Background: Caveolins affect signaling by G protein-coupled receptors (GPCRs).
Results: Interaction between β3a-adrenoceptors and caveolin-1 facilitates Gs-mediated responses but prevents the receptor from coupling to inhibitory Gi/o proteins.
Conclusion: Association of the β3a-adrenoreceptor with caveolin-1 is important in determining the selectivity and efficiency of G protein coupling and signaling.
Significance: We demonstrate the functional impact of a GPCR-caveolin association.
Keywords: Adenylate Cyclase (Adenylyl Cyclase), Adipocyte, Adrenergic Receptor, Caveolin, G Proteins
Abstract
Caveolins act as scaffold proteins in multiprotein complexes and have been implicated in signaling by G protein-coupled receptors. Studies using knock-out mice suggest that β3-adrenoceptor (β3-AR) signaling is dependent on caveolin-1; however, it is not known whether caveolin-1 is associated with the β3-AR or solely with downstream signaling proteins. We have addressed this question by examining the impact of membrane rafts and caveolin-1 on the differential signaling of mouse β3a- and β3b-AR isoforms that diverge at the distal C terminus. Only the β3b-AR promotes pertussis toxin (PTX)-sensitive cAMP accumulation. When cells expressing the β3a-AR were treated with filipin III to disrupt membrane rafts or transfected with caveolin-1 siRNA, the cyclic AMP response to the β3-AR agonist CL316243 became PTX-sensitive, suggesting Gαi/o coupling. The β3a-AR C terminus, SP384PLNRF389DGY392EGARPF398PT, resembles a caveolin interaction motif. Mutant β3a-ARs (F389A/Y392A/F398A or P384S/F389A) promoted PTX-sensitive cAMP responses, and in situ proximity assays demonstrated an association between caveolin-1 and the wild type β3a-AR but not the mutant receptors. In membrane preparations, the β3b-AR activated Gαo and mediated PTX-sensitive cAMP responses, whereas the β3a-AR did not activate Gαi/o proteins. The endogenous β3a-AR displayed Gαi/o coupling in brown adipocytes from caveolin-1 knock-out mice or in wild type adipocytes treated with filipin III. Our studies indicate that interaction of the β3a-AR with caveolin inhibits coupling to Gαi/o proteins and suggest that signaling is modulated by a raft-enriched complex containing the β3a-AR, caveolin-1, Gαs, and adenylyl cyclase.
Introduction
The plasma membrane is not a random or uniform array of lipids and proteins but instead has physical heterogeneity as well as higher order structures that are critical to the functioning of receptors, ion channels, and signaling proteins. Membrane rafts, or lipid rafts, are liquid-ordered lipid domains of 5–10 nm that are enriched in cholesterol and sphingolipids (1, 2). Rafts display reduced lateral diffusion relative to the liquid-disordered phase, providing nucleation sites for further membrane organization to produce larger structures of 50–150 nm. These higher order structures are enriched in multiprotein complexes, acting as signaling platforms that govern association between receptors and effector proteins (reviewed in Ref. 3). Caveolae represent a subset of membrane rafts that have a distinctive membrane structure delineated by the presence of caveolin proteins as well as the protein cavin (4, 5). Caveolin-1, -2, and -3 consist of a cytoplasmic N terminus, a 21-amino acid hairpin structure that inserts into the cell membrane, and a cytoplasmic C terminus with three palmitoylation sites. Caveolins interact with signaling proteins via a conserved scaffolding domain (for example, amino acids 82–101 of caveolin-1). Caveolae are thought to contain 100–200 caveolin molecules (6); however, caveolins may form smaller noncaveolar oligomers of at least 15 molecules that have been termed caveolin scaffolds (7). Noncaveolar caveolins may also modulate signaling (4), for example by growth factor receptors (8) and G protein-coupled receptors (GPCRs)3 (9).
The three β-adrenoreceptor subtypes (β-ARs) are highly conserved GPCRs that share common determinants for coupling to the α subunit of the stimulatory guanine nucleotide-binding protein (Gαs); however, functional diversity is generated by sequence-specific protein-protein interactions and by differential enrichment in membrane domains. For example, interaction of the β2-AR with inhibitory guanine nucleotide-binding proteins (Gαi/o) is dependent on the presence of a functional type 1 PSD-95/Drosophila Discs Large/ZO-1 (PDZ) docking site at the receptor C terminus (DSLL) (10), whereas the β1-AR C-terminal PDZ motif (ESKV) inhibits receptor internalization and Gαi coupling (11). The signaling properties of the β2-AR are clearly regulated by partitioning in membrane rafts or in caveolae (12). In cardiac myocytes, disruption of caveolae has no effect on the inotropic response to β1-AR stimulation, although it significantly enhances β2-AR-mediated Ca2+ transients and L-type Ca2+ channel currents (13, 14).
Although no studies to date have reported localization of the β3-AR in membrane rafts or caveolae, there is firm evidence that caveolin-1 regulates β3-AR signaling in adipocytes. In both white and brown adipocytes, β3-ARs stimulate the Gαs/adenylyl cyclase/protein kinase A (PKA) pathway, promoting breakdown of fat (lipolysis) via phosphorylation of perilipin and hormone-sensitive lipase. Brown adipocytes also display β3-AR-mediated thermogenesis via induction of the mitochondrial uncoupling protein UCP1. The role of caveolin-1 in both white and brown adipocytes has been examined using caveolin-1−/− mice. Stimulation of lipolysis by the β3-AR selective agonist CL316243 is reduced substantially in white adipocytes isolated from caveolin-1−/− mice compared with wild type mice, due to disruption of a signaling complex that normally includes caveolin-1, the catalytic subunit of PKA and perilipin (15). A similar pattern is seen in differentiated 3T3-L1 adipocytes treated with caveolin-1 siRNA (16). In control cells, CL316243 promotes phosphorylation of perilipin, hormone-sensitive lipase, and also the phosphorylation, activation, and recruitment of phosphodiesterase 3B into complexes that contain caveolin-1, β3-AR, and PKA regulatory subunit RII. Knockdown of caveolin-1 blocks the activation of PDE3B and its recruitment into plasma membrane signaling complexes. In brown adipose tissue from caveolin-1−/− mice, perilipin phosphorylation and the mobilization of triglycerides usually associated with fasting/cold exposure are substantially reduced (17). Upstream cAMP responses are also reduced, in part due to decreased adenylyl cyclase activity and β3-AR abundance (18, 19). It cannot be determined from these studies, however, whether caveolin-1 is associated functionally with the β3-AR itself or whether the diminished responses in knock-out mice are due solely to effects on downstream signaling, for example via PKA and perilipin.
We have been able to address this question by taking advantage of the distinct signaling properties of two mouse β3-AR isoforms generated by alternative splicing (20, 21). The β3a- and β3b-AR isoforms differ only in their distal C-terminal tail, yet cAMP accumulation mediated by the β3b-AR is increased following pretreatment of cells with pertussis toxin (PTX), whereas the β3a-AR response is PTX-insensitive. Use of cell-permeable peptides corresponding to the unique β3a- and β3b-AR C termini demonstrated that the β3a-AR C-terminal tail interacts with a distinct protein or signaling complex (22). We proposed that binding of proteins such as caveolin or other scaffolding proteins to the β3a-AR C terminus may localize the receptor to membrane microdomains or intracellular compartments where it cannot couple to Gαi/o.
We demonstrate here that when CHO-K1 cells expressing the β3a-AR are treated with filipin III to disrupt membrane rafts, the cyclic AMP response to CL316243 becomes PTX-sensitive. In contrast, there is no change in the PTX sensitivity of the β3b-AR response. This suggests that residues present in the β3a-AR C-terminal tail may direct localization of the receptor to membrane rafts, and this in turn may govern its capacity to couple to Gαi/o proteins. The β3a-AR C terminus, SP384PLNRF389DGY392EGARPF398PT, contains a motif that is similar to the caveolin interaction motif of many proteins (φXφXXXXφ or φXXXXφXXφ, where φ is an aromatic residue (23)). We show that cAMP accumulation is PTX-sensitive in cells expressing β3a-ARs carrying mutations in the putative caveolin-binding site. Knockdown of caveolin-1 in CHO-K1 cells expressing the wild type β3a-AR or in mouse brown adipocytes expressing endogenous β3a-ARs also alters the PTX sensitivity of cAMP accumulation and glucose uptake. We demonstrate that caveolin-1 interacts with the wild type β3a-AR but not with mutant β3a-ARs lacking key residues within the interaction motif. Our findings also indicate that PTX treatment increases cAMP responses in membranes derived from cells expressing the β3b-AR via inhibition of receptor-Gαo coupling.
EXPERIMENTAL PROCEDURES
Expression of the Mouse β3a- and β3b-AR and Receptor Mutants in CHO-K1 Cells
Plasmids (pcDNA3.1+) carrying the coding region for each of the β3a- and β3b-AR, a truncated β3-AR, and the mutant Y392A were as described previously (21, 22). Five additional mutants were created to examine the potential importance of residues in the C terminus for G protein coupling. A construct for expression of the F389A,Y392A,F398A mutant was made by replacing a 561-bp XhoI/XbaI fragment from the wild type β3a-AR plasmid with a PCR fragment generated using the primers mb3.TF, 5′-CGTCTATGCTCGAGTGTTCGTTGTGG-3′ and mb3.FYF-AAA, 5′-CGGTTCTAGACCCTTCACGTGGGAGCCGGACGCGCACCTTCAGCGCCATCAGCCCTGTTGAGC-3′ (restriction sites are underlined and mutated nucleotides are bold). The same strategy was used for the other four mutants, using mb3.TF as the forward primer and the reverse primers mb3.F389A, 5′-CGGTTCTAGACCCTTCACGTGGGAAACGGACGCGCACCTTCATAGCCATCAGCCCTGTTGAGC-3′; mb3.F389A/Y392A, 5′-CGGTTCTAGACCCTTCACGTGGGAAACGGACGCGCACCTTCAGCGCCATCAGCCCTGTTGAGC-3′; mb3.A395E, 5′-CGGTTCTAGACCCTTCACGTGGGAAACGGACGCTCACCTTCATAGCCATC-3′; mb3.P384S, 5′-CGGTTCTAGACCCTTCACGTGGGAAACGGACGCGCACCTTCATAGCCATCAAACCTGTTGAGCGGTGAACTCTGCCTG-3′; and mb3.P384S/F389A, 5′-CGGTTCTAGACCCTTCACGTGGGAAACGGACGCGCACCTTCATAGCCATCAGCCCTGTTGAGCGGTGAACTCTGCCTG-3′. All PCRs were carried out as described before (22), using Platinum Pfx High Fidelity DNA polymerase (Invitrogen). The complete insert and junctions with pcDNA3.1 were confirmed for each of the β3-AR constructs by DNA sequencing on both strands (Micromon, Monash University, Victoria, Australia).
Cell Culture of CHO-K1 Cells Expressing Mouse β3-ARs
Chinese hamster ovary (CHO-KI) cells were grown in 50:50 Dulbecco's modified Eagle's medium (DMEM)/Ham's F-12 medium supplemented with 10% (v/v) fetal bovine serum (FBS), glutamine (2 mm), penicillin (100 units/ml), and streptomycin (100 μg/ml) at 37 °C with 5% CO2. Clonal cell lines expressing the wild type β3a-AR, β3b-AR, and truncated β3-AR were described previously (21, 22). The cells were maintained in DMEM/Ham's F-12 (50:50) medium containing 10% FBS and 400 μg/ml G418 under 5% CO2 at 37 °C. CHO-K1 cells expressing mutant β3a-ARs were generated by transient transfection using Lipofectamine, and cAMP assays or the Duolink in situ proximity ligation assay were performed 48 h after transfection.
Cell Culture and Transient Transfection of siRNA-Cav1
For transfection, CHO-K1 cells stably expressing the β3a- or β3b-AR were seeded overnight at 3.5 × 105 cells per well in 6-well plates. siRNA constructs were obtained from Dr. Debbie C. Thurmond (Indiana University School of Medicine, Indianapolis) and consisted of siRNA-directed against canine caveolin-1 (GCCCAACAACAAGGCCATG) or siRNA containing a control sequence (GCGCGCTTTGTAGGATTCG) (24). The caveolin-1 siRNA was chosen as it corresponded to a region that was identical across all available mammalian sequences and had been used successfully to knock down expression in CHO-K1 cells (24). 1.5 μg of the caveolin-1 siRNA or control plasmid was transfected using LipofectamineTM (Invitrogen). For cAMP accumulation assays, cells were plated into 96-well plates, and PTX was added to half the wells 16 h before experiments. Western blotting and cAMP accumulation assays were performed 48 h after the start of transfection.
Immunoblotting to Detect β3-ARs
Transfected cells were grown in 12-well plates at 1 × 105 cells per well in DMEM/Ham's F-12 medium containing 0.5% FBS overnight. Cells were lysed directly in each well by the addition of 80 μl of 65 °C SDS sample buffer (62.5 mm Tris-HCl, pH 6.8, 2% SDS, 10% glycerol, 50 mm dithiothreitol, and 0.1% bromophenol blue). Cells were scraped, transferred to an Eppendorf tube on ice, and sonicated for 5 s followed by heating to 95 °C for 5 min. Aliquots of the samples were separated on a 10% polyacrylamide gel and electrotransferred to Hybond-P polyvinylidene difluoride membranes (pore size 0.45 μm; Amersham Biosciences) with a semidry electroblotter. After transfer, the membranes were allowed to soak in Tris-buffered saline for 5 min, followed by quenching of nonspecific binding (1 h at room temperature in 5% nonfat dry milk and 0.1% Tween 20 in Tris-buffered saline). Membranes were incubated overnight at 4 °C with primary antibody, β3-AR (Santa Cruz Biotechnology) diluted 1:1000, or total-AKT (Cell Signaling Technology) diluted 1:1000. This was detected using a secondary antibody (HRP-linked anti-goat IgG, Cell Signaling) diluted 1:2000 and enhanced chemiluminescence (ECL, Amersham Biosciences).
