Abstract
AIM
To identify electroencephalographic (EEG) biomarkers for the analgesic effect of pregabalin in patients with chronic visceral pain.
METHODS
This was a double-blind, placebo-controlled study in 31 patients suffering from visceral pain due to chronic pancreatitis. Patients received increasing doses of pregabalin (75 mg–300 mg twice a day) or matching placebo during 3 weeks of treatment. Pain scores were documented in a diary based on a visual analogue scale. In addition, brief pain inventory-short form (BPI) and quality of life questionnaires were collected prior to and after the study period. Multi-channel resting EEG was recorded before treatment onset and at the end of the study. Changes in EEG spectral indices were extracted, and individual changes were classified by a support vector machine (SVM) to discriminate the pregabalin and placebo responses. Changes in individual spectral indices and pain scores were correlated.
RESULTS
Pregabalin increased normalized intensity in low spectral indices, most prominent in the theta band (3.5–7.5 Hz), difference of −3.18, 95% CI −3.57, −2.80; P = 0.03. No changes in spectral indices were seen for placebo. The maximum difference between pregabalin and placebo treated patients was seen in the parietal region, with a classification accuracy of 85.7% (P = 0.009). Individual changes in EEG indices were correlated with changes in pain diary (P = 0.04) and BPI pain composite scores (P = 0.02).
CONCLUSIONS
Changes in spectral indices caused by slowing of brain oscillations were identified as a biomarker for the central analgesic effect of pregabalin. The developed methodology may provide perspectives to assess individual responses to treatment in personalized medicine.
Keywords: analgesic, electroencephalographic, pain, pattern recognition, pregabalin
WHAT IS ALREADY KNOWN ABOUT THIS SUBJECT
Pregabalin is an anticonvulsive agent prescribed as a secondary analgesic for patients when standard pain treatment is insufficient.
The analgesic effect resides in the central nervous system.
The central analgesic effect can be evaluated by electroencephalography.
WHAT THIS STUDY ADDS
The analgesic effect of pregabalin is reflected as a slowing of brain oscillations.
The slowing of brain oscillations for each individual patient is correlated with subjective pain scores.
The developed methodology may be used as a mechanistic approach to monitor the analgesic effect of pregabalin in pharmacological studies.
Introduction
Pregabalin is an anticonvulsive agent prescribed as a secondary analgesic in various chronic painful diseases [1]. Traditionally pregabalin has been used in somatic neuropathic pain such as diabetic polyneuropathy, post stroke pain and post herpetic neuralgia [2]. Furthermore, pregabalin was recently shown to be effective for visceral pain associated with irritable bowel syndrome and chronic pancreatitis (CP) [3, 4]. Although widely used, the complex mechanisms of action underlying pregabalin analgesia remain to be elucidated. Animal experiments have shown that the analgesic effect is obtained by selectively binding to the alpha-2-delta subunit of voltage-dependent calcium channels. This blocks the calcium influx into the presynaptic nerve terminals and hence reduces the release of excitatory neurotransmitters such as glutamate, norepinephrine and substance P [5–7].
There are, however, several limitations in animal studies due to fundamental differences in the neurobiology of the nociceptive receptors between humans and animals. Accordingly, results from animal experiments do not directly translate to human conditions [8]. To study the central analgesic effect of pregabalin in human pain patients, pharmaco-electroencephalography (EEG) provides a sensitive method to study neuronal cortical activity [9]. However, previous studies have demonstrated that drugs affecting glutamatergic neurotransmission, such as pregabalin, may cause a general sedative effect, which also influences EEG characteristics [10]. This may lead to misinterpretation of the results obtained from the EEG. To verify that the described neuronal cortical activities are linked to the analgesic effect and not caused by sedation, a correlation between EEG characteristics and subjective pain scores should be demonstrated.
Most previous pharmaco-EEG studies have been based on spectral analysis, where oscillations in the EEG signals are divided into predefined frequency bands. The most widely used method is the Fourier transform, which gives a rough estimate of frequency content in the signals. To improve the analysis and extract more detailed information, advanced methods such as the continuous wavelet transform (CWT) are needed [11]. The individual changes in the spectral indices before and after pregabalin or placebo could then be correlated with subjective pain scores, to test whether the observed EEG changes are linked to the analgesic effect. However, it may be that the analgesic effect of pregabalin can only be described by the interaction between all frequency bands simultaneously, an approach which requires more advanced methods of analysis. This can be achieved using algorithms based on statistical learning such as the support vector machine (SVM), which estimates the discriminative capacity between changes in the pregabalin and placebo responses without making any theoretical a priori assumptions [12, 13].
We hypothesized that pregabalin treatment as compared with placebo induces a slowing of brain oscillations and that this is associated with clinical pain relief. Using advanced signal analysis methods based on CWT and SVM, the aims of the study were 1) To investigate whether pregabalin as compared with placebo alters EEG spectral indices and 2) to investigate whether changes in individual quantitative EEG indices are related to the analgesic effect of pregabalin.
Methods
Study subjects
Thirty-one patients with CP visiting the gastrointestinal outpatient clinic at Aalborg Hospital, Denmark between October 2008 and April 2010 were enrolled in the study [4]. Inclusion criteria were a diagnosis of CP based on The Mayo Clinic diagnostic criteria [14], abdominal pain typical for CP (i.e. dull epigastric pain, eventually radiating to the back) and chronic pain (i.e. pain 3 days or more per week for the past 3 months). Patients taking concomitant medication were included only if the pain treatment was stable. Concomitant medication included both stable opioids and non-opioid analgesics. New analgesic therapies or other medications with effects in the central nervous system were not allowed to be initiated at any time during the study period.
