3. To evaluate this possibility, two different experimental approaches were exploited. (i) We first evaluated the effect of neuraminidase and MAA on αvβ3/Tat interaction. Preliminary SPR analyses demonstrated that neuraminidase treatment of the integrin, as well as the presence of MAA, significantly inhibits its interaction with Tat. It is important to note that MAA and neuraminidase do not bind directly to Tat (Fig. 2C). In a second set of experiments, increasing concentrations of native or neuraminidase-treated αvβ3 were injected onto the Tat surface in the absence or in the presence of MAA. Then, the values of steady-state SPR data were used to generate the saturation curves shown in Fig. 2D. Scatchard plot analysis demonstrated that neuraminidase treatment, as well as MAA, decreases the affinity of the αvβ3/Tat interaction (Table 1). Interestingly, the reduction of the affinity observed for αvβ3/Tat interaction in the absence or in the presence of MAA (2.48 times) is in the same order of magnitude as the difference of the affinity measured for the interaction of αvβ3 with wild type GST-Tat or with GST-Tat R→A (2.53 times) (Table 1), suggesting that MAA hampers the interaction of NeuAc residues of αvβ3 to the basic domain of Tat. (ii) The two Tat mutants GST-Tat 1e and GST-Tat R→A where evaluated for their capacity to induce EC adhesion in the presence of MAA. Similarly to what was observed with synthetic Tat, at 250 nm, MAA partially inhibits the binding of αvβ3 to wild type GST-Tat (Fig. 2E). At the same concentration, MAA exerts a weak inhibition on αvβ3/GST-Tat R→A interaction (possibly because this occurs only via the RGD sequence), whereas it efficiently inhibits αvβ3/GST-Tat 1e interaction (possibly because this can occur only via the basic domain) (Fig. 2E). Taken together, these data indicate that at low concentrations, MAA binds to NeuAc residues of αvβ3, inhibiting the interaction of the integrin with the basic domain of Tat but leaving unaffected that with the RGD motif.
NeuAc Mediates αvβ3-dependent EC Adhesion to Substrate-immobilized Tat
Substrate-immobilized Tat induces EC adhesion in an αvβ3-dependent manner (25). Accordingly, by using specific anti-αvβ3 or anti-VEGFR2 antibodies, here we confirmed that EC adhesion to Tat specifically depends on the integrin but not on VEGFR2 (Fig. 3A). Also, our unpublished experiments with silencing RNAs directed against enzymes of the biosynthetic pathway of heparan sulfate demonstrated that these receptors are not involved in EC adhesion to Tat (data not shown).
FIGURE 3.
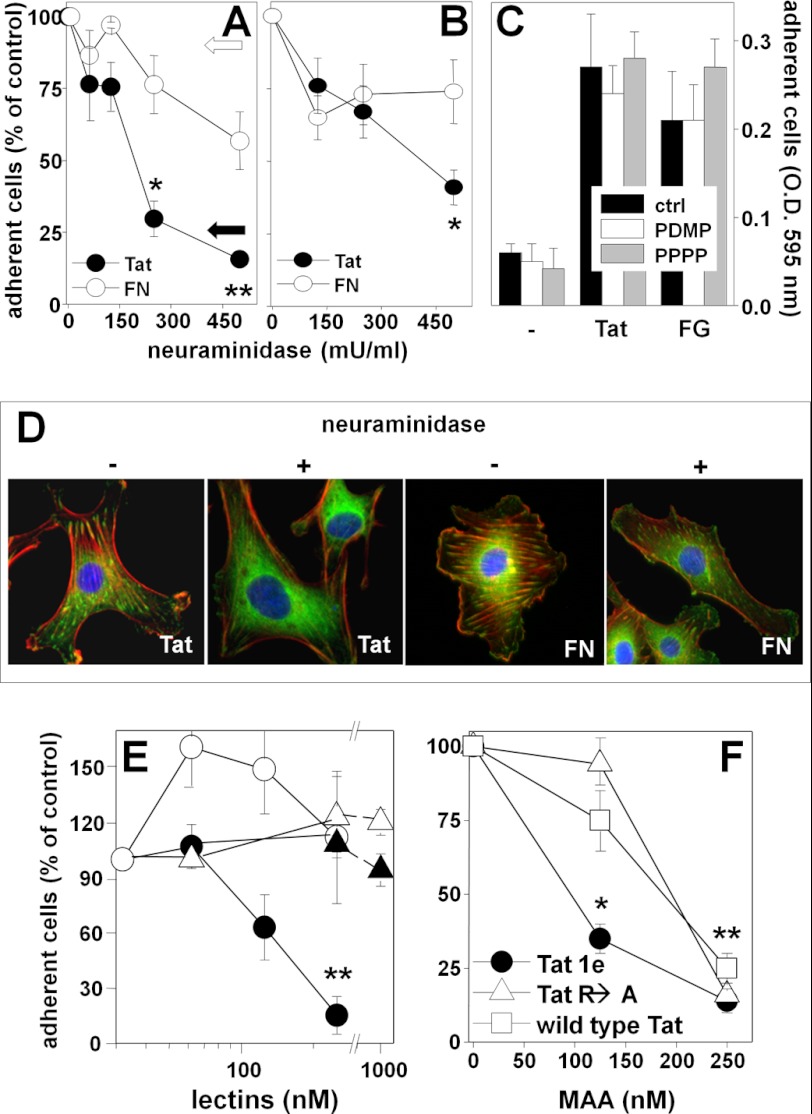
Role of NeuAc in αvβ3-dependent EC adhesion to Tat and cytoskeleton organization. A, GM7373 ECs were treated with the indicated concentrations of neuraminidase and subjected to cell adhesion assay on Tat or FN. Alternatively, cells were subjected to adhesion assay in the presence of anti-VEGFR2 antibody (400 μg/ml, white arrow) or with anti-αvβ3 antibody (100 μg/ml, black arrow). B, GM7373 ECs were allowed to adhere to Tat or FN, incubated for 1 h with increasing concentrations of neuraminidase, and then further incubated in the absence of the enzyme for 24 h. C, GM7373 ECs were treated with PDMP (10 μm for 72 h), PPPP (1 μm for 48 h), or vehicle (ctrl) and subjected to cell adhesion assay on wells without coating (−) or coated with Tat or fibrinogen (FG). At the end of the incubations, adherent cells were counted. D, GM7373 ECs were treated with neuraminidase, allowed to adhere to Tat or FN, co-stained for nuclei (blue), paxillin (green), and actin (red), and photographed (630×). E, GM7373 ECs were incubated for 2 h at 37 °C with increasing concentrations of MAA (circles) or UEA (triangles) and then allowed to adhere to Tat (black symbols) or FN (white symbols). F, GM7373 ECs were incubated for 2 h at 37 °C with increasing concentrations of MAA and allowed to adhere onto the indicated GST-Tat proteins. In panels A–C, E, and F, each point is the mean ± S.E. of 3–4 independent experiments in duplicate (* = p < 0.05, ** = p < 0.01, with respect to untreated controls, Student's t test).
