Abstract
When the cost of reproduction for males and variance in female quality are high, males are predicted to show adaptive mate choice. Using Drosophila melanogaster, we test this prediction and show that sperm limited males preferentially mated with young and/or well fed females. The preferred females had higher reproductive output – direct evidence of adaptive precopulatory male mate choice. Our most striking finding is the strong positive correlation between the degree of mating bias showed by the males and the variance in the fitness of the females. We discuss the possible mechanism for such adaptive male mate choice and propose that such choice has important consequences with respect to the existing understanding of the mating system and the evolution of aging.
Male mate choice is defined as differential male sexual response to different reproductively mature conspecific females1. In contrast with the age old perception2, results from studies over the past few decades indicate that males pay a non-trivial cost related to sexual reproduction and consequently, male-mate choice, either in the form of mating decisions or post-copulatory events (differential ejaculate investment), may evolve as an adaptive strategy2,3.
As males can derive at least some fitness from each additional mating4, variance in the quality of females is thought to be one of the prerequisites for the evolution of male mate choice, ensuring fitness returns in spite of the cost associated with rejecting available mating opportunities5. Fecundity is one of the most important components of fitness in females and thus mating efforts of males in most promiscuous species are both expected and observed to be sensitive to some indicators of female fecundity, e.g., body size, fatness, gravid or non-gravid condition etc.1,5,6,7,8,9,10,11. In general, factors having strong influence on, and perceivable phenotypic correlation with female fecundity are expected to serve as honest indicators of female quality. In addition, non-trivial amount of resources utilized by the males for the production of ejaculate, courtship and other mating related activities provide a favourable condition for the evolution of male mate choice12,13,14,15.
Although both age and immediate nutritional status have been shown to affect female fecundity16,17, there are very few studies looking at male mating behaviour towards females differing in these two factors. Here we report the effect of age and nutritional status on female fitness in an outbred population of Drosophila melanogaster. We then examine the behaviour of the males of this population when subjected to two-way choice conditions across a range of variance in female fitness (generated by altering the two above mentioned factors). We also report the male behaviour under no-choice conditions.
Results
Fitness (number of progeny produced) of the experimental females
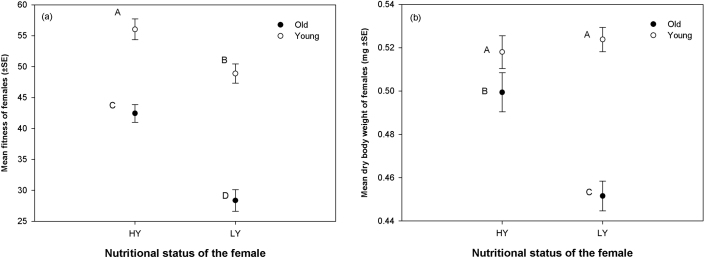
The experiment was done with four kinds of females differing in two factors, viz. age and nutritional status - Young High Yeasted (Y/HY), Young Low Yeasted (Y/LY), Old High Yeasted (O/HY) and Old Low Yeasted (O/LY). These four kinds of females are hereafter referred to as "Experimental Females". Young females were 3 days old post-eclosion and old females were 13 days old post eclosion. High and Low Yeasting status were created by supplying 5 mg and 15 mg of live Yeast per 10 females respectively. Fitness of the above mentioned females was measured by combining 10 females of a given type (Y/HY or Y/LY or O/HY or O/LY) with ten 3 days old virgin males in a food vial. For each type of experimental female 11–13 such vials were set up. The experimental females were allowed to mate and interact with males for an hour and then held in single sex groups for one day following which they were allowed to oviposit individually in test tubes. The progeny count was taken as fitness of the females (for details see Methods section). We found significant main effects of age and nutritional status of the females on their fitness, with old females having 32.6% lesser fitness compared to young females and females from the low nutritional regime having 21% lower fitness than females from the high nutritional regime (Table 1a, Figure 1a). However, young females were relatively less affected by the difference in the nutritional status while the level of nutrition strongly affected the fitness of old females, leading to a significant interaction between the two factors (Table 1a, Figure 1a).
Table 1. Summary of two factor ANOVA for (a) Fitness, (b) Dry body weight, (c) Mating latency and (d) Copulation duration of experimental females with age and nutritional status as fixed factors. p-value marked with * indicates statistically significant value.
| Trait | Effect | Numerator df | Denominator df | SS | F | p |
|---|---|---|---|---|---|---|
| (a) Fitness | Age | 1 | 44 | 3476.33 | 110.77 | <0.0001* |
| Nutritional status | 1 | 44 | 1348.46 | 42.97 | <0.0001* | |
| Age×Nutritional status | 1 | 44 | 143.51 | 4.57 | 0.04* | |
| (b) Dry Body Weight | Age | 1 | 34 | 0.02 | 37.55 | <0.0001* |
| Nutritional status | 1 | 34 | 0.004 | 8.04 | 0.008* | |
| Age×Nutritional status | 1 | 34 | 0.007 | 13.09 | 0.001* | |
| (c) Mating Latency | Age | 1 | 32 | 3.54 | 2.11 | 0.16 |
| Nutritional status | 1 | 32 | 0.07 | 0.04 | 0.84 | |
| Age×Nutritional status | 1 | 32 | 0.21 | 0.12 | 0.73 | |
| (d) Copulation Duration | Age | 1 | 32 | 1.70 | 0.32 | 0.57 |
| Nutritional status | 1 | 32 | 2.60 | 0.50 | 0.48 | |
| Age×Nutritional status | 1 | 32 | 2.31 | 0.44 | 0.50 |
Figure 1. Effect of Nutritional status and Age of the females on: (a) Fitness (number of progeny produced) of the females; (b) Dry Body Weight.
