Abstract
Arteries in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) are susceptible to smooth muscle loss and fibrosis, but the molecular components underlying these dramatic vascular changes are not well characterized. The purpose of this study was to investigate the distribution of collagen isoforms in the cerebral vessels of North American CADASIL patients with classical NOTCH3 mutations. Expression of type I-VI collagen in brains obtained at autopsy from six CADASIL patients with cysteine-altering mutations in NOTCH3 was compared to control brain expression. We identified a consistent increase of type I, III, IV, and VI collagen in CADASIL brains. Strong accumulation of type I, III, IV and VI collagen was noted in all calibers of vessels, including small and medium-sized leptomeningeal arteries, small penetrating white matter arteries, and capillaries. Within leptomeningeal arteries, where we could define the three tunicae of each vessel, we found distinct collagen subtype distribution patterns in CADASIL. Type I and III collagen were largely found in either adventitial/medial or transmural locations. Type IV collagen was strictly intimal/medial. Type VI collagen was adventitial or adventitial/medial. Within the thickened penetrating arteries of CADASIL patients, all four collagens extended through most of the arterial wall. We observed increased staining of capillaries in CADASIL for type I, IV, and VI collagen. In conclusion, brain vascular collagen subtypes are increased in CADASIL in multiple layers of all sizes of arteries, with disease-specific changes most prominent in the tunica media and thickened small penetrating vessels. In diseased arteries, type I, III, and VI collagen spreads from an external location (adventitia) into the vascular media, while type IV collagen accumulates in an internal pattern (intima and media). These observations are consistent with a pathological role for collagen accumulation in the vascular media in CADASIL.
1. INTRODUCTION
Vascular dementia is a common form of progressive cognitive dysfunction, with a prevalence of 1.6% in European subjects over 65 years (Lobo et al., 2000); vascular dementia may account for one-third or more of dementia in Asian populations (Chiu et al., 1998; Shaji et al., 1996). However, understanding this disorder has been challenging due to the pervasive coexistence of cerebrovascular disease in common disorders such as Alzheimer’s disease (Schneider et al., 2007a; Schneider et al., 2007b; Schneider et al., 2009). Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL), the most common cause of hereditary small vessel disease (Chabriat et al., 2009), usually occurs in isolation of other neurological diseases and has therefore has been adopted as model disorder to understand vascular dementia.
CADASIL is caused by mutations in NOTCH3 (Joutel et al., 1996), whose expression in the cerebral cortex is restricted to vascular smooth muscle cells (Joutel et al., 2000). Unlike other neurodegenerative diseases, injury to the nervous system in CADASIL likely results from impaired blood flow to the deep white matter (Chabriat et al., 2009). Accordingly, symptoms of CADASIL also include repeated ischemic attacks which occur in midlife and generally spare the gray matter (Kalimo et al., 2002). Migraine with aura is another common feature of the disease, occurring in up to half of CADASIL patients (Desmond et al., 1999). Since vascular impairment underlies each of these primary clinical manifestations in CADASIL, defining the sequence of events leading to blood vessel degeneration in CADASIL may be a key step in developing therapies that target the cardinal manifestations of the disease.
The pathogenic changes in the vasculature associated with CADASIL have been extensively described over the last two decades. The most often cited features include loss of vascular smooth muscle cells of the small arteries and arterioles (Kalimo et al., 2002; Miao et al., 2004; Ruchoux and Maurage, 1997). Vascular protein accumulation is an additional prominent component of CADASIL pathology and is manifested by accumulation of PAS-positive material around smooth muscle cells (including granular osmiophilic material — GOM) and marked accumulation of proteins such as NOTCH3 (Joutel et al., 2000) and von Willebrand factor [DOI: 10.1007/s12975-011–0112-2]. Dramatic vascular fibrosis has also been consistently described in the penetrating arteries of CADASIL (Kalimo et al., 2002; Miao et al., 2004; Ruchoux and Maurage, 1997).
Fibrosis is hallmarked by the replacement or displacement of normal cellular components with high concentrations of matrix materials, including collagen. Collagens are extracellular matrix proteins which provide both structural integrity and signaling functions. In vessels, the best characterized of this extensive family include collagen types I, III, IV, V and VI. Types I, III, and V belong to a group of fibrillar collagens found in most connective tissues. Types I and III have been localized to vascular smooth muscle cells (VSMC), tendons, skin, muscle, bone and other tissues. Type IV is a network-forming collagen organized into sheets and is the primary component of basement membranes which envelop individual vascular smooth muscle cells. Type VI collagen forms filaments that anchor a multitude of proteins to cells. Significantly, all of the most prevalent arterial disorders in the aging brain, including cerebral atherosclerosis, small vessel disease (SVD), cerebral amyloid angiopathy (CAA) and age-related medial 1degeneration and sclerosis, feature collagen deposition, though the magnitude and type of protein deposition varies (Grinberg and Thal, 2010; Sawabe, 2010; Tian et al., 2006).