The removal of NeuAc from the EC surface by neuraminidase prevents EC adhesion to Tat, only slightly affecting that to the α5β1-ligand FN, here used as a control (Fig. 3A). Neuraminidase also causes the detachment of ECs already adhered to Tat over a 24-h period of incubation (Fig. 3B), without causing significant cell death (as assessed by MTS assay, data not shown).
Besides integrins, NeuAc is also associated with gangliosides. To evaluate the possible involvement of these structures in EC adhesion to Tat, the cells were treated with PDMP or PPPP (which prevent ganglioside biosynthesis without affecting intracellular levels of ceramide (34)) and then evaluated for their capacity to adhere to Tat. When used at doses that effectively hamper the expression of NeuAc-bearing gangliosides (34), PDMP and PPPP do not affect ECs adhesion to Tat or to fibrinogen (another αvβ3 ligand here used as a control, Fig. 3C). These results rule out the possibility that ganglioside-associated NeuAc is responsible for the observed EC adhesion to Tat.
EC adhesion to Tat induces cytoskeleton organization with the assembly of actin stress fibers and focal adhesion plaques containing integrins and paxillin (35). We then evaluated the involvement of NeuAc in cytoskeleton organization of Tat-adherent ECs. When tested at concentrations that do not hamper EC adhesion (125 milliunits/ml), neuraminidase prevents the proper organization of actin stress fibers and paxillin-containing focal adhesion plaques. The effect is specific because neuraminidase did not alter focal adhesion plaque formation in FN-adherent ECs (Fig. 3D).
In a second set of experiments, we also evaluated whether MAA affects αvβ3-dependent EC adhesion to Tat. As shown in Fig. 3E, MAA inhibits EC adhesion to Tat in a dose-dependent way. The inhibition is specific because MAA does not affect EC adhesion to FN and UEA (a lectin that specifically binds to α-linked fucose) does not affect EC adhesion to Tat. In agreement with the results of the SPR analyses, MAA inhibits EC adhesion to GST-Tat 1e with a potency (ID50 < 125 nm) that is higher than those with which it inhibits EC adhesion to GST-Tat R→A and to wild type GST-Tat (ID50 = 198 and 182, respectively) (Fig. 3F).
NeuAc Is Required for Signal Transduction Triggered by Tat/αvβ3 Interaction in ECs
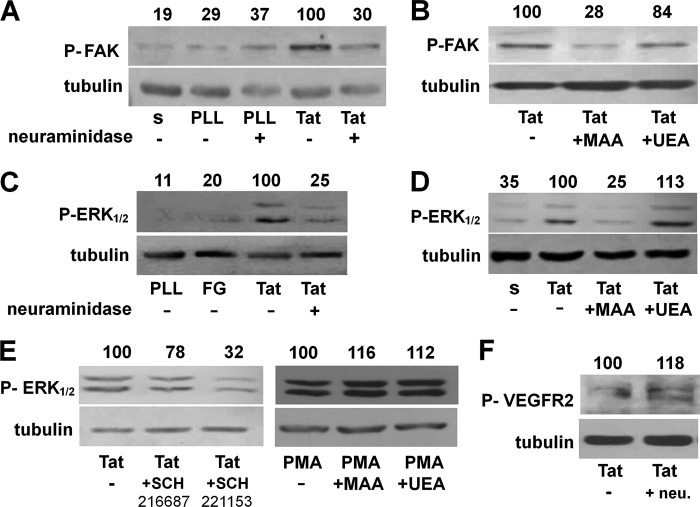
We evaluated the role of NeuAc in Tat/αvβ3-dependent phosphorylation of FAK, a second messenger involved in Tat/αvβ3-dependent EC cytoskeleton organization and proangiogenic activation (25, 36). Preliminary experiment confirmed that FAK undergoes phosphorylation in ECs adherent to Tat but not in ECs maintained in suspension or adherent to poly-l-lysine (an integrin-independent adhesive molecule). When used at 125 milliunits/ml (a dose that prevents cytoskeleton organization without hampering EC adhesion to Tat; Fig. 3), neuraminidase inhibits FAK phosphorylation in Tat-adherent ECs (Fig. 4A). Accordingly, at 62 nm, MAA, but not UEA, prevents FAK phosphorylation (Fig. 4B). It is relevant to note that at 62 nm, MAA does not hamper EC adhesion to Tat (Fig. 3E). Besides FAK, EC adhesion to Tat induces the activation of ERK1/2, another second messenger involved in Tat-dependent EC proangiogenic activation (37). Neuraminidase pretreatment (Fig. 4C) and MAA, but not UEA (Fig. 4D), prevent ERK1/2 phosphorylation in Tat-adherent EC.
FIGURE 4.
Role of NeuAc in Tat/αvβ3-triggered signal transduction in ECs. A–E, serum-starved GM7373 ECs were allowed to adhere to the indicated proteins in the presence of MAA or UEA (62 nm) or treated with neuraminidase (neu., 125 milliunits/ml) and then maintained in suspension (s) for 30 min or allowed to adhere for 1 h to the indicated proteins. Alternatively, cells were allowed to adhere to Tat and incubated in the presence of the αvβ3 antagonist SCH221153 or of its inactive analog SCH216687 (0.3 μm) or allowed to adhere to tissue culture plates and treated with free phorbol myristate acetate (PMA, 10 ng/ml) in the presence of MAA or UEA (62 nm) (E). At the end of the incubations, cells were lysed and analyzed for FAK (A and B), ERK1/2 (C–E), or VEGFR2 (F) phosphorylation (indicated by P). Tubulin was used as a loading control. The integrated densities of the bands were evaluated, normalized to tubulin, and expressed as the percentage with respect to the bands from EC adherent to Tat in the absence of any inhibitor. The data shown are representative of 2–3 additional experiments that gave similar results. PLL, poly-l-lysine.