Points not connected by common letters are significantly different. Open circles ( ): Young females; Closed circles (
): Young females; Closed circles ( ): Old females.
): Old females.
Dry body weight of experimental females
Both age and nutritional status had significant effect on dry body weight (see Methods for measurement of dry weight) of the females along with a significant interaction between the two factors (Table 1b, Figure 1b). However, multiple comparisons using Tukey's HSD suggested that only dry body weight of O/LY – female was significantly less than that of the other three types.
Components of reproductive behaviour under no-choice condition
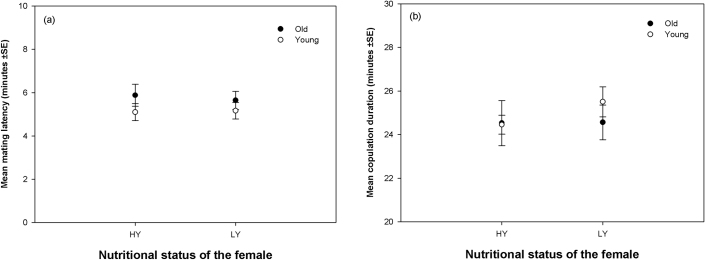
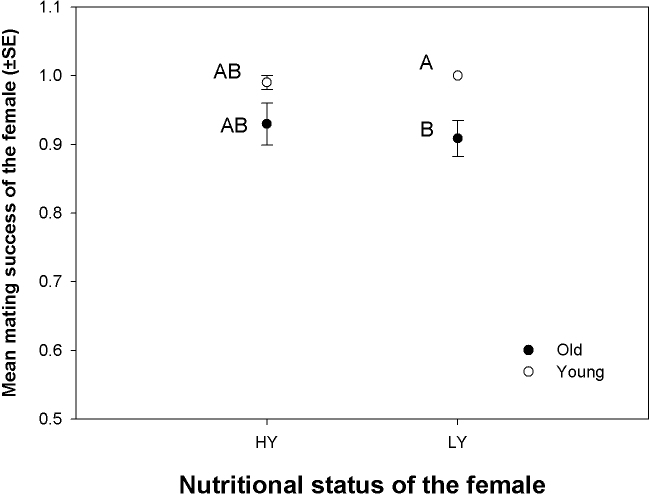
Ten Experimental females of a given type were combined with ten virgin males that were 3 days old in a food vial. This vial was then observed for components of reproductive behaviour for one hour (see Methods section for details). Neither age nor nutritional status had any significant effect on mating latency (time taken to initiate mating) and copulation duration (time for which the copulation lasted) (Table 1c,d; Figure 2a,b). Analyses of the intrinsic mating success (proportion of females successfully mating within one hour after being combined with young, virgin males, Table 2, Figure 3) suggested that both young and old females were not significantly affected by their nutritional status (see comparisons - O/HY vs. O/LY and Y/HY vs. Y/LY, Table 2). We did four additional pair-wise comparisons of mating success (Table 2, Figure 3) thus completing all possible comparisons between the four types of females mentioned. Only Y/LY had significantly higher mating success than O/LY. The comparison - Y/HY vs. O/LY was marginally not significant, whereas rest of the comparisons were not statistically significant (see Methods section for statistical details).
Table 2. Summary of Kruskal-Wallis analyses of all six comparisons between the mating successes of the four types of females. p-value marked with * indicates statistically significant value after sequential Bonferroni correction.
| Comparison | χ2-square | Numertor df | Denominator df | p |
|---|---|---|---|---|
| O/HY vs. O/LY | 0.31 | 1 | 17 | 0.58 |
| O/HY vs. Y/HY | 3.21 | 1 | 17 | 0.07 |
| O/HY vs. Y/LY | 4.76 | 1 | 17 | 0.03 |
| O/LY vs. Y/HY | 6.78 | 1 | 17 | 0.009 |
| O/LY vs. Y/LY | 8.10 | 1 | 17 | 0.004* |
| Y/HY vs. Y/LY | 0.90 | 1 | 17 | 0.34 |
Figure 2. Effect of Nutritional status and Age of the female on the components of reproductive behaviour: (a) Mating latency; (b) Copulation duration.
None of the points are significantly different from each other.
Figure 3. Effect of Nutritional status and Age of the females on Mating Success (proportion of virgin females inseminated within 1 hour under no-choice condition) of the females.
Points not connected by common letters are significantly different. Open circles ( ): Young females; Closed circles (
): Young females; Closed circles ( ): Old females.
): Old females.