The subtypes of collagen that accumulate in CADASIL have not been systematically studied in patients with genetically defined disease. To our knowledge, previous studies of collagen subtypes in CADASIL are derived from examination of only one patient or one collagen subtype (Low et al., 2007; Miao et al., 2004; Miao et al., 2006a; Miao et al., 2006b; Oide et al., 2008; Szpak et al., 2007). Moreover, earlier studies have examined patients prior to identification of NOTCH3 mutations as a cause of CADASIL. At least one of these published cases was retrospectively shown to have a disorder distinct from CADASIL (Low et al., 2007). To definitively describe collagen subtype distribution in CADASIL, we investigated the vascular distribution of collagen subtypes in a cohort of North American CADASIL patients with cysteine-altering NOTCH3 mutations.
2. RESULTS
We will describe our findings on blood vessels in three locations: 1) arteries of the leptomeninges 2) small penetrating arteries of the white matter; and 3) capillaries. Collagen staining patterns were remarkably consistent between all six CADASIL patients. Control staining patterns were similar to one another and were easily differentiated from CADASIL. Type II and V collagen did not give satisfactory staining. The distinctive features of CADASIL patients are noted below for the remaining four vascular collagens.
2.1 Type I Collagen
2.1.1 Leptomeningeal arteries
Leptomeningeal arteries generally retained three layers that could be easily distinguished to reveal regional collagen expression. In CADASIL, three main patterns of type I collagen staining were observed: adventitial/medial (Fig 1B), adventitial/intimal, and transmural (all three layers). Tunica media staining was increased in vessels in which significant smooth muscle cell loss had occurred. Adventitial staining was intense and tightly packed in concentric bands of fibers. In controls, the predominant pattern was adventitial staining (Fig 1A); in contrast to CADASIL, staining was observed in loosely arranged fibers. In larger control vessels, light intimal staining was sometimes observed. The principle differences between CADASIL and control were the overall intensity of staining and extension of type I collagen into the degenerating media of many CADASIL arteries.
Figure 1.

Type I and III collagen in CADASIL. Control arteries (A, C, E, and G) and CADASIL arteries (B, D, F, and H) were analyzed using immunohistochemistry. A–D demonstrate type I collagen staining in leptomeningeal vessels (A–B) and small penetrating arteries (C–D). Although controls and CADASIL patients have adventitial type I collagen staining, larger CADASIL vessels also stain for type I collagen in part of the media. In contrast to controls (C), penetrating small arteries of CADASIL (D) contain larger amounts of immunoreactivity through the width of the vessel. Similar changes were observed for type III collagen, except that the extent of medial staining was increased in most vessels (E–H).
2.1.2 Small penetrating arteries
In CADASIL, the main pattern was dark transmural staining (Fig 1D) with a minority of vessels exhibiting sparing of the intima. In controls, penetrating vessels had much lighter staining that was primarily localized to the adventitia (Fig 1C). Overall, the white matter penetrating vessels in CADASIL exhibited an increase in both the extent and strength of staining.
2.1.3 Capillaries
In CADASIL, there was frequent expression in selected gray and white matter capillaries. This was seen in controls but at much lower frequency and intensity.
2.2 Type III Collagen
2.2.1 Leptomeningeal arteries
In CADASIL, type III collagen exhibited three patterns of expression that always included adventitial staining. The most common pattern was robust transmural staining in leptomeningeal vessels (Fig 4D and 4I). In some vessels, the adventitia and media were very avidly stained, while there was notably less immunoreactivity in the intima (Fig 1F). The fibers of type III collagen, like type I collagen, appeared densely packed in concentric whirls. In a minority of vessels, there was adventitial/intimal staining with relative sparing of the media; vessels without medial type III collagen contained intact smooth muscle cells, while the media of transmurally stained vessels were largely devoid of healthy smooth muscle cells. Controls also demonstrated strong staining, but signal was preferential found in the adventitia with only light media staining (Fig 1E); positive fibers were arrayed in a looser arrangement compared to CADASIL. When medial staining was seen, it was mostly in a reticular pattern which delicately surrounded intact smooth muscle. Control vessels also occasionally accumulated type III collagen in the intima in large vessels. Overall, the principle finding in CADASIL was strongly increased staining of the media that correlated with areas of smooth muscle degeneration. Compared with type I collagen, type III collagen consistently covered a higher percentage of the vessel wall.
Figure 4.
Comparison of patterns of immunoreactivity in a CADASIL artery. The same cerebral artery was investigated using the following stains, shown at low and high power: (A and F) NOTCH3; (B and G) smooth muscle actin; (C and H) von Willebrand factor; (D and I) type III collagen; and (E and J) type IV collagen. In high power images (F–J), vascular landmarks are denoted as i (intima), e (elastic lamina), and m (media; a thin smooth muscle layer). The internal elastic lamina separates the pathologically thickened intima from the degenerating, discontinuous tunica media. NOTCH3 staining was focused in the thin remnant vascular media, which is situated just outside the elastic lamina (F). Smooth muscle actin (SMA) was strongly expressed in the neointima (B and G); SMA was present in rare surviving smooth muscle cells (punctate accumulations in B) but absent from the media in areas of profound muscle cell loss (just outside the elastic lamina in G). vWF was expressed on the inner aspect of the thickened intima (C and H). Type III collagen was transmurally deposited (D and I), while type IV collagen localized to the neointima and the remnant vascular media. Additional stains of this artery are presented in Supplementary Figure 1.