In the same experimental conditions, ERK1/2 phosphorylation is inhibited by the specific αvβ3 antagonist SCH221153 but not by its inactive analog SCH21668, indicating that ERK1/2 activation is dependent, at least in part, on αvβ3. Also, MAA does not affect ERK1/2 phosphorylation induced by phorbol myristate acetate in EC adherent to tissue culture plastic (Fig. 4E), suggesting that the specificity of the inhibitory effect exerted by MAA is likely due to its interaction with αvβ3, which prevents the binding of the integrin to Tat (Fig. 2).
Besides integrin αvβ3, the proangiogenic activity of Tat is also mediated by VEGFR2 activation (38). Interestingly, this receptor bears NeuAc residues (39). On these bases, we evaluated the effect of the removal of NeuAc residues of VEGFR2 on its phosphorylation driven by Tat. As shown in Fig. 4F, neuraminidase effectively removes NeuAc from VEGFR2, as assessed by the decrease of the molecular mass of the band corresponding to the receptor. However, neuraminidase treatment does not hamper VEGFR2 phosphorylation in response to Tat, which is instead even increased (Fig. 4F).
EC NeuAc Is Involved in αvβ3-dependent Proangiogenic Activity of Tat
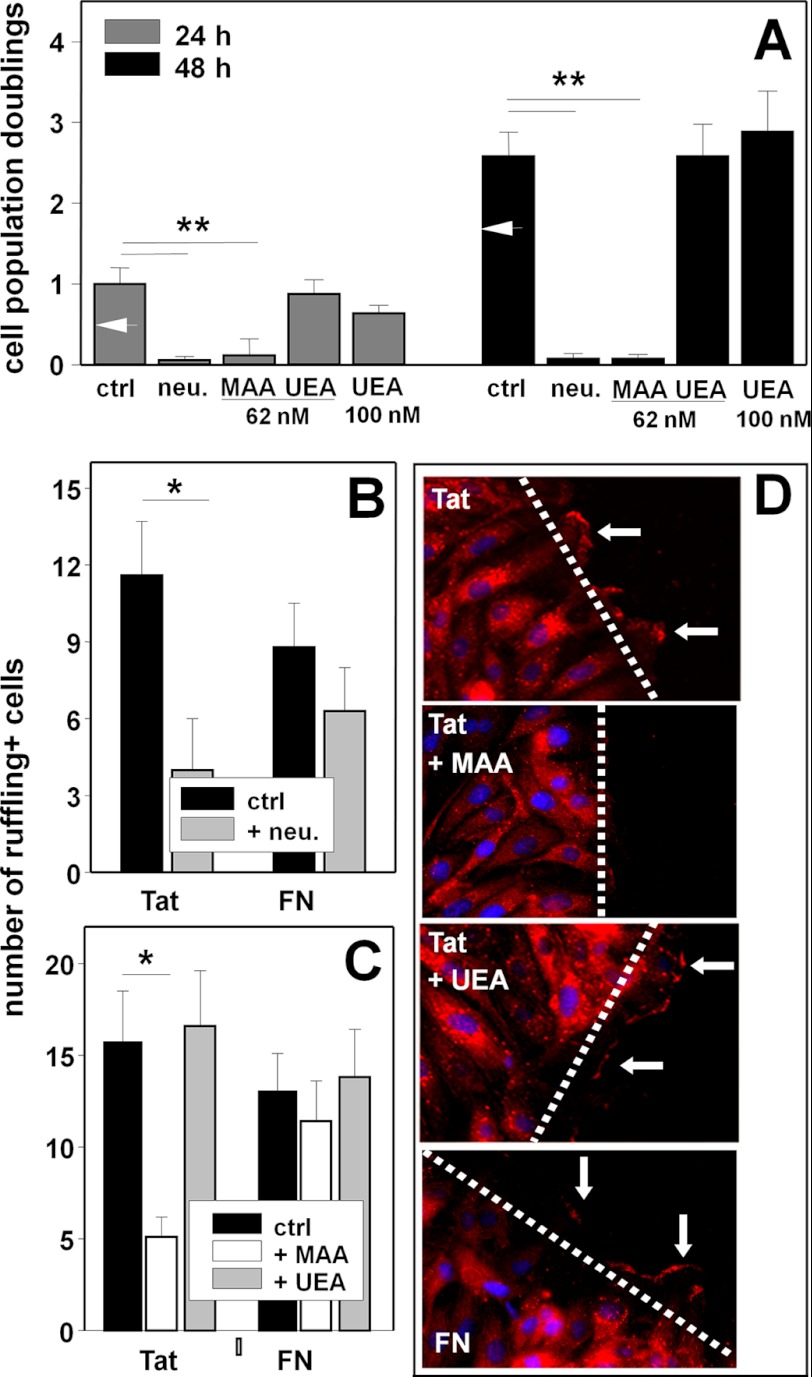
EC adhesion, proliferation, and migration represent essential steps of neovascularization (40). Accordingly, ECs adherent to substrate-immobilized Tat are induced to migrate and proliferate in an αvβ3-dependent way (27). Preliminary experiments confirmed that Tat-adherent ECs proliferate more efficiently than those adherent to FN both at 24 h and at 48 h (Fig. 5A). Pretreatment with neuraminidase (125 milliunits/ml) inhibits the proliferation of Tat-adherent cells in a specific way because it leaves unaffected the basal proliferation of FN-adherent ECs or of ECs adherent on tissue culture plates (data not shown). Besides neuraminidase, MAA (62 nm), but not UEA (assayed at both 62 nm and 100 nm), inhibits the proliferation of Tat-adherent ECs (Fig. 5A).
FIGURE 5.
Role of NeuAc in Tat/αvβ3-dependent proliferation and migration of ECs. GM7373 ECs adherent to the indicated proteins were treated as follows. A, cells were treated with neuraminidase (neu., 125 milliunits/ml) and incubated in the absence of the enzyme for an additional 24 or 48 h. Alternatively, cells were directly incubated for 24 or 48 h with the indicated concentrations of MAA or UEA. B, cells were incubated in the absence (ctrl) or in the presence of neuraminidase (125 milliunits/ml), wounded, and further incubated for 30 min. C, cells were wounded and incubated for 30 min in the absence (ctrl) or in the presence of MAA or UEA (62 nm). At the end of the incubations, EC proliferation (A) or the number of ruffling-positive ECs (B and C) were evaluated. Each point is the mean ± S.E. of 3–4 independent experiments in duplicate (* = p < 0.01, ** = p < 0.001, Student's t test). In panel A, white arrowheads point to the proliferation measured in cells adherent to FN. D, representative epifluorescence microphotographs (630×) of ECs at the edge of a wounded monolayer incubated in the absence (ctrl) or in the presence of MAA or UEA (62 nm) and stained with DAPI (blue) and with anti-fascin antibody (red). Arrows point to the most prominent ruffles.