Exclusion criteria were acute or chronic pain syndromes other than CP (e.g. irritable bowel syndrome and lower back pain), patients previously diagnosed with moderate to severe renal impairment, clinically significant abnormal cardiac rhythm, evidence of untreated myocardial ischaemia or injury, hypersensitivity or history of major depression. Furthermore, patients were not eligible if they had been treated with pregabalin up to 4 months before study start or if they had any known allergic response to pregabalin.
The local Ethics Committee approved the study (N-20080028MCH), which was conducted according to the Declaration of Helsinki and monitored by the GCP-unit, Aarhus University Hospital, Denmark. All patients gave oral and written consent before the start of the study.
Experimental protocol
The study was a 3 week double-blind placebo-controlled study with 31 patients stratified depending on the occurrence of diabetes mellitus. A computer generated pseudo-random code randomized patients to receive either pregabalin or matching placebo. Based on response and tolerability, patients received increasing doses of pregabalin (75 mg–300 mg) twice daily or matching placebo titrated at 3 day intervals. All patients followed the same oral dosing schedule (daily dose was split into two equivalent doses administered in the morning and in the evening). In case of unacceptable adverse effects, the dose was titrated downwards, and this dose was then kept for the remainder of the study period. If the patient could not tolerate an end dose of at least 150 mg twice daily, the subject was withdrawn from the study. Pfizer Clinical Research Operations, UK prepared identical coded medication bottles containing identical capsules of pregabalin or placebo.
Clinical pain scores
A pain diary and questionnaires were used to evaluate the clinical efficacy of pregabalin treatment. As the study was not designed or powered to study clinical efficacy of pregabalin per se, the retrieved changes in clinical pain scores were used for correlation analysis only. Pain intensity was assessed by a pain diary based on a visual analogue scale (VAS) with 0 = no pain and 10 = worst pain imaginable. The pain diary started 1 week before medication onset and continued during the entire study period of 3 weeks. Brief pain inventory-short form (BPI) was used to explore further aspects of the pain and how it interfered with daily activities prior to medication and after 3 weeks of treatment. BPI is a 14-item questionnaire where patients on a 0 to 10 scale rated pain and the degree to which it interfered with activities during the last week. The 14 items were summarized in a pain and an interference composite score [15, 16]. The patient's quality of life (QL) was assessed before and after treatment by a part of the European Organization for Research and Treatment of Cancer questionnaire (EORTCQLQ-C30) [17, 18].
EEG recordings
EEG was recorded in the resting condition to obtain spontaneous brain activity during a 2 min period before drug administration and at the end of the treatment period, i.e. after 3 weeks of pregabalin or placebo treatment. Signals were recorded using a SynAmp2 system (Neuroscan Compumedics, El Paso, Texas, USA) and a standard 62 channel cap (Quick-Cap International, Neuroscan, El Paso, Texas, USA) with Ag/AgCl surface electrodes mounted according to the extended 10–20 system [19]. The impedance of all electrodes was kept below 5 kOhm by applying Electro-gel (Electrocap international, Inc., Eaton, Ohio, USA). Recordings were performed in AC mode with sampling frequency of 1000 Hz and online band pass filter from 0.05 to 200 Hz.
The recordings took place in a quiet room with dimmed light and all unnecessary equipment was turned off to avoid artifacts and noise. Instructions and recordings were performed by a medical doctor and qualified research nurses, and patients were instructed to rest in a supine position with eyes open during the recordings. All patients were instructed to refrain from caffeine beverages before recordings to avoid decreased theta activity induced by caffeine [20].
Post-processing
The EEG signals were offline post-processed (Neuroscan 4.3.1, Compumedics, El Paso, Texas, USA). The processing included the following steps: (i) re-reference to linked-ear, (ii) visual inspection of data quality for each of the channels. Channels displaying abnormal signals in respect to level and/or shape were discarded and replaced by a new signal interpolated from the four neighbouring electrodes (on average 1.4 of the channels were replaced per subject), (iii) artifact rejection by visual inspection, leaving at least 1 min of valid recording for further analysis, (iv) notch filtering to remove 50 Hz noise and (v) high-pass filtering to remove DC-offset and linear detrends by a first order Butterworth filter with cut-off frequency 0.5 Hz (this part of the post-processing was performed in MATLAB®).
Spectral indices
To study the behaviour of the electrical activity in the brain, a CWT was applied to calculate the time–frequency characteristics in all channels. The CWT is based on a mother wavelet function, which can be chosen from a set of infinite functions [21]. To obtain a trade-off between time and frequency resolution, a complex Morlet wavelet function was chosen for this study [22]. The Morlet function is defined by two design parameters, which were set to have a band width parameter of 128 Hz and wavelet centre frequency of 0.5 Hz [23, 24]. The coefficients were calculated over dyadic scales (although the CWT does not impose this limitation), in order to present the energy distribution in the following band widths: delta (0.5–3.5 Hz), theta (3.5–7.5 Hz), alpha1 (7.5–10.5 Hz), alpha2 (10.5–13.5 Hz), beta1 (13.5–18.5 Hz), beta2 (18.5–24.5 Hz) and beta3 (24.5–32 Hz). The coefficients in each band were rectified and integrated over time to obtain the marginal distribution in the spectral indices (intensity of the signal in each sub-band). The marginals were calculated separately for each channel and the intensities in the indices were normalized into percentage of the total power (0.5–32 Hz). The mean spectral distribution over all channels was used to calculate the changes in spectral indices for each patient, to identify alterations induced by pregabalin or placebo.
