Abstract
We investigated the mechanism whereby expression of a transgene encoding a locally acting isoform of insulin-like growth factor 1 (mIGF-1) enhances repair of skeletal muscle damage. Increased recruitment of proliferating bone marrow cells to injured MLC/mIgf-1 transgenic muscles was accompanied by elevated bone marrow stem cell production in response to distal trauma. Regenerating MLC/mIgf-1 transgenic muscles contained increased cell populations expressing stem cell markers, exhibited accelerated myogenic differentiation, expressed markers of regeneration and readily converted cocultured bone marrow to muscle. These data implicate mIGF-1 as a powerful enhancer of the regeneration response, mediating the recruitment of bone marrow cells to sites of tissue damage and augmenting local repair mechanisms.
Skeletal muscle regeneration involves the activation of quiescent satellite cells, which participate in the reconstitution of damaged tissues. Recent studies have identified another source of muscle stem cells (SC), named side population (SP) on the basis of their Hoechst dye exclusion properties (1). Originating in the bone marrow (2), these cells can home to various tissues, differentiating into multiple cell types (3–8). Although in lethally irradiated mice, bone marrow-derived SCs replenish the depleted satellite cell pool and subsequently incorporate effectively into exercised skeletal muscle (9), less is known about the ability of bone-marrow-derived SCs to ameliorate muscle damage under more clinically relevant conditions. Indeed, the transplantation of bone marrow-derived SCs into the mdx dystrophic mouse model had a limited impact on muscle cell replacement (10), suggesting that the poor recruitment of circulating SCs is one of the limiting factors for tissue repair (10, 11). Subsequent studies in nonirradiated mice have identified resident stem-like cell populations in skeletal muscle distinct from satellite cells (12–14), which undergo myogenesis by means of myocyte-mediated inductive interactions (15).
We have previously reported that postmitotic expression of a local isoform of insulin-like growth factor 1 (mIGF-1) induces myocyte hypertrophy (16), increases mass and strength of postnatal muscle, and preserves regenerative capacity in senescent and dystrophic mice (17–19). In the present study, enhanced muscle regeneration was accompanied by increased recruitment of marked, transplanted bone marrow SCs to sites of muscle injury after lethal irradiation. In nonirradiated MLC/mIgf-1 transgenic mice, muscle injury expanded the SP compartment in the bone marrow. Transgenic animals also had elevated levels of cells coexpressing markers of SC lineage and myogenic commitment at sites of muscle damage. When isolated from regenerating muscles, these cells exhibited accelerated myogenic differentiation in culture and readily induced muscle-specific markers in a subset of cocultured bone marrow cells. These results establish mIGF-1 as a potent regenerative agent, increasing bone marrow and local SC pools and providing a mechanistic explanation for the dramatic effects of supplemental MLC/mIgf-1 transgene expression on muscle mass and integrity both in vitro and in vivo.
Materials and Methods
c-kit/GFP Transgenic Mice. Transgenic mice were generated carrying the GFP gene driven by a 6.7-kb mouse c-kit promoter linked to fragments derived from the first intron including five chromatin DNase I hypersensitive sites (50). The GFP transgene was highly expressed in the majority of c-kit+ cells in embryonic sites of haematopoiesis, such as the aorta gonad mesonephros region, fetal liver, and bone marrow.
Bone Marrow Transplantation and SC Analysis. The SP from bone marrow cells was isolated from femur and tibia of 8-week-old c-kit/GFP transgenic mice by fluorescence-activated cell sorter (FACS) analysis. WT and MLC/mIgf-1 transgenic recipient mice were lethally irradiated with 9.8 Gy, and each recipient received 1 × 103 SP-derived-c-kit/GFP cells by tail-vein injection. Recipient mice also received 1.5 × 105 non-SP cells isolated from WT mice. Irradiated mice were maintained on acidified water and in virus-pathogen-free conditions. Mice exposed to marrow-ablative doses of γ irradiation survive 12–14 days in the absence of bone marrow transplantation. After 15 days of irradiation, the tibialis anterior (TA) muscle was injected with cardiotoxin (CTX), and muscles were harvested 2 days after the injection. Controlateral TA was used as control. Muscle-derived cells were isolated and analyzed by FACS, as described below.
Muscle Regeneration and Immunofluorescence Analysis. TA muscle from 3-month-old WT and MLC/mIgf-1 transgenic mice were injected with 30 μl of 10 μM CTX, and muscles were harvested 2 days after injection. Controlateral TA was used as control. Cryosections (7 μm) were fixed in 4% paraformaldehyde and processed for immunofluorescence (18).
Antibodies. Antibodies were used against Sca-1, c-kit, CD-45, CD11b (Becton Dickinson Biosciences), desmin and tubulin (Sigma), MF-20 (myosin heavy chain), and Pax-7 (Hybridoma Bank, University of Iowa, Iowa City).