Confirmation of Caveolin-1 siRNA Efficacy by Immunoblotting
Lysates from siRNA-treated cells were prepared as above, resolved on 10% polyacrylamide gels, and transferred to Bio-Rad PVDF membranes. Blots were blocked using 5% BSA dissolved in PBS plus 0.1% Tween 20 (PBS-T) and then probed with a mixture of 1 μg/ml each of rabbit anti-caveolin-1 (AbCam ab2910) and mouse anti-β-actin (ab8226) in 1% BSA in PBS-T plus 0.02% sodium azide. Following washing, blots were probed with mixtures of 1 μg/ml goat anti-mouse AF647 (Molecular Probes A21236) and goat anti-rabbit AF532 (Molecular Probes A11009) in PBS-T containing 0.02% sodium azide. After washing, blots were imaged on a Typhoon Trio (GE Healthcare) using 532 laser 555/20 emission (caveolin-1) and 633 laser 670/30 emission (β-actin).
Animals and Genotyping
All animal studies were approved by the Monash University Animal Ethics Committee. Animals used for experimentation were anesthetized with 80% CO2, 20% O2. 3–4-Week-old FVB mice of either sex were bred at Mouseworks (Monash University). Caveolin-1+/+ and Caveolin-1−/− mice of either sex (3–4 weeks old) were obtained from Dr. Robin L Anderson (Peter MacCallum Cancer Centre, Melbourne, Australia) with permission from Dr. T. Kurzchalia (25). These mice had been backcrossed for at least 10 generations on a pure BALB/c background (Dr. R. Anderson). Offspring were the product of +/− × +/− or +/− × −/− matings because −/− × −/− matings were not effective. Genomic DNA analysis was conducted on mouse tails to determine the genotype of all mice used for experimental studies before animals were obtained. Genomic DNA was isolated by proteinase K digestion overnight followed by extraction of DNA using a commercial kit (Wizard SV Genomic DNA Purification System, Promega Corp., Alexandria, New South Wales, Australia). PCR was performed on ∼20 ng of DNA (50 °C annealing, 35 cycles) using primers designed to indicate the presence/absence of neomycin disruption to the caveolin-1 allele using Go TaqDNA polymerase according to the manufacturer's instructions (Promega). Primers to amplify the caveolin-1 knock-out fragment were forward, 5′-TATTCTGCCTTCCTGATGATAACTG-3′, and reverse, 5′-CCTGCGTGCAATCCATCTTGTTCAATG-3′, and primers to amplify the caveolin-1 wild type fragment were forward, 5′-TTTACCGCTTGTTGTCTACGA-3′, and reverse, 5′-TATCTCTTTCTGCGTGCTGA-3′ (primers from Invitrogen). This generated a wild type product of 240-bp and a knock-out product of 1450 bp. PCR products were run on a 1.3% agarose gel and the bands digitally captured.
Cell Isolation and Culture of Mouse Brown Adipocytes
Brown fat precursor cells were isolated and cultured as described previously (26) with modifications as outlined in Ref. 27. The interscapular, axillary, and cervical brown adipose tissue depots were dissected out under sterile conditions, minced, and transferred to a solution containing 123 mm NaCl, 5 mm KCl, 1.3 mm CaCl2, 5 mm glucose, 1.5% (w/v) crude bovine serum albumin, 100 mm Hepes, pH 7.4, and 0.2% (w/v) crude collagenase type II. Routinely, pooled tissue from two mice was digested in 10 ml of the Hepes-buffered solution. The tissue was digested (30 min, 37 °C) with vortexing every 5 min, and the digest was filtered through a 250-μm filter into sterile tubes. The solution was placed on ice for 15 min to allow the mature brown fat cells and lipid droplets to float. The infranatant was filtered through a 25-μm filter and collected, and the precursor cells were pelleted by centrifugation (10 min, 700 × g), resuspended in Dulbecco's modified Eagle's medium (DMEM) (4.5 g glucose/liter), and re-centrifuged. The pellet was resuspended in 12 ml of DMEM, and the cells were seeded into 24-well plates and grown in DMEM supplemented with 10% (v/v) newborn calf serum, 2.4 nm insulin, 10 mm Hepes, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 25 μg/ml sodium ascorbate. Brown adipocytes were used for experiments following 7 days in culture.
Radioligand Binding Assay
Cell membranes were prepared, and saturation-binding experiments were performed as described previously (21). Briefly, the homogenate (∼10–20 μg of protein) was incubated with [125I]iodo-(−)-cyanopindolol (100–2000 pm) for 60 min at room temperature in the absence or presence of (−)-alprenolol (1 mm) to define nonspecific binding. Reactions were terminated by rapid filtration through GF/C filters presoaked for 30 min in 0.5% (v/v) polyethyleneimine using a Packard Cell Harvester, and radioactivity was measured using a Packard Top Count.
cAMP Accumulation Studies
CHO-K1 cells (1 × 104 cells per well) expressing β3-ARs were grown in 96-well plates in DMEM/Ham's F-12 medium containing 0.5% (v/v) FBS for 2 days. In studies where brown adipocytes were used, all experiments were performed on day 7 of cell culture. On day 6, the cells were serum-starved overnight in DMEM/Nutrient Mix F-12 (1:1) with 4 mm l-glutamine, 0.5% BSA, 2.4 nm insulin, 10 mm Hepes, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 50 μg/ml sodium ascorbate.
On the day of experiment, media were aspirated, and appropriate drugs diluted in stimulation buffer (1 mg/ml BSA, 0.5 mm IBMX, 0.5 m Hepes, pH 7.4, in Hanks' balanced salt solution) were added. After 30 min of incubation at 37 °C, media were removed, and 100 μl of lysis buffer (1 mg/ml BSA, 0.3% (v/v) Tween 20, 0.5 m Hepes, 0.5 mm IBMX, pH 7.4) were added. Samples were rapidly frozen at −70 °C to lyse cells prior to measurement of cAMP. To examine the effect of PTX, cells were treated with PTX (100 ng/ml) for 16 h before stimulation with appropriate drugs. The effects of filipin III were examined by addition of 1 μg/ml filipin III for 1 h prior to stimulation of cells with CL316243 for 30 min. cAMP accumulation was measured utilizing the cAMP αScreen kit (PerkinElmer Life Sciences) as detailed previously (22). All results are expressed as the percentage of the forskolin response (100 μm) in a given experiment.
Glucose Uptake Measurements
Glucose uptake studies were performed as described previously (28, 29). All experiments were performed on day 7 of brown adipocyte culture. On day 6, the cells were serum-starved overnight in DMEM/Nutrient Mix F-12 (1:1) with 4 mm l-glutamine, 0.5% BSA, 2.4 nm insulin, 10 mm Hepes, 50 IU/ml penicillin, 50 μg/ml streptomycin, and 50 μg/ml sodium ascorbate, plus or minus PTX (100 ng/ml). On the morning of day 7, the medium was changed to DMEM without insulin (containing 0.5% BSA, 50 μg/ml of sodium ascorbate) for at least 30 min. Drugs were added and the samples incubated for 110 min in a final volume of 500 μl, and then the medium was discarded, and cells washed with prewarmed PBS (10 mm phosphate buffer, 2.7 mm KCl, 137 mm NaCl, pH 7.4). Glucose-free DMEM (containing 0.5% BSA, 50 μg/ml sodium ascorbate) was added, and the drug concentrations were re-added with 50 nm 2-deoxy-d-[1-3H]glucose (Amersham Biosciences, specific activity 9.5–12 Ci/mmol) in a total volume of 500 μl for 10 min. Reactions were terminated by rapid aspiration and washing cells twice in ice-cold PBS. Cells were lysed (500 μl of 0.2 m NaOH, 1 h at 55 °C), and the incorporated radioactivity was determined by liquid scintillation counting (the contents of the entire well were transferred to a single scintillation vial). All results are expressed as the percentage of the basal response (defined as 100%) in a given experiment.
cAMP Accumulation and GTPγ35S Binding in Crude Membranes
CHO-K1 cells stably expressing the mouse β3a- or β3b-AR and primary cultures of mouse brown adipocytes were grown to confluence prior to the preparation of crude membranes. Growth media were removed, and cells were washed once in room temperature PBS. Cells were removed with a cell scraper in ice-cold buffer A (20 mm Tris, pH 7.5, 2 mm EDTA, 0.4 mm PMSF, protease inhibitor mixture) and lysed using a 22-gauge needle. The cell suspension was centrifuged (39,000 × g, 20 min, 4 °C) before re-homogenization in ice-cold buffer B (50 mm Tris, pH 7.5, 1 mm EDTA, protease inhibitor mixture) using a 25-gauge needle. Protein concentration was measured using a BCA protein assay (Pierce) prior to snap freezing and storage of the crude membranes at −80 °C.
cAMP generated by the crude membranes was measured using an AlphaScreen cAMP accumulation kit according to the manufacturer's instructions (PerkinElmer Life Sciences). Crude membranes were diluted to 1 μg of protein per well in a white 384-well plate in stimulation buffer (50 mm Tris, pH 7.4, 150 mm NaCl, 10 mm MgCl2, 1.5 mm CaCl2, 100 μm ATP, 1 μm GDP, and 1 nm GTP), mixed with anti-cAMP acceptor beads, and incubated for 30 min at room temperature in the dark. Membranes were stimulated with vehicle or CL316243 for 30 min at room temperature in the dark, before the addition of streptavidin-donor beads and biotinylated cAMP diluted in detection buffer (5 mm Hepes, pH 7.4, 0.1% BSA, 0.3% Tween 20). Samples were incubated for 4 h in the dark at room temperature prior to reading the plates on a Fusion-α microplate reader. Data were analyzed against a cAMP standard curve and are expressed as the amount of cAMP generated per μg of crude membrane protein. To examine the effect of pertussis toxin, crude membranes were incubated with 20 μg/ml activated pertussis toxin (5 mm ATP, 5 mm DTT, 30 min at 30 °C, as per manufacturer's instructions) (30, 31) for 15 min at room temperature.
[35S]GTPγS immunoprecipitation was performed to assess direct activation of Gαs, Gαi1, Gαi2, Gαi3, or Gαo following stimulation of the mouse β3a- or β3b-adrenergic receptors (32, 33). Reaction tubes were maintained at 30 °C and contained crude membranes (75 μg per reaction) diluted in assay buffer (10 mm Hepes, 100 mm NaCl, 10 mm MgCl2, 1% BSA, 0.01% saponin, pH 7.4). Crude membranes were preincubated for 5 min with 1 μm (Gαs immunoprecipitations) or 10 μm (Gαi1, Gαi2, Gαi3, and Gαo immunoprecipitations) GDP at 30 °C, prior to addition of vehicle or 3 μm CL316243 and 1 nm [35S]GTPγS (PerkinElmer Life Sciences) for 20 min at 30 °C. The final reaction volume was 100 μl. Reactions were terminated by placing the tubes on ice, and membranes were completely solubilized by the addition of 100 μl of ice-cold solubilization buffer (100 mm Tris, 200 mm NaCl, 1 mm EDTA, 1.25% Nonidet P-40, 0.1% SDS, pH 7.5). Samples were precleared for 90 min at 4 °C by the addition of 10 μl of 10% (v/v) preimmune serum (normal rabbit IgG for Gαs and Gαi3 immunoprecipitations; normal mouse IgG for Gαi1, Gαi2, and Gαo immunoprecipitations, diluted in assay buffer) and 30 μl of 20% (v/v) protein G-agarose (Pierce) that had been diluted in solubilization buffer containing 2% BSA and 0.1% NaN3. The precleared supernatant (200 μl) was combined with 5 μl of the appropriate Gα antibody (1:40 dilution) and rotated at 4 °C overnight. 70 μl of 20% protein G-agarose (as described above) was added, and samples were rotated at 4 °C for 90 min. Beads were washed three times by adding 1 ml of ice-cold solubilization buffer (without SDS), inverting the tubes, and then centrifuging at 500 × g for 30 s at 4 °C. After the final wash, the beads were resuspended in 100 μl of solubilization buffer, and scintillation mixture was added, and samples were counted using a liquid scintillation counter.
DuolinkTM in Situ Proximity Ligation Assay
The assay was carried out according to the manufacturer's protocol (Olink Biosciences). 24 h after transfection, CHO-K1 cells (1 × 104 cells per well) expressing β3a-ARs as well as the F389, Y392,F398A β3a-AR and P384S,F389A β3a-AR were plated in polystyrene vessel tissue culture-treated glass slides (FalconTM) in DMEM/Ham's F-12 medium containing 0.5% (v/v) FBS and incubated at 37 °C for 24 h. On the day of the experiment, media were aspirated, and cells were fixed with 4% paraformaldehyde in PBS for 20 min. Cells were washed with PBS and quenched in 50 mm glycine for 5 min. After washing with PBS, cells were permeabilized with 0.25% Triton X-100 for 15 min. Cells were washed with PBS and then blocked in Duolink blocking solution overnight at 4 °C. After cells were washed with PBS, primary antibodies, anti-caveolin-1 (rabbit, 1:1000 dilution, Cell Signaling), and anti-β3-AR antibody (goat, 1:1000 dilution, Santa Cruz Biotechnology) were added and incubated overnight at 4 °C. Cells were washed with PBS and then secondary antibodies conjugated with oligonucleotides (PLA probe anti-rabbit MINUS and anti-goat PLUS) were added and incubated for 2 h at 37 °C. Cells were washed with PBS and then incubated with Hybridization solution, consisting of two oligonucleotides, for 15 min at 37 °C. After washing with TBS-T, the Ligation solution was added together with ligase for 30 min at 37 °C. Cells were washed with TBS-T, and the Amplification solution, consisting of nucleotides, was added together with polymerase for 90 min at 37 °C. The cells were washed with TBS-T, and the Detection solution, consisting of fluorescently labeled oligonucleotides plus Hoechst 33342 nuclear stain, was added for 1 h at 37 °C. Slides were washed successively in 2× SSC (0.3 m sodium chloride, 0.03 M sodium citrate) for 2 min, 1× SSC for 2 min, 0.2× SSC for 2 min, 0.02× SSC for 2 min, and 70% EtOH for 1 min and mounted with ProLong Gold antifade reagent (Invitrogen), and a cover glass was placed on the sample. Cells were observed in a Leica DMLB epifluorescence microscope. Photographs were taken at ×63 magnification, and images were acquired by using a DC350F camera and IM500 software (Leica Microsystems AB; Kista, Sweden).