Clinical correlations
To investigate how the simultaneous interaction of changes in all spectral indices was correlated to the clinical scores, the individual changes for each single channel were used as input to a SVM. The overall strategy of the SVM is presented in Figure 1. In brief, the input to the classifier was the changes in spectral indices for each patient along with a label indicating to which class (pregabalin or placebo) the patient belonged. Based on these features the SVM calculated a linear decision rule to separate the classes in the most optimal way. Once the decision rule was determined, any new patient could be classified without an indication of class membership [12]. The accuracy was determined by extracting one patient for test and using the remaining patients to calculate the optimal linear decision rule, which minimized the probability of error estimated from the training set. When the optimal decision rule was determined, the patient extracted for test was classified and the agreement to the correct class was registered. This procedure was repeated for all patients and all channels, and hence it was possible to determine the classification accuracy for each single channel.
Figure 1.
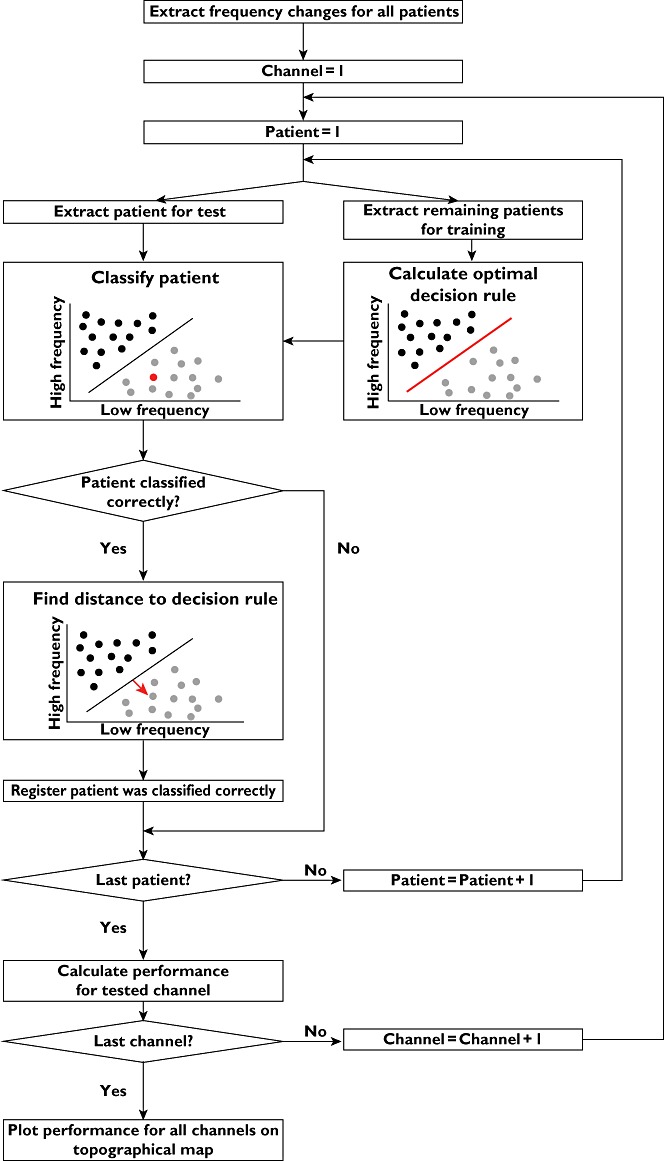
Schematic illustration of the principle in SVM classification and regression. For simplicity the features are illustrated as the portion of low and high frequencies extracted from the EEG signals, although the input space in this study was a seven dimensional space representing all frequency bands. For each subject, the input features were calculated and given as input to the SVM classifier with a label indicating if the patient was treated with pregabalin or placebo. The SVM calculated an optimal decision rule to separate the pregabalin (grey) group from the placebo (black) group. For patients classified correctly, the distance to the decision rule (representing the overall change in the EEG) was determined. The overall purpose of applying the SVM is to assess changes in all frequency bands simultaneously. This complies with the complexity in the EEG signals where multiple sources in the pain matrix generate oscillations in different bands, and the interaction between these sources might reflect the analgesic effect of pregabalin
The channel presenting the highest accuracy to discriminate between the pregabalin and placebo changes was used for further correlation analysis. Besides generating a categorical output (pregabalin or placebo), the SVM may also be applied in regression mode, where the output represents the distance to the decision rule as demonstrated with the red arrow in Figure 1. This output is a real value on a continuous scale and indicates how well the patient was classified to the particular class. A high regression value implies that the subject is far from the decision rule and hence with very high probability belongs to the assigned class, while a low value indicates that the patient had a response more similar to the other class (i.e. assigned to pregabalin, but with some similarities to the placebo group and vice versa). By using SVM in regression mode, the interaction of changes in spectral indices generated a single value estimating the overall changes of the EEG response. This value was correlated to the four clinical scores for all patients classified correctly for both classes (pregabalin and placebo).
Statistical analysis
All data are presented as mean ± SD and normally distributed unless otherwise indicated. Data in Table 1 were compared by Student's t-test or Fisher's exact test as appropriate. The relative changes in spectral indices were calculated as the average over all channels. The changes were compared by a mixed anova model with electrode as within subjects factor and treatment group (i.e. pregabalin vs. placebo) as between subjects factor. The classification accuracy was examined by Fisher's exact test. The correlation between clinical outcomes and changes in EEG were examined by a Pearson product-moment correlation coefficient. All reported P values were two sided and a P value below 0.05 was considered to indicate statistical significance.
Table 1.
Characteristics of patients in each stratum (pregabalin or placebo)
| Pregabalin (n = 14) | Placebo (n = 14) | |
|---|---|---|
| Mean age (range) (years) | 50 (23–61) | 53 (35–67) |
| Males/Females | 6/8 | 11/3 |
| BMI (kg m−2) | 23 ± 5 | 22 ± 3 |
| Aetiology (alcohol/others) | 7/7 | 8/6 |
| Duration of CP (months) | 102 ± 73 | 130 ± 106 |
| Diabetes mellitus | 5 | 4 |
| Exocrine insufficiency | 7 | 7 |
| Opioid treatment | 9 | 10 |
| Mean pain score prior to treatment (VAS) | 4.0 ± 2.5 | 3.6 ± 2.1 |
| Final pregabalin dose 300 mg/600 mg | 6/8 | N/A |
BMI, body mass index; CP, chronic pancreatitis; VAS, visual analogue scale; N/A, not applicable.