Flow Cytometry. Whole bone marrow cells (9) or muscle-derived cells (20) were overlaid on a Percoll gradient (70–40% in PBS) (2). Cell sorting and analyses were performed on a MoFlo triple-laser flow cytometer and summit software (Cytomation, Fort Collins, CO). For SP analysis, cells were stained with 5 μg/ml Hoechst 33342 (Sigma–Aldrich) for 90 min at 37°C (1). A 350-nm argon laser was used to excite the Hoechst dye, and fluorescence emission was collected with 405/30 and 670/40 BP bandpass filters. A 488-nm argon laser was used for FITC, phycoerythrin (PE), and PE-Cy5 excitation.
Muscle Primary Cultures. Muscle cultures were prepared as described (20). Sca-1+/c-kit+ and Sca-1+ cells were isolated by FACS analysis and cultured in growth medium (GM) (DMEM supplemented with 20% horse serum/3% chick embryonic extract). After 5 days of culture, primary muscle cells were shifted in differentiation medium (DMEM supplemented with 5% horse serum/3% chick embryonic extract). Muscle primary cultures were harvested at different time points, fixed in 4% paraformaldehyde, and processed for immunofluorescence and/or β-galactosidase analysis as described (16, 21).
Bone Marrow–Myoblast Coculture. Myoblasts were cultured as described above. After 3 days in GM, total bone marrow cells or c-kit+/Sca-1+ enriched cells isolated from desmin/nLacZ transgenic mice (22) were added to a monolayer of proliferating myoblasts. Nonadherent cells were removed with PBS washes after 40 min. Adherent bone marrow cells and myoblasts were maintained for 24 h in GM and then shifted in differentiation medium. After 4 days of coculture, cells were harvested, fixed in 4% paraformaldehyde, and stained for β-galactosidase (18).
5-Fluorouracil (5-FU) Treatment. WT and MLC/mIgf-1 transgenic mice were injected i.v. with 250 mg of 5-FU per kg of body weight (Sigma). TA muscle was injected with CTX 24 h after 5-FU administration; bone marrow SP and muscle cells were analyzed by FACS after 48 h.
RT-PCR. Muscle-derived cells were purified from injured and control muscle of both WT and MLC/mIgf-1 transgenic mice, and the cell population Sca-1+/c-kit+, c-kit/Sca-1+, and Sca-1bright+ cells were isolated by a FACS-sorter instrument. Total RNA were prepared as described (18), and 0.2 μg was used in each RT-PCR assay. The following oligonucleotides were used: Flk1 sense 5′-TCTGTGGTTCTGCGTGGAGA-3′ and antisense 5′-GTATCATTTCCAACCACCCT-3′, MyoD sense 5′-CACTACAGTGGCGACTCAGACGCG-3′ and antisense 5′-CCTGGACTCGCGCACCGCCTCACT-3′, and Emx2 sense 5′-CCCAGCTTTTAAGGCTAGAG-3′ and antisense 5′-CTCCGGTTCTGAAACCATAC-3′.
Western Blot Analysis. TA muscles injected with CTX and controlateral control TA were homogenized in modified lysis buffer (10 mM Tris·HCl, pH 7.4/150 mM NaCl/1% Nonidet P-40/1% sodium deoxycholate/0.1% SDS/10% glycerol) as described (23). Equal amounts of protein were separated by 12% SDS/PAGE, and standard blotting procedures were used.