Data Analysis
All results are expressed as a means ± S.E. of n. Data were analyzed using nonlinear curve fitting (GraphPad PRISM version 5.0) to obtain pEC50 values where appropriate or using a one-site fit to obtain KD and Bmax values (saturation binding experiments). Statistical significance was determined using two-way ANOVA tests or Student's t test. Probability values less than or equal to 0.05 were considered significant.
Drugs and Reagents
Drugs and reagents were purchased as follows: 2-deoxy-d-[1-3H]glucose (specific activity 9.5–12 Ci/mmol) (Amersham Biosciences); G418 (Calbiochem); HRP-linked anti-rabbit IgG, Akt antibody, caveolin-1 antibody (Cell Signaling Technology, Beverly, MA); anti-β3-AR antibody, anti-Gαs, Gαi1, Gαi2, Gαi3, and Gαo antibodies (Santa Cruz Biotechnology); normal rabbit/mouse IgG, protein-G agarose (Pierce); DuolinkTM in situ proximity ligation assay kit (Olink Bioscience, Uppsala, Sweden); [125I]iodo-(−)-cyanopindolol (2200 Ci/mmol, ProSearch International Australia Pty Ltd., Melbourne, Australia); aprotinin, leupeptin, and pepstatin A (ICN, Costa Mesa, CA); Lipofectamine, OptiMEM® (Invitrogen); insulin (Actrapid®) (Novo Nordisk, Bagsvaerd, Denmark); cAMP α-screen kit, GTPγ35S (PerkinElmer Life Sciences); (−)-alprenolol, bacitracin, CL316243 ((R,R)-5-[2-[[2-(3-chlorophenyl)-2-hydroxyethyl]-amino]-propyl]1,3-benzodioxole-2,2-decarboxylate), filipin III, IBMX, pertussis toxin, polyethyleneimine, (Sigma). All cell culture media and supplements were obtained from Trace Biosciences (Castle Hill, New South Wales, Australia). All other drugs and reagents were of analytical grade.
RESULTS
Differential PTX Sensitivity of cAMP Accumulation Mediated by β3-AR Isoforms
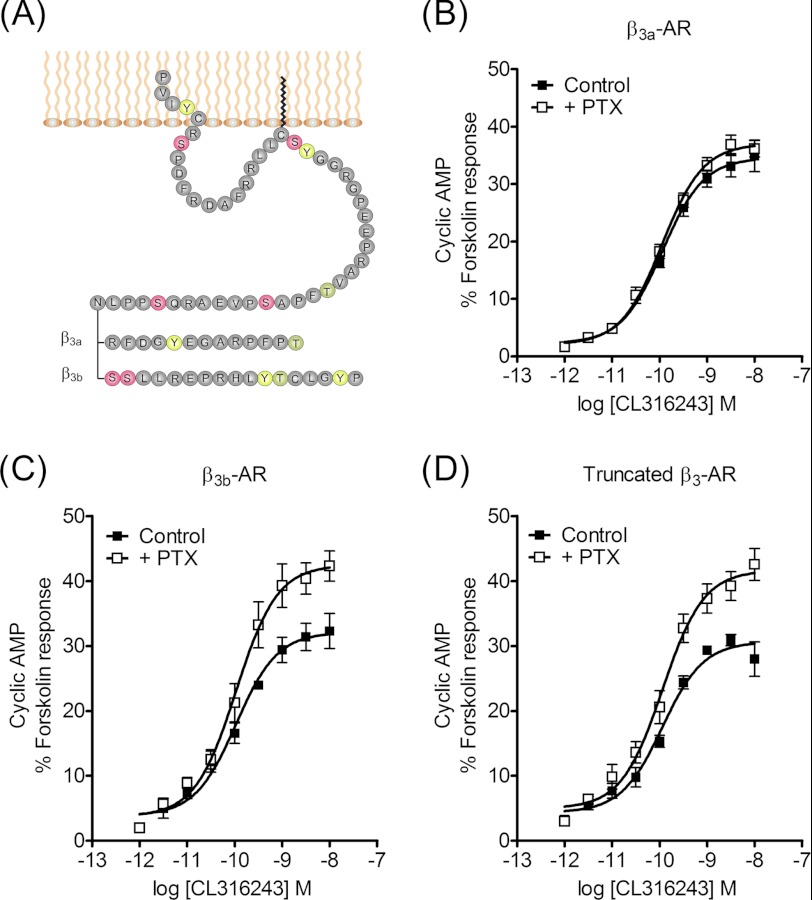
The two β3-AR isoforms share a common proximal C-terminal region but differ at the distal C terminus (Fig. 1A). We examined the properties of the wild type β3a-AR, β3b-AR, and a truncated β3-AR that lack the C-terminal tail of either splice variant in clonal CHO-K1 cell lines with equivalent receptor densities (Bmax 1148 ± 241, 1309 ± 128, and 1224 ± 105 fmol/mg protein, respectively). Maximal cAMP responses to the selective β3-AR agonist CL316243 in cells expressing the β3a-AR were unaffected by pretreatment of cells with PTX (100 ng/ml, 16 h; Fig. 1B and supplemental Table S1), whereas responses in cells expressing the β3b-AR increased by 36% following pretreatment with PTX (p < 0.0001; Fig. 1C), confirming our previous results (22). The pEC50 values for CL316243 at β3a- and β3b-AR were not significantly different and were not altered significantly by PTX pretreatment (supplemental Table S1). The truncated β3-AR behaved similarly to the β3b-AR and displayed PTX sensitivity (Fig. 1D and supplemental Table S1). This suggested that rather than the β3b-AR containing a motif that enables coupling to Gαi/o, the C terminus of the β3a-AR contains a motif that disables coupling to the inhibitory G protein. Our previous study indicated that this β3a-AR motif interferes with Gαi/o coupling by effects on receptor localization and/or protein-protein interactions (22). In essence, the PTX resistance of β3a-AR responses provides a functional readout of the capacity of the C terminus to direct this localization or interaction.
FIGURE 1.
Functional responses following activation of β3-AR isoforms. A, snake diagram of the C-terminal tail of β3-AR isoforms. Note that the truncated mutant terminates at residue Asn-387. B–D, concentration-response curves for stimulation of cAMP accumulation by the β3a-AR (B), β3b-AR (C), and truncated β3-AR (D) in the presence or absence of pretreatment with PTX (100 ng/ml, 16 h). CHO-K1 cells stably expressing each β3-AR were exposed to CL316243 for 30 min in stimulation buffer containing 0.5 mm IBMX to inhibit phosphodiesterases. Responses to forskolin (10−4 m) were determined in parallel with agonist-stimulated cAMP accumulation for each batch of cells, and results are expressed as a % of the response to forskolin. Basal cAMP and responses to 100 μm forskolin (fsk) were in control basal 0.33 ± 0.13 and fsk 17.2 ± 4.2 pmol/104 cells; +PTX basal 0.33 ± 0.13 and fsk 18.1 ± 5.5 pmol/104 cells (B); control basal 0.27 ± 0.16 and fsk 22.4 ± 4.2 pmol/104 cells; +PTX basal 0.41 ± 0.12 and fsk 21.8 ± 2.7 pmol/104 cells (C); control basal 0.78 ± 0.21 and fsk 22.5 ± 4.1 pmol/104 cells; +PTX basal 0.68 ± 0.17 and fsk 23.1 ± 3.3 pmol/104 cells (D). cAMP accumulation responses were PTX-sensitive in cells expressing the β3b-AR and truncated receptor (p < 0.001 determined by two-way ANOVA) but not the β3a-AR. Values are means ± S.E. of 4–5 independent experiments.
Signaling by β3-AR Isoforms Is Differentially Affected by Filipin Treatment
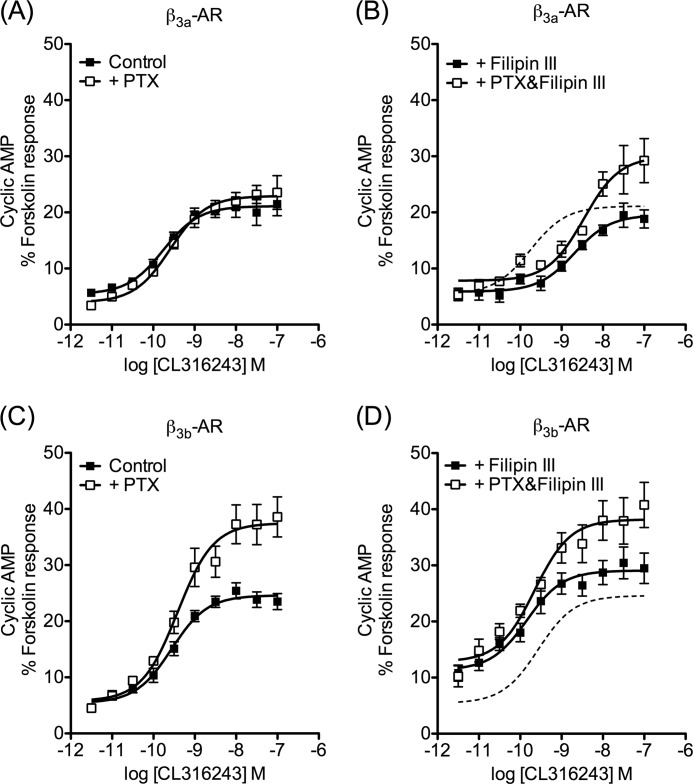
As the behavior of the β2-AR and other GPCRs are affected by localization in membrane raft domains (12), we examined the effect of disrupting membrane rafts using filipin III. In cells expressing the β3a-AR, PTX pretreatment had no effect on cAMP accumulation in response to CL316243 as described previously (21, 22). However, after treatment with filipin III (1 μg/ml), the cAMP response to CL316243 became PTX-sensitive, with the maximum response being increased by 55% (supplemental Table S1 and Fig. 2B). Interestingly, the concentration-response curve was also markedly shifted to the right in the presence of filipin III (pEC50 control 9.76 ± 0.14, + filipin III 8.69 ± 0.15, p < 0.01), and an equivalent negative effect of filipin III on the potency of CL316243 was seen in the presence of PTX (supplemental Table S1).
FIGURE 2.
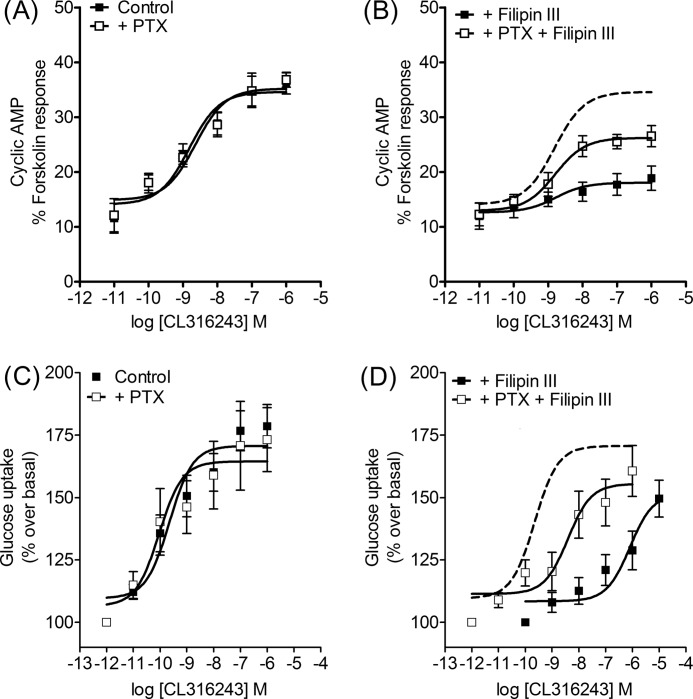
Disruption of caveolae by filipin III alters functional responses of β3-AR isoforms. Concentration-response curves were constructed for stimulation of cAMP accumulation by CL316243 in the presence or absence of filipin III (1 μg/ml, 1 h) or following pretreatment of cells with PTX (100 ng/ml, 16 h). In cells stably expressing the β3a-AR, cAMP accumulation was not altered by PTX treatment (A), but the addition of filipin III caused the response to become PTX-sensitive (B, p < 0.001 determined by two-way ANOVA). In cells expressing the β3b-AR isoform, filipin III did not have a significant effect on PTX-sensitivity (C and D). Basal cAMP and responses to 100 μm fsk were in control basal 1.23 ± 0.47 and fsk 24.4 ± 9.7 pmol/104 cells; +PTX basal 0.85 ± 0.36 and fsk 24.6 ± 10.4 pmol/104 cells (A); control basal 1.45 ± 0.64 and fsk 23.0 ± 9.5 pmol/104 cells; +PTX basal 1.35 ± 0.62 and fsk 22.5 ± 10.6 pmol/104 cells (B); control basal 1.06 ± 0.40 and fsk 23.1 ± 8.3 pmol/104 cells; +PTX basal 1.35 ± 0.55 and fsk 27.1 ± 9.3 pmol/104 cells (C); control basal 2.56 ± 0.88 and fsk 27.0 ± 9.6 pmol/104 cells; +PTX basal 2.29 ± 0.80 and fsk 26.1 ± 9.5 pmol/104 cells (D). B and D, dotted line shows the concentration-response curve for CL316243 in the absence of filipin III or PTX pretreatment, taken from A or C, respectively, for comparison. In CHO-β3a-AR cells filipin III caused a right shift of the curve, whereas in CHO-β3b-AR cells, basal and maximal cAMP accumulation were increased, but there was no change in the potency of CL316243. Results are expressed as a % of the response to forskolin (10−4 m), and values are means ± S.E. of 4–6 independent experiments.