Results
Thirty-one patients fulfilled the inclusion criteria and were randomized to study medication. Two patients in the pregabalin group had unacceptable side effects (dizziness and confusion) at a dose of 150 mg twice daily and were withdrawn from the study. The EEG data for one of the subjects in the placebo group was noisy and did not enable extraction of at least 1 min of valid traces. Hence, 28 subjects (mean age 51.5, range 23–67 years) were considered for the analysis with 14 subjects in each group. The characteristics of the two groups displayed similar demographic and clinical characteristics (all P > 0.05), and are presented in Table 1.
Clinical pain scores
The changes in clinical pain scores were obtained from all patients (Table 2). These scores showed that patients treated with pregabalin had greater pain relief than patients in the placebo group, although the difference did not reach statistical significance (all P > 0.07).
Table 2.
Individual regression values (representing the overall change in EEG spectral indices) and changes in clinical pain scores
| Treatment | Subject | Regression value | ΔVAS pain score | ΔBPI, pain | ΔBPI, interference | ΔQL |
|---|---|---|---|---|---|---|
| Pregabalin | 1 | 1.60 | −0.25 | 0.50 | −0.43 | −8.33 |
| 2 | 0.71 | −0.63 | 2.50 | 5.00 | 16.67 | |
| 3 | 0.67 | −2.43 | −1.00 | −2.71 | −8.33 | |
| 4 | 0.79 | −1.89 | −2.00 | −0.43 | 50.00 | |
| 5 | 3.51 | −0.88 | −4.50 | −2.14 | 25.00 | |
| 6 | 2.68 | −5.00 | −2.00 | −3.86 | −16.67 | |
| 7 | x | −1.57 | −2.00 | −8.00 | 50.00 | |
| 8 | 0.13 | 0.80 | −1.25 | −0.43 | 0.00 | |
| 9 | 1.64 | 1.16 | −1.00 | −0.57 | −16.67 | |
| 10 | 1.11 | 0.00 | −0.50 | 0.00 | −8.33 | |
| 11 | 0.25 | −2.07 | −1.50 | −3.50 | 16.67 | |
| 12 | 3.36 | −9.00 | −8.75 | −2.00 | 58.33 | |
| 13 | 3.01 | −4.89 | −2.00 | −1.29 | 50.00 | |
| 14 | 2.70 | −5.05 | −3.75 | −2.14 | 41.67 | |
| Placebo | 1 | 0.98 | 0.00 | −1.00 | 0.02 | 0.00 |
| 2 | x | −5.32 | −1.00 | 0.50 | 50.00 | |
| 3 | 1.44 | −0.45 | −1.25 | −5.71 | 25.00 | |
| 4 | 0.98 | 0.07 | 0.50 | −1.29 | −8.33 | |
| 5 | 0.88 | −2.57 | 0.00 | −2.43 | 33.33 | |
| 6 | 1.89 | −3.42 | −3.25 | −4.00 | 8.33 | |
| 7 | 1.17 | −3.46 | −2.25 | −2.14 | −8.33 | |
| 8 | x | −0.70 | −0.50 | −2.43 | −8.33 | |
| 9 | 2.22 | −0.14 | −0.25 | 0.86 | −16.67 | |
| 10 | 1.60 | −0.42 | 0.25 | 0.29 | 8.33 | |
| 11 | 0.22 | 0.52 | 1.00 | 0.29 | 50.00 | |
| 12 | 0.27 | −1.57 | −1.00 | −2.00 | −8.33 | |
| 13 | x | −1.86 | −0.75 | −2.00 | 16.67 | |
| 14 | 2.55 | −1.71 | 5.00 | 0.71 | −16.67 |
(x) indicates misclassified subject.Δ = absolute change in pain scores and symptoms from initial values. VAS, visual analogue scale; BPI, brief pain inventory-short form; QL, quality of life.
EEG spectral indices
A significant increase in theta activity was seen after pregabalin treatment, difference between the means of −3.18, 95% CI −3.57, −2.80, (F = 5.12, P = 0.03), while no changes were seen for the other frequency bands (all P > 0.1) (Figure 2). No changes in EEG spectral indices were seen following placebo treatment (all P > 0.24).
Figure 2.
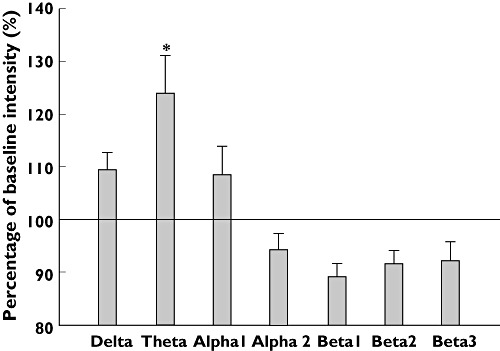
Relative grand mean frequency shifts measured as the mean over all electrodes for the pregabalin treated patients. *P < 0.05
Clinical correlations
The individual changes in all spectral indices were used as input to the SVM for classification. The classification accuracy for each channel is presented in a topographic map in Figure 3, where 50% indicates that patients were randomly assigned to one of the classes, while an increasing accuracy indicates improved performance. The classification had the highest performance at electrode P1, with an accuracy of 85.7%, which was above chance level compared with random performance at 50% (P = 0.009). This accuracy was obtained with 13 patients correctly classified in the pregabalin group in comparison with 11 patients classified correctly in the placebo group.
Figure 3.