Results
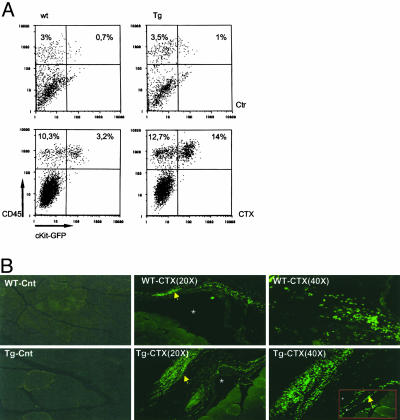
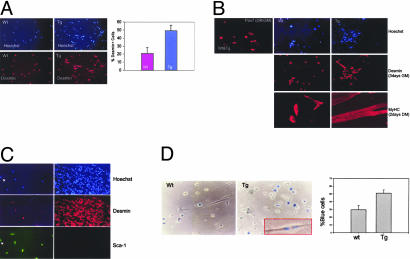
The mIGF-1 Isoform Increases Recruitment of Bone Marrow-Derived SCs to Injured Muscle. Among growth factors, IGF-1 plays a key role in muscle regeneration after injury (24). To determine whether local expression of the MLC/mIgf-1 transgene enhances recruitment of bone marrow SCs observed after lethal irradiation and toxin-induced muscle damage (9), we transplanted lethally irradiated 3-month-old MLC/mIgf-1 and nontransgenic siblings with 103 bone marrow SP cells derived from a transgenic mouse expressing GFP driven by the c-kit promoter (25, 26). Using radiation doses that ablate bone marrow cell reserves as well as muscle satellite cell reserves (ref. 9 and T. Partridge, personal communication), we followed the recruitment of transplanted c-kit/GFP bone marrow cells by induction of local myonecrosis with a single CTX injection in the TA muscle 15 days posttransplant (18, 27). The effects of MLC/mIgf-1 transgene expression on bone marrow cell recruitment were assessed 48 h later by FACS analysis of cells expressing the c-kit/GFP transgene and CD45, a cell surface marker expressed exclusively by the hematopoietic lineage (2, 28). Immediate recruitment of CD45+/GFP+ cells to injured muscle was increased 4-fold above WT levels by local expression of mIGF-1 (Fig. 1). Similar results were obtained from three additional mice in which muscle injury was induced 6 weeks posttransplant (data not shown), as predicted from previous studies (9). Transplanted bone marrow-derived non-SP cells from c-kit/GFP transgenic mice did not rescue irradiated bone marrow (data not shown), confirming that the reconstituting SC population is mainly localized in the SP tail (1). Although lethal irradiation is a significant confounder in defining parameters of SC recruitment because preirradiation of skeletal muscle induces local proliferation and enhances migration of transplanted myogenic precursors (29), expression of the MLC/mIgf-1 transgene in nonirradiated regenerating muscle also enhanced c-kit/GFP cell recruitment when compared to c-kit/GFP littermate lacking mIGF-1 (Fig. 1B).
Fig. 1.
Local mIGF-1 expression enhances the recruitment of bone marrow SP cells to injured muscle. (A) TA muscle of lethally irradiated WT and MLC/mIgf-1 transgenic (Tg) mice reconstituted with c-kit/GFP bone marrow were injected with CTX, and GFP/CD45+ cells were analyzed by FACS analysis. Controlateral TA was used as control muscle (Ctr). (B) Increased GFP+ cells (yellow arrow) in nonirradiated MLC/mIgf-1 × c-kit/GFP double transgenic mice compared to c-kit/GFP littermates. Necrotic areas are indicated by asterisks. (Inset) GFP+ cells near the muscle fibers.
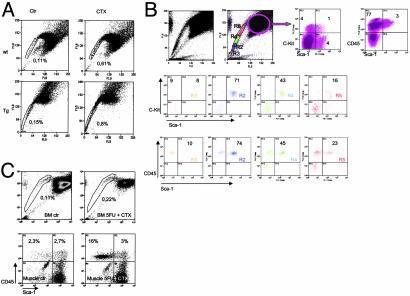
Bone Marrow SC Expansion Induced by Distal Muscle Injury Is Enhanced by mIGF-1. Muscle injury induced a 5- to 6-fold increase in bone marrow SP cells of both WT and MLC/mIgf-1 mice (Fig. 2A), not seen in other tissues (data not shown). The bone marrow-SP tail of injured MLC/mIgf-1 muscle included a thickened region expressing high levels of hematopoietic cell surface markers CD45, c-kit, and Sca-1 (2, 30) (Fig. 2B, R2, blue). Treatment of injured mice with 5-FU, a cytotoxic agent that depletes cycling SCs (26), was sufficient to block proliferation of bone marrow SP (Fig. 2C Upper) and expansion of the CD45+/Sca-1+ population in injured muscle, whereas the CD45+/Sca-1– population, representing nonproliferating monocyte infiltration at the site of tissue damage, remained unaffected (Fig. 2C Lower). Thus, even under nonirradiating conditions, mIGF-1-enhanced signals emanating from the site of injury, inducing proliferation of SC populations both in the damaged muscle and in distal bone marrow.
Fig. 2.
Regenerating muscle augments local and distal SC compartments. (A) Increased bone marrow SP in WT and MLC/mIgf-1 (Tg) mice after injection with CTX (Right). (B) Fractionation of the bone marrow SP tail from Tg injured muscle. (C) Ablation of the Sca-1+/CD45+ compartment with 5-FU (mean of four separate experiments).