Cell membrane ruffling, which precedes the migration of EC body, is considered a morphological phenotype of motile cells (41) and has been already exploited to characterize the migration of Tat-adherent ECs (27). As shown in Fig. 5D, when adherent to Tat or to FN, ECs rapidly form membrane ruffles at the edge of a wounded monolayer. Pretreatment with neuraminidase (125 milliunits/ml) inhibits membrane ruffling in Tat-adherent ECs and, to a lesser extent, in FN-adherent ECs (Fig. 5B). Accordingly, MAA (62 nm), but not UEA, inhibits membrane ruffling only in Tat- but not FN-adherent ECs (Fig. 5C).
The ability of a substrate-immobilized protein to stimulate proliferation and motility in adherent ECs leads to an increased capacity of a mechanically wounded EC monolayer to cover the denuded area, a biological activity referred to as “motogenic activity” that has been used as a surrogate marker of angiogenesis (31). Substrate-immobilized Tat induces motogenesis of adherent ECs in an αvβ3-dependent way (25).
When allowed to adhere to Tat, treated with neuraminidase (250 milliunits/ml), and wounded, ECs show a decreased motogenic activity. The effect is specific because, in the same experimental conditions, neuraminidase does not affect the motogenic activity of FN-adherent ECs (Fig. 6A). In this regard, it is important to recall that when added after EC adhesion has occurred, neuraminidase at 250 nm does not cause the detachment of Tat-adherent ECs (Fig. 3B) nor affect their viability over a 48-h period of time (data not shown). MAA (62 nm) inhibits the motogenic activity of Tat. The effect is specific because MAA does not affect viability of Tat-adherent ECs (data not shown) nor the motogenic activity of FN-adherent ECs (Fig. 6, B and C). Also, UEA is ineffective on Tat-adherent ECs (Fig. 6, B and C).
FIGURE 6.
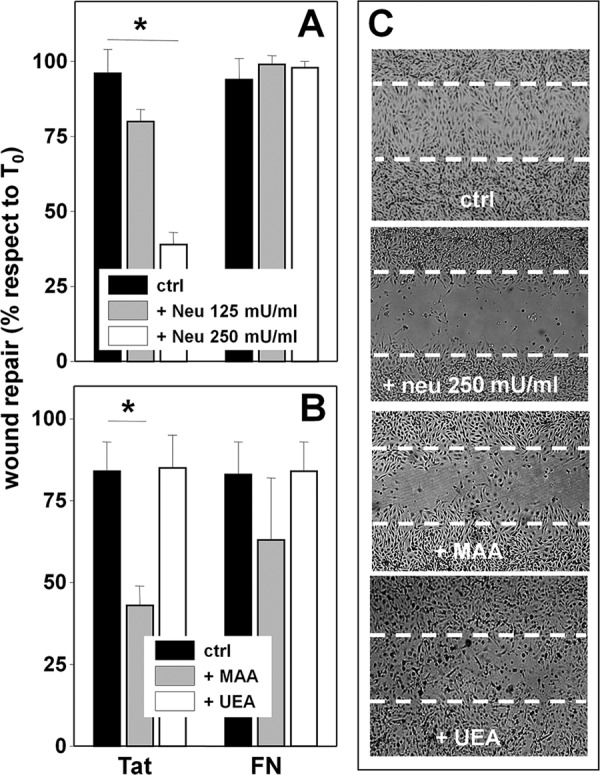
Role of NeuAc in Tat/αvβ3-dependent motogenesis of ECs. A, GM7373 ECs adherent to the indicated proteins were left untreated (ctrl) or treated with neuraminidase (neu) at 125 or 250 milliunits/ml, wounded, and further incubated for 48 h. B, GM7373 ECs adherent to the indicated proteins were wounded and incubated for 48 h in the absence (ctrl) or in the presence of MAA or UEA (62 nm). At the end of the incubations, the extension of the area of the wound repaired was evaluated. Each point is the mean ± S.E. of 3–5 fields measured in one experiment out of 2–3 that gave similar results (* = p < 0.05, Student's t test). C, microphotographs (50×) of wounded monolayers of Tat-adherent GM7373 ECs taken at the end of the 48-h period of incubation with the indicated treatments. Dashed lines mark the edge of the wound at the beginning of the experiment.
Tat induces neovascularization via αvβ3 (27). We then decided to evaluate the involvement of NeuAc in Tat proangiogenic activity by exploiting the human artery ring sprouting assay, an ex vivo model used to characterize the pro- or antiangiogenic properties of various angiogenic growth factors and inhibitors (32). As shown in Fig. 7, Tat effectively induces an increase of the number of EC sprouts that generate from a human artery ring, and this activity can be inhibited by MAA, but not by UEA.
FIGURE 7.
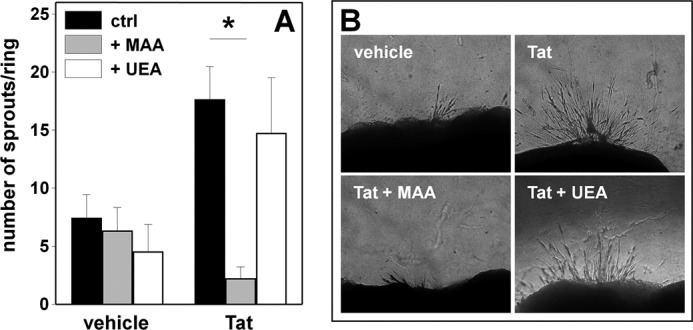
Effect of MAA on Tat-induced neovascularization. A, human umbilical artery rings were embedded in fibrin gel and incubated without (vehicle) or with Tat in the absence (ctrl) or in the presence of MAA or UEA. After 6 days, EC sprouts were counted. Each point is the mean ± S.E. of 10–29 artery rings randomly chosen (* = p < 0.001, Student's t test). B, representative aorta rings in the different experimental conditions photographed under an inverted microscope at 200× magnification.
DISCUSSION
αvβ3 mediates EC adhesion and proangiogenic activation by binding cationic angiogenic growth factors including FGF2 and HIV-1 Tat (27, 30). On the other hand, NeuAc has so far been identified on αvβ3 of melanoma metastatic cell surface, where it regulates cell adhesion (18, 20). We thus decided to investigate whether NeuAc is also associated with αvβ3 integrin of ECs and whether it is involved in αvβ3/Tat interaction and in the consequent proangiogenic activation of ECs. These possibilities were investigated by two complementary approaches, consisting of the use of neuraminidase from C. perfringens (an enzyme that removes NeuAc from the cell surface) and of MAA (a lectin that specifically binds NeuAc residues).