Two way choice experiment
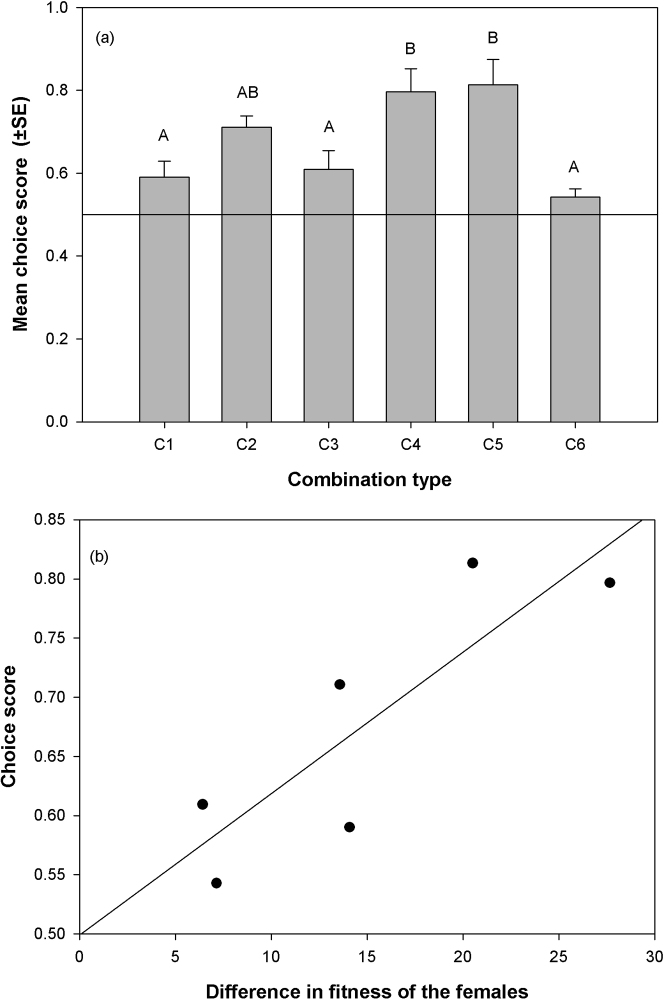
Six different combinations (C1-6, Table 3, see Methods for details) of females were assayed in the choice experiment. Each combination was assayed using a two-way choice design. We combined 10 sperm depleted, 3 days old males with 20 experimental females of which 10 females were of one type while 10 others were of a different type (see Table 3) in a standard vial (95 mm height × 25 mm diameter) with food. Twelve such vials were set up for each combination. We then counted the number of females of each type mated within each vial within half an hour of combining with the males. From each of the experimental vials, the raw mating data was used to generate a "Choice Score" (CS) – a measurement of the intensity of mating bias (1≥CS≥0, see Methods for calculation and details). A CS = 0.5 indicates no-choice, 0.5>CS≥0 indicates bias for lower-fitness females and 1≥CS>0.5 indicates bias for higher-fitness females. Choice scores were tested using a one sample t-test (two-tailed) with a null hypothesis of mean-CS = 0.5, i.e., no-choice. Apart from C6, choice scores from all other combinations were significantly greater than 0.5 (p≤0.04 for all combination types) indicating a bias in favour of higher-fitness females (Figure 4a). The choice score of C6 ( = 0.54) was marginally not significantly different (p = 0.053) from the expected CS of 0.5.
Table 3. Choice combinations: C1-6 are combinations assayed during the two-way choice experiment. Each combination had two types of females differing with respect to their age (young/old) and/or nutritional status (high/low Yeasted): Young High Yeasted (Y/HY), Young Low Yeasted (Y/LY), Old High Yeasted (O/HY) and Old Low Yeasted (O/LY). Based on fitness measurement these females are categorized as higher-fitness female and lower-fitness female in each combination type.
| Combination type | Females involved | Higher-fitness female | Lower-fitness female |
|---|---|---|---|
| C1 | O/HY vs. O/LY | O/HY | O/LY |
| C2 | O/HY vs. Y/HY | Y/HY | O/HY |
| C3 | O/HY vs. Y/LY | Y/LY | O/HY |
| C4 | Y/HY vs. O/LY | Y/HY | O/LY |
| C5 | O/LY vs. Y/LY | Y/LY | O/LY |
| C6 | Y/HY vs. Y/LY | Y/HY | Y/LY |
Figure 4. Choice scores and variance in female quality: (a) Mean Choice Scores of all the combinations (C1: O/HY vs.O/LY, C2: Y/HY vs. O/HY, C3: Y/LY vs. O/HY, C4: Y/HY vs. O/LY, C5: Y/LY vs. O/LY, C6: Y/HY vs. Y/LY).
Bars not sharing common letters are significantly different. The horizontal line indicates expected choice score if there is no mating bias (CS = 0.5). Except C6, in all combinations Choice Score was significantly greater than this expected value of 0.5 (p<0.04 for all combination). Choice score in C6 was marginally not-significantly different from 0.5 (p = 0.053); (b) Regression between Choice Score and difference in fitness of the females. Slope = 0.012, r2 = 0.73, p = 0.03.
The expected choice score of 0.5 assumes equal intrinsic mating successes of the two types of females under choice assay. Due to the difference in intrinsic mating success between Y/LY and O/LY-females (see mating success results earlier), the expected mean CS for C5 was revised considering the experimentally derived intrinsic mating successes of these experimental females using the formula:
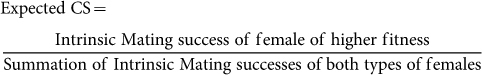 |
The revised expected choice score (indicating absence of mate choice) for C5 was 0.523. The observed CS of C5 was then analysed using one sample t-test (two tail), this time considering the revised hypothesised mean ( = 0.523). The analysis revealed a significant (p = 0.0011) difference, indicating mating bias towards Y/LY females.
We then analysed the choice score to see whether it is different across different choice combinations (C1-6). A one factor ANOVA was done with combination type as fixed factor. Choice score was significantly affected by combination type (df = 5, SS = 0.69, F = 6.82, p<0.0001). Multiple comparisons using Tukey's HSD showed that C4 and C5 were significantly different from C1, C3 and C6, whereas C2 had intermediate value and was not significantly different from any of the combinations (Figure 4a).
Choice score and variance in female fitness
A linear regression of mean choice scores from each of the combination on the difference in fitness of the females corresponding to the combination yielded a significant positive slope (Slope = 0.012, r2 = 0.73, p = 0.03, Figure 4b).