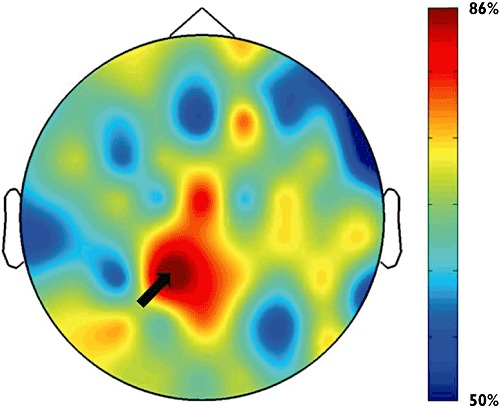
Topographical distribution of classification accuracy between pregabalin and placebo responses. An accuracy of 50% indicates random assignment while increased accuracy indicates improved performance. The system had the highest classification accuracy at electrode P1 (indicated by the black arrow), which was 85.7%
The regression value at the best performing electrode (P1) was determined for each of the correctly classified patients and correlated with the changes in scores for clinical pain relief (VAS), composite scores for pain and interference in the modified BPI, and the patient's QL. The individual regression values (representing the overall change in the EEG) and clinical pain scores are presented in Table 2. These values were used to calculate the correlation coefficients presented in Table 3. Significant correlations between changes in EEG spectral indices and changes in VAS and BPI pain scores were evident in the pregabalin treated group, while no correlations were seen for BPI interference and QL (Figure 4). No significant correlations between EEG changes and clinical scores were seen in the placebo group.
Table 3.
Pearsons product-moment correlation coefficients between the column ‘regression value’ and changes in clinical pain scores in Table 2. For VAS and BPI pain the negative correlation coefficient indicates that a high value for regression (high degree of alteration in the EEG) results in a high reduction of pain after treatment
| Treatment | VAS | BPI, pain | BPI, interference | QL |
|---|---|---|---|---|
| Pregabalin | −0.618* | −0.671* | −0.332 | 0.406 |
| Placebo | −0.190 | −0.279 | 0.112 | 0.499 |
P < 0.05. VAS, visual analogue scale; BPI, brief pain inventory-short form; QL, quality of life.
Figure 4.
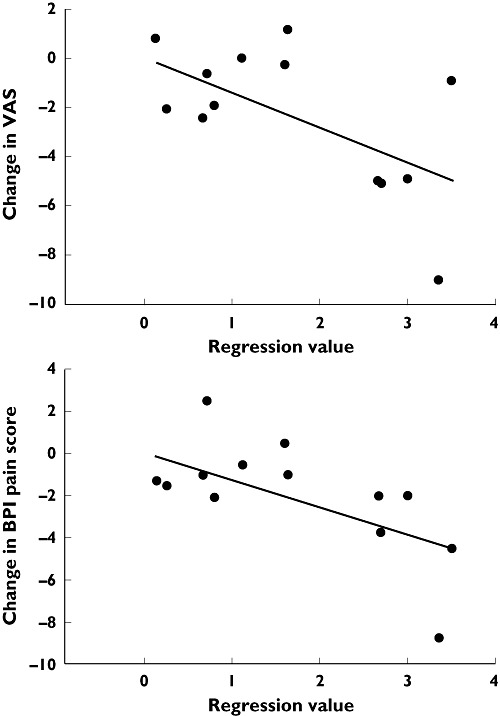
Correlation between regression value (representing the overall alteration of the EEG) and changes in clinical scores for pain diary based on the visual analogue scale (VAS) and the pain composite score in brief inventory short-form (BPI) for patients receiving pregabalin
Discussion
We investigated changes in EEG spectral indices before and after treatment with pregabalin or placebo in patients with visceral pain due to chronic pancreatitis. Pregabalin induced increased normalized marginal intensity in the low frequency spectral indices evident as increased theta band (3.5–7.5 Hz) activity (P = 0.03). Individual changes in EEG indices showed a correlation with changes in clinical pain scores in the pregabalin but not the placebo-treated group, whereas there was no relation to daily activities or quality of life. These findings suggest that slowing of EEG oscillations reflects the analgesic mechanisms underlying pregabalin treatment.
Methodological considerations
EEG signals were recorded in the resting state independent of external noxious stimuli, since previous studies have demonstrated that spontaneous brain activity in chronic pain conditions (especially neuropathic pain) involve specific brain circuitry different from brain activity commonly observed in acute pain [25]. The recordings were performed with open eyes, which has been shown to decrease the signal power without altering the frequency distribution [26]. However, recordings with open eyes minimize the risk of the subject being drowsy, which might induce alterations of alpha and theta oscillations [26]. The only important disadvantage of open eyes is that recordings are more prone to artifacts and noise, but as the data were cleaned by manual inspection, this aspect was not considered to interfere with the results. Furthermore, recordings with open eyes are in agreement with the standard operating procedures formulated by the International Pharmaco-EEG Group [27, 28].
The frequency analysis was performed by a CWT, which showed stable and consistent results for all subjects. The CWT has advantages over the Fourier transform, as it does not assume the signals are stationary, and has demonstrated improved extraction of time–frequency coefficients in several previous studies [21, 29, 30]. Furthermore, a consequence of the CWT algorithm is that low frequency oscillations are detected with high frequency resolution, while high frequency oscillations are detected with high time resolution, which mimics the properties of the EEG very well [21]. The CWT was based on a complex Morlet wavelet function, which is characterized by having a Gaussian shape in both time and frequency domain and hence provides optimal time–frequency resolution [24]. This wavelet has in other studies been used successfully to extract detailed physiological features from EEG signals [23].