In ECs, the removal of αvβ3-associated NeuAc by neuraminidase is rapid and efficient, occurring in less than 2 h. The biochemical features of bacterial neuraminidase surely contribute to this (43), but so does the rapid recycling of integrins that are classically internalized to the early endosomes to be immediately returned to the plasma membrane (44, 45). In this way, endogenous αvβ3 remains continuously exposed at the cell surface, accessible to exogenous neuraminidase.
Fully sialylated αvβ3 integrins are re-exposed on ECs only 48 h after neuraminidase treatment. The long-lasting nature of αvβ3 desialylation is likely due to the fact that after de novo synthesis, integrins must undergo sialylation by sialyltransferases in the Golgi apparatus (46). Whatever its cause, the long-lasting desialylation of αvβ3 by neuraminidase is in agreement with the capacity of the enzyme to inhibit processes, such as EC proliferation and motogenesis, that occur over 24–48-h periods.
MAA immunoprecipitates both αv and β3 subunits from ECs, indicating the presence of α(2→3)-linked NeuAc on the two chains. We also obtained similar results with the lectin from Sambucus nigra that binds instead to α(2→6)-linked NeuAc (data not shown). Accordingly, neuraminidase from C. perfringens, which hydrolyzes both α((2→3)-linked and α((2→6)-linked NeuAc, causes a decrease of the molecular mass of both αv subunit (−7 kDa, corresponding to about 25 NeuAc residues) and β3 subunit (−3 kDa, corresponding to about 10 NeuAc residues). These data are in agreement with the observations that αv and β3 subunits possess 13 and 6 putative N-glycosylation sites, respectively, with biantennary structures (UniProt accession number P05106) (18).
Neuraminidase cleaves α(2→3)-linked NeuAc residues faster than α((2→6) linkages (47). Accordingly, neuraminidase operates a complete removal of αvβ3-associated NeuAc only when used at high concentration (>250 milliunits/ml), whereas when used at suboptimal concentrations (125 milliunits/ml), desialylation of αvβ3 remains incomplete. Interestingly, when used at higher concentrations, neuraminidase directly prevents (and even disrupts) αvβ3-dependent EC adhesion to Tat (Fig. 3, A and B), whereas at lower concentrations, it leaves EC adhesion unaffected, although inhibiting Tat/αvβ3-dependent proangiogenic activation of Tat-adherent ECs. Taken together, these data suggest that the complete removal of αvβ3-associated NeuAc prevents the binding of the integrin to Tat and the consequent EC adhesion. Instead, a partial removal of NeuAc residues allows an “unproductive” Tat/αvβ3 interaction enough for cell adhesion, but that does not mediate the signal transduction cascade required for EC proangiogenic activation.
By removing NeuAc residues from αvβ3, neuraminidase causes a decrease of the affinity of αvβ3/Tat interaction and its inhibition (Fig. 2). Accordingly, by binding NeuAc residues of αvβ3, MAA causes the same effects, also if at a lesser extent. The inhibition exerted by neuraminidase and MAA on αvβ3/Tat interaction is mirrored by their capacity to inhibit several αvβ3-dependent biological activities of Tat, connecting the two processes. In effect, MAA shares with neuraminidase the capacity to differently affect Tat/αvβ3 interaction and consequent biological activities in a concentration-dependent way. At higher concentrations (125–250 nm), it directly inhibits αvβ3-mediated EC adhesion to Tat, whereas at a lower concentration (less than 62 nm), it leaves cell adhesion unaffected, although inhibiting the αvβ3-dependent signal transduction and proangiogenic activation of Tat-adherent ECs. Interestingly, MAA scarcely affects αvβ3 interaction with (and EC adhesion to) GST-Tat R→A (in which the intact RGD motif mediates integrin interaction and EC adhesion), whereas it exerts a stronger inhibition on GST-Tat 1e that, lacking the RGD motif, binds to integrin and mediates EC adhesion via its basic domain. These findings, together with the notion that αvβ3 interaction occurs via both the RGD motif and the basic domain of Tat (25) (Fig. 2B), suggest that at appropriate concentrations, MAA succeeds in inhibiting the interaction of the basic domain of Tat with NeuAc residues of αvβ3, leaving unaffected the RGD-dependent αvβ3/Tat interaction. This generates an unproductive Tat/αvβ3 interaction enough to promote cell adhesion but not adequate to trigger signal transduction and hence EC proangiogenic activation.
Besides integrin αvβ3, a wide variety of cell surface sialoglycoproteins can be affected by neuraminidase and MAA, possibly impacting the phenomena reported here. Although we cannot completely rule out this possibility, it is, however, relevant to point out that neuraminidase does not significantly affect (or even increase) Tat-driven phosphorylation of VEGFR2, a tyrosine kinase receptor that bears NeuAc residues (39) and whose activation is required for Tat proangiogenic activity (38). Curiously, the proangiogenic fibroblast growth factor (FGF) receptor-1 also possesses NeuAc-bearing glycans whose removal leads to an increased capacity to bind its ligand FGF2 (48). Also, hypo- or desialylation of α5β1 integrin increases its affinity for the natural ligand FN (49). Relevant to this point, FN acts as a proangiogenic factor (50), and α5β1 can act as a Tat receptor on ECs (26). Taken together, these results indicate that although neuraminidase and MAA can act on sialoglycoproteins different from αvβ3, the overall inhibitory effect exerted on the Tat proangiogenic potential depends on their action on NeuAc linked to αvβ3.
The results presented in this work open new fields of research, some of which have been totally unexplored so far. First, mammals have four types of endogenous sialidases, among which the plasma membrane-associated sialidase NEU3 and the secreted form of NEU2 (51) could be involved in the fine-tuning of the acidic sugar on the cell surface. On the other hand, sialidases are expressed by ECs (52), and neutrophil-derived endogenous sialidase(s) have already been demonstrated to induce desialylation of the EC surface with implications in the process of inflammation (53). Taken together, these observations point to a possible role of neuraminidases as regulators of the process of neovascularization. Second, besides Tat, VEGF (54) and FGF2 (14, 15) also bind to integrins and to NeuAc-bearing gangliosides (55), suggesting a broader involvement of integrin-associated NeuAc in angiogenesis. Third, sialyltransferases are a family of enzymes located in the Golgi apparatus that mediate sialylation of various glycoconjugates (56) including integrins (46). Also, they are expressed by ECs (57). Thus, besides neuraminidases, sialyltransferases may also be involved in the regulation of angiogenesis. Fourth, integrin αvβ3 is a target for antitumor therapies based on antibodies or RGD-based compounds that prevent the interaction of the integrin with its proangiogenic ligands (58). The αvβ3 antagonist activity of MAA points to NeuAc-binding lectins as templates for the design of novel integrin antagonists endowed with antiangiogenic potential. Fifth, in the field of virology, integrins act as entry receptors for several viruses (59), suggesting the possibility of blocking viral infection by means of NeuAc-binding lectin-like compounds or by NeuAc analogues. Relevant to this point, the NeuAc derivative NMSO3 has been demonstrated to exert a potent inhibition against HIV-1 (42).