In cells expressing the β3b-AR, pretreatment with PTX caused a 36% increase in the maximum cAMP response to CL316243, with no change in the pEC50 value (Fig. 2C). Filipin III treatment (1 μg/ml) significantly increased the basal level of cAMP; however, PTX pretreatment followed by filipin III still caused an increase in the maximum response to CL316243 by 38% compared with cells treated with filipin III alone (Fig. 2D and supplemental Table S1). Filipin III had no significant effect on the pEC50 of CL316243 relative to control cells, indicating that the rightward shift in β3a-AR-expressing cells was not an artifact associated with overall effects on cell signaling or viability. Thus, treatment with filipin III facilitated coupling of the β3a-AR isoform to Gαi/o and possibly reduced efficiency of coupling to the cAMP pathway, but it had no effect on agonist-stimulated β3b-AR responses.
Note that the Emax and pEC50 values for the filipin experiment are lower than those seen in Fig. 1 for both the β3a- and β3b-AR expressing cells. We are making comparisons only within a given experiment, however, where cells at equivalent confluence and passage number were treated in parallel with PTX/vehicle and then with filipin III/vehicle.
Effect of siRNA Knockdown of Caveolin-1 on cAMP Signaling Mediated by the β3-AR
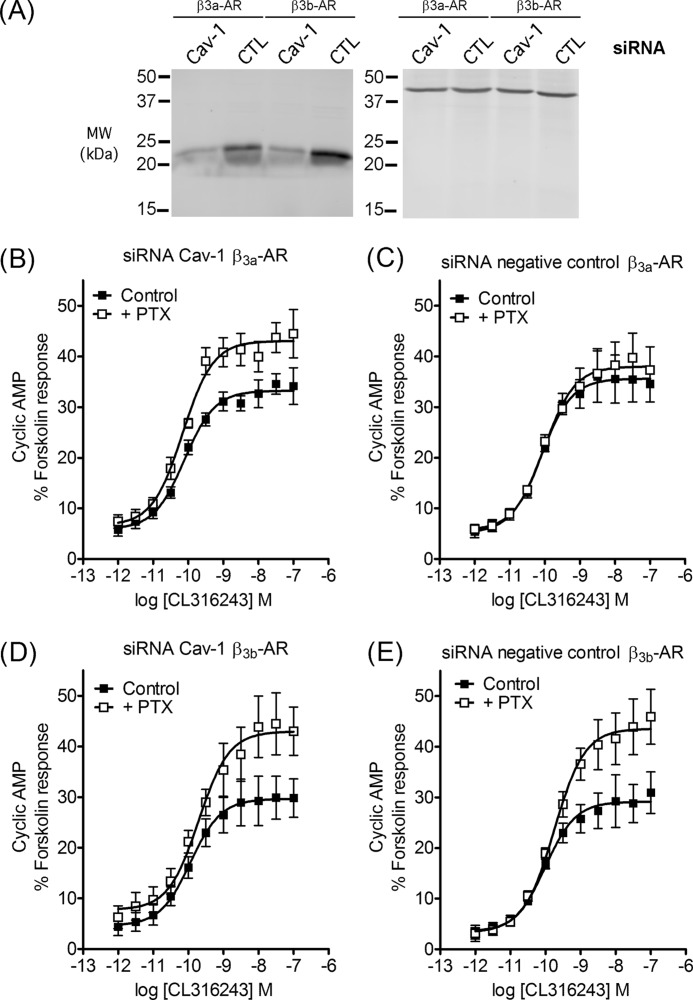
Treatment of cells with filipin III disrupts membrane rafts globally, so we next used a more targeted approach by reducing caveolin-1 expression. CHO-K1 cells stably expressing either the β3a- or the β3b-AR were transiently transfected with siRNA directed against caveolin-1 or an siRNA comprising a scrambled form of the same sequence (24). After 48 h of incubation, cells were used for cAMP accumulation assays and analyzed for abundance of caveolin-1 protein. Transfection of cells expressing either the β3a- or the β3b-AR with the siRNA directed against caveolin-1 but not the control sequence caused knockdown of caveolin-1 expression as shown in Fig. 3A. Caveolin-1 siRNA treatment of β3a-AR-expressing cells caused the cAMP responses to become PTX-sensitive (Fig. 3B), whereas cAMP responses in cells expressing β3a-AR and treated with the negative control sequence were unaltered (Fig. 3C). In contrast, transfection of cells expressing the β3b-AR with either the caveolin-1 scrambled sequence or caveolin-1 siRNA did not affect the PTX sensitivity of cAMP responses (Fig. 3, D and E). Treatment of cells expressing the β3a-AR with filipin III caused a 10-fold reduction in the potency of CL316243 (Fig. 2B and supplemental Table S1), whereas knockdown of caveolin-1 had no effect on potency in the presence or absence of PTX (Fig. 3, B and D, and supplemental Table S1). Thus filipin III and knockdown of caveolin-1 both promote PTX sensitivity of β3a-AR responses, but filipin III has additional effects on the efficiency of signaling to the cAMP pathway.
FIGURE 3.
Knockdown of caveolin-1 by siRNA influences responses mediated by β3a- but not β3b-AR. A, siRNA constructs were tested by immunoblotting protein extracts using a caveolin-1 antibody (left panel) or β-actin antibody (right panel). Equal volumes of cell lysate were subjected to 10% SDS-PAGE prior to immunoblotting. Approximate molecular masses are indicated on the left in kDa. A typical immunoblot from four experiments is shown. The left panel shows that the caveolin-1 siRNA produced substantial knockdown of caveolin-1 compared with the control siRNA in cells expressing either the β3a-AR or β3b-AR. B–E, concentration-response curves were constructed for stimulation of cAMP accumulation by CL316243 in CHO-K1 cells stably expressing β3a- or β3b-AR and transiently transfected with a negative control (CTL) or caveolin-1 (Cav-1) siRNA construct. Basal cAMP and responses to 100 μm fsk were in control basal 1.47 ± 0.33 and fsk 27.9 ± 3.7 pmol/104 cells; +PTX basal 1.49 ± 0.24 and fsk 25.4 ± 5.6 pmol/104 cells (B); control basal 0.97 ± 0.19 and fsk 22.0 ± 3.5 pmol/104 cells; +PTX basal 1.22 ± 0.09 and fsk 24.2 ± 2.9 pmol/104 cells (C); control basal 1.08 ± 0.47 and fsk 21.7 ± 2.0 pmol/104 cells; +PTX basal 1.75 ± 0.73 and fsk 23.0 ± 2.3 pmol/104 cells (D); control basal 0.76 ± 0.23 and fsk 26.7 ± 4.1 pmol/104 cells; +PTX basal 0.65 ± 0.29 and fsk 22.6 ± 4.8 pmol/104 cells (E). In CHO-β3a-AR cells, knockdown of caveolin-1 caused cAMP accumulation to become PTX-sensitive (B, p < 0.001 determined by two-way ANOVA), whereas the negative control siRNA had no effect (C). In CHO-β3b-AR cells, neither the caveolin-1 siRNA nor the negative control siRNA had any effect on PTX sensitivity (D and E). Results are expressed as a % of the response to forskolin (10−4 m), and values are means ± S.E. of six independent experiments.
Mutations of a Putative Caveolin-binding Site in the C Terminus of the β3a-AR Alter Signaling Properties
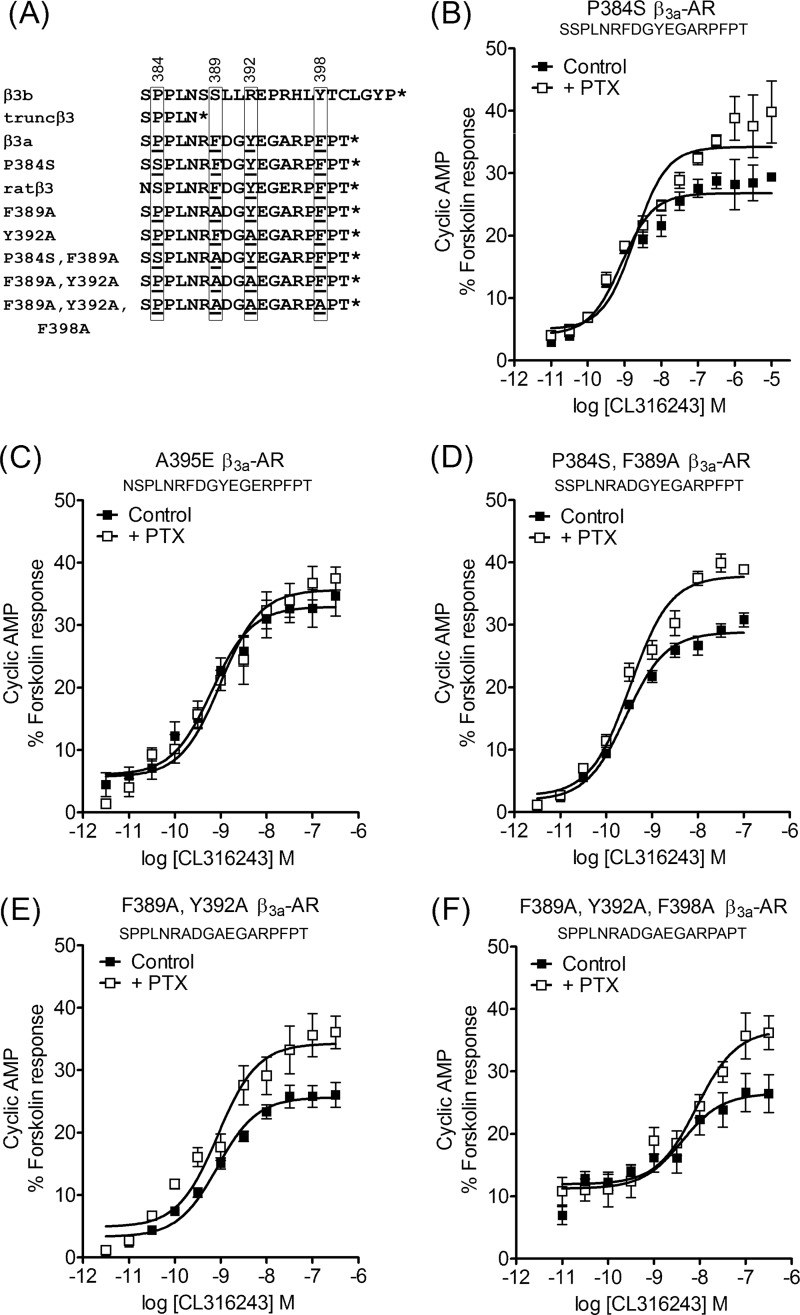
The capacity of caveolin-1 siRNA to alter the signaling properties of the β3a-AR indicated that there were amino acid residues or a motif unique to the β3a-AR C terminus that confers interaction with caveolin-1. The β3a-AR C terminus includes the sequence RF389DGY392EGARPF398PT (Fig. 4A), which has three aromatic residues and resembles the caveolin interaction motif of many proteins (23). We previously showed that mutation of Tyr-392 to Ala does not affect β3a-AR cAMP responses, whereas removal of the unique C terminus causes cAMP responses to become PTX-sensitive (22). In this study we have extended this observation by comparing the β3a-, β3b-, and truncated β3-AR with new mutant receptors lacking single or multiple residues implicated in caveolin-1 binding. As seen with the Y392A β3a-AR, PTX sensitivity was not conferred by the single mutation F389A (supplemental Table S2) or by mutation of the four C-terminal residues (PFPT) to alanine (data not shown). In cells expressing β3a-ARs mutated at either two or three of the aromatic residues, namely F389A,Y392A, or F389A,Y392A,F398A, CL316243-stimulated cAMP accumulation was increased by 39 or 46%, respectively, in the presence of PTX (Fig. 4, E and F). Although this indicates that the aromatic residues participate in an interaction that prevents Gαi/o coupling, this finding did not appear at first sight to be consistent with a previous demonstration that the rat β3-AR expressed in the same cell background does couple to Gαi/o (34). The rat β3-AR C terminus (RF389DGY392EGERPF398PT) only differs from the mouse β3a-AR sequence at position 395, where it has a glutamate residue instead of alanine. However, when we made a mutant A395E mouse β3a-AR, cAMP accumulation was still PTX-resistant (Fig. 4C and supplemental Table S2). We therefore tested the idea that there must be additional residues outside the unique β3a-AR tail that influence Gαi/o coupling. One possibility is that the caveolin-1 interaction motif actually starts within a region common to the β3a- and β3b-AR isoforms. Although there are no aromatic residues in the region upstream of Phe-389, there is a hydrophobic proline residue at position 384 in the mouse β3a-AR that creates a motif with the spacing PXXXXFXXY (mouse SP384PLNRF389DGY392EGARPFPT; rat NS384PLNRF389DGY392EGERPFPT). Therefore, we made a P384S mutation in the mouse β3a-AR, either alone or in combination with F389A. The P384S,F389A double mutant and even the P384S single mutant receptor displayed 30 and 28% increases in cAMP accumulation following PTX treatment (Fig. 4, B and D), slightly less than the increases seen with the F389A,Y392A (Fig. 4E) and F389A,Y392A,F398A (Fig. 4F) mutants (supplemental Table S2). The combined mutation data suggest that the motif PXXXXFXXY is the dominant factor in determining Gαi/o coupling properties of the mouse β3a-AR (Fig. 4A).
FIGURE 4.