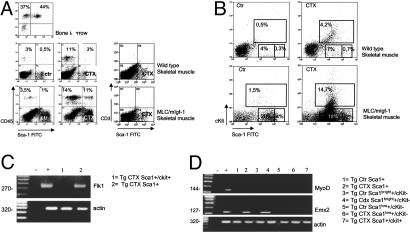
Quantitative and Qualitative Effects of Supplemental mIGF-1 in Regenerating Muscle. Both WT and transgenic muscles accumulated CD45+ cells 48 h after muscle injury (Fig. 3A), but higher levels of CD45+/Sca-1+ and kit+/Sca-1+ cells were found in MLC/mIgf-1 injured muscle than in WT (Fig. 3 A and B, CTX). These cells did not express CD3, a lymphoid lineage marker (31) (Fig. 3A Right). Because the combination of c-kit, Sca-1, and CD-45 expression is limited to SCs and precursor cells (2, 28, 30, 32–35), enhancement of the CD45+/Sca-1+ and c-kit+/Sca-1+ cells in MLC/mIgf-1 transgenic muscle marked a population of cells capable of host tissue regeneration, self renewal, and transdifferentiation (36). A c-kit-negative subpopulation with high affinity for the Sca-1 antibody, termed Sca-1bright, comprised 12% of the isolated cells from regenerating transgenic muscle (Fig. 3B Lower).
Fig. 3.
Transgenic mIGF-1 alters local cell populations mobilized by injury. (A) FACS analysis of CD45+/Sca-1+ cells in bone marrow SP and CD45+/Sca-1+, CD3+/Sca-1+ cells in muscle-derived cells from WT and Tg mice before (Ctr) and after (CTX) CTX injection. (B) c-kit+/Sca-1+ cells in WT and Tg muscle before (Left) and after (Right) CTX injection. Note the appearance of a Sca-1bright population in regenerating Tg muscle (Lower Right). (C and D) RT-PCR analysis of cell populations isolated by FACS from TA muscles injected with CTX and from controlateral TA (Ctr). (–, RT-PCR buffer without RNA sample; +, positive control; total RNA from embryonic day 9 mouse embryo analyzed for Flk1 and Emx2, and RNA from C2C12 myoblasts analyzed for MyoD). Data are the mean of six separate experiments.
Sca-1+ cells expressed the endothelial marker Flk1 (Fig. 3C), whereas Sca-1bright cells activated the expression of Emx2 (Fig. 3D), a vertebrate homeobox gene controlling neuronal SC proliferation (37–39). Thus, local mIGF-1 induces qualitative as well as quantitative changes in proliferating cell populations from regenerating muscle.
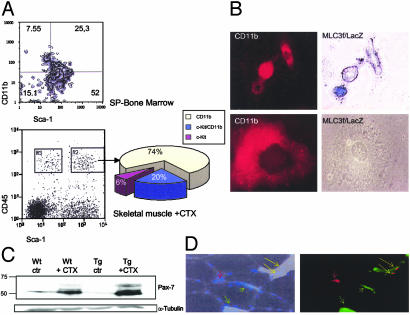
Markers of Muscle Regeneration. CD11b is an early myeloid lineage marker (40, 41), also prominent on human SP cells (42). Indeed, 25 ± 2.1% of mouse bone marrow SP cells were CD11b/Sca-1 positive (Fig. 4A). Of the CD45+ cell populations from injured MLC/mIgf-1 muscle that expressed Sca-1 (Fig. 4A, gate R2), 74 ± 1.9% were CD11b+ and 20 ± 0.9% were c-kit+/CD11b+. In contrast, cells expressing CD45 but not Sca-1 (gate R3) were 90 ± 0.8% CD11b+ with no c-kit+ cells (data not shown). Similar results were obtained from WT muscle (data not shown). Thus, expression of CD11b on CD45+Sca-1+ c-kit+ cells in the injured muscle bed defines a subgroup of progenitors that may contribute to the process of muscle regeneration. Indeed, when myoblast cultures were generated from the injured muscles of MLC3f/nLacZ mice (21, 43), a subset of small cells expressing low levels of CD11b coexpressed the muscle-specific transgene (Fig. 4B).
Fig. 4.
Transgenic mIGF-1 augments markers of muscle regeneration. (A) FACS analysis of CD11b+/Sca-1+ cells in bone marrow (Upper) and in muscle-derived cells from injured MLC/mIgf-1 (Tg) mice (Lower and chart). (B) CD11b and β-galactosidase (β-gal) expression in representative primary cells isolated from TA muscle of MLC3f/nLacZ reporter mice. Note muscle-specific β-gal expression only in a subset of small mononucleate cells (Upper). Large cells with flattened morphology never expressed the MLC3f/nLacZ transgene (Lower). (C) Western blot analysis of protein lysates from injured (CTX) and control (Ctr) TA muscles of WT and Tg mice analyzed with Pax-7 antibody. (D) Immunocytochemical analysis of Sca-1 and Pax-7 in transverse sections of TA muscles from WT and Tg mice after CTX injection. Note vessel expressing only Sca-1 (green arrow) and satellite cell expressing Pax7 (red arrow). Nuclei were visualized by Hoechst dye. Data are the mean of six separate experiments.