Pattern recognition by SVM was applied to discriminate pregabalin induced changes in the EEG from those induced by placebo. This approach was chosen to comply with the multi-dimensional characteristics of the EEG, where the electrical activity recorded on the scalp is a summation of activity from multiple sources. As the pain matrix includes several sources oscillating in different frequency bands, simple linear regression models analyzing one frequency band at a time are not sufficient to explore how pregabalin influences the CNS. SVM enabled a robust analysis of the complex interaction between changes in EEG spectral indices, where all bands were taken into consideration simultaneously. The approach to classify the individual changes in spectral indices between pregabalin and placebo treated patients is a novel way to assess the analgesic effect in pharmaco-EEG. By applying the SVM in regression mode, a single score was obtained (regression value) representing the overall changes in the EEG. Interestingly, the regression value correlated with pain related clinical scores. A similar approach has been used in a pilot study of pharmaco-EEG, where pre-treatment resting EEG was used to predict treatment outcome of a selective serotonin re-uptake inhibitor [31]. In this study, a classification accuracy of 86.6% was obtained, and similar to our study, the high performance was only obtained by including features from multiple bands simultaneously.
The method proposed in this study assessed subjects on an individual basis before and after drug intake, and identified a potential biomarker for analgesic effect of pregabalin. This might in the future provide a useful tool to monitor the analgesic effect of various agents and thereby optimize individualized analgesic treatment. Although it has to be investigated in a new study, it might also provide a useful tool to predict the analgesic efficacy for individual patients.
Slowing of brain oscillations and the analgesic effect of pregabalin
Patients treated with pregabalin were characterized by a slowing of brain oscillations most prominent as an increase in normalized theta band activity. A comparable response has been observed in a study of healthy volunteers treated with the NAALADase-inhibitor GPI 5693 used for treatment of neuropathic pain [32]. As patients with chronic pancreatitis have many neuropathic features this may be of interest in future studies [33].
Increased theta band activity of the EEG in the resting state has also been reported after treatment with ketamine, an N-methyl-D-aspartate (NMDA) receptor glutamatergic antagonist [34]. Although speculative, the alteration of brain oscillations in the present study may reflect that the main analgesic effect of pregabalin is caused by reduced NMDA activation due to dampening of the excitatory neurotransmitter glutamate. As NMDA receptor activation has been shown to play a key role in hyperalgesia and enhancement of pain signalling seen in chronic pain states including inflammation and neuropathic pain conditions, the underlying mechanisms of pregabalin could be a reduction in central sensitization [35, 36].
The increased theta activity and correlations with clinical outcome should also be seen in light of other factors which could disrupt the findings. One such factor is that we allowed opioid intake on a stable regime, although opioids have been shown to highly influence the EEG [37]. However, as we only assessed the changes in spectral indices before and after pregabalin treatment, we do not believe this could have influenced the results. This is further supported by a previous study on all patients from this study, which were compared with age and gender matched healthy volunteers. In this previous study we extracted spectral indices by the same CWT methodology as for the present study, and found that opioids as well as diabetes mellitus and alcohol aetiology only modified the delta band over the centro-frontal brain regions, while no effect was seen for the theta band [38]. Another factor is the gender difference between the two groups with a majority of males in the placebo treated group. We have, however, refrained from subgroup analysis on gender differences, since the subgroups were too small to power the statistical analysis.
Furthermore, attention should be drawn to the fact that the results obtained for the patients treated with pregabalin were validated by non-significant results for the placebo treated group. In the placebo group, no changes were found in spectral indices before and after treatment and no correlation was seen between the regression value and changes in clinical scores. The non-significant change in spectral indices indicates that although some placebo treated patients reported pain relief at similar magnitude to those in the pregabalin group, this was not observable as common alterations in the resting EEG.
The proposed method is suitable to look into mechanistic aspects of analgesia, hence encompassing the influence of other factors such as the placebo effect, psychological and social factors that normally confound clinical evaluation of analgesics [39, 40]. The non-significance in pain scores between the pregabalin and placebo treated groups could be due to the low sample size confirmed by the fact that patients in the present study were a part of a larger parallel group study in Netherlands and Denmark with 34 patients treated with pregabalin and 30 patients treated with placebo. In this enlarged study population a statistical difference was found for the average pain diary score [4]. Hence, we believe that the resting EEG is a powerful tool to assess the mechanistic alterations caused by pharmacological intervention independent of the placebo effect.
In conclusion, this study showed that quantitative pharmaco-EEG can be used to monitor the central analgesic mechanisms of pregabalin. In future studies this approach may be used to predict effect of treatment leading to pharmaco-diagnostic testing.
Acknowledgments
This study was supported by the Danish Council for Strategic Research (MultiPain) and a free grant from Pfizer Research and Development.
Competing Interests
There are no competing interests to declare.