Site-directed mutagenesis of putative sites in the β3a-AR C terminus involved in interactions with caveolin-1. The C-terminal sequences of all relevant mouse β3-AR isoforms and mutants, plus the rat β3-AR, are shown in A. The residue at position 384 and aromatic residues at positions 389, 392, and 398 are boxed. Concentration-response curves were constructed for stimulation of cAMP accumulation by CL316243 in CHO-K1 cells transiently transfected with mutant β3a-ARs. Pretreatment with PTX (100 ng/ml, 16 h) increased maximal cAMP accumulation in cells expressing the P384S β3a-AR (B), P384S,F389A β3a-AR (D), F389A,Y392A β3a-AR (E), or F389A,Y392A,F398A β3a-AR (F) mutants (p < 0.001 determined by two-way ANOVA), but not the A395E β3-AR (C). Basal cAMP and responses to 100 μm fsk were as follows: B, control basal 0.13 ± 0.03 and fsk 9.3 ± 2.3 pmol/104 cells; +PTX basal 0.16 ± 0.06 and fsk 9.9 ± 2.5 pmol/104 cells; C, control basal 0.58 ± 0.25 and fsk 8.5 ± 1.1 pmol/104 cells; +PTX basal 0.18 ± 0.07 and fsk 8.1 ± 1.3 pmol/104 cells; D, control basal 0.11 ± 0.02 and fsk 12.6 ± 5.9 pmol/104 cells; +PTX basal 0.08 ± 0.01 and fsk 11.5 ± 5.5 pmol/104 cells; E, control basal 0.17 ± 0.05 and fsk 8.5 ± 0.9 pmol/104 cells; +PTX basal 0.19 ± 0.05 and fsk 10.0 ± 1.3 pmol/104 cells; F, control basal 0.60 ± 0.24 and fsk 9.1 ± 4.3 pmol/104 cells; +PTX basal 0.87 ± 0.44 and fsk 10.0 ± 4.3 pmol/104 cells. Results are expressed as a % of the response to forskolin (10−4 m), and values are means ± S.E. of 4–6 independent experiments. The 389PXXXXF389XXY392 motif appears to be dominant in modulating Gαi/o coupling of the β3-AR isoforms.
It is important to note that all mutation studies were done in transiently transfected CHO-K1 cells. The key comparison in each case was between control and PTX-treated samples derived from the same population of transfected cells, and we can therefore make direct comparisons of maximal responses and pEC50 values in the absence or presence of PTX for a given cell population but not between cells transfected with different mutant receptors. Variation in the potency of CL316243 between mutants most likely reflects differences in surface expression of the receptors (supplemental Table S2), as we have seen previously (35). It is clear, however, that there is no consistent correlation between pEC50 values and the impact of mutations on PTX sensitivity.
Interaction between β3a-AR and Caveolin-1 Requires an Intact Caveolin-1-binding Site
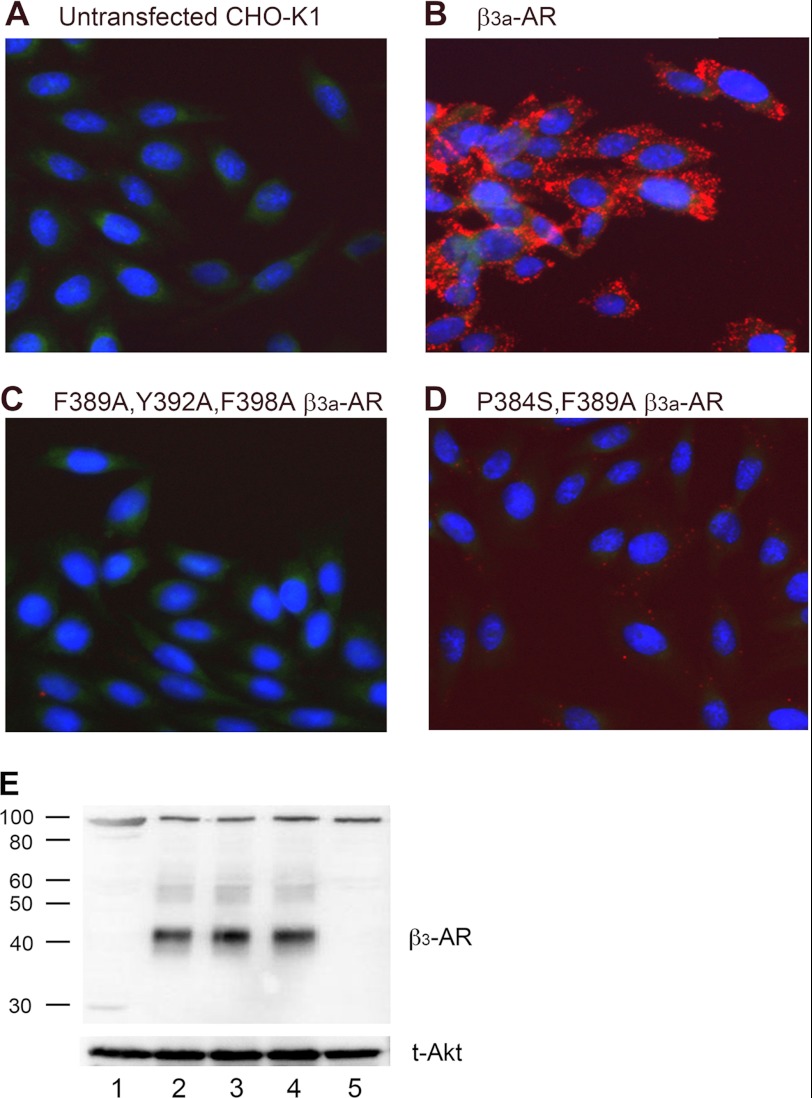
The functional studies described here indicate that mutation of the putative caveolin-binding site enables the modified β3a-AR to couple to both Gαs and Gαi/o and suggest that an interaction between the β3a-AR and caveolin-1 modulates cAMP signaling and disables coupling between the β3a-AR and Gαi/o. To examine interactions between the β3a-AR and caveolin-1, we used a Duolink in situ proximity ligation assay (36). We compared untransfected CHO-K1 cells, cells expressing wild type β3a-AR (cAMP responses not sensitive to PTX), and cells expressing either F389A,Y392A,F398A β3a-AR or the P384S,F389A β3a-AR (both cAMP responses PTX-sensitive but to slightly different degrees). Only cells expressing the β3a-AR produced a robust red fluorescent signal (Fig. 5B) suggesting that only receptors possessing an intact caveolin-1-binding motif were able to interact with caveolin. Untransfected CHO-K1 cells (Fig. 5A) or cells expressing the F389A,Y392A,F398A β3a-AR (Fig. 5C) produced no reaction product with the in situ proximity ligation assay. Consistent with a somewhat diminished PTX sensitivity, the P384S,F389A β3a-AR showed very low levels of red fluorescence, suggesting a weak interaction with caveolin-1 (Fig. 5D). We confirmed expression of wild type and mutant β3a-ARs in the cells used for this assay by immunoblotting (Fig. 5E). Note that this experiment could not be performed with the β3b-AR as the β3-AR antibody used recognizes the C-terminal tail of wild type and mutant β3a-ARs but not the β3b-AR (Fig. 5E).
FIGURE 5.
Detection of interactions between β3-ARs and caveolin-1 using the DuolinkTMin situ proximity ligation assay. A, untransfected CHO-K1 cells; B, CHO-K1 cells expressing the wild type β3a-AR; C, F389A,Y392A,F398A β3a-AR; or D, P384S,F389A β3a-AR. Cells were fixed on glass slides and incubated with anti-β3-AR and anti-caveolin-1 primary antibodies and then PLA probe MINUS and PLUS secondary antibodies, as described under “Experimental Procedures.” Cells were then treated with hybridization solution containing appropriate oligonucleotides that hybridize to the two PLA probes if they are in close proximity. Hybridized oligonucleotides were ligated and subjected to rolling-circle amplification, and then the concatameric product was detected using fluorescently labeled oligonucleotides. The detection solution also contained Hoechst 33342 nuclear stain. After washing, fluorescence was detected using a Leica DMLB epifluorescence microscope. Photographs were taken at ×63 magnification, and images were acquired using a DC350F camera and IM500 software (Leica Microsystems AB). Cells expressing the wild type β3a-AR displayed robust red fluorescence indicating interaction with caveolin-1 (B), whereas nontransfected cells or those expressing the F389A,Y392A,F398A mutant gave no signal (A and C). There was detectable fluorescence in cells expressing the P384S,F389A β3a-AR, suggesting a weak caveolin-1 interaction (D). E, expression of each receptor and recognition by the β3-AR antibody was verified by immunoblotting protein extracts using the β3-AR antibody (upper panel) or t-Akt antibody (lower panel). Samples are numbered as follows: lane 1, untransfected CHO-K1 cells; lane 2, wild type β3a-AR; lane 3, F389A,Y392A,F398A β3a-AR; lane 4, P384S,F389A β3a-AR; and lane 5, β3b-AR. Approximate molecular masses are indicated on the left in kDa. Note that the wild type and both mutant β3a-ARs are recognized by the β3-AR antibody, whereas the β3b-AR does not cross-react with this antibody.
Functional Studies in Brown Adipocytes That Physiologically Express High Levels of β3a-AR
The β3a-AR mRNA transcripts make up >92% of total β3-AR transcripts in mouse brown adipocytes (20, 37). BAT was therefore chosen as a suitable tissue to test the likely physiological significance of the findings. CL316243 caused a concentration-dependent increase in cAMP levels in primary brown adipocytes from FVB mice, which was unaffected by pretreatment with PTX (Fig. 6A). Treatment with filipin III (1 μg/ml) significantly reduced the cAMP response to CL316243 (p < 0.0001), indicating that disruption of membrane rafts reduced the efficiency of the Gs/AC/cAMP pathway. However, addition of filipin III to cells pretreated with PTX caused a 40% increase in the maximum cAMP response compared with cells treated with filipin III alone (p < 0.0001) (Fig. 6B and supplemental Table S3). Thus, as in CHO β3a-AR cells, treatment with filipin III caused cAMP responses in BAT to become PTX-sensitive, suggesting enabling of coupling to Gαi/o.
FIGURE 6.
Disruption of caveolae by filipin III alters responses to CL316243 in mouse brown adipocytes that express endogenous β3a-ARs. Concentration-response curves were constructed for stimulation of cAMP accumulation by CL316243 or glucose uptake in the presence or absence of filipin III (1 μg/ml, 1 h) or following pretreatment of cells with PTX (100 ng/ml, 16 h). In brown adipocytes, cAMP accumulation was not altered by PTX treatment (A), but the addition of filipin III (B) significantly reduced the maximum response (p < 0.0001) but also caused the response to become PTX-sensitive (p < 0.001 determined by two-way ANOVA). cAMP accumulation is expressed as a % of the response to forskolin (10−4 m), and values are means ± S.E. of seven independent experiments. Basal cAMP and responses to 100 μm fsk were as follows: A, control basal 1.3 ± 0.4 and fsk 12.2 ± 1.3 pmol/well; +PTX basal 1.1 ± 0.3 and fsk 12.0 ± 2.7 pmol/well; B, filipin III basal 1.2 ± 0.2 and fsk 13.9 ± 2.1 pmol/well; +PTX basal 1.3 ± 0.3 and fsk 14.0 ± 1.7 pmol/well. Examination of a downstream response, glucose uptake, expressed as % over basal, also showed a lack of PTX sensitivity (C)., whereas filipin III treatment (D) caused a marked shift to the right of the concentration-response curve that was significantly shifted back to the left by PTX pretreatment (p < 0.001 determined by two-way ANOVA). Values are means ± S.E. of 8–13 independent experiments. Basal glucose uptake and maximum responses to CL316243 were in control basal 810 ± 64, maximum 1411 ± 118 dpm/well; +PTX basal 760 ± 82, maximum 1332 ± 107 dpm/well (C); filipin III basal 1076 ± 121, maximum 1547 ± 164 dpm/well; +PTX basal 883 ± 115, maximum 1475 ± 92 dpm/well (D). B and D, dotted line shows for comparison the concentration-response curve for CL316243 in the absence of filipin III or PTX pretreatment, taken from A or C, respectively.
We also examined a downstream consequence of β3a-AR activation in BAT, namely the facilitation of glucose uptake. CL316243 increased glucose uptake in FVB brown adipocytes as described previously (27, 37), and this response was not significantly affected by PTX pretreatment (Fig. 6C). After filipin III treatment (1 μg/ml), the concentration-response relationship for increases in glucose uptake with CL316243 was substantially shifted to the right (Fig. 6D). However, in the presence of filipin III and after pretreatment with PTX, the concentration-response curve was shifted back to the left. Thus, in this case, the PTX influence on the sensitivity of the response is manifested as an increase in CL316243 potency rather than as a greater maximum response, perhaps reflecting the difference between an output that is proximal to receptor-Gα coupling versus glucose uptake, a downstream signaling event that would display much greater signal amplification.
We also utilized brown adipocytes isolated from WT BALB/c mice and caveolin-1 knock-out (cav-1−/−) mice. Whereas brown adipocytes from cav-1+/+ mice displayed the normal PTX-insensitive β3a-AR cAMP response, brown adipocytes from cav-1−/− mice displayed cAMP responses that were PTX-sensitive (Fig. 7). Thus, these studies conducted in primary brown adipocytes isolated from WT and cav-1−/− mice provide supporting evidence that interaction between the β3a-AR and caveolin determines the signaling characteristics of this receptor.
FIGURE 7.