2.2.2 Small penetrating arteries
In CADASIL, we found intense transmural staining in the penetrating vessels of the white matter (Fig 1H). Staining in the vessels of controls was principally limited to the periphery of the vessel (Fig 1G), though we occasionally found transmurally stained arteries. The overall staining in CADASIL vessels was significantly stronger in the white matter.
2.2.3 Capillaries
Both CADASIL and control capillaries were positive for type III collagen in gray and white matter. There was heterogeneity of capillary staining in both groups.
2.3 Type IV Collagen
2.3.1 Leptomeningeal arteries
Type IV collagen was deposited in very large quantities in leptomeningeal vessels of CADASIL patients. The protein was densely expressed in both the hyperplastic intima and the tunica media (Fig 2B and 2D). In the media, immunoreactivity was deposited as large, clumps of inhomogeneous deposits in a matrix that was devoid of cells except occasional balloon cells. These balloon cells were sharply outlined with very high levels of type IV collagen (Fig 2D and 2I). Of note, type IV collagen and NOTCH3 overlapped in distribution; NOTCH3 reactivity, when present, also outlined balloon cells in the media and co-localized to degenerating media that included type IV collagen (Fig 2I and 2J, 4A and 4E, 4F and 4J); type IV collagen was also found in the intima, where substantially lower amounts of NOTCH3 were found. In most but not all vessels, type IV collagen in the media was stronger than in the intima. In controls, type IV collagen was deposited in a fine basement membrane pattern in leptomeningeal arteries (Fig 2A and 2C), clearly outlining the subendothelium and smooth muscle profiles which were seen as a delicate network of immunoreactivity in the vascular media. Significant quantities of type IV collagen were not present in the adventitia of controls or CADASIL patients.
Figure 2.

Type IV collagen in CADASIL. Control (A, C, E, G) and CADASIL vessels (B, D, F, H–L) were analyzed using immunohistochemistry. Control leptomeningeal arteries (A, C) exhibit fine basement membrane staining for type IV collagen in the vascular media, which is markedly increased in intensity and breadth in CADASIL arteries (B, D). In CADASIL, strong staining of the pathologically thickened neointima was seen. Focal deposits of staining around ballooned degenerating smooth muscle cells were also notable in CADASIL (D). In small penetrating arteries, immunoreactivity was limited to a fine basement membrane distribution in controls (E) but was heavy and transmurally distributed in CADASIL arteries (F). Type IV collagen reactivity was weak in control capillaries (G), but strongly increased in CADASIL (H). (I–L) show comparative staining of serial sections of CADASIL arteries for type IV collagen (I, K) and NOTCH3 (J, L) in leptomeningeal arteries (I–J) and penetrating small arteries (K–L). Type IV collagen and NOTCH3 overlap in expression in the vascular media, which features cell degeneration and balloon cells (I–J). Media (m) and intima (i) are labeled.
2.3.2 Small penetrating arteries
In CADASIL, penetrating arteries contained strong type IV collagen immunoreactivity and defined the entire width of the thickened arterial wall except adventitia when present (Fig 2F). Type IV collagen reactivity appeared to be more intense immediately adjacent to the lumen, contrasting type I and III collagen which tended have the highest concentration near the periphery of the vessels. When present, NOTCH3 reactivity exhibited a similar pattern as type IV collagen in small, thickened CADASIL vessels (Fig 2K and 2L). In penetrating small arteries of controls, type IV collagen formed a fine line beneath the endothelium in both the white and gray matter (Fig 2E) in many vessels.
2.3.3 Capillaries
In CADASIL, almost all capillaries in both gray and white matter could be easily recognized using type IV collagen antibodies (Fig 2H). Under identical staining conditions, capillaries of controls were faintly reactive (Fig 2G).
2.4 Type VI Collagen
2.4.1 Leptomeningeal arteries
In CADASIL, type VI collagen was distributed in both adventitia and media (Fig 3B). The medial involvement was confirmed by observing type VI collagen staining encircling balloon cells in the degenerated arterial wall. In some vessels, the staining was only seen in the external circumference of the vessel (including the adventitia and the most peripheral rim of degenerating media). A minority of vessels had dark medial staining with less reactivity in the adventitia. In controls, there was light adventitial staining and no detectable reactivity of the media or intima (Fig 3A). In sum, CADASIL arteries (compared to controls) showed increased adventitial type VI collagen and disease-specific spreading of immunoreactivity to the vascular media.
Figure 3.