REFERENCES
- 1.Chauhan S, Forsmark CE. Pain management in chronic pancreatitis: a treatment algorithm. Best Pract Res Clin Gastroenterol. 2010;24:323–35. doi: 10.1016/j.bpg.2010.03.007. [DOI] [PubMed] [Google Scholar]
- 2.Finnerup NB, Sindrup SH, Jensen TS. The evidence for pharmacological treatment of neuropathic pain. Pain. 2010;150:573–81. doi: 10.1016/j.pain.2010.06.019. [DOI] [PubMed] [Google Scholar]
- 3.Houghton LA, Fell C, Whorwell PJ, Jones I, Sudworth DP, Gale JD. Effect of a second-generation alpha2delta ligand (pregabalin) on visceral sensation in hypersensitive patients with irritable bowel syndrome. Gut. 2007;56:1218–25. doi: 10.1136/gut.2006.110858. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Olesen SS, Bouwense SA, van Goor H, Wilder-Smith OH, Drewes AM. Pregabalin reduces pain in patients with chronic pancreatitis, in a randomized, controlled trial. Gastroenterology. 2011;14:536–43. doi: 10.1053/j.gastro.2011.04.003. [DOI] [PubMed] [Google Scholar]
- 5.Dooley DJ, Donovan CM, Pugsley TA. Stimulus-dependent modulation of [(3)H]norepinephrine release from rat neocortical slices by gabapentin and pregabalin. J Pharmacol Exp Ther. 2000;295:1086–93. [PubMed] [Google Scholar]
- 6.Fehrenbacher JC, Taylor CP, Vasko MR. Pregabalin and gabapentin reduce release of substance P and CGRP from rat spinal tissues only after inflammation or activation of protein kinase C. Pain. 2003;105:133–41. doi: 10.1016/s0304-3959(03)00173-8. [DOI] [PubMed] [Google Scholar]
- 7.Fink K, Dooley DJ, Meder WP, Suman-Chauhan N, Duffy S, Clusmann H, Gothert M. Inhibition of neuronal Ca(2+) influx by gabapentin and pregabalin in the human neocortex. Neuropharmacology. 2002;42:229–36. doi: 10.1016/s0028-3908(01)00172-1. [DOI] [PubMed] [Google Scholar]
- 8.Le BD, Gozariu M, Cadden SW. Animal models of nociception. Pharmacol Rev. 2001;53:597–652. [PubMed] [Google Scholar]
- 9.Cohen AF, Ashby L, Crowley D, Land G, Peck AW, Miller AA. Lamotrigine (BW430C), a potential anticonvulsant. Effects on the central nervous system in comparison with phenytoin and diazepam. Br J Clin Pharmacol. 1985;20:619–29. doi: 10.1111/j.1365-2125.1985.tb05120.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Saletu B, Grunberger J, Anderer P, Linzmayer L, Konig P. On the cerebro-protective effects of caroverine, a calcium-channel blocker and antiglutamatergic drug: double-blind, placebo-controlled, EEG mapping and psychometric studies under hypoxia. Br J Clin Pharmacol. 1996;41:89–99. doi: 10.1111/j.1365-2125.1996.tb00165.x. [DOI] [PubMed] [Google Scholar]
- 11.Akin M. Comparison of wavelet transform and FFT methods in the analysis of EEG signals. J Med Syst. 2002;26:241–7. doi: 10.1023/a:1015075101937. [DOI] [PubMed] [Google Scholar]
- 12.Vapnik VN. An overview of statistical learning theory. IEEE Trans Neural Netw. 1999;10:988–99. doi: 10.1109/72.788640. [DOI] [PubMed] [Google Scholar]
- 13.Lee Y. Support vector machines for classification: a statistical portrait. Methods Mol Biol. 2010;620:347–68. doi: 10.1007/978-1-60761-580-4_11. [DOI] [PubMed] [Google Scholar]
- 14.Layer P, Yamamoto H, Kalthoff L, Clain JE, Bakken LJ, DiMagno EP. The different courses of early- and late-onset idiopathic and alcoholic chronic pancreatitis. Gastroenterology. 1994;107:1481–7. doi: 10.1016/0016-5085(94)90553-3. [DOI] [PubMed] [Google Scholar]
- 15.Castel LD, Abernethy AP, Li Y, Depuy V, Saville BR, Hartmann KE. Hazards for pain severity and pain interference with daily living, with exploration of brief pain inventory cutpoints, among women with metastatic breast cancer. J Pain Symptom Manage. 2007;34:380–92. doi: 10.1016/j.jpainsymman.2006.12.007. [DOI] [PubMed] [Google Scholar]
- 16.Jensen MP, Turner JA, Romano JM, Fisher LD. Comparative reliability and validity of chronic pain intensity measures. Pain. 1999;83:157–62. doi: 10.1016/s0304-3959(99)00101-3. [DOI] [PubMed] [Google Scholar]
- 17.Fayers PM. Interpreting quality of life data: population-based reference data for the EORTC QLQ-C30. Eur J Cancer. 2001;37:1331–4. doi: 10.1016/s0959-8049(01)00127-7. [DOI] [PubMed] [Google Scholar]
- 18.Fitzsimmons D, Kahl S, Butturini G, van Wyk M, Bornman P, Bassi C, Malfertheiner P, George SL, Johnson CD. Symptoms and quality of life in chronic pancreatitis assessed by structured interview and the EORTC QLQ-C30 and QLQ-PAN26. Am J Gastroenterol. 2005;100:918–26. doi: 10.1111/j.1572-0241.2005.40859.x. [DOI] [PubMed] [Google Scholar]
- 19.Knott VJ. Quantitative EEG methods and measures in human psychopharmacological research. Hum Psychopharmacol. 2000;15:479–98. doi: 10.1002/1099-1077(200010)15:7<479::AID-HUP206>3.0.CO;2-5. [DOI] [PubMed] [Google Scholar]
- 20.Landolt HP, Retey JV, Tonz K, Gottselig JM, Khatami R, Buckelmuller I, Achermann P. Caffeine attenuates waking and sleep electroencephalographic markers of sleep homeostasis in humans. Neuropsychopharmacology. 2004;29:1933–9. doi: 10.1038/sj.npp.1300526. [DOI] [PubMed] [Google Scholar]
- 21.Samar VJ, Bopardikar A, Rao R, Swartz K. Wavelet analysis of neuroelectric waveforms: a conceptual tutorial. Brain Lang. 1999;66:7–60. doi: 10.1006/brln.1998.2024. [DOI] [PubMed] [Google Scholar]