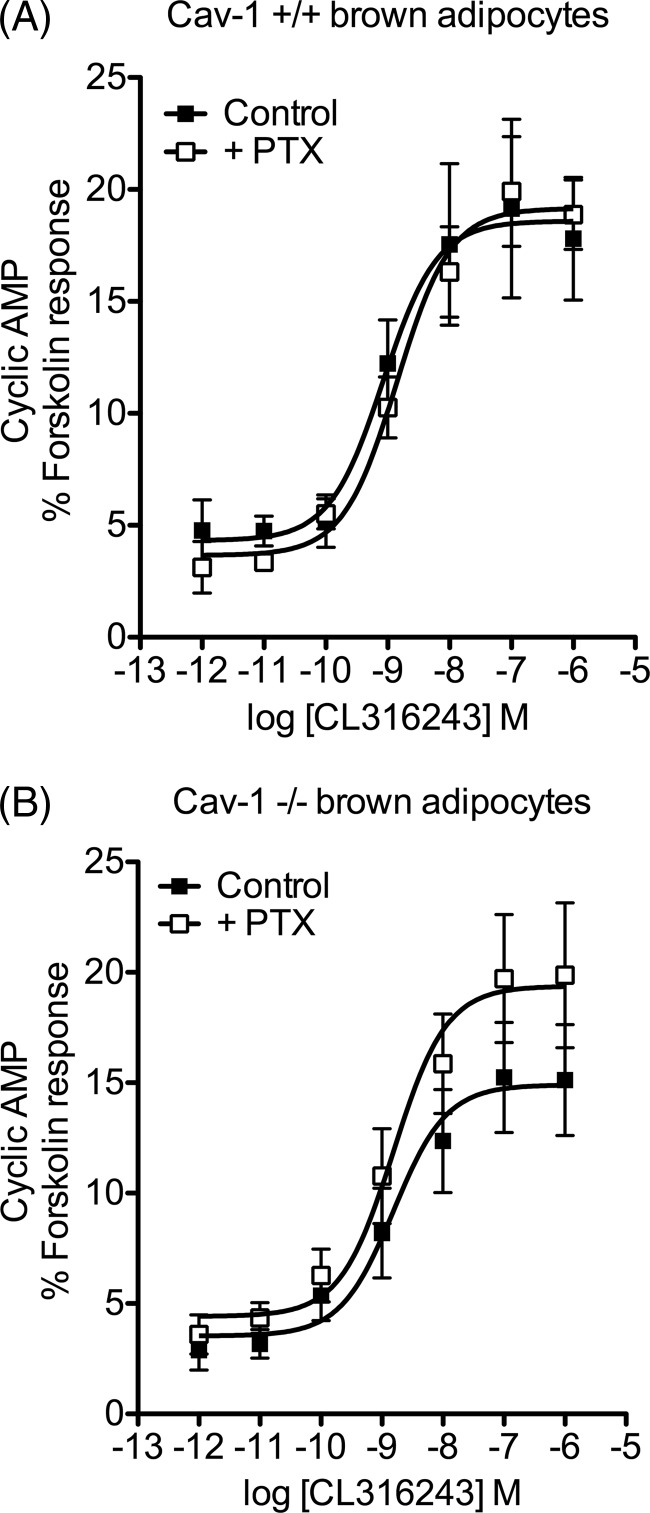
Brown adipocytes isolated from caveolin-1 knock-out mice display altered signaling characteristics. Concentration-response curves were constructed for stimulation of cAMP accumulation by CL316243 in brown adipocytes cultured from wild type (+/+, A) or caveolin-1 knock-out (−/−, B) mice. Pretreatment of adipocytes with PTX (100 ng/ml, 16 h) increased CL316243-stimulated cAMP accumulation in adipocytes from the caveolin-1−/− mice (p < 0.05 determined by two-way ANOVA) but not in those from wild type mice. Results are expressed as a % of the response to forskolin (10−4 m), and values are means ± S.E. of 6–7 independent experiments. Basal cAMP and responses to 100 μm fsk were as follows: A, control basal 0.70 ± 0.26 and fsk 16.7 ± 2.0 pmol/well; +PTX basal 0.42 ± 0.25 and fsk 15.8 ± 2.5 pmol/well; B, basal 0.45 ± 0.20 and fsk 14.5 ± 3.4 pmol/well; +PTX basal 0.55 ± 0.24 and fsk 14.3 ± 3.2 pmol/well.
Confirmation That PTX Sensitivity of the β3b-AR Response Is Due to Gαi/o Coupling
Our data demonstrate that membrane localization and caveolin interaction influence whether cAMP accumulation is sensitive to pretreatment of cells with PTX. We sought to confirm that the PTX sensitivity of the β3b-AR response does reflect coupling of this isoform to Gαi/o proteins, as an alternative explanation is that PTX acts primarily by relieving tonic inhibition of adenylyl cyclase (AC) by Gαi/o. The β3b-AR may occupy cellular compartments enriched for AC and/or Gαi/o isoforms that display enhanced tonic inhibition of AC activity, unlike the β3a-AR that is confined to membrane rafts/caveolae.
To examine preferential Gαi/o coupling to the β3b-AR, we performed [35S]GTPγS immunoprecipitation to measure activation of specific Gα subunits following receptor stimulation with CL316243. As these experiments must be done using membranes rather than whole cells, we first characterized the system by measuring cAMP responses. CL316243 stimulated cAMP accumulation in crude membranes prepared from CHO-β3a-AR or CHO-β3b-AR cells, albeit with lower potency than in whole cells (pEC50 β3a-AR, 7.10 ± 0.26; β3b-AR 7.29, ± 0.38; see Fig. 8, A and B). Maximum responses were reached only at 3 μm CL316243. When β3a-AR or β3b-AR membranes were treated with activated PTX, there was a substantial increase in basal cAMP, showing that in this experimental paradigm PTX does indeed remove tonic inhibition of adenylyl cyclase.
FIGURE 8.
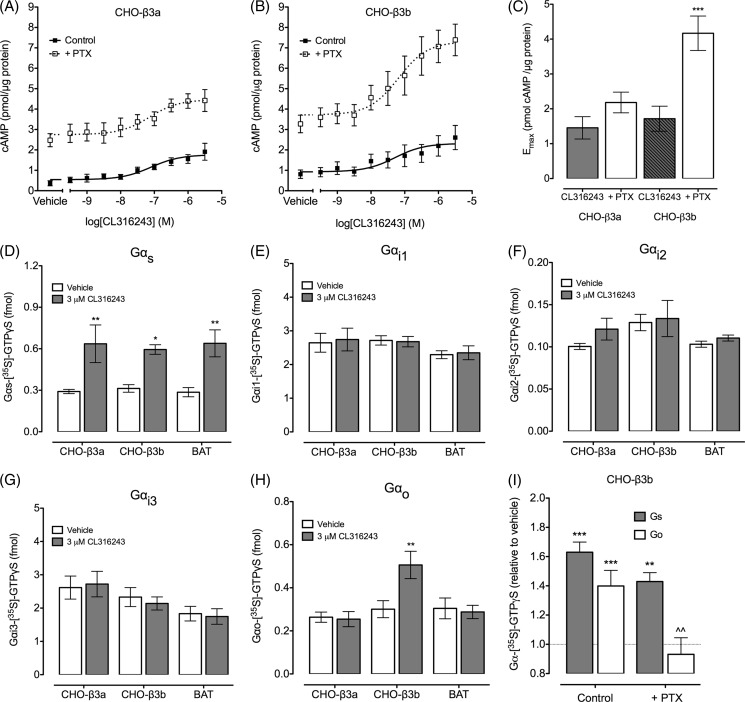
Crude membranes isolated from CHO-K1 cells expressing the β3a-AR or β3b-AR display distinct receptor coupling and signaling. Concentration-response curves were constructed for CL316243-stimulated cAMP accumulation by membranes prepared from CHO-β3a-AR (A) or CHO-β3b-AR cells (B). To test the effect of PTX, membranes were treated with activated PTX (20 μg/ml) for 15 min at room temperature. The data are expressed as pmol of cAMP/μg of protein, with no correction for basal cAMP levels. Values are means ± S.E. of 4–6 experiments (using independent crude membrane preparations) performed in triplicate. C shows the data from each concentration-response curve expressed as the maximal cAMP accumulation minus basal cAMP for membranes derived from CHO-β3a- or CHO-β3b-AR cells. Maximal CL316243-induced cAMP accumulation in β3a-AR membranes was not significantly different with or without PTX treatment. In contrast, PTX-treated membranes from β3b-AR cells show a significant increase in the maximal CL316243-induced cAMP response compared with untreated membranes (p < 0.001, one-way ANOVA with Newman Keuls multiple comparison test). [35S]GTPγS immunoprecipitation was performed to assess direct activation of Gαs (D), Gαi1 (E), Gαi2, (F), Gαi3 (G), or Gαo (H) following stimulation of the β3a-AR (in CHO-K1 cells or BAT) or the β3b-AR expressed in CHO-K1 cells. Reactions contained 100 fmol of [35S]GTPγS in 100 μl. Data are expressed as femtomoles of [35S]GTPγS; values are means ± S.E. of 4–6 experiments (using independent crude membrane preparations). 3 μm CL316243 stimulated a significant increase in Gαs activation in all three membrane samples (**, p < 0.01; *, p < 0.05; two-way ANOVA with Bonferroni multiple comparison tests). The CHO-β3b-AR membranes also displayed a significant increase in Gαo activation (**, p < 0.01; two-way ANOVA with Bonferroni multiple comparison tests). I, pretreatment of β3b-AR membranes with activated PTX abolished Gαo activation but did not have a significant effect on Gαs activation (***, p < 0.001; **, p < 0.01, response significantly different to vehicle; ^^, p < 0.01, CL316243 response in the presence of PTX significantly different from the CL316243 response in untreated membranes; two-way ANOVA with Bonferroni multiple comparison tests).
Nevertheless, the increase in CL316243-induced cAMP accumulation in β3a-AR membranes was not significantly different with or without PTX pretreatment (Fig. 8C; Emax minus basal cAMP, control 1.66 ± 0.28 pmol/μg protein; PTX, 2.18 ± 0.30). In contrast, the membranes from β3b-AR cells show not only the increased basal cAMP in the presence of PTX but also a significant increase in the maximal CL316243-induced cAMP response compared with untreated cells (Fig. 8C; Emax minus basal cAMP, control 1.72 ± 0.36 pmol/μg protein; PTX, 4.17 ± 0.50, p < 0.001). These experiments show that the distinct properties of β3-AR isoforms seen in whole cells are recapitulated in crude membrane preparations. Furthermore, these data suggest that PTX not only removes tonic inhibition of adenylyl cyclase but also permits additional CL316243-stimulated cAMP accumulation via the β3b-AR but not the β3a-AR.
We then sought to verify that the increase in CL316243-stimulated cAMP following PTX pretreatment in β3b-AR membranes was due to inhibition of receptor-Gαi/o coupling. We incubated membranes with GDP for 5 min at 30 °C, prior to addition of vehicle or 3 μm CL316243 and 1 nm [35S]GTPγS for 20 min at 30 °C. Each Gα subunit was immunoprecipitated with an isoform-selective antibody, and the bound [35S]GTPγS was measured (Fig. 8, D–H). Both sets of membranes displayed activation of Gαs, but the β3b-AR membranes showed additional activation of Gαo that was sensitive to PTX treatment (Fig. 8I). These experiments indicate that the β3b-AR, but not the β3a-AR, is able to couple to Gαo and that inhibition of this coupling is associated with increased cAMP accumulation. We also found that BAT membranes displayed robust activation of Gαs, but not any Gαi/o isoforms (Fig. 8, D–H), consistent with the cAMP studies done in whole brown adipocytes.
DISCUSSION
We show here that the β3b-AR is able to couple to both Gαs and Gαo, in agreement with the PTX sensitivity of CL316243-stimulated cAMP accumulation in whole cells and in crude membranes. In contrast, the β3a-AR is unable to couple to Gαi/o in either native mouse brown adipocytes or recombinant CHO-K1 cells. Our previous study indicated that residues present in the unique β3a-AR C-terminal tail may interfere with Gαi/o coupling due to interaction with other proteins such as caveolin (22). This notion was reinforced by the observation that the β3a-AR C-terminal tail contains a motif that is similar to the caveolin interaction motif of many proteins (φXφXXXXφ or φXXXXφXXφ (23)). We investigated this idea by treating CHO-K1 cells expressing the β3-AR isoforms with filipin III to disrupt membrane rafts or with a caveolin-1 siRNA and by examining the coupling of mutant β3a-ARs. We have also demonstrated a direct association between the β3a-AR and caveolin-1 using a proximity ligation assay, and we show that our findings in recombinant CHO-K1 cells are recapitulated in brown adipocytes derived from wild type or caveolin-1 knock-out mice.
CHO-K1 cells express caveolin-1 and exhibit caveolar structures (38–40). As knockdown of caveolin-1 in CHO-K1 cells promoted coupling of the β3a-AR to Gαi/o, we sought further evidence that caveolin-1 interacts with the receptor C-terminal tail. Our first step was to make a series of β3a-ARs with mutations in single or multiple amino acids that might contribute to the caveolin-1 interaction. Single mutations of Phe-389 or Tyr-392 to alanine did not promote Gαi/o coupling of the β3a-AR, whereas cAMP responses mediated by the combined mutants F389A,Y392A or F389A,Y392A, F398A became PTX-sensitive (Fig. 4). We also mutated Pro-384 to serine to mimic the rat β3-AR sequence, as this receptor does couple to Gαi/o (34). Interestingly, both the P384S single mutant and a P384S,F389A double mutant showed PTX sensitivity, albeit slightly less than the other composite mutants. This study indicated that the motif PXXXXFXXY is dominant in preventing Gαi/o coupling of the β3a-AR, and it gave us the opportunity to test directly whether the mutants differed from the wild type receptor in their capacity to interact with caveolin-1. We examined this interaction using the Duolink in situ proximity ligation assay, which is based on close juxtaposition of antibodies directed against the two interacting partners (36). We were unable to perform this experiment with the β3b-AR because the β3-AR antibody is directed toward the β3a-AR C-terminal tail. We did show, however, that the antibody detected the F389A,Y392A,F398A and the P384S,F389A mutants as well as the wild type β3a-AR (Fig. 5E). The wild type receptor displayed robust interaction with caveolin-1 in this assay, whereas there was no signal in nontransfected CHO-K1 cells or in cells expressing the F389A,Y392A,F398A mutant. There was a very low signal in cells expressing the P384S,F389A β3a-AR, suggesting that this receptor retained weak association with caveolin-1. In combination with the functional properties of mutant receptors, the Duolink data indicate that an interaction with caveolin-1 does modulate the G protein coupling of the β3a-AR.