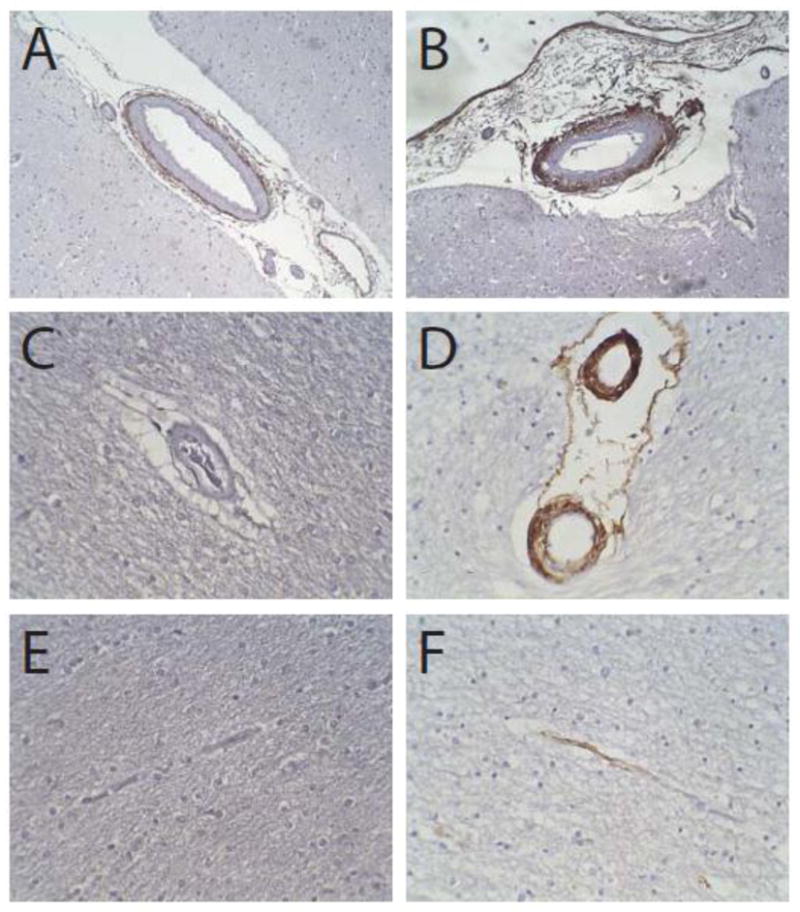
Type VI collagen in CADASIL. Control (A, C, and E) and CADASIL arteries (B, D, and F) were analyzed using immunohistochemistry. Control leptomeningeal arteries (A) exhibit adventitial staining, which is markedly increased in intensity and breadth in CADASIL arteries (B); in diseased arteries, staining for type VI collagen encroaches into the tunica media. In small penetrating arteries, immunoreactivity was limited to adventitia in controls (C), but was strong and nearly transmural in CADASIL arteries (D). Capillaries were negative for type VI collagen in controls (E); 3 of 6 CADASIL patients had sporadic reactive capillaries (F).
2.4.2 Small penetrating arteries
In CADASIL, the penetrating vessels had a thick layer of adventitial staining (Fig 3D). In most vessels, the staining penetrated towards the center of the vessel involving most, but not all, of the width of the arterial wall. In some cases, in the smallest vessels, there was transmural staining. In controls, there was occasional light adventitial staining, but no staining within the remainder of the arterial wall (Fig 3C).
2.4.3 Capillaries
In CADASIL, there was a dichotomy in capillary staining. Three of six CADASIL patients had no significant capillary staining, while the remaining three patients had staining in a subset of capillaries of both the gray and white matter (Fig 3F). No controls had capillary staining (Fig 3E).
3. DISCUSSION
We describe the most comprehensive study to date of collagen subtypes in CADASIL patients with cysteine-altering mutations in NOTCH3; Table 1 summarizes our findings. Our study is notable for the following: 1) core vascular collagens are sharply and consistently upregulated in cerebral arteries in CADASIL; 2) the arterial distribution of type IV collagen in CADASIL overlaps with NOTCH3 and follows a distinct pattern vis-à-vis other subtypes; 3) type VI collagen specifically deposits in the vascular media in CADASIL; and 4) all core vascular collagens encroach upon and accumulate in the vascular media of CADASIL in both large and small vessels.
Table 1.
| Collagen type | Leptomeningeal arteries
|
White matter arteries | Capillaries | |||
|---|---|---|---|---|---|---|
| Intima | Media | Adventitia | ||||
| Type I | Control | + | + | +++ | + | + |
| CADASIL | ++ | ++ | +++ | +++ | ++ | |
| Type III | Control | + | + | +++ | + | ++ |
| CADASIL | ++ | ++ | +++ | +++ | ++ | |
| Type IV | Control | + | + | − | + | + |
| CADASIL | +++ | +++ | − | +++ | +++ | |
| Type VI | Control | − | − | + | + | − |
| CADASIL | − | +++ | +++ | ++ | +/− | |
+++ Consistent dark staining
++ Sporadic dark staining
+ Moderate or light staining
+/− Moderate or light staining only in some samples
Previous studies of collagen in CADASIL have been largely based on single cases or have focused on only one type of collagen. A unified view of collagen subtypes in CADASIL has therefore not been possible until now. Since the six patients studied here, who have independent NOTCH3 mutations, showed extremely consistent staining patterns, we have high confidence in generalizing our findings. Three observations should be highlighted.
First, collagen subtypes in CADASIL tended to follow two main patterns of distribution: 1) an external pattern of expression seen for type I, III, and VI collagen, and 2) an internal pattern seen with type IV collagen. Among large vessels, types I, III, and VI were predominantly found in the adventitia of vessels; reactivity appeared to infuse into the media, starting with externally located smooth muscle. The external collagens did not encroach upon the media of arteries with equal propensity; type III collagen was the most likely to deposit in the media, followed by type I and type VI. On the other hand, type IV collagen was found at high levels in the inner layers of the artery. Its expression begins at the inner aspect of the neointima, which accumulates proteins such as vWF (Fig 4C and 4H) [DOI: 10.1007/s12975-011-0112-2], and continues outward with nearly universal encroachment into the tunica media. Unlike other vascular collagens, type IV collagen was rarely found in the adventitia.