Discussion
Theory predicts that under certain conditions, viz. considerable variation in female quality and sufficiently high cost of mating to the males, it will be advantageous for the males to bias their reproductive effort1. In our experiment, we manipulated both these factors. First, we generated female quality variation by manipulating the age and nutritional status which are known to have major effects on female fitness in D. melanogaster16,17. Second, we assessed mate choice in sperm depleted males, which are expected to have greater propensity to show mating bias11. Our results indicate that males tend to mate with females of higher potential reproductive fitness with the intensity of mating bias being positively correlated with the variance in the female fitness. Age as well as nutritional status of the females were found to be important determinants of male mating preference.
Apart from male mate choice, there are at least two alternative explanations for the observed pattern of mating bias in males. First, since mating decision depends on both males and females18, the bias in mating pattern observed in our experiment can potentially be explained in terms of higher receptivity of the young females compared to the old females. However, we found no significant difference in the receptivity, measured in terms of mating latency of the four different classes of females. The data suggests that O/LY-females, with an intrinsic mating success of 0.93, were significantly less successful in mating compared to Y/LY-females. However, the choice score in C5, where males were allowed to choose between O/LY and Y/LY females, was 0.81. This score was significantly different from the expected choice score (even after considering the difference in mating success of the females), indicating mating bias in favour of Y/LY (see revised choice score in Results section).
Second, males can learn19 to mate with the type of females they were exposed to during sperm depletion treatment. However, we rule this possibility out as the females used during sperm depletion came from a different age class (7 days post eclosion) compared to the two age classes (old = 13 days and young = 3 days post eclosion) used in the experiment.
Thus it seems very likely that the observed bias in mating can be attributed to male mate choice rather than female receptivity or male learning. To summarise, we found no difference in mating latency and insufficient difference in the mating success under no-choice situation. However, a mating bias towards one type of females was evident under choice conditions. Thus we conclude that the observed pattern of results offer strong evidence for male mate choice. We did not find any evidence of post-copulatory male mate choice in the form of variation in copulation duration, which is often used as an indicator of male ejaculate investment in females and has been found to vary in a number of situations20,21. However, a recent study by Lupold et al. (2011) using D. melanogaster suggests that males might vary their ejaculate investment, especially sperms, without varying the copulation duration22. This study showed that males invested more sperm in young and larger females. Thus at present we cannot completely rule out the possibility of post copulatory, cryptic male mate choice within our experimental regime.
In all the six choice combinations tested, fitness of the preferred females was significantly higher. Hence, at least in our experimental system, male mate choice is adaptive. More interestingly, the difference in the fitness of the two types of females within each of the six mate-choice combinations varied greatly and so did the degree of mating bias exhibited by the males (i.e., there was a significant effect of mating combination on choice score). A regression fit of choice scores on the difference in fitness of the experimental females resulted in a significantly positive slope indicating a positive correlation between variance in female fitness and degree of mating bias in line with the theories of adaptive male mate choice. Although theoretically indicated, effect of variance in female quality on male mating decision has rarely been addressed. Males in a bush cricket species were shown to be more likely to reject mates when perceived mate quality variance and mate encounter rate were both high23. However, the organism studied has reversed sex roles, at least under nutrition deprived condition, and thus males are expected to be choosy23. Here, we have shown that males of D. melanogaster, a species with conventional sex roles, adaptively vary their degree of mating bias according to the prevailing mate quality variance. Our finding is the only the second empirical evidence of its kind, supporting, with even greater strength, theories of evolution of male mate choice. In addition, the pattern of choice scores in our experiment suggests males' ability to respond to both age as well as nutritional status of the females.
Although one previous study24 suggested that young and well fed Drosophila females receive higher amount of courtship, the study lacked any evidence regarding the actual mating success and fitness of the females and could only resolve the extreme difference. Thus our study is the first unambiguous evidence of adaptive precopulatory male mate choice based on female age and nutritional status in Drosophila and one of the very few evidences for adaptive precopulatory male mate choice in general. It should also be noted that the population used in this study was maintained under optimum laboratory conditions for a large number of generations and it is likely that the mate quality variation (at least with respect to the two factors of age and nutritional status addressed here) experienced by the males is relatively low. The fact that we could show adaptive male mate choice based on experimentally generated mate quality variation even in this population points to mechanisms ingrained over the course of evolutionary history, prior to their laboratory adaptation and/or sufficient variation in mate quality even under laboratory conditions.
There can be several potential mechanisms by which males can differentiate between the two types of females. Theoretically, male mate choice is expected to depend on male's ability to assess the quality of females based on certain signals as well as how honest the signals are in indicating female quality1. In the present experiment, there are two factors affecting female quality- age and nutritional status. There are potentially, multiple signals that can be associated with ageing. Specifically, the cuticular hydrocarbon profile of a female is known to change with age25 and males of Drosophila can differentiate females based on cuticular hydrocarbon profiles20. In an elegant study, Byrne and Rice (2006) showed that males of D. melanogaster that were resource limited preferentially mate with females of a larger body size11. The body size variation among the females was generated by altering larval density. In our study, we varied the amount of yeast supplement available to the adults and found that nutritional manipulation significantly affected dry body weight of the females, specifically that of the old flies. Since all the adults in our experiment came from standard density cultures, body size, measured as thorax length, is not expected to change due to nutritional manipulation. Therefore, body weight, but not body size, is one factor that can potentially explain the mechanism of the observed choice in the experiment. However, as suggested by the multiple comparison (Tukey's HSD), only O/LY females were significantly lighter than all the other type of females and thus only combinations (C1, C4 and C5) which had this type of females can be explained. Abdominal distension, although we did not quantify it, can be a potent cue for the nutritional status.