The homeodomain protein Pax7 has been implicated in the specification of satellite cells from multipotent SCs (44). Pax 7 products in regenerating muscles were dramatically increased in MLC/mIgf-1 transgenic mice (Fig. 4C). Although cells expressing Sca-1 were abundant in the region of mononuclear infiltration and in blood vessels of damaged muscle (Fig. 4D, green arrow), only a subpopulation of these cells coexpressed Pax7 (Fig. 4D, yellow arrow), suggesting a stepwise transition of progenitor cells to a myogenic phenotype. Notably, satellite cells positive for Pax-7 did not express Sca-1 (Fig. 4D, red arrow) (15).
Myogenic Potential of Cell Subpopulations. In primary cultures, MLC/mIgf-1 transgene expression expanded the myoblast population in response to muscle damage, containing 50% more desmin-expressing cells than did WT after 5 days in cultures (Fig. 5A). A subset of Sca-1+ cells isolated by FACS also expressed markers of the myogenic program, such as Pax-7 and desmin, exhibiting accelerated myogenic differentiation and hypertrophy (Fig. 5B). Whereas primary MLC/mIgf-1 muscle cells cultured at low cell density contained a subpopulation cells coexpressing Sca-1 and desmin (Fig. 5C), the same muscle cultures expressed exclusively desmin when cultured at increased cell density, which triggers a differentiated muscle phenotype (45, 46). Although 80% of the Sca-1+ cells displayed a myogenic phenotype, the remaining 20% differentiated spontaneously into cells with adipocyte, cardiomyocyte, and neuronal-like characteristics (data not shown). In the context of damaged muscle, Sca-1 therefore marks multipotent progenitor cells endowed with myogenic potential.
Fig. 5.
Sca-1 expression defines early muscle cell precursors. (A) Relative desmin expression in primary cultures from injured WT and MLC/mIgf-1 (Tg) muscles. (B) Pax-7, desmin, and MyHC expression in Sca-1+ cells isolated by FACS from injured WT and Tg muscle and cultured in GM. (C) Coexpression of Sca-1 and desmin in primary Tg muscle cultures. (Left) Cells harvested after 24 h in GM. (Right) Cells plated at high density and harvested after 4 days in GM. (D) Bone marrow cells isolated from desmin/nLacZ transgenic mice cocultured with primary myoblast monolayers prepared from WT and MLC/mIgf-1 (Tg) mice and stained for β-galactosidase after 4 days. (Right) Representative percentage of blue cells (of a total of 70 cells) in WT and Tg cocultures (mean of six separate experiments).
Previous studies have demonstrated that skeletal myoblasts can stimulate myogenic conversion in cocultured muscle SP cells (15). When total bone marrow cells or the c-kit+/Sca-1+ fraction were harvested from mice carrying a transgenic desmin/nLacZ marker (22) and added to a monolayer of proliferating WT and MLC/mIgf-1 transgenic myoblasts, adherent desmin/nLacZ cells converted efficiently to a myogenic lineage in vitro after 4 days of coculture (Fig. 5D) and incorporated into multinucleate myotubes (Fig. 5D Inset). Myogenic conversion was significantly more efficient on MLC/mIgf-1 myoblast monolayers (Fig. 5D). Notably, c-kit+/Sca-1+ cells sorted from desmin/nLacZ bone marrow or muscle tissue did not undergo myogenic differentiation on serum withdrawal when cultured alone (data not shown). Thus c-kit+/Sca-1+ cells are not inherently myogenic; their conversion to a myogenic lineage by exposure to myoblasts is enhanced by MLC/mIgf-1 transgene expression.
Discussion
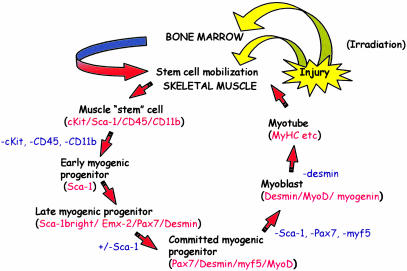
Although SC-mediated tissue regeneration has promising therapeutic potential, bone marrow-derived SC incorporation into skeletal muscle has heretofore been clinically insignificant (2, 3, 11, 47). Here we show that enhanced mobilization and homing of bone marrow-derived cells by local mIGF-1 is triggered by damage and presumably contributes to increases in muscle mass, strength, and resistance in age-related atrophy and degenerative disease (17–19). In MLC/mIgf-1 transgenic muscle, the dramatic increase in the bone marrow SP immediately after local muscle injury suggests a potential feedback mechanism whereby this rich source of SCs is mobilized in response to distal trauma. The 4-fold increase in c-kit/GFP+ cells migrating to the injured muscle of MLC/mIgf-1 transgenic mice early in the regeneration process suggests that mIGF-1 induces the production of local signals to increase preferential recruitment of circulating SCs to regenerating tissue. The results in this study are consistent with a model (Fig. 6) whereby injury in the context of irradiation stimulates the mobilization of uncommitted cell subsets in the bone marrow, which migrate to sites of tissue damage and participate in the regeneration process. The enhancement of c-kit+ cells in regenerating MLC/mIgf-1 muscle tissue does not depend on irradiation, as confirmed by the increase in c-kit/GFP+ cells after muscle injury of nonirradiated double transgenic mice.