In conclusion, the results presented in this study open up the possibility that modulation of integrin glycosylation could be a promising strategy for regulating angiogenesis and viral infection.
Acknowledgments
We thank Mauro Giacca (International Centre for Genetic Engineering and Biotechnology, Trieste, Italy) for the E. coli strain expressing the various GST-Tat mutants, Pasqua Oreste for the K5NOSH, Alessandra Armato for technical assistance, and Eugenio Monti and Marco Presta (University of Brescia) for helpful discussion.
This work was supported by grants from Ministero dell'Istruzione, dell'Università e della Ricerca, Istituto Superiore di Sanità (AIDS Project), and Cassa di Risparmio delle Provincie Lombarde (to M. R.).
- EC
- endothelial cell
- FAK
- focal adhesion kinase
- FN
- fibronectin
- MAA
- lectin from M. amurensis
- UEA
- lectin from U. europaeus
- PDMP
- d-threo-1-phenyl-2-decanoylamino-3-morpholino-1-propanol
- PPPP
- d-1-threo-1-phenyl-2-hexadecanoylamino-3-pyrrolidino-1-propanol-HCl
- SPR
- surface plasmon resonance
- VEGFR2
- vascular endothelial growth factor receptor-2
- WB
- Western blot
- TRITC
- tetramethylrhodamine isothiocyanate
- MTS
- 3-(4,5-dimethylthiazol-2-yl)-5-(3-carboxymethoxyphenyl)-2-(4-sulfophenyl)-2H-tetrazolium, inner salt.
REFERENCES
- 1. Urbinati C., Chiodelli P., Rusnati M. (2008) Polyanionic drugs and viral oncogenesis: a novel approach to control infection, tumor-associated inflammation, and angiogenesis. Molecules 13, 2758–2785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2. dos Santos W. L., Rahman J., Klein N., Male D. K. (1995) Distribution and analysis of surface charge on brain endothelium in vitro and in situ. Acta Neuropathol. 90, 305–311 [DOI] [PubMed] [Google Scholar]
- 3. Vorbrodt A. W. (1989) Ultracytochemical characterization of anionic sites in the wall of brain capillaries. J. Neurocytol. 18, 359–368 [DOI] [PubMed] [Google Scholar]
- 4. Traving C., Schauer R. (1998) Structure, function, and metabolism of sialic acids. Cell. Mol. Life Sci. 54, 1330–1349 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5. Schauer R. (2009) Sialic acids as regulators of molecular and cellular interactions. Curr. Opin. Struct. Biol. 19, 507–514 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Murea̧n V., Simionescu N. (1987) High and low molecular weight tracers for the electron microscopical detection of sialoglycoconjugates. Histochem. J. 19, 170–178 [DOI] [PubMed] [Google Scholar]
- 7. Welim H. B., Thies M., Herken R. (1989) Appearance of lectin-binding sites during vascularization of the primordium of the central nervous system in 10–12-day-old mouse embryos. Cell Tissue Res. 255, 627–630 [DOI] [PubMed] [Google Scholar]
- 8. Henry C. B., DeFouw D. O. (1996) Distribution of anionic sites on microvascular endothelium of the chick chorioallantoic membrane. Tissue Cell 28, 449–454 [DOI] [PubMed] [Google Scholar]
- 9. Doiron A. L., Kirkpatrick A. P., Rinker K. D. (2004) TGF-β and TNF-α affect cell surface proteoglycan and sialic acid expression on vascular endothelial cells. Biomed. Sci. Instrum. 40, 331–336 [PubMed] [Google Scholar]
- 10. Karlsson K. A. (1991) Glycobiology: a growing field for drug design. Trends Pharmacol. Sci. 12, 265–272 [DOI] [PubMed] [Google Scholar]
- 11. Banks W. A., Robinson S. M., Wolf K. M., Bess J. W., Jr., Arthur L. O. (2004) Binding, internalization, and membrane incorporation of human immunodeficiency virus-1 at the blood-brain barrier is differentially regulated. Neuroscience 128, 143–153 [DOI] [PubMed] [Google Scholar]
- 12. Banks W. A., Kastin A. J. (1998) Characterization of lectin-mediated brain uptake of HIV-1 GP120. J. Neurosci. Res. 54, 522–529 [DOI] [PubMed] [Google Scholar]
- 13. Chung T. W., Kim S. J., Choi H. J., Kim K. J., Kim M. J., Kim S. H., Lee H. J., Ko J. H., Lee Y. C., Suzuki A., Kim C. H. (2009) Ganglioside GM3 inhibits VEGF/VEGFR2-mediated angiogenesis: direct interaction of GM3 with VEGFR2. Glycobiology 19, 229–239 [DOI] [PubMed] [Google Scholar]
- 14. Rusnati M., Tanghetti E., Urbinati C., Tulipano G., Marchesini S., Ziche M., Presta M. (1999) Interaction of fibroblast growth factor-2 (FGF-2) with free gangliosides: biochemical characterization and biological consequences in endothelial cell cultures. Mol. Biol. Cell 10, 313–327 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Rusnati M., Urbinati C., Tanghetti E., Dell'Era P., Lortat-Jacob H., Presta M. (2002) Cell membrane GM1 ganglioside is a functional coreceptor for fibroblast growth factor 2. Proc. Natl. Acad. Sci. U.S.A. 99, 4367–4372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Kajiji S., Tamura R. N., Quaranta V. (1989) A novel integrin (αEβ4) from human epithelial cells suggests a fourth family of integrin adhesion receptors. EMBO J. 8, 673–680 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Graham K. L., Halasz P., Tan Y., Hewish M. J., Takada Y., Mackow E. R., Robinson M. K., Coulson B. S. (2003) Integrin-using rotaviruses bind α2β1 integrin α2I domain via VP4 DGE sequence and recognize αXβ2 and αVβ3 by using VP7 during cell entry. J. Virol. 77, 9969–9978 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Kremser M. E., Przybyło M., Hoja-Łukowicz D., Pocheć E., Amoresano A., Carpentieri A., Bubka M., Lityńska A. (2008) Characterization of α3β1 and αvβ3 integrin N-oligosaccharides in metastatic melanoma WM9 and WM239 cell lines. Biochim. Biophys. Acta 1780, 1421–1431 [DOI] [PubMed] [Google Scholar]