The prototypical caveolin-binding motif consists of the sequences, φXφXXXXφ, φXXXXφXXφ, or a combination of the two (23). These consensus sequences were elucidated by screening phage display libraries using the caveolin scaffolding domain to select random peptide fragments. The most commonly occurring peptides were found to conform to one of the motifs above; however, there were many less abundant peptides in which the spacing or number of aromatic residues varied from the consensus (23). Among proteins known to interact with caveolin, there are exceptions to the strict requirement for a consensus caveolin-binding motif with 3 or 4 aromatic residues. For example, the motif φXφXXXXφXXφ is present in Gαi and Gαo proteins, but the sequence of other G proteins known to interact with caveolin varies at one or more key positions; for example, Gαs has the sequence TKFQVDKVNFHMFDA, and Gαq has YFDLQSVIFRMVDA (23). Our data indicate that the β3a-AR C terminus interacts with caveolin-1 despite lacking one of the consensus aromatic residues. More broadly, there may be many GPCRs that interact with the caveolin scaffolding domain of caveolin-1 despite lacking any cytoplasmic sequences that conform strictly to the φXφXXXXφ or φXXXXφXXφ consensus sites.
It has been pointed out that there is no single sorting signal that directs localization of GPCRs to membrane rafts (41). In addition, this localization may be unaffected, increased, or decreased by agonist treatment (3). For example, the δ-opioid receptor redistributes into raft domains upon agonist activation (42), possibly because the activated receptor adopts a longer conformation that has higher affinity for areas of the membrane bilayer such as rafts that are thickened due to enrichment with sphingomyelin (41). The α1A-AR, on the other hand, colocalizes with raft markers both before and immediately after agonist stimulation, but it moves from membrane rafts within 3–10 min (43). Another study has shown that signaling by the β2-AR is constrained by exclusion from cholesterol-rich raft nanodomains that are enriched in other components of the signaling machinery, including Gαs and AC (12). Increasing the abundance of liquid-ordered raft domains by increasing cholesterol content or overexpressing caveolin-3 inhibits β2-AR-mediated cAMP responses, whereas disruption of rafts by cholesterol extraction with methyl-β-cyclodextrin increases both basal and maximal agonist-stimulated cAMP accumulation. Similar effects have been demonstrated in C6 glioma cells that express endogenous β2-ARs, where disruption of membrane rafts with methyl-β-cyclodextrin or knockdown of caveolin-1/caveolin-2 by siRNA led to increased cAMP accumulation (44). Our Duolink proximity assay suggests that the β3a-AR interacts with caveolin-1 in the absence of agonist, and in contrast to the β2-AR studies, our functional data with filipin III indicate that signaling is more efficient in the presence of membrane rafts, both in CHO-K1 cells expressing the β3a-AR and in brown adipocytes with endogenous receptors.
Chimeric Gαq/Gαs and Gαq/Gαi chimeras have been used as an alternative tool to test the coupling of mouse β3a-AR and β3b-AR isoforms (34). The Gα constructs consisted of Gαq with the C-terminal five amino acids that determine receptor coupling replaced by those from Gαs or Gαi (45, 46). This provides a single readout (increased intracellular Ca2+) to measure the relative efficiency of coupling to Gαs and Gαi subunits. The β3a-AR and β3b-AR both coupled less efficiently to Gαi than to Gαs, but there was no difference in the relative coupling of each isoform to Gαi. This result is entirely consistent with our data, as we have also shown that there is no inherent difference in the capacity of the β3a-AR and β3b-AR to couple to Gαi/o (22). Instead, the difference between the two isoforms resides in their differential interaction with caveolin-1 and localization in membrane rafts/caveolae. In the study by Lenard et al. (34), membrane localization of the chimeric Gα subunits would be determined by the common Gαq component. It has been shown previously that Gαq interacts with caveolin-1 (47, 48) and is enriched in membrane raft fractions (49), although others have shown that the raft localization of Gαq is dependent on the extraction procedure used (43). Even if the Gαq chimeras were enriched in membrane rafts relative to bulk membrane, the high abundance of these proteins (34) would likely mask any differences in the Gαq/i coupling of the β3a-AR and β3b-AR isoforms.
In CHO-K1 cells, membrane rafts are enriched in Gαi/o and Gαs relative to the bulk membrane (50–52). The two predominant adenylyl cyclase isoforms expressed are AC6 and AC7 (53), with AC7 excluded from membrane rafts (54). In contrast, the AC6 isoform is enriched in membrane rafts, and this localization is known to be functionally important (12, 54, 55). We have shown that in CHO-K1 cells expressing the β3a-AR, filipin treatment not only enhances PTX sensitivity but also causes a right shift of the concentration-response curve to CL316243. On the other hand, filipin treatment of cells expressing the β3b-AR increases basal and maximal cAMP accumulation. In cardiomyocytes and S49 lymphoma cells expressing low levels of endogenous β-ARs, on the order of 30 fmol/mg protein, the molar ratio of receptor/Gαs protein/AC has been estimated as 1:100:3 (50). Our recombinant CHO-K1 cells have a 30-fold higher abundance of receptors, so AC6 is almost certainly the limiting step in cAMP accumulation, and the enrichment of AC6 in membrane rafts may be another key difference between β3a-AR and β3b-AR responses. In the presence of filipin, the β3a-AR may display reduced responsiveness because it loses its co-localization with AC6, whereas the β3b-AR becomes more responsive because the AC6 is redistributed throughout the membrane and has higher availability. Another key question is why the β3a-AR does not couple to Gαi/o even though these subunits are also enriched in membrane rafts (51). We suggest that despite their close proximity within rafts, coupling may be suppressed because the activity of Gαi/o is inhibited by interaction with caveolin (56). In the presence of filipin, the β3a-AR would not only lose co-localization with AC6 but also gain the ability to interact with Gαi/o that is no longer associated with caveolin.
We have shown that the effects of filipin III treatment or caveolin-1 knockdown observed in CHO-K1 cells expressing the β3a-AR are seen also in cultured brown adipocytes. These cells express the β3-AR at ∼400 fmol/mg of protein (57–59). In untreated brown adipocytes, CL316243 potency is 10-fold lower than in CHO-K1 cells, despite only a 2.5-fold lower β3-AR abundance, suggesting that the efficiency of cAMP generation is also decreased in the adipocytes. In CHO-β3a-AR cells, filipin III treatment reduced the pEC50 of CL316243 without affecting the maximum response. In contrast, filipin III treatment of brown adipocytes had no effect on the pEC50 of CL316243, but the maximum cAMP response was reduced. This is consistent with the overall lower potency of CL316243 in adipocytes, evidence that a 10-fold higher receptor occupancy is required in adipocytes to achieve a maximum cAMP response. When signaling efficiency is reduced further in the presence of filipin III, high concentrations of CL316243 cannot produce the same maximum response as that seen without filipin III. It has been shown that caveolin-1 knock-out mice have compromised cAMP accumulation in response to CL316243, due to composite effects on β3-AR abundance and AC activity (18). Our data extend these findings by demonstrating that both filipin III treatment and knockdown of caveolin-1 in brown adipocytes cause cAMP responses to become PTX-sensitive, indicating that the β3a-AR acquires coupling to Gαi/o proteins.
In conclusion, our study demonstrates that the β3a-AR interacts with caveolin-1 and that the interaction affects functional coupling of the receptor to Gαi/o and Gαs subunits. Our work and that of others indicates that the reduced β3-AR signaling in mice lacking caveolin-1 is due to disruption of a signaling complex containing caveolin-1, the β3a-AR, Gαs, and adenylyl cyclase and not just due to reduced β3-AR abundance (16–18). Physiologically, the β3-AR plays a major role in white and brown adipocytes, where caveolin-1 association serves to potentiate receptor-mediated cAMP accumulation and downstream responses such as lipolysis or thermogenesis. For other receptors such as the β2-AR, raft localization and caveolin interaction undergo dynamic regulation due to requirements for strict spatial and temporal control of signaling (12). Thus, although membrane raft localization is a critical factor in organizing GPCR signaling complexes, the functional impact varies between receptors (41). Continuing studies on GPCRs will provide novel insights into the determinants that dictate association of receptors with caveolins, promote localization in membrane rafts, and thereby modulate receptor signaling.
Acknowledgments
We thank Dr. Debbie Thurmond for providing the caveolin-1 siRNA constructs and Dr. Robin Anderson (with permission from Dr. T. Kurzchalia) for the caveolin-1+/+ and caveolin-1−/− mice.
This work was supported by National Health and Medical Research Council of Australia Program Grant 519461 (to P. M. Sexton, A. Christopoulos, and R. J.), National Health and Medical Research Council of Australia Career Development Award 545952 (to D. S. H.), National Health and Medical Research Council of Australia C. J. Martin Fellowship 519581 (to M. L. H.), and by Australian Research Council Linkage International Fellowship LX0989791 (to M. S.).

This article contains supplemental Tables S1–S3.
- GPCR
- G protein-coupled receptor
- AC
- adenylyl cyclase
- β-AR
- β-adrenoceptor
- β3-AR
- β3-adrenoceptor
- BAT
- brown adipose tissue
- cav-1
- caveolin-1
- Gαs
- stimulatory guanine-nucleotide binding protein
- Gαi/o
- inhibitory guanine nucleotide-binding protein
- PTX
- pertussis toxin
- GTPγS
- guanosine 5′-3-O-(thio)triphosphate
- IBMX
- 3-isobutyl-1-methylxanthine
- ANOVA
- analysis of variance
- fsk
- forskolin.