Fig. 6.
Model of SC-mediated muscle regeneration enhanced by mIGF-1. Supplemental mIGF-1 enhances the expression of signals from injured muscle (yellow arrows), inducing distal SC mobilization in transplanted bone marrow of irradiated animals and migration to the injured tissue (green–red arrow) or expanding local muscle SC pools. Thereafter, sequential stages of cell commitment to the myogenic lineage (markers in red) are promoted and accelerated by muscle-specific mIGF-1 expression, which induces new markers of regeneration in Sca-1bright cells. Subsequent loss of SC markers (blue) correlates with activation of myogenic commitment and differentiation programs.
Our results establish proliferation as an important property of regeneration, because the dramatic increase in cells coexpressing CD45, c-kit, CD11b, and Sca-1 in MLC/mIgf-1 injured muscles was ablated by administration of 5-FU, a potent repressor of the cell cycle. The present study establishes expression of Sca-1 as another marker of enhanced muscle repair. Although expression of Sca-1 in satellite cells remains controversial, (ref. 15 and G. Pavlath, personal communication), the present study suggests that mIGF-1 improves muscle regeneration by increasing the Sca-1+ cell population and promoting their differentiation toward a myogenic lineage. The present study, in which a high proportion (80%) of CD45–Sca-1+ cells harvested from regenerating muscle expressed myogenic markers such as Pax7, desmin, and MyHC, contrasts with a previous report in which only 4% of CD45-Sca-1+ cells from regenerating muscles coexpressed a Myf5nLacZ transgene (48). Isolation of the CD45-Sca-1+ population earlier after injury (48 h vs. 96 h, ref. 48), and subsequent cultivation in promyogenic conditions for a longer period, may capture a larger population of regenerative cells with myogenic potential.
The inability of c-kit+/Sca-1+ cells from injured muscle to express myogenic markers unless cocultured with committed myoblasts suggests that c-kit expression marks a block in the initiation of the myogenic program. Indeed, Flk-1, a marker of endothelial and mesenchymal precursors, is expressed only in cells lacking c-kit. In contrast to previous studies (15), cultured Sca-1+ cells readily expressed Pax7, a homeobox-containing transcription factor critical for the specification of satellite cells from a multipotent SC pool (44) but not essential for full myogenic differentiation (15). Muscle injury dramatically induced the levels and complexity of Pax7 protein isoforms, which were further augmented in the MLC/mIgf-1 transgenic background, and a population of cells in injured MLC/mIgf-1 transgenic muscle expressed high levels of Sca-1 and Emx-2, a gene encoding a Hox protein controlling symmetry of neural SC division (37–39). Notably, Emx2 expression is associated with the regeneration of the newt limb amputation in a region giving rise to the blastema, a territory composed of mesenchymal multipotent cells (49). Although the functional significance of Emx-2 activation in mammalian regeneration remains to be explored, its expression reflects a qualitative change in the damage response of MLC/mIgf-1 transgenic mice. Current studies are in progress to define the roles played by these factors in enhancement of muscle regeneration by mIGF-1.
Acknowledgments
We thank Marcello Coletta, Carmine Nicoletti, Pierpaolo Coluccia, Stefania Straino, and Esfir Slonimsky for technical assistance, and Bianca Maria Scicchitano for critical comments on the manuscript. This work was supported by grants from Telethon-Italy (Grant GP0098Y01 to A.M.); Ministero della Salute; the Muscular Dystrophy Association (to N.R.); Ministero dell'Istruzione, dell'Università e della Ricerca (to M.M.); and Telethon and Associazione Italiana per la Ricerca sul Cancro (to S.O.).
This paper was submitted directly (Track II) to the PNAS office.
Abbreviations: IGF, insulin-like growth factor; mIGF-1, local isoform of IGF-1; SC, stem cells; SP, side population; TA, tibialis anterior; CTX, cardiotoxin; GM, growth medium; 5-FU, 5-fluorouracil.