The contrasting external and internal patterns of expression strongly suggest independent cellular origins of collagen subtypes. Because of heavy adventitial expression, type I, III, and VI collagens likely derive (at least in part) from fibroblasts surrounding the vessel wall. Demonstration of these collagens in the outer rim of vessels is consistent with previous studies that show that type I and III proteins are frequently found together and in covalently bound complexes (Henkel and Glanville, 1982). Type IV collagen, on the other hand, may be derived from smooth muscle cells based on its heavy expression in the intima and media, which contain dedifferentiated and differentiated smooth muscle cells, respectively.
The patterns of collagen expression in small vessels of CADASIL strongly resemble those seen in limited studies of hypertensive vascular dementia. As in CADASIL, pathological descriptions of collagen deposition in Binwangers disease are largely single case reports, except two studies (Lin et al., 2000; Zhang and Olsson, 1997). In each of these studies, type I, III, and VI collagen were noted to more likely deposit in the outer edges of penetrating small arteries, while type IV collagen accumulated from the inside to the outside, much like what we observed in our CADASIL patients. It is noteworthy that we also found type VI collagen in capillaries in some (3 of 6) CADASIL patients and did not find it in controls. Likewise, Roggendorf et al found only 2 of 6 patients with chronic hypertension had capillary type VI collagen and also failed to detect expression in controls (Roggendorf et al., 1988). Similarities of collagen expression between CADASIL and other forms of cerebrovascular disease may indicate a common, convergent mechanism of vessel pathology.
Second, among the core vascular collagens, type IV is most consistently upregulated in vascular media and partly colocalizes with NOTCH3. Thus, our localization studies suggest that type IV collagen may be best positioned to play a mechanistic role in CADASIL. This is not surprising, since among all the core vascular collagens, type IV collagen is the only subtype that is made selectively in vascular smooth muscle (Khoshnoodi et al., 2008). While other collagens partly encroach into the vascular media, type IV collagen extensively colocalizes with the degenerating smooth muscle cells, universally appearing in large quantities in the tunica media (Fig 2 and Fig 4E and 4J) which frequently exhibits profound loss of smooth muscle actin (Fig 4B and 4G). Type IV protein was always present in areas of NOTCH3 expression, whilst other collagens had weaker or incomplete overlap with NOTCH3 positive material. Moreover, among the collagen types examined, type IV (and type I) collagen was most notably upregulated in all three classes of vessels studied. Finally, it is worthwhile noting that protean genetic disorders have been attributed to mutations in all of the collagen types investigated in our study; however, type IV collagen is the only subtype genetically linked to leukoencephalopathy and stroke (Alamowitch et al., 2009; Gould et al., 2006; Jeanne et al., 2011; Lanfranconi and Markus, 2010; Shah et al., 2010; Vahedi et al., 2007).
Third, our study confirms that CADASIL strongly affects vessels of all calibers throughout the brain. Importantly, for the first time, we show that collagen subtype patterns are similar in leptomeningeal arteries and small penetrating vessels. For example, type I, III, and VI collagen were clearly expressed prominently on the outer edges of penetrating small vessels and leptomeningeal arteries. In addition, type IV collagen, which was clearly seen in the inside of larger arteries, was also found most prominently in the inner aspect of penetrating small vessels. The increased proportion of small vessels (compared to medium sized vessels) with transmural expression of the collagens is consistent with a model in which collagen is synthesized in the same region of vessels, regardless of caliber, and then spreads throughout the vessel. In small vessels, collagen needs only to transit a short distance before penetrating the full thickness of a vessel. A similar caliber-dependent distribution of the endothelial-specific protein vWF has recently been described in our lab [DOI: 10.1007/s12975-011-0112-2]. These patterns suggest that the mechanisms of fibrosis and protein production and deposition could be similar in leptomeningeal vessels and small vessels; but small vessels may be preferentially affected by disease processes since thinner-walled structures are more likely to be fully embued by encroaching proteins made in different layers of arteries.
Future directions
Our observations raise fundamental questions about the role of collagen in CADASIL. Are specific collagen types protective or harmful for patients? On the one hand, type IV and VI collagen have been shown to be associated with decreased amyloid deposition and protection from amyloid pathology (Barnes and Farndale, 1999; Cheng et al., 2009; Kiuchi et al., 2002). Potentially, these collagens could also protect the brain from protein extravasation in CADASIL. In atherosclerosis, collagen networks in the cap of the plaque may prevent rupture and promote stability. Lastly, type IV collagen has been shown to promote smooth muscle maturation and prevent maladaptive dedifferentiation in some studies (Orr et al., 2009).