Our finding is important in the understanding of the mechanism of maintenance of fitness variation in females of a population. As shown by Long et al. (2009), in an organism, like D. melanogaster, that experience sexual selection and sexual conflict simultaneously, the distribution of female fitness in a population is determined by both the distribution of the intrinsic fitness of the females and the distribution of the amount of fitness depressing male interactions26. The latter factor is a direct outcome of male mate choice. Preferred females are expected to attract more male attention and thus higher mate-harm. If males can vary the degree of mate choice depending upon female fitness variance, it will scale the intensity of the effect proposed by Long et al. (2009). Greater fecundity variance will lead to stronger male mate choice and thus greater reduction in the fecundity of preferred females (due to sexually antagonistic effects). On the other hand lesser fitness variance will lead to weaker male mate choice and consequently less fitness depression of the preferred females. Hence, all else being equal, the positive correlation between intensity of adaptive male mate choice and variance in female fitness together with sexually antagonistic interactions can potentially maintain variation in female fecundity and other fitness related traits much more effectively (compared to a situation where adaptive male mate choice is not plastic).
Our results have important consequences for the evolution of senescence. Empirical and theoretical studies suggest that female mate choice for older males27 has the potential to significantly lower the mortality rate in a population28. We propose that male mate choice based on female age is very likely to have major consequences for the evolution of mortality rates. However, this has not received sufficient attention either theoretically or empirically. It is interesting to note that our study raises the possibility that the preferences of males and females with respect to the age of their mates might be in the opposite directions. However, the relative importance of male-mate choice will depend on two factors. First, if mate choice is exhibited mostly by males that are subjected to ejaculate depletion, then, this may not be common in the wild since ejaculate depletion is unlikely to be common. However, resource limitation, in terms of nutritional limitations, can affect males' ability to produce sperm (or ejaculate as a whole), given that they are energetically costly and such situation of resource limitation is expected to be common. Secondly, since intensity of male mate choice scales with the variance in the female quality, its relative importance is also expected to depend on factors affecting female quality (e.g., availability and distribution of food, age structure of the natural populations etc.). When variance in female quality is high male mate choice is expected to be very important compared to when it is low.
Methods
Experimental organism
The experiments are done using a large (N 9,000), outbred, laboratory population of Drosophila melanogaster – LH29. The population was founded by Larry Harshman in 1991 from 400 wild caught inseminated females from central California and since then have been maintained as a laboratory population with an effective population size of >5,00030.The flies are maintained on a 14-day discrete generation cycle, at 25°C temperature, 50% RH and 12 hour light and 12 hour dark condition on cornmeal-molasses food. The flies are cultured in vials (95 mm height × 25 mm diameter), with standard cornmeal-molasses food. Each generation, approximately 150 eggs are cultured per vial with 8-10 ml of food. A total of 60 such vials are maintained. After 12 days, by which time almost all adults would have eclosed, adult flies from different vials are mixed and redistributed into 60 fresh food vials with 16 pairs (16 males and 16 females) in each vial. The vials are supplied with a limiting quantity of live Yeast supplement. Two days later they are transferred to fresh vials with food and allowed to oviposit for 18 hrs to start the next generation.
9,000), outbred, laboratory population of Drosophila melanogaster – LH29. The population was founded by Larry Harshman in 1991 from 400 wild caught inseminated females from central California and since then have been maintained as a laboratory population with an effective population size of >5,00030.The flies are maintained on a 14-day discrete generation cycle, at 25°C temperature, 50% RH and 12 hour light and 12 hour dark condition on cornmeal-molasses food. The flies are cultured in vials (95 mm height × 25 mm diameter), with standard cornmeal-molasses food. Each generation, approximately 150 eggs are cultured per vial with 8-10 ml of food. A total of 60 such vials are maintained. After 12 days, by which time almost all adults would have eclosed, adult flies from different vials are mixed and redistributed into 60 fresh food vials with 16 pairs (16 males and 16 females) in each vial. The vials are supplied with a limiting quantity of live Yeast supplement. Two days later they are transferred to fresh vials with food and allowed to oviposit for 18 hrs to start the next generation.
Experimental females and their dry body weight
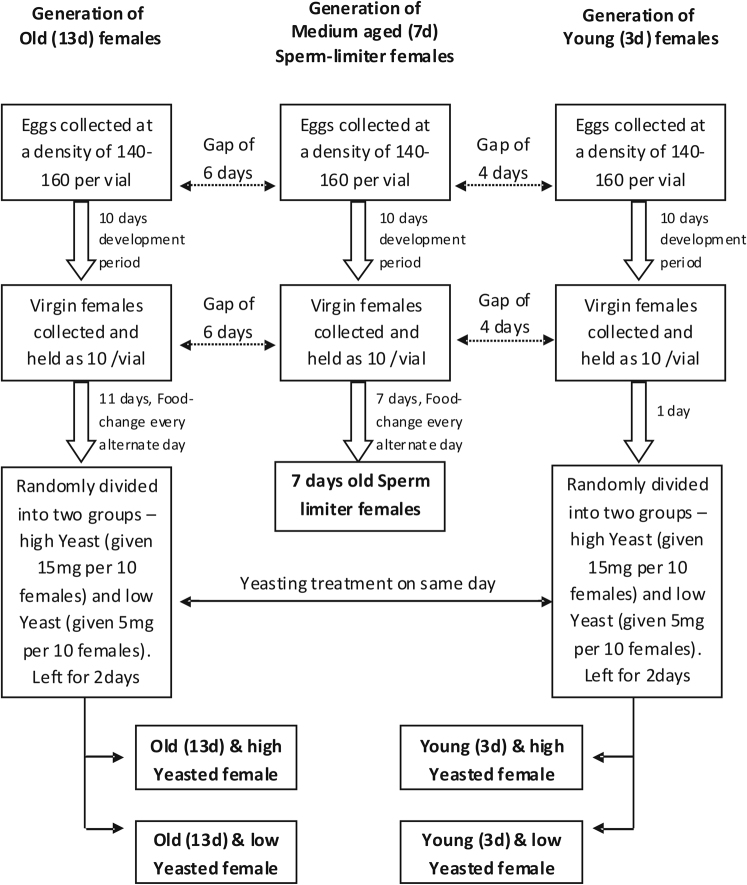