References
- 1.Goodell, M. A., Brose, K., Paradis, G., Conner, A. S. & Mulligan, R. C. (1996) J. Exp. Med. 183, 1797–1806. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.McKinney-Freeman, S. L., Jackson, K. A., Camargo, F. D., Ferrari, G., Mavilio, F. & Goodell, M. A. (2002) Proc. Natl. Acad. Sci. USA 99, 1341–1346. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Ferrari, G., Cusella De Angelis, M. G., Coletta, M., Stornaioulo, A., Paolucci, E., Cossu, G. & Mavilio, F. (1998) Science 279, 1528–1530. [DOI] [PubMed] [Google Scholar]
- 4.Gussoni, E., Soneoka, Y., Strickland, C. D., Buzney, E. A., Khan, M. K., Flint, A. F., Kunkel, L. M. & Mulligan, R. C. (1999) Nature 401, 390–394. [DOI] [PubMed] [Google Scholar]
- 5.Jackson, K. A., Majka, S. M., Wang, H., Pocius, J., Hartley, C. J., Majesky, M. W., Entman, M. L., Michael, L. H., Hirschi, K. K. & Goodell, M. A. (2001) J. Clin. Invest. 107, 1395–1402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Lagasse, E., Connors, H., Al-Dhalimy, M., Reitsma, M., Dohse, M., Osborne, L., Wang, X., Finegold, M., Weissman, I. L. & Grompe, M. (2000) Nat. Med. 6, 1229–1234. [DOI] [PubMed] [Google Scholar]
- 7.Krause, D. S., Theise, N. D., Collector, M. I., Henegariu, O., Hwang, S., Gardner, R., Neutzel, S. & Sharkis, S. J. (2001) Cell 105, 369–377. [DOI] [PubMed] [Google Scholar]
- 8.Korbling, M., Katz, R. L., Khanna, A., Ruifrok, A. C., Rondon, G., Albitar, M., Champlin, R. E. & Estrov, Z. (2002) N. Engl. J. Med. 346, 738–746. [DOI] [PubMed] [Google Scholar]
- 9.LaBarge, M. A. & Blau, H. M. (2002) Cell 111, 589–601. [DOI] [PubMed] [Google Scholar]
- 10.Ferrari, G., Stornaiuolo, A. & Mavilio, F. (2001) Nature 411, 1014–1015. [DOI] [PubMed] [Google Scholar]
- 11.Wagers, A. J., Sherwood, R. I., Christensen, J. L. & Weissman, I. L. (2002) Science 297, 2256–2259. [DOI] [PubMed] [Google Scholar]
- 12.Torrente, Y., Tremblay, J. P., Pisati, F., Belicchi, M., Rossi, B., Sironi, M., Fortunato, F., El Fahime, M., D'Angelo, M. G., Caron, N. J., et al. (2001) J. Cell Biol. 152, 335–348. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Tamaki, T., Akatsuka, A., Ando, K., Nakamura, Y., Matsuzawa, H., Hotta, T., Roy, R. R. & Edgerton, V. R. (2002) J. Cell Biol. 157, 571–577. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Qu-Petersen, Z., Deasy, B., Jankowski, R., Ikezawa, M., Cummins, J., Pruchnic, R., Mytinger, J., Cao, B., Gates, C., Wernig, A. & Huard, J. (2002) J. Cell Biol. 157, 851–864. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Asakura, A., Seale, P., Girgis-Gabardo, A. & Rudnicki, M. A. (2002) J. Cell Biol. 159, 123–134. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Musaro, A., McKullagh, K. J. A., Naya, F. J., Olson, E. N. & Rosenthal, N. (1999) Nature 400, 581–585. [DOI] [PubMed] [Google Scholar]
- 17.Barton-Davis, E., Shoturma, D. I., Musaro, A., Rosenthal, N. & Sweeney, H. L. (1998) Proc. Natl. Acad. Sci. USA 95, 15603–15607. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Musaro A., McCullagh K., Paul A., Houghton L., Dobrowolny G., Molinaro M., Barton E. R., Sweeney H. L. & Rosenthal N. (2001) Nat. Genet. 27, 195–200. [DOI] [PubMed] [Google Scholar]
- 19.Barton, E. R., Morris, L., Musaro, A., Rosenthal, N. & Sweeney, H. L. (2002) J. Cell Biol. 157, 137–148. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Rando, T. A. & Blau, H. M. (1994) J. Cell Biol. 125, 1275–1287. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Kelly, R., Alonso, S., Tajbakhsh, S., Cossu, G. & Buckingham, M. (1995) J. Cell Biol. 129, 383–396. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lescaudron, L., Creuzet, S. E., Li, Z., Paulin, D. & Fontaine-Perus, J. (1997) J. Muscle Res. Cell Motil. 18, 631–641. [DOI] [PubMed] [Google Scholar]