On the other hand, increased collagen may interfere with the mechanical properties of vessels, impairing dynamic regulation of blood flow. Moreover, preliminary studies from our lab have shown that increased type IV collagen is associated with downregulation of mature smooth muscle genes, an activity that may promote vascular pathology. Future studies, potentially exploiting a growing number of murine models of CADASIL, should be pursued.
In conclusion, we demonstrate distinct and consistent patterns of collagen subtype deposition in cerebral arteries in CADASIL. Of the collagen subtypes identified, type IV collagen is best positioned play a potential role in disease pathogenesis. Given the link between CADASIL and type IV collagen, which has been shown to participate in other inherited small vessel diseases, interactions between mutant NOTCH3 and collagen deserve additional study.
4. METHODS AND MATERIALS
Patients and controls
We studied brains from six deceased North American CADASIL patients from independent families (mean age 66.3±12.8, range 46–83 years) with genetically confirmed NOTCH3 mutations. These patients have been described in prior work [DOI: 10.1007/s12975-011-0112-2]. Controls included 6 deceased patients (mean age 65.5 ± 14.5 years, range 54–82 years) free of known cerebrovascular disease.
Histological methods
Samples of the anterior temporal lobe were selected for this study. Paraffin-embedded blocks were sectioned (5-μm thickness) and included cortex, subcortical white matter, and leptomeninges. Hematoxylin and eosin and Movat’s Pentachrome (Petrovic et al., 2011) staining were used to define general vascular structure. For immunohistochemical studies, we used established primary monoclonal antibodies to specific subtypes of collagen (Table 2). Anti-H antigen (BRIC; Santa Cruz) was used as a positive control for vessels and identified endothelial cells in every control and CADASIL sample. NOTCH3, smooth muscle actin, and von Willebrand factor antibodies were respectively obtained from Abnova (M01 mouse monoclonal), Dako (1A4 mouse monoclonal), and Dako (A0082 rabbit polyclonal). Staining was performed using standard published methods employing either the Vectastain ABC Elite or Dako Envision autostaining systems.
Table 2.
| Protein | Mouse Monoclonal Antibody | Source |
|---|---|---|
| Collagen I | I-8H5 | MP Biomedical |
| Collagen II | CIIC1 | DSHB |
| Collagen III | III-53 | MP Biomedical |
| Collagen IV | M3F7 | DSHB |
| Collagen V | V-3C9 | MP Biomedical |
| Collagen VI | 5C6 | DSHB |
| NOTCH3 | M01 | Abnova |
| SM Actin | 1A4 | Dako |
Supplementary Material
Highlights.
Fibrotic arteries form the core of CADASIL pathology, but the molecular components of the vessels are not known. This is the first systematic study of collagen types in this unique inherited cerebral vasculopathy.
Type I, III, and VI collagen build up in arteries in CADASIL from the outside and move into the arterial media
The most dramatically increased collagen, type IV collagen, accumulates in the intima and media in robust amounts in CADASIL. Type IV collagen is the only isotype whose mutations cause cerebral small vessel disease, raising the possibility that it plays a core role in brain vascular disease.
Collagen builds up in all caliber vessels in CADASIL, and the pattern recapitulates other cerebral vasculopathies.
Acknowledgments
Funding: We gratefully acknowledge NIH grants NS052681, NS054724, and NS062816 to MMW. The ADRC is supported by grant P50-AG08671 and supplied control tissues for studies. Other human tissues were obtained from the NICHD Brain and Tissue Bank for Developmental Disorders at the University of Maryland, Baltimore, MD, contract HHSN275200900011C, Ref. No. NO1-HD-9-0011. We express deep thanks to the patients and families whose precious tissue donations were used in this study. We thank the UM Comprehensive Cancer Center Histology Core for providing excellent technical support for experiments.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Alamowitch S, Plaisier E, Favrole P, Prost C, Chen Z, Van Agtmael T, Marro B, Ronco P. Cerebrovascular disease related to COL4A1 mutations in HANAC syndrome. Neurology. 2009;73:1873–82. doi: 10.1212/WNL.0b013e3181c3fd12. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barnes MJ, Farndale RW. Collagens and atherosclerosis. Exp Gerontol. 1999;34:513–25. doi: 10.1016/s0531-5565(99)00038-8. [DOI] [PubMed] [Google Scholar]
- Chabriat H, Joutel A, Dichgans M, Tournier-Lasserve E, Bousser MG. Cadasil. Lancet Neurol. 2009;8:643–53. doi: 10.1016/S1474-4422(09)70127-9. [DOI] [PubMed] [Google Scholar]