Four different types of experimental females, Young High Yeasted (Y/HY), Young Low Yeasted (Y/LY), Old High Yeasted (O/HY) and Old Low Yeasted (O/LY), were generated following a scheme described in Figure 5. To generate young experimental females (3days old), eggs were cultured at a density of approximately 150 per vial (8–10 ml of corn meal-molasses food) and were incubated at 25°C under 12:12 LD condition similar to the standard culture condition of the LH population. 10 days later, when pupae started darkening the vials were observed every 3–4 hours. Adult virgin females were collected during the peak of their eclosion cycle using light CO2 anaesthesia. Virginity was ensured by collecting flies within 6 hours of eclosion. Collected females were held in single sex groups at a density of 10 individuals per vial. One day later they were divided into two sets - "HighYeast" and "Low Yeast" and were transferred to food-vials with 15 mg and 5 mg of Yeast respectively and were left undisturbed for 2 days. Experiments were done when they were 3 days old. Old females were generated following a similar protocol but eggs were cultured 10days before culturing eggs for generation of the young females. Virgin old females were held in single sex vials till they were 11 days old with food changes every alternate day and at the age of 11 days they were given the similar Yeast-treatment as that given to young females. Experiments were done when they were 13 days old (same day when young females were 3 days old). Eggs were collected for the generation of medium aged - sperm limiter females 4 days before collecting eggs for the generation of young females. Virgin females were collected from these vials in the similar way and were held as virgins till they were approximately 7 days old before using them for sperm-limitation.
Figure 5. Method of generation of experimental females.
Just prior to conducting experiment 1, 45–50 experimental females of each type (O/HY, O/LY, Y/HY and Y/LY) were randomly chosen and flash frozen for measurement of body weights. These flies were then dispensed into clean dry vials in groups of 5, dried at 70°C for 24 hours and weighed in a high precision electronic balance (Sartorius CPA225D) to the nearest 0.01 mg.
Experimental males
Males used in Experiment-1 and 2 (described below) were generated using a similar protocol as that followed while generating young females. They were collected as virgins and were held in single sex groups of 10 per vial. All the males used in this study were 3 days old at the time of experiment. For Experiment 2, males were made sperm limited by keeping them with excess of 7 days old virgin (sperm limiter, described earlier) females (male:female = 10:30 in each vial) for approximately 12 hours, just before the start of the experiment. Females and males were combined in food vials without using anaesthesia and after 12 hours, the males were separated from the females under light CO2 anaesthesia. This treatment ensured multiple mating opportunities for each of the males and was sufficient to make them resource (sperm and other components of ejaculate) depleted31,11. These males were kept undisturbed for 30 – 45 minutes to allow them to recover from the effect of CO2 anaesthesia before starting the experiment.
Experiment 1: Effect of age and nutritional status on fitness (number of progeny produced) and reproductive behaviour of the females under no-choice condition
Fitness of the four different types of experimental females (O/HY, O/LY, Y/HY and Y/LY) was assayed using the following protocol. 10 females of a given type were combined with 10 males (3 days old, virgin) without using anaesthesia and were allowed to interact for 1 hour in a food vial. For each type of experimental female, we set up 11–13 such vials. The females were then separated under light CO2 anaesthesia. One day later, they were transferred (under mild CO2 anaesthesia) individually into test tubes (12 mm×75 mm) containing media and allowed to oviposit for 18 hours. Females were discarded and the test tubes were incubated at 25°C. Emerging progeny were counted after 13 days by which time all progeny had completed development. The progeny count was taken as the measure of their fitness. The one hour interaction time between the males and females ensures a single mating. This is because the mean copulation duration in our flies measured under similar laboratory conditions is typically 20–30 minutes. Additionally, there is a latency period preceding the mating and a refractory period succeeding the mating. We also ensured a single mating by observing the vials continuously.
Under similar conditions, we combined 10 females of a given type (O/HY or O/LY or Y/HY or Y/LY) with ten 3 days old virgin males in a food-vial and their mating behaviour was continuously observed (manually) for 1 hour. 9–10 such vials were observed for each female type (O/HY, O/LY, Y/HY and Y/LY). We recorded the number of mating pairs over time which yielded start and end time for copulation. Using this data, we calculated average mating latency (time taken by a pair of virgin flies to initiate mating), copulation duration (time taken to complete mating) and mating success (proportion of females inseminated within 1 hour under no-choice condition) for each vial. In this experiment, in each vial males were exposed to only one type of experimental female (i.e., either O/HY or O/LY or Y/HY or Y/LY). Hence we term this as "no-choice" condition.
The entire protocol to measure fitness and reproductive behaviour of the four different types of experimental females was designed to closely mimic the natural maintenance protocol of this population. Hence experiment was done during the light phase of their 12:12 LD cycle at 25°C temperature and uniform overhead laboratory lighting.