- 19. Woodard-Grice A. V., McBrayer A. C., Wakefield J. K., Zhuo Y., Bellis S. L. (2008) Proteolytic shedding of ST6Gal-I by BACE1 regulates the glycosylation and function of α4β1 integrins. J. Biol. Chem. 283, 26364–26373 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20. Chiang C. H., Wang C. H., Chang H. C., More S. V., Li W. S., Hung W. C. (2010) A novel sialyltransferase inhibitor AL10 suppresses invasion and metastasis of lung cancer cells by inhibiting integrin-mediated signaling. J. Cell Physiol. 223, 492–499 [DOI] [PubMed] [Google Scholar]
- 21. Morova J., Osicka R., Masin J., Sebo P. (2008) RTX cytotoxins recognize β2 integrin receptors through N-linked oligosaccharides. Proc. Natl. Acad. Sci. U.S.A. 105, 5355–5360 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Mousa S. A. (2008) Cell adhesion molecules: potential therapeutic and diagnostic implications. Mol. Biotechnol. 38, 33–40 [DOI] [PubMed] [Google Scholar]
- 23. Eliceiri B. P., Cheresh D. A. (1998) The role of αv integrins during angiogenesis. Mol. Med. 4, 741–750 [PMC free article] [PubMed] [Google Scholar]
- 24. Noonan D., Albini A. (2000) From the outside in: extracellular activities of HIV Tat. Adv. Pharmacol. 48, 229–250 [DOI] [PubMed] [Google Scholar]
- 25. Urbinati C., Bugatti A., Giacca M., Schlaepfer D., Presta M., Rusnati M. (2005) αvβ3 integrin-dependent activation of focal adhesion kinase mediates NF-κB activation and motogenic activity by HIV-1 Tat in endothelial cells. J. Cell Sci. 118, 3949–3958 [DOI] [PubMed] [Google Scholar]
- 26. Barillari G., Sgadari C., Fiorelli V., Samaniego F., Colombini S., Manzari V., Modesti A., Nair B. C., Cafaro A., Stürzl M., Ensoli B. (1999) The Tat protein of human immunodeficiency virus type-1 promotes vascular cell growth and locomotion by engaging the α5β1 and αvβ3 integrins and by mobilizing sequestered basic fibroblast growth factor. Blood 94, 663–672 [PubMed] [Google Scholar]
- 27. Urbinati C., Mitola S., Tanghetti E., Kumar C., Waltenberger J., Ribatti D., Presta M., Rusnati M. (2005) Integrin αvβ3 as a target for blocking HIV-1 Tat-induced endothelial cell activation in vitro and angiogenesis in vivo. Arterioscler. Thromb. Vasc. Biol. 25, 2315–2320 [DOI] [PubMed] [Google Scholar]
- 28. Rusnati M., Tulipano G., Urbinati C., Tanghetti E., Giuliani R., Giacca M., Ciomei M., Corallini A., Presta M. (1998) The basic domain in HIV-1 Tat protein as a target for polysulfonated heparin-mimicking extracellular Tat antagonists. J. Biol. Chem. 273, 16027–16037 [DOI] [PubMed] [Google Scholar]
- 29. Grinspan J. B., Mueller S. N., Levine E. M. (1983) Bovine endothelial cells transformed in vitro by benzo(a)pyrene. J. Cell Physiol. 114, 328–338 [DOI] [PubMed] [Google Scholar]
- 30. Rusnati M., Tanghetti E., Dell'Era P., Gualandris A., Presta M. (1997) αvβ3 integrin mediates the cell-adhesive capacity and biological activity of basic fibroblast growth factor (FGF-2) in cultured endothelial cells. Mol. Biol. Cell 8, 2449–2461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. Lauder H., Frost E. E., Hiley C. R., Fan T. P. (1998) Quantification of the repair process involved in the repair of a cell monolayer using an in vitro model of mechanical injury. Angiogenesis 2, 67–80 [DOI] [PubMed] [Google Scholar]
- 32. Mitola S., Moroni E., Ravelli C., Andres G., Belleri M., Presta M. (2008) Angiopoietin-1 mediates the proangiogenic activity of the bone morphogenic protein antagonist Drm. Blood 112, 1154–1157 [DOI] [PubMed] [Google Scholar]
- 33. Urbinati C., Bugatti A., Oreste P., Zoppetti G., Waltenberger J., Mitola S., Ribatti D., Presta M., Rusnati M. (2004) Chemically sulfated Escherichia coli K5 polysaccharide derivatives as extracellular HIV-1 Tat protein antagonists. FEBS Lett. 568, 171–177 [DOI] [PubMed] [Google Scholar]
- 34. Kopitz J., Bergmann M., Gabius H. J. (2010) How adhesion/growth-regulatory galectins-1 and -3 attain cell specificity: case study defining their target on neuroblastoma cells (SK-N-MC) and marked affinity regulation by affecting microdomain organization of the membrane. IUBMB Life 62, 624–628 [DOI] [PubMed] [Google Scholar]
- 35. Urbinati C., Ravelli C., Tanghetti E., Belleri M., Giacopuzzi E., Monti E., Presta M., Rusnati M. (2012) Substrate-immobilized HIV-1 Tat drives VEGFR2/αvβ3 integrin complex formation and polarization in endothelial cells. Arterioscler. Thromb. Vasc. Biol. 32, e25–e34 [DOI] [PubMed] [Google Scholar]
- 36. Angelucci A., Bologna M. (2007) Targeting vascular cell migration as a strategy for blocking angiogenesis: the central role of focal adhesion protein tyrosine kinase family. Curr. Pharm. Des. 13, 2129–2145 [DOI] [PubMed] [Google Scholar]
- 37. Rusnati M., Urbinati C., Musulin B., Ribatti D., Albini A., Noonan D., Marchisone C., Waltenberger J., Presta M. (2001) Activation of endothelial cell mitogen-activated protein kinase ERK(1/2) by extracellular HIV-1 Tat protein. Endothelium 8, 65–74 [DOI] [PubMed] [Google Scholar]