- Cheng JS, Dubal DB, Kim DH, Legleiter J, Cheng IH, Yu GQ, Tesseur I, Wyss-Coray T, Bonaldo P, Mucke L. Collagen VI protects neurons against Abeta toxicity. Nat Neurosci. 2009;12:119–21. doi: 10.1038/nn.2240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chiu HF, Lam LC, Chi I, Leung T, Li SW, Law WT, Chung DW, Fung HH, Kan PS, Lum CM, Ng J, Lau J. Prevalence of dementia in Chinese elderly in Hong Kong. Neurology. 1998;50:1002–9. doi: 10.1212/wnl.50.4.1002. [DOI] [PubMed] [Google Scholar]
- Desmond DW, Moroney JT, Lynch T, Chan S, Chin SS, Mohr JP. The natural history of CADASIL: a pooled analysis of previously published cases. Stroke. 1999;30:1230–3. doi: 10.1161/01.str.30.6.1230. [DOI] [PubMed] [Google Scholar]
- Gould DB, Phalan FC, van Mil SE, Sundberg JP, Vahedi K, Massin P, Bousser MG, Heutink P, Miner JH, Tournier-Lasserve E, John SW. Role of COL4A1 in small-vessel disease and hemorrhagic stroke. N Engl J Med. 2006;354:1489–96. doi: 10.1056/NEJMoa053727. [DOI] [PubMed] [Google Scholar]
- Grinberg LT, Thal DR. Vascular pathology in the aged human brain. Acta Neuropathol. 2010;119:277–90. doi: 10.1007/s00401-010-0652-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henkel W, Glanville RW. Covalent crosslinking between molecules of type I and type III collagen. The involvement of the N-terminal, nonhelical regions of the alpha 1 (I) and alpha 1 (III) chains in the formation of intermolecular crosslinks. Eur J Biochem. 1982;122:205–13. doi: 10.1111/j.1432-1033.1982.tb05868.x. [DOI] [PubMed] [Google Scholar]
- Jeanne M, Labelle-Dumais C, Jorgensen J, Kauffman WB, Mancini GM, Favor J, Valant V, Greenberg SM, Rosand J, Gould DB. COL4A2 Mutations Impair COL4A1 and COL4A2 Secretion and Cause Hemorrhagic Stroke. Am J Hum Genet. 2011 doi: 10.1016/j.ajhg.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Joutel A, Corpechot C, Ducros A, Vahedi K, Chabriat H, Mouton P, Alamowitch S, Domenga V, Cecillion M, Marechal E, Maciazek J, Vayssiere C, Cruaud C, Cabanis EA, Ruchoux MM, Weissenbach J, Bach JF, Bousser MG, Tournier-Lasserve E. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–10. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- Joutel A, Andreux F, Gaulis S, Domenga V, Cecillon M, Battail N, Piga N, Chapon F, Godfrain C, Tournier-Lasserve E. The ectodomain of the Notch3 receptor accumulates within the cerebrovasculature of CADASIL patients. J Clin Invest. 2000;105:597–605. doi: 10.1172/JCI8047. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kalimo H, Ruchoux MM, Viitanen M, Kalaria RN. CADASIL: a common form of hereditary arteriopathy causing brain infarcts and dementia. Brain Pathol. 2002;12:371–84. doi: 10.1111/j.1750-3639.2002.tb00451.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khoshnoodi J, Pedchenko V, Hudson BG. Mammalian collagen IV. Microsc Res Tech. 2008;71:357–70. doi: 10.1002/jemt.20564. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kiuchi Y, Isobe Y, Fukushima K. Type IV collagen prevents amyloid beta-protein fibril formation. Life Sci. 2002;70:1555–64. doi: 10.1016/s0024-3205(01)01528-4. [DOI] [PubMed] [Google Scholar]
- Lanfranconi S, Markus HS. COL4A1 mutations as a monogenic cause of cerebral small vessel disease: a systematic review. Stroke. 2010;41:e513–8. doi: 10.1161/STROKEAHA.110.581918. [DOI] [PubMed] [Google Scholar]
- Lin JX, Tomimoto H, Akiguchi I, Matsuo A, Wakita H, Shibasaki H, Budka H. Vascular cell components of the medullary arteries in Binswanger's disease brains: a morphometric and immunoelectron microscopic study. Stroke. 2000;31:1838–42. doi: 10.1161/01.str.31.8.1838. [DOI] [PubMed] [Google Scholar]
- Lobo A, Launer LJ, Fratiglioni L, Andersen K, Di Carlo A, Breteler MM, Copeland JR, Dartigues JF, Jagger C, Martinez-Lage J, Soininen H, Hofman A. Prevalence of dementia and major subtypes in Europe: A collaborative study of population-based cohorts. Neurologic Diseases in the Elderly Research Group. Neurology. 2000;54:S4–9. [PubMed] [Google Scholar]
- Low WC, Junna M, Borjesson-Hanson A, Morris CM, Moss TH, Stevens DL, St Clair D, Mizuno T, Zhang WW, Mykkanen K, Wahlstrom J, Andersen O, Kalimo H, Viitanen M, Kalaria RN. Hereditary multi-infarct dementia of the Swedish type is a novel disorder different from NOTCH3 causing CADASIL. Brain. 2007;130:357–67. doi: 10.1093/brain/awl360. [DOI] [PubMed] [Google Scholar]