- 23.Musaro, A. & Rosenthal, N. (1999) Mol. Cell. Biol. 19, 3115–3124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Sommerland, H., Ullman, M., Jennische, E., Skottner, A. & Oldfors, A. (1989) Acta Neuropathol. 78, 264–269. [DOI] [PubMed] [Google Scholar]
- 25.Ikuta, K. & Weissman, I. L. (1992) Proc. Natl. Acad. Sci. USA 89, 1502–1506. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Heissig, B., Hattori, K., Dias, S., Friedrich, M., Ferris, B., Hackett, N. R., Crystal, R. G., Besmer, P., Lyden, D., Moore, M. A., et al. (2002) Cell 109, 625–637. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Garry, D. J., Yang, Q., Bassel-Duby, R. & Williams, R. S. (1997) Dev. Biol. 188, 280–294. [DOI] [PubMed] [Google Scholar]
- 28.Trowbridge, I. S. & Thomas, M. L. (1994) Annu. Rev. Immunol. 12, 85–116. [DOI] [PubMed] [Google Scholar]
- 29.Morgan, J. E., Gross, J. G., Pagel, C. N., Beauchamp, J. R., Fassati, A., Thrasher, A. J., Di Santo, J. P., Fisher, I. B., Shiwen, X., Abraham, D. J., et al. (2002) J. Cell Biol. 157, 693–702. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Spangrude, G. J., Heimfeld, S. & Weissman, I. L. (1988) Science 241, 58–62. [DOI] [PubMed] [Google Scholar]
- 31.Miescher, G. C., Schreyer, M. & MacDonald, H. R. (1989) Immunol. Lett. 23, 113–118. [DOI] [PubMed] [Google Scholar]
- 32.Uchida, N. & Weissman, I. L. (1992) J. Exp. Med. 175, 175–184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Morrison, S. J. & Weissman, I. L. (1994) Immunity 1, 661–673. [DOI] [PubMed] [Google Scholar]
- 34.Osawa, M., Hanada, K., Hamada, H. & Nakauchi, H. (1996) Science 273, 242–245. [DOI] [PubMed] [Google Scholar]
- 35.Jackson, K. A., Mi, T. & Goodell, M. A. (1999) Proc. Natl. Acad. Sci. USA 96, 14482–14486. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Blau, H. M., Brazelton, T. R. & Weimann, J. M. (2001) Cell 105, 829–841. [DOI] [PubMed] [Google Scholar]
- 37.Gangemi, R. M., Daga, A., Marubbi, D., Rosatto, N., Capra, M. C. & Corte, G. (2001) Mech. Dev. 109, 323–329. [DOI] [PubMed] [Google Scholar]
- 38.Heins, N., Cremisi, F., Malatesta, P., Gangemi, R. M., Corte, G., Price, J., Goudreau, G., Gruss, P. & Gotz, M. (2001) Mol. Cell Neurosci. 18, 485–502. [DOI] [PubMed] [Google Scholar]
- 39.Galli, R., Fiocco, R., De Filippis, L., Muzio, L., Gritti, A., Mercurio, S., Broccoli, V., Pellegrini, M., Mallamaci, A. & Vescovi, A. L. (2002) Development (Cambridge, U.K.) 129, 1633–1644. [DOI] [PubMed] [Google Scholar]
- 40.Lichanska, A. M., Browne, C. M., Henkel, G. W., Murphy, K. M., Ostrowski, M. C., McKercher, S. R., Maki, R. A. & Hume, D. A. (1999) Blood 94, 127–138. [PubMed] [Google Scholar]
- 41.Drexler, H. G. (1987) Leukemia 1, 697–705. [PubMed] [Google Scholar]
- 42.Preffer, F. I., Dombkowski, D., Sykes, M., Scadden, D. & Yang, Y. G. (2002) Stem Cells 20, 417–427. [DOI] [PubMed] [Google Scholar]
- 43.Kelly, R. G., Zammit, P. S., Schneider, A., Alonso, S., Biben, C. & Buckingham, M. E. (1997) Dev. Biol. 187, 183–199. [DOI] [PubMed] [Google Scholar]
- 44.Seale, P., Sabourin, L. A., Girgis-Gabardo, A., Mansouri, A., Gruss, P. & Rudnicki, M. A. (2000) Cell 102, 777–786. [DOI] [PubMed] [Google Scholar]
- 45.Gurdon, J. B. (1988) Nature 336, 772–774. [DOI] [PubMed] [Google Scholar]
- 46.Standley, H. J., Zorn, A. M. & Gurdon, J. B. (2002) Int. J. Dev. Biol. 46, 279–283. [PubMed] [Google Scholar]
- 47.Kuehnle, I. & Goodell, M. A. (2002) Br. Med. J. 325, 372–376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Polesskaya, A., Seale, P. & Rudnicki, M. A. (2003) Cell 113, 841–852. [DOI] [PubMed] [Google Scholar]
- 49.Beauchemin, M., Del Rio-Tsonis, K., Tsonis, P. A., Tremblay, M. & Savard, P. (1998) J. Mol. Biol. 279, 501–511. [DOI] [PubMed] [Google Scholar]
- 50.Cairns, L. A., Moroni, E., Levantini, E., Giorgetti, A., Klinger, F. G., Ronzoni, S., Tatangelo, L., Tiveron, C., De Felici, M., Dolci, S., et al. (2003) Blood 102, 3954–3962. [DOI] [PubMed] [Google Scholar]