REFERENCES
- 1. Simons K., Ikonen E. (1997) Functional rafts in cell membranes. Nature 387, 569–572 [DOI] [PubMed] [Google Scholar]
- 2. Sharma P., Varma R., Sarasij R. C., Ira, Gousset K., Krishnamoorthy G., Rao M., Mayor S. (2004) Nanoscale organization of multiple GPI-anchored proteins in living cell membranes. Cell 116, 577–589 [DOI] [PubMed] [Google Scholar]
- 3. Patel H. H., Murray F., Insel P. A. (2008) G-protein-coupled receptor-signaling components in membrane raft and caveolae microdomains. Handb. Exp. Pharmacol. 186, 167–184 [DOI] [PubMed] [Google Scholar]
- 4. Parton R. G., Simons K. (2007) The multiple faces of caveolae. Nat. Rev. Mol. Cell Biol. 8, 185–194 [DOI] [PubMed] [Google Scholar]
- 5. Hill M. M., Bastiani M., Luetterforst R., Kirkham M., Kirkham A., Nixon S. J., Walser P., Abankwa D., Oorschot V. M., Martin S., Hancock J. F., Parton R. G. (2008) PDRF-Cavin, a conserved cytoplasmic protein required for caveola formation and function. Cell 132, 113–124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6. Parton R. G., Hanzal-Bayer M., Hancock J. F. (2006) Biogenesis of caveolae. A structural model for caveolin-induced domain formation. J. Cell Sci. 119, 787–796 [DOI] [PubMed] [Google Scholar]
- 7. Lajoie P., Goetz J. G., Dennis J. W., Nabi I. R. (2009) Lattices, rafts, and scaffolds. Domain regulation of receptor signaling at the plasma membrane. J. Cell Biol. 185, 381–385 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8. Lajoie P., Partridge E. A., Guay G., Goetz J. G., Pawling J., Lagana A., Joshi B., Dennis J. W., Nabi I. R. (2007) Plasma membrane domain organization regulates EGFR signaling in tumor cells. J. Cell Biol. 179, 341–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Head B. P., Insel P. A. (2007) Do caveolins regulate cells by actions outside of caveolae? Trends Cell Biol. 17, 51–57 [DOI] [PubMed] [Google Scholar]
- 10. Xiang Y., Kobilka B. (2003) The PDZ-binding motif of the β2-adrenoceptor is essential for physiologic signaling and trafficking in cardiac myocytes. Proc. Natl. Acad. Sci. U.S.A. 100, 10776–10781 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11. Xiang Y., Devic E., Kobilka B. (2002) The PDZ-binding motif of the β1-adrenergic receptor modulates receptor trafficking and signaling in cardiac myocytes. J. Biol. Chem. 277, 33783–33790 [DOI] [PubMed] [Google Scholar]
- 12. Pontier S. M., Percherancier Y., Galandrin S., Breit A., Galés C., Bouvier M. (2008) Cholesterol-dependent separation of the β2-adrenergic receptor from its partners determines signaling efficacy: insight into nanoscale organization of signal transduction. J. Biol. Chem. 283, 24659–24672 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13. Balijepalli R. C., Foell J. D., Hall D. D., Hell J. W., Kamp T. J. (2006) Localization of cardiac L-type Ca2+ channels to a caveolar macromolecular signaling complex is required for β2-adrenergic regulation. Proc. Natl. Acad. Sci. U.S.A. 103, 7500–7505 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14. Calaghan S., White E. (2006) Caveolae modulate excitation-contraction coupling and β2-adrenergic signaling in adult rat ventricular myocytes. Cardiovasc. Res. 69, 816–824 [DOI] [PubMed] [Google Scholar]
- 15. Cohen A. W., Razani B., Schubert W., Williams T. M., Wang X. B., Iyengar P., Brasaemle D. L., Scherer P. E., Lisanti M. P. (2004) Role of caveolin-1 in the modulation of lipolysis and lipid droplet formation. Diabetes 53, 1261–1270 [DOI] [PubMed] [Google Scholar]
- 16. Ahmad F., Lindh R., Tang Y., Ruishalme I., Ost A., Sahachartsiri B., Strålfors P., Degerman E., Manganiello V. C. (2009) Differential regulation of adipocyte PDE3B in distinct membrane compartments by insulin and the β3-adrenergic receptor agonist CL316243. Effects of caveolin-1 knockdown on formation/maintenance of macromolecular signaling complexes. Biochem. J. 424, 399–410 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17. Cohen A. W., Schubert W., Brasaemle D. L., Scherer P. E., Lisanti M. P. (2005) Caveolin-1 expression is essential for proper nonshivering thermogenesis in brown adipose tissue. Diabetes 54, 679–686 [DOI] [PubMed] [Google Scholar]
- 18. Mattsson C. L., Andersson E. R., Nedergaard J. (2010) Differential involvement of caveolin-1 in brown adipocyte signaling. Impaired β3-adrenergic, but unaffected LPA, PDGF, and EGF receptor signaling. Biochim. Biophys. Acta 1803, 983–989 [DOI] [PubMed] [Google Scholar]
- 19. Mattsson C. L., Csikasz R. I., Shabalina I. G., Nedergaard J., Cannon B. (2010) Caveolin-1-ablated mice survive in cold by nonshivering thermogenesis despite desensitized adrenergic responsiveness. Am. J. Physiol. Endocrinol. Metab. 299, E374–E383 [DOI] [PubMed] [Google Scholar]
- 20. Evans B. A., Papaioannou M., Hamilton S., Summers R. J. (1999) Alternative splicing generates two isoforms of the β3-adrenoceptor which are differentially expressed in mouse tissues. Br. J. Pharmacol. 127, 1525–1531 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21. Hutchinson D. S., Bengtsson T., Evans B. A., Summers R. J. (2002) Mouse β3a- and β3b-adrenoceptors expressed in Chinese hamster ovary cells display identical pharmacology but utilize distinct signaling pathways. Br. J. Pharmacol. 135, 1903–1914 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Sato M., Hutchinson D. S., Bengtsson T., Floren A., Langel U., Horinouchi T., Evans B. A., Summers R. J. (2005) Functional domains of the mouse β3-adrenoceptor associated with differential G protein coupling. J. Pharmacol. Exp. Ther. 315, 1354–1361 [DOI] [PubMed] [Google Scholar]
- 23. Couet J., Li S., Okamoto T., Ikezu T., Lisanti M. P. (1997) Identification of peptide and protein ligands for the caveolin-scaffolding domain. Implications for the interaction of caveolin with caveolae-associated proteins. J. Biol. Chem. 272, 6525–6533 [DOI] [PubMed] [Google Scholar]
- 24. Nevins A. K., Thurmond D. C. (2006) Caveolin-1 functions as a novel Cdc42 guanine nucleotide dissociation inhibitor in pancreatic beta-cells. J. Biol. Chem. 281, 18961–18972 [DOI] [PubMed] [Google Scholar]
- 25. Drab M., Verkade P., Elger M., Kasper M., Lohn M., Lauterbach B., Menne J., Lindschau C., Mende F., Luft F. C., Schedl A., Haller H., Kurzchalia T. V. (2001) Loss of caveolae, vascular dysfunction, and pulmonary defects in caveolin-1 gene-disrupted mice. Science 293, 2449–2452 [DOI] [PubMed] [Google Scholar]
- 26. Néchad M., Kuusela P., Carneheim C., Björntorp P., Nedergaard J., Cannon B. (1983) Development of brown fat cells in monolayer culture. I. Morphological and biochemical distinction from white fat cells in culture. Exp. Cell Res. 149, 105–118 [DOI] [PubMed] [Google Scholar]
- 27. Chernogubova E., Hutchinson D. S., Nedergaard J., Bengtsson T. (2005) α1- and β1-adrenoceptor signaling fully compensates for β3-adrenoceptor deficiency in brown adipocyte norepinephrine-stimulated glucose uptake. Endocrinology 146, 2271–2284 [DOI] [PubMed] [Google Scholar]
- 28. Nevzorova J., Bengtsson T., Evans B. A., Summers R. J. (2002) Characterization of the β-adrenoceptor subtype involved in mediation of glucose transport in L6 cells. Br. J. Pharmacol. 137, 9–18 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Tanishita T., Shimizu Y., Minokoshi Y., Shimazu T. (1997) The β3-adrenergic agonist BRL37344 increases glucose transport into L6 myocytes through a mechanism different from that of insulin. J. Biochem. 122, 90–95 [DOI] [PubMed] [Google Scholar]
- 30. Kaslow H. R., Lim L. K., Moss J., Lesikar D. D. (1987) Structure-activity analysis of the activation of pertussis toxin. Biochemistry 26, 123–127 [DOI] [PubMed] [Google Scholar]
- 31. Moss J., Stanley S. J., Burns D. L., Hsia J. A., Yost D. A., Myers G. A., Hewlett E. L. (1983) Activation by thiol of the latent NAD glycohydrolase and ADP-ribosyltransferase activities of Bordetella pertussis toxin (islet-activating protein). J. Biol. Chem. 258, 11879–11882 [PubMed] [Google Scholar]
- 32. Halls M. L., van der Westhuizen E. T., Wade J. D., Evans B. A., Bathgate R. A., Summers R. J. (2009) Relaxin family peptide receptor (RXFP1) coupling to Gαi3 involves the C-terminal Arg-752 and localization within membrane Raft microdomains. Mol. Pharmacol. 75, 415–428 [DOI] [PubMed] [Google Scholar]
- 33. Mistry R., Dowling M. R., Challiss R. A. (2011) [35S]GTPγS binding as an index of total G-protein and Gα-subtype-specific activation by GPCRs. Methods Mol. Biol. 746, 263–275 [DOI] [PubMed] [Google Scholar]
- 34. Lenard N. R., Prpic V., Adamson A. W., Rogers R. C., Gettys T. W. (2006) Differential coupling of β3A- and β3B-adrenergic receptors to endogenous and chimeric Gαs and Gαi. Am. J. Physiol. Endocrinol. Metab. 291, E704–E715 [DOI] [PubMed] [Google Scholar]
- 35. Hutchinson D. S., Sato M., Evans B. A., Christopoulos A., Summers R. J. (2005) Evidence for pleiotropic signaling at the mouse β3-adrenoceptor revealed by SR59230A [3-(2-ethylphenoxy)-1-[(1S)-1,2,3,4-tetrahydronapth-1-ylamino]-2S-2-propanol oxalate]. J. Pharmacol. Exp. Ther. 312, 1064–1074 [DOI] [PubMed] [Google Scholar]
- 36. Söderberg O., Gullberg M., Jarvius M., Ridderstråle K., Leuchowius K. J., Jarvius J., Wester K., Hydbring P., Bahram F., Larsson L. G., Landegren U. (2006) Direct observation of individual endogenous protein complexes in situ by proximity ligation. Nat. Methods 3, 995–1000 [DOI] [PubMed] [Google Scholar]
- 37. Chernogubova E., Cannon B., Bengtsson T. (2004) Norepinephrine increases glucose transport in brown adipocytes via β3-adrenoceptors through a cAMP, PKA, and PI 3-kinase-dependent pathway stimulating conventional and novel PKCs. Endocrinology 145, 269–280 [DOI] [PubMed] [Google Scholar]
- 38. Abrami L., Fivaz M., Kobayashi T., Kinoshita T., Parton R. G., van der Goot F. G. (2001) Cross-talk between caveolae and glycosylphosphatidylinositol-rich domains. J. Biol. Chem. 276, 30729–30736 [DOI] [PubMed] [Google Scholar]
- 39. Zeng Y., Tao N., Chung K. N., Heuser J. E., Lublin D. M. (2003) Endocytosis of oxidized low density lipoprotein through scavenger receptor CD36 utilizes a lipid raft pathway that does not require caveolin-1. J. Biol. Chem. 278, 45931–45936 [DOI] [PubMed] [Google Scholar]
- 40. Cheng Z. J., Singh R. D., Holicky E. L., Wheatley C. L., Marks D. L., Pagano R. E. (2010) Co-regulation of caveolar and Cdc42-dependent fluid phase endocytosis by phosphocaveolin-1. J. Biol. Chem. 285, 15119–15125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bethani I., Skånland S. S., Dikic I., Acker-Palmer A. (2010) Spatial organization of transmembrane receptor signaling. EMBO J. 29, 2677–2688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42. Alves I. D., Salamon Z., Hruby V. J., Tollin G. (2005) Ligand modulation of lateral segregation of a G-protein-coupled receptor into lipid microdomains in sphingomyelin/phosphatidylcholine solid-supported bilayers. Biochemistry 44, 9168–9178 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43. Morris D. P., Lei B., Wu Y. X., Michelotti G. A., Schwinn D. A. (2008) The α1a-adrenergic receptor occupies membrane rafts with its G protein effectors but internalizes via clathrin-coated pits. J. Biol. Chem. 283, 2973–2985 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Allen J. A., Yu J. Z., Dave R. H., Bhatnagar A., Roth B. L., Rasenick M. M. (2009) Caveolin-1 and lipid microdomains regulate Gs trafficking and attenuate Gs/adenylyl cyclase signaling. Mol. Pharmacol. 76, 1082–1093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45. Conklin B. R., Herzmark P., Ishida S., Voyno-Yasenetskaya T. A., Sun Y., Farfel Z., Bourne H. R. (1996) C-terminal mutations of Gqα and Gsα that alter the fidelity of receptor activation. Mol. Pharmacol. 50, 885–890 [PubMed] [Google Scholar]
- 46. Coward P., Chan S. D., Wada H. G., Humphries G. M., Conklin B. R. (1999) Chimeric G proteins allow a high throughput signaling assay of Gi-coupled receptors. Anal. Biochem. 270, 242–248 [DOI] [PubMed] [Google Scholar]
- 47. de Weerd W. F., Leeb-Lundberg L. M. (1997) Bradykinin sequesters B2 bradykinin receptors and the receptor-coupled Gα subunits Gαq and Gαi in caveolae in DDT1 MF-2 smooth muscle cells. J. Biol. Chem. 272, 17858–17866 [DOI] [PubMed] [Google Scholar]
- 48. Bhatnagar A., Sheffler D. J., Kroeze W. K., Compton-Toth B., Roth B. L. (2004) Caveolin-1 interacts with 5-HT2A serotonin receptors and profoundly modulates the signaling of selected Gαq-coupled protein receptors. J. Biol. Chem. 279, 34614–34623 [DOI] [PubMed] [Google Scholar]
- 49. Sugawara Y., Nishii H., Takahashi T., Yamauchi J., Mizuno N., Tago K., Itoh H. (2007) The lipid raft proteins flotillins/reggies interact with Gαq and are involved in Gq-mediated p38 mitogen-activated protein kinase activation through tyrosine kinase. Cell. Signal. 19, 1301–1308 [DOI] [PubMed] [Google Scholar]
- 50. Ostrom R. S., Post S. R., Insel P. A. (2000) Stoichiometry and compartmentation in G protein-coupled receptor signaling: implications for therapeutic interventions involving G(s). J. Pharmacol. Exp. Ther. 294, 407–412 [PubMed] [Google Scholar]
- 51. Razani B., Woodman S. E., Lisanti M. P. (2002) Caveolae. From cell biology to animal physiology. Pharmacol. Rev. 54, 431–467 [DOI] [PubMed] [Google Scholar]
- 52. Head B. P., Patel H. H., Roth D. M., Murray F., Swaney J. S., Niesman I. R., Farquhar M. G., Insel P. A. (2006) Microtubules and actin microfilaments regulate lipid raft/caveolae localization of adenylyl cyclase signaling components. J. Biol. Chem. 281, 26391–26399 [DOI] [PubMed] [Google Scholar]
- 53. Varga E. V., Stropova D., Rubenzik M., Wang M., Landsman R. S., Roeske W. R., Yamamura H. I. (1998) Identification of adenylyl cyclase isoenzymes in CHO and B82 cells. Eur. J. Pharmacol. 348, R1–R2 [DOI] [PubMed] [Google Scholar]
- 54. Crossthwaite A. J., Seebacher T., Masada N., Ciruela A., Dufraux K., Schultz J. E., Cooper D. M. (2005) The cytosolic domains of Ca2+-sensitive adenylyl cyclases dictate their targeting to plasma membrane lipid rafts. J. Biol. Chem. 280, 6380–6391 [DOI] [PubMed] [Google Scholar]
- 55. Thangavel M., Liu X., Sun S. Q., Kaminsky J., Ostrom R. S. (2009) The C1 and C2 domains target human type 6 adenylyl cyclase to lipid rafts and caveolae. Cell. Signal. 21, 301–308 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 56. Xu W., Yoon S. I., Huang P., Wang Y., Chen C., Chong P. L., Liu-Chen L. Y. (2006) Localization of the κ-opioid receptor in lipid rafts. J. Pharmacol. Exp. Ther. 317, 1295–1306 [DOI] [PubMed] [Google Scholar]
- 57. Sillence M. N., Moore N. G., Pegg G. G., Lindsay D. B. (1993) Ligand binding properties of putative β3-adrenoceptors compared in brown adipose tissue and in skeletal muscle membranes. Br. J. Pharmacol. 109, 1157–1163 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 58. Adli H., Bazin R., Vassy R., Perret G. Y. (1997) Effects of triiodothyronine administration on the adenylyl cyclase system in brown adipose tissue of rat. Am. J. Physiol. 273, E247–E253 [DOI] [PubMed] [Google Scholar]
- 59. Malo A., Puerta M. (2001) Oestradiol and progesterone change β3-adrenergic receptor affinity and density in brown adipocytes. Eur. J. Endocrinol. 145, 87–91 [DOI] [PubMed] [Google Scholar]