- Miao Q, Paloneva T, Tuominen S, Poyhonen M, Tuisku S, Viitanen M, Kalimo H. Fibrosis and stenosis of the long penetrating cerebral arteries: the cause of the white matter pathology in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Brain Pathol. 2004;14:358–64. doi: 10.1111/j.1750-3639.2004.tb00078.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Miao Q, Kalimo H, Bogdanovic N, Kostulas K, Borjesson-Hanson A, Viitanen M. Cerebral arteriolar pathology in a 32-year-old patient with CADASIL. Neuropathol Appl Neurobiol. 2006a;32:455–8. doi: 10.1111/j.1365-2990.2006.00746.x. [DOI] [PubMed] [Google Scholar]
- Miao Q, Paloneva T, Tuisku S, Roine S, Poyhonen M, Viitanen M, Kalimo H. Arterioles of the lenticular nucleus in CADASIL. Stroke. 2006b;37:2242–7. doi: 10.1161/01.STR.0000236838.84150.c2. [DOI] [PubMed] [Google Scholar]
- Oide T, Nakayama H, Yanagawa S, Ito N, Ikeda S, Arima K. Extensive loss of arterial medial smooth muscle cells and mural extracellular matrix in cerebral autosomal recessive arteriopathy with subcortical infarcts and leukoencephalopathy (CARASIL) Neuropathology. 2008;28:132–42. doi: 10.1111/j.1440-1789.2007.00864.x. [DOI] [PubMed] [Google Scholar]
- Orr AW, Lee MY, Lemmon JA, Yurdagul A, Jr, Gomez MF, Bortz PD, Wamhoff BR. Molecular mechanisms of collagen isotype-specific modulation of smooth muscle cell phenotype. Arterioscler Thromb Vasc Biol. 2009;29:225–31. doi: 10.1161/ATVBAHA.108.178749. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovic A, Abramovic M, Mihailovic D, Gligorijevic J, Zivkovic V, Mojsilovic M, Ilic I. Multicolor counterstaining for immunohistochemistry ? a modified Movat's pentachrome. Biotech Histochem. 2011;86:429–435. doi: 10.3109/10520295.2010.528026. [DOI] [PubMed] [Google Scholar]
- Roggendorf W, Opitz H, Schuppan D. Altered expression of collagen type VI in brain vessels of patients with chronic hypertension. A comparison with the distribution of collagen IV and procollagen III. Acta Neuropathol. 1988;77:55–60. doi: 10.1007/BF00688243. [DOI] [PubMed] [Google Scholar]
- Ruchoux MM, Maurage CA. CADASIL: Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. J Neuropathol Exp Neurol. 1997;56:947–64. [PubMed] [Google Scholar]
- Sawabe M. Vascular aging: from molecular mechanism to clinical significance. Geriatr Gerontol Int. 2010;10(Suppl 1):S213–20. doi: 10.1111/j.1447-0594.2010.00603.x. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007a;69:2197–204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Boyle PA, Arvanitakis Z, Bienias JL, Bennett DA. Subcortical infarcts, Alzheimer's disease pathology, and memory function in older persons. Ann Neurol. 2007b;62:59–66. doi: 10.1002/ana.21142. [DOI] [PubMed] [Google Scholar]
- Schneider JA, Arvanitakis Z, Leurgans SE, Bennett DA. The neuropathology of probable Alzheimer disease and mild cognitive impairment. Ann Neurol. 2009;66:200–8. doi: 10.1002/ana.21706. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shah S, Kumar Y, McLean B, Churchill A, Stoodley N, Rankin J, Rizzu P, van der Knaap M, Jardine P. A dominantly inherited mutation in collagen IV A1 (COL4A1) causing childhood onset stroke without porencephaly. Eur J Paediatr Neurol. 2010;14:182–7. doi: 10.1016/j.ejpn.2009.04.010. [DOI] [PubMed] [Google Scholar]
- Shaji S, Promodu K, Abraham T, Roy KJ, Verghese A. An epidemiological study of dementia in a rural community in Kerala, India. Br J Psychiatry. 1996;168:745–9. doi: 10.1192/bjp.168.6.745. [DOI] [PubMed] [Google Scholar]
- Szpak GM, Lewandowska E, Wierzba-Bobrowicz T, Bertrand E, Pasennik E, Mendel T, Stepien T, Leszczynska A, Rafalowska J. Small cerebral vessel disease in familial amyloid and non-amyloid angiopathies: FAD-PS-1 (P117L) mutation and CADASIL. Immunohistochemical and ultrastructural studies. Folia Neuropathol. 2007;45:192–204. [PubMed] [Google Scholar]
- Tian J, Shi J, Smallman R, Iwatsubo T, Mann DM. Relationships in Alzheimer's disease between the extent of Abeta deposition in cerebral blood vessel walls, as cerebral amyloid angiopathy, and the amount of cerebrovascular smooth muscle cells and collagen. Neuropathol Appl Neurobiol. 2006;32:332–40. doi: 10.1111/j.1365-2990.2006.00732.x. [DOI] [PubMed] [Google Scholar]
- Vahedi K, Kubis N, Boukobza M, Arnoult M, Massin P, Tournier-Lasserve E, Bousser MG. COL4A1 mutation in a patient with sporadic, recurrent intracerebral hemorrhage. Stroke. 2007;38:1461–4. doi: 10.1161/STROKEAHA.106.475194. [DOI] [PubMed] [Google Scholar]
- Zhang WW, Olsson Y. The angiopathy of subcortical arteriosclerotic encephalopathy (Binswanger's disease): immunohistochemical studies using markers for components of extracellular matrix, smooth muscle actin and endothelial cells. Acta Neuropathol. 1997;93:219–24. doi: 10.1007/s004010050607. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.