Experiment 2: Assay of precopulatory male mate choice
During the choice experiments, different types of females were identified by feeding them yeast suspension with non-toxic food colours (commercially available, green and red). Females were allowed to feed on Yeast-colour suspension for half an hour prior to the choice trial, upon which the abdomen of the females were coloured. To control for the effect of the abdomen-colours on mate choice, we replicated the experiments with reciprocal colouring of females. All combinations of female types (Table 3) were used in the experiment – O/HY vs. O/LY (C1), Y/HY vs. O/HY (C2), Y/LY vs. O/HY (C3), Y/HY vs. O/LY (C4), Y/LY vs. O/LY (C5), Y/HY vs. Y/LY (C6). Mating vials were set up by combining two types of females (10 individuals of each type) and the sperm limited 3 days old males (10 individuals) without anaesthesia in vials containing food. As mentioned before, the two types of females in a given vial were identified by red and green colouration of their abdomen. 6 such vials for each combination type and each colour-code were setup (see following section: Experimental replications and data analyses). The mating vials were left undisturbed for 30 minutes. Mating was stopped by mechanically disturbing the vials and then females were immediately sorted on the basis of their abdomen-colour using light CO2 – anaesthesia and transferred singly into test tubes (12 mm × 75 mm) containing food. Females were allowed to lay eggs for 48 hrs, after which they were discarded and the test tubes were incubated at 25°C. After two days, the test tubes were observed for the presence of larvae. Females in the test tubes with live larvae were scored as “mated” and those with none as “unmated”.
Our own observations with these flies suggest that usually virgin pairs take 3-9 minutes to start mating and then if not disturbed they mate for 20–30 minutes. Thus 30 minute exposure is sufficient to ensure single mating per male. More exposure time might have allowed at least some males to start second round of mating as excess virgin females were present in the mating vials. Another study using a derivative of the LH population11 also followed the same protocol to successfully to ensure single mating per male.
The experiment was done during the light phase of the 12:12 LD cycle of the flies at 25°C temperature and uniform overhead laboratory lighting
Experimental Replications and Data Analysis
Fitness (11–13 replicate vials per female type), mating latency (9–10 replicate vials per female type), copulation duration (9–10 replicate vials per female type) and dry body weight (9–10 replicate vials per female type) data were analysed using two factor ANOVA with age and nutritional status as fixed factors. Mating success (9–10 replicate vials per female type) data were not normally distributed, so they were analysed using Kruskal-Wallis tests. Total of six tests were done, corresponding to all possible comparisons between the four types of females used in the experiment. A sequential Bonferroni test was used in these pair wise comparisons. As there were a total of six comparisons (Table 2), for each of them the level of significance was revised to 0.008 following Bonferroni method32. Experiment 2 had 12 replicate vials for each combination type. Of these, 6 vials had a particular abdominal colour code (for identifying the two different types of females) while the other six vials had the reciprocal colour code. The raw choice data (proportion of each type of female inseminated during the choice trial) were converted into choice score (CS) following the equation given below:
Where P is the proportion of higher-fitness females fertilized and Q is the proportion of lower-fitness females fertilized. This was possible because, for every combination, the two kinds of females could be categorised as higher fitness and lower fitness, based on earlier fitness measurement results (Table 3). In the absence of any mating bias, this score is expected to be equal to 0.5, assuming no difference in intrinsic mating success of the females. If there is a bias towards the females of higher fitness, CS should range between 0.5 and 1, with higher values indicating stronger bias. If the bias is towards lower fitness females, CS should range between 0.5 and 0, with lower values indicating stronger bias. Effects of food colour and combination type on CS were analysed by modelling food colour and combination type as a fixed factors in a two factor ANOVA. Since, food colour had no significant main effect or interaction (p>0.4 for both), choice scores from reciprocal colour combinations were pooled for the rest of the analyses. For each combination, choice scores were analysed using one sample t-test (two tail) with hypothesised mean as 0.5. For all the statistical tests (ANOVA and t-tests) data were tested for normality using Shapiro-Wilk W tests, and, unless otherwise mentioned, the data were found to not be significantly different from normality. Level of significance (α) was taken as 0.05 in all the tests done.
Author Contributions
BN designed the study, carried out experiments, analysed data and wrote the manuscript. AJ, ZSA and SS carried out experiments. NGP designed the study, analysed data and wrote the manuscript. All authors reviewed the manuscript.
Acknowledgments
We thank Adam K. Chippindale for helpful comments on the design of the experiment. We thank Amitabh Joshi and Stephanie Bedhomme for their comments on an earlier version of the manuscript. We also thank the two anonymous reviewers and the handling editor for their helpful remarks which improved the clarity of the manuscript to a great extent. We thank Indian Institute of Science Education and Research Mohali for continued funding for the project. We thank Department of Science and Technology, Govt. of India for financial support for the project. We are also thankful to the undergraduate students of IISER, Kolkata, and IISER, Mohali, for their help in the lab. BN thanks the Council for Scientific and Industrial Research, Govt. of India, for financial assistance in the form of a Junior Research Fellowship. AJ and ASZ thanks the Department of Science and Technology, Govt. of India, for financial assistance in the form of Kishore Vaigyanik Protsahan Yojana fellowship. SS thanks Department of Science and Technology, Govt. of India for INSPIRE scholarship.
References
- Bonduriansky R. The evolution of male mate choice in insects: a synthesis of ideas and evidence. Biol. Rev. Camb. Phil. Soc. 76, 305–339 (2001). [DOI] [PubMed] [Google Scholar]
- Trivers R. L. in Sexual Selection and The Descent of Man, B. Campbell, Ed. (Chicago: Aldine, 1972), pp. 136–179.