- 22.Storti SF, Formaggio E, Beltramello A, Fiaschi A, Manganotti P. Wavelet analysis as a tool for investigating movement-related cortical oscillations in EEG-fMRI coregistration. Brain Topogr. 2010;23:46–57. doi: 10.1007/s10548-009-0117-2. [DOI] [PubMed] [Google Scholar]
- 23.Digiacomo MR, Marco-Pallares J, Flores AB, Gomez CM. Wavelet analysis of the EEG during the neurocognitive evaluation of invalidly cued targets. Brain Res. 2008;1234:94–103. doi: 10.1016/j.brainres.2008.07.072. [DOI] [PubMed] [Google Scholar]
- 24.Tallon-Baudry C, Bertrand O, Delpuech C, Permier J. Oscillatory gamma-band (30–70 Hz) activity induced by a visual search task in humans. J Neurosci. 1997;17:722–34. doi: 10.1523/JNEUROSCI.17-02-00722.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Baliki MN, Geha PY, Apkarian AV. Spontaneous pain and brain activity in neuropathic pain: functional MRI and pharmacologic functional MRI studies. Curr Pain Headache Rep. 2007;11:171–7. doi: 10.1007/s11916-007-0187-3. [DOI] [PubMed] [Google Scholar]
- 26.Sarnthein J, Stern J, Aufenberg C, Rousson V, Jeanmonod D. Increased EEG power and slowed dominant frequency in patients with neurogenic pain. Brain. 2006;129:55–64. doi: 10.1093/brain/awh631. [DOI] [PubMed] [Google Scholar]
- 27.Versavel M, Leonard JP, Herrmann WM. Standard operating procedure (SOP) for the registration and computer-supported evaluation of pharmaco-EEG data. Working Team ‘EEG in Phase I’ of CIPS. Pharmacopsychiatry. 1995;28:245–8. doi: 10.1055/s-2007-979610. [DOI] [PubMed] [Google Scholar]
- 28.Versavel M, Leonard JP, Herrmann WM. Standard operating procedure for the registration and computer-supported evaluation of pharmaco-EEG data. ‘EEG in Phase I’ of the Collegium Internationale Psychiatriae Scalarum (CIPS) Neuropsychobiology. 1995;32:166–70. doi: 10.1159/000119230. [DOI] [PubMed] [Google Scholar]
- 29.Akay M, Akay YM, Cheng P, Szeto HH. Time-frequency analysis of the electrocortical activity during maturation using wavelet transform. Biol Cybern. 1994;71:169–76. doi: 10.1007/BF00197320. [DOI] [PubMed] [Google Scholar]
- 30.Schiff SJ, Aldroubi A, Unser M, Sato S. Fast wavelet transformation of EEG. Electroencephalogr Clin Neurophysiol. 1994;91:442–55. doi: 10.1016/0013-4694(94)90165-1. [DOI] [PubMed] [Google Scholar]
- 31.Khodayari-Rostamabad A, Reilly JP, Hasey G, Debruin H, Maccrimmon D. Using pre-treatment EEG data to predict response to SSRI treatment for MDD. Conf. Proc. IEEE Eng Med. Biol. Soc. 2010;1:6103–6. doi: 10.1109/IEMBS.2010.5627823. [DOI] [PubMed] [Google Scholar]
- 32.van der Post JP, de Visser SJ, de Kam ML, Woelfler M, Hilt DC, Vornov J, Burak ES, Bortey E, Slusher BS, Limsakun T, Cohen AF, van Gerven JM. The central nervous system effects, pharmacokinetics and safety of the NAALADase-inhibitor GPI 5693. Br J Clin Pharmacol. 2005;60:128–36. doi: 10.1111/j.1365-2125.2005.02396.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Drewes AM, Krarup AL, Detlefsen S, Malmstrom ML, Dimcevski G, Funch-Jensen P. Pain in chronic pancreatitis: the role of neuropathic pain mechanisms. Gut. 2008;57:1616–27. doi: 10.1136/gut.2007.146621. [DOI] [PubMed] [Google Scholar]
- 34.Lazarewicz MT, Ehrlichman RS, Maxwell CR, Gandal MJ, Finkel LH, Siegel SJ. Ketamine modulates theta and gamma oscillations. J Cogn Neurosci. 2010;22:1452–64. doi: 10.1162/jocn.2009.21305. [DOI] [PubMed] [Google Scholar]
- 35.Willert RP, Woolf CJ, Hobson AR, Delaney C, Thompson DG, Aziz Q. The development and maintenance of human visceral pain hypersensitivity is dependent on the N-methyl-D-aspartate receptor. Gastroenterology. 2004;126:683–92. doi: 10.1053/j.gastro.2003.11.047. [DOI] [PubMed] [Google Scholar]
- 36.D'Mello R, Dickenson AH. Spinal cord mechanisms of pain. Br J Anaesth. 2008;101:8–16. doi: 10.1093/bja/aen088. [DOI] [PubMed] [Google Scholar]
- 37.Quante M, Scharein E, Zimmermann R, Langer-Brauburger B, Bromm B. Dissociation of morphine analgesia and sedation evaluated by EEG measures in healthy volunteers. Arzneimittelforschung. 2004;54:143–51. doi: 10.1055/s-0031-1296951. [DOI] [PubMed] [Google Scholar]
- 38.Olesen SS, Hansen TM, Graversen C, Steimle K, Wilder-Smith OH, Drewes AM. Slowed EEG rhythmicity in patients with chronic pancreatitis: evidence of abnormal cerebral pain processing? Eur J Gastroenterol Hepatol. 2011;23:418–24. doi: 10.1097/MEG.0b013e3283457b09. [DOI] [PubMed] [Google Scholar]
- 39.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing analgesic actions of opioids by experimental pain models in healthy volunteers – an updated review. Br J Clin Pharmacol. 2009;68:149–68. doi: 10.1111/j.1365-2125.2009.03456.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40.Staahl C, Olesen AE, Andresen T, Arendt-Nielsen L, Drewes AM. Assessing efficacy of non-opioid analgesics in experimental pain models in healthy volunteers: an updated review. Br J Clin Pharmacol. 2009;68:322–41. doi: 10.1111/j.1365-2125.2009.03433.x. [DOI] [PMC free article] [PubMed] [Google Scholar]