- 38. Albini A., Soldi R., Giunciuglio D., Giraudo E., Benelli R., Primo L., Noonan D., Salio M., Camussi G., Rockl W., Bussolino F. (1996) The angiogenesis induced by HIV-1 tat protein is mediated by the Flk-1/KDR receptor on vascular endothelial cells. Nat. Med. 2, 1371–1375 [DOI] [PubMed] [Google Scholar]
- 39. Nacev B. A., Grassi P., Dell A., Haslam S. M., Liu J. O. (2011) The antifungal drug itraconazole inhibits vascular endothelial growth factor receptor 2 (VEGFR2) glycosylation, trafficking, and signaling in endothelial cells. J. Biol. Chem. 286, 44045–44056 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Folkman J., Klagsbrun M. (1987) Angiogenic factors. Science 235, 442–447 [DOI] [PubMed] [Google Scholar]
- 41. Ridley A. J., Paterson H. F., Johnston C. L., Diekmann D., Hall A. (1992) The small GTP-binding protein rac regulates growth factor-induced membrane ruffling. Cell 70, 401–410 [DOI] [PubMed] [Google Scholar]
- 42. Terada M., Fujita S., Suda I., Mastico R. (2005) Polysulfated sialic acid derivatives as anti-human immunodeficiency virus. Biomed. Pharmacother. 59, 423–429 [DOI] [PubMed] [Google Scholar]
- 43. Bouwstra J. B., Deyl C. M., Vliegenthart J. F. (1987) Purification and kinetic properties of sialidase from Clostridium perfringens. Biol. Chem. Hoppe Seyler 368, 269–275 [DOI] [PubMed] [Google Scholar]
- 44. Caswell P. T., Vadrevu S., Norman J. C. (2009) Integrins: masters and slaves of endocytic transport. Nat. Rev. Mol. Cell Biol. 10, 843–853 [DOI] [PubMed] [Google Scholar]
- 45. Roberts M., Barry S., Woods A., van der Sluijs P., Norman J. (2001) PDGF-regulated rab4-dependent recycling of αvβ3 integrin from early endosomes is necessary for cell adhesion and spreading. Curr. Biol. 11, 1392–1402 [DOI] [PubMed] [Google Scholar]
- 46. Christie D. R., Shaikh F. M., Lucas J. A., 4th, Lucas J. A., 3rd, Bellis S. L. (2008) ST6Gal-I expression in ovarian cancer cells promotes an invasive phenotype by altering integrin glycosylation and function. J. Ovarian Res. 1, 3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Corfield A. P., Higa H., Paulson J. C., Schauer R. (1983) The specificity of viral and bacterial sialidases for α(2→3)- and α(2→6)-linked sialic acids in glycoproteins. Biochim. Biophys. Acta 744, 121–126 [DOI] [PubMed] [Google Scholar]
- 48. Duchesne L., Tissot B., Rudd T. R., Dell A., Fernig D. G. (2006) N-Glycosylation of fibroblast growth factor receptor 1 regulates ligand and heparan sulfate co-receptor binding. J. Biol. Chem. 281, 27178–27189 [DOI] [PubMed] [Google Scholar]
- 49. Semel A. C., Seales E. C., Singhal A., Eklund E. A., Colley K. J., Bellis S. L. (2002) Hyposialylation of integrins stimulates the activity of myeloid fibronectin receptors. J. Biol. Chem. 277, 32830–32836 [DOI] [PubMed] [Google Scholar]
- 50. Scatena M., Almeida M., Chaisson M. L., Fausto N., Nicosia R. F., Giachelli C. M. (1998) NF-κB mediates αvβ3 integrin-induced endothelial cell survival. J. Cell Biol. 141, 1083–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 51. Monti E., Bonten E., D'Azzo A., Bresciani R., Venerando B., Borsani G., Schauer R., Tettamanti G. (2010) Sialidases in vertebrates: a family of enzymes tailored for several cell functions. Adv. Carbohydr. Chem. Biochem. 64, 403–479 [DOI] [PubMed] [Google Scholar]
- 52. Renkonen R., Mattila P., Majuri M. L., Räbinä J., Toppila S., Renkonen J., Hirvas L., Niittymäki J., Turunen J. P., Renkonen O., Paavonen T. (1997) In vitro experimental studies of sialyl Lewis x and sialyl Lewis a on endothelial and carcinoma cells: crucial glycans on selectin ligands. Glycoconj. J. 14, 593–600 [DOI] [PubMed] [Google Scholar]
- 53. Sakarya S., Rifat S., Zhou J., Bannerman D. D., Stamatos N. M., Cross A. S., Goldblum S. E. (2004) Mobilization of neutrophil sialidase activity desialylates the pulmonary vascular endothelial surface and increases resting neutrophil adhesion to and migration across the endothelium. Glycobiology 14, 481–494 [DOI] [PubMed] [Google Scholar]
- 54. Lang Z., Guerrera M., Li R., Ladisch S. (2001) Ganglioside GD1a enhances VEGF-induced endothelial cell proliferation and migration. Biochem. Biophys. Res. Commun. 282, 1031–1037 [DOI] [PubMed] [Google Scholar]
- 55. Rusnati M., Presta M. (2006) Extracellular angiogenic growth factor interactions: an angiogenesis interactome survey. Endothelium 13, 93–111 [DOI] [PubMed] [Google Scholar]
- 56. Harduin-Lepers A., Mollicone R., Delannoy P., Oriol R. (2005) The animal sialyltransferases and sialyltransferase-related genes: a phylogenetic approach. Glycobiology 15, 805–817 [DOI] [PubMed] [Google Scholar]
- 57. Brockhausen I., Lehotay M., Yang J. M., Qin W., Young D., Lucien J., Coles J., Paulsen H. (2002) Glycoprotein biosynthesis in porcine aortic endothelial cells and changes in the apoptotic cell population. Glycobiology 12, 33–45 [DOI] [PubMed] [Google Scholar]
- 58. Auzzas L., Zanardi F., Battistini L., Burreddu P., Carta P., Rassu G., Curti C., Casiraghi G. (2010) Targeting αvβ3 integrin: design and applications of mono- and multifunctional RGD-based peptides and semipeptides. Curr. Med. Chem. 17, 1255–1299 [DOI] [PubMed] [Google Scholar]
- 59. Stewart P. L., Nemerow G. R. (2007) Cell integrins: commonly used receptors for diverse viral pathogens. Trends Microbiol. 15, 500–507 [DOI] [PubMed] [Google Scholar]