- Edward D. A. & Chapman T. The evolution and significance of male mate choice. TREE, 26(12), 647– 654 (2011). [DOI] [PubMed] [Google Scholar]
- Bateman A. J. Intra-sexual selection in Drosophila. Heredity 2, 349–368 (1948). [DOI] [PubMed] [Google Scholar]
- Bonduriansky R. & Brooks R. J. Male antler flies (Protopiophila litigata ; Diptera: Piophilidae) are more selective than females in mate choice. Can. J. Zoolog. 76, 1277–1285 (1998). [Google Scholar]
- Pitafi K. D., Simpson R. & Day T. Male mate choice for fecund females in seaweed flies. Pak. J. Zool. 27, 233–240 (1995). [Google Scholar]
- Gage A. R. & Barnard C. J. Male crickets increase sperm number in relation to competition and female size. Behav. Ecol. Sociobiol. 38, 349–353 (1996). [Google Scholar]
- Wedell N. & Cook P. A. Butterflies tailor their ejaculate in response to sperm competition risk and intensity. Proc. R. Soc. Lond. [Biol] 266, 1033–1039 (1999). [Google Scholar]
- Lefranc A. & Bundgaard J. The influence of male and female body size on copulation duration and female fecundity. Proc. R. Soc. Lond. [Biol] 132, 243–247 (2000). [DOI] [PubMed] [Google Scholar]
- Katvala M. & Kaitala A. Male choice for current egg fecundity in a polyandrous egg-carrying bug. Anim. Behav. 62, 133–137 (2001). [Google Scholar]
- Byrne P. G. & Rice W. R. Evidence for adaptive male mate choice in the fruit fly Drosophila melanogaster. Proc. R. Soc. Lond.[Biol] 273, 917–922 (2006). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cordts R. & Partridge L. Courtship reduces longevity of male Drosophila melanogaster. Anim. Behav. 52, 269–278 (1996). [Google Scholar]
- Dewsbury D. A. Ejaculate cost and male choice. Am. Nat. 119, 601–610 (1982). [Google Scholar]
- Pitnick S. & Markow T. A. Male Gametic Strategies: Sperm Size, Testes Size, and the Allocation of Ejaculate among Successive Mates by the Sperm-Limited Fly Drosophila pachea and its relatives. Am. Nat. 143, 785–819 (1994). [Google Scholar]
- Pitnick S. & Markow T. A. Large male advantage associated with the cost of sperm production in Drosophila hydei, a species with giant sperm. Proc.Natl. Acad. Sc. U.S.A. 91, 9277–9281 (1994). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boorman E. & Parker G. A. Sperm (ejaculate) competition in Drosophila melanogaster, and the reproductive value of females to males in relation to female age and mating status. Ecol. Entomol. 1, 145–155 (1976). [Google Scholar]
- Stewart A. D., Morrow E. H. & Rice W. R. Assessing putative interlocus sexual conflict in Drosophila melanogaster using experimental evolution. Proc. R. Soc. Lond.[Biol] 272, 2029–2035 (2005). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Moehring A. J. & Mackay T. F. C. The quantitative genetic basis of male mating behaviour in Drosophila melanogaster. Genetics 167, 1249–1263 (2004). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reif M., Linsenmair K. E. & Heisenberg M. Evolutionary significance of courtship conditioning in Drosophila melanogaster. Anim. Behav. 63, 143–155 (2002). [Google Scholar]
- Friberg U. Male perception of female mating status: its effect on copulation duration, sperm defence and female fitness. Anim. Behav. 72, 1259–1268 (2006). [Google Scholar]
- Nandy B. & Prasad N. G. Reproductive behaviour and fitness components in male Drosophila are non-linearly affected by the number of male co-inhabitants early in adult life. J. Insect Sci. 11, 67 (2011), available online: insectscience.org/11.67. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lüpold S., Manier M. K., Ala-Honkola O., Belote J. M. & Pitnick S. Male Drosophila melanogaster adjust ejaculate size based on female mating status, fecundity and age. Behav Ecol 22,184–191(2011). [Google Scholar]
- Kvarnemo C. & Simmons L. W. Variance in female quality, operational sex ratio and male mate choice in a bushcricket. .Behav Ecol Sociobiol 45, 245–252 (1999). [Google Scholar]
- Cook R. & Cook A. The attractiveness to males of females Drosophila melanogaster: effect of mating, age and diet. Anim. Behav. 23, 521–526 (1975). [DOI] [PubMed] [Google Scholar]
- Ferveur J. Cuticular hydrocarbons: Their evolution and role in Drosophila pheromonal communication. Behav. Genet. 35, 279–295 (2005). [DOI] [PubMed] [Google Scholar]
- Long T. A. F., Pischedda A., Stewart A. D. & Rice W. R. A cost of sexual attractiveness to high-fitness females. .PloS Biol 7(12), e1000254 (2009) 10.1371/journal.pbio.1000254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beck C. W. & Powell L. A. Evolution of female choice based on male age: Are older males better mates? Evol. Ecol. Res. 2, 107–118 (2000). [Google Scholar]
- Beck C. W., Shapiro B., Choksi S. & Promislow D. A genetic algorithm approach to study the evolution of female preference based on male age. Evol. Ecol. Res. 4, 275–292 (2002). [Google Scholar]
- Prasad N. G., Bedhomme S., Dey T. & Chippindale A. K., An evolutionary cost of separate genders revealed by male-limited evolution.. Am. Nat. 169, 29–37 (2007). [DOI] [PubMed] [Google Scholar]
- Chippindale A. K. & Rice W. R. Y chromosome polymorphism is a strong determinant of male fitness in Drosophila melanogaster. Proc. Natl. Acad. Sci. U.S.A. 98, 5677–5682 (2001). [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markow T. A., Quaid M. & Kerr S. Male mating experience and competitive courtship success. .Nature, 276, 821–822 (1978). [Google Scholar]
- Sokal R. R. & Rohlf F. J. in Biometry, Third Ed., W.H. Freeman and Company New York, 1995, pp. 238